Abstract
Introduction
Single-agent immune checkpoint inhibitors (ICIs) like pembrolizumab or atezolizumab have been approved as first-line monotherapy for advanced non-small cell lung cancer (NSCLC) patients with PD-L1 ≥ 50%. However, emerging evidences have showed that ICI combinations (chemoimmunotherapy or dual-agent ICIs) argue to offer a higher response rate. In this network meta-analysis, we aimed to evaluate the efficacy and toxicity of first-line single-agent ICIs versus ICI combinations for advanced NSCLC patients with PD-L1 ≥ 50%.
Methods
PubMed, Embase, Cochrane Library and the Clinicaltrials.gov were systematically searched to extract eligible literature until December 2020. Outcomes included overall survival (OS), progression free survival (PFS), objective response rate (ORR) and treatment related adverse events (TRAEs) of grades 3–5.
Results
Fourteen studies with 3448 patients were included. The results showed that chemotherapy plus ICIs significantly improved PFS and ORR compared to chemotherapy, and sinti-chemo (HR: 0.31, 95% CI: 0.20–0.49) and pembro-chemo (OR: 4.2, 95% CI: 2.6–6.7) ranked first. In terms of OS, cemiplimab provided the best benefit versus chemotherapy (HR: 0.57, 95% CI: 0.43–0.77), followed by atezolizumab and pembro-chemo. In the subgroup analysis of histological type, pembro-chemo and sinti-chemo showed the best benefit of PFS in squamous and nonsquamous NSCLC, respectively, while there was no significant difference between ICI combinations with single-agent ICIs in OS. Moreover, the addition of chemotherapy to ICIs elevated toxicity compared to chemotherapy.
Conclusion
The study suggested that chemotherapy plus ICIs might improve PFS and ORR than single-agent ICIs for advanced NSCLC patients with PD-L1 ≥ 50%. However, it did not lead to OS benefit.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00262-021-03089-x.
Keywords: Non-small cell lung cancer, Network meta-analysis, Immune checkpoint inhibitors, PD-(L)1 inhibitors, PD-L1 high expression
Introduction
Lung cancer is the leading cause of cancer-related death in both men and women worldwide [1]. The choice of first-line treatment for advanced non-small cell lung cancer (NSCLC) depends on the presence of oncogene-driven mutations, such as mutations of epidermal growth factor receptor (EGFR) and translocations of anaplastic lymphoma kinase (ALK). However, only 10–20% of NSCLC patients have these actionable mutations [2, 3]. For the remaining patients, treatment options are limited to platinum-based cytotoxic chemotherapy with only moderate benefit and moderate-to-severe toxicities [4]. There exists a considerable unmet need for more efficacious and tolerable therapies for advanced non-oncogene-driven NSCLC.
In recent years, substantial progress has been made in the first- or second-line immunotherapy of advanced non-oncogene-driven NSCLC, especially immune checkpoint inhibitors (ICIs), such as programmed death-1 (PD-1) inhibitors, programmed death-ligand 1 (PD-L1) inhibitors, and cytotoxic T lymphocyte associated antigen-4 (CTLA-4) inhibitors [5–7]. Monotherapy is an appealing approach for patients with PD-L1 ≥ 50%. In the Keynote-024 study, pembrolizumab (anti-PD-1 antibody) single agent showed a superior median OS of 30 months compared to 14.2 months with chemotherapy [8]. Atezolizumab (anti-PD-L1 antibody) was also approved by the FDA for first-line treatment of metastatic NSCLC with PD-L1 ≥ 50% after the IMpower110 trial showed a median OS of 20.2 months for patients in the atezolizumab arm compared to 13.1 months in the chemotherapy arm [9]. More recently, dual-checkpoint blockade with ipilimumab (anti-CTLA-4 antibody) and nivolumab (anti-PD-1 antibody) has been shown to be superior to chemotherapy independent of PD-L1 status [10–12]. Moreover, chemotherapy plus ICIs (chemo-ICIs) has emerged as another first-line treatment option based on the results of recent trials demonstrating an OS benefit with chemo-ICIs over platinum-based doublet chemotherapy, regardless of PD-L1 expression [13–19]. Other chemo-ICIs trials similarly have reported promising preliminary survival data compared to platinum doublets [20, 21]. Based on the available data, both single-agent ICIs and ICI combinations (chemo-ICIs or dual-agent ICIs) appear to be efficacious in first-line treatments, as reflected in the current guideline recommendations [22]. However, in the absence of head-to-head trials comparing single-agent ICIs with ICI combinations, it is unclear which regimen is superior for advanced NSCLC patients with PD-L1 ≥ 50%.
Therefore, the objective of the current study was to evaluate the relative efficacy of first-line single-agent ICIs versus ICI combinations in advanced NSCLC patients with PD-L1 ≥ 50% by performing a systematic review and network meta-analysis (NMA).
Methods
The current NMA was performed based on the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline [23, 24]. The study protocol was prospectively registered with the National Institute for Health Research PROSPERO registration site (CRD42021232403).
Literature search
The initial literature search was conducted through PubMed, Embase, Cochrane Library and the Clinicaltrials.gov until December 2020. In addition, we performed an individual search of abstract listings from the annual meetings of the American Society of Clinical Oncology (ASCO), the European Society of Medical Oncology (ESMO) and the World Conference of Lung Cancer (WCLC) (2015–2020) to identify potentially relevant studies. Key search terms included “non-small cell lung cancer”, “immunotherapy”, “immune checkpoint inhibitors”, “PD-(L)1 inhibitor”, and “randomized clinical trial”. References from review articles, commentaries, included studies, and conference publications were hand searched and cross referenced to ensure a comprehensive search. Three reviewers (MFH, YP, and XYZ) independently carried out the literature retrieval.
Study selection
A study was considered acceptable according to the following inclusion criteria: (a) it represented a prospective phase 3 randomized trial that evaluated the efficacy of first-line ICIs or chemo-ICIs in the treatment of patients with advanced NSCLC; (b) reported outcomes of overall survival (OS), progression free survival (PFS), objective response rate (ORR) and treatment related adverse events (TRAEs) of grades 3–5; (c) English was the language of the publication. Studies failing to meet these criteria were excluded. When multiple articles describing the same trial were retrieved, the most recent or most complete publications were selected.
