Abstract
Previously, we found that dysfunctional natural killer (NK) cells with low interferon gamma (IFN-γ) were restored in acute myeloid leukemia (AML) by the FLT4 antagonist MAZ51. Here, we developed 12 peptides targeting FLT4 for clinical application and examined whether they restored the frequency of lymphocytes, especially T cells and NK cells, and high IFN-γ expression, as MAZ51 treatment did in our previous study. Although clinical data from using peptides are currently available, peptides targeting FLT4 to modulate immune cells have not been fully elucidated. In this study, we focus on novel peptide 4 (P4) from the intracellular domain of FLT4 because it had dominant negative activity. Similar to MAZ51, high IFN-γ levels were expressed in AML-mononuclear cells exposed to P4. Additionally, T and NK cell levels were restored, as were high IFN-γ levels, in a leukemic environment when P4 was treated. Interestingly, the regulatory T cells were significantly decreased by P4, implying the role of peptide in tumor niche. Overall, we demonstrated the therapeutic value of functionally modulating lymphocytes using a peptide targeting FLT4 and proposed the development of advanced therapeutic approaches against AML by using immune cells.
Keywords: FLT4, Peptide, AML, Interferon-γ, Natural killer cells, T cells
Introduction
Acute myeloid leukemia (AML) is defined as an increase in myeloid lineage cells with aberrant genetic changes that result in abnormal hematopoietic and immune procedures [1]. Among many trials to cure AML, peptide-based combinational therapy is emerging as an important strategy for treating cancer [2]. Because peptides having many advantages, including their small size, easy synthesis, rapid clinic application, and access to the tumor, are [2, 3] small, with a 12/18 mer structure, they can penetrate cells and function as bioactive peptides that target internalized signaling pathways, making them highly efficient in regulating molecular movements. Since Veli-Matti L et al. reported the crystal structure of VEGF-C/Fms-related tyrosine kinase-4 (FLT4) and provided mechanistic insights about its binding and activation [4], the use of peptides targeting FLT4 kinase activity is also emerging as a substance that blocks tumors by enhancing IFN-γ in immune cells [5]. The VEGF-C/FLT4 axis is involved in the high rate of growth and survival of leukemia cells, as well as with dysfunctional natural killer (NK) cells [6], our group previously focused on the role of the FLT4 inhibitor MAZ51 in restoring NK cell functioning, and we demonstrated the therapeutic effects of blocking FLT4 by showing that it increased IFN-γ in NK cells in AML [6–9]. We here designed novel peptides targeting FLT4 that could replicate the effect of MAZ51 in previous studies, and we confirmed that our FLT4-targeting peptide synergistically restored dysfunctional AML-NK cells when it was administered together with cytosine β-D-arabinofuranoside (ara-C), suggesting the therapeutic potential of the peptide in AML.
Materials and methods
Peptides
The sequence of used peptides is listed in Table 1 (Peptron, Korea). Twenty milligrams per kg of the peptides was used in vivo, and 25 μM was used in vitro. P4 was intraperitoneally injected into tumor-bearing mice.
Table 1.
Sequence of the peptides selected by binding to FLT4
| Peptide ID | Peptide sequence |
|---|---|
| P4, 440 | RQALTCTAYGVP |
| P6, 440 mutation | RQATAYGVPLPLS |
| P11, 121 mutation | KSIDNEWRKGCMPREVA |
| P12, 121 mutation | KSIDNEWRKLGCMPREVA |
Human samples
Human bone marrow (BM) and peripheral blood (PB) AML samples were collected from ten patients. The clinical characteristics and experimental information for the AML patients enrolled in this study are listed in Table 2. The cells (1 × 106 cells) were grown in RPMI 1640 medium (Gibco) supplemented with 10% FBS (Gibco) for use in the peptides, IL-2 (Peprotech, 500 U/ml), and IL-15 (Peprotech, 50 ng/ml) treatments.
Table 2.
