Abstract
Background
Anti-angiogenic drugs increase anti-tumor efficacy of immune checkpoint inhibitors (ICIs). However, the optimal dose of anti-angiogenic drugs remains unclear.
Methods
We retrospectively analyzed efficacy and safety data from patients diagnosed with advanced or metastatic non-small cell lung cancer (NSCLC) that received PD-1 blockade with low-doses of anlotinib, a highly selective receptor tyrosine kinase inhibitor mainly targeting vascular endothelial growth factor receptors, as second or later line therapy. The primary endpoint was progression-free survival (PFS). Secondary endpoints included overall survival (OS), overall response rate (ORR), disease control rate (DCR), and safety profile. Univariate and multivariate analyses were used to identify prognostic factors.
Results
A total of 40 eligible patients were included. The median PFS was 11.4 months. The median OS of the entire cohort was 27.0 months. ORR was achieved in 16 patients (40.0%) and DCR was maintained in 33 patients (82.5%). The overall incidence of adverse events (AEs) was 52.5%, and the most common all grade AE was gastrointestinal reactions, which occurred in four patients (10.0%). Treatment-related grade 3/4 toxicity was observed in one patient (2.5%).
Conclusions Low-dose anlotinib may be an effective and well-tolerated anti-angiogenesis partner for combination therapy with ICIs in second-line and later settings for advanced NSCLC.
Keywords: NSCLC, ICIs, Anti-angiogenic drugs, Combination therapy, Second-line treatment
Background
Immune checkpoint inhibitors (ICIs) have changed the paradigm of advanced non-small cell lung cancer (NSCLC) treatment. ICIs, such as antibodies against programmed cell death-1 (PD-1) or to its ligand, programmed cell death-ligand 1 (PD-L1), are evolving treatment options for advanced NSCLC. Accumulating evidence, however, demonstrates that not all patients benefit from single-agent ICIs, suggesting that combination regimens may be necessary to improve treatment efficacy in broader groups of patients. Therefore, multiple studies exploring combination regimens of various ICIs have emerged [1].
Angiogenesis has a critical role in tumor growth and metastasis, and blocking tumor angiogenic pathways as a therapeutic strategy has demonstrated clinical success in the treatment of cancer. Anlotinib is a selective receptor tyrosine kinase inhibitor (TKI) that targets vascular endothelial growth factor receptors, c-Kit, platelet-derived growth factor receptor-α, and fibroblast growth factor receptors [2]. Anlotinib is approved by the China National Medical Products Administration for the treatment of third-line advanced NSCLC based on the overall survival (OS) benefit observed with anlotinib over a placebo in a randomized, double-blind, multicenter, phase III trial, ALTER-0303 [3]. Although anlotinib is not currently approved in the United States or Europe, relevant clinical studies are ongoing. The APROMISS study explored the efficacy and safety of dacarbazine and anlotinib in patients with synovial sarcoma who had received ≥ 2 lines of therapy. Compared with dacarbazine in the treatment of advanced synovial sarcoma, the progression free survival (PFS) of anlotinib is longer (2.9 months vs 1.6 months), and the incidence of adverse reactions are tolerable [4].
Recently, a series of studies have found that anlotinib has a synergistic effect when used in combination with PD-1 blockade. In a phase IB clinical study, using anlotinib plus PD-1 blockade as first-line therapy had good efficacy and tolerability in patients with advanced NSCLC without epidermal growth factor receptor (EGFR) mutations [5]. In real-world practice, anlotinib plus PD-1 blockade yielded a higher objective response rate (ORR) and longer PFS than PD-1 monotherapy in second-line or later settings [6]. Anlotinib plus PD-1 blockade is also beneficial when used in previously-treated advanced NSCLC patients positive for the EGFR mutation and in patients with liver or brain metastases [7]. Studies to date thus indicate that anlotinib has the potential to improve the efficacy of PD-1 blockade in NSCLC treatment; however, in these cases, anlotinib is typically used at a high dose (12 mg once daily, 2-weeks on/1-week off).