Two independent reviewers (BXT, XJ) performed an independent review of all of the obtained abstracts to assess their eligibility according to the inclusion criteria. Each trial that fulfilled the inclusion criteria was assessed for methodological quality using the Cochrane Collaboration tool [25]. All disagreements between reviewers were resolved by consensus.
Data extraction
The data on study identification, baseline characteristics, therapeutic regimen, number of patients, and clinical outcomes were retrieved and summarized separately by two reviewers (YSH, THZ). The preferred survival outcomes, including OS and PFS, were evaluated by independent review committees rather than investigators to reduce potential assessment bias. The TRAEs were assessed in the as-treated population, which included all patients who underwent randomization and received at least one dose of the assigned combination treatment. The original tests, supplementary materials and data in conference proceedings were evaluated to obtain the most extensive and updated data.
Statistical analysis
The primary efficacy outcomes of interest were OS and PFS, and the secondary outcomes were ORR and TRAEs of grades 3–5. The hazard ratio (HR) or odds ratio (OR) and their 95% confidence intervals (95% CIs) were used to measure outcomes and safety. For a specific comparison, an agent with an HR less than 1 for OS and PFS or an OR greater than 1 for ORR was deemed preferable, while an OR greater than 1 for TRAEs of grades 3–5 indicated a greater likelihood of toxic effects.
First, we performed a Bayesian network meta-analysis with R version 3.5.1 (R Project for Statistical Computing; gemtc package) with identical parameter settings. For each outcome measure, a fixed or random effects consistency model was used depending on the amount of heterogeneity observed, and analyses were performed using Markov chain Monte Carlo methods. The 95% CIs of either the pooled HR or OR excluding 1 or a 2-sided P < 0.05 were considered statistically significant. Second, we performed a pairwise meta-analysis on indirect comparisons and subgroup analysis based on histological type. Moreover, the Bayesian approach also provided overall ranking probabilities for each treatment, making it possible to rank each outcome measurement from the best to the worst, and were then visualized by calculating the surface under the cumulative ranking curves (SUCRAs) based on the ranking profiles [26, 27].
We considered the distribution that might affect outcomes to be similar in all of the pairwise comparisons according to the transitivity assumption, and Node-Splitting analysis was used to evaluate the inconsistency within the multiple treatment comparison. A P < 0.05 was considered significant inconsistency [28]. Statistical heterogeneity was assessed using the Q test and the statistical inconsistency index (I2). An I2 value > 50% is generally considered to indicate a substantial level of heterogeneity, which consequently initiates sensitivity analysis to identify the source [29].
Egger’s regression test with a funnel plot was used to assess the publication bias, and a P-value of < 0.10 was considered to indicate significant asymmetry and publication bias.
Results
Study selection
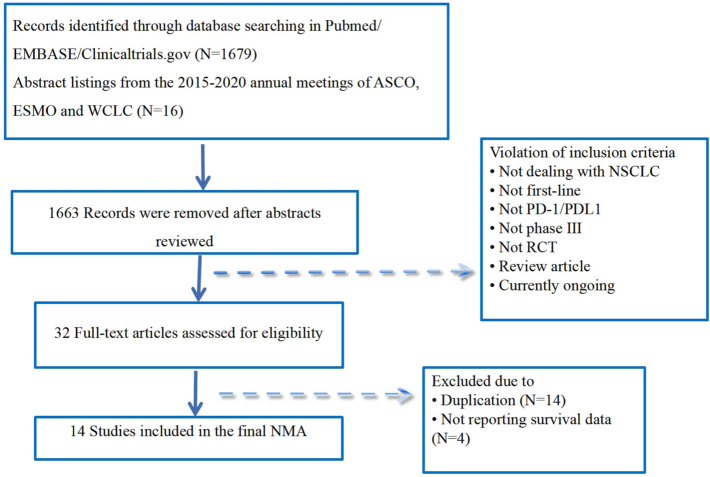
The literature search identified 1695 unique references. After a full-text review of 32 articles, we finally included 14 trials (Fig. 1), which included 3448 patients for advanced NSCLC with PD-L1 ≥ 50%. There were 4 trials of the Keynote series, 2 trials compared pembrolizumab (pembro) with chemotherapy (chemo) [8, 30–32] and 2 trials compared pembrolizumab plus chemotherapy (pembro-chemo) with chemotherapy [13–15]. There were 4 trials of the IMpower series, 1 trial compared atezolizumab (atezo) with chemotherapy [9] and 3 trials compared atezolizumab plus chemotherapy (atezo-chemo) with chemotherapy [17–19]. One trial compared nivolumab with chemotherapy [33], and 1 trial compared the combination of ipilimumab and nivolumab (nivo-ipi) with chemotherapy [10, 34]. One trial compared the combination of durvalumab plus tremelimumab (durva-treme) with chemotherapy or durvalumab (durva) with chemotherapy [35]. One trial compared cemiplimab with chemotherapy [36]. One trial compared sintilimab plus chemotherapy (sinti-chemo) with chemotherapy [20], and 1 trial compared camrelizumab plus chemotherapy (camre-chemo) with chemotherapy [21]. Detailed information on all the included studies is presented in Table 1 and Supplementary Table 1.
Fig. 1.
Flow diagram for study review and inclusion
Table 1.
