Abstract
Metformin has been found to have inhibitory effects on a variety of tumors. However, its effects on non-small cell lung cancer (NSCLC) remain unclear. We demonstrated that metformin could inhibit the proliferation of A549 and H1299 cells. RNA transcriptome sequencing revealed that PDL1 was significantly downregulated in both cell types following treatment with metformin (P < 0.001). Jaspar analysis and chromatin immunoprecipitation showed that CEBPB could directly bind the promoter region of PDL1. Western blotting showed that protein expression of the isoforms CEBPB-LAP*, CEBPB-LAP, and CEBPB-LIP was significantly upregulated and the LIP/LAP ratio was increased. Gene chip analysis showed that PDL1 was significantly upregulated in A549-CEBPB-LAP cells and significantly downregulated in A549-CEBPB-LIP cells (P < 0.05) compared with CEBPB-NC cells. Dual-luciferase reporter gene assay showed that CEBPB-LAP overexpression could promote transcription of PDL1 and CEBPB-LIP overexpression could inhibit the process. Functional assays showed that the changes in CEBPB isoforms affected the function of NSCLC cells. Western blotting showed that metformin could regulate the function of NSCLC cells via AMPK–CEBPB–PDL1 signaling. Animal experiments showed that tumor growth was significantly inhibited by metformin, and atezolizumab and metformin had a synergistic effect on tumor growth. A total of 1247 patients were retrospectively analyzed, including 166 and 1081 patients in metformin and control groups, respectively. The positive rate of PDL1 was lower than that of the control group (HR = 0.338, 95% CI = 0.235–0.487; P < 0.001). In conclusion, metformin inhibited the proliferation of NSCLC cells and played an anti-tumor role in an AMPK–CEBPB–PDL1 signaling-dependent manner.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00262-021-03116-x.
Keywords: Metformin, CEBPB, PDL1, NSCLC
Introduction
Non-small cell lung cancer (NSCLC) is the most common type of lung cancer, accounting for approximately 85% of all lung cancers [1, 2]. The development of programmed cell death-1/programmed cell death ligand-1 (PD-1/PDL1) inhibitors has brought treatment of lung cancer to a new stage [3]. However, most of the current studies have focused on anti-tumor immunity, especially in T cells, and the PDL1 signal inherent in tumors has not been extensively studied [4]. Recently, the regulation of tumor cell proliferation by PDL1 and its interaction with other carcinogenic pathways has attracted attention. While studies have found that endogenous PDL1 has a wide range of biological effects on various tumors [4, 5], few studies have reported the function of endogenous PDL1 in NSCLC.
CCAAT-enhancer-binding protein beta (CEBPB) is a transcription factor with three isoforms (LAP*, LAP, and LIP) [6, 7]. CEBPB-LAP* and CEBPB-LAP have 346 and 323 amino acids, respectively, and act as transcriptional activators [8]. CEBPB-LAP is a more important transcription factor [9]. CEBPB-LIP has 148 amino acids and plays a transcriptional inhibitory role due to its lack of transcriptional activation domain [10]. The LIP/LAP ratio determines whether transcription is activated or inhibited [11]. Maehara et al. [11] believed that metformin could regulate the function of hepatoma carcinoma cells by regulating CEBPB-mediated signaling. The relationship between metformin and CEBPB deserves further study.
Our study found that metformin could regulate the expression of PDL1 in NSCLC cell lines by transcriptome sequencing analysis and experimental validation. However, the mechanism and the inhibitory effects of metformin have not been clearly identified. Therefore, this study aimed to explore the regulatory mechanism of metformin on PDL1 and clarify the intrinsic function of PDL1 in NSCLC.
Methods
Determination of IC50 concentration
A549 and H1299 cells in logarithmic growth phase were prepared as cell suspensions of 15,000 cells/mL; a suspension of 100 µL was added to each well of a 96-well plate. Eight metformin (S1741, Beyotime Biotechnology) concentrations were evaluated: 0, 0.1, 0.2, 0.5, 1.0, 2.0, 5.0, and 10.0 mmol/L. After 72 h of incubation, 10 μL of Alamar Blue solution (40202ES60, YEASEN) was added to each well and the plate was incubated for 3 h at 37 °C. A microplate reader was used to assess fluorescence intensity at excitation and emission wavelengths of 545 nm and 590 nm, respectively.
Sample preparation
A549 and H1299 cells were cultured with Dulbecco’s modified Eagle medium (DMEM; Thermo Fisher Scientific) mixed with metformin at the IC50 concentration for 72 h. The cells were lysed using Trizol solution (R0016, Beyotime Biotechnology). Cell lysates were collected, and RNA transcriptome sequencing was performed.
The tissues were cut into small pieces and lysed using Trizol reagent. Lysates were collected, and RNA transcriptome sequencing was performed.
Cells were lysed using RIPA buffer (containing 1% protease and phosphatase inhibitors; Beyotime Biotechnology), and phosphorylated protein mass spectrometry identification was performed.
In this study, the detection time of mRNA and protein is consistent with the time we determined the IC50 of concentration. We treated the cells with metformin for 72 h and then performed the gene expression experiments and signaling experiments.
Construction of cell lines
Cell lines of A549-CEBPB-LAP*-overexpression (OE), A549-CEBPB-LAP-OE, A549-CEBPB-LIP-OE, A549 empty vector control, A549-PDL1-OE, H1299-CEBPB-LAP*-OE, H1299-CEBPB-LAP-OE, H1299-CEBPB-LIP-OE, and H1299 empty vector control were constructed. The plasmid was purchased from Heyuan Biotechnology (Shanghai) Co., Ltd., and 293 T cells were used as the experimental cells. The Lenti-Easy Packaging Mix and the target gene (CEBPB-LAP*, CEBPB-LAP, CEBPB-LIP, CEBPB-NC, and PDL1) lentiviral vector plasmids were co-transfected into 293 T cells using Lipofectamine 8000. The virus supernatant was collected, concentrated, and purified followed by lentiviral transfection. Proteins were extracted and verified by western blots.
Western blotting
The BCA method was used to determine protein concentrations. The protein samples were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE) and transferred to a nitrocellulose membrane (FFP24, Beyotime Biotechnology). The membranes were blocked with QuickBlock™ Blocking Buffer (P0252, Beyotime Biotechnology) and incubated with the following primary antibodies: CEBPB (Ab53138, Abcam), p-CEBPB (phosphor T235 + T188) (Ab52194, Abcam), AMPKα1 (Ab3759, Abcam), p-AMPKα1 (phosphor S48) (Ab131357, Abcam), PDL1 (13,684, Cell Signaling Technology), and actin (AA128, Beyotime Biotechnology). Then, the membranes were incubated with the appropriate horseradish peroxidase (HRP)-conjugated secondary antibody (goat anti-rabbit IgG, 1:4000; goat anti-mouse IgG, 1:5000; CWBio, China). Bands were detected using the ECL Western Blotting Detection System.
Real-time fluorescent quantitative polymerase chain reaction (qPCR)
Total RNA was extracted using TRIzol reagent (R0016, Beyotime Biotechnology). The PrimeScript™ RT reagent kit (Tiangen Biotech (Beijing) Co., Ltd.) was used to convert RNA into cDNA. Quantitative real-time polymerase chain reaction analysis (qRT-PCR) was conducted using 2 × HotStart Taq PCR MasterMix (Tiangen Biotech (Beijing) Co., Ltd.) on a fluorescent PCR device (7500, Thermo Fisher Scientific). The primers were as follows: CEBPB (forward: 5′-TACTACGAGGCGGACTGCTTGG-3′ and reverse: 5′-TCTCGTCCTGACCATAGT TCGGAGAAGAGGTCGGAGAGGA-3'), AMPKα1 (forward: 5′- TTTGCGTGTACGAAGGAAGAAT-3′ and reverse: 5′- CTCTGTGGAGTAGCAGTCCCT-3′), and PDL1 (forward: 5'-GCTGCACTAATTGTCTATTGGGA-3′ and reverse: 5′- AATTCGCTTGTAGTCGGCACC-3′). The results were expressed using the 2−△△Ct method.
