Abstract
Background
Hepatic immune-related adverse events (irAE) including elevated liver function tests (transaminases) occur in 1.4–22.3% of melanoma patients receiving immune checkpoint inhibitors (ICPI) and constitute a potentially serious toxicity that is challenging to treat. In contrast to the liver transaminases alanine aminotransferase (ALT) and aspartate aminotransferase (AST), only little is known about the frequency and impact of gamma-glutamyl transferase (GGT) elevations.
Methods
GGT determined prior to and during therapy of metastatic melanoma patients treated with ICPI were retrospectively assessed in two independent cohorts (PD-1: n = 218, Ipi + Nivo: n = 148). Overall survival (OS) and best objective response were analyzed according to baseline and immune-related GGT (irGGT) elevations during treatment.
Results
In multivariate analysis, OS was reduced in patients with elevated baseline GGT (PD-1 group: hazard ratio [HR] 1.76, p = .0073; Ipi + Nivo group: HR 1.77, p = .032). Immune-related GGT elevation was recorded in 17% (PD-1 group) and 38.5% (Ipi + Nivo group). Of these patients, the majority (81 and 68%, respectively) had normal ALT and AST and showed no clinical signs of hepatotoxicity. Patients who experienced irGGT elevation had superior response (PD-1 group: odds ratio [OR] 3.57, p = .00072; Ipi + Nivo group: OR 1.74, p = .12) and OS (PD-1 group: HR 0.37, p = .0016; Ipi + Nivo group: HR 0.33, p = .00050).
Conclusions
The frequency of hepatic irAE is currently underestimated. The addition of the sensitive enzyme GGT to the laboratory panel before and during therapy with ICPI allows to detect two to three times more patients developing hepatic or hepatobiliary toxicity than known so far. Immune-related GGT elevations correlate with response and favorable survival.
Precis for use in the Table of Contents
The frequency of hepatotoxicity under immune checkpoint blockade is currently underestimated. We suggest the addition of gamma-glutamyl transferase to the laboratory panel in checkpoint inhibitor patients for the detection of hepatobiliary toxicity.
Keywords: Gamma-glutamyl transferase, Melanoma, Immune checkpoint inhibitors, PD-1, Immune-related adverse events, Hepatotoxicity
Introduction
Immune-related adverse events (irAE) are a common phenomenon in cancer patients receiving immune checkpoint inhibitors (ICPI). In metastatic melanoma, clinically serious grade irAE (grade 3 or higher according to CTCAE criteria) occur in 10–16% of patients receiving PD-1 inhibitors [1, 2] and in 55–56% receiving combined immunotherapy with the CTLA-4 antibody ipilimumab and the PD-1 antibody nivolumab [3, 4]. Although irAE constitute a challenge for the clinician, their occurrence is now considered being related with a favorable outcome, even in cases when the treatment with ICPI must be quit and corticosteroids are necessary [5–7]. The prognostic impact of irAE differs between the distinct sites affected by this excessive immune response, with the skin being most clearly associated with favorable prognosis [8–11]. Hepatic irAE are less frequent compared to cutaneous irAE or diarrhea and colitis, yet they can run complicated and should be handled carefully to preserve the long-term function of this crucial metabolic organ [12–14]. In contrast to cutaneous or endocrine irAE, the prognostic impact of hepatic irAE remains elusive.
Liver metastasis is considered an unfavorable prognostic factor in melanoma patients treated with ICPI which is associated with lower CD8 + T-cell infiltration at the invasive tumor margin [15]. Moreover, the intracellular enzyme lactate dehydrogenase (LDH), a widely used serum biomarker in metastatic melanoma, increases exceptionally strong in patients with advanced hepatic metastases. Besides its well-known function as a parameter for cholestasis, the biliary enzyme gamma-glutamyl transferase (GGT) plays a fundamental role in the metabolism of glutathione [16]. In contrast to this function as an anti-oxidant enzyme, it has been shown that, under certain conditions, GGT is also able to exert pro-oxidant effects, promoting tumor formation and progression [17]. GGT is mainly expressed on the luminal surface of secretory epithelial cells, especially in epithelial cells of the hepato-biliary tract, the pancreas, and the kidneys [16]. In various malignancies, such as colorectal carcinoma [18, 19], urothelial carcinoma [20], endometrial carcinoma [21], renal cell carcinoma [22], and others, elevated levels of GGT correlated with impaired survival, higher disease stages, or presence of hepatic metastases. However, in metastatic uveal melanoma, the prognostic role of liver function tests (LFTs) including GGT in detecting metastasis was contradictory [23–26]. In metastatic cutaneous melanoma and in other cancer patients receiving ICPI, it is still unknown, whether LFTs are prognostically relevant. We conducted the present study to evaluate the association between baseline serum levels of GGT and survival of advanced melanoma patients receiving ICPI. The second aim of this study was to characterize GGT serum levels over time during treatment with ICPI. Based on the experience that GGT elevations occur frequently in patients receiving ICPI, our hypothesis before conducting this study was that GGT elevations constitute a hitherto underreported and undescribed hepatic or hepatobiliary irAE. We aimed at evaluating its association with response and survival in melanoma patients receiving either PD-1 antibodies or the combined immunotherapy with CTLA-4 and PD-1 antibodies.
Methods
Patients
From October 2013 to May 2019, 366 patients with unresectable melanoma were treated with pembrolizumab or nivolumab (referred to as PD-1 group, n = 218 patients) or ipilimumab plus nivolumab (also denoted as Ipi + Nivo group, n = 148 patients) and were enrolled retrospectively in this study. The two cohorts were analyzed separately to account for the major differences of PD-1 monotherapy and the combined PD-1 plus CTLA-4 blockade in respect of efficacy and toxicity profiles [3]. The study was carried out in accordance with the Declaration of Helsinki of 1975 and succeeding amendments. Approval to conduct this study was obtained from the local ethics committee of the Medical Faculty of University Tübingen (project No. 436/2017BO2).