Characteristics and main outcomes of the studies included in the meta-analysis
| Study | Histology | Treatment characteristics | No. of total patient | TRAEs 3–5 No./total no | No. of patient with PD-L1 ≥ 50%/total no. (%) | No. of squamous/nonsquamous | No. of ORR (%) | PFS HR (95% CI) | OS HR (95% CI) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Squamous | Non squamous | Squamous | Non squamous | ||||||||
| Keynote024 [8, 30] | NSCLC | Pembrolizumab | 154 | 41/154 | 154 (100) | 29/125 | 69 (44.8) | 0.50(0.39–0.65) | 0.62(0.48–0.81) | ||
| Chemotherapy | 151 | 80/150 | 151 (100) | 27/124 | 42 (27.8) | 0.45 (0.26–0.77) | 0.55 (0.39–0.76) | 0.73 (0.38–1.39) | 0.58 (0.41–0.83) | ||
| Keynote042 [32] | NSCLC | Pembrolizumab | 637 | 113/636 | 299 (46.9) | NR | 118 (39.5) | 0.81 (0.67–0.99) | 0.69 (0.56–0.85) | ||
| Chemotherapy | 637 | 252/615 | 300 (47.1) | 96 (32.0) | |||||||
| Keynote407 [15] | Squamous NSCLC |
Pembrolizumab + Chemotheropy |
278 | 206/278 | 73 (26.3) | 73/0 | 44 (60.3) | 0.37 (0.24–0.58) | 0.79 (0.52–1.21) | ||
| Chemotherapy | 281 | 195/280 | 73 (26.0) | 73/0 | 24 (32.9) | ||||||
| Keynote189 [13, 14] | Non squamous NSCLC |
Pembrolizumab + Chemotherapy |
410 | 291/405 | 132 (32.2) | 0/132 | 81 (61.4) | 0.36 (0.25–0.52) | 0.42 (0.26–0.68) | ||
| Chemotherapy | 206 | 135/202 | 70 (34.0) | 0/70 | 16 (22.9) | ||||||
| IMpower110 [9] | NSCLC | Atezolizumab | 277 | 97/286 | 107 (38.6) | NR | 41 (38.3) | 0.63 (0.45–0.88) | 0.59 ( 0.40–0.89) | ||
| Chemotherapy | 277 | 149/263 | 98 (35.4) | 28 (28.6) | |||||||
| IMpower130 [17] | Non squamous NSCLC |
Atezolizumab + Chemotherapy |
451 | 354/473 | 88 (19.5) | 0/88 | NR | 0.51 (0.34–0.77) | 0.84 (0.51–1.39) | ||
| Chemotherapy | 228 | 141/232 | 42 (18.4) | 0/42 | |||||||
| IMpower131 [18] | Squamous NSCLC |
Atezolizumab + Chemotherapy |
343 | 231/334 | 47 (13.7) | 47/0 | 29 (61.7) | 0.41 (0.25–0.68) | 0.48 (0.29–0.81) | ||
| Chemotherapy | 340 | 195/334 | 44 (12.9) | 44/0 | 14 (31.8) | ||||||
| IMpower132 [19] | Non squamous NSCLC |
Atezolizumab + Chemotherapy |
292 | 202/291 | 25 (8.6) | 0/25 | 18 (72.0) | 0.46 (0.22–0.96) | 0.73 (0.31–1.73) | ||
| Chemotherapy | 286 | 161/274 | 20 (7.0) | 0/20 | 11 (55.0) | ||||||
| CheckMate 227 [10, 34] | NSCLC |
Nivolumab + Ipilimumab |
583 | 189/576 | 205 (35.2) | NR | 91 (44.4) | 0.62 (0.49–0.79) | 0.70 (0.55–0.90) | ||
| Chemotherapy | 583 | 205/570 | 192 (32.9) | 68 (35.4) | |||||||
| CheckMate 026 [33] | NSCLC | Nivolumab | 271 | 47/267 | 88 (32.5) | NR | 30 (34.1) | 1.07 (0.77–1.49) | 0.90 (0.63–1.29) | ||
| Chemotherapy | 270 | 131/263 | 126 (46.7) | 49 (38.9) | |||||||
| Mystic study [35] | NSCLC |
Durvalumab + Tremelimuma |
372 | 194/371 | 108 (29.0) | NR | NR | NR | 0.77 (0.56–1.07) | ||
| Chemotherapy | 372 | 166/352 | 107 (28.8) | ||||||||
| Durvalumab | 374 | 153/369 | 118 (31.6) | 0.76 (0.55–1.04) | |||||||
| Chemotherapy | 372 | 166/352 | 107 (28.8) | ||||||||
| EMPOWER-Lung 01 [36] | NSCLC | Cemiplimab | 356 | 132/355 | 283 (79.5) | 122/161 | 111 (39.2) | 0.54 (0.43–0.68) | 0.57 (0.42–0.77) | ||
| Chemotherapy | 354 | 166/342 | 280 (79.1) | 121/159 | 57 (20.4) | 0.48 (0.34–0.67) | 0.60 (0.44–0.81) | 0.48 (0.30–0.77) | 0.64 (0.43–0.96) | ||
| ORIENT 11 [20] | Non squamous NSCLC |
Sintilimab + Chemotherapy |
266 | 164/266 | 107 (40.2) | 0/107 | 73 (68.2) | 0.31 (0.20–0.49) | NR | ||
| Chemotherapy | 131 | 77/131 | 61 (46.6) | 0/61 | 24 (39.3) | ||||||
| CAMEL [21] | Non squamous NSCLC |
Camrelizumab + Chemotherapy |
205 | 141/205 | 30 (14.6) | 0/30 | NR | 0.39 (0.14–0.99) | NR | ||
| Chemotherapy | 207 | 98/207 | 20 (9.7) | 0/20 | |||||||
Abbreviations: 95% CI 95% confidence interval, HR hazard ratio, NR not reported, TRAEs treatment related adverse events, ORR overall response rate, OS overall survival, PFS progression free survival, PD-L, programmed death–ligand 1
Risk of bias
The studies were considered adequate for performing random sequence generation and allocation concealment as well as having a low risk of detection and reporting bias. Most studies were open-label trials, and two studies had incomplete outcome data (Supplementary Fig. 1). Egger’s regression test was carried out to determine publication bias, and a p-value of 0.25 was obtained, suggesting the absence of publication bias in the included studies (Supplementary Fig. 2).
Comparisons of OS, PFS and ORR
The evidence formed connected star-shaped network plots (Fig. 2). The NMA included 13 studies for OS, 12 studies for PFS, and 11 studies for ORR.
Fig. 2.
