Abstract
CD19-specific chimeric antigen receptor T (CAR T) immunotherapy is used to treat B-cell malignancies. However, antigen-escape mediated relapse following CAR T therapy has emerged as a major concern. In some relapsed cases, especially KMT2A rearrangement-positive B-acute lymphoblastic leukemia (KMT2A-r B-ALL), most of the B-cell antigens are lost via lineage conversion to the myeloid phenotype, rendering multi-B-cell-antigen-targeted CAR T cell therapy ineffective. Fms-related tyrosine kinase-3 (FLT3) is highly expressed in KMT2A-r B-ALL; therefore, in this study, we aimed to evaluate the antitumor efficacy of CAR T cells targeting both CD19 and FLT3 in KMT2A-r B-ALL cells. We developed piggyBac transposon-mediated CAR T cells targeting CD19, FLT3, or both (dual) and generated CD19-negative KMT2A-r B-ALL models through CRISPR-induced CD19 gene-knockout (KO). FLT3 CAR T cells showed antitumor efficacy against CD19-KO KMT2A-r B-ALL cells both in vitro and in vivo; dual-targeted CAR T cells showed cytotoxicity against wild-type (WT) and CD19-KO KMT2A-r B-ALL cells, whereas CD19 CAR T cells demonstrated cytotoxicity only against WT KMT2A-r B-ALL cells in vitro. Therefore, targeting FLT3-specific CAR T cells would be a promising strategy for KMT2A-r B-ALL cells even with CD19-negative relapsed cases.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00262-022-03303-4.
Keywords: CAR T cell therapy, FLT3, piggyBac transposon, KMT2A gene rearrangement, Lineage switch
Introduction
CD19-targeted chimeric antigen receptor (CAR) T cell therapies have radically improved the outcomes for children and young adults with relapsed/refractory B-acute lymphoblastic leukemia (B-ALL); however, despite impressive initial responses, long-term remission occurs only in half of the patients with B-ALL [1–5]. CD19-negative relapse with CD19 surface expression loss is the predominant cause of treatment failure in patients treated with CD19-targeted CAR T cells; therefore, multi-B-cell antigen-targeted CAR T cells have been developed to overcome this issue [6–11], including CD19/CD22 [7], CD19/CD20 [9], and CD19/BAFF-R [11] dual-specific CAR T cells.
Despite improvements in the clinical management and survival of B-ALL, B-ALL patients with 11q23 translocations/KMT2A rearrangement (KMT2A-r), especially infants, continue to demonstrate the poorest outcomes [12–15]. Furthermore, relapsed cases with loss of B-cell antigens due to a lineage switch from B-ALL to the myeloid phenotype in KMT2A-r positive ALL (KMT2A-r ALL) following CD19-targeted immunotherapy have been reported [16–19]. In such relapsed cases, because of the loss of B lymphoid lineage, B-cell antigen-targeted immunotherapies including CAR T treatment are ineffective.
Fms-related tyrosine kinase-3 (FLT3) is highly expressed in KMT2A-r B-ALL [20, 21]. Using CRISPR-interference functional genomic screening, Nix et al. [22] revealed that FLT3 is the only protein that shows specific upregulation and significant genetic dependence in KMT2A-r B-ALL; therefore, FLT3 is an optimal immunotherapeutic target for KMT2A-r B-ALL. This study aimed to generate CAR T cells with dual targeting of CD19 and FLT3 and evaluate their antitumor efficacy in KMT2A-r B-ALL both in vitro and in vivo.
Materials and methods
Ethics approval statement
This study was approved by the Institutional Review Board of Kyoto Prefectural University of Medicine (Approval Numbers: ERB-C-669 and ERB-C-1406), and recombinant DNA experiments were approved by the safety committee of the Kyoto Prefectural University of Medicine (Approval Numbers 2019-111 and 2019-112). All experiments involving human participants were performed in accordance with the Declaration of Helsinki, and all participants provided written informed consent. All animal experiments and procedures were approved by the Kyoto Prefectural University of Medicine Institutional Review Board (Approval Number: M2020-13).
Blood sample collection and cell lines
Blood samples from healthy donors were obtained with written informed consent (3 donors). Peripheral blood mononuclear cells (PBMCs) were isolated from whole blood samples using density gradient centrifugation and lymphocyte separation medium 1077 (FUJIFILM Wako Pure Chemical Corporation, Osaka, Japan), followed by multiple washes with Dulbecco’s phosphate-buffered saline (D-PBS; Nakarai Tesque Inc., Kyoto, Japan). The number of living cells was determined using standard trypan blue staining and an automated cell counter (model R1; Olympus, Tokyo, Japan). The human B-cell precursor leukemia (REH), human acute myeloid leukemia (AML) with KMT2A-r (THP-1 and MV4-11), and human T cell leukemia (JURKAT) cell lines were purchased from the American Type Culture Collection (Manassas, VA, USA). Infant B-acute lymphoblastoid leukemia with KMT2A-r cell lines (KOCL44 and KOCL58) were established in the department of pediatrics, school of medicine, university of Yamanashi [23]. MV4-11 had FLT3-internal tandem duplication (ITD) mutations, whereas other cell lines had no FLT3 mutations. All cell lines expressing firefly luciferase (FFLuc) and green fluorescent protein (GFP) were obtained by introducing piggyBac transposon (PB)-based pIRII-FFLuc-puroR-GFP [24] into the cells and subsequent fluorescent-activated cell sorting. All cells were cultured in RPMI-1640 medium (Nacalai Tesque Inc.) supplemented with 10% fetal bovine serum (Thermo Fisher Scientific, Inc., Waltham, MA, USA) and maintained in a humidified incubator at 37 °C in a 5% CO2 atmosphere.
Generation of CD19-knockout cell lines
CD19-knockout cell lines (KOCL44 CD19-KO and KOCL58 CD19-KO) were generated using the CRISPR-Cas9 system and CD19 sgRNA CRISPR All-in-One Lentivirus product (Applied Biological Materials Inc., Richmond, Canada) according to the manufacturer’s instructions. Briefly, a lentiviral plasmid (pLenti-U6-sgRNA-SFFV-Cas9-2A-Puro) was transfected into cells via lentiviral infection. The infected cells were selected for stable expression using puromycin selection and expanded. CD19-deficient clones were sorted, and stable CD19-KO cells were verified using flow cytometry. These CD19-KO cell lines were also transfected with the PB-based pIRII-FFLuc-puroR-GFP plasmid to express FFLuc and GFP.