Laboratory and clinical parameters
Clinical characteristics, including age and sex, American Joint Committee on Cancer (AJCC) clinical staging, and other factors, were extracted from clinical records. Laboratory tests, including gamma-glutamyl-transferase (GGT; upper limit of normal [ULN], male 60 U/l, female 40 U/l), alanine aminotransferase (ALT; normal range, male 10–50 U/l, female 10–34 U/l), aspartate aminotransferase (AST; normal range, male 10–50 U/l, female 10–35 U/l), total bilirubin (upper limit of normal 1.1 mg/dl), alkaline phosphatase (ALP; normal range 40–130 U/l), lactate dehydrogenase (LDH; upper limit of normal 250 U/l), were evaluated. Grading of immune-related adverse events (irAE) was done in accordance with the common toxicity criteria for adverse events (CTCAE) of the National Cancer Institute, version 5.0. For increased GGT, grade 1 refers to > ULN—2.5 × ULN, grade 2 refers to > 2.5—5.0 × ULN, grade 3 refers to > 5.0—20.0 × ULN, and grade 4 refers to > 20.0 × ULN. Treatment-related hepatitis was diagnosed based on ALT, AST, and bilirubin, irrespective of presence of hepatitis-related clinical symptoms or other related findings. Increases of liver enzymes during therapy due to hepatic metastasis, comedication, or infections were not considered as being immune related.
Immune checkpoint inhibitors
The patients in the PD-1 group were treated with either nivolumab (until 2018: 3 mg/kg intravenously every 2 weeks, since 2018: 480 mg flat dose every 4 weeks or 240 mg flat dose every 2 weeks) or pembrolizumab (until 2018: 2 mg/kg intravenously every 3 weeks, since 2018: 200 mg flat dose every 3 weeks), whereas the patients in the Ipi + Nivo group were treated with combined therapy with nivolumab 1 mg/kg followed by ipilimumab 3 mg/kg every 3 weeks for up to four cycles, continued with nivolumab 3 mg/kg every 2 weeks or 240 mg every 2 weeks (since 2018).
Statistical analysis
Overall survival (OS) was calculated from start of therapy with ICPI to death (event) or last follow-up (censored). Univariate analysis of OS was performed utilizing Kaplan–Meier estimator and two-sided log-rank test. Three-month landmark analysis as well as time-dependent Cox regression were applied to account for guarantee-time bias [27]. Multivariate analysis of OS was performed utilizing Cox regression. Besides baseline GGT (≤ ULN vs. > ULN) or immune-related GGT (irGGT) elevation (absence vs. occurrence), the known prognostic factors liver metastasis (absence vs. presence), LDH (normal vs. > ULN), number of metastatic sites (1 vs. 2–4 vs. 5 or more), and number of prior therapies (0 vs. 1–2 vs. 3 or more) were included in the multivariate models. Associations between categorial variables were compared with Fisher’s exact test. Differences between continuous numeric variables were calculated with Wilcoxon rank-sum test (Mann–Whitney U test). All analyzes were carried out with R, version 3.6.1 and the ‘survival’ package. Analysis of statistical power was performed using the powerCT.default0 function of the powerSurvEpi package for R. All reported tests were two sided, and P values < 0.05 were considered significant.
Results
218 of 220 patients receiving anti-PD-1 monotherapy with either nivolumab or pembrolizumab (henceforth referred to as PD-1 group) and 148 of 150 patients receiving combination therapy with ipilimumab plus nivolumab (henceforth referred to as Ipi + Nivo group) had at least one available baseline GGT measurement and could therefore be included in the analyzes. Detailed clinicopathologic information is summarized in Table 1.
Table 1.
Patient Demographics and Clinical Characteristics
| Characteristic | PD-1 group | Ipi + Nivo group |
|---|---|---|
| No. of Patients (%) | No. of Patients (%) | |
| Total no | 218 | 148 |
| Age: Median [range], y | 64.5 [27–94] | 62 [28–87] |
| Sex | ||
| Male | 131 (60) | 86 (58) |
| Female | 87 (40) | 62 (42) |
| Mutational status | ||
| BRAF mutant | 73 (33) | 44 (30) |
| NRAS mutant | 44 (20) | 28 (19) |
| BRAF/NRAS wt | 101 (46) | 76 (51) |
| LDH | ||
| Normal | 119 (55) | 82 (55) |
| Elevated | 99 (45) | 66 (45) |
| AJCC v7a | ||
| IIIB | 2 (1) | 1 (1) |
| IIIC | 8 (4) | 7 (5) |
| M1a | 8 (4) | 4 (3) |
| M1b | 31 (14) | 10 (7) |
| M1c | 169 (78) | 126 (85) |
| Liver metastasis | ||
| No | 151 (69) | 84 (57) |
| Yes | 67 (31) | 64 (43) |
| Brain metastasis | ||
| No | 151 (69) | 108 (73) |
| Yes | 67 (31) | 40 (27) |
| No. of metastatic sites | ||
| 1 | 29 (13) | 29 (20) |
| 2 | 71 (33) | 25 (17) |
| 3 | 48 (22) | 42 (28) |
| 4 | 30 (14) | 20 (14) |
| 5 or more | 40 (18) | 32 (22) |
| Prior lines of therapy | ||
| 0 | 69 (32) | 59 (40) |
| 1 | 60 (28) | 48 (32) |
| 2 | 47 (22) | 19 (13) |
| 3 | 36 (17) | 14 (9) |
| 4 | 6 (3) | 8 (5) |
| Prior therapy regimens | ||
| Anti-CTLA-4 | 96 (44) | 17 (11) |
| Anti-PD-1 | 5 (2) | 48 (32) |
| BRAFi ± MEKi | 46 (21) | 26 (18) |
| Chemotherapy | 40 (18) | 13 (9) |
| Radiotherapy | 92 (42) | 48 (32) |
| Other | 5 (2) | 0 (0) |
AJCC American Joint Committee on Cancer, version 7; Ipi ipilimumab; LDH lactate dehydrogenase; Nivo nivolumab; no. number of patients; y years
aStaging included LDH according to AJCC 2009 classification
Baseline GGT
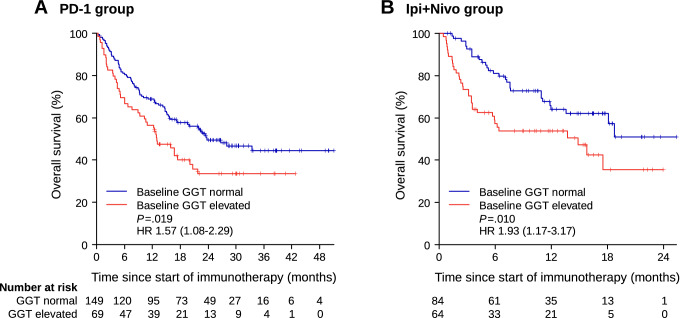
Baseline GGT was elevated in 69 patients (31.7%) in the PD-1 group and in 64 patients (43.2%) in the Ipi + Nivo group. Presence of liver metastasis was overrepresented in patients with elevated baseline GGT in the PD-1 group (43.5% vs. 24.8%, p = 0.0072), but not in the Ipi + Nivo group (50.0% vs. 38.1%, p = 0.18). Univariate analysis of overall survival (OS) showed a significant association of impaired OS with elevated baseline GGT in the PD-1 group (hazard ratio [HR] 1.57, 95% confidence interval [CI] 1.08–2.29, p = 0.019) as well as in the Ipi + Nivo group (HR 1.93, 95% CI 1.17–3.17, p = 0.010) (Fig. 1). In multivariate Cox regression analysis including baseline GGT, presence or absence of liver metastasis, lactate dehydrogenase (LDH), number of metastatic sites, and number of prior therapies, OS was significantly reduced for patients with elevated baseline GGT (Table 2).