Antiangiogenic drugs induce normalization of tumor vessels, which directly promotes delivery of anti-tumor drugs and the adhesion, infiltration and activity of immune cells [8]. A number of studies have shown that targeting tumor vasculature with lower vascular-normalizing doses, as opposed to high doses of antiangiogenic drugs, results in a more homogeneous distribution of functional tumor vessels [9–12]. Furthermore, low doses are superior to high doses for reprogramming the tumor microenvironment from an immunosuppressive to an immune permissive one [8, 9]. Specifically, a recent report has shown that apatinib, another VEGFR TKI drug similar to anlotinib, can better normalize tumor blood vessels when used in low doses [12]. When used in combination with the anti-PD-L1 antibody, the synergistic effect of low-dose apatinib was significantly higher than that of high doses [12]. These findings suggest that applying low-dose antiangiogenic drugs with PD-1 blockade may enhance anti-tumor synergistic effects better than high doses of antiangiogenic drugs with PD-1 blockade.
In clinical trials and real-world clinical practice, the dosage of anlotinib monotherapy for cancer treatment typically includes one of three standard doses of 8 mg, 10 mg or 12 mg [13]. Its anti-tumor efficacy is positively correlated with the dose [14]. Presumably for this reason, the 12 mg dose is used in combination with PD-1 blockade in most studies. However, based on the anti-tumor characteristics of the aforementioned anti-angiogenic drugs, we speculate that the application of low-dose anlotinib and PD-1 blockade may bring better effectiveness and tolerability. In this study, we retrospectively analyzed the clinical outcomes and toxicity profile of lower doses of anlotinib (8 mg or 10 mg once daily, 2-weeks on/1-week off) in combination with PD-1 blockade in NSCLC patients in second-line and later settings.
Methods
Study design and patients
We carried out a single-center, observational, retrospective cohort study. Patients diagnosed with advanced NSCLC who received low-dose anlotinib in combination with PD-1 blockade as second-line or later treatment between January 2019 and December 2020 at the Affiliated Cancer Hospital of Zhengzhou University and Henan Cancer Hospital in China were included. All relevant medical records were assessed for eligibility. Inclusion criteria were: diagnosis of advanced NSCLC (unresectable stage III or IV disease based on the 8th edition of the TNM classification [15], including postoperative recurrence); diagnosis confirmed by histology or cytology; ECOG performance status (PS) of grade 0–2; treatment with at least four cycles of anlotinib (10 mg or 8 mg once daily, 2-weeks on/1-week off) in combination with PD-1 blockade every 3 weeks until disease progression or intolerance to treatment. Exclusion criteria were: mixed tumors of small cell and non-small cell lung cancer; a history of hemoptysis; any prior use of anlotinib; and any other current or recent malignancy in the past 5 years. The cut-off date for follow-up was December 25th, 2021. Research was conducted in accordance with the principles of the Declaration of Helsinki and the Guidelines for Good Clinical Practice of the World Health Organization.
Endpoints and assessment
Clinical responses were defined according to the Response Evaluation Criteria in Solid Tumors 1.1. PFS is defined as the time between the date of the start of treatment with anlotinib and PD-1 blockade and documented disease progression or death from any cause. OS is defined as the time from the start of therapy with anlotinib with PD-1 blockade to death or last follow-up. ORR is defined as the proportion of patients who have a partial or complete response to therapy. Disease control rate (DCR) is defined as the percentage of patients with a complete response (CR), partial response (PR), or stable disease (SD). AEs were recorded and graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 5.0.
Statistical analysis
Continuous and categorical data were summarized as medians (range) and numbers (percentage), respectively. We used Kaplan–Meier analysis to obtain PFS and OS estimates along with corresponding 95% confidence intervals (CIs). The Mantel-Cox log-rank test was used for statistical inference in the formal comparison between groups. Analysis of the variables associated with clinical outcomes was carried out by univariate analysis using the Cox-regression model. For all analyses, two-tailed p-values < 0.05 were considered statistically significant. All statistical analyses were carried out using R and SPSS.
Results
Patient characteristics
Forty advanced NSCLC patients were eligible as per inclusion and exclusion criteria. Detailed information for each patient is listed in supplementary Table 1, including prior lines of treatments, mutations, PD-L1 expression, duration of treatment, change from baseline, and reasons for drug discontinuation. The baseline characteristics of the included patients are summarized in Table 1. The median age of the study population was 63 years. The patients were 30.0% (12/40) male and 70.0% (28/40) female. Patient pathological diagnosis included lung squamous cell carcinoma (37.5%; 15/40), adenocarcinoma (60.0%; 24/40), and adenosquamous carcinoma (2.5%; 1/40). At the time of diagnosis, 32.5% (13/40) of patients carried mutations, and 10.0% (4/40) had brain metastases. 60.0% (24/40) of patients had undergone a second-line treatment prior to the treatment of this study.