FLT3 CAR design
We generated PB transposon-mediated transgenes pIRII-FLT3 scFv-CAR-28z and pIRII-FLT3LG-CAR-28z, which enable transduced cells to express FLT3-CAR. For the specific recognition of FLT3, we utilized either FLT3 single-chain fragment variable (scFv) or the natural ligand of FLT3, FLT3LG. We synthesized cDNA containing the VH and VL chains from the single-chain variable regions (scFv) of the FLT3 monoclonal antibody (EB10) [25] fused with the IgG1-derived CH2CH3 sequence and the extracellular domain of human FLT3LG (Accession Number: NP_001450) encoded by FLT3LG (Accession Number: NM_001459), respectively. These were cloned into the PB transposon vector by replacing the CD19 scFv portion of pIRII-CD19-CAR (Supplementary Fig. 1) [26]. To reduce the non-specific recognition of the spacer region by FcγR-expressing macrophages, we replaced the IgG1 CH2CH3 spacer region of the FLT3-CAR vectors with either the IgG1 CH3 region (CH3) [27] or three repeated sequences of Gly-Gly-Gly-Gly-Ser (G4S)3 [28, 29]. The truncated CD34 sequence (Accession Number: NM_001025109) was cloned into pIRII-FLT3-G4S with T2A self-cleaving sites for the detection of CAR expression (Supplementary Fig. 2a). To produce antigen-presenting feeder cells, we generated an antigen-presenting feeder plasmid (pIRII-tFLT3-CD80-41BBL) that enabled transduced cells to express truncated FLT3 protein, CD80, and 4-1BBL. The truncated FLT3 sequence encoded the extracellular, transmembrane, and 20 amino-acid long intracellular portions of the FLT3 protein (Accession Number: NM_004119) and was cloned into the PB transposon vector by replacing the CD19 portion of pIRII-tCD19-CD80-41BBL (Supplementary Fig. 1) [26].
Generating PB-mediated CAR T cells
PB-FLT3 CAR T cells were generated from healthy donor PBMCs using PB transposon-mediated gene transfer, as described previously [24, 26]. Briefly, approximately 20 × 106 PBMCs were electroporated with 7.5 µg of the PB transposase and pIRII-FLT3 CAR transposon plasmids (Supplementary Fig. 2a) using the P3 Primary Cell 4D-Nucleofector™ X kit (Lonza, Basel, Switzerland, Program; FI-115). Concurrently, the antigen-presenting feeder plasmid (pIRII-tFLT3-CD80-41BBL; 15 μg) (Supplementary Fig. 1) was introduced into approximately 10 × 106 PBMCs via electroporation. For FLT3-CD19 dual CAR T cells, approximately 20 × 106 PBMCs were electroporated with 5 µg of the PB transposase plasmid, 7 µg of the pIRII-FLT3 CAR transposon plasmid, and 3 µg of the pIRII-CD19-28z CAR transposon plasmid. Approximately 10 × 106 PBMCs were electroporated with 10 µg of the pIRII-tFLT3-CD80-41BBL plasmid and 5 µg of the pIRII-tCD19-CD80-41BBL plasmid for feeder cells (Supplementary Fig. 1). Following electroporation, CAR T and feeder cells were cultured in a complete culture medium consisting of ALyS™705 Medium (Cell Science & Technology Institute Inc., Miyagi, Japan) supplemented with 5% artificial serum (Animal-free; Cell Science & Technology Institute Inc., Miyagi, Japan), IL-7 (10 ng/mL; Miltenyi Biotec, Bergisch Gladbach, Germany), and IL-15 (5 ng/mL; Miltenyi Biotec, Bergisch Gladbach, Germany). Feeder cells were irradiated with ultraviolet light to inactivate them 24 h after electroporation and co-cultured with CAR T cells for 14 days as described previously [24, 26, 30]. CAR T cells redirected to the EPHB4 receptor were generated using the PB transposon system as described previously [30]; these cells were used as control CAR T cells in the in vitro experiments (Supplementary Fig. 1).
Flow cytometry
The expression of FLT3-CH3-CAR on the T cell surface was measured using flow cytometry with a goat anti-human IgG Fc fragment specific antibody (Merck Millipore, Burlington, MA, USA) and an anti-goat IgG specific antibody conjugated to phycoerythrin (PE) to detect the CH3 region. The expression of FLT3-G4S-CAR was measured using fluorescein an isothiocyanate (FITC)-conjugated anti-CD34 antibody (BioLegend, San Diego, CA, USA). CD19-CAR expression was measured using an FITC-labeled monoclonal anti-FMC63 scFv antibody (ACROBiosystems Inc., Newark, DE, USA). Allophycocyanin (APC)-conjugated anti-PD-1, FITC-conjugated anti-CD45RA, and APC-conjugated anti-CCR7 antibodies (all from BioLegend) were used to characterize CAR T-cell phenotypes. PE-conjugated anti-FLT3 and FITC-conjugated anti-CD19 antibodies were used to determine expression on cell line surfaces, and APC-conjugated anti-CD3 antibody was used to identify CAR T cells in vivo and in vitro (all from BioLegend). All flow cytometry data were acquired using BD Accuri™ C6 Plus (BD Biosciences, Franklin Lakes, NJ, USA) and analyzed using FlowJo™ software (BD Biosciences).
In vitro cytotoxicity
FLT3 CAR T or EPHB4 CAR T (control CAR T) cells and GFP-expressing tumor cells were co-cultured with effector-to-target (E:T) ratios of 0:1 (tumor cells only), 1:10, 1:5, 1:2, and 1:1 in 24-well cell culture plates. Three days later, the cytotoxicity of CAR T cells was assessed using flow cytometry, and the survival of tumor cells was determined by measuring GFP expression and defined by the exclusion of 7-amino-actinomycin D and the absence of CD3 expression.
Cytokine production assay
The levels of interferon (IFN)-γ, tumor necrosis factor (TNF), and IL-2 were measured using a cytometric bead array (CBA) kit (BD Biosciences). Briefly, CAR T cells were co-cultured with tumor cells at a ratio of 1:1. After 24 h of co-culture, the cell culture supernatant was collected, and cytokine levels were determined and analyzed. Data were acquired with a BD Accuri C6 Plus system (BD Biosciences) and analyzed using FCAP Array v.3.0 (BD Biosciences).
In vivo experiments
Six-week-old female NOD.Cg-PrkdcscidIl2rgtm1Wjl/SzJ (NSG) mice were purchased from Jackson Laboratory (Bar Harbor, ME, USA) and housed at the Kyoto Prefectural University of Medicine for over a week before the start of the experiments. Food and water were provided ad libitum. Then, 5 × 105 KOCL44 CD19-KO-FFLuc-GFP cells suspended in D-PBS were injected into mice via the tail vein. Three and six days later, 7.5 × 106 FLT3 CAR T, dual CAR T, CD19 CAR T cells, or D-PBS was infused via the tail vein, and tumor burdens were monitored using the IVIS Lumina Series III system (PerkinElmer, Inc., Waltham, MA, USA), and regions of interest on the displayed images were quantified in photons per second (ph/s) using Living Image v2 (PerkinElmer, Inc.) as described previously [30]. Bone marrow (BM) cells were obtained by sequential BM aspiration from tibias at several time points. The BM cells were stained with an APC-conjugated anti-human CD3 antibody (BioLegend), and the long-term persistence of human T cells was evaluated using flow cytometry. The mice were euthanized at predefined endpoints under conditions that met the euthanasia criteria stipulated by the Center for Comparative Medicine at the Kyoto Prefectural University of Medicine.
Statistical analysis
Statistical comparisons between groups were determined using two-tailed parametric or non-parametric tests (Mann–Whitney U-test) for unpaired data or two-tailed paired Student's t-tests for matched samples. All data are presented as the mean ± standard deviation. The log-rank test was used to compare survival curves obtained using the Kaplan–Meier method. Statistical significance was set at P < 0.05. All statistical analyses were performed using Prism 9 software (GraphPad Software, San Diego, USA).