Fig. 1.
Overall survival expressed by Kaplan–Meier estimator according to baseline levels of gamma-glutamyl transferase (GGT) in a patients receiving PD-1 antibodies, and in b patients receiving combined therapy with ipilimumab plus nivolumab. GGT gamma-glutamyl transferase; HR hazard ratio; P p value
Table 2.
Multivariate Cox regression analysis of baseline GGT regarding overall survival
| PD-1 group (n = 217) |
Ipi + Nivo group (n = 146) |
|||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Baseline GGT | ||||
| Normal | 1 | 1 | ||
| Elevated | 1.76 (1.16–2.67) | 0.0073 | 1.77 (1.05–2.99) | 0.032 |
| Liver metastasis | ||||
| No | 1 | 1 | ||
| Yes | 1.50 (1.00–2.24) | 0.049 | 1.89 (1.09–3.30) | 0.024 |
| LDH | ||||
| Normal | 1 | 1 | ||
| Elevated | 2.20 (1.51–3.20) | < 0.0001 | 1.60 (0.94–2.70) | 0.083 |
| Metastatic sites | ||||
| 1 | 1 | 1 | ||
| 2–4 | 1.26 (0.66–2.43) | 0.48 | 1.32 (0.58–3.00) | 0.51 |
| 5 or more | 2.54 (1.19–5.40) | 0.016 | 2.48 (1.01–6.10) | 0.048 |
| Prior therapies | ||||
| 0 | 1 | 1 | ||
| 1–2 | 1.24 (0.77–2.01) | 0.38 | 2.12 (1.18–3.80) | 0.012 |
| 3 or more | 1.87 (1.06–3.30) | 0.030 | 2.48 (1.11–5.50) | 0.026 |
Three cases of the PD-1 group and four cases of the Ipi + Nivo group were excluded due to missing information on one or more of the analyzed factors
CI confidence interval; GGT gamma-glutamyl transferase; HR hazard ratio; LDH lactate dehydrogenase; n number of patients; P p value
Immune-related elevations of GGT
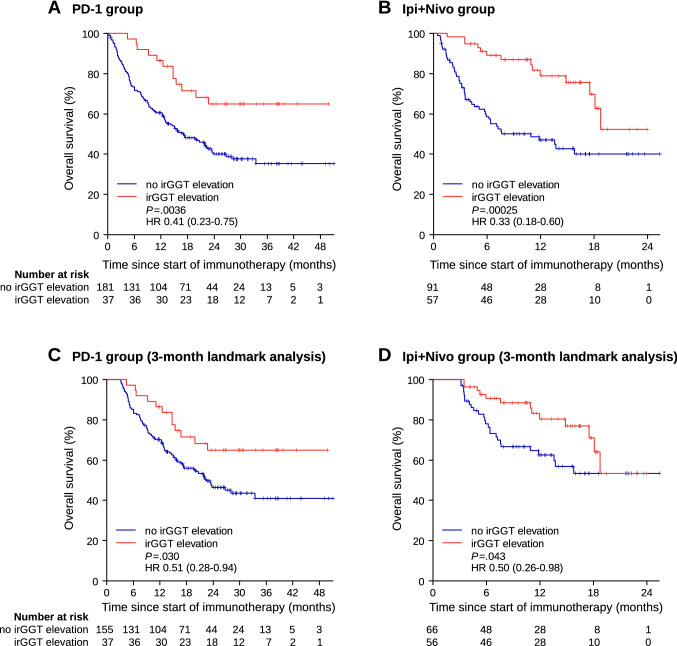
Immune-related GGT (irGGT) increase during ICPI therapy was found in 37 of 218 patients (17.0%) in the PD-1 group and in 57 of 148 patients (38.5%) in the Ipi + Nivo group, respectively. The median time to onset of irGGT elevation was 6.0 weeks (IQR 3.0–9.0, range 0.86–42.9) in the PD-1 group and 4.4 weeks (IQR 3.0–6.6, range 1.0–13.7) in the Ipi + Nivo group. In univariate analysis of OS, patients who experienced an irGGT elevation showed significantly favorable survival in the PD-1 group (HR 0.41, 95% CI 0.23–0.75, p = 0.0036) as well as in the Ipi + Nivo group (HR 0.33, 95% CI 0.18–0.60, p = 0.00025) (Fig. 2a, b). Importantly, irGGT elevation retained significance in a time-dependent Cox model (PD-1 group: HR 0.46, 95% CI 0.25–0.85, p = 0.012; Ipi + Nivo group: HR 0.38, 95% CI 0.21–0.70, p = 0.0019) as well as in a 3-month landmark analysis (Fig. 2c, d) which both account for guarantee-time bias. Power analysis based on the irGGT data obtained in the Ipi + Nivo group, revealed a statistical power of 0.93. In multivariate Cox regression analysis of OS, irGGT elevation was shown to be significantly associated with favorable survival in both cohorts (PD-1 group: HR 0.37, 95% CI 0.20–0.69, p = 0.0016; Ipi + Nivo group: HR 0.33, 95% CI 0.18–0.62, p = 0.00050) (Table 3). Presence of liver metastasis and prior therapies were also significantly related with OS in both cohorts, whereas lactate dehydrogenase (LDH) and number of metastatic sites were only significant prognostic factors in one of the two cohorts each. In the PD-1 group, 73% (27 of 37 cases) of irGGT elevations were CTCAE grade 1 whereas only five cases (13.5%) each were CTCAE grade 2 or 3. In the Ipi + Nivo group, CTCAE grade 2 or 3 irGGT elevations were more common (15 of 57 cases = 26%, and 11 of 57 cases = 19%, respectively) than in the PD-1 group, and CTCAE grade 1 was noticed in 31 cases (54%). However, these differences between the PD-1 group and the Ipi + Nivo group were not statistically significant (Fisher’s exact test: p = 0.19). No grade 4 irGGT elevation was registered in each of the two cohorts. Moreover, there were no statistically significant differences in OS according to the distinct CTCAE grades (data not shown).