Clinical characteristics and experimental information for AML patients
| Patients | Cell source | Age at diagnosis | Sex | WBS count [10^9/L] | Cytogenetic anomalies |
|---|---|---|---|---|---|
| 1 | BM | 50 | F | 116.6 | 46, XX[20] |
| 2 | BM | 20 | M | 190.2 | 46, XY[20] |
| 3 | BM | 33 | M | 2.78 | 46, XY, t(6;9) (p23;q34) [18] /46, xy[2] |
| 4 | BM | 71 | M | 2.33 | 46, XY[20] |
| 5 | BM, PB | 62 | M | 61.47 | 46, XY[20] |
| 6 | PB | 41 | M | 208.55 | 46, XY, t(9;22) (q34;q11,2) [2] /53, idem, + 3, + 8, + 10, + 10, + 15, + 19, + der(22)t9;22)[18] |
| 7 | PB | 84 | M | 345.38 | 45,X, -Y[20] |
| 8 | PB | 59 | M | 10.84 | 46,XY, inv(16) (p13,1q22) [20] |
| 9 | PB | 52 | F | 406.24 | 46, XX, t(9;22) (q34;q11,2) [18] /47, idem, + 19[2] |
| 10 | PB | 47 | M | 335.41 | 46, XY, inv(9) (p12q13) [20] |
Flow cytometry and MACS sorting
The following human antibodies were used: APC-labeled mouse anti-human CD56 (555, 518; BD Biosciences), FITC-labeled mouse anti-human CD3 (555, 339; BD Biosciences), and PE-labeled anti-human FLT4 (356, 204; BioLegend, San Diego, CA, USA). In the in vivo model, PE-labeled mouse anti-mouse NK-1.1 (553, 165; BD Biosciences), perCP-conjugated mouse CD8 (ThermoFisher, 45-0081-82), APC anti-mouse CD4 antibody (100, 412; BioLegend), FITC anti-mouse CD4 antibody (553, 729, BD Biosciences), APC rat anti-mouse CD25 (558, 643; BD Biosciences), FITC anti-mouse TCR γ/δ antibody (118, 106; BioLegend), biotin-labeled FLT4 monoclonal antibody (AFL4; 13-5988-82; Invitrogen, Carlsbad, CA, USA), APC-labeled IFN-γ monoclonal antibody (XMG1.2; 17-7311-82; Invitrogen), and FOXP3 monoclonal antibody (FJK-16 s; PE, eBioscience™; 12–5773-82; Invitrogen) were used as primary antibodies. The proper secondary antibodies were used. For NK isolation, isolation of NK cells was performed by magnetic-activated cell sorting (MACS) using the NK Micro-Bead Kit (130–092-657, Miltenyi Biotec). The cells were collected using a FACSCanto II flow cytometer (BD Biosciences), and the data were analyzed using FlowJo software (v10; Tree Star Inc., Ashland, OR, USA).
Statistical analysis
All data are presented as the mean ± standard error (SE). The means were used to compare the data. The results were analyzed by the Kruskal–Wallis test. GraphPad Prism, version 7, software was used for the analyses. P-values of < 0.05 were considered to indicate statistical significance.