Results
Generation and characterization of FLT3‐specific CAR T cells via PB transposon‐based gene transfer
First, to identify optimal anti-FLT3 specific CAR T cells, we generated two types of antigen recognition sites for FLT3, the EB10 single-chain variable fragments (scFv) and the natural ligand of FLT3 (FLT3LG), and two types of spacer sites (an IgG1 CH3 region and a G4S linker) (Supplementary Fig. 2a). After 14 days of CAR T cells culture, the total cell number and CAR expression on T cells of these FLT3 CAR T cells were assessed using flow cytometry (Supplementary Fig. 2b, c). FLT3 scFv CH3 CAR T cells showed superior expansion capacity and highest CAR expression after expansion for 14 days; therefore, FLT3 scFv CH3 CAR T cells were selected as the lead CARs for further development (Fig. 1a). PB-FLT3 scFv CH3 CAR T cells were successfully generated with a positivity rate of 47.7% ± 2.6% for FLT3-CAR at day 14 after transfection. PD-1 was scarcely expressed on PB- FLT3 scFv CH3 CAR T cells (1.4% ± 0.7% in CAR-positive T cells), and PB-FLT3 scFv CH3 CAR T cells predominantly comprised the naïve/stem cell memory fraction (CD45RA+CCR7+ fraction; 83.4% ± 4.8%; Fig. 1b).
Fig. 1.
Generation of PB-FLT3 CAR T cells and their characteristics. a Schematic representation of a transposon plasmid expressing the FLT3-CAR construct. ITR, internal tandem repeat; TM, transmembrane domain; cyto, cytoplasmic domain. b Representative flow cytometry dot plots of PB-FLT3 CAR T cell characteristics regarding CAR expression, programmed cell death-1 (PD-1) expression, and differentiation profiles in CAR T cells on day 14 after transfection
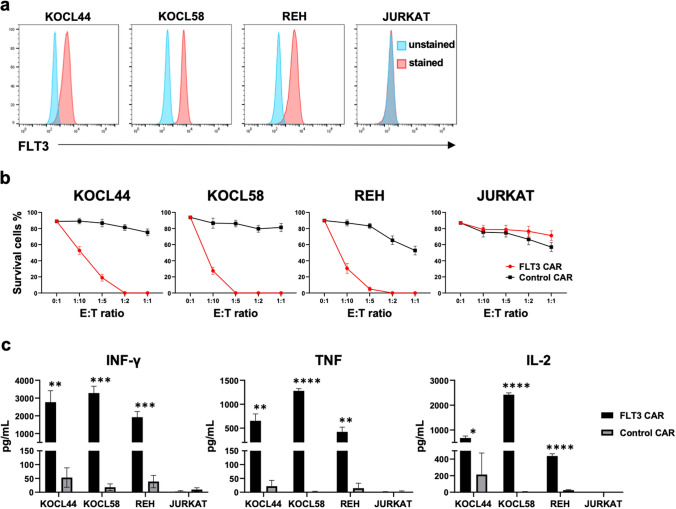
Cytotoxic effects of PB-FLT3 CAR T cells in FLT3-positive leukemia cells in vitro
We evaluated the cytotoxic effects of PB-FLT3 CAR T cells on leukemic cells using co-culture assays. First, we assessed the expression of FLT3 in leukemic cell lines using flow cytometry; FLT3 was positive in KOCL44, KOCL58, REH, THP-1, and MV4-11 cells and negative in JURKAT cells (Fig. 2a, Supplementary Fig. 3a). PB-FLT3 CAR T and control CAR T cells were co-cultured with these cell lines at various E:T ratios, and the survival of tumor cells was measured using flow cytometry. PB-FLT3 CAR T cells exhibited antigen-specific cytotoxicity against FLT3 positive leukemic cells, in proportion to the E:T ratio (Fig. 2b, Supplementary Fig. 3b). We also evaluated the production of inflammatory cytokines in the cell culture supernatant in response to co-culture with leukemia cells; production of IFN-γ, TNF, and IL-2 was observed only in PB-FLT3 CAR T cells with FLT3-positive leukemia cells (Fig. 2c, Supplementary Fig. 3c).
Fig. 2.
Cytotoxic effects of PB-FLT3 CAR T cells on FLT3-positive ALL cells in vitro. a FLT3 expression in ALL cell lines analyzed using flow cytometry. b Cytotoxicity of PB-FLT3 CAR T cells co-cultured for 72 h with ALL cell lines at the indicated effector-to-target (E:T) ratio. The survival of ALL cells was assessed using flow cytometry (n = 3). c Cytokine levels in the co-culture supernatant containing CAR T cells and ALL cells after 24 h of co-culture (n = 3). All data are presented as the mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001
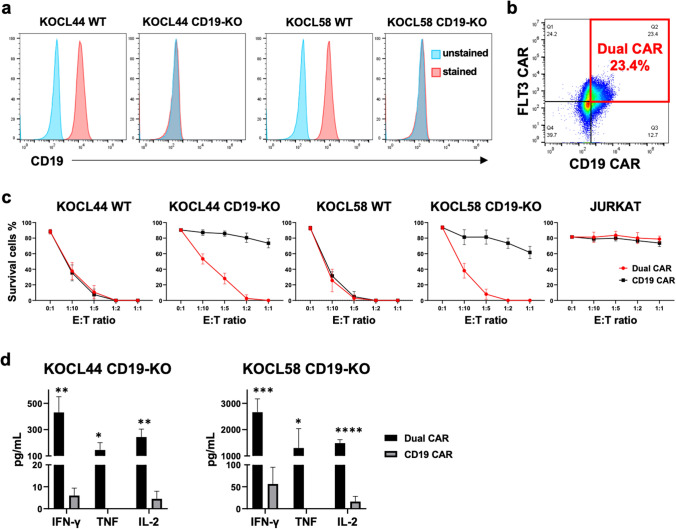
In vitro therapeutic effects of PB-FLT3 and CD19-dual CAR T cells in CD19-KO infant lymphoblastic leukemia cell lines with KMT2A-r
CD19-negative immune escape is frequently observed in relapsed KMT2A-r B-ALL cells after CD19-targeted therapy [16]. Therefore, we modeled CD19-negative KMT2A-r B-ALL cells via CRISPR-induced CD19 gene-knockout (KO) (KOCL44 CD19-KO and KOCL58 CD19-KO) and confirmed stable and complete CD19-KO cells using flow cytometry (Fig. 3a). To evaluate the efficacy of FLT3-targeted cell therapy against the CD19-negative immune escape model of KMT2A-r B-ALL cells, we introduced the FLT3 and CD19 CAR plasmids into T cells to target both CD19 and FLT3 (dual CAR T). Dual CAR T cells were generated with a positivity rate of 23.0% ± 0.5% for both FLT3 and CD19 CAR, 14 days after transfection (Fig. 3b). We then evaluated the cytotoxicity of either dual CAR T or CD19 CAR T cells against wild-type (WT), CD19-KO KMT2A-r B-ALL, and JURKAT (control) cells in vitro. CD19 CAR T cells showed cytotoxic effects only in WT KMT2A-r B-ALL cells, whereas dual CAR T cells showed cytotoxic effects in both WT and CD19-KO KMT2A-r B-ALL cells, in proportion to the E:T ratio (Fig. 3c). In addition, IFN-γ, TNF, and IL-2 production were observed only in dual CAR T cells with CD19-KO KMT2A-r B-ALL cells (Fig. 3d). These data indicate that FLT3-targeted CAR T cells have a cytotoxic effect in CD19-negative immune escape models of KMT2A-r B-ALL cells.