Fig. 2.
Overall survival expressed by Kaplan–Meier estimator according to occurrence of immune-related elevations of gamma-glutamyl transferase (irGGT elevation) in a patients receiving PD-1 antibodies, and in b patients receiving combined therapy with ipilimumab plus nivolumab. Three-month landmark analysis of overall survival c in the PD-1 group and d in the Ipi + Nivo group excluding patients who died or were lost to follow-up within the first three months after start of therapy. HR hazard ratio; irGGT elevation immune-related gamma-glutamyl transferase elevation; P p value
Table 3.
Multivariate Cox regression analysis of irGGT elevation regarding overall survival
| PD-1 group (n = 217) |
Ipi + Nivo group (n = 146) |
|||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| irGGT elevation | ||||
| No | 1 | 1 | ||
| Yes | 0.37 (0.20–0.69) | 0.0016 | 0.33 (0.18–0.62) | 0.00050 |
| Liver metastasis | ||||
| No | 1 | 1 | ||
| Yes | 1.74 (1.17–2.57) | 0.0057 | 2.25 (1.28–3.94) | 0.0047 |
| LDH | ||||
| Normal | 1 | 1 | ||
| Elevated | 2.40 (1.64–3.50) | < 0.0001 | 1.48 (0.87–2.51) | 0.15 |
| Metastatic sites | ||||
| 1 | 1 | 1 | ||
| 2–4 | 1.08 (0.57–2.07) | 0.81 | 1.74 (0.76–4.01) | 0.19 |
| 5 or more | 1.80 (0.85–3.83) | 0.12 | 2.93 (1.19–7.18) | 0.019 |
| Prior therapies | ||||
| 0 | 1 | 1 | ||
| 1–2 | 1.40 (0.87–2.26) | 0.17 | 1.83 (1.03–3.25) | 0.040 |
| 3 or more | 1.78 (1.00–3.15) | 0.049 | 2.13 (0.95–4.77) | 0.066 |
Three cases of the PD-1 group and four cases of the Ipi + Nivo group were excluded due to missing information on one or more of the analyzed factors
CI confidence interval; GGT gamma-glutamyl transferase; HR hazard ratio; irGGT elevation, immune-related gamma-glutamyl transferase elevation; LDH lactate dehydrogenase; n number of patients; P p value
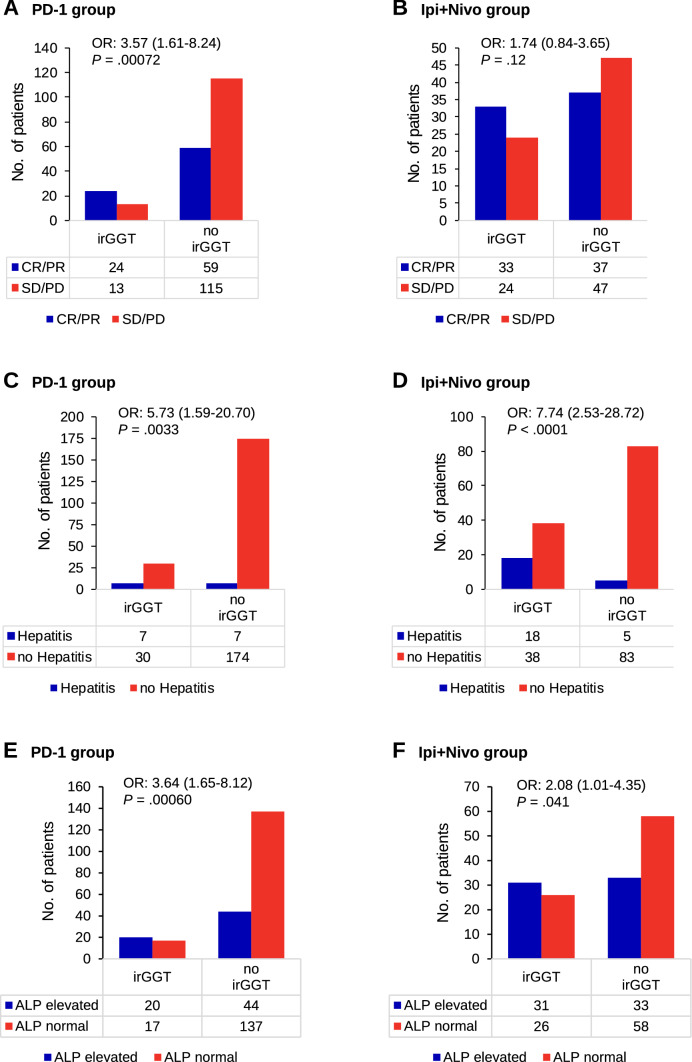
Analysis of best objective response and presence or absence of irGGT elevation revealed an odds ratio (OR) of 3.57 (1.61–8.24), p = 0.00072 to develop an objective response (CR/PR) compared to no response (SD/PD) for the patients with irGGT elevations in the PD-1 group (Fig. 3a). For disease control (CR/PR/SD vs. PD) the results were similar (OR: 2.88 [1.26–7.09], p = 0.0067) (data not shown). In the Ipi + Nivo group, likelihood for response (OR: 1.74 [0.84–3.65], p = 0.12) and disease control (OR: 2.11 [0.98–4.68], p = 0.052) was also higher in the patients developing irGGT elevations, but not reaching statistical significance (Fig. 3b).
Fig. 3.