Table 1.
Baseline characteristics (n = 70)
| Characteristics | No. of patients (%) |
|---|---|
| Doses of anlotinib | |
| 8 mg | 8 (20.0) |
| 10 mg | 32 (80.0) |
| Gender | |
| Male | 12 (30.0) |
| Female | 28 (70.0) |
| Age | |
| < 65 | 22 (55.0) |
| ≥ 65 | 18 (45.0) |
| ECOG score | |
| 0–1 | 33 (82.5) |
| 2 | 7 (17.5) |
| Smoking history | |
| Yes | 25 (62.5) |
| No | 15 (37.5) |
| Pathological type | |
| Squamous carcinoma | 15 (37.5) |
| Adenocarcinoma | 24 (60.0) |
| Adenosquamous | 1 (2.5) |
| Gene | |
| No mutation | 18 (45.0) |
| Gene mutation | 13 (32.5) |
| No test | 9 (22.5) |
| Metastatic organs | |
| ≥ 3 | 25 (62.5) |
| < 3 | 15 (37.5) |
| Brain metastasis | |
| Yes | 4 (10.0) |
| No | 36 (90.0) |
| Treatment baseline | |
| Second-line treatment | 20 (50.0) |
| Above second-line treatment | 20 (50.0) |
Efficacy
Sixteen patients achieved a PR, and no patients attained a CR. The ORR was 40.0% (16/40). Seventeen patients (42.5%) had a SD and DCR was 82.5% (33/40). Seven patients (17.5%) had progressive disease (Fig. 1). At the cutoff date, the median PFS (mPFS) of the entire cohort was 11.4 months (95% CI: 8.5–NR; Fig. 2) and the median OS (mOS) was 27.0 months (95% CI: 12.0–NR; Fig. 3). PFS was determined using the Cox proportional hazards regression model.
Fig. 1.
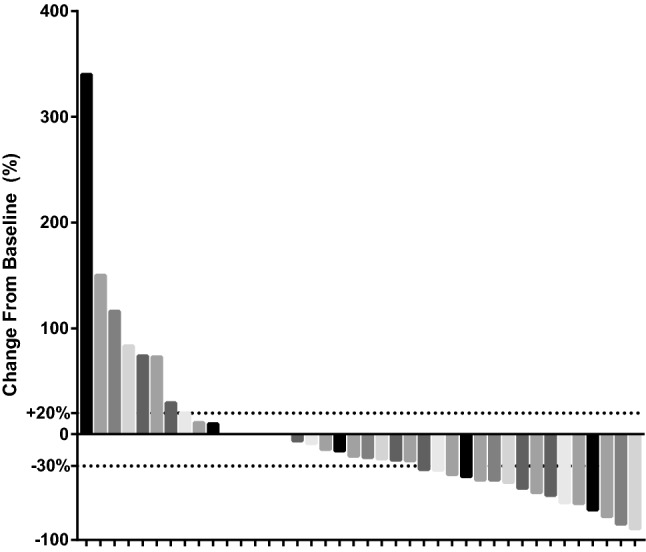
Waterfall plot illustrating maximum change in target lesion size (n = 40)
Fig. 2.
Kaplan–Meier curves depict median PFS in the anlotinib combination with immune checkpoint inhibitor
Fig. 3.
Kaplan–Meier curves depict median OS in the anlotinib combination with immune checkpoint inhibitor
We further performed subgroup analysis among the patients to investigate whether any clinical or pathologic features would impact the mPFS or mOS, including gender (i.e. male or female), age (i.e. ˂65 vs ≥ 65), ECOG score (i.e. 0–1 vs 2), smoking history (i.e. yes vs no), pathological type (i.e. squamous cell carcinoma vs adenocarcinoma vs. adenosquamous carcinoma), gene mutation status (i.e. no mutation vs mutation), organ metastasis (i.e. ˂3 vs ≥ 3), brain metastasis (i.e. yes vs no) and treatment lines (i.e. second-line vs second-line above). Our analysis revealed no statistical difference in PFS in any of the clinical factors investigated, although it appeared that patients with an ECOG score of 0–1 had a better mPFS than those with an ECOG score of 2 (hazard ratio, HR = 0.55, 95% CI: 0.18–1.67, p = 0.19) and patients with adenocarcinoma had a better mPFS than those with squamous cell carcinoma (HR = 1.88, 95% CI: 0.83–4.3, p = 1.88; Figs. 4 and 5). The mOS in patients with adenocarcinoma (n = 15) was not yet reached and 11.0 months for patients with squamous cell carcinoma (n = 24; HR = 3.98, 95% CI: 1.49–10.6, p = 0.0047; Figs. 6 and 7). Thus, individuals with adenocarcinoma had an increased mOS. Other clinical or pathologic features did not significantly affect mOS (Figs. 6 and 7).