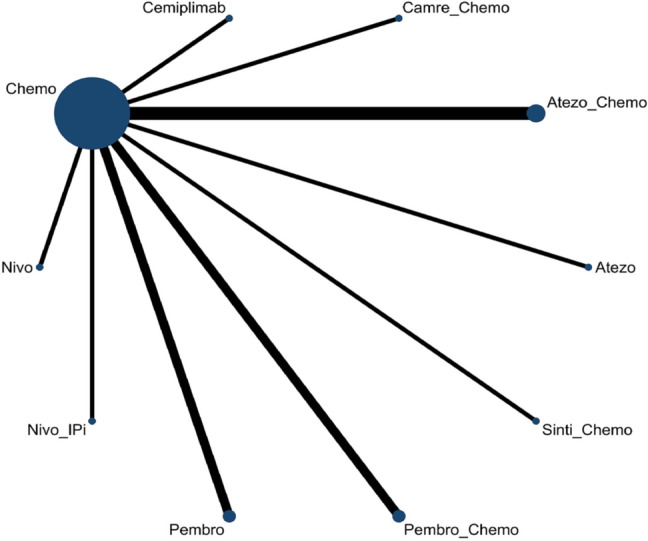
Network plot of multiple therapies in the first-line treatment of advanced NSCLC with PD-L1 ≥ 50%
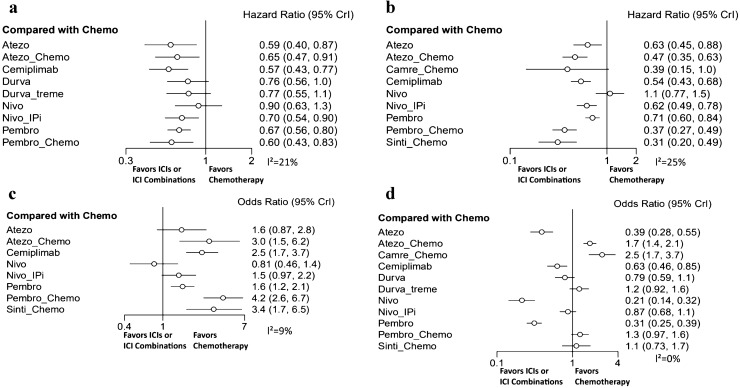
In terms of OS (Fig. 3a), except for durva-treme and nivolumab, ICIs and ICI combinations showed a significant OS benefit compared to standard chemotherapy. Cemiplimab provided the best OS benefit versus chemotherapy (HR: 0.57, 95% CI: 0.43–0.77), followed by atezolizumab and pembro-chemo. Similar efficacies were noted in atezo-chemo, pembrolizumab and nivo-ipi, with HRs of 0.65, 0.67 and 0.70, respectively. The efficacy of durvalumab showed a boundary significant relationship with chemotherapy (HR: 0.76, 95% CI: 0.56–1.0). In indirect comparisons, there were no significant differences among single-agent ICIs, chemo-ICIs and dual-agent ICIs (Supplementary Fig. 3a).
Fig. 3.
Forest plots for advanced non-small cell lung cancer patients with PD-L1 ≥ 50%. a Hazard ratio for overall survival; b hazard ratio for progression free survival; c response ratio for objective response rate; d risk ratio for TRAEs of grades 3–5
In terms of PFS (Fig. 3b), a significantly improved PFS was also observed in ICIs or ICI combinations compared to standard chemotherapy. Sinti-chemo yielded the best PFS benefit (HR: 0.31, 95% CI: 0.20–0.49), and pembro-chemo showed to be comparable to sinti-chemo in providing PFS benefit (HR: 0.37, 95% CI: 0.27–0.49). In addition, chemo-ICIs were more likely to obtain a greater PFS benefit than single-agent ICIs or dual-agent ICIs. However, the efficacy of camre-chemo showed a boundary significant difference (HR: 0.39, 95% CI: 0.15–1.0), and nivolumab was likely to show a worse effect than chemotherapy (HR: 1.1, 95% CI: 0.77–1.5). In indirect comparisons, sinti-chemo and pembro-chemo showed a significantly superior benefit compared to single-agent ICIs and nivo-ipi (Supplementary Fig. 3b).
In terms of ORR (Fig. 3c), pembro-chemo was observed to be the best treatment regarding the objective response (OR: 4.2, 95% CI: 2.6–6.7), which was followed by sinti-chemo and atezo-chemo. While atezolizumab and nivo-ipi did not show significant benefit in improving ORR over standard chemotherapy, and similar to PFS, nivolumab was likely to show a worse effect than chemotherapy (OR: 0.81, 95% CI: 0.46–1.4). In indirect comparisons, pembro-chemo showed a significantly superior benefit compared to single-agent ICIs and nivo-ipi (Supplementary Fig. 3c).
Safety and toxicity
All studies were included in the NMA for TRAEs of grades 3–5 (Fig. 3d). The toxicity was found to be lower for the single-agent ICIs across all treatments, and nivolumab (OR: 0.21, 95% CI: 0.14–0.32) was likely to be the lowest. The addition of chemotherapy to ICIs elevated toxicity compared to standard chemotherapy (Supplementary Fig. 3d). Pembro-chemo and sinti-chemo were associated with relatively fewer TRAEs of grades 3–5 than the other chemo-ICIs. No new safety signals were identified with the combinations. The TRAEs that were frequently reported for the ICI combinations included fatigue, vomiting, anorexia, neutropenia, anemia, diarrhea and constipation.
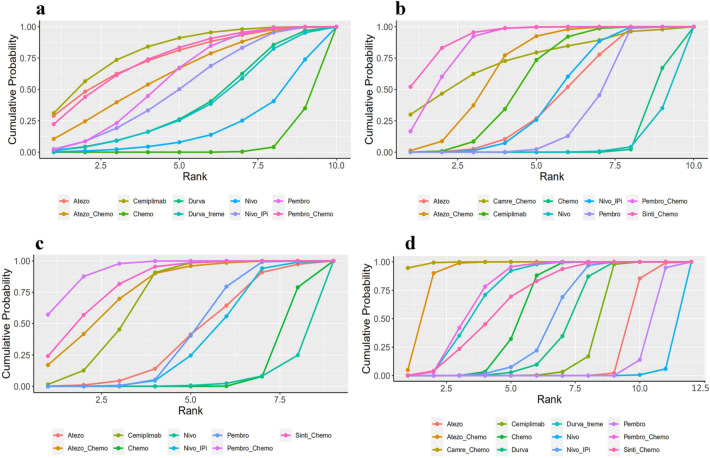
Rankings
The ranking profiles of comparable treatments indicated the probability of each regimen with the best outcomes and safety profiles. The ranking results were similar to those of the pooled analyses using HRs and ORs, implying the stability and reliability of the framework (Fig. 4). For advanced NSCLC patients with PD-L1 ≥ 50%, chemo-ICIs was more likely to improve PFS (Fig. 4b) and ORR (Fig. 4c), the ranking first was sinti-chemo (cumulative probability of 52.0%) and pembro-chemo (57.0%), respectively, followed by pembro-chemo, atezo-chemo for PFS, and sinti-chemo, atezo-chemo for ORR. In terms of OS (Fig. 4a), single-agent ICIs was likely to show superior benefit in improving OS than ICI combinations, and the ranking first was cemiplimab (31.1%), followed by atezolizumab and pembro-chemo. In terms of toxicity, camre-chemo (94.8%) displayed the highest probability of ranking first in causing TRAEs of grades 3–5, and nivolumab (94.3%) ranked last (Fig. 4d).