Associations of immune related gamma-glutamyl transferase elevations with a and b best objective response, c and d occurrence of hepatitis considering cases with clinical signs of hepatitis or elevations of transaminases alone, and e and f elevations of alkaline phosphatase (ALP). Statistical differences were calculated utilizing two-sided Fisher’s exact test. ALP alkaline phosphatase; CR complete response; irGGT immune-related gamma-glutamyl transferase elevation; OR odds ratio (given together with 95% confidence interval); P p value, PD progressive disease; PR partial response; SD stable disease
Analyzing factors associated with occurrence of irGGT elevation, median baseline eosinophil count was identified as being significantly higher in the patients developing irGGT elevation in the PD-1 group (absolute eosinophils: 180/µl vs. 120/µl, p = 0.0067; relative eosinophils: 2.4% vs. 1.7%, p = 0.0060; data not shown). In the Ipi + Nivo group, elevations of eosinophils after the first cycle of therapy correlated significantly with occurrence of irGGT elevation (absolute eosinophils: + 100/µl vs. + 50/µl, p = 0.037; relative eosinophils: + 1.7% vs. + 0.6%, p = 0.023; data not shown). Occurrence of irGGT elevation correlated significantly with the occurrence of autoimmune hepatitis (defined as elevated transaminases with or without clinical symptoms of hepatitis) in both cohorts (PD-1 group: OR 5.73, 95% CI 1.59–20.70, p = 0.0033; Ipi + Nivo group: OR 7.74, 95% CI 2.53–28.72, p < 0.0001) (Fig. 3c, d). Interestingly, 30 of 37 (81%) patients in the PD-1 group and 38 of 56 (68%) patients in the Ipi + Nivo group experienced an irGGT elevation but showed no elevations of transaminases or other signs of hepatitis (occurrence of irGGT elevation vs. occurrence of hepatitis defined by other LFTs: PD-1 group: OR 2.97 [1.51–6.15], p = 0.00089; Ipi + Nivo group: OR 3.33 [1.86–6.13], p < 0.0001). However, elevation of alkaline phosphatase (ALP) correlated with irGGT elevation (PD-1 group: OR 3.64, 95% CI 1.65–8.12, p = 0.00060; Ipi + Nivo group: OR 2.08, 95% CI 1.01–4.35, p = 0.041) (Fig. 3e, f). Elevation of bilirubin was not significantly correlated with irGGT elevation but showed a strong correlation with hepatitis (data not shown).
Discussion
Immune-related adverse events (irAE) are common in patients receiving immune checkpoint inhibitors (ICPI). With combined ICPI therapy with ipilimumab plus nivolumab 55–56% of the patients experience grade 3–4 treatment-related adverse events (AE), while this proportion is between 10 and 16% in ICI monotherapy with PD-1 antibodies nivolumab or pembrolizumab [1–4]. Autoimmune hepatitis of any grade was recorded in 1% of patients receiving pembrolizumab and in 3.2% of patients receiving the combination of ipilimumab plus nivolumab [2, 4]. Transaminases (i.e. alanine aminotransferase [ALT] and aspartate aminotransferase [AST]) were reported to increase in 1.4–5% of patients receiving pembrolizumab or nivolumab and in 15.3–22.3% of patients receiving ipilimumab + nivolumab [1, 3, 4]. In the present study, these proportions were 6.4% (PD-1 group) and 16.4% (Ipi + Nivo group), respectively. Interestingly, 17.0% (PD-1 group) and 38.5% (Ipi + Nivo group) experienced a treatment-related increase of gamma-glutamyl transferase (irGGT) in our study which is in sharp contrast to 1.1% reported incidence (0 cases with grade 3–4 GGT increase) receiving ipilimumab plus nivolumab in the CheckMate 069 trial [4]. In a pooled analysis of advanced melanoma patients receiving nivolumab monotherapy, Weber et al. reported only 0.2% treatment-related increases of gamma-glutamyl transferase [5]. And a meta-analysis of 48 studies by Xing et al. reported incidence rates of GGT elevations of 1.02% (95% CI 0.37–2.80) for nivolumab monotherapy and 5.13% (3.51–7.43) for ipilimumab plus nivolumab in patients with advanced solid tumors [8]. Of note, the study protocols of all large phase 1–3 melanoma trials on PD-1-based immunotherapy did not include GGT measurements in the prerequisite on-study laboratory tests [1–4]. Thus, it is not surprising that in detail characterization of GGT at baseline and during treatment with ICPI is lacking.
Immune-related GGT elevations
To the best of our knowledge, our study is the first to describe irGGT elevations as a commonly occurring adverse event that is associated with response to ICPI and favorable survival in metastatic melanoma. Patients with occurrence of irGGT elevation showed favorable OS in both cohorts. Importantly, we also found statistically significant results for irGGT in the 3-month landmark analysis and the time-dependent extended Cox model which both account for guarantee-time bias [27]. In the PD-1 group, irGGT elevations were also strongly associated with objective response.
Unlike in monotherapy with the CTLA-4 antibody ipilimumab alone, treatment-related hepatotoxicity in PD-1-based regimens is predominantly characterized by a cholestatic or mixed cholestatic-hepatocellular pattern compared to a pan-hepatocellular pattern [28–34]. In a large two-center study from Japan, markedly elevated levels of ALP and GGT but only mild increases of the liver enzymes ALT and AST were observed in patients developing pathology-proven hepatotoxicity which further supports the notion that immune-related cholangitis and cholestatic hepatitis are more frequent than hepatocellular hepatitis [34]. Importantly, the discrimination between hepatocellular and cholestatic liver injury, e.g. by means of the biliary enzymes GGT and ALP in contrast to the hepatocellular enzymes ALT and AST, stratifies patients with different response to corticosteroids [32, 35]. Many of these cases with cholangitis with non-obstructive dilation of the bile ducts resemble primary sclerosing cholangitis (PSC) and show resistance to steroid therapy [34, 35]. Nevertheless, Imoto et al. reported that most patients with grade ≤ 2 liver injury improved spontaneously and five of eight patients with grade ≥ 3 liver injury required prednisolone or additional immunosuppressants, or ursodeoxycholic acid [33]. Regarding the severity of liver injury, this study reported a statistically significant difference in the distribution between hepatocellular type (11% in grade 1 or 2 liver injury, 55% in grade 3 or 4 liver injury) and cholestatic or mixed type of liver injury (64% in grade 1 or 2 liver injury, 45% in grade 3 or 4 liver injury) [33]. These findings are in accordance with our data with most cases being mild or moderate grades 1–2 and that GGT elevations were significantly more frequent than ALT/AST elevations.
Based on the finding that most cases of immune-related hepatotoxicity do not present with clinical manifestations, Tan et al. concluded that close monitoring of liver function tests is mandatory [36]. Although only the hepatocellular enzymes ALT and AST together with bilirubin are recommended by the CTCAE to assess hepatotoxicity, these LFTs should be complemented with the regular assessment of GGT in patients receiving therapy with ICPI, thus, accounting for the diverse clinical features of hepatic irAE, particularly microscopic biliary liver injury [33]. The results of the present study underline the importance of GGT measurements to avoid missing silent cholestatic hepatotoxicity. However, most irGGT elevations were temporary and self-limited and the extent of liver damage cannot be estimated based on our data.