Fig. 3.
Cytotoxic effects of dual-targeted CAR T cells on CD19-knockout KMT2A-r ALL cells in vitro. a Flow cytometry analysis of CD19 expression in wild-type (WT) and CD19-KO KMT2A-r ALL cells. b Representative flow cytometry dot plots of CAR expression in dual CAR T cells. c Cytotoxicity of dual CAR T cells co-cultured for 72 h with leukemic cell lines at the indicated effector-to-target (E:T) ratio compared to CD19 CAR T cells. The survival of the leukemic cells was assessed using flow cytometry (n = 3). d Cytokine levels in the co-culture supernatant containing CAR T cells and CD19-KO KMT2A-r ALL cells after 24 h of co-culture (n = 3). All data are presented as the mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001
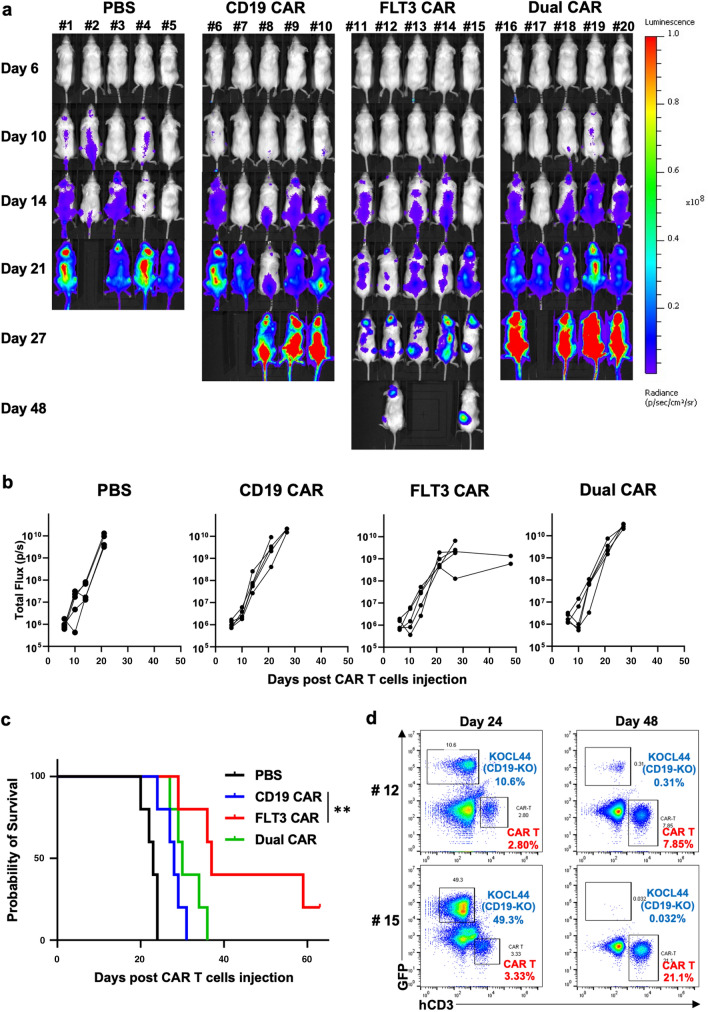
FLT3-targeted CAR T cells prolong the survival of CD19-KO KMT2A-r B-ALL bearing mice
To evaluate the antitumor efficacy of FLT3-targeted CAR T cells in vivo, KOCL44 CD19-KO-FFLuc bearing NSG female mice were divided into four groups (n = 5 each) and were treated with intravenous injections of FLT3 CAR T, dual CAR T, CD19 CAR T cells, or PBS. FLT3 CAR T cells induced more significant tumor reduction and prolonged median survival compared to CD19 CAR T cells (Fig. 4a–c). Furthermore, in two of the long-lived mice in the FLT3 CAR group, human CD3+ T cells were expanded, and leukemic cells were controlled even after 48 days of treatment, as confirmed by sequential BM studies (Fig. 4d), which indicated the antigen-induced proliferation and long-term functionality of FLT3 CAR T cells in vivo. In contrast, dual CAR T cells did not demonstrate beneficial antitumor efficacy over CD19-CAR T cells or FLT3-CAR T cells in vivo (Fig. 4a–c).
Fig. 4.
PB-FLT3 CAR T cells prolong the survival of CD19-knockout KMT2A-r ALL cells in vivo. We injected 5 × 105 KOCL44 CD19-KO-FFLuc cells into NSG mice via tail vein. Three and six days later, 7.5 × 106 FLT3 CAR T, dual CAR T, CD19 CAR T cells, or D-PBS was injected into the tail vein of each mouse. a Bioluminescence images of groups of five NSG mice after the first intravenous CAR T cell injection. b Tumor volumes of each mouse in each group measured as the total flux (p/s). The FLT3 CAR T cell group demonstrates a significant tumor reduction, measured as the mean total flux at day 27, compared with the CD19 CAR T cell group. P < 0.001. c The Kaplan–Meier plot of overall survival (n = 5 per group). The FLT3 CAR T cell group achieves prolonged tumor control compared to the CD19 CAR T cell group. Log-rank test: **P < 0.01. d Most long-lived mice (#12 and #15) were injected with FLT3 CAR T cells and leukemic cells. Representative dot plots of their bone marrow on days 24 and 48 showing decrease of leukemic cells and expansion of CAR T cells
Discussion
In this study, we generated PB transposon-mediated CAR T cells targeting FLT3, which optimized the antigen recognition portion of FLT3 and the spacer region. PB-FLT3 scFv CH3 CAR was selected as the lead CAR, and these CAR T cells showed antitumor efficacy against the CD19-negative immune escape model of KMT2A-r B-ALL cells, both in vitro and in vivo. Furthermore, we generated CAR T cells with dual targeting of CD19 and FLT3, which showed cytotoxic effects against both WT and CD19-KO KMT2A-r B-ALL cells, whereas CD19 CAR T cells demonstrated cytotoxic effects only against WT KMT2A-r B-ALL cells in vitro. Although dual CAR T cells should be further optimized to increase their in vivo cytotoxicity, targeting FLT3-specific CAR T cells would be a promising strategy for KMT2A-r B-ALL cells even with a CD19-negative relapsed model.
KMT2A-r B-ALL is clearly distinguished from both conventional ALL and AML by gene expression profiles and has the feature of constitutive wild-type FLT3 overexpression [20, 21]. In primary KMT2A-r infantile B-ALL samples, high-level FLT3 expression is associated with FLT3 ligand-independent FLT3 activation [31] and is associated with worse outcomes [32]. Even though FLT3 is an optimal target for KMT2A-r B-ALL and FLT3 inhibitor (FLT3i) has been investigated for the treatment of KMT2A-r B-ALL, there was no difference in 3-year event-free survival (EFS) between the KMT2A-r B-ALL patients treated with chemotherapy plus FLT3i lestaurtinib compared to chemotherapy alone [33]. The effectiveness of FLT3i against KMT2A-r B-ALL patients correlated with the pharmacodynamics (PD) profiles and ex vivo sensitivity (EVS) of FLT3i which would be associated with the activation or mutation status of FLT3 on the leukemic blasts. Since FLT3 is highly expressed in KMT2A-r B-ALL regardless of mutations of FLT3 gene [20, 21], and CAR T cells directly recognize FLT3 regardless of its activation or mutation status, FLT3 is an optimal target of CAR T cells for KMT2A-r B-ALL.