Results
Peptides targeting FLT4 increased IFN-γ expressions on AML-NK cells in BM/PB
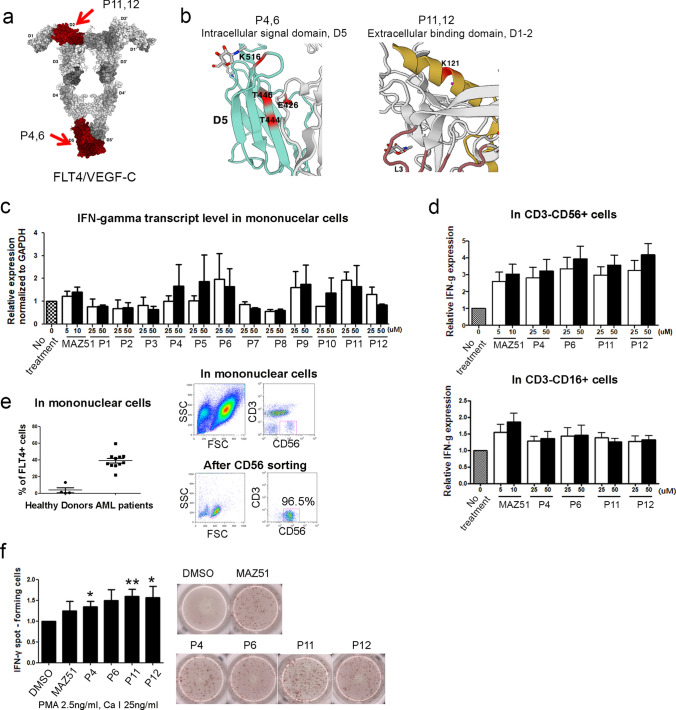
We designed functional peptides based on previous reports [4] on the crystal structure and mechanistic insights of VEGF-C/FLT4 for peptide binding and activation. Four peptides were selected to further examine their function in immune cells (P4, P6: targeting intracellular signal domain, P11, P12: targeting binding site) (Fig. 1a). The detailed loci for these peptides are depicted in Fig. 1(b). To screen proper peptides in increasing the IFN-γ on mononuclear cells (MNCs) in AML patients, 12 designed peptides were tested their effects in MNCs with dose-dependent manners. The transcriptional IFN-γ was increased by P4, P6, and P11 in a dose-dependent manner, compared with that of other type of peptides (Fig. 1c). In protein level, IFN-γ level was increased in CD3−CD56+ cytokine released NK cells in high concentration, compared with CD3−CD16+ cytolytic NK cells in treatment with four peptides (Fig. 1d). As shown in Fig. 1(e), the expression of FLT4 in MNCs was highly increased in AML patients, compared with that of normal donors. To acquire accurate data, ELISpot assay for IFN-γ was performed using isolated high NK cells from leukapheresis. P4, P11, and P12 showed a significant increase in IFN-γ spot-forming cells (Fig. 1f). Finally, we found that designed four peptides (including 4, 6, 11, and 12) may similar or superior efficacy with FLT4 antagonist, MAZ51 in increasing the expression of IFN-γ in vitro treatment.
Fig. 1.
Crystal structure of the VEGF-C/FLT4 D1-2 complex and the homodimer of FLT4 D4-5. (a) The red in the cartoon depicts the designed peptide locations. (b) Detailed depiction of the peptides. (c) qRT-PCR showed that the expression level of IFN-γ in AML-MNCs was significantly increased by the peptide treatment. (d) FACS data showed that cytolytic NK cells and cytokine-releasing NK cells. (e) The expression of FLT4 was higher in AML-MNCs than in healthy donors. To perform further experiments, purity of more than 96% should be confirmed after NK cell sorting. (f) The ELISpot assay showed a high level of IFN-γ in AML-NK cells, suggesting the relevance of the peptides to IFN-γ expression. (**P < 0.01, *P < 0.05 vs. DMSO-injected group)
Effects of peptides on increasing the frequency of AML-NK cells and T cells
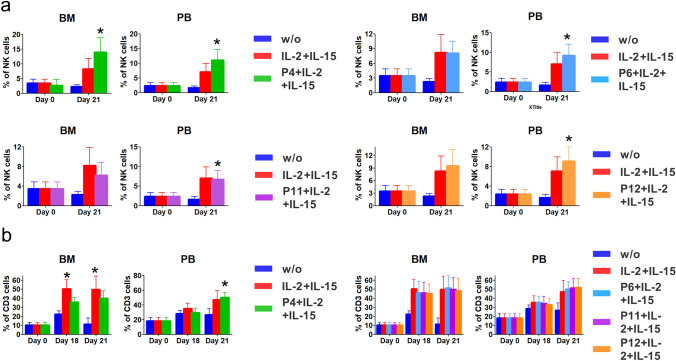