The median time to onset of irGGT elevation (median: 6.0 in the PD-1 group, median 4.4 in the Ipi + Nivo group) was slightly shorter than the median time to onset of treatment-related select hepatic AEs (median 7.7 weeks, range 2.0–38.9) as reported by Weber et al. for PD-1 blockade with nivolumab [5]. In the present study, irGGT elevation was recorded prior to the first staging in 86% of the patients. Therefore, irGGT elevation is a suitable early prognostic marker that enables the clinician to gain prognosis-related information days or weeks before the first radiological staging. As GGT is highly expressed not only in melanoma but also in several other cancer cells like colorectal, breast, and lung cancer as well as in astrocytic glioma and Ewing’s sarcoma, it is likely that the results of our study can be translated to anti-PD-1 treatment of other tumor entities [37].
Immune-related adverse events are considered an antibody-driven autoimmune effect of the host. Thus, it is important to consider that immune-related hepatotoxicity is likely to serve as a prognostic marker only in patients with an intact immune system.
Baseline GGT
Our study is the first to analyze baseline GGT and its prognostic impact on response and survival in metastatic melanoma and in patients receiving therapy with ICPI. Increased levels of GGT have been described in the context of hepatocellular carcinoma, viral hepatitis, chronic alcoholism, and several diseases related to increased oxidative stress like cardiovascular disease, Alzheimer’s disease, and diabetes mellitus [38–46]. Although it has been shown that elevated GGT is a prognostic marker for liver metastasis in breast cancer and colorectal cancer, its prognostic impact in metastatic melanoma had not been uncovered so far. In mice with transplanted B16 melanomas, serum levels of GGT correlated with tumor growth [47]. Moreover, Melezinek et al. showed in their mouse model that the B16 melanoma cells express a soluble isoform of GGT which was the exclusive driver of the observed serum GGT elevations [47]. Obrador et al. demonstrated that overexpression of GGT leads to altered glutathione metabolism and increased metastatic growth in a B16 melanoma mouse model [48]. Specific isoforms of GGT complexed with low density lipoproteins (LDL) and very low density lipoproteins (VLDL) have been demonstrated to discriminate liver tumor patients from patients with chronic hepatitis or liver cirrhosis [49, 50]. In our data, multivariate Cox regression analysis including presence or absence of liver metastasis, lactate dehydrogenase (LDH), number of metastatic sites, and number of prior therapies, elevated baseline GGT was an independent predictor for impaired overall survival (OS) in both cohorts. As its determination can be routinely done and is cheap, GGT could amend other prognostic biomarkers and should be measured before starting therapy with ICPI.
We conclude that the sensitive enzyme GGT is worth to be determined at baseline and during therapy with ICPI in addition to the hepatocellular enzymes ALT and AST, and bilirubin. By continuous monitoring of GGT, it is possible to detect two to three times more patients with hepatotoxicity than with the widely utilized LFTs. After exclusion of liver metastasis or other confounding factors like viral or steatohepatitis, irGGT elevation can be considered an independent prognostic factor for response and favorable survival.
Acknowledgements
We thank the whole team of the melanoma unit for their passionate patient care and support in data collection.
Author contributions
JW: collected the clinical data, analyzed the data, revised the article, and approved the submission. MML, MG, AF, UL, BW, MTP, LF, AC, MR, CG, TKE: analyzed the data, revised the article, and approved the submission. NBW: conceived and designed the study, collected the clinical data, analyzed the data, wrote the article, revised the article, and approved the submission.
Funding
The project was funded, in part, by a joint program of the Swiss Academy of Medical Sciences and the Gottfried and Julia Bangerter-Rhyner Foundation (NBW).
Data availability
Data can be obtained upon request (nikolausbenjamin.wagner@kssg.ch).
Compliance with ethical standards
Conflicts of interest
The authors declare no conflicts of interest.
Ethics approval
This study was approved by the Medical Faculty of University Tübingen ethical committee (436/2017BO2). The study was conducted in accordance with the Declaration of Helsinki.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. 2015;372:2521–2532. doi: 10.1056/NEJMoa1503093. [DOI] [PubMed] [Google Scholar]
- 2.Ribas A, Puzanov I, Dummer R, Schadendorf D, Hamid O, Robert C, et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol. 2015;16:908–918. doi: 10.1016/S1470-2045(15)00083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373:23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Postow MA, Chesney J, Pavlick AC, Robert C, Grossmann K, McDermott D, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med. 2015;372:2006–2017. doi: 10.1056/NEJMoa1414428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weber JS, Hodi FS, Wolchok JD, Topalian SL, Schadendorf D, Larkin J, et al. Safety profile of nivolumab monotherapy: a pooled analysis of patients with advanced melanoma. J Clin Oncol. 2017;35:785–792. doi: 10.1200/JCO.2015.66.1389. [DOI] [PubMed] [Google Scholar]
- 6.Postow MA, Sidlow R, Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med. 2018;378:158–168. doi: 10.1056/NEJMra1703481. [DOI] [PubMed] [Google Scholar]