Fig. 4.
Subgroup analysis of progression-free survival
Fig. 5.
Forest plot of hazard ratios (HR) of baseline predictors of PFS
Fig. 6.
Subgroup analysis of overall survival
Fig. 7.
Forest plot of hazard ratios (HR) of baseline predictors of OS
Safety
The overall incidence of AEs was 52.5%. Among these, the most common all-grade AEs were gastrointestinal reactions, which occurred in four patients (10.0%), followed by hand-foot syndrome (5.0%), hypertension (5.0%), hemoptysis (5.0%), thyroid dysfunction (5.0%), abnormal liver function (5.0%) and weakness (5.0%). Treatment-related grade 3/4 toxicity was observed in one patient (2.5%). No fatalities were related to AEs. Detailed AEs are listed in Table 2.
Table 2.
Adverse event of different anlotinib dose groups
| Adverse events (n = 21) | Any grade (n, %) | Grade 1 (n, %) | Grade 2 (n, %) | Grade 3 or 4 (n, %) |
|---|---|---|---|---|
| Hand-foot syndrome | 2 (5.00) | 0 | 2 (5.00) | 0 |
| Hypertension | 2 (5.00) | 1 (2.50) | 0 | 1 (2.50) |
| Hemoptysis | 2 (5.00) | 0 | 2 (5.00) | 0 |
| Thyroid dysfunction | 2 (5.00) | 0 | 2 (5.00) | 0 |
| Diarrhea | 1 (2.50) | 1 (2.50) | 0 | 0 |
| Immune hepatitis | 1 (2.50) | 1 (2.50) | 0 | 0 |
| Gastrointestinal reactions | 4 (10.00) | 2 (5.00) | 2 (5.00) | 0 |
| Abnormal liver function | 2 (5.00) | 2 (5.00) | 0 | 0 |
| Weakness | 2 (5.00) | 0 | 2 (5.00) | 0 |
| Oral mucosa syndrome | 1 (2.50) | 1 (2.50) | 0 | 0 |
| Hyperglycemia | 1 (2.50) | 1 (2.50) | 0 | 0 |
| Immune myocarditis | 1 (2.50) | 0 | 1 (2.50) | 0 |
Discussion
In this study, the ORR of low doses of anlotinib plus PD-1 blockade was 40.0%, the mPFS was 11.4 months, and the mOS was 27.0 months. Comparing these results to previous studies suggests that the efficacy of the combination treatment is better than either PD-1 blockade or anlotinib monotherapy for second-line and later treatment of NSCLC. For example, trials using PD-1 blockade monotherapy had ORR, mPFS, and mOS values of 20%, 3.5 months, and 9.2 months, respectively (CheckMate 017); 19.0%, 2.3 months, and 12.2 months, respectively (CheckMate 057); and 19.4%, 3.7 months, and 12.0 months, respectively (KEYNOTE-001) [16–18]. Similarly, in the clinical trial CheckMate 078, in which enrolled patients were mainly Chinese, the ORR, mPFS, and mOS were 17%, 2.8 months and 12.0 months, respectively [19, 20]. In each of these trials, all of the clinical outcomes were approximately 50% lower than in our study using combined therapy. The ALTER0303 study of anlotinib monotherapy in the treatment of NSCLC in second-line or later treatments reported an ORR of 9.2%, a mPFS of 5.4 and a mOS of 9.6 months [3]. Thus, the efficacy of PD-1 blockade or anlotinib monotherapies for the treatment of NSCLC in second-line or later appear limited relative to using low-dose anlotinib combined with PD-1 blockade.