Construction of the A549 CEBPB gene chip
Affymetrix GeneChip Human Gene 1.0 ST Array was performed by Genminix Informatics Co., Ltd.
Cell proliferation assay
The Alamar Blue Assay was used to measure cell proliferation. A total of 1.5 × 103 cells were seeded into 96-well plates and cultured in DMEM medium. Cell proliferation was measured every day for 8 consecutive days. Then, 10 µL of Alamar Blue reagent was added and the plates were incubated for 3 h at 37 °C. Alamar Blue reduction was monitored using a fluorescence-based plate reader with excitation and emission wavelengths of 545 and 590 nm, respectively.
Cell scratch test
Cells at a concentration of 300,000/mL in logarithmic growth phase were seeded in a 6-well plate and incubated overnight. The cells were scratched with a 1-mL sterile pipette tip and incubated with 2 mL DMEM without serum. Mobility was calculated with the following equation: (0 h scratch width–24 h scratch width)/0 h scratch width × 100%.
Transwell assays
For the Transwell migration assay, 2 × 104 cells were seeded in the top chamber of each insert and cultured in serum-free medium. DMEM with 5% fetal bovine serum was added to the lower chamber. After 24 h, migrating cells were fixed, stained, and quantified for a total of five fields per membrane.
Chromatin immunoprecipitation (ChIP) assays
A ChIP assay was performed using the ChIP Assay Kit (P2078, Beyotime) according to the manufacturer’s instructions. Anti-CEBPB antibody was used to immunoprecipitate crosslinked protein–DNA complexes. The immunoprecipitated DNA was purified for qPCR analyses with specific primers, and the products were subjected to DNA electrophoresis.
Dual-luciferase reporter gene assays
The JASPAR database was used to predict the potential binding site of CEBPB and PDL1 (from − 1000 bp to + 200 bp); the PDL1 promoter region was cloned into the pGL3-basic fluorescein enzyme plasmid vector (Promega). Then, the constructed CEBPB-LAP overexpression (LAP-OE), CEBPB-LIP overexpression (LIP-OE), and control plasmid CEBPB-NC vectors and Renilla pRLTK plasmid (Promega) were transfected into 293 T cells. Luciferase detection reagent II (LARII) was used to detect RLU1. Stop&Glo reagent was used to detect RLU2. The ratio of RLU1/RLU2 was calculated.
Animal experiments
SPF nude and C57BL/6 J mice were provided and raised by the animal room of Zhongshan Hospital Fudan University, and passed the animal ethics audit. A total of 36 male nude mice aged approximately 4 weeks were used as experimental animals and randomly divided into six groups. To evaluate the effect of metformin on tumor growth, control or LAP- or PDL1-overexpressing A549 tumor cells (2 × 106) were inoculated subcutaneously into mice. Mice were given metformin (300 mg/kg per day) or saline through gastric perfusion once a day in subsequent days.
Tumor size was recorded every day beginning at day 8. The maximum tumor diameter (a) and width by perpendicular (b) were measured every 3 days using calipers, and tumor volume was estimated using the following formula: V = 0.5 × a × b2. Mice were euthanized at day 25, and subcutaneous tumors were resected and weighed.
Twenty-four C57BL/6 J mice were randomly divided into four groups. A total of 2 × 106 Lewis lung carcinoma cells (LLCs) were inoculated subcutaneously. Mice were given saline, metformin (300 mg/kg per day), atezolizumab (anti-PDL1) (1 mg/kg per day by intraperitoneal injection; A2004, Selleck, China), or metformin combined with atezolizumab once a day in subsequent days.
Histopathological analysis
Tumor tissues were fixed in 4% paraformaldehyde and embedded in paraffin. Paraffin tumor sections (5 μm) were stained with hematoxylin and eosin (H&E). For immunohistochemistry assays, paraffin sections were incubated with primary antibodies against CEBPB (1:200; Ab53138, Abcam) and PDL1 (1:200; 13,684, Cell Signaling Technology) followed by HRP-conjugated secondary antibodies (goat anti-rabbit IgG) at room temperature for 2 h. Five random fields of each section were photographed under the light microscope.
Retrospective analysis
Patient tissue samples were collected from the Department of Thoracic Surgery, Zhongshan Hospital Fudan University. The basic information and medication of the 12 patients with lung adenocarcinoma were queried before surgery, and patients were divided into an oral metformin group and untreated control group. Transcriptome sequencing was performed with the tumor tissues.
From January 2016 to January 2018, 1247 patients who received surgery and underwent PDL1 gene detection in our department were enrolled in our study. The results of PDL1 gene expression in NSCLC tissues were acquired from patient archives. The positive PDL1 was defined as tumor cells ≥ 1% or tumor stromal cells ≥ 1%. All patients included in this study provided informed consent, which was approved by the ethics (no. B2018-137R). The clinical and pathological data of the patients, including sex, age, smoking status, tumor location, surgical margin, tumor TNM staging, metformin taking, and PDL1 expression level, were analyzed. The Kaplan–Meier plotter Web site was used to explore the effect of certain genes on the prognosis of lung cancer.
Bioinformatics analysis
The raw data were standardized, and the limma package of R language software was used to analyze gene differences between the metformin and control group. |log10 (fold change [FC])|> 0.5 and P < 0.05 were set as the standard for differences between groups. The volcano maps and heat maps to visualize differentially expressed genes were drawn using the ggplot2 and pheatmap packages. The topGO package was used to perform Gene Ontology (GO) function enrichment analysis of the differentially expressed genes (DEGs) between the two groups and draw bubble charts. Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis was performed on the DEGs.
Statistical analysis
The line graph and histogram were generated using Graphpad 6.0, R language (version 3.61) was used for bioinformatics analysis, and SPSS 24.0 was used for statistical analysis. The two groups of continuous variables were compared by Student’s t-test. The Chi-square test was used for categorical variables. Two-sided P < 0.05 was considered statistically significant.
Results
Metformin inhibited proliferation of NSCLC cells
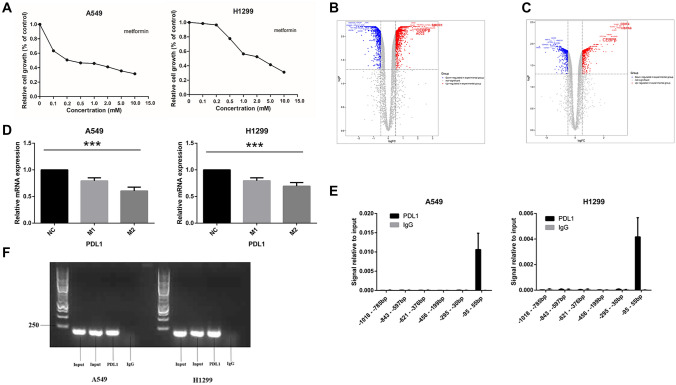
According to dose–response analysis, we found that metformin has a concentration-dependent inhibitory effect on NSCLC cells with IC50 values of 0.25 mmol/L and 2.0 mmol/L in A549 and H1299 cells, respectively (Fig. 1A).
Fig. 1.