Fig. 4.
Cumulative ranking probability for different treatments. a Overall survival; b progression free survival; c objective response rate; d TRAEs of grades 3–5
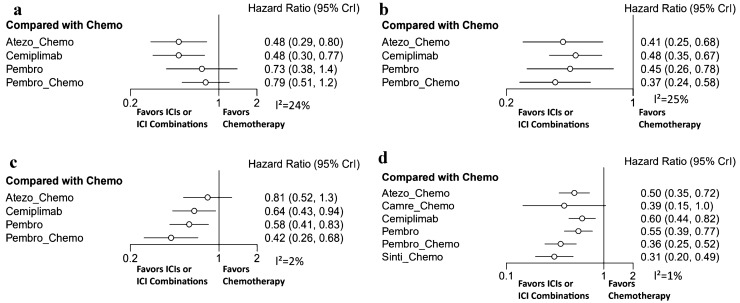
Subgroup analysis
We conducted a subgroup analysis based on histological type. Four studies reported the outcomes of OS and PFS in squamous NSCLC. Cemiplimab and atezo-chemo showed a significant benefit in improving OS compared to chemotherapy, and their HRs were both 0.48 (Fig. 5a). In terms of PFS, atezo-chemo, pembro-chemo, cemiplimab and pembrolizumab showed a significant benefit in improving PFS (Fig. 5b), and pembro-chemo was likely to be the best. In indirect comparisons, there was no significant difference between chemo-ICIs and single-agent ICIs in OS or PFS (Supplementary Fig. 4a–b).
Fig. 5.
Forest plots for subgroup. a Overall survival of squamous NSCLC; b progression free survival of squamous NSCLC; c overall survival of nonsquamous NSCLC; d progression free survival of nonsquamous NSCLC
Five studies reported the outcome of OS, and seven studies reported the outcome of PFS in nonsquamous NSCLC. Pembro-chemo, pembrolizumab and cemiplimab showed a significant benefit in improving OS compared to chemotherapy, and pembro-chemo was likely to show a better benefit (Fig. 5c). In terms of PFS, atezo-chemo, cemiplimab, pembrolizumab, pembro-chemo and sinti-chemo showed a significant benefit of improving PFS, except camre-chemo (Fig. 5d), and sinti-chemo was likely to be the best. In indirect comparisons, sinti-chemo showed a significant difference in PFS compared to single-agent ICIs, while there was no significant difference between chemo-ICIs and single-agent ICIs in OS (Supplementary Fig. 4c-d).
Heterogeneity, inconsistency, and transitivity assessment
Assessment of heterogeneity using the Q test and the I2 statistic also signified minimal (I2 = 0%) or low heterogeneity (I2 ≤ 25%) across the included trials (Fig. 3). The included studies did not form loops in the network and ultimately no inconsistency and coherence analyses were performed. The assumption of transitivity was accepted because no significant variability was identified in the study and population baselines (Supplementary Table 1).
Discussion
The study showed superior PFS and ORR with chemo-ICIs, with sinti-chemo and pembro-chemo ranking first, respectively. Studies have previously demonstrated a synergy between platinum-based chemotherapy and ICIs by modulating the immune response, such as increasing the potential for antigen cross-presentation by dendritic cells after the destruction of tumor cells, inhibiting myeloid-derived suppressor cells, increasing the ratio of cytotoxic lymphocytes to regulatory T cells, and blocking the STAT6 pathway to enhance dendritic cell activity [37–40]. However, the OS advantages were not observed in ICI combinations. Single-agent ICIs were likely to show a superior benefit in improving OS than ICI combinations. Cemiplimab ranked first, followed by atezolizumab and pembro-chemo, while in indirect comparisons, there were no significant differences among single-agent ICIs and ICI combinations. Importantly, the median follow-up period of OS reported for most studies was 8–13 months, thus making it almost impossible to obtain 5-year OS data. Moreover, 11 of 14 studies [8, 13, 15, 19–21, 32–36] allowed the chemotherapy arm to cross over to the immunotherapy arm after disease progression. And the duration of immunotherapy was different in each study, for example, immunotherapy was discontinued after 2 years in most studies, while it was discontinued after disease progression or unable to tolerate in IMpower studies. This limited data availability and cross-trial comparisons might affect the final results of OS.
In the subgroup analysis of histological type, the results showed that pembro-chemo and sinti-chemo were likely to have the best benefit of PFS in squamous and nonsquamous NSCLC, respectively. In terms of OS, cemiplimab and atezo-chemo showed a similar benefit in improving OS compared to chemotherapy in squamous NSCLC, and pembrolizumab, cemiplimab and pembro-chemo showed a better OS than chemotherapy in nonsquamous NSCLC. In indirect comparisons, there was no significant difference between chemo-ICIs and single-agent ICIs in OS, which were similar to upfront results. The higher response rate of chemo-ICIs therapy suggested that patients may benefit from it when suffering a rapidly progressive disease, such as an oncologic emergency, functional decline, or limiting additional therapy within 6 weeks [41, 42]. However, until direct prospective trial results are available, the decision to offer chemo-ICIs versus ICIs alone for PD-L1 high expression patients should be made on a case-by-case basis, taking attention to disease burden, functional status, comorbidities, and patient preference. A head-to-head comparison study (PERSEE, ClinicalTrials.gov identifier NCT04547504) is ongoing [43].
