Abstract
Heparanase has been identified as a universal tumor-associated antigen, but heparanase epitope peptides are difficult to recognize. Therefore, it is necessary to explore novel strategies to ensure efficient delivery to antigen-presenting cells. Here, we established a novel immunotherapy model targeting antigens to dendritic cell (DC) receptors using a combination of heparanase CD4+ and CD8+ T-cell epitope peptides to achieve an efficient cytotoxic T-cell response, which was associated with strong activation of DCs. First, pegylated poly(lactic-coglycolic acid) (PLGA) nanoparticles (NPs) were used to encapsulate a combined heparanase CD4+ and CD8+ T-cell epitope alone or in combination with Toll-like receptor 3 and 7 ligands as a model antigen to enhance immunogenicity. The ligands were then targeted to DC cell-surface molecules using a DEC-205 antibody. The binding and internalization of these PLGA NPs and the activation of DCs, the T-cell response and the tumor-killing effect were assessed. The results showed that PLGA NPs encapsulating epitope peptides (mHpa399 + mHpa519) could be targeted to and internalized by DCs more efficiently, stimulating higher levels of IL-12 production, T-cell proliferation and IFN-γ production by T cells in vitro. Moreover, vaccination with DEC-205-targeted PLGA NPs encapsulating combined epitope peptides exhibited higher tumor-killing efficacy both in vitro and in vivo. In conclusion, delivery of PLGA NP vaccines targeting DEC-205 based on heparanase CD4+ and CD8+ T-cell epitopes are suitable immunogens for antitumor immunotherapy and have promising potential for clinical applications.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00262-022-03209-1.
Keywords: Heparanase, Tumor immunotherapy, Dendritic cells, Targeting, Nanoparticles
Introduction
Cancer immunotherapy aims to activate and expand cancer-specific immune cells to eliminate cancer cells by recognizing antigen targets expressed on cancer cells and is considered an effective treatment. The host immune system, consisting of innate and adaptive immunity, plays an essential role in immunosurveillance and the recognition and destruction of cancer cells [1, 2]. To develop tumor-specific immunity for cancer therapy, it is extremely important to identify cancer antigens that are recognized by immune cells. Many tumor-associated antigens (TAAs) have been identified using tumor-reactive T cells [3–6]. However, due to the expression patterns of many TAAs, most of them are not suitable for immunotherapy due to potential toxicities associated with self-antigens or cross-reactivity with unanticipated antigens in essential tissues. An ideal TAA should have the following three characteristics to elicit effective and safe T-cell-mediated antitumor immunity in cancer patients: (1) cancer-specific expression of the TAA. An ideal TAA must be frequently overexpressed in cancer tissues but must not be expressed in normal tissues: (2) oncogenic characteristics. It is well known that tumors escape host antitumor immunity through multiple mechanisms, such as the loss of HLA class I molecule or TAA expression. With respect to losing TAA expression, TAAs involved in oncogenesis are mostly lost during the process of tumor progression: (3) immunogenicity. This characteristic requires that TAAs have the capacity to induce an immune response in cancer patients [7]. Therefore, it is critical to identify suitable and universal TAAs for tumor immunotherapy.
Heparanase (Hpa) is an endogenous endoglycosidase that degrades heparan sulfate proteoglycans, thereby remodeling the extracellular matrix. Heparanase promotes tumor metastasis by destroying the extracellular matrix and basement membrane, playing a key role in tumor progression [8–10]. Previous studies have consistently indicated that the level of heparanase expression in cancer is positively correlated with malignant potential, tumor volume increase, enhanced metastasis, and poor prognosis [11–13]. Heparanase is universally overexpressed in a wide variety of cancers, which may facilitate both the tumor cell invasion and neovascularization that are critical for cancer progression. Our previous studies demonstrated that heparanase may represent a novel H-2Kb-restricted cytotoxic T lymphocytes (CTL) epitope capable of inducing heparanase-specific CTLs suitable for tumor immunotherapy, having the advantages of being broad spectrum, highly effective, highly specific, and safe [14, 15].
CTLs are considered the chief mediators of tumor immunosurveillance through the recognition of TAAs as cognate peptides bound to MHC molecules expressed on the surface of tumor cells. CTL epitopes comprising 8 to 10 amino acids bind to MHC to induce CTL activation [16]. We previously predicted and identified 3 HLA-A2-restricted CTL epitopes, Hpa277 (277–285, KMLKSFLKA), Hpa405 (405–413, WLSLLFKKL) and hHpa525 (525–533, PAFSYSFFV), in humans and 2 H-2Kb-restricted CTL epitopes, mHpa398 (398–405, LSLLFKKL) and mHpa519 (519–526, FSYGFFVI), in mice. These heparanase epitopes induce heparanase-specific CTLs to lyse various tumor cells [14, 17, 18]. To overcome the weakness of small peptide vaccines, including their low molecular weight, single structure, weak immunogenicity, and rapid degradation, we designed a multiple antigenic peptide (MAP) based on HLA-A2-restricted CTL epitopes [15].
Given the importance of CD4+ T cells in antitumor immunity, many MHC class II-restricted tumor antigens capable of stimulating CD4+ T helper (Th) cells have been identified [19]. Accumulating evidence indicates that the development of cancer-specific immunity requires antigen-presenting cell (APC) capture and antigenic peptides for CD8+ T-cell and CD4+ T-cell recognition [20]. Thus, it is important to specifically transport the antigen to APCs to activate CD8+ T cells and CD4+ T cells. Cell surface receptors are essential for the delivery of CD4+ T cells to CTLs to enhance their antitumor responses [21]. Among these receptors, Toll-like receptors (TLRs) are pattern recognition receptors that play a key role in the innate immune response. The engagement of TLRs in DCs is vital for the proliferation of antigen specific CD4+ and CD8+ T cells. DC-based vaccines primarily contain TNFα, IL-1β, IL-6, and prostaglandin E2 (PGE2), which produce very little IL-12p70 [22]. Therefore, it is necessary to develop new platforms that can activate effector T-cell responses against tumor-associated antigens.
Recently, most DC-based vaccines contain pathogen- and/or damage-associated molecular patterns (PAMPs and DAMPs), which bind to pattern recognition receptors (PRRs), such as TLRs expressed by DCs. TLR7 can be activated by several synthetic agonists, such as imiquimod and resiquimod (R848), which have been demonstrated to have immune response properties in terms of antiviral and antitumor activities. TLR3 can be activated by polvriboinsine–polyribocyaidylic acid [poly(I:C)], which is a synthetic analog of double-stranded RNA that induces the production of IL-12 and IFN-γ during the antitumor immune response [23]. More importantly, nanotechnology for antigen packing and delivery has become ubiquitous in tumor immunotherapy in recent years. By associating the antigen with a nanoparticulate carrier, the antigen is protected from degradation, which increases its half-life, stabilizes its biological activity, and increases its solubility [24–26]. Various delivery systems, including liposomes, poly(propylene) sulfide nanoparticles, gold nanoparticles, PLGA nanoparticles, exosomes, micelles and dendrimers, have achieved cytoplasmic delivery of exogenous antigens into DCs and enhanced immune responses [27]. Targeting DCs through their cell surface receptors, such as DC205, CD40 or CD11c, for the delivery of subunit vaccine components can increase the efficiency of cross-presentation and induce more potent CD8+ T cells [24].
In this study, we aimed to construct an improved DEC-205-targeting PLGA nanoparticle vaccine that encapsulates the heparanase CD4+ and CD8+ epitope peptides R848 and poly(I:C). Our study demonstrated the importance of targeting cell surface molecules on DCs, which enhanced the internalization of NPs by DCs and induced immune activation. The targeted PLGA NPs and nontargeted controls were evaluated using in vitro and in vivo tumor-killing assays. The results indicated that the DEC-205-targeted PLGA NP vaccine based on heparanase CD4+ and CD8+ T-cell epitope peptides is promising for tumor immunotherapy and has potential clinical application prospects.