Metformin inhibited the proliferation of NSCLC. A Metformin has a concentration-dependent inhibitory effect on NSCLC cells. B, C The volcano map and heatmap show the differential expressed genes. D qPCR showed the mRNA of PDL1 in A549 and H1299 significantly reduced after metformin treatment. E The DNA fragment pulled by the CEBPB antibody was the fragment of − 95 to + 55 bp. F The PCR products were used for electrophoresis. Metformin concentration. NC: no metformin treatment. M1: 0.15 mmol/L (A549), 1 mmol/L (H1299). M2: 0.25 mmol/L (A549), 2 mmol/L (H1299)
According to transcriptome sequencing results, as shown in the volcano map and heat map (Fig. 1B), 1103 genes were upregulated (logFC > 0.5, P < 0.05) and 829 genes downregulated (logFC < − 0.5, P < 0.05) in A549 cells treated with metformin. GO and KEGG analyses results are shown in Fig. S1A and S1B. A total of 759 genes were upregulated (logFC > 0.5, P < 0.05) and 532 genes downregulated (logFC < − 0.5, P < 0.05) in H1299 cells treated with metformin (Fig. 1C). GO and KEGG analysis results are shown in Figure S1C and S1D.
Based on the DEG analysis, the expression of PDL1 was significantly downregulated in A549 cells (logFC = − 1.093, P = 0.036) and H1299 cells (logFC = − 1.624, P = 0.024) following treatment with metformin. mRNA changes were verified by qPCR (Fig. 1D). These results indicated that metformin inhibited PDL1 expression.
CEBPB regulates the transcription of PDL1
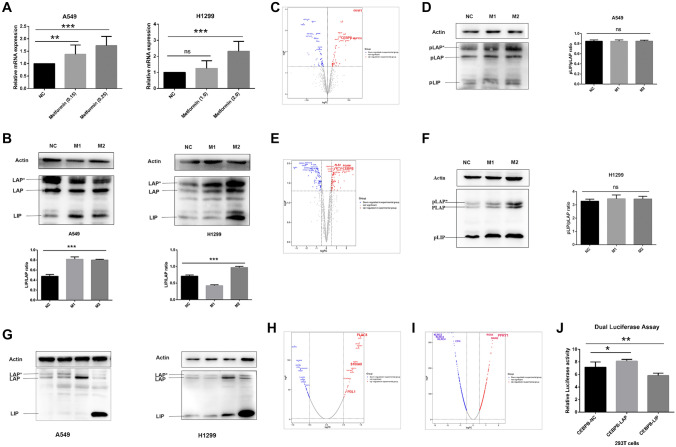
The potential CEBPB binding site for the PDL1 sequence (− 1000 to + 200 bp) was predicted using Jaspar (http://jaspar.genereg.net/). According to the transcriptome sequencing results, CEBPB expression was significantly upregulated in A549 cells (logFC = 2.058, P < 0.001) and H1299 cells (logFC = 2.133, P < 0.001) following treatment with metformin (Fig. 1B, C). qPCR confirmed these results (Fig. 2A). Therefore, by bioinformatics analysis and validation of mRNA and protein levels, we choose CEBPB as the target gene.
Fig. 2.
Overexpression of LAP and LIP had opposite effects on PDL1 expression. A qPCR showed that the expression of CEBPB-mRNA was increased significantly in both A549 and H1299. B Western blot analysis showed that both the expression of CEBPB-LAP and CEBPB-LIP increased. The ratio of LIP/LAP increased. C The phosphorylated protein profile of A549 treated with metformin was analyzed. D The expression of pLAP*, pLAP, and pLIP phosphorylated levels in A549 cells was upregulated, but the ratio of pLIP/pLAP did not change significantly (0.849 vs. 0.849 vs. 0.850; P = 0.998). E The phosphorylated protein profile of H1299 treated with metformin was analyzed. F The expression of pLAP*, pLAP, and pLIP phosphorylated levels in H1299 cells was upregulated, but the ratio of pLIP/pLAP did not change significantly (3.282 vs. 3.458 vs. 3.443; P = 0.589). G CEBPB-LAP*, CEBPB-LAP, and CEBPB-LIP and control cell line CEBPB-NC cell lines were constructed. (H, I) Gene-chip results showed the differential expressed genes in A549-LAP-OE cells and LIP-OE cells. h Dual-luciferase reporter assays showed the RLU1/RLU2 of the LAP-OE group was significantly increased (7.146 vs. 8.128, P = 0.023), and the RLU1/RLU2 of the LIP-OE group was significantly reduced (7.146 vs. 5.830, P = 0.006). Metformin concentration. NC: no metformin treatment. M1: 0.15 mmol/L (A549), 1 mmol/L (H1299). M2: 0.25 mmol/L (A549), 2 mmol/L (H1299)
Using the CEBPB DNA binding sequence as a substrate, six pairs of primers were designed for qPCR; the input and IgG group were used as controls. The DNA fragment pulled by the CEBPB antibody was the sixth fragment (− 95 to + 55 bp) (Fig. 1E). The PCR products were visualized by agarose gel electrophoresis (Fig. 1F).
The potential CEBPB binding site was located in the sixth fragment with a sequence of CTGGCGCAAC (score = 11.3283, relative score = 0.938232, start 1999, end 2008, strand +). In addition, ChIP-Seq analysis of GSM2421500, GSM2421662, GSM2421664, GSM2421852, GSM2421853, and GSM2421896 obtained from the GEO database showed that CEBPB had binding affinity to the PDL1 promoter region in A549 cells, consistent with the ChIP and Jaspar prediction results. In summary, CEBPB may be a transcription factor that can directly regulate PDL1 transcription by binding a specific sequence.
Cell construction
Following treatment of A549 and H1299 cells with metformin at the IC50 value, the expression of CEBPB-mRNA was increased significantly in both A549 and H1299 cells; this was verified by qPCR (Fig. 2A). This indicated that metformin could promote mRNA expression of CEBPB at the IC50 value of metformin. Western blot analysis showed that both the protein expression of CEBPB-LAP and CEBPB-LIP increased. Notably, the ratio of LIP/LAP was also increased (Fig. 2B).
The phosphorylated protein profile showed that, in A549 cells, by analyzing the distribution of all modified sites, a total of 1319 phosphorylated proteins were identified. According to analysis of differences in protein phosphorylation levels (Fig. 2C), the phosphorylation of 63 proteins was upregulated (logFC > 0.5, P < 0.05) and that of 55 proteins was downregulated (logFC < − 0.5, P < 0.05). GO and KEGG analyses are shown in Fig. 2SA and 2B. In H1299 cells, 1321 phosphorylated proteins were identified (Fig. 2E). Among them, the phosphorylation of 83 proteins was upregulated (logFC > 0.5, P < 0.05) and that of 96 proteins was downregulated (logFC < − 0.5, P < 0.05). GO and KEGG analyses are shown in Figure S2C and S2D.
In the phosphorylated protein profile analysis, following treatment with metformin, the phosphorylation level of CEBPB increased significantly in A549 cells (logFC = 0.765, P = 0.003) and H1299 cells (logFC = 1.578, P < 0.001). The site of CEBPB phosphorylation was Thr235. Western blot analysis showed that phosphorylation levels of pLAP*, pLAP, and pLIP were upregulated, but the ratio of pLIP/pLAP did not change significantly in A549 cells (0.849 vs. 0.849 vs. 0.850; P = 0.998; Fig. 2D) and H1299 cells (3.282 vs. 3.458 vs. 3.443; P = 0.589; Fig. 2F). Therefore, we considered that the inhibitory effect of metformin on cells is mainly through an increase in the LIP/LAP ratio rather than that of pLIP/pLAP.
To further investigate CEBPB-dependent transcriptional regulation of PDL1, we constructed A549 and H1299 cell lines overexpressing CEBPB-LAP*, CEBPB-LAP, and CEBPB-LIP as well as the control cell line CEBPB-NC. The cell lines were verified by western blot analysis (Fig. 2G).