Moreover, the role of TMB as a predictive biomarker for anti-PD(L)1 therapy is still being determined [44, 45]. In the NMA, nivolumab single-agent treatment failed in advanced NSCLC patients with PD-L1 TPS ≥ 50%, and dual-agent ICIs (nivo-ipi or durva-treme) did not show a better advantage than chemo-ICIs. On the other hand, durva-treme combination showed clinical activity in patients with blood-based TMB (bTMB) ≥ 20 mut/Mb [35], and the nivo-ipi showed the greatest benefit in patients with a high TMB [10]. Emerging data have shown promising results for using bTMB as a predictive biomarker [46], but many of the challenges related to regulatory approval and variance among laboratories, in addition to unclear cutoffs for patient selection, currently limit the use of this approach in clinical practice. Therefore, further understanding of the role of the TMB as a biomarker is warranted before the integration of this factor into clinical practice [47].
Unlike previous meta-analyses investigating treatments of patients with advanced NSCLC [48, 49], our network meta-analysis compared more extensive therapy regimens and ranked efficacy and safety for each treatment. In the absence of head-to-head clinical trials, our study may help clinicians make better decisions from multiple promising treatment regimens for advanced NSCLC patients with PD-L1 ≥ 50%. The latest data available were considered for this NMA, including trials such as the EMPOWER Lung-01 [36] and the long-term follow-up of Keynote-024 [30], the results of which were recently presented. Moreover, we conducted subgroup analysis based on histological type to further assess the robustness of the results.
Finally, the current study also had several limitations. (1) Although we attempted an exhaustive literature search and only phase 3 trials were included, the influence of factors such as differences in ICIs and chemotherapy regimens could have introduced some intransitivity. (2) The PD-L1 assay methods and sensitivity were not consistent across all studies. A previous study showed that 22C3 and SP263 PD-L1 assays were highly concordant, whereas the SP142 assay was less sensitive for staining both tumor cells and tumor-infiltrating immune cells. In the clinical setting, the 22C3 and SP263 assays evaluate PD-L1 expression on tumor cells only, whereas the SP142 assay evaluates expression on both tumor cells and tumor-infiltrating immune cells [50, 51]. In IMpower studies [9, 16–19], although PD-L1 expression in tumor cells was used to reclassify patients into their corresponding TPS cohorts, we recognized the potential for the misclassification of some patients using this approach. (3) About 35% of trails included were less than 100 participants per group, especially the IMpower studies and Camel study, which may introduce bias due to small study effects. (4) Due to the use of the study-level data, the subgroup analysis based on histology were limited, and in the Keynote-024 study [8], the majority of patients in both groups had nonsquamous disease (82%). Moreover, we were unable to examine the impact of individual patient characteristics such as age, smoking status or the presence of liver or brain metastases on the efficacy outcomes. (5) Additionally, putative differences between PD-1 and PD-L1 inhibitors should be considered.
Conclusions
In the current NMA, it was found that the addition of chemotherapy to ICIs might improve PFS and ORR in advanced NSCLC patients with PD-L1 ≥ 50%. However, there was no OS benefit for chemo-ICIs compared to single-agent ICIs or dual-agent ICIs. In terms of PFS and ORR, pembro-chemo, sinti-chemo and atezo-chemo might be superior choices, while in terms of OS, cemiplimab, atezolizumab and pembro-chemo might be superior choices. However, further studies of head-to-head comparisons are required.
Supplementary Information
Below is the link to the electronic supplementary material.
Authors’ contributions
ZZY, MFH: protocol development; MFH and THZ: data analysis and manuscript writing; MFH, XYZ and YP: literature search; BXT and XJ: study selection; MFH, YSH, THZ: data extraction. All authors have read and agreed to the published version of the manuscript.
Funding
No specific funding was disclosed.
Data availability
The authors confirm that all data and material analyzed during this study are included in this article.
Declarations
Conflict of interest
The authors declare no conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71:7–33. doi: 10.3322/caac.21654. [DOI] [PubMed] [Google Scholar]
- 2.Planchard D, Popat S, Kerr K, Van Schil PE, Hellmann MD, Peters S, et al. Metastatic non-small cell lung cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2019;30:863–870. doi: 10.1093/annonc/mdy474. [DOI] [PubMed] [Google Scholar]
- 3.Abdel KN, Kelly K. role of targeted therapy and immune checkpoint blockers in advanced non-small cell lung cancer: a review. Oncologist. 2019;24:1270–1284. doi: 10.1634/theoncologist.2018-0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arbour KC, Riely GJ. Systemic therapy for locally advanced and metastatic non-small cell lung cancer: a review. JAMA. 2019;322:764–774. doi: 10.1001/jama.2019.11058. [DOI] [PubMed] [Google Scholar]
- 5.Chen R, Manochakian R, James L, Zhao Y, Zhou K, Lou Y, et al. Emerging therapeutic agents for advanced non-small cell lung cancer. J Hematol Oncol. 2020;13:58. doi: 10.1186/s13045-020-00881-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Verma V, Shrimali RK, Ahmad S, Mkrtichyan M, Gupta S, Khleif SN, et al. PD-1 blockade in subprimed CD8 cells induces dysfunctional PD-1CD38 cells and anti-PD-1 resistance. Nat Immunol. 2019;20:1231–1243. doi: 10.1038/s41590-019-0441-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu Y, Zheng P. Preserving the CTLA-4 Checkpoint for safer and more effective cancer immunotherapy. Trends Pharmacol Sci. 2020;41:4–12. doi: 10.1016/j.tips.2019.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reck M, Rodriguez-Abreu D, Robinson AG, Shentu Y, Rangwala R, Brahmer JR, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non–small-cell lung cancer. N Engl J Med. 2016;375:1823–1833. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 9.Herbst RS, Giaccone G, de Marinis F, Mocci S, Jassem J, Spigel DR, et al. Atezolizumab for first-line treatment of PD-L1-selected patients with NSCLC. N Engl J Med. 2020;383:1328–1339. doi: 10.1056/NEJMoa1917346. [DOI] [PubMed] [Google Scholar]