Materials and methods
Mice, cell lines, reagents, and antibodies
C57BL/6(H-2Kb) mice were purchased from Army Medical University (Chongqing, PR China), housed in a specific pathogen-free environment, and were used at ages 8–12 weeks. Animal experiments were approved by the Ethics Committee of the Army Medical University. Murine-originated cell lines, including Lewis lung cancer cell line and B16 melanoma cell line, were purchased from American Type Culture Collection (ATCC, USA). Mouse primary aortic endothelial cells were purchased from Cell Biologics (Chicago, USA). All the cells were cultured in RPMI-1640 media containing 10% fetal calf serum (FCS), streptomycin (100 mg/mL), and penicillin (200 units/mL). Cells were kept at 37 °C at 5% CO2, and medium was refreshed every 2 days. Recombinant Mouse GM-CSF (catalog: 415-ML-020), recombinant Mouse IL-4 (catalog: 404-ML-025/CF), recombinant Mouse TNF-alpha (catalog: AFL410) were obtained from R&D Systems (USA). CFSE (catalog: 65-0850-84) was purchased from eBioscience (USA). Poly (dl-lactide-co-glycolide) polymer (PLGA) (50:50, approximately 50,000 g/mol) was purchased from Durect (Pelham, AL, USA). Methylene chloride and avidin were purchased from Thermo Fisher Scientific (Waltham, MA, USA) and Invitrogen (Carlsbad, CA, USA), respectively. FITC-anti-mouse CD11c (Catalog 11-0114-82), PerCP-anti-mouse CD4 (Catalog 45-0048-42), PE-anti-mouse CD8α (Catalog 12-0081-82), APC-anti-mouse CD3 (Catalog:17-0032-82) were purchased from eBioscience (USA). Anti-mouse DEC-205 monoclonal antibody (Clone: NLDC-145, Catalog: ABCA0054268), rat IgG2a isotype control (Clone: YTH71.3, Catalog: ABCA0064773), and mouse IgG2b isotype control (Clone: MPC-11, Catalog: BE0086) were purchased from Bio X Cell Antibody Production and Purification (USA). DEC-205 anti-body and isotype control antibodies were labeled with the Lightning-Link® Rapid DyLight® 680 antibody labeling kit (Innova Biosciences).
Lentiviral constructs and transfection
Mouse heparanase cDNA was subcloned in the sense direction into pIRES2-enhanced green fluorescent protein vectors. The heparanase overexpression plasmid or control plasmid was designed and synthesized by Cyagen (Cyagen Biosciences Inc., Guangzhou, China). Lentiviral constructs containing heparanase expression vector were obtained. Mouse primary aortic endothelial cells were cultured in DMEM media containing 10% fetal calf serum. The titer of lentivirus was determined with serial dilution method. Mouse primary aortic endothelial cells were seeded into 96-well plates, followed by addition of 1 × 108 TU/ml lentivirus (10 μl), 5 μg/ml polybrene (Sigma, St. Louis, MO, USA) and complete medium. Cells were subsequently selected for antibiotic resistance and observed under a fluorescence microscope to evaluate the transfection efficiency.
Epitope prediction and synthesizing
We used the NetMHCII 2.3 Server (http://www.cbs.dtu.dk/services/NetMHCII/) to predict the candidate CD4+ T cell epitopes from the mHpa antigen. The predicted CD4+ T cell epitope peptides mHpa399 (Hpa399-413, SLLFKKLVGPRVLLS), mHpa61 (Hpa61-75, PRFLTFLGSPRLRAL), mHpa171(Hpa171-185, CSGLDLIFGLNALLR), mHpa521 (Hpa521-535, YGFFVIRNAKIAACI) were synthesized by Shanghai C-Strong Co. Ltd (Shanghai, China) with a purity of > 90% (as determined by high-performance liquid chromatography and amino acid analysis). The CD8+ cell epitopes were previously identified mHpa519 (mHpa519-526, FSYGFFVI) and were also synthesized by Shanghai C-Strong Co. Ltd with a purity of > 90% (as determined by HPLC). An HLA-A0201-restricted epitope peptide (KMLKSFLKA) derived from human influenza virus was served as negative control peptide (NC), was synthesized by Shanghai C-Strong Co. Ltd with a purity of > 90% (NP-(NC)-aDec205). The molecular weight of the peptides was validated by mass spectrometry. Lyophilized peptides were stored at − 20 °C. The CD4+ T cell epitopes were screened by T cell proliferation assay; the results showed mHpa399 is the best one to induce CD4+ T cell proliferation in four candidate epitopes (Supplementary material).
Dendritic cell isolation and generation
Dendritic cells from mouse bone marrow (mDC) were isolated and generated according to the protocol described previously [14, 15]. In brief, bone marrow was flushed from the tibias and femurs of C57BL/6 mice and depleted of erythrocytes with commercial lysis buffer (Sigma). The cells were washed twice in serum free RPMI 1640 and cultured in a six-well plate at 1 × 105 cells per well with RPMI 1640 containing 10 ng/mL recombinant murine granulocyte macrophage colony stimulating factor (rmGM-CSF) and 10 ng/mL recombinant murine interleukin (IL)-4 (rmIL-4). On days 7 of culture, 2000U/mL recombinant murine TNF-α was added to the medium. On day 8, nonadherent cells obtained from these cultures were considered to be mature bone marrow-derived mDCs. The phenotypic markers of mDCs were confirmed by flow cytometry.
Preparation of PLGA-NP encapsulating epitope peptides and DEC-205 ligand
We used the water-in-oil-in-water double emulsion method to prepare the PLGA-(Hpa) nanoparticles as previously described [34]. 250 μl of CD4+ and CD8+ T cell epitope peptides-free endotoxin (1 mg/ml in PBS) and Poly I:C (4 mg) and R848 (1 mg), or 250 μl of CD8+ T cell epitope peptides and Poly I:C (4 mg) and R848 (1 mg), or negative control peptide and Poly I:C (4 mg) and R848 (1 mg) were emulsified with the prepared PLGA solution and sonicated (Jencons, VCX130, UK) at 70% amplitudes for 60 s, with one-pulse-per-second cycles in an ice-cold water bath. The emulsion was then added slowly and dropwise into 10 ml of 2% (w/v) polyvinyl alcohol (PVA), and the mixture was sonicated for 5 min as above. The double emulsion (w/o/w) was then added to 0.5% PVA to a final volume of 20 ml. The solvent was evaporated by magnetic stirring at 1000 rpm for 4 h at room temperature. Finally, the nanoparticles were centrifuged at 14,000g for 30 min at 4 °C and washed three times in PBS. Control NPs (blanks) were synthesized with negative control epitope replacing the heparanase epitope peptides. Labeled DEC-205 monoclonal antibody or isotype control antibody was added to PLGA NPs (10 μg/mg) in phosphate-buffered saline and rotated for 40 min at room temperature. The particles were then centrifuged at 6500 rpm for 5 min, washed twice in phosphate-buffered saline. A dynamic light scattering instrument (Nano-ZS, Malvern, UK) was used to determine the average size distribution, and zeta potential. The particle morphology was analyzed by transmission electron microscope (TEM) (JEM-2100, JEOL, Japan).
Quantifying encapsulated peptides, poly I:C, and R848
Biodegradable PLGA NP was hydrolyzed with 0.8 M NaOH overnight at 37 °C. The encapsulation of epitope peptides, poly I:C and R848 was analyzed by reversed-phase high-performance liquid chromatography-quadrupole-time-of flight mass spectrometry (RP-HPLC-QTOF/MS). The analysis was performed using an HPLC system 1100 (Agilent Technologies, Santa Clara, USA) coupled to a quadrupole-time-of flight mass spectrometer (QTOF/MS) Agilent 6530 equipped with an orthogonal electrospray ionization (ESI) source (Agilent Jet Steam, AJS). The HPLC instrument was equipped with an auto sampler, a quaternary solvent pump, and a column heater. Agilent Mass Hunter Workstation software B07.00 was using for HPLC and MS control, data acquisition, and data analysis. The separation was carried out using a porous-shell fused-core Ascentis Express C18 analytical column (150 mm × 2.1 mm, particle size 2.7 μm) with an Ascentis Express C18 guard column (0.5 cm × 2.1 mm, 2.7 μm particle size) (Supelco, USA). The mass spectrometer was operated in positive ion mode, and the mass range was from 100 to 1700 m/z. MS parameters were the following: capillary voltage, 3500 V; nebulizer pressure, 50 psig; drying gasflow rate, 12 L/min; gas temperature, 350 °C. The fragmentor voltage (cone voltage after capillary) was set at 80 V. The skimmer and octapole voltage were 60 V and 750 V, respectively. Source sheath gas temperature and flow were 400 °C and 12 L/min, respectively. MS/MS was performed employing the auto mode. The analyses were conducted in triplicate. Tandem MS/MS spectra were obtained for the molecular ion with the highest abundance. Every sample was injected in triplicate into the MS system. Data analysis was performed by de novo sequencing tool.