Immune escape due to loss of antigen surface expression is a major concern in CAR T cell therapy [34]. In recent clinical trials, relapse after antigen-based immunotherapy or CAR T cell therapy due to loss or diminished expression of the targeted antigen has been observed [1–6], which indicates the importance of targeting multiple antigens on tumor cells to reduce antigen loss-induced relapse. There are several approaches to overcome this issue, such as targeting two different antigens on tumor cells. The most straightforward approach is cocktail therapy with a mixture of varying CAR T cells (pooled CAR) [8], which requires two different CAR T cells products for manufacturing. Another approach is the co-transduction of two other CAR vectors or a bicistronic CAR vector to produce T cells expressing two CARs targeting distinct antigens [35, 36]. However, expressing both CARs at a similar density level is challenging [37], and this dual transduction strategy usually results in a mixture of cells expressing both single and dual transduced CAR T cells. The transduction strategy of a bicistronic CAR vector allows both CARs to be expressed in all transduced cells at the same density level; however, the expression of two different CARs in one vector may require codon optimization, and the combination of co-stimulatory domains in bicistronic CAR T cells also requires optimization [38]. Another alternative approach is to design a receptor with two distinct antigen recognition domains and a single co-stimulatory domain in a tandem CAR [6, 39, 40]. Compared with bicistronic CARs, tandem CARs have the advantage of a smaller transgene size but require optimization of the spacer length and linker sequence between the two antigen recognition domains. Hegde et al. revealed that compared to pooled CAR T cells, bicistronic CAR T cells induced more significant tumor reduction and prolonged median survival, and compared to bicistronic CAR T cells, tandem CAR T cells induced greater tumor reduction and prolonged median survival in vivo [35, 39]. Furthermore, in a recent clinical trial, CAR T cells with dual targeting of CD19 and CD22 treatment in pediatric and young adult patients with relapsed or refractory B-ALL were not found superior to the standard approved CD19 CAR T cell therapy [1, 7]. Therefore, the function of dual-targeted CAR T cells should be improved. In this study, we aimed to demonstrate the importance of dual-targeted CAR T cells in a CD19-KO relapsed model of KMT2A-r B-ALL cells, thus generating dual CAR T cells using a dual transduction strategy. Although dual CAR T cells showed cytotoxicity against CD19-KO KMT2A-r B-ALL cells in vitro, they did not show beneficial antitumor efficacy over CD19 CAR T or FLT3 CAR T cells in vivo, which is a limitation of our study. One of the conceivable factors of inefficiency of dual CAR T cells in vivo is low CAR expression in T cells for both FLT3 and CD19 CAR; only positivity rate of 23.0% ± 0.5% (Fig. 3b). These differences of FLT3 CAR expression in T cells between FLT3 CAR T and dual CAR T may cause differences of in vivo efficacy, so we need to improve the manufacturing of dual CAR T cells to achieve high levels of CAR expression as dual CAR T. Furthermore, a recent study revealed that the combination of a spacer region and transmembrane and co-stimulatory domains should be customized to produce an effective multivalent CAR. Hirabayashi et al. [38] demonstrated that the optimal configuration of dual CAR, in GD2/B7-H3 dual CAR T cells, comprised CD8-derived transmembrane domain with CD28 co-stimulatory domain and CD3ζ chain for one CAR construct, and CD8-derived transmembrane domain with only 4-1BB co-stimulatory domain (no CD3ζ chain) for another CAR construct. Therefore, by exploring the different combinations of each CAR component, the in vivo efficacy of our CD19/FLT3 dual CAR T cells can be further improved.
Although several studies have reported the efficacy of FLT3-targeted CAR T cells in the treatment of AML [41–43], its efficacy in ALL has not been well-studied. Niswander et al. have recently reported that targeting FLT3 by CAR T cells was highly effective against FLT3-mutant AML and KMT2A-r B-ALL. They demonstrated the anti-tumor potency of FLT3 CAR T cells for KMT2A-r B-ALL, including a post-tisagenlecleucel ALL-to-AML lineage switch patient-derived xenograft model [44]. Furthermore, they also demonstrated significant in vitro and in vivo anti-tumor efficacy of bispecific CD19xFLT3 CAR T against KMT2A-r B-ALL. They utilized anti-FLT3-scFv (NC7) as an antigen binding domain with a lower affinity than EB10 anti-FLT3-scFv we used in this study [25]. Moreover, they included CD28 for CD19 CAR and 4-1BB for FLT3 CAR as a co-stimulatory domain. All of these components are strongly associated with the function of CAR T cells, which could be one of the explanations for the differences in the anti-tumor efficacy of CD19xFLT3 CAR T cells between their study and ours. Therefore, the binding affinity of scFv, the co-stimulatory domain for each CAR construct, and the expression vector configuration should be optimized for the better function of dual-targeting CAR T cells.
Since FLT3 is expressed on normal hematopoietic stem and progenitor cells (HSPCs) [45], the off-target cytotoxicity of FLT3 CAR T therapies against HSPCs must be considered. FLT3 CAR T cells have minor cytotoxic effects on normal HSPCs [41, 42]. Furthermore, Sommer et al. [43] revealed that FLT3 CAR T cells showed sensitivity and on-target off-tumor toxicity against HSPCs in vitro, and a correlation between the magnitude of on-tumor activity and the off-tumor effects in vivo. Although we did not estimate the off-target cytotoxicity of FLT3 CAR T cells against normal HSPCs, we suggest the use of suicide switches that inactivate CAR T cell function or rescue hematopoietic stem cell transplantation following FLT3 CAR T cell therapy.
In conclusion, we demonstrated the utility of FLT3 CAR T cells in the treatment of KMT2A-r B-ALL. Targeting myeloid-lineage marker by CAR-T cells is a promising strategy for KMT2A-r B-ALL and would be a potent therapeutic option even for CD19-negative, lineage-switch relapsed cases.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors acknowledge Ms. Kumiko Yamashima and Ms. Mami Kotoura for their valuable technical assistance and Ms. Mika Tanimura, Ms. Ryoko Murata, and Ms. Yasuko Hashimoto for their secretarial assistance. The authors also thank Editage (http://www.editage.com) for proofreading, editing, and reviewing this manuscript. This work was supported by the Japan Agency for Medical Research and Development (AMED) (18ck0106413h0001), JSPS KAKENHI (19K08326) and Kawano Masanori Memorial Public Interest Incorporated Foundation for Promotion of Pediatrics.
Author contributions
Conceptualization, SY, YN, SO, TI, and TI; Methodology, MS, SY, HY, and YN; Investigation, MS, SY; Writing—Original Draft, MS, and SY; Writing—Review & Editing, MS, SY, SO, HY, TI, YN, TI, and TI; Funding Acquisition, SY and SO; Resources, SY, TI, and TI; Supervision, SY, TI, and TI.