Overexpression of LAP and LIP had opposite effects on PDL1 expression
Gene-chip technology was applied to investigate the effect of overexpressed CEBPB-LAP and LIP on A549 gene expression. A total of 33 upregulated genes (logFC > 0.5, P < 0.05) and 50 downregulated genes (logFC < 0.5, P < 0.05) were identified in A549-LAP-OE cells (Fig. 2H), while 63 upregulated genes (logFC > 0.5, P < 0.05) and 56 downregulated genes (logFC < − 0.5, P < 0.05) were identified in LIP-OE cells (Fig. 2i). Among these, PDL1 was upregulated in A549-LAP-OE cells (logFC = 0.624, P < 0.05), but downregulated in A549-LIP-OE cells (logFC = − 0.598, P < 0.05). Therefore, PDL1 was proposed as a downstream regulatory gene of CEBPB.
LAP and LIP overexpression had opposite effects on PDL1 promoter activity
We used a dual-luciferase reporter system to detect the effect of LAP and LIP isoforms on the activity of the PDL1 promoter. Compared with the CEBPB-NC group, RLU1/RLU2 of the LAP-OE group was significantly increased (7.146 vs. 8.128, P = 0.023) and RLU1/RLU2 of the LIP-OE group was significantly reduced (7.146 vs. 5.830, P = 0.006; Fig. 2J). This indicated that LAP overexpression combined with the PDL1 transcription factor binding site to promote the expression of PDL1, while CEBPB-LIP overexpression inhibited this transcription.
CEBPB regulated the function of A549 and H1299 cells.
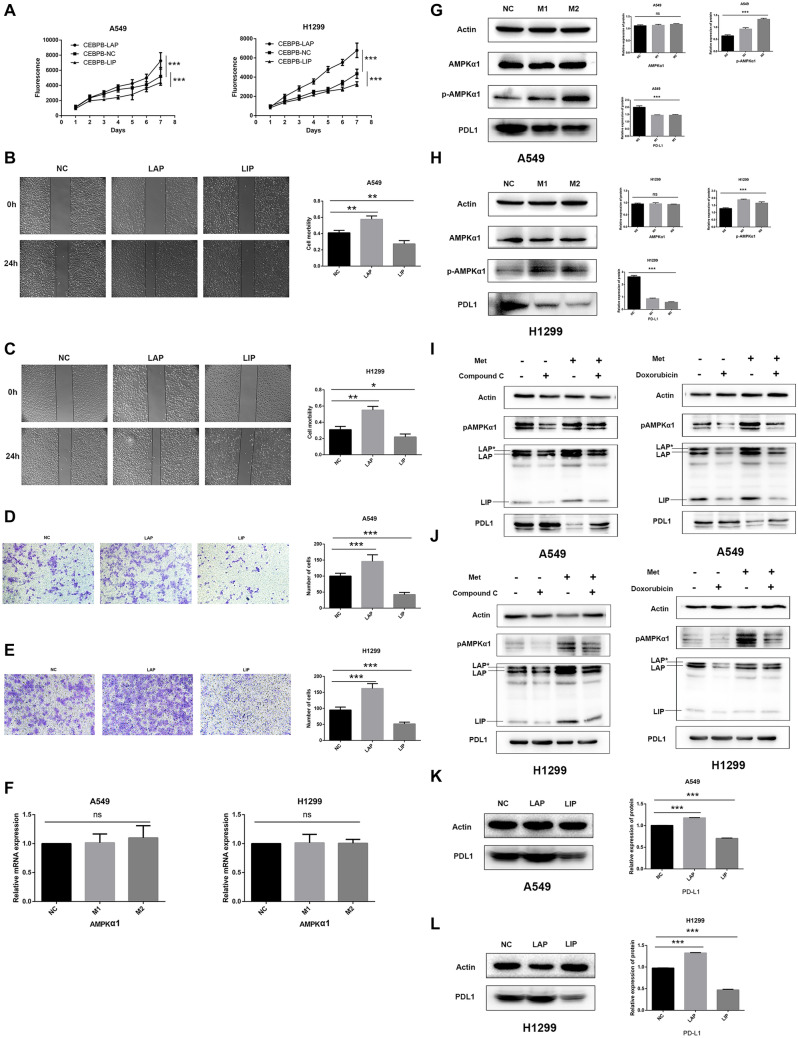
As shown in Fig. 3A, the proliferation of A549-LAP and H1299-LAP overexpressed (LAP-OE) cells increased significantly (P < 0.05), while the proliferation of A549-LIP-OE and H1299-LIP-OE cells decreased significantly (P < 0.001) compared with NC cells. The average mobility of A549-NC, LAP-OE, and LIP-OE cells was 0.41, 0.58, and 0.27, respectively. Compared with A549-CEBPB-NC cells, the mobility of LAP-OE cells was significantly increased (P = 0.0046), while the mobility of LIP-OE cells was significantly decreased (P = 0.0093; Fig. 3B). Similarly, the mobility of H1299-LAP-OE cells was significantly increased (0.55 vs. 0.31, P = 0.0024), and the mobility of H1299-LIP-OE cells was significantly reduced (0.22 vs. 0.31, P = 0.0444) compared with control cells (Fig. 3C). Transwell migration assays showed that the migration of A549-LAP-OE cells was significantly increased (145.8 vs. 58.5; P < 0.001), while the migration of A549-LIP-OE cells was significantly reduced (42.2 vs. 58.5; P = 0.0029) compared with A549-NC cells (Fig. 3D). In addition, similar results were observed in H1299-NC, LAP-OE, and LIP-OE cells (Fig. 3E). Thus, we conclude that overexpression of LAP promotes cell proliferation and migration, while overexpression of LIP works in an opposite manner.
Fig. 3.
Metformin inhibited NSCLC through AMPK–CEBPB–PDL1 signaling pathway. A The proliferation of LAP-OE cells increased significantly (P < 0.05), and the proliferation of LIP-OE decreased significantly (P < 0.001) compared with NC cells. b, C The mobility of LAP-OE was significantly increased, while the mobility of LIP-OE was significantly decreased. D, E The cell migration of LAP-OE significantly increased, while the cell migration of LIP-OE significantly reduced. F No significant change in AMPKα1 mRNA expression was observed after being treated by metformin by qPCR. G, H The phosphorylation level of AMPKα1 (pAMPKα1) was significantly increased (P < 0.001), and the PDL1 protein was significantly downregulated in 549 cells and H1299 cells treated with metformin (P < 0.001). I, J After the addition of AMPK inhibitor (Compound C and Doxorubicin) in A549 cells and H1299 cells, AMPKα1 phosphorylation was significantly downregulated, and the expression of three isoforms of CEBPB, namely LAP*, LAP, and LIP, were all downregulated, while the expression of PDL1 was upregulated. The metformin reversed the inhibitory effect of AMPK inhibitors. K, l Western blot analysis showed that PDL1 expression was significantly increased LAP-OE cells, but was significantly downregulated in LIP-OE cells (P < 0.001). Metformin concentration. NC: no metformin treatment. M1: 0.15 mmol/L (A549), 1 mmol/L (H1299). M2: 0.25 mmol/L (A549), 2 mmol/L (H1299)
Metformin inhibited NSCLC cells via AMPK–CEBPB–PDL1 signaling.
The qPCR showed that following treatment with metformin, there was no significant change in AMPKα1 mRNA expression in A549 cells (P = 0.2268) or H1299 cells (P = 0.9544; Fig. 3F). Western blot analysis showed that there was no significant change in AMPKα1 protein expression in A549 or H1299 cells treated with metformin (P > 0.05), but the phosphorylation level of AMPKα1 (pAMPKα1) was significantly increased (P < 0.001), indicating that phosphorylation of AMPKα1 is required for the anti-tumor effect of metformin. PDL1 was also significantly downregulated in A549 and H1299 cells treated with metformin (P < 0.001; Fig. 3G, H).