Analysis of the uptake of NP by DC
To assess the uptake of NPs using confocal microscopy, BMDCs were seeded into 35 mm glass-bottom dishes at a concentration of 1 × 105/mL. NP at a concentration of 20 μg/mL were added to the wells in triplicate and incubated at 37 °C for 3 h. Then, the DCs were stained by FITC-anti-mouse CD11c and DAPI. The cells were then washed twice by PBS. Uptake of NP in DCs was visualized examined with a Leica fluorescence microscope (Leica TCS-SP5). The images analyzed using Leica LAS AF Lite 2.6 software. WT C57BL/6 bone marrow dendritic cells (BMDC) (100,000/well) were plated into a 48-well flat bottom plate and incubated for 3 h at either 4 ℃ (binding analysis) or 37 °C (internalization analysis) with 5, 10 and 20 μg/mL of (mHpa519 + 399, or mHpa399, or NC)-DEC-205-targeted or non-targeted formulations, respectively. Cells were washed twice to remove residual non-bound NP. For internalization assay, the dendritic cells were washed by acidic PBS (pH = 2) to remove the bound but not internalized NP. Binding and internalization of NP by DC was determined on a BD FACsAria cytometer. The mean fluorescence intensity (MFI) was measured. The internalization ratio was calculated following the formula: %internalization = [(MFI in 37 °C − background)]/[(MFI on ice) − background] × 100%.
Analysis of DC activation using enzyme-linked immunosorbent assay (ELISA)
Dendritic cells (100,000/well) were plated into a 96-well round bottom plate and incubated for 24 h with titrated amounts of PLGA-NPs. Supernatants were harvested and tested for IL-12 p70 (BD OptEIA™ MOUSE IL-12), following manufacturer's instructions.
Analysis of T cell expansion and proliferation in vitro
Matured BMDCs obtained from the above-mentioned method were seed in 48-well plate (1 × 106/well), BMDCs were incubated for 4 h with the NP-(mHpa519 + 399, or mHpa519, or NC)-αDEC-205, or NP-(mHpa519)-isotype at different concentrations. Spleen cells were labeled at 107 cells/mL with 5 μM carboxyfluorescein diacetate succinimidyl ester (CFSE), and then, spleen cells were incubated with NPs pulsed dendritic cells for 6 days. Thereafter, T cells were stained with APC-anti-mouse CD3, PerCP-anti-mouse CD4 and PE-anti-mouse CD8, the cells were analyzed on a BD FACsAria cytometer, and data were analyzed by FlowJo software (FlowJo, Eugene, OR). The activated T cells were used for ELISPOT assay. The CD4+ or CD8+ T cells were sorted separately by flow cytometry. Briefly, CD4+ or CD8+ incubated by different concentration NP pulsed dendritic cells were added to ImmunoSpot plates (Dakewe, China) precoated with anti-IFN-γ monoclonal antibody, and the plates were incubated overnight at 37 °C in 5% humidity incubator. The wells were washed twice with deionized water and thrice with wash buffer (0.05% Tween 20 with PBS). The wells were then incubated with biotin-conjugated anti- IFN-γ mAb for 1 h at 37 °C. After thrice washes, streptavidin–alkaline phosphatase was added to each well and incubated for 1 h at room temperature. Wash the plate three times; 30 μL activator solution was added to develop spots. After 20 min, the plates were washed with distilled water to stop reaction. Experiments were performed in triplicate. The number of spots in each well was counted by the Bioreader 4000 PRO-X (Bio-Sys, Germany).
Cytotoxicity assays
Standard 4-h 51Cr-release assays were used to evaluate the ability of various PLGA-NPs-induced CTLs to lyse tumor cells. The experiment was conducted as we described previously [14, 17]. Briefly, target cells were labeled by 51Cr (PerkinElmer). Then, target cells were resuspended in RPMI-1640, 104 per well in 96-well plate; the effector cells were added to target cells at effector-to-target (E/T) ratios of 10:1, 20:1, 40:1, or 80:1. Target cells and effector cells were incubated for 4 h in 37 °C. After 4 h incubation, 100 mL supernatants of each well were harvested and detected by gamma counter (FM-1000 g counter). For mouse primary aortic endothelial cells, the cells were transfected with heparanase expression plasmid or control vectors, and then, the cells were labeled by 51Cr (PerkinElmer) and treated same as the above procedure. The specific lysis rate was calculated following the formula: %Specific killing = [(CPM test-background)]/[(CPM max killing-background)] * 100%.
Inhibition of tumor growth and metastasis assay
Inhibition of tumor growth assays was performed according to our previous research [14, 15, 17]. For protective vaccination, 8-week-old C57BL/6 mice were used and randomly divided into four groups, vaccinated by s.c. injection of PLGA-NPs encapsulating various epitope peptides or control NPs 3 times at 7-day intervals. Seven days after the last injection, 1 × 106 B16 cells in 200 μL PBS were inoculated into each mouse. Tumor growth was monitored with a digital caliper every 4 days, and tumor volume was calculated according to the formula V = L × S2/2, where L is the longest side and S is the shortest. On day 28 post-tumor induction, the animals were killed using humane methods. The tumors were obtained for further analysis at indicated days.
For tumor metastasis assay, mice were vaccinated as above. Lewis lung cancer cells (1 × 106 cells in 200 μL PBS) were injected through tail vein. On day 28 post-tumor injection, mice were killed, the lungs were filled with 2 mL of a 10% India Ink solution (Winsor & Newton, London, UK) in PBS, through the trachea with the help of a cannula. The trachea was blocked by surgical thread, and the lungs were extracted and washed 3 times with 10 mL of Fekete’s solution (85 mL 70% ethanol, 10 mL 10% paraformaldehyde, and 5 mL acetic acid), and the lungs were fixed in the same solution overnight. The macroscopic foci were counted and photographed with a stereoscopic microscope, OLYMPUS SZ CTV (Olympus, Tokyo, Japan).
Survival analysis
Mice were randomly divided into 4 groups, vaccinated by s.c. injection of PLGA-NPs encapsulating various epitope peptides or control NPs 3 times at 7-day intervals. Seven days after the last injection, 1 × 105 B16 cells in 200 mL PBS were s.c. or 1 × 105 Lewis lung cancer cells were i.v. injected. Survival rates of mice bearing tumors were observed over a 80-day period.
Statistical analysis
Prism 6.0 (GraphPad Prism) software was used for statistical analyses. Experimental data were shown as the mean ± S.E.M. One-way analysis of variance (ANOVA) followed by Tukey’s post hoc test was used to evaluate the statistical significance of differences among three or more groups. All data shown were representative results from at least three independent experiments. Significance was defined as p < 0.05.
Results
Design, preparation, and characterization of PLGA-NPs
PLGA-NP vaccines were prepared using the biodegradable polymer PLGA. Figure 1A shows a schematic diagram of the heparanase-targeted NP vaccine. The PLGA NP surface was encapsulated with a polyethylene glycol (PEG)-lipid layer to reduce nonspecific binding to cells and to coat the DC receptor-specific DEC-205 antibody to specifically target mouse DCs. The size distribution, polydispersity index and zeta potential of the PLGA NPs are shown in Table 1. The size of the PLGA NPs ranged from 198 ± 12.6 nm to 204 ± 10.2 nm. Figure 1B shows a representative image and spherical shape of the PLGA NPs. The imaging clearly shows the presence of the PEG-lipid layer on the PLGA NPs (Fig. 1B).
Fig. 1.
Schematic diagram of PLGA NP encapsulating heparanase epitope peptides targeting DEC-205 on mouse DCs. A PLGA NP vaccines were generated in combination with different heparanase epitope peptides as indicated. B Image analysis revealed the morphology of NP (scale bar, 0.5 μm; magnification, 25,000×)
Table 1.