- 10.Hellmann MD, Paz-Ares L, Bernabe Caro RB, Kasinathan RS, Nathan FE, Ramalingam SS, et al. Nivolumab plus ipilimumab in advanced non-small-cell lung cancer. N Engl J Med. 2019;381:2020–2031. doi: 10.1056/NEJMoa1910231. [DOI] [PubMed] [Google Scholar]
- 11.Paz-Ares L, Ciuleanu TE, Cobo M, Oukessou A, Yan J, Reck M, et al. First-line nivolumab plus ipilimumab combined with two cycles of chemotherapy in patients with non-small-cell lung cancer (CheckMate 9LA): an international, randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22:198–211. doi: 10.1016/S1470-2045(20)30641-0. [DOI] [PubMed] [Google Scholar]
- 12.US Food and Drug Administration (2020) FDA approves nivolumab plus ipilimumab for first-line mNSCLC (PD-L1 tumor expression ≥1%). https:// www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-nivolumab-plus-ipilimumab-first-line-mnsclc-pd-l1-tumor-expression-1
- 13.Gandhi L, Rodriguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018;378:2078–2092. doi: 10.1056/NEJMoa1801005. [DOI] [PubMed] [Google Scholar]
- 14.Gadgeel S, Rodríguez-Abreu D, Speranza G, Esteban E, Felip E, Dómine M, et al. Updated analysis from KEYNOTE-189: pembrolizumab or placebo plus pemetrexed and platinum for previously untreated metastatic nonsquamous non-small-cell lung cancer. J Clin Oncol. 2020;38:1505–1517. doi: 10.1200/JCO.19.03136. [DOI] [PubMed] [Google Scholar]
- 15.Paz-Ares L, Luft A, Vicente D, Tafreshi A, Gümüş M, Mazières J, et al. Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med. 2018;379:2040–2051. doi: 10.1056/NEJMoa1810865. [DOI] [PubMed] [Google Scholar]
- 16.Socinski MA, Jotte RM, Cappuzzo F, Orlandi F, Stroyakovskiy D, Nogami N, et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med. 2018;378:2288–2301. doi: 10.1056/NEJMoa1716948. [DOI] [PubMed] [Google Scholar]
- 17.West H, McCleod M, Hussein M, Morabito A, Rittmeyer A, Conter HJ, et al. Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019;20:924–937. doi: 10.1016/S1470-2045(19)30167-6. [DOI] [PubMed] [Google Scholar]
- 18.Jotte R, Cappuzzo F, Vynnychenko I, Stroyakovskiy D, Rodríguez-Abreu D, Hussein M, et al. Atezolizumab in combination with carboplatin and Nab-Paclitaxel in advanced squamous NSCLC (IMpower131): results from a randomized phase III Trial. J Thorac Oncol. 2020;15:1351–1360. doi: 10.1016/j.jtho.2020.03.028. [DOI] [PubMed] [Google Scholar]
- 19.Papadimitrakopoulou V, Cobo M, Bordoni R, Dubray-Longeras P, Szalai Z, Ursol G, et al. OA05.07 IMpower132: PFS and safety results with 1L atezolizumab + carboplatin/cisplatin + pemetrexed in stage IV non-squamous NSCLC. J Thorac Oncol. 2018;13:S332–S333. doi: 10.1016/j.jtho.2018.08.262. [DOI] [Google Scholar]
- 20.Yang Y, Wang Z, Fang J, Yu Q, Han B, Cang S, et al. Efficacy and safety of sintilimab plus pemetrexed and platinum as first-line treatment for locally advanced or metastatic nonsquamous NSCLC: a randomized, double-blind, Phase 3 Study (Oncology pRogram by InnovENT anti-PD-1-11) J Thorac Oncol. 2020;15:1636–1646. doi: 10.1016/j.jtho.2020.07.014. [DOI] [PubMed] [Google Scholar]
- 21.Zhou C, Chen G, Huang Y, Zhou J, Lin L, Feng J, et al. Camrelizumab plus carboplatin and pemetrexed versus chemotherapy alone in chemotherapy-naive patients with advanced non-squamous non-small-cell lung cancer (CameL): a randomised, open-label, multicentre, phase 3 trial. Lancet Respir Med. 2021;9:305–314. doi: 10.1016/S2213-2600(20)30365-9. [DOI] [PubMed] [Google Scholar]
- 22.NCCN clinical practice guidelines in oncology, Version 5 (2021) Non-small cell lung cancer. https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1450
- 23.Moher D, Liberati A, Tetzlaff J, Altman DG (2009) PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 339:b2535. 10.1136/bmj.b2535 [PMC free article] [PubMed]
- 24.Phan K, Tian DH, Cao C, Black D, Yan TD. Systematic review and meta-analysis: techniques and a guide for the academic surgeon. Ann Cardiothorac Surg. 2015;4:112–122. doi: 10.3978/j.issn.2225-319X.2015.02.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Sterne JA (2011) Cochrane Bias Methods Group; Cochrane Statistical Methods Group. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 343: d5928. 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed]
- 26.Rucker G. Network meta-analysis, electrical networks and graph theory. Res Synth Methods. 2012;3:312–324. doi: 10.1002/jrsm.1058. [DOI] [PubMed] [Google Scholar]
- 27.Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol. 2011;64:163–171. doi: 10.1016/j.jclinepi.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 28.Van Valkenhoef G, Dias S, Ades AE, Welton NJ. Automated generation of node-splitting models for assessment of inconsistency in network meta-analysis. Res Syn Methods. 2016;7:80–93. doi: 10.1002/jrsm.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brahmer JR, Rodriguez-Abreu D, Robinson AG, Hui R, Csszi T, Fülp A et al (2020) LBA51 KEYNOTE-024 5-year OS update: First-line (1L) pembrolizumab (pembro) vs. platinum-based chemotherapy (chemo) in patients (pts) with metastatic NSCLC and PD-L1 tumour proportion score (TPS) ≥50%. Ann Oncol 31: S1181-S1182. https://www.sciencedirect.com/science/article/pii/S092375342042366X
- 31.Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, et al. Updated analysis of KEYNOTE-024: Pembrolizumab versus platinum-based chemotherapy for advanced non-small-cell lung cancer with PD-L1 tumor proportion score of 50% or greater. J Clin Oncol. 2019;37:537–546. doi: 10.1200/JCO.18.00149. [DOI] [PubMed] [Google Scholar]