Based on these findings, we explored whether AMPKα1 phosphorylation affected the expression of CEBPB. We showed the reversal of the metformin effect from two AMPK inhibitors in A549 and H1299 cells, including Compound C and Doxorubicin. After the addition of AMPK inhibitor (Compound C and Doxorubicin) in A549 cells and H1299 cells, AMPKα1 phosphorylation was significantly downregulated, and the expression of three isoforms of CEBPB, namely LAP*, LAP, and LIP, was all downregulated, while the expression of PDL1 was upregulated. The metformin could reverse the inhibitory effect of AMPK inhibitors (Fig. 3I and J).
Western blot analysis showed that PDL1 expression was significantly increased in A549-LAP-OE and H1299-LAP-OE cells, but significantly downregulated in A549-LIP-OE and H1299-LIP-OE cells (P < 0.001; Fig. 3K, L) compared with NC cells. In summary, AMPKα1 upstream of CEBPB and AMPKα1 phosphorylation could regulate the expression of CEBPB and PDL1.
Metformin inhibited the growth of NSCLC xenografts in a mouse model
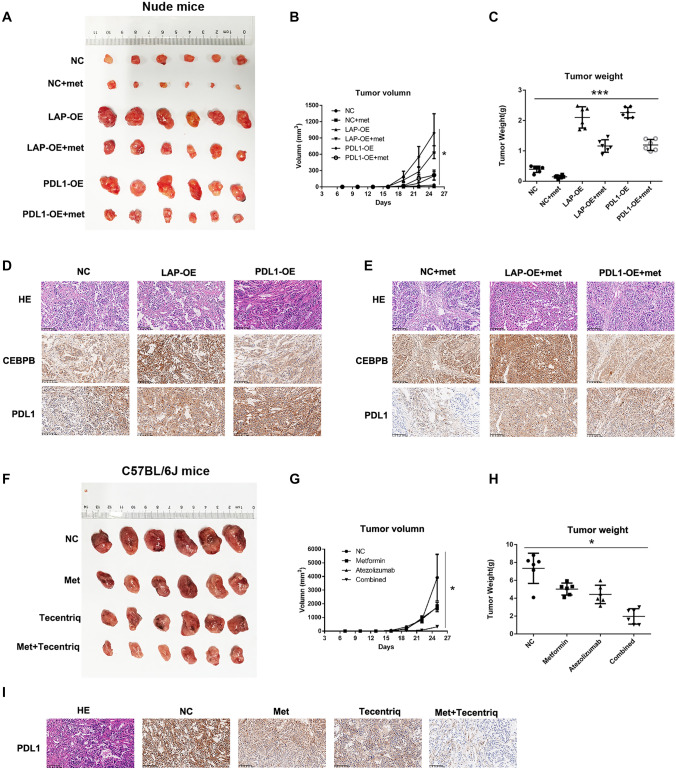
In untreated cells, A549-CEBPB-LAP-OE and A549-PDL1-OE tumor growth was significantly faster than that of A549-CEBPB-NC (P < 0.001). Tumor growth of groups treated with metformin was significantly slower than that of groups not treated with metformin (P < 0.05) (Fig. 4A, B), indicating that overexpression of CEBPB-LAP and PDL1 significantly promoted the proliferation of A549 cells, while metformin significantly inhibited tumor growth in vivo. Tumor weight was in accordance with tumor proliferation (Fig. 4C).
Fig. 4.
Metformin could inhibit the tumor growth in vivo. A, B, C The tumor growth of groups treated with metformin was significantly slower than that of groups not treated with metformin in nude mice. d, e Metformin could upregulate the CEBPB expression but downregulate the PDL1 expression in tumor tissues. f–h Metformin and atezolizumab could inhibit the tumor growth in C57BL/6 J mice, respectively, and atezolizumab and metformin has synergistic effect. i The immunohistochemistry staining showed that the expression PDL1 decreased in tumor of metformin group and atezolizumab group and decreased most significantly compared with those of control group
Tumor tissue was subjected to immunohistochemistry for CEBPB and PDL1. Compared with untreated A549-NC cells, expression of CEBPB and PDL1 was increased in A549-CEBPB-LAP-OE cells as well as A549-PDL1-OE cells. Compared with untreated NC cells, expression of CEBPB increased, while expression of PDL1 decreased in NC cells. Compared with untreated A549-CEBPB-LAP-OE cells, expression of CEBPB in A549-CEBPB-LAP-OE cells treated with metformin increased, while expression of PDL1 was downregulated. Compared with untreated A549-PDL1-OE cells, expression of PDL1 decreased in A549-PDL1-OE cells treated with metformin (Fig. 4D, E).
For the xenografts in C57BL/6 J mice, tumors in the control group grew the fastest, followed by tumors in the metformin and atezolizumab groups; tumors in the combined group grew the slowest (P < 0.05; Fig. 4F, G). The results suggested that atezolizumab and metformin had a synergistic effect on LLC tumor growth in vivo. Tumor weights were consistent with the tumor proliferation results (Fig. 4H). Immunohistochemistry staining showed that expression of PDL1 was decreased in tumors of the metformin and atezolizumab groups and most significantly decreased compared with those of the control group (Fig. 4i).
Clinical analysis
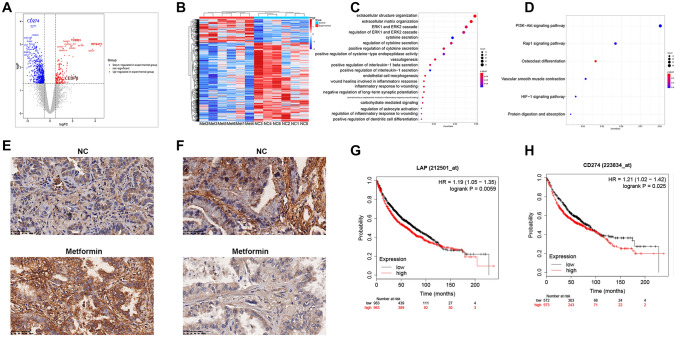
Twelve patients with adenocarcinoma were enrolled and divided into a metformin group and NC group. Clinical characteristics of the patients were analyzed (Supplementary Table 1). RNA transcriptome sequencing was performed on cancer tissue samples from these patients, and 9,423 genes were identified. DEG analysis showed that 192 genes were upregulated (logFC > 0.5, P < 0.05) and 653 genes were downregulated (logFC < -0.5, P < 0.05) compared with the control group (Fig. 5A, B). GO and KEGG analysis results are shown in Fig. 5C and D.
Fig. 5.
Clinical analysis. A, B RNA transcriptome sequencing was performed on cancer tissue samples and identified the DEGs. C, D GO and KEGG analyses were performed. e, F Immunohistochemistry results showed that the expression of CEBPB in the metformin group was higher than that in the control group, and the expression of PDL1 was lower than that in the control group. G The survival analysis showed that patients with low expression of CEBPB-LAP had significantly better prognosis than those with high expression of CEBPB-LAP (HR = 1.19, 95% CI = 1.05–1.35, P = 0.0059). (H) Better prognosis was observed in patients with low PDL1 expression than high PDL1 expression (HR = 1.21, 95% CI = 1.02–1.42, P = 0.025)
Compared with the control group, expression of CEBPB in cancer tissues of the metformin group was significantly upregulated (241.10 vs. 149.00, logFC = 0.6717, P = 0.045), while expression of PDL1 was significantly decreased (16.82 vs. 5.10, logFC = − 1.6215, P < 0.001). Immunohistochemistry showed that expression of CEBPB in the metformin group was higher than that in the control group (Fig. 5E) and expression of PDL1 was lower than that in the control group (Fig. 5F).
Among 1247 patients included in the study, 166 patients were taking metformin and 1081 patients did not take metformin (including patients who did not receive any hypoglycemic prescription, or were taking other hypoglycemic drugs, and patients using insulin). There were no significant differences between the two groups in terms of gender, smoking, tumor size, resection margin, tumor location, and tumor stage (all P > 0.05). In the metformin group, the average age of the patients was older (58.23 vs. 61.08; P = 0.002) and the positive rate of PDL1 was lower than that of the control group (HR = 0.338, 95% CI = 0.235–0.487; P < 0.001) (Table 1).