Physicochemical characterization of targeted and non-targeted PLGA NPs, size distribution and zeta potential
| Samples | Poly I:C (μg/mg NP) (w/w) R848 (μg/mg NP) (w/w) |
Peptide (μg/mg NP) (w/w) |
NP diameter ± S.D. (nm) | Polydispersity index ± S.D | Zeta potential ± S.D. (mV) | mAbs (μg/mg NP) |
|---|---|---|---|---|---|---|
| NP-(mHpa399 + 519 + poly I:C + R848)-αDEC205 |
21 ± 3.5 2.9 ± 0.3 |
41.2 ± 6.4 | 204 ± 10.2 | 0.084 ± 0.013 | − 32.4 ± 6.1 | 32.1 ± 3.6 |
| NP-(mHpa519 + poly I:C + R848)-αDEC205 |
23 ± 2.6 2.6 ± 0.5 |
37.8 ± 4.9 | 198 ± 12.6 | 0.106 ± 0.022 | − 30.4 ± 4.6 | 34.4 ± 2.7 |
| NP-(mHpa519 + poly I:C + R848)-Isotype |
26 ± 2.9 2.6 ± 0.4 |
35.3 ± 5.7 | 203 ± 11.4 | 0.085 ± 0.014 | − 29.7 ± 3.8 | 31.5 ± 1.8 |
| NP-(NC + poly I:C + R848)-αDEC205 |
24 ± 3.7 2.4 ± 0.4 |
38.4 ± 6.2 | 201 ± 12.3 | 0.092 ± 0.054 | − 38.4 ± 4.5 | 32.5 ± 2.4 |
Dynamic light scattering instrument was use to characterized the PLGA NPs’ size and zeta potential measurements. PLGA NP’s diameter was determined by DLS measurements, the size data represent the mean value ± SD of 6 readings from dynamic light scattering measurements. Zeta potential data present the mean value ± SD of six readings. The encapsulation efficiency of peptides (μg/mg NP), TLR ligands (μg Poly I:C or R848 per mg NP) was determined by reversed-phase high-performance liquid chromatography-quadrupole-time-of-flight mass spectrometry (RP-HPLC-QTOF/MS). The amount of anti-DEC-205 antibody introduced into the PLGA NPs was determined by Nano-Drop 2000 Spectrophotometer and present the mean value ± SD of six measurements
NP targeting and DC uptake of NP in vitro
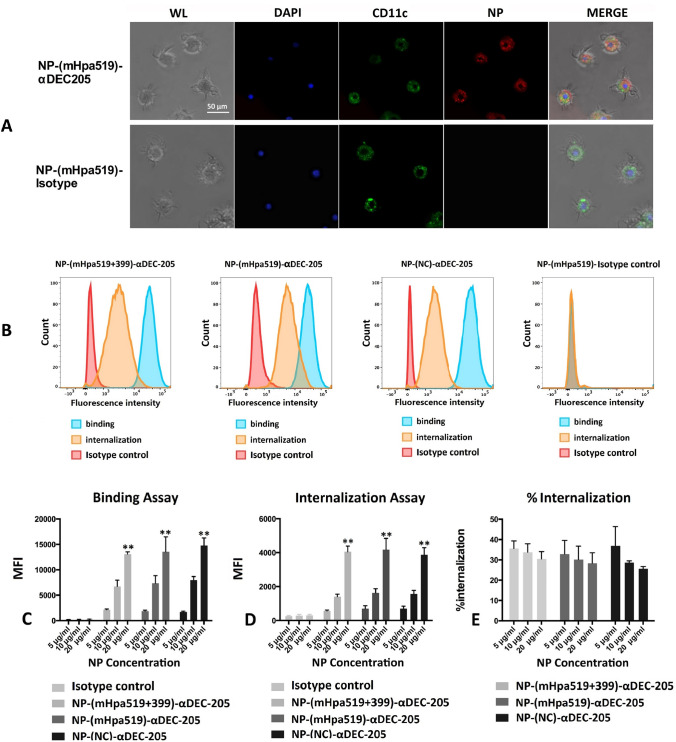
To monitor the intercellular distribution of NPs, BMDCs were incubated with 20 μg/mL NP-(mHpa519)-αDEC-205 or NP-(mHpa519)-isotype at 37 °C for 3 h. Then, BMDCs were stained with FITC-anti-mouse CD11c and DAPI, followed by observation under a confocal scanning microscope. Figure 2A shows the internalization state of red-fluorescent NP-(mHpa519)-αDEC-205 by green-labeled CD11c+ cells following 3 h of incubation. We also used flow cytometry to assess the internalization of NP by DCs. BMDCs incubated with NP at 4 °C were washed with PBS to remove unbound NP, BMDCs incubated at 37 °C were washed with acidic PBS at pH = 2 to remove NPs that were not internalized, and then, the fluorescence intensity was measured by flow cytometry (Fig. 2B). We evaluated the uptake efficiency of NP-(mHpa519 + 399)-αDEC-205, NP-(mHpa519)-αDEC-205, NP-(mHpa519)-isotype, and NP-(NC)-αDEC-205 by BMDCs. BMDCs were incubated with 5, 10, and 20 μg/mL PLGA NPs (encapsulating different epitope peptides) targeted to DEC205 receptors or isotype control (nontargeted) at 4 °C or 37 °C for 3 h. BMDCs incubated with NP at 4 °C were washed with PBS to remove unbound NP, BMDCs incubated at 37 °C were washed with acidic PBS at pH = 2 to remove NPs that were not internalized, and then, the mean fluorescence intensity was measured by flow cytometry. DEC-205-targeted PLGA NPs exhibited better binding and internalization capacity than their nontargeted counterparts (Fig. 2C, D). Furthermore, the internalization ratio of PLGA NPs by DCs was calculated (Fig. 2E).
Fig. 2.
Uptake efficacy of NPs. A BMDCs were incubated with Lightning-Link® Rapid DyLight® 680 labeled NP-(mHpa519)-αDEC-205 or NP-(mHpa519)-isotype (20 μg/mL) at 37 °C for 3 h, and then stained with FITC-anti-mouse CD11c and DAPI. Images were captured with a Leica scanning microscope (Leica TCS-SP5). WL panel (white light), DAPI panel (BMDCs nuclear stained with DAPI); CD11c panel (FITC-anti-mouse CD11c fluorescent images); NP panel (Lightning-Link® Rapid DyLight® 680 labeled NP fluorescent images); merge panel, merged images. B The uptake efficacy was evaluated by histograms. BMDCs were incubated for 3 h at either 4 °C (binding analysis) or 37 °C (internalization analysis) with 20 μg/mL NPs. Then washed to remove unbound NPs. For internalization assay, the BMDCs were washed by acidic PBS (pH = 2) to remove the bound but not internalized NP. The fluorescence intensity was measured by a BD FACsAria cytometer. (C and D) BMDCs were incubated for 3 h at either 4 °C (binding analysis) or 37 °C (internalization analysis) with 1, 5, 10, 20 μg/mL NPs. Then washed to remove unbound NPs. For internalization assay, the BMDCs were washed by acidic PBS (pH = 2) to remove the bound but not internalized NPs. The mean fluorescence intensity (MFI) was measured by a BD FACsAria cytometer. (E) The internalization ratio was calculated following the formula: %internalization = [(MFI in 37 °C-background)]/[(MFI on ice)-background] × 100%. Data are representative of three independent experiments. *p < 0.05; **p < 0.01 (by student’s t test)
Activation of DCs in vitro by targeted PLGA NPs
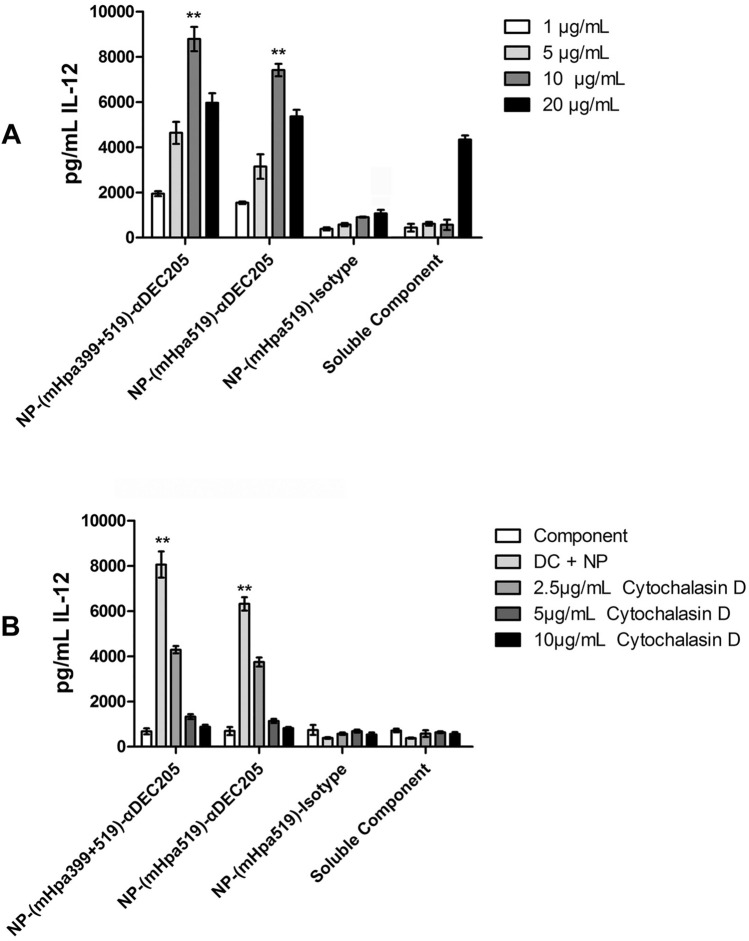
The in vitro production of IL-12 was examined to evaluate the activation of DCs by PLGA NPs. Our results showed that DCs loaded with PLGA NPs targeted to DC-205 receptors displayed much higher IL-12 expression than the controls (Fig. 3A). However, much lower IL-12 production was detected using the isotype control at all concentrations (Fig. 3A). Additionally, there was a direct relationship between the targeted PLGA NP concentration and binding capacity. However, no differences were detected among the different epitope peptide formulations of targeted PLGA NPs (Fig. 3A). Furthermore, DCs treated with increasing concentrations of cytochalasin D, an inhibitor of actin polymerization that disrupts the phagocytic uptake of exogenous material, exhibited a marked decrease in IL-12 production when cultured in the presence of targeted NPs (Fig. 3B). In contrast, treatment with cytochalasin D did not reduce the IL-12 production of DCs cultured with nontargeted NPs or soluble components (a mixture of mHpa399 peptide, mHpa519 peptide, Poly I:C and R848) at any of the concentrations tested (Fig. 3B).