Next, we examined that peptides have a role to support cell survival and to increase the frequency of immune cells such as NK and T cells. As adjuvant materials, IL-2 and IL-15 were treated with peptides and were used in culture of AML-BM/PB cells. Data showed that BM/PB-NK cells were dramatically increased in only P4 at 21 days after culture (Fig. 2a), suggesting the relevance of immune cells releasing IFN-γ by peptide 4 in cells. In CD3 T cells, peptide 4 only showed a significant increase in cell frequency from 12 days after culture both in IL-2/IL-15-treated group of BM, compared with non-treated group. (Day 18, 49.9 ± 9.7%, day 21, 49.4 ± 13.5%) Meanwhile, CD3 T of PB cells were increased in peptide 4-treated group with significant difference at day 21 only (50.0 ± 6.5%) (Fig. 2b). Other peptides were detected with no significant difference in cells. PB-, BM-NK, and PB-T cells were dramatically expanded by peptide 4 under exposure of cytokines such as IL-2 and IL-15 at day 21.
Fig. 2.
The effect of peptides in increasing the frequency of AML-NK cells and T cells during in vitro cell proliferation. (a) Under IL-2 and IL-15 exposure, peptides 4, 6, 11, and 12 helped maintain BM and PB cell viability. *P < 0.05. (b) Unlike the data for PB-NK cells, the frequency of CD3 T cells increased only in the P4-treated group, compared with that with the other peptides. *P < 0.05
The effects of peptide with conventional chemotherapy on the expression of IFN-γ from leukemic mice
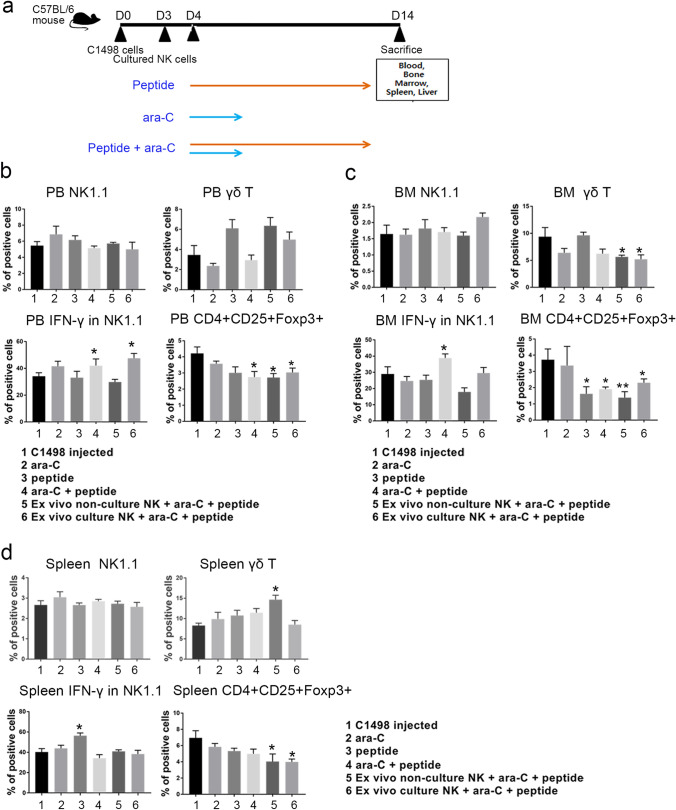
To address effects of peptide with conventional chemotherapeutic agents, a syngeneic leukemic mouse model was developed using C1498 leukemic cells and ex vivo cultured NK cells with or without peptide were used. Due to the importance of functional NK production with high IFN-γ in AML, ex vivo expanded NK cells with functionality and proliferation are regarded as an essential cell source in anti-tumor therapeutic strategies [10–12]. Thus, we injected expanded NK cells, which applied the novel P4, into tumor-bearing mice with exogenous P4 and ara-C together. It was used for in vivo experiments based on the protocol shown in Fig. 3a. FACS data showed that IFN-γ expression was increased in NK cells in the ara-C treatment and the NK cells-injected group co-cultured with the ex vivo peptide (Fig. 3b–c). We also found that CD4+CD25+Foxp3+ Treg cells in PB, BM, and spleen were dramatically decreased in all peptides and ara-C-treated groups, compared with that of leukemic group (Fig. 3b–d). It implied the role for P4 functioning in increasing IFN-γ from the activation of CD3 and NK cells along with regression of Treg cells in tumor microenvironments.