- 32.Mok TSK, Wu YL, Kudaba I, Kowalski DM, Cho BC, Turna HZ, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet. 2019;393:1819–1830. doi: 10.1016/S0140-6736(18)32409-7. [DOI] [PubMed] [Google Scholar]
- 33.Carbone DP, Reck M, Paz-Ares L, Creelan B, Horn L, Steins M, et al. First-Line Nivolumab in Stage IV or recurrent non-small-cell lung cancer. N Engl J Med. 2017;376:2415–2426. doi: 10.1056/NEJMoa1613493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ramalingam SS, Ciuleanu TE, Pluzanski A, Lee JS, Schenker M, Caro RB et al (2021) OA03.03 Nivolumab (NIVO) + ipilimumab (IPI) Versus Platinum-Doublet Chemotherapy (Chemo) as first-line (1L) Treatment for advanced non-small cell lung cancer (aNSCLC): 3-year update from CheckMate 227 Part 1. J Thorac Oncol 16. https://www.sciencedirect.com/science/article/abs/pii/S1556086420308893
- 35.Rizvi NA, Cho BC, Reinmuth N, Lee KH, Luft A, Ahn MJ, et al. Durvalumab with or without tremelimumab vs standard chemotherapy in first-line treatment of metastatic non-small cell lung cancer: The MYSTIC Phase 3 randomized clinical trial. JAMA Oncol. 2020;6:661–674. doi: 10.1001/jamaoncol.2020.0237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sezer A, Kilickap S, Gümüş M, Bondarenko I, Özgüroğlu M, Gogishvili M, et al. Cemiplimab monotherapy for first-line treatment of advanced non-small-cell lung cancer with PD-L1 of at least 50%: a multicentre, open-label, global, phase 3, randomised, controlled trial. Lancet. 2021;397:592–604. doi: 10.1016/S0140-6736(21)00228-2. [DOI] [PubMed] [Google Scholar]
- 37.Bracci L, Schiavoni G, Sistigu A, Belardelli F. Immune-based mechanisms of cytotoxic chemotherapy: implications for the design of novel and rationale-based combined treatments against cancer. Cell Death Differ. 2014;21:15–25. doi: 10.1038/cdd.2013.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang Z, Till B, Gao Q. Chemotherapeutic agent-mediated elimination of myeloid-derived suppressor cells. Oncoimmunology. 2017;6:e1331807. doi: 10.1080/2162402X.2017.1331807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roselli M, Cereda V, di Bari MG, Formica V, Spila A, Jochems C, et al. Effects of conventional therapeutic interventions on the number and function of regulatory T cells. Oncoimmunology. 2013;2:e27025. doi: 10.4161/onci.27025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lesterhuis WJ, Punt CJ, Hato SV, Eleveld-Trancikova D, Jansen BJ, Nierkens S, et al. Platinum-based drugs disrupt STAT6- mediated suppression of immune responses against cancer in humans and mice. J Clin Invest. 2011;121:3100–3108. doi: 10.1172/JCI43656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lisberg A, Garon EB. Does platinum-based chemotherapy still have a role in first-line treatment of advanced non-small-cell lung cancer? J Clin Oncol. 2019;37:529–536. doi: 10.1200/JCO.18.01534. [DOI] [PubMed] [Google Scholar]
- 42.Goldberg SB, Herbst RS. Should chemotherapy plus immune check point inhibition be the standard front-line therapy for patients with metastatic non-small cell lung cancer? Cancer. 2018;124:4592–4596. doi: 10.1002/cncr.31681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.ClinicalTrials.gov (2020) PEmbRolizumab verSus chEmotherapy and pEmbrolizumab in Non-small-cell Lung Cancers (NSCLC) With PDL1 ≥ 50 % (PERSEE). https://clinicaltrials.gov/ct2/show/NCT04547504
- 44.Herbst RS, Lopes G, Kowalski DM, Nishio M, Wu YL, Junior G, et al (2019) Association between tissue TMB (tTMB) and clinical outcomes with pembrolizumab monotherapy (pembro) in PD-L1-positive advanced NSCLC in the KEYNOTE-010 and -042 trials. Ann Oncol 30: v916–v917. https://www.sciencedirect.com/science/article/pii/S0923753419604370
- 45.Peters S, Creelan B, Hellmann MD, Socinski MA, Carbone DP (2017) Abstract CT082: Impact of tumor mutation burden on the efficacy of first-line nivolumab in stage iv or recurrent non-small cell lung cancer: An exploratory analysis of CheckMate 026. Cancer Res 77:CT082–CT082. 10.1158/1538-7445.AM2017-CT082
- 46.Gandara DR, Paul SM, Kowanetz M, Schleifman E, Zou W, Li Y, et al. Blood-based tumor mutational burden as a predictor of clinical benefit in non-small-cell lung cancer patients treated with atezolizumab. Nat Med. 2018;24:1441–1448. doi: 10.1038/s41591-018-0134-3. [DOI] [PubMed] [Google Scholar]
- 47.Sholl LM, Hirsch FR, Hwang D, Botling FJ, Lopez-Rios FF, Lb H, et al. The promises and challenges of tumor mutation burden as an immunotherapy biomarker: a perspective from the International Association for the Study of Lung Cancer Pathology Committee. J Thorac Oncol. 2020;15:1409–1424. doi: 10.1016/j.jtho.2020.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim R, Keam B, Hahn S, Ock CY, Kim M, Kim TM, et al. First-line pembrolizumab versus pembrolizumab plus chemotherapy versus chemotherapy alone in non-small-cell lung cancer: a systematic review and network meta-analysis. Clin Lung Cancer. 2019;20:331–338. doi: 10.1016/j.cllc.2019.05.009. [DOI] [PubMed] [Google Scholar]
- 49.Addeo A, Banna GL, Metro G, Di Maio M. chemotherapy in combination with immune checkpoint inhibitors for the first-line treatment of patients with advanced non-small cell lung cancer: a systematic review and literature-based meta-analysis. Front Oncol. 2019;9:264. doi: 10.3389/fonc.2019.00264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hirsch FR, McElhinny A, Stanforth D, Ranger-Moore J, Jansson M, Kulangara, et al. PD-L1 immunohistochemistry assays for lung cancer: results from phase 1 of the Blueprint PD-L1 IHC assay comparison project. J Thorac Oncol. 2017;12:208–222. doi: 10.1016/j.jtho.2016.11.2228. [DOI] [PubMed] [Google Scholar]
- 51.Vennapusa B, Baker B, Kowanetz M, Boone J, Menzl I, Bruey JM, et al. Development of a PD-L1 complementary diagnostic immunohistochemistry assay (SP142) for atezolizumab. Appl Immunohistochem Mol Morphol. 2019;27:92–100. doi: 10.1097/PAI.0000000000000594. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors confirm that all data and material analyzed during this study are included in this article.