Fig. 3.
Activation of DCs in vitro by targeted PLGA NP. C57BL/6 BMDCs (100,000 cells/well) were incubated with increasing concentration of NP-(mHpa519 + 399)-αDEC-205, or NP-(mHpa519)-αDEC-205, or NP-(NC)- αDEC-205, NP-(mHpa519)-isotype, for 24 h at 37 °C. Culture supernatants were harvested, and the amount of IL-12 was determined by ELISA (A). Differences in cytokine production were analyzed applying two-way ANOVA analysis. Data shown are mean ± SD from one representative experiment out of 3 independent experiments. B Dendritic cells were pre-incubated for 1 h at 37 °C with titrated amounts of Cytochalasin D followed by a 24 h incubation with 10 μg/ml of NP-(mHpa399 + 519)-αDEC-205, NP-(mHpa519)-αDEC-205, or non-targeted. Indicated amounts of Cytochalasin D were maintained during the 24 h incubation with NP. After incubation, culture supernatants were harvested and analyzed for IL-12 amounts by ELISA
PLGA NPs induce T-cell activation in vitro
Next, DCs treated with different PLGA NPs were used as APCs in cocultures with splenocytes to measure T-cell proliferation and IFN-γ production. T-cell proliferation was efficiently induced by DCs loaded with targeted NPs but not by the nontargeted counterparts or negative control peptides (Fig. 4A–C). Significant differences in T-cell proliferation were observed among DCs loaded with different epitope peptides. Then, IFN-γ levels in culture supernatants were measured using an ELISPOT assay. We found that targeted PLGA NPs increased IFN-γ levels compared to the nontargeted counterparts or targeted negative controls (Fig. 4D, E).
Fig. 4.
DEC-205 targeted NP containing heparanase epitope peptides, poly I:C, and R848 improved T cell stimulatory capacity. A BMDCs were incubated for 3 h at 37 °C with 20 μg/mL NP-(mHpa519 + 399)-αDEC-205, or NP-(mHpa519)-αDEC-205, or NP-(mHpa519)-isotype, or NP-(NC)-αDEC-205. Splenocytes were labeled at 107 cells/mL with 5 μM CFSE and then incubated with NPs loaded BMDCs for 6 days at (1 BMDC: 5 splenocytes). Thereafter, T cells were stained with APC-anti-mouse CD3, PerCP-anti-mouse CD4 and PE-anti-mouse CD8. T cell proliferation was measured by a BD FACsAria cytometer. B, C NP-(mHpa519 + 399)-αDEC-205, or NP-(mHpa519)-αDEC-205 was taken up by BMDCs and mediated activation of heparanase-specific T cells. Data shown represent the mean ± SD from triplicates of characteristic of three independent experiments (*p < 0.05, **p < 0.01). (D and E) CD8+ T cells or CD4+ T cells were sorted separately by flow cytometry. IFN-γ secreted by CD8+ T cells or CD4+ T cells was evaluated. Data shown represent the mean ± SD from triplicates of characteristic of three independent experiments (*p < 0.05, **p < 0.01)
Induction of CTLs specific to mouse heparanase antigen through targeted PLGA NPs
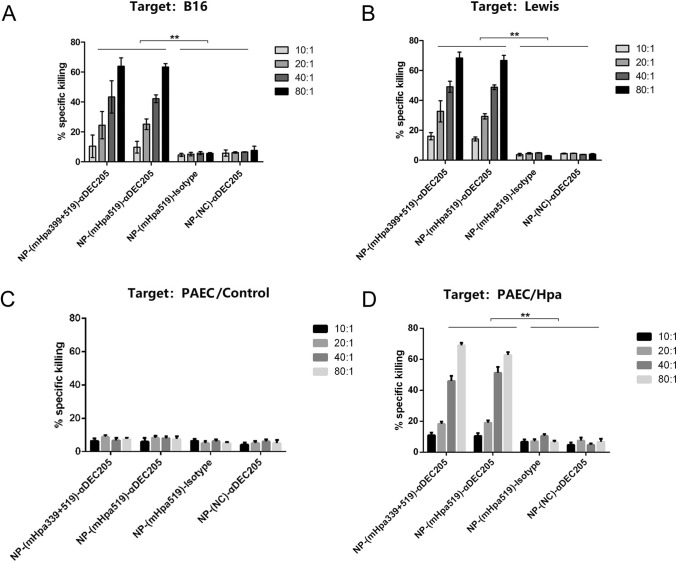
To determine whether the NPs induced cytotoxic activity in vitro, we used heparanase-positive B16 melanoma cells and Lewis lung cancer cells as targets. Our results revealed that B16 cells could be killed by effectors immunized by targeted PLGA NPs but not by their nontargeted counterparts or nonepitope peptides (Fig. 5A). The combined epitope (mHpa399 + mHpa519) did not generate a higher killing efficiency than either epitope alone in vitro (Fig. 5A). Further study indicated that in addition to B16 cells, a similar killing effect was observed in Lewis cells (Fig. 5B). The results indicated that the CD4+ epitope did not improve the killing performance of CD8+ cells during in vitro stimulation (Fig. 5A, B). To further validate the specificity of heparanase in cytotoxic activity, we utilized a mouse primary aortic endothelial cell line with negative heparanase expression. We observed a similar killing effect for primary aortic endothelial cells transfected with heparanase vectors, whereas no effects were observed for primary aortic endothelial cells transfected with control vectors (Fig. 5C, D), suggesting specificity of the heparanase antigen in cytotoxic activity by targeted PLGA NPs.
Fig. 5.