Fig. 3.
The therapeutic potential of P4 and its accompanying high frequency of NK and T cells and low number of Treg cells in leukemic mice. (a) Schematic diagram of the in vivo experiment using ara-C and the peptide together. The frequency of NK, γδ T cells, IFN-γ in NK cells, and Treg cells are shown in the PB (b), BM (c), and spleens (d) of leukemic mice. (b-d) **P < 0.01, *P < 0.05
Discussion
Based on our previous studies [7, 8], we developed novel peptides that can bind soluble FLT4 and replace MAZ51, and we found that P4 effectively increased IFN-γ by inhibiting FLT4 activation. Chang et al. [13] also confirmed the antagonistic action of several peptides as an antibody concept that can suppress FLT4 activity by binding soluble FLT4, and they showed the therapeutic potential of peptides by inhibiting FLT4 kinase activity in tumor progression. However, therapeutic suggestions for a peptide that inhibits FLT4 activity in the dysfunctional immune cells in tumor microenvironments remain still to be developed. Because the signaling domain of FLT4 is susceptible to disruption by homodimers, FLT4 activation can be hampered without antibodies by continuing interactions with other molecules such as peptides that target the wild-type intracellular domain of FLT4 [14]. Therefore, we carefully assumed that a peptide with a dominant negative action that binds to the wild-typed subunit could make an inactive oligomer that inhibits signal activation [15]. Aberrant expression of FLT4 on immune cells leads to dysfunctions that can decrease abnormal signaling by blocking the FLT4 signal cascade using the instability of the homodimer structure, which ultimately results in functional immune cell restoration. The discovery of peptide for NK activation is challenging in modulating cancer niche. Unlike MAZ51, P4 was restricted to FLT4 signaling, with no effects on other VEGFRs [16]. Consistent with H Nishikawa’s data, the ex vivo cultured NK cells in this study also functioned as effectors to increase IFN-γ expression and induce immunity in tumor microenvironments [17].
Although a significant progress has been made in applying next-generation sequencing to the detection and monitoring of multiple mutations, including FLT3-ITD and NPM1, from the initial diagnosis to the long-term follow-up period even after allogeneic hematopoietic stem cell transplantation, we found no definite correlative clue in our cohort of patients with AML due to mostly the small sample size. In addition, we tried to make a depicted heatmap with cluster analysis in association with FLT4 levels, but there was no good finding either (data not shown).
Taken together, these results suggest that the intracellular domain of a peptide targeting FLT4 can be used in combination with conventional chemotherapy to increase IFN-γ expression in NK cells and T cells. Furthermore, these results provide clues about advanced therapeutic approaches that could be used to correct the tumor microenvironment.
Author contributions
JY Lee, S Park, AR Han, and HS Hwang performed the experiments and analyzed the data. JY Lee and HJ Kim wrote the manuscripts.
Funding
This work was financially supported by a grant from the National Research Foundation (NRF, 2018R1D1A1A09083557), Ministry of Education, Republic of Korea.
Declarations
Conflict of interest
The authors declare no conflicts of interest.