We also compared our study with others that have reported on the efficacy of anlotinib in combination with PD-1 blockade in second-line or later treatments of NSCLC. In the multi-tumor study reported by Yuan et al. [21], a total of nine patients with lung cancer were included, and all received 12 mg of anlotinib plus PD-1 blockade. The ORR and mPFS were 11.1% and 4.3 months, respectively. Although the study did not report OS, in terms of ORR and PFS, the efficacy is relatively poor compared to that achieved in our study. In a real-world retrospective analysis reported by Zhang [6], 11 mutation-positive relapsed NSCLC patients treated with 12 mg of anlotinib plus PD-1 blockade had a PFS of 5.0 months. In contrast, Wang et al. [7] found the ORR, mPFS, and mOS for patients receiving anlotinib plus PD-1 blockade were 28.4%, 6.9 months, and 14.5 months, respectively. In Wang et al.’s study, the 67 patients enrolled received 8 (n = 7), 10 (n = 32), and 12 (n = 28) mg of anlotinib. While Wang et al. [7] did not conduct a subgroup analysis based on the dose of anlotinib, the majority of patients (58.2%) taking low-dose anlotinib (8 mg and 10 mg) may be related to the increased efficacy observed. Zhai et al. [22] found that the combination treatment of anlotinib and PD-1 blockade in 22 NSCLC patients had an ORR of 36.4%, mPFS of 6.8 months and a mOS of 17.3 months; here again, the majority of patients (16 people, 72.7%) used a lower dose (10 mg) of anlotinib in this study. In the study we conducted, all patients received 8 or 10 mg of anlotinib, and the ORR, mPFS and mOS observed were similar or better at 40.0%, 11.4 months and 27.0 month, respectively. Although the above studies are not directly comparable, the results among them suggest a trend that lower doses of anlotinib can improve the prognosis of patients more than higher dose anlotinib when applied in combination with PD-1 blockade.
A recent study explored the regulating effect of anlotinib on tumor blood vessels and its possible mechanism for increasing the anti-tumor efficacy of PD-1 blockade [23]. Anlotinib facilitates tumor vessel normalization at least partially through CD4+ T cells, reprograms immunosuppressive tumor microenvironments into immunostimulatory tumor microenvironments, significantly inhibits tumor growth and effectively prevents systemic immunosuppression. Moreover, the combination of anlotinib with PD-1 blockade counteracts the immunosuppression caused by the upregulation of PD-L1 after monotherapy, extends the period of vascular normalization, and finally induces tumor regression. However, the study [23] used a mouse model of neurofibroma, which differs from lung cancer in many aspects, including cell origin, biological characteristics, and sensitivity to small molecule targeted drugs and immunotherapy. Another study that used mouse models of lung cancer showed that anlotinib increased infiltration of the innate immune cells, including natural killer cells and antigen presenting cells, whereas the percentage of tumor associated macrophages was reduced. In combination with PD-1 blockade, anlotinib conferred significantly synergistic therapeutic benefits [24]. Clinically, the effect and mechanism of anlotinib on lung cancer blood vessels and PD-1 efficacy and its mechanism need further study.
The overall incidence of AEs using low-dose anlotinib combined with PD-1 blockade was 52.5%. The most common AE was gastrointestinal reactions, all of which were grade 1 or 2. Only one patient (2.5%) had a grade 3–4 AE. It is especially worth mentioning that the incidence of hypertension, one of the most common adverse reactions for anlotinib, was only 5.0% in the present study. These figures of AEs are significantly lower than for data where 12 mg anlotinib was combined with PD-1 in the treatment of lung cancer. In the study reported by Wang et al. [7], the overall incidence of AEs was 85.0%. Grade 3–4 treatment-related AEs occurred in 27 patients (40.0%), of which hypertension accounted for 18.0%. Zhai et al. [22] found the most common grade 1–2 AEs were hypertension (45.5%) and fatigue (45.5%); common grade 3–4 AEs were hypertension (9.1%) and rash (9.1%). Yuan et al. [21] reported that 46.2% of patients experienced treatment-related AEs; the most serious AE was grade 3 hand-foot syndrome and hypertension, which affected three patients (11.5%).