Table 1.
The clinical and pathological characteristics
| Characteristics | Metformin (−) | Metformin ( +) | HR | 95%CI | P-value |
|---|---|---|---|---|---|
| Gender | |||||
| Male | 404 | 64 | 0.951 | 0.680–1.331 | 0.770 |
| Female | 677 | 102 | |||
| Age | 58.23 ± 11.139 | 61.08 ± 10.765 | 0.002 | ||
| > 65 years | 293 | 101 | 1.731 | 1.233–2.430 | 0.001 |
| ≤ 65 years | 788 | 65 | |||
| Smoking | |||||
| No | 955 | 144 | 1.158 | 0.713–1.882 | 0.554 |
| Yes | 126 | 22 | |||
| Tumor size (cm) | 1.88 ± 1.409 | 1.73 ± 1.080 | 0.214 | ||
| Residual | |||||
| R0 | 1071 | 163 | 0.971 | 0.537–7.238 | 0.297 |
| R1/2 | 10 | 3 | |||
| Tumor location | 0.098 | ||||
| Left | 439 | 53 | |||
| Right | 628 | 110 | |||
| Bilateral | 14 | 3 | |||
| Stage | 0.191 | ||||
| 0/I | 883 | 146 | |||
| II | 78 | 8 | |||
| III | 96 | 8 | |||
| IV | 24 | 4 | |||
| PDL1 | |||||
| ( +) | 558 | 44 | 0.338 | 0.235–0.487 | < 0.001 |
| (−) | 523 | 122 | |||
The Kaplan–Meier plotter Web site was used to explore the effect of certain genes on the prognosis of lung cancer. To explore the relationship between CEBPB and the prognosis of lung cancer, 1,926 lung cancer patients were enrolled, including 963 patients with high expression of CEBPB-LAP in lung cancer tissue and 963 patients with low expression of CEBPB-LAP (Affy id/Gene symbol “212501_at”). The survival analysis showed that patients with low expression of CEBPB-LAP had significantly better prognoses than those with high expression of CEBPB-LAP (HR = 1.19, 95% CI = 1.05–1.35, P = 0.0059) (Fig. 5G). Similarly, to explore the relationship between the expression of CD274 (PDL1) and lung cancer prognosis, 1,145 lung cancer patients were enrolled, including 573 patients with high PDL1 expression in lung cancer tissues and 572 patients with low PDL1 expression (Affy id/Gene symbol “223834_at”). Better prognosis was observed in patients with low PDL1 expression than high PDL1 expression (HR = 1.21, 95% CI = 1.02–1.42, P = 0.025) (Fig. 5H).
Discussion
Studies have shown that metformin can inhibit NSCLC via the AMP-activated protein kinase (AMPK) pathway. In NSCLC cells, metformin can activate liver kinase B1, which is upstream of AMPK, resulting in negative regulation of mTOR activity and contributing to cell growth inhibition [12, 13]. In this study, we explore the metformin–AMPK–CEBPB–PDL1 pathway, but do not delve into the way that metformin regulates AMPK. As we know, A549 cell line is LKB1 null. In our study, the regulation of AMPK by metformin may not be STK11/LKB1 dependent. More and more evidence shows that metformin also inhibits cells through a pathway independent of AMPK mechanism. It was reported that LKB1-inactivated lung adenocarcinoma is highly sensitive to metformin, which, as a safe and low-cost anti-diabetic compound, can inhibit mitochondrial oxidative phosphorylation [14, 15]. Metformin can also prevent cisplatin resistance in an inactive LKB1 in vivo model of lung adenocarcinoma [15]. Shackelford et al. [16] found that metformin can reduce the tumor burden of tumor-bearing mice and increase the survival rate of LKB1-deficient lung tumor mice. Although there has been evidence that metformin has an inhibitory effect on NSCLC, its mechanism remains unclear.
In the current study, according to dose–response analysis, we found that metformin has a concentration-dependent inhibitory effect on NSCLC cells with an IC50 of 0.25 mmol/L and 2.0 mmol/L on A549 and H1299 cells, respectively. Extensive experimental results in vitro show that millimolar (mM) levels of metformin have antiproliferative effects. However, the direct clinical anti-tumor activity of this concentration is impractical, because the diabetic patients have plasma level of metformin in the 10 μM range, which are unlikely to mimic the in vivo microenvironment [17, 18]. Therefore, Chandel et al. [19] believe that the requirement for the concentration of metformin in vitro may not be helpful in predicting the concentration of the drug required for in vivo or clinical activities. Maybe the metformin has other potential mechanisms to exert effects, for example, oxidative stress. Metformin can directly inhibit cell proliferation by regulating the cell cycle, upregulating tumor suppressor genes, and promoting cell death mediated by oxidative stress and thus exhibit antiproliferative effects indirectly [14]. It is worth to further study.
PD-1/PDL1 inhibitors have shown extraordinary efficacy in inhibition of a variety of tumors [20]. Tumor-expressed PDL1 transmits negative signals to anti-tumor T cells expressing PD-1, changing the pathogenesis of tumor immunity [21]. However, emerging evidence has shown that PDL1 also has intrinsic tumor functions [22, 23]. Zheng et al. [24] showed that PDL1 promoted proliferation of head and neck squamous cell carcinoma via mTOR signaling; blocking PDL1 was beneficial. Other studies have shown that PDL1 is also involved in the regulation of epithelial interstitial transformation [25, 26] and is closely related to cell cycle progression in human breast cancer and expression of the proliferation marker Ki-67 [27]. In ovarian cancer cells and melanoma, high expression of PDL1 increased autophagy through mTOR signaling [4]. PDL1 inhibitors can also reverse the immune metabolic dysfunction of monocytes in chronic lymphocytic leukemia through BTK signaling [28]. In this study, metformin and atezolizumab could inhibit tumor growth in vivo and decrease the expression of PDL1. Furthermore, the two drugs had a synergistic effect. In the manual of atezolizumab provided by Selleck, it is reported that atezolizumab can apply to humanized mice, non-humanized mice (e.g., C57BL/6 mice), peripheral blood, and other related assays. In a study by Sanjeev Mariathasan, C57BL6 mice were inoculated with MC38 (a mouse tumor cell line) tumor cells subcutaneously, and then, atezolizumab was used to study the therapeutic effect of the blockade of PD-L1, similar to our study [29].