The killing effect of CTLs induced by DEC-205 targeted NP containing heparanase epitope. C57BL/6 mice were immunized thrice at 7-day intervals by s.c. injection of 5 × 105 NP-(mHpa399 + 519)-αDEC-205, or PLGA-(mHpa519)-αDEC-205, or non-targeted NP-pulsed dendritic cells. On day 28, mice splenocytes were served as effectors. Standard 51Cr-release assays were performed to test for their cytotoxic activity against B16 melanoma cells (A) and Lewis lung cancer cells (B) at various effector: target (E/T) ratios. Data shown are mean ± SEM of three independent experiments (**p < 0.01). C, D Mouse primary aortic endothelial cells (PAEC) were transfected with heparanase expression plasmid and control vectors. Standard 51Cr-release assays were performed as above
Heparanase epitope targeting under immunizing conditions reduces tumor growth and metastasis
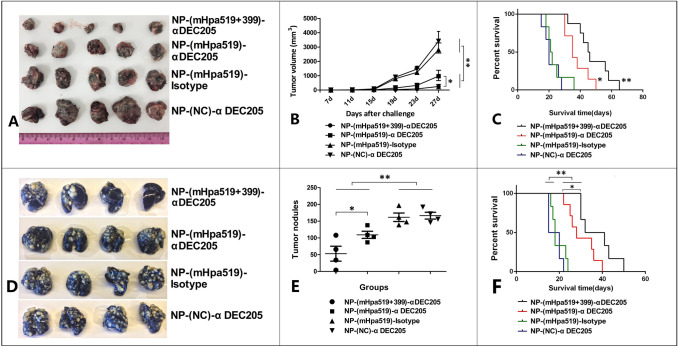
The delivery of heparanase epitopes led to strong CD8+ T-cell responses, accompanied by specific CD4+ T-cell responses (Fig. 4). We next investigated whether the induced immune responses would be sufficient to inhibit tumor growth and metastasis. We immunized mice with targeted NP (encapsulating heparanase epitope peptides), the nontargeted counterparts or targeted negative control (targeted NP encapsulating negative control epitope, NC) three times per week for 3 weeks. Next, we established a subcutaneous tumor model and lung metastasis model by s.c. injection of 1 × 106 viable B16 melanoma cells or i.v. injection of 1 × 106 Lewis lung cancer cells. We observed that mice treated with NP-(mHpa519 + 399)-αDEC-205 or NP-(mHpa519)-αDEC-205 in conjunction with Poly I:C and R848 maturation stimulus exhibited markedly delayed B16 melanoma growth and Lewis lung cancer metastasis (Fig. 6A, B, D, E). In contrast, mice treated with NP- (NC)-αDEC-205 or NP- (mHpa519)-isotype combined with poly I:C and R848 had no inhibition of tumor growth or metastasis (Fig. 6A, B, D, E).
Fig. 6.
DEC-205 targeted NP containing heparanase epitope peptides vaccination reduce tumor burden. Mice were vaccinated by NP-(mHpa519 + 399)-αDEC-205, or NP-(mHpa519)-αDEC-205, or NP-(NC)- αDEC-205, or NP-(mHpa519)-isotype loading BMDCs 3 times at 7-day intervals. Seven days after the last vaccination, mice were challenged with 1 × 106 B16 melanoma cells by inoculated s.c. in the left flank. Tumor growth (A, B) and animal survival (C) were monitored. Mice were immunized as (A), and then mice were challenged with 1 × 106 Lewis cancer cells by i.v. injection through tail vein. Lewis cancer pulmonary metastasis (D, E) and animal survival (F) were monitored. Differences in tumor sizes and metastasis nodules per group were analyzed by regular two-way ANOVA with Bonferroni posttests to calculate the difference in mean values at each time point. Animal survival per group was assessed using log-rank (Mantel–Cox) test, **p < 0.001, *p < 0.05
For survival analysis, mice received three vaccinations as mentioned above, and the tumor growth and metastasis model were established using the same method described above. The survival rates of mice bearing tumors were then observed over an 80-day period. Mice treated with NP-(mHpa519 + 399)-αDEC-205- or NP-(mHpa519)-αDEC-205-loaded dendritic cells had a prolonged lifespan; however, mice treated with either NP-(NC)-αDEC-205- or NP-(mHpa519) displayed no survival benefit with a corresponding high tumor burden (Fig. 6). Mice treated with nontargeted counterparts or negative control epitope peptides began dying 22 days after tumor cell injection, and all mice died by day 38 (Fig. 6C, F). In contrast, mice immunized with targeted NP-pulsed dendritic cells began dying at 40, and several mice survived until the end of the 60-day period (Fig. 6C, F).
Discussion
DC-based immunotherapy has become an effective strategy to treat malignant tumors due to its advantages of strong immunogenicity, mild side effects and broad applicability [28]. A key mechanism of tumor immunotherapy is to identify suitable tumor-associated antigens (TAAs) to load dendritic cells. By targeting TAAs, immunotherapy is tumor specific, less toxic, and can have a long-lasting effect [18]. Our previous study revealed that heparanase may represent a novel TAA epitope capable of inducing heparanase-specific CTLs that is suitable for tumor immunotherapy due to advantages such as being broad spectrum, highly effective, highly specific, and safe [14, 15, 17]. Unfortunately, peptides also have a short half-life in the body, partly due to enzyme degradation, but primarily because their small size means that they are filtered out of the blood by the kidneys, usually within minutes. To extend the half-life of the heparanase epitope and to ensure efficient delivery of the antigen to DCs, we encapsulated heparanase epitope peptides in NPs coated with DEC-205 antibodies.
Our previous study primarily focused on the CD8+ T-cell-based heparanase epitope while ignoring the function of CD4+ T cells in tumor immunotherapy. CD4+ T cells play a key role in generating effective immune responses by sustaining CD8+ T-cell proliferation, preventing exhaustion, and establishing long-lived functional T-cell memory [29]. Several reports have also suggested that CD4+ T cells enhance CD8+ T-cell infiltration into tumors [30, 31]. Here, we used combined CD8+ T and CD4+ T-cell-based heparanase epitopes to evaluate their antitumor effect. We observed that the provision of CD4+ T cells enhanced antitumor activity in vivo, while having minimal effects on killing efficiency in vitro. These findings might explain the importance of CD4+ T cells in the accumulation of CD8+ T cells in tumor tissues, which may involve multiple mechanisms, including enhanced proliferation, trafficking, and infiltration, as well as reduced apoptosis of CTLs in vivo. We also demonstrated that both CD4+ and CD8+ T cells must be primed by DCs to generate the most efficient antitumor response. Possible mechanisms include CD4+ T cells being brought into close proximity to CD8+ T cells because the same DC presents both epitopes, and CD4+ T cells may activate DCs, which then subsequently prime CD8+ T cells [32].
In our study, we demonstrated that DC vaccines with PLGA nanoparticles encapsulating CD4+ and CD8+ T-cell epitope peptides and a combination of R848 and poly(I:C) display features crucial for triggering efficient T-cell-mediated antitumor responses. Both R848 and poly (I:C) generate a CD8+ T-cell immune response through different mechanisms [33], suggesting R848 and poly(I:C) as immunomodulators to improve cellular immune responses. Previous studies reported that R848 and poly (I:C) markedly affect the subpopulation of CD4+ and CD8+ T cells when the two TLR agonists were administered together [23, 47]. Therefore, the two immunoregulatory materials could work together to improve cellular responses in tumor immunotherapy. Moreover, to increase DC targeting, the nanoparticle complex was conjugated to a DEC-205 monoclonal antibody targeting DEC-205 receptors expressed on the surface of dendritic cells. Although there are other DC-specific surface molecules, such as CD11c and CD40, a previous study compared the delivery efficiencies of targeting these molecules and showed that there were no significant differences [34].
Previous studies have shown that binding of DEC-205 receptors facilitates internalization and activation of signaling pathways that induce the maturation of DCs and ultimately increase antigen presentation to enhance the induction of T-cell responses [35, 36]. Nevertheless, the different epitope peptides did not affect the binding or internalization of PLGA NPs, implying the broad activity of PLGA NP particles. DC-targeting vaccine delivery strategies increase the amount of vaccine uptake by DCs, improving the efficiency of the vaccine [37, 38]. Activation of DCs by targeted NPs was significantly inhibited by cytochalasin D, a potent inhibitor of actin polymerization that disrupts actin microfilaments associated with phagocytosis, suggesting that internalization of NPs requires actin polymerization to translocate NPs toward antigen processing compartments to produce peptides for presentation by MHC class I and MHC class II.
Targeting strategies to improve vaccine efficacy and to achieve increased delivery to DCs have been thoroughly investigated [36, 37]. DCs targeting the endocytic lectin receptor DEC-205 with mAb conjugated to PLGA NPs induced strong CD8+ T-cell responses [39, 40]. DEC-205-targeted antigens have been shown to be routed to late endosomes, which are compartments associated with suboptimal MHC class I presentation [41]. Our results showed that DEC-205-targeted PLGA NPs induced T-cell proliferation and IFN-γ production but not their nontargeted counterparts or nonepitope peptides. Moreover, the combination of CD4+ and CD8+ T-cell epitope peptides induced higher activation of T cells than the CD8+ T-cell epitope alone, suggesting that DEC-205 targeting of DCs is the major point to induce T-cell proliferation and activation.