- 7.Schadendorf D, Wolchok JD, Hodi FS, Chiarion-Sileni V, Gonzalez R, Rutkowski P, et al. Efficacy and safety outcomes in patients with advanced melanoma who discontinued treatment with nivolumab and ipilimumab because of adverse events: a pooled analysis of randomized phase II and III trials. J Clin Oncol. 2017;35:3807–3814. doi: 10.1200/JCO.2017.73.2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xing P, Zhang F, Wang G, Xu Y, Li C, Wang S, et al. Incidence rates of immune-related adverse events and their correlation with response in advanced solid tumours treated with NIVO or NIVO+IPI: a systematic review and meta-analysis. J Immunother Cancer. 2019;7:341. doi: 10.1186/s40425-019-0779-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berner F, Bomze D, Diem S, Ali OH, Fassler M, Ring S, et al. Association of checkpoint inhibitor-induced toxic effects with shared cancer and tissue antigens in non-small cell lung cancer. JAMA Oncol. 2019;5:1043–1047. doi: 10.1001/jamaoncol.2019.0402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sanlorenzo M, Vujic I, Daud A, Algazi A, Gubens M, Luna SA, et al. Pembrolizumab cutaneous adverse events and their association with disease progression. JAMA Dermatol. 2015;151:1206–1212. doi: 10.1001/jamadermatol.2015.1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hua C, Boussemart L, Mateus C, Routier E, Boutros C, Cazenave H, et al. Association of vitiligo with tumor response in patients with metastatic melanoma treated with pembrolizumab. JAMA Dermatol. 2016;152:45–51. doi: 10.1001/jamadermatol.2015.2707. [DOI] [PubMed] [Google Scholar]
- 12.Reddy HG, Schneider BJ, Tai AW. Immune checkpoint inhibitor-associated colitis and hepatitis. Clin Transl Gastroenterol. 2018;9:180. doi: 10.1038/s41424-018-0049-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McGuire HM, Shklovskaya E, Edwards J, Trevillian PR, McCaughan GW, Bertolino P, et al. Anti-PD-1-induced high-grade hepatitis associated with corticosteroid-resistant T cells: a case report. Cancer Immunol Immunother. 2018;67:563–573. doi: 10.1007/s00262-017-2107-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spankuch I, Gassenmaier M, Tampouri I, Noor S, Forschner A, Garbe C, et al. Severe hepatitis under combined immunotherapy: Resolution under corticosteroids plus anti-thymocyte immunoglobulins. Eur J Cancer. 2017;81:203–205. doi: 10.1016/j.ejca.2017.05.018. [DOI] [PubMed] [Google Scholar]
- 15.Tumeh PC, Hellmann MD, Hamid O, Tsai KK, Loo KL, Gubens MA, et al. Liver metastasis and treatment outcome with anti-PD-1 monoclonal antibody in patients with melanoma and NSCLC. Cancer Immunol Res. 2017;5:417–424. doi: 10.1158/2326-6066.CIR-16-0325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Whitfield JB. Gamma glutamyl transferase. Crit Rev Clin Lab Sci. 2001;38:263–355. doi: 10.1080/20014091084227. [DOI] [PubMed] [Google Scholar]
- 17.Corti A, Franzini M, Paolicchi A, Pompella A. Gamma-glutamyltransferase of cancer cells at the crossroads of tumor progression, drug resistance and drug targeting. Anticancer Res. 2010;30:1169–1181. [PubMed] [Google Scholar]
- 18.Wu XZ, Ma F, Wang XL. Serological diagnostic factors for liver metastasis in patients with colorectal cancer. World J Gastroenterol. 2010;16:4084–4088. doi: 10.3748/wjg.v16.i32.4084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.He WZ, Guo GF, Yin CX, Jiang C, Wang F, Qiu HJ, et al. Gamma-glutamyl transpeptidase level is a novel adverse prognostic indicator in human metastatic colorectal cancer. Colorectal Dis. 2013;15:e443–452. doi: 10.1111/codi.12258. [DOI] [PubMed] [Google Scholar]
- 20.Takemura K, Fukushima H, Ito M, Kataoka M, Nakanishi Y, Sakamoto K, et al. Prognostic significance of serum gamma-glutamyltransferase in patients with advanced urothelial carcinoma. Urol Oncol. 2019;37:108–115. doi: 10.1016/j.urolonc.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 21.Seebacher V, Polterauer S, Grimm C, Rahhal J, Hofstetter G, Bauer EM, et al. Prognostic significance of gamma-glutamyltransferase in patients with endometrial cancer: a multi-centre trial. Br J Cancer. 2012;106:1551–1555. doi: 10.1038/bjc.2012.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hofbauer SL, Stangl KI, de Martino M, Lucca I, Haitel A, Shariat SF, et al. Pretherapeutic gamma-glutamyltransferase is an independent prognostic factor for patients with renal cell carcinoma. Br J Cancer. 2014;111:1526–1531. doi: 10.1038/bjc.2014.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eskelin S, Pyrhonen S, Summanen P, Prause JU, Kivela T. Screening for metastatic malignant melanoma of the uvea revisited. Cancer. 1999;85:1151–1159. doi: 10.1002/(SICI)1097-0142(19990301)85:5<1151::AID-CNCR20>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 24.Kaiserman I, Amer R, Pe'er J. Liver function tests in metastatic uveal melanoma. Am J Ophthalmol. 2004;137:236–243. doi: 10.1016/j.ajo.2003.08.045. [DOI] [PubMed] [Google Scholar]
- 25.Hendler K, Pe'er J, Kaiserman I, Baruch R, Kalickman I, Barak V, et al. Trends in liver function tests: a comparison with serum tumor markers in metastatic uveal melanoma (part 2) Anticancer Res. 2011;31:351–357. [PubMed] [Google Scholar]
- 26.Mouriaux F, Diorio C, Bergeron D, Berchi C, Rousseau A. Liver function testing is not helpful for early diagnosis of metastatic uveal melanoma. Ophthalmology. 2012;119:1590–1595. doi: 10.1016/j.ophtha.2012.01.045. [DOI] [PubMed] [Google Scholar]
- 27.Giobbie-Hurder A, Gelber RD, Regan MM. Challenges of guarantee-time bias. J Clin Oncol. 2013;31:2963–2969. doi: 10.1200/JCO.2013.49.5283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim KW, Ramaiya NH, Krajewski KM, Jagannathan JP, Tirumani SH, Srivastava A, et al. Ipilimumab associated hepatitis: imaging and clinicopathologic findings. Invest New Drugs. 2013;31:1071–1077. doi: 10.1007/s10637-013-9939-6. [DOI] [PubMed] [Google Scholar]
- 29.Johncilla M, Misdraji J, Pratt DS, Agoston AT, Lauwers GY, Srivastava A, et al. Ipilimumab-associated hepatitis: clinicopathologic characterization in a series of 11 cases. Am J Surg Pathol. 2015;39:1075–1084. doi: 10.1097/PAS.0000000000000453. [DOI] [PubMed] [Google Scholar]