The transcription factor CEBPB is involved in various physiological and pathophysiological processes, including the development and progression of malignant tumors. Increased CEBPB expression has been observed in many human tumors, including breast cancer [30], gastric cancer [31], ovarian cancer [32], colorectal cancer [33], and liver cancer [34]. Studies have shown that regulation of the LAP/LIP ratio plays a key role in liver regeneration, acute phase response, bone homeostasis, and breast development [35, 36], and the downstream genes are involved in many physiological and pathological processes. Maehara et al. [11] found that metformin could upregulate the expression of CEBPB, both LAP and LIP were upregulated, and the LIP/LAP ratio was increased. In this study, we found that metformin upregulated phosphorylation of AMPKα1, thereby affecting the expression of downstream CEBPB. Gene chip analysis revealed that PDL1 was significantly upregulated in A549 CEBPB-LAP-OE cells and downregulated in CEBPB-LIP-OE cells. Our ChIP results and ChIP-Seq data from the GEO database and Jaspar Web site argued that CEBPB can directly bind the PDL1 promoter to activate transcription. Dual-luciferase reporter assay showed that overexpression of CEBPB-LAP could promote PDL1 transcription; this was inhibited by overexpression of CEBPB-LIP. Following metformin treatment of NSCLC cells, the CEBPB-LIP/LAP ratio was increased, transcription of PDL1 was inhibited, and expression of PDL1 was downregulated. In this study, both pLAP and pLIP were increased following metformin treatment, but the ratio of pLAP/pLIP showed no significance. Metformin increased the phosphorylation of CEBPB at Thr235. Three kinases, namely MAPK, CDK2, and RPS6KA1, have been reported to phosphorylate CEBPB and promote its transcriptional activity [37–39]. However, there is no evidence that CEBPB is a substrate of AMPK. As both LAP and LIP were similarly phosphorylated by metformin, it appears that the LAP/LIP ratio, rather than pLAP/LIP, is important in regulation of the expression of PDL1; one possibility is that phosphorylation of CEBPB increases the stability of the three isoforms. Based on our results, metformin can regulate NSCLC cells via AMPK–CEBPB–PDL1 signaling. We also demonstrated that metformin inhibited tumor growth, and atezolizumab and metformin had a synergistic effect on LLC tumor growth in vivo. Immunohistochemistry staining verified that metformin could upregulate the expression of CEBPB and downregulate the expression of PDL1. In this study, we used a CEBPB antibody to detect the total protein, but could not differentiate the LAP or LIP protein because there was no LAP or LIP isoform antibody available. We demonstrated that CEBPB was highly expressed, but we were unable to detect the LAP/LIP ratio.
There are some limitations in our study. Firstly, in our experiment of Dual-luciferase reporter gene assay, we used 293 T cells to prove that LAP or LIP overexpression has opposite effects on PD-L1 promoter activity, because A549 or H1299 cells had low transfection efficiency and are hard to be transfected using plasmids. Secondly, we used the antibody of CEBPB to detect the total protein, but we could not differentiate the LAP or LIP protein because there was no commercial LAP or LIP isoform antibody for IHC. We only demonstrated that the CEBPB highly expressed, but we were unable to detect the ratio of LAP/LIP. This needs to be further studied.
Conclusions
Our study showed that metformin had an inhibitory effect on the NSCLC cell lines A549 and H1299. As a transcription factor, CEBPB directly regulated the transcription of PDL1. The inhibitory effect of metformin on NSCLC may be mediated by metformin–AMPK–CEBPB–PDL1 signaling.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
This work was supported by grants from National Natural Science Foundation of China (No. 81401875) and the Research Program of Shanghai Health Commission (Grant No. 20204Y022). We have asked the International Science Editing Corporation for editing the language.
Abbreviations
- AMPK
AMP-activated protein kinase.
- ChIP
Chromatin immunoprecipitation
- DEG
Differential expressed genes
- EMT
Epithelial interstitial transformation
- FC
Fold change
- HR
Hazard ratio
- NSCLC
Non-small cell lung cancer
- OE
Overexpressed
- PDL1
Programmed cell death ligand-1
- qPCR
Quantitative polymerase chain reaction
- SPF
Specific pathogen-free
Author contributions
CZ and WJ take responsibility for the integrity of the work as a whole. CZ, WJ, and TL conceived the study. TL, ML, and MNZ performed most of the bioinformatics analysis and wrote the manuscript. GSB, YWH, JQL, ZCC, and YSZ collected the tumor samples and analyzed the data of clinical characteristics. JJX, ZWL, and QW helped project design and manuscript editing. LLT supervised this study.
Declarations
Conflict of interest
The authors declare no conflict of interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Tao Lu, Ming Li and Mengnan Zhao have contributed equally to this work.
Contributor Information
Cheng Zhan, Email: czhan10@fudan.edu.cn.
Wei Jiang, Email: jiang.wei1@zs-hospital.sh.cn.
References
- 1.Yang P, Allen MS, Aubry MC, et al. Clinical features of 5,628 primary lung cancer patients: experience at mayo clinic from 1997 to 2003. Chest. 2005;128:452–462. doi: 10.1378/chest.128.1.452. [DOI] [PubMed] [Google Scholar]
- 2.Wistuba II, Gelovani JG, Jacoby JJ, Davis SE, Herbst RS. Methodological and practical challenges for personalized cancer therapies. Nat Rev Clin Oncol. 2011;8:135–141. doi: 10.1038/nrclinonc.2011.2. [DOI] [PubMed] [Google Scholar]
- 3.Li JX, Huang JM, Jiang ZB, Li RZ, Sun A, Lai-Han Leung E, Yan PY. Current clinical progress of PD-1/PD-L1 immunotherapy and potential combination treatment in non-small cell lung cancer. Integr Cancer Ther. 2019;18:1534735419890020. doi: 10.1177/1534735419890020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clark CA, Gupta HB, Sareddy G, et al. Tumor-intrinsic PD-L1 signals regulate cell growth, pathogenesis, and autophagy in ovarian cancer and melanoma. Cancer Res. 2016;76:6964–6974. doi: 10.1158/0008-5472.CAN-16-0258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang JJ, Zhang QS, Li ZQ, Zhou JW, Du J. Metformin attenuates PD-L1 expression through activating hippo signaling pathway in colorectal cancer cells. Am J Trans Res. 2019;11:6965–6976. [PMC free article] [PubMed] [Google Scholar]
- 6.Xiong W, Hsieh CC, Kurtz AJ, Rabek JP, Papaconstantinou J. Regulation of CCAAT/enhancer-binding protein-beta isoform synthesis by alternative translational initiation at multiple AUG start sites. Nucl Acids Res. 2001;29:3087–3098. doi: 10.1093/nar/29.14.3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Won C, Kim BH, Yi EH, et al. Signal transducer and activator of transcription 3-mediated CD133 up-regulation contributes to promotion of hepatocellular carcinoma. Hepatology. 2015;62:1160–1173. doi: 10.1002/hep.27968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Akira S, Isshiki H, Sugita T, Tanabe O, Kinoshita S, Nishio Y, Nakajima T, Hirano T, Kishimoto T. A nuclear factor for IL-6 expression (NF-IL6) is a member of a C/EBP family. EMBO J. 1990;9:1897–1906. doi: 10.1002/j.1460-2075.1990.tb08316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burgess-Beusse BL, Timchenko NA, Darlington GJ. CCAAT/enhancer binding protein alpha (C/EBPalpha) is an important mediator of mouse C/EBPbeta protein isoform production. Hepatology. 1999;29:597–601. doi: 10.1002/hep.510290245. [DOI] [PubMed] [Google Scholar]
- 10.Descombes P, Chojkier M, Lichtsteiner S, Falvey E, Schibler U. LAP, a novel member of the C/EBP gene family, encodes a liver-enriched transcriptional activator protein. Genes Dev. 1990;4:1541–1551. doi: 10.1101/gad.4.9.1541. [DOI] [PubMed] [Google Scholar]