To verify whether the targeted PLGA NPs encapsulating different epitope peptides could induce CTL responses, dendritic cells generated from mouse bone marrow were pulsed with the targeted NPs or their nontargeted counterparts. We found that B16 melanoma cells and Lewis cells were killed by effectors induced by targeted PLGA NPs at much higher levels than their nontargeted counterparts. Moreover, the combined epitope (mHpa399 + mHpa519) generated much stronger killing effects than mHpa519 alone. Taken together, these results indicate that the DEC-205 target PLGA NP encapsulating epitope peptides (mHpa399 + mHpa519) induces more effective heparanase-specific CTL responses. Furthermore, the capacity to induce specific in vivo killing was significantly different between targeted and nontargeted NPs in vivo, indicating that targeted NPs prime specific cytotoxic CD8+ T cells from the endogenous naïve T-cell repertoire. Thus, our present study indicates that PLGA NPs targeting DEC205 with combined epitope peptides are capable of inducing heparanase CTLs in vitro and in vivo.
Recently, a major obstacle to the transformation of nanomedicine is the removal of nanoparticles from the bloodstream. Once the nanomedicines were injected intravenously, various serum proteins adhered to the surface of the nanoparticles. The immune system recognizes the NPs as invaders and removes the particles to the filter organs for clearance [42]. PLGA is an optimal biocompatible and biodegradable material that is used for efficient drug delivery. Therapeutics encapsulated in PLGA NPs displayed enhanced activity due to their protection of cargo from degradation [43]. The hydrophobicity of PLGA resulted in a reduction in the degradation rate, slowed drug release kinetics and reduced clearance by increasing the hydrophilicity of the particle surface. NPs based on PLGA are biocompatible and biodegradable [44]. Previous studies have shown that NPs improve memory T-cell generation, induce stronger immune responses, and are effective antigen delivery vehicles for tumor immunotherapy [45, 46]. Here, our data revealed that the PLGA NPs in our study induced significant T-cell activation and may also generate a T-cell memory response. We will focus on this point in future studies.
In conclusion, our present study demonstrates that using PLGA NP vaccines containing a combined epitope of CD4+ T and CD8+ T-cell antigen with mAb targeted specific DEC-205 receptors on DCs. The designed PLGA NP vaccines induced stronger responses and better effects than vaccination with their nontargeted counterparts or a single CD8+ T-cell epitope peptide. Thus, our findings indicate that PLGA NP vaccines targeting DEC-205 based on CD4+ T and CD8+ T-cell heparanase epitopes are suitable immunogens for antitumor immunotherapy and suggest their potential for clinical applications in humans.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (No. 81372470), and the Chongqing Science and Technology Commission Frontier and Applied Basic Research General Project (Project Number Nos. CSTCjcyjmsxmX0634, CSTC2014jcyjA10100, and cstc2019jcyj-msxmX0791).
Declarations
Ethical approval
This study was approved by the Ethics Committee of the Army Medical University.
Conflict of interest
The authors declare that there are no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Xu-Dong Tang, Kui-Lin Lü and Jin Yu contributed equally to this study.
Contributor Information
Chao-Qiang Fan, Email: fcqxhkwjs@126.com.
Lei Chen, Email: chenlei1977603@126.com.
References
- 1.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science. 2011;331(6024):1565–1570. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 2.Vesely MD, Kershaw MH, Schreiber RD, Smyth MJ. Natural innate and adaptive immunity to cancer. Annu Rev Immunol. 2011;29:235–271. doi: 10.1146/annurev-immunol-031210-101324. [DOI] [PubMed] [Google Scholar]
- 3.Finn OJ. Vaccines for cancer prevention: a practical and feasible approach to the cancer epidemic. Cancer Immunol Res. 2014;2(8):708–713. doi: 10.1158/2326-6066.CIR-14-0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coulie PG, Van den Eynde BJ, van der Bruggen P, Boon T. Tumour antigens recognized by T lymphocytes: at the core of cancer immunotherapy. Nat Rev Cancer. 2014;14(2):135–146. doi: 10.1038/nrc3670. [DOI] [PubMed] [Google Scholar]
- 5.Hinrichs CS, Restifo NP. Reassessing target antigens for adoptive T-cell therapy. Reassessing target antigens for adoptive T-cell therapy. Nat Biotechnol. 2013;31(11):999–1008. doi: 10.1038/nbt.2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Novellino L, Castelli C, Parmiani G. A listing of human tumor antigens recognized by T cells: March 2004 update. Cancer Immunol Immunother. 2005;54(3):187–207. doi: 10.1007/s00262-004-0560-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nishimura Y, Tomita Y, Yuno A, Yoshitake Y, Shinohara M. Cancer immunotherapy using novel tumor-associated antigenic peptides identified by genome-wide cDNA microarray analyses. Cancer Sci. 2015;106(5):505–511. doi: 10.1111/cas.12650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hammond E, Khurana A, Shridhar V, Dredge K. The role of heparanase and sulfatases in the modification of heparan sulfate proteoglycans within the tumor microenvironment and opportunities for novel cancer therapeutics. Front Oncol. 2014;4:195. doi: 10.3389/fonc.2014.00195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barash U, Cohen-Kaplan V, Dowek I, Sanderson RD, Ilan N, Vlodavsky I. Proteoglycans in health and disease: new concepts for heparanase function in tumor progression and metastasis. FEBS J. 2010;277(19):3890–3903. doi: 10.1111/j.1742-4658.2010.07799.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rivara S, Milazzo FM, Giannini G. Heparanase: a rainbow pharmacological target associated to multiple pathologies including rare diseases. Future Med Chem. 2016;8(6):647–680. doi: 10.4155/fmc-2016-0012. [DOI] [PubMed] [Google Scholar]
- 11.Masola V, Secchi MF, Gambaro G, Onisto M. Heparanase as a target in cancer therapy. Curr Cancer Drug Targets. 2014;14(3):286–293. doi: 10.2174/1568009614666140224155124. [DOI] [PubMed] [Google Scholar]
- 12.Pisano C, Vlodavsky I, Ilan N, Zunino F. The potential of heparanase as a therapeutic target in cancer. Biochem Pharmacol. 2014;89(1):12–19. doi: 10.1016/j.bcp.2014.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vlodavsky I, Beckhove P, Lerner I, Pisano C, Meirovitz A, Ilan N, Elkin M. Significance of heparanase in cancer and inflammation. Cancer Microenviron. 2012;5(2):115–132. doi: 10.1007/s12307-011-0082-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tang XD, Wan Y, Chen L, Chen T, Yu ST, Xiong Z, Fang DC, Liang GP, Yang SM. H-2Kb-restricted CTL epitopes from mouse heparanase elicit an antitumor immune response in vivo. Cancer Res. 2008;68(5):1529–1537. doi: 10.1158/0008-5472.CAN-07-5965. [DOI] [PubMed] [Google Scholar]
- 15.Tang XD, Wang GZ, Guo J, Lü MH, Li C, Li N, Chao YL, Li CZ, Wu YY, Hu CJ, Fang DC, Yang SM. Multiple antigenic peptides based on H-2K(b)-restricted CTL epitopes from murine heparanase induce a potent antitumor immune response in vivo. Mol Cancer Ther. 2012;11(5):1183–1192. doi: 10.1158/1535-7163.MCT-11-0607. [DOI] [PubMed] [Google Scholar]
- 16.Rammensee HG, Falk K, Rötzschke O. Peptides naturally presented by MHC class I molecules. Annu Rev Immunol. 1993;11:213–244. doi: 10.1146/annurev.iy.11.040193.001241. [DOI] [PubMed] [Google Scholar]