Declarations
Conflict of interest
The authors have no financial relationship to declare related to this study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Maude SL, Laetsch TW, Buechner J, Rives S, Boyer M, Bittencourt H, Bader P, Verneris MR, Stefanski HE, Myers GD, Qayed M, De Moerloose B, Hiramatsu H, Schlis K, Davis KL, Martin PL, Nemecek ER, Yanik GA, Peters C, Baruchel A, Boissel N, Mechinaud F, Balduzzi A, Krueger J, June CH, Levine BL, Wood P, Taran T, Leung M, Mueller KT, Zhang Y, Sen K, Lebwohl D, Pulsipher MA, Grupp SA. Tisagenlecleucel in children and young adults with B-Cell lymphoblastic leukemia. N Engl J Med. 2018;378:439–448. doi: 10.1056/NEJMoa1709866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Park JH, Rivière I, Gonen M, Wang X, Sénéchal B, Curran KJ, Sauter C, Wang Y, Santomasso B, Mead E, Roshal M, Maslak P, Davila M, Brentjens RJ, Sadelain M. Long-term follow-up of CD19 CAR therapy in acute lymphoblastic leukemia. N Engl J Med. 2018;378:449–459. doi: 10.1056/NEJMoa1709919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shah NN, Lee DW, Yates B, Yuan CM, Shalabi H, Martin S, Wolters PL, Steinberg SM, Baker EH, Delbrook CP, Stetler-Stevenson M, Fry TJ, Stroncek DF, Mackall CL. Long-term follow-up of CD19-CAR T-cell therapy in children and young adults with B-ALL. J Clin Oncol. 2021;39:1650–1659. doi: 10.1200/jco.20.02262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang X, Lu XA, Yang J, Zhang G, Li J, Song L, Su Y, Shi Y, Zhang M, He J, Song D, Lv F, Li W, Wu Y, Wang H, Liu H, Zhou X, He T, Lu P. Efficacy and safety of anti-CD19 CAR T-cell therapy in 110 patients with B-cell acute lymphoblastic leukemia with high-risk features. Blood Adv. 2020;4:2325–2338. doi: 10.1182/bloodadvances.2020001466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ghorashian S, Kramer AM, Onuoha S, Wright G, Bartram J, Richardson R, Albon SJ, Casanovas-Company J, Castro F, Popova B, Villanueva K, Yeung J, Vetharoy W, Guvenel A, Wawrzyniecka PA, Mekkaoui L, Cheung GW, Pinner D, Chu J, Lucchini G, Silva J, Ciocarlie O, Lazareva A, Inglott S, Gilmour KC, Ahsan G, Ferrari M, Manzoor S, Champion K, Brooks T, Lopes A, Hackshaw A, Farzaneh F, Chiesa R, Rao K, Bonney D, Samarasinghe S, Goulden N, Vora A, Veys P, Hough R, Wynn R, Pule MA, Amrolia PJ. Enhanced CAR T cell expansion and prolonged persistence in pediatric patients with ALL treated with a low-affinity CD19 CAR. Nat Med. 2019;25:1408–1414. doi: 10.1038/s41591-019-0549-5. [DOI] [PubMed] [Google Scholar]
- 6.Fry TJ, Shah NN, Orentas RJ, Stetler-Stevenson M, Yuan CM, Ramakrishna S, Wolters P, Martin S, Delbrook C, Yates B, Shalabi H, Fountaine TJ, Shern JF, Majzner RG, Stroncek DF, Sabatino M, Feng Y, Dimitrov DS, Zhang L, Nguyen S, Qin H, Dropulic B, Lee DW, Mackall CL. CD22-targeted CAR T cells induce remission in B-ALL that is naive or resistant to CD19-targeted CAR immunotherapy. Nat Med. 2018;24:20–28. doi: 10.1038/nm.4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cordoba S, Onuoha S, Thomas S, Pignataro DS, Hough R, Ghorashian S, Vora A, Bonney D, Veys P, Rao K, Lucchini G, Chiesa R, Chu J, Clark L, Fung MM, Smith K, Peticone C, Al-Hajj M, Baldan V, Ferrari M, Srivastava S, Jha R, Arce Vargas F, Duffy K, Day W, Virgo P, Wheeler L, Hancock J, Farzaneh F, Domning S, Zhang Y, Khokhar NZ, Peddareddigari VGR, Wynn R, Pule M, Amrolia PJ. CAR T cells with dual targeting of CD19 and CD22 in pediatric and young adult patients with relapsed or refractory B cell acute lymphoblastic leukemia: a phase 1 trial. Nat Med. 2021;27:1797–1805. doi: 10.1038/s41591-021-01497-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang N, Hu X, Cao W, Li C, Xiao Y, Cao Y, Gu C, Zhang S, Chen L, Cheng J, Wang G, Zhou X, Zheng M, Mao X, Jiang L, Wang D, Wang Q, Lou Y, Cai H, Yan D, Zhang Y, Zhang T, Zhou J, Huang L. Efficacy and safety of CAR19/22 T-cell cocktail therapy in patients with refractory/relapsed B-cell malignancies. Blood. 2020;135:17–27. doi: 10.1182/blood.2019000017. [DOI] [PubMed] [Google Scholar]
- 9.Shah NN, Johnson BD, Schneider D, Zhu F, Szabo A, Keever-Taylor CA, Krueger W, Worden AA, Kadan MJ, Yim S, Cunningham A, Hamadani M, Fenske TS, Dropulić B, Orentas R, Hari P. Bispecific anti-CD20, anti-CD19 CAR T cells for relapsed B cell malignancies: a phase 1 dose escalation and expansion trial. Nat Med. 2020;26:1569–1575. doi: 10.1038/s41591-020-1081-3. [DOI] [PubMed] [Google Scholar]
- 10.Qin H, Dong Z, Wang X, Cheng WA, Wen F, Xue W, Sun H, Walter M, Wei G, Smith DL, Sun X, Fei F, Xie J, Panagopoulou TI, Chen CW, Song JY, Aldoss I, Kayembe C, Sarno L, Müschen M, Inghirami GG, Forman SJ, Kwak LW. CAR T cells targeting BAFF-R can overcome CD19 antigen loss in B cell malignancies. Sci Transl Med. 2019 doi: 10.1126/scitranslmed.aaw9414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang X, Dong Z, Awuah D, Chang WC, Cheng WA, Vyas V, Cha SC, Anderson AJ, Zhang T, Wang Z, Szymura SJ, Kuang BZ, Clark MC, Aldoss I, Forman SJ, Kwak LW, Qin H. CD19/BAFF-R dual-targeted CAR T cells for the treatment of mixed antigen-negative variants of acute lymphoblastic leukemia. Leukemia. 2022 doi: 10.1038/s41375-021-01477-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pui CH, Gaynon PS, Boyett JM, Chessells JM, Baruchel A, Kamps W, Silverman LB, Biondi A, Harms DO, Vilmer E, Schrappe M, Camitta B. Outcome of treatment in childhood acute lymphoblastic leukaemia with rearrangements of the 11q23 chromosomal region. Lancet. 2002;359:1909–1915. doi: 10.1016/s0140-6736(02)08782-2. [DOI] [PubMed] [Google Scholar]
- 13.Pieters R, Schrappe M, De Lorenzo P, Hann I, De Rossi G, Felice M, Hovi L, LeBlanc T, Szczepanski T, Ferster A, Janka G, Rubnitz J, Silverman L, Stary J, Campbell M, Li CK, Mann G, Suppiah R, Biondi A, Vora A, Valsecchi MG. A treatment protocol for infants younger than 1 year with acute lymphoblastic leukaemia (Interfant-99): an observational study and a multicentre randomised trial. Lancet. 2007;370:240–250. doi: 10.1016/s0140-6736(07)61126-x. [DOI] [PubMed] [Google Scholar]