- 30.Tirumani SH, Ramaiya NH, Keraliya A, Bailey ND, Ott PA, Hodi FS, et al. Radiographic profiling of immune-related adverse events in advanced melanoma patients treated with ipilimumab. Cancer Immunol Res. 2015;3:1185–1192. doi: 10.1158/2326-6066.CIR-15-0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kawakami H, Tanizaki J, Tanaka K, Haratani K, Hayashi H, Takeda M, et al. Imaging and clinicopathological features of nivolumab-related cholangitis in patients with non-small cell lung cancer. Invest New Drugs. 2017;35:529–536. doi: 10.1007/s10637-017-0453-0. [DOI] [PubMed] [Google Scholar]
- 32.Doherty GJ, Duckworth AM, Davies SE, Mells GF, Brais R, Harden SV, et al. Severe steroid-resistant anti-PD1 T-cell checkpoint inhibitor-induced hepatotoxicity driven by biliary injury. ESMO Open. 2017;2:e000268. doi: 10.1136/esmoopen-2017-000268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Imoto K, Kohjima M, Hioki T, Kurashige T, Kurokawa M, Tashiro S, et al. Clinical features of liver injury induced by immune checkpoint inhibitors in japanese patients. Can J Gastroenterol Hepatol. 2019;2019:6391712. doi: 10.1155/2019/6391712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mizuno K, Ito T, Ishigami M, Ishizu Y, Kuzuya T, Honda T, et al. Real world data of liver injury induced by immune checkpoint inhibitors in Japanese patients with advanced malignancies. J Gastroenterol. 2020;55:653–661. doi: 10.1007/s00535-020-01677-9. [DOI] [PubMed] [Google Scholar]
- 35.Onoyama T, Takeda Y, Yamashita T, Hamamoto W, Sakamoto Y, Koda H, et al. Programmed cell death-1 inhibitor-related sclerosing cholangitis: a systematic review. World J Gastroenterol. 2020;26:353–365. doi: 10.3748/wjg.v26.i3.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tan B, Li Y, Xu Y, Chen M, Wang M, Qian J. Recognition and management of the gastrointestinal and hepatic immune-related adverse events. Asia Pac J Clin Oncol. 2020;16:95–102. doi: 10.1111/ajco.13317. [DOI] [PubMed] [Google Scholar]
- 37.Pompella A, De Tata V, Paolicchi A, Zunino F. Expression of gamma-glutamyltransferase in cancer cells and its significance in drug resistance. Biochem Pharmacol. 2006;71:231–238. doi: 10.1016/j.bcp.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 38.Hann HW, Wan S, Myers RE, Hann RS, Xing J, Chen B, et al. Comprehensive analysis of common serum liver enzymes as prospective predictors of hepatocellular carcinoma in HBV patients. PLoS ONE. 2012;7:e47687. doi: 10.1371/journal.pone.0047687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lin YJ, Lee MH, Yang HI, Jen CL, You SL, Wang LY, et al. Predictability of liver-related seromarkers for the risk of hepatocellular carcinoma in chronic hepatitis B patients. PLoS ONE. 2013;8:e61448. doi: 10.1371/journal.pone.0061448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhu J, Jiang F, Ni HB, Xiao MB, Chen BY, Ni WK, et al. Combined analysis of serum gamma-glutamyl transferase isoenzyme II, alpha-L-fucosidase and alpha-fetoprotein detected using a commercial kit in the diagnosis of hepatocellular carcinoma. Exp Ther Med. 2013;5:89–94. doi: 10.3892/etm.2012.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu XS, Wan Y, Song SD, Chen W, Miao RC, Zhou YY, et al. Model based on gamma-glutamyltransferase and alkaline phosphatase for hepatocellular carcinoma prognosis. World J Gastroenterol. 2014;20:10944–10952. doi: 10.3748/wjg.v20.i31.10944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu SJ, Lin YX, Ye H, Xiong XZ, Li FY, Cheng NS. Prognostic value of alkaline phosphatase, gamma-glutamyl transpeptidase and lactate dehydrogenase in hepatocellular carcinoma patients treated with liver resection. Int J Surg. 2016;36:143–151. doi: 10.1016/j.ijsu.2016.10.033. [DOI] [PubMed] [Google Scholar]
- 43.Huang R, Yang CC, Liu Y, Xia J, Su R, Xiong YL, et al. Association of serum gamma-glutamyl transferase with treatment outcome in chronic hepatitis B patients. World J Gastroenterol. 2015;21:9957–9965. doi: 10.3748/wjg.v21.i34.9957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yavuz BB, Yavuz B, Halil M, Cankurtaran M, Ulger Z, Cankurtaran ES, et al. Serum elevated gamma glutamyltransferase levels may be a marker for oxidative stress in Alzheimer's disease. Int Psychogeriatr. 2008;20:815–823. doi: 10.1017/S1041610208006790. [DOI] [PubMed] [Google Scholar]
- 45.Koehler EM, Sanna D, Hansen BE, van Rooij FJ, Heeringa J, Hofman A, et al. Serum liver enzymes are associated with all-cause mortality in an elderly population. Liver Int. 2014;34:296–304. doi: 10.1111/liv.12311. [DOI] [PubMed] [Google Scholar]
- 46.Koenig G, Seneff S. Gamma-glutamyltransferase: a predictive biomarker of cellular antioxidant inadequacy and disease Risk. Dis Markers. 2015;2015:818570. doi: 10.1155/2015/818570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Melezinek I, Borovansky J, Elleder M, Bubnova E. Tumour tissue is a source of gamma-glutamyl transpeptidase sialoform in the sera of melanoma-bearing mice. Melanoma Res. 1998;8:39–45. doi: 10.1097/00008390-199802000-00007. [DOI] [PubMed] [Google Scholar]
- 48.Obrador E, Carretero J, Ortega A, Medina I, Rodilla V, Pellicer JA, et al. Gamma-glutamyl transpeptidase overexpression increases metastatic growth of B16 melanoma cells in the mouse liver. Hepatology. 2002;35:74–81. doi: 10.1053/jhep.2002.30277. [DOI] [PubMed] [Google Scholar]
- 49.Sacchetti L, Castaldo G, Cimino L, Budillon G, Salvatore F. Diagnostic efficiency in discriminating liver malignancies from cirrhosis by serum gamma-glutamyltransferase isoforms. Clin Chim Acta. 1988;177:167–172. doi: 10.1016/0009-8981(88)90138-6. [DOI] [PubMed] [Google Scholar]
- 50.Castaldo G, Oriani G, Lofrano MM, Cimino L, Topa M, Budillon G, et al. Differential diagnosis between hepatocellular carcinoma and cirrhosis through a discriminant function based on results for serum analytes. Clin Chem. 1996;42:1263–1269. doi: 10.1093/clinchem/42.8.1263. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data can be obtained upon request (nikolausbenjamin.wagner@kssg.ch).