- 17.Chen T, Tang XD, Wan Y, Chen L, Yu ST, Xiong Z, Fang DC, Liang GP, Yang SM. HLA-A2-restricted cytotoxic T lymphocyte epitopes from human heparanase as novel targets for broad-spectrum tumor immunotherapy. Neoplasia. 2008;10(9):977–986. doi: 10.1593/neo.08576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tang XD, Liang GP, Li C, Wan Y, Chen T, Chen L, Yu ST, Xiong Z, Fang DC, Wang GZ, Yang SM. Cytotoxic T lymphocyte epitopes from human heparanase can elicit a potent anti-tumor immune response in mice. Cancer Immunol Immunother. 2010;59(7):1041–1047. doi: 10.1007/s00262-010-0829-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang HY, Wang RF. Enhancing cancer immunotherapy by intracellular delivery of cell-penetrating peptides and stimulation of pattern-recognition receptor signaling. Adv Immunol. 2012;114:151–176. doi: 10.1016/B978-0-12-396548-6.00006-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang RF, Wang HY. Immune targets and neoantigens for cancer immunotherapy and precision medicine. Cell Res. 2017;27(1):11–37. doi: 10.1038/cr.2016.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Borst J, Ahrends T, Bąbała N, Melief CJM, Kastenmüller W. CD4+ T cell help in cancer immunology and immunotherapy. Nat Rev Immunol. 2018;18(10):635–647. doi: 10.1038/s41577-018-0044-0. [DOI] [PubMed] [Google Scholar]
- 22.Lövgren T, Sarhan D, Truxová I, et al. Enhanced stimulation of human tumor-specific T cells by dendritic cells matured in the presence of interferon-γ and multiple toll-like receptor agonists. Cancer Immunol Immunother. 2017;66(10):1333–1344. doi: 10.1007/s00262-017-2029-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou CX, Li D, Chen YL, et al. Resiquimod and polyinosinic-polycytidylic acid formulation with aluminum hydroxide as an adjuvant for foot-and-mouth disease vaccine. BMC Vet Res. 2014;10:2. doi: 10.1186/1746-6148-10-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kapadia CH, Perry JL, Tian S, Luft JC, DeSimone JM. Nanoparticulate immunotherapy for cancer. J Control Release. 2015;219:167–180. doi: 10.1016/j.jconrel.2015.09.062. [DOI] [PubMed] [Google Scholar]
- 25.Cho K, Wang X, Nie S, Chen ZG, Shin DM. Therapeutic nanoparticles for drug delivery in cancer. Clin Cancer Res. 2008;14(5):1310–1316. doi: 10.1158/1078-0432.CCR-07-1441. [DOI] [PubMed] [Google Scholar]
- 26.Zamboni WC, Torchilin V, Patri AK, Hrkach J, Stern S, Lee R, Nel A, Panaro NJ, Grodzinski P. Best practices in cancer nanotechnology: perspective from NCI nanotechnology alliance. Clin Cancer Res. 2012;18(12):3229–3241. doi: 10.1158/1078-0432.CCR-11-2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zang X, Zhao X, Hu H, Qiao M, Deng Y, Chen D. Nanoparticles for tumor immunotherapy. Eur J Pharm Biopharm. 2017;115:243–256. doi: 10.1016/j.ejpb.2017.03.013. [DOI] [PubMed] [Google Scholar]
- 28.Decker WK, Xing D, Shpall EJ. Dendritic cell immunotherapy for the treatment of neoplastic disease. Biol Blood Marrow Transplant. 2006;12(2):113–125. doi: 10.1016/j.bbmt.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 29.Bevan MJ. Helping the CD8(+) T-cell response. Nat Rev Immunol. 2004;4(8):595–602. doi: 10.1038/nri1413. [DOI] [PubMed] [Google Scholar]
- 30.Bos R, Sherman LA. CD4+ T-cell help in the tumor milieu is required for recruitment and cytolytic function of CD8+ T lymphocytes. Cancer Res. 2010;70(21):8368–8377. doi: 10.1158/0008-5472.CAN-10-1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakanishi Y, Lu B, Gerard C, Iwasaki A. CD8(+) T lymphocyte mobilization to virus-infected tissue requires CD4(+) T-cell help. Nature. 2009;462(7272):510–513. doi: 10.1038/nature08511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhu Z, Cuss SM, Singh V, et al. CD4+ T cell help selectively enhances high-avidity tumor antigen-specific CD8+ T cells. J Immunol. 2015;195(7):3482–3489. doi: 10.4049/jimmunol.1401571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wick DA, Martin SD, Nelson BH, Webb JR. Profound CD8+ T cell immunity elicited by sequential daily immunization with exogenous antigen plus the TLR3 agonist poly(I:C) Vaccine. 2011;29(5):984–993. doi: 10.1016/j.vaccine.2010.11.036. [DOI] [PubMed] [Google Scholar]
- 34.Cruz LJ, Rosalia RA, Kleinovink JW, Rueda F, Löwik CW, Ossendorp F. Targeting nanoparticles to CD40, DEC-205 or CD11c molecules on dendritic cells for efficient CD8(+) T cell response: a comparative study. J Control Release. 2014;192:209–218. doi: 10.1016/j.jconrel.2014.07.040. [DOI] [PubMed] [Google Scholar]
- 35.Geijtenbeek TB, Gringhuis SI. Signalling through C-type lectin receptors: shaping immune responses. Nat Rev Immunol. 2009;9(7):465–479. doi: 10.1038/nri2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Elgueta R, Benson MJ, de Vries VC, Wasiuk A, Guo Y, Noelle RJ. Molecular mechanism and function of CD40/CD40L engagement in the immune system. Immunol Rev. 2009;229(1):152–172. doi: 10.1111/j.1600-065X.2009.00782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang Z, Tongchusak S, Mizukami Y, Kang YJ, Ioji T, Touma M, Reinhold B, Keskin DB, Reinherz EL, Sasada T. Induction of anti-tumor cytotoxic T cell responses through PLGA-nanoparticle mediated antigen delivery. Biomaterials. 2011;32(14):3666–3678. doi: 10.1016/j.biomaterials.2011.01.067. [DOI] [PubMed] [Google Scholar]
- 38.Manolova V, Flace A, Bauer M, Schwarz K, Saudan P, Bachmann MF. Nanoparticles target distinct dendritic cell populations according to their size. Eur J Immunol. 2008;38(5):1404–1413. doi: 10.1002/eji.200737984. [DOI] [PubMed] [Google Scholar]
- 39.Raghuwanshi D, Mishra V, Suresh MR, Kaur K. A simple approach for enhanced immune response using engineered dendritic cell targeted nanoparticles. Vaccine. 2012;30(50):7292–7299. doi: 10.1016/j.vaccine.2012.09.036. [DOI] [PubMed] [Google Scholar]
- 40.Tacken PJ, Figdor CG. Targeted antigen delivery and activation of dendritic cells in vivo: steps towards cost effective vaccines. Semin Immunol. 2011;23(1):12–20. doi: 10.1016/j.smim.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 41.Burgdorf S, Kurts C. Endocytosis mechanisms and the cell biology of antigen presentation. Curr Opin Immunol. 2008;20(1):89–95. doi: 10.1016/j.coi.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 42.Tsoi KM, MacParland SA, Ma XZ, Spetzler VN, Echeverri J, Ouyang B, Fadel SM, Sykes EA, Goldaracena N, Kaths JM, Conneely JB, Alman BA, Selzner M, Ostrowski MA, Adeyi OA, Zilman A, McGilvray ID, Chan WC. Mechanism of hard-nanomaterial clearance by the liver. Nat Mater. 2016;15(11):1212–1221. doi: 10.1038/nmat4718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Danhier F, Ansorena E, Silva JM, Coco R, Le Breton A, Préat V. PLGA-based nanoparticles: an overview of biomedical applications. J Control Release. 2012;161(2):505–522. doi: 10.1016/j.jconrel.2012.01.043. [DOI] [PubMed] [Google Scholar]
- 44.Min Y, Roche KC, Tian S, Eblan MJ, McKinnon KP, Caster JM, Chai S, Herring LE, Zhang L, Zhang T, DeSimone JM, Tepper JE, Vincent BG, Serody JS, Wang AZ. Antigen-capturing nanoparticles improve the abscopal effect and cancer immunotherapy. Nat Nanotechnol. 2017;12(9):877–882. doi: 10.1038/nnano.2017.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu L, Cao F, Liu X, Wang H, Zhang C, Sun H, Wang C, Leng X, Song C, Kong D, Ma G. Hyaluronic acid-modified cationic lipid-PLGA hybrid nanoparticles as a nanovaccine induce robust humoral and cellular immune responses. ACS Appl Mater Interfaces. 2016;8(19):11969–11979. doi: 10.1021/acsami.6b01135. [DOI] [PubMed] [Google Scholar]
- 46.Jahan ST, Sadat SMA, Yarahmadi M, Haddadi A. Potentiating antigen specific immune response by targeted delivery of the PLGA-based model cancer vaccine. Mol Pharm. 2019;16(2):498–509. doi: 10.1021/acs.molpharmaceut.8b00700. [DOI] [PubMed] [Google Scholar]
- 47.Hänel G, Angerer C, Petry K, Lichtenegger FS, Subklewe M. Blood DCs activated with R848 and poly(I:C) induce antigen-specific immune responses against viral and tumor-associated antigens. Cancer Immunol Immunother. 2021;CII:1–14. doi: 10.1007/s00262-021-03109-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.