- 11.Maehara O, Ohnishi S, Asano A, Suda G, Natsuizaka M, Nakagawa K, Kobayashi M, Sakamoto N, Takeda H. Metformin regulates the expression of CD133 Through the AMPK-CEBPbeta pathway in hepatocellular carcinoma cell lines. Neoplasia. 2019;21:545–556. doi: 10.1016/j.neo.2019.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vallianou NG, Evangelopoulos A, Kazazis C. Metformin and cancer. Rev Diabet Stud: RDS. 2013;10:228–235. doi: 10.1900/RDS.2013.10.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin CC, Yeh HH, Huang WL, Yan JJ, Lai WW, Su WP, Chen HH, Su WC. Metformin enhances cisplatin cytotoxicity by suppressing signal transducer and activator of transcription-3 activity independently of the liver kinase B1-AMP-activated protein kinase pathway. Am J Respir Cell Mol Biol. 2013;49:241–250. doi: 10.1165/rcmb.2012-0244OC. [DOI] [PubMed] [Google Scholar]
- 14.Queiroz EA, Puukila S, Eichler R, et al. Metformin induces apoptosis and cell cycle arrest mediated by oxidative stress, AMPK and FOXO3a in MCF-7 breast cancer cells. PLoS ONE. 2014;9:e98207. doi: 10.1371/journal.pone.0098207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vernieri C, Signorelli D, Galli G, et al. Exploiting fasting-mimicking diet and metformin to improve the efficacy of platinum-pemetrexed chemotherapy in advanced LKB1-inactivated lung adenocarcinoma: the FAME trial. Clin Lung Cancer. 2019;20:e413–e417. doi: 10.1016/j.cllc.2018.12.011. [DOI] [PubMed] [Google Scholar]
- 16.Shackelford DB, Abt E, Gerken L, et al. LKB1 inactivation dictates therapeutic response of non-small cell lung cancer to the metabolism drug phenformin. Cancer Cell. 2013;23:143–158. doi: 10.1016/j.ccr.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Graham GG, Punt J, Arora M, et al. Clinical pharmacokinetics of metformin. Clin Pharmacokinet. 2011;50:81–98. doi: 10.2165/11534750-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 18.Gravel SP, Hulea L, Toban N, et al. Serine deprivation enhances antineoplastic activity of biguanides. Cancer Res. 2014;74:7521–7533. doi: 10.1158/0008-5472.CAN-14-2643-T. [DOI] [PubMed] [Google Scholar]
- 19.Chandel NS, Avizonis D, Reczek CR, et al. Are metformin doses used in murine cancer models clinically relevant? Cell Metab. 2016;23:569–570. doi: 10.1016/j.cmet.2016.03.010. [DOI] [PubMed] [Google Scholar]
- 20.Zhou H, Liu J, Zhang Y, Zhang L. Inflammatory bowel disease associated with the combination treatment of nivolumab and metformin: data from the FDA adverse event reporting system. Cancer Chemother Pharmacol. 2019;83:599–601. doi: 10.1007/s00280-018-03763-5. [DOI] [PubMed] [Google Scholar]
- 21.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kleffel S, Posch C, Barthel SR, et al. Melanoma cell-Intrinsic PD-1 receptor functions promote tumor growth. Cell. 2015;162:1242–1256. doi: 10.1016/j.cell.2015.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chang CH, Qiu J, O'Sullivan D, et al. Metabolic competition in the tumor microenvironment is a driver of cancer progression. Cell. 2015;162:1229–1241. doi: 10.1016/j.cell.2015.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zheng A, Li F, Chen F, Zuo J, Wang L, Wang Y, Chen S, Xiao B, Tao Z. PDL1 promotes head and neck squamous cell carcinoma cell growth through mTOR signaling. Oncol Rep. 2019;41:2833–2843. doi: 10.3892/or.2019.7053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cao Y, Zhang L, Kamimura Y, Ritprajak P, Hashiguchi M, Hirose S, Azuma M. B7–H1 overexpression regulates epithelial-mesenchymal transition and accelerates carcinogenesis in skin. Cancer Res. 2011;71:1235–1243. doi: 10.1158/0008-5472.CAN-10-2217. [DOI] [PubMed] [Google Scholar]
- 26.Alsuliman A, Colak D, Al-Harazi O, Fitwi H, Tulbah A, Al-Tweigeri T, Al-Alwan M, Ghebeh H. Bidirectional crosstalk between PD-L1 expression and epithelial to mesenchymal transition: significance in claudin-low breast cancer cells. Mol Cancer. 2015;14:149. doi: 10.1186/s12943-015-0421-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ghebeh H, Tulbah A, Mohammed S, Elkum N, Bin Amer SM, Al-Tweigeri T, Dermime S. Expression of B7–H1 in breast cancer patients is strongly associated with high proliferative Ki-67-expressing tumor cells. Int J Cancer. 2007;121:751–758. doi: 10.1002/ijc.22703. [DOI] [PubMed] [Google Scholar]
- 28.Qorraj M, Bruns H, Bottcher M, Weigand L, Saul D, Mackensen A, Jitschin R, Mougiakakos D. The PD-1/PD-L1 axis contributes to immune metabolic dysfunctions of monocytes in chronic lymphocytic leukemia. Leukemia. 2017;31:470–478. doi: 10.1038/leu.2016.214. [DOI] [PubMed] [Google Scholar]
- 29.Mariathasan S, Turley SJ, Nickles D, et al. TGFbeta attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature. 2018;554:544–548. doi: 10.1038/nature25501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Russell A, Boone B, Jiang A, Sealy L. Genomic profiling of C/EBPbeta2 transformed mammary epithelial cells: a role for nuclear interleukin-1beta. Cancer Biol Ther. 2010;10:509–519. doi: 10.4161/cbt.10.5.12725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Regalo G, Forster S, Resende C, et al. C/EBPbeta regulates homeostatic and oncogenic gastric cell proliferation. J Mol Med. 2016;94:1385–1395. doi: 10.1007/s00109-016-1447-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sundfeldt K, Ivarsson K, Carlsson M, Enerback S, Janson PO, Brannstrom M, Hedin L. The expression of CCAAT/enhancer binding protein (C/EBP) in the human ovary in vivo: specific increase in C/EBPbeta during epithelial tumour progression. Br J Cancer. 1999;79:1240–1248. doi: 10.1038/sj.bjc.6690199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rask K, Thorn M, Ponten F, Kraaz W, Sundfeldt K, Hedin L, Enerback S. Increased expression of the transcription factors CCAAT-enhancer binding protein-beta (C/EBBeta) and C/EBzeta (CHOP) correlate with invasiveness of human colorectal cancer. Int J Cancer. 2000;86:337–343. doi: 10.1002/(sici)1097-0215(20000501)86:3<337::aid-ijc6>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 34.Li Y, Bevilacqua E, Chiribau CB, Majumder M, Wang C, Croniger CM, Snider MD, Johnson PF, Hatzoglou M. Differential control of the CCAAT/enhancer-binding protein beta (C/EBPbeta) products liver-enriched transcriptional activating protein (LAP) and liver-enriched transcriptional inhibitory protein (LIP) and the regulation of gene expression during the response to endoplasmic reticulum stress. J Biol Chem. 2008;283:22443–22456. doi: 10.1074/jbc.M801046200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wethmar K, Begay V, Smink JJ, Zaragoza K, Wiesenthal V, Dorken B, Calkhoven CF, Leutz A. C/EBPbetaDeltauORF mice–a genetic model for uORF-mediated translational control in mammals. Genes Dev. 2010;24:15–20. doi: 10.1101/gad.557910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smink JJ, Begay V, Schoenmaker T, Sterneck E, de Vries TJ, Leutz A. Transcription factor C/EBPbeta isoform ratio regulates osteoclastogenesis through MafB. EMBO J. 2009;28:1769–1781. doi: 10.1038/emboj.2009.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Buck M, Poli V, Hunter T, Chojkier M. C/EBPbeta phosphorylation by RSK creates a functional XEXD caspase inhibitory box critical for cell survival. Mol Cell. 2001;8:807–816. doi: 10.1016/s1097-2765(01)00374-4. [DOI] [PubMed] [Google Scholar]
- 38.Tang QQ, Gronborg M, Huang H, Kim JW, Otto TC, Pandey A, Lane MD. Sequential phosphorylation of CCAAT enhancer-binding protein beta by MAPK and glycogen synthase kinase 3beta is required for adipogenesis. Proc Natl Acad Sci USA. 2005;102:9766–9771. doi: 10.1073/pnas.0503891102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li X, Kim JW, Gronborg M, Urlaub H, Lane MD, Tang QQ. Role of cdk2 in the sequential phosphorylation/activation of C/EBPbeta during adipocyte differentiation. Proc Natl Acad Sci USA. 2007;104:11597–11602. doi: 10.1073/pnas.0703771104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.