Ethics approval and consent to participate
All experiments were performed with authorization from the Institutional Review Board for Human Research at the Catholic University of Korea (KC19TESI0462). The patients/participants provided their written informed consent to participate in this study. All protocols for testing the animals were approved by the Catholic University of Korea's Institutional Animal Care and Use Committee (CUMC-2019-0135-01).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Ji Yoon Lee and Soojin Park have contributed equally to this article.
References
- 1.Chen SJ, Shen Y, Chen Z (2013) A panoramic view of acute myeloid leukemia. Nat Genet 45(6):586–587 [DOI] [PubMed] [Google Scholar]
- 2.Wu D, Gao Y, Qi Y et al (2014) Peptide-based cancer therapy: opportunity and challenge. Cancer Lett 351(1):13–22 [DOI] [PubMed] [Google Scholar]
- 3.Baxter AA, Lay FT, Poon IKH et al (2017) Tumor cell membrane-targeting cationic antimicrobial peptides: novel insights into mechanisms of action and therapeutic prospects. Cell Mol Life Sci 74(20):3809–3825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leppanen VM, Tvorogov D, Kisko K et al (2013) Structural and mechanistic insights into VEGF receptor 3 ligand binding and activation. Proc Natl Acad Sci U S A 110(32):12960–12965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park S, Kim HJ, Hwang HS et al (2021) Peptides targeting FMS-related tyrosine kinase-4 activate the function of natural killer cells in acute myeloid Leukemia. Int J Stem Cells 14(4):400–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pizzolo G, Trentin L, Vinante F et al (1988) Natural killer cell function and lymphoid subpopulations in acute non-lymphoblastic leukaemia in complete remission. Br J Cancer 58(3):368–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee JY, Park S, Kim DC et al (2013) A VEGFR-3 antagonist increases IFN-gamma expression on low functioning NK cells in acute myeloid leukemia. J Clin Immunol 33(4):826–837 [DOI] [PubMed] [Google Scholar]
- 8.Lee JY, Park S, Min WS et al (2014) Restoration of natural killer cell cytotoxicity by VEGFR-3 inhibition in myelogenous leukemia. Cancer Lett 354(2):281–289 [DOI] [PubMed] [Google Scholar]
- 9.Lee JY, Kim HJ (2014) (Lymph)angiogenic influences on hematopoietic cells in acute myeloid leukemia. Exp Mol Med 46:e122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Terren I, Orrantia A, Vitalle J et al (2020) CFSE dilution to study human T and NK cell proliferation in vitro. Methods Enzymol 631:239–255 [DOI] [PubMed] [Google Scholar]
- 11.Choi YH, Lim EJ, Kim SW et al (2019) IL-27 enhances IL-15/IL-18-mediated activation of human natural killer cells. J Immunother Cancer 7(1):168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Waldmann TA (2006) The biology of interleukin-2 and interleukin-15: implications for cancer therapy and vaccine design. Nat Rev Immunol 6(8):595–601 [DOI] [PubMed] [Google Scholar]
- 13.Chang YW, Su CM, Su YH et al (2014) Novel peptides suppress VEGFR-3 activity and antagonize VEGFR-3-mediated oncogenic effects. Oncotarget 5(11):3823–3835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herskowitz I (1987) Functional inactivation of genes by dominant negative mutations. Nature 329(6136):219–222 [DOI] [PubMed] [Google Scholar]
- 15.Dorrity MW, Queitsch C, Fields S (2019) High-throughput identification of dominant negative polypeptides in yeast. Nat Methods 16(5):413–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kirkin V, Thiele W, Baumann P et al (2004) MAZ51, an indolinone that inhibits endothelial cell and tumor cell growth in vitro, suppresses tumor growth in vivo. Int J Cancer 112(6):986–993 [DOI] [PubMed] [Google Scholar]
- 17.Nishikawa H, Kato T, Tawara I et al (2005) IFN-gamma controls the generation/activation of CD4+CD25+ regulatory T cells in antitumor immune response. J Immunol 175(7):4433–4440 [DOI] [PubMed] [Google Scholar]