- 14.Koh K, Tomizawa D, Moriya Saito A, Watanabe T, Miyamura T, Hirayama M, Takahashi Y, Ogawa A, Kato K, Sugita K, Sato T, Deguchi T, Hayashi Y, Takita J, Takeshita Y, Tsurusawa M, Horibe K, Mizutani S, Ishii E. Early use of allogeneic hematopoietic stem cell transplantation for infants with MLL gene-rearrangement-positive acute lymphoblastic leukemia. Leukemia. 2015;29:290–296. doi: 10.1038/leu.2014.172. [DOI] [PubMed] [Google Scholar]
- 15.Pieters R, De Lorenzo P, Ancliffe P, Aversa LA, Brethon B, Biondi A, Campbell M, Escherich G, Ferster A, Gardner RA, Kotecha RS, Lausen B, Li CK, Locatelli F, Attarbaschi A, Peters C, Rubnitz JE, Silverman LB, Stary J, Szczepanski T, Vora A, Schrappe M, Valsecchi MG. Outcome of Infants Younger Than 1 Year With Acute Lymphoblastic Leukemia Treated With the Interfant-06 Protocol: Results From an International Phase III Randomized Study. J Clin Oncol. 2019;37:2246–2256. doi: 10.1200/jco.19.00261. [DOI] [PubMed] [Google Scholar]
- 16.Gardner R, Wu D, Cherian S, Fang M, Hanafi LA, Finney O, Smithers H, Jensen MC, Riddell SR, Maloney DG, Turtle CJ. Acquisition of a CD19-negative myeloid phenotype allows immune escape of MLL-rearranged B-ALL from CD19 CAR-T-cell therapy. Blood. 2016;127:2406–2410. doi: 10.1182/blood-2015-08-665547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rayes A, McMasters RL, O'Brien MM. Lineage switch in MLL-rearranged infant leukemia following CD19-directed therapy. Pediatr Blood Cancer. 2016;63:1113–1115. doi: 10.1002/pbc.25953. [DOI] [PubMed] [Google Scholar]
- 18.Lamble AJ, Myers RM, Taraseviciute A, John S, Yates B, Steinberg SM, Sheppard J, Kovach AE, Wood BL, Borowitz M, Stetler-Stevenson M, Yuan CM, Pillai V, Foley T, Chung P, Chen L, Lee DW, Annesley C, DiNofia AM, Grupp SA, Verneris MR, Gore L, Laetsch TW, Bhojwani D, Brown PA, Pulsipher MA, Rheingold SR, Gardner RA, Shah NN. KMT2A rearrangements are associated with lineage switch following CD19 targeting CAR T-cell therapy. Blood. 2021;138:256–256. doi: 10.1182/blood-2021-153336. [DOI] [Google Scholar]
- 19.Semchenkova A, Mikhailova E, Komkov A, Gaskova M, Abasov R, Matveev E, Kazanov M, Mamedov I, Shmitko A, Belova V, Miroshnichenkova A, Illarionova O, Olshanskaya Y, Tsaur G, Verzhbitskaya T, Ponomareva N, Bronin G, Kondratchik K, Fechina L, Diakonova Y, Vavilova L, Myakova N, Novichkova G, Maschan A, Maschan M, Zerkalenkova E, Popov A. Lineage conversion in pediatric B-cell precursor acute leukemia under blinatumomab therapy. Int J Mol Sci. 2022 doi: 10.3390/ijms23074019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Armstrong SA, Staunton JE, Silverman LB, Pieters R, den Boer ML, Minden MD, Sallan SE, Lander ES, Golub TR, Korsmeyer SJ. MLL translocations specify a distinct gene expression profile that distinguishes a unique leukemia. Nat Genet. 2002;30:41–47. doi: 10.1038/ng765. [DOI] [PubMed] [Google Scholar]
- 21.Ferrando AA, Armstrong SA, Neuberg DS, Sallan SE, Silverman LB, Korsmeyer SJ, Look AT. Gene expression signatures in MLL-rearranged T-lineage and B-precursor acute leukemias: dominance of HOX dysregulation. Blood. 2003;102:262–268. doi: 10.1182/blood-2002-10-3221. [DOI] [PubMed] [Google Scholar]
- 22.Nix MA, Mandal K, Geng H, Paranjape N, Lin YT, Rivera JM, Marcoulis M, White KL, Whitman JD, Bapat SP, Parker KR, Ramirez J, Deucher A, Phojanokong P, Steri V, Fattahi F, Hann BC, Satpathy AT, Manglik A, Stieglitz E, Wiita AP. Surface proteomics reveals CD72 as a target for in vitro-evolved nanobody-based CAR-T cells in KMT2A/MLL1-rearranged B-ALL. Cancer Discov. 2021;11:2032–2049. doi: 10.1158/2159-8290.Cd-20-0242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Furuichi Y, Goi K, Inukai T, Sato H, Nemoto A, Takahashi K, Akahane K, Hirose K, Honna H, Kuroda I, Zhang X, Kagami K, Hayashi Y, Harigaya K, Nakazawa S, Sugita K. Fms-like tyrosine kinase 3 ligand stimulation induces MLL-rearranged leukemia cells into quiescence resistant to antileukemic agents. Cancer Res. 2007;67:9852–9861. doi: 10.1158/0008-5472.Can-07-0105. [DOI] [PubMed] [Google Scholar]
- 24.Nakamura K, Yagyu S, Hirota S, Tomida A, Kondo M, Shigeura T, Hasegawa A, Tanaka M, Nakazawa Y. Autologous antigen-presenting cells efficiently expand piggyBac transposon CAR-T cells with predominant memory phenotype. Mol Ther Methods Clin Dev. 2021;21:315–324. doi: 10.1016/j.omtm.2021.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yiwen Li (2009) ANTI-FLT3 ANTIBODIES. https://patents.google.com/patent/US8071099B2/en. Accessed 28 May 2009
- 26.Suematsu M, Yagyu S, Nagao N, Kubota S, Shimizu Y, Tanaka M, Nakazawa Y, Imamura T. PiggyBac transposon-mediated CD19 chimeric antigen receptor-T cells derived from CD45RA-positive peripheral blood mononuclear cells possess potent and sustained antileukemic function. Front Immunol. 2022 doi: 10.3389/fimmu.2022.770132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mamonkin M, Rouce RH, Tashiro H, Brenner MK. A T-cell-directed chimeric antigen receptor for the selective treatment of T-cell malignancies. Blood. 2015;126:983–992. doi: 10.1182/blood-2015-02-629527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bishop DC, Xu N, Tse B, O'Brien TA, Gottlieb DJ, Dolnikov A, Micklethwaite KP. PiggyBac-engineered T cells expressing CD19-specific CARs that Lack IgG1 Fc spacers have potent activity against B-ALL xenografts. Mol Ther. 2018;26:1883–1895. doi: 10.1016/j.ymthe.2018.