Our study has obvious limitations. First, it is a small retrospective analysis at a single center. Comparing the anti-tumor efficacy and adverse reactions observed in our study with other studies should thus be done with caution, keeping especially in mind the composition ratio of adenocarcinoma and squamous cell carcinoma in NSCLC, gene mutation status, liver and brain metastasis ratio, smoking status, number of treatment lines, and whether other treatments targeting VEGF were used. Second, in this study, our main criterion for screening cases is that patients had used the low-dose anlotinib combined with PD-1 blockade combination regimen for at least four cycles. We also did not take into account which drugs they used after the combination regimen failed, so the influence of other factors such as follow-up treatments on the prognosis of patients cannot be ruled out. Third, in this study, the PFS and OS of low-dose anlotinib combined with PD-1 blockade in the treatment of NSCLC were much better than the efficacy of high-dose anlotinib combined with PD-1 blockade reported in other studies. This still needs to be interpreted with caution, especially given the demographic differences in the included populations between studies. For example, in our study, most patients had an ECOG score of 0–1 (82.5%), and more than half of the patients were younger than 65 years (55.0%). It is not unreasonable to observe that a healthier population could be a contributor to the PFS and OS observed in this study. Fourth, the dose of anlotinib in this study includes both 8 and 10 mg and, because of sample size limitations, the two were not analyzed separately. To confirm results of this retrospective study, we are currently conducting two prospective clinical trials. The first is to simply observe the efficacy of anlotinib at a dose of 10 mg combined with PD-1 blockade in advanced solid tumors (ClinicalTrials.gov Identifier: NCT03975036). The second is to observe the efficacy of 8 mg, 10 mg and 12 mg anlotinib combined with PD-1 blockade in the treatment of advanced NSCLC (under ethics review).
In summary, most previous studies used high-dose anlotinib, but in the present study, we explored the efficacy and safety of low-dose anlotinib combined with ICI in the treatment of advanced NSCLC patients. The combination of low-dose anlotinib and PD-1 blockade exhibited efficacy, durability, and tolerability in patients with advanced NSCLC. Additional work is needed, and we propose that prospective trial designs with more accurate drug dosages are necessary to answer remaining questions related to ICIs plus anti-angiogenic approaches in NSCLC treatment.
Abbreviations
- AEs
Adverse events
- Cis
Confidence intervals
- CR
Complete response
- DCR
Disease control rate
- ECOG
Eastern cooperative oncology group
- FGFR
Fibroblast growth factor receptors
- HR
Hazard ratio
- ICIs
Immune checkpoint inhibitors
- mOS
Mean overall survival
- mPFS
Mean progression-free survival
- NR
Not reached
- NSCLC
Non-small cell lung cancer
- ORR
Overall response rate
- OS
Overall survival
- PD-1
Programmed cell death-1
- PD-L1
Programmed cell death-ligand 1
- PFS
Progression-free survival
- PR
Partial response
- PS
Performance status
- SD
Stable disease
- TKI
Tyrosine kinase inhibitor
- VEGFR
Vascular endothelial growth factor receptors
Author contributions
SY, LP, and YL performed experiments, analyzed data, and wrote the manuscript. BT, LZ, JZ, XY, HW, SZ analyzed data and edited the manuscript. ZW, QG, and HL designed and supervised the study.
Funding
This work was supported by the National Natural Science Foundation of China (Grant No. 81972690) and Medical Science and Technology Research Project of Health Commission of Henan Province (YXKC2021007, SBGJ202101008). The funding bodies played no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Data availability
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical approval
This is an observational study. The ethics committees of the Affiliated Cancer Hospital of Zhengzou University & Henan Cancer Hospital has confirmed that no ethical approval is required.
Consent to participate
Due to the retrospective nature of the study and because no patient specimens were used, the requirement for informed consent was waived by the ethics committees.
Consent for publication
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Shumin Yuan, Ling Peng and Yuqing Liu These authors contributed equally to this work.
Contributor Information
Hongle Li, Email: llhl73@163.com.
Quanli Gao, Email: zlyygql0855@zzu.edu.cn.
Zibing Wang, Email: zlyywzb2118@zzu.edu.cn.
References
- 1.Remon J, Passiglia F, Ahn M, Barlesi F, Forde P, Garon E, Gettinger S, Goldberg S, Herbst R, Horn L, Kubota K, Lu S, Mezquita L, Paz-Ares L, Popat S, Schalper K, Skoulidis F, Reck M, Adjei A, Scagliotti G. Immune checkpoint inhibitors in thoracic malignancies: review of the existing evidence by an