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hasegawa A, Saito S, Narimatsu S, Nakano S, Nagai M, Ohnota H, Inada Y, Morokawa H, Nakashima I, Morita D, Ide Y, Matsuda K, Tashiro H, Yagyu S, Tanaka M, Nakazawa Y. Mutated GM-CSF-based CAR-T cells targeting CD116/CD131 complexes exhibit enhanced anti-tumor effects against acute myeloid leukaemia. Clin Transl Immunol. 2021;10:e1282. doi: 10.1002/cti2.1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kubo H, Yagyu S, Nakamura K, Yamashima K, Tomida A, Kikuchi K, Iehara T, Nakazawa Y, Hosoi H. Development of non-viral, ligand-dependent, EPHB4-specific chimeric antigen receptor T cells for treatment of rhabdomyosarcoma. Mol Ther Oncol. 2021;20:646–658. doi: 10.1016/j.omto.2021.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stam RW, den Boer ML, Schneider P, Nollau P, Horstmann M, Beverloo HB, van der Voort E, Valsecchi MG, de Lorenzo P, Sallan SE, Armstrong SA, Pieters R. Targeting FLT3 in primary MLL-gene-rearranged infant acute lymphoblastic leukemia. Blood. 2005;106:2484–2490. doi: 10.1182/blood-2004-09-3667. [DOI] [PubMed] [Google Scholar]
- 32.Stam RW, Schneider P, de Lorenzo P, Valsecchi MG, den Boer ML, Pieters R. Prognostic significance of high-level FLT3 expression in MLL-rearranged infant acute lymphoblastic leukemia. Blood. 2007;110:2774–2775. doi: 10.1182/blood-2007-05-091934. [DOI] [PubMed] [Google Scholar]
- 33.Brown PA, Kairalla JA, Hilden JM, Dreyer ZE, Carroll AJ, Heerema NA, Wang C, Devidas M, Gore L, Salzer WL, Winick NJ, Carroll WL, Raetz EA, Borowitz MJ, Small D, Loh ML, Hunger SP. FLT3 inhibitor lestaurtinib plus chemotherapy for newly diagnosed KMT2A-rearranged infant acute lymphoblastic leukemia: children's oncology Group trial AALL0631. Leukemia. 2021;35:1279–1290. doi: 10.1038/s41375-021-01177-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guedan S, Calderon H, Posey AD, Jr, Maus MV. Engineering and design of chimeric antigen receptors. Mol Ther Methods Clin Dev. 2019;12:145–156. doi: 10.1016/j.omtm.2018.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hegde M, Corder A, Chow KK, Mukherjee M, Ashoori A, Kew Y, Zhang YJ, Baskin DS, Merchant FA, Brawley VS, Byrd TT, Krebs S, Wu MF, Liu H, Heslop HE, Gottschalk S, Yvon E, Ahmed N. Combinational targeting offsets antigen escape and enhances effector functions of adoptively transferred T cells in glioblastoma. Mol Ther. 2013;21:2087–2101. doi: 10.1038/mt.2013.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ruella M, Barrett DM, Kenderian SS, Shestova O, Hofmann TJ, Perazzelli J, Klichinsky M, Aikawa V, Nazimuddin F, Kozlowski M, Scholler J, Lacey SF, Melenhorst JJ, Morrissette JJ, Christian DA, Hunter CA, Kalos M, Porter DL, June CH, Grupp SA, Gill S. Dual CD19 and CD123 targeting prevents antigen-loss relapses after CD19-directed immunotherapies. J Clin Invest. 2016;126:3814–3826. doi: 10.1172/jci87366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van der Schans JJ, van de Donk N, Mutis T. Dual targeting to overcome current challenges in multiple myeloma CAR T-Cell treatment. Front Oncol. 2020;10:1362. doi: 10.3389/fonc.2020.01362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hirabayashi K, Du H, Xu Y, Shou P, Zhou X, Fuca G, Landoni E, Sun C, Chen Y, Savoldo B, Dotti G. Dual targeting CAR-T cells with optimal costimulation and metabolic fitness enhance antitumor activity and prevent escape in solid tumors. Nat Cancer. 2021;2:904–918. doi: 10.1038/s43018-021-00244-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hegde M, Mukherjee M, Grada Z, Pignata A, Landi D, Navai SA, Wakefield A, Fousek K, Bielamowicz K, Chow KK, Brawley VS, Byrd TT, Krebs S, Gottschalk S, Wels WS, Baker ML, Dotti G, Mamonkin M, Brenner MK, Orange JS, Ahmed N. Tandem CAR T cells targeting HER2 and IL13Rα2 mitigate tumor antigen escape. J Clin Invest. 2016;126:3036–3052. doi: 10.1172/jci83416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grada Z, Hegde M, Byrd T, Shaffer DR, Ghazi A, Brawley VS, Corder A, Schönfeld K, Koch J, Dotti G, Heslop HE, Gottschalk S, Wels WS, Baker ML, Ahmed N. TanCAR: a novel bispecific chimeric antigen receptor for cancer immunotherapy. Mol Ther Nucleic Acids. 2013;2:e105. doi: 10.1038/mtna.2013.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen L, Mao H, Zhang J, Chu J, Devine S, Caligiuri MA, Yu J. Targeting FLT3 by chimeric antigen receptor T cells for the treatment of acute myeloid leukemia. Leukemia. 2017;31:1830–1834. doi: 10.1038/leu.2017.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang Y, Xu Y, Li S, Liu J, Xing Y, Xing H, Tian Z, Tang K, Rao Q, Wang M, Wang J. Targeting FLT3 in acute myeloid leukemia using ligand-based chimeric antigen receptor-engineered T cells. J Hematol Oncol. 2018;11:60. doi: 10.1186/s13045-018-0603-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sommer C, Cheng HY, Nguyen D, Dettling D, Yeung YA, Sutton J, Hamze M, Valton J, Smith J, Djuretic I, Chaparro-Riggers J, Sasu BJ. Allogeneic FLT3 CAR T cells with an off-switch exhibit potent activity against AML and can be depleted to expedite bone marrow recovery. Mol Ther. 2020;28:2237–2251. doi: 10.1016/j.ymthe.2020.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Niswander LM, Graff ZT, Chien CD, Chukinas JA, Meadows CA, Leach LC, Loftus JP, Kohler ME, Tasian SK, Fry TJ. Potent preclinical activity of FLT3-directed chimeric antigen receptor T cell immunotherapy against FLT3-mutant acute myeloid leukemia and KMT2A-rearranged acute lymphoblastic leukemia. Haematologica. 2022 doi: 10.3324/haematol.2022.281456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kikushige Y, Yoshimoto G, Miyamoto T, Iino T, Mori Y, Iwasaki H, Niiro H, Takenaka K, Nagafuji K, Harada M, Ishikawa F, Akashi K. Human Flt3 is expressed at the hematopoietic stem cell and the granulocyte/macrophage progenitor stages to maintain cell survival. J Immunol. 2008;180:7358–7367. doi: 10.4049/jimmunol.180.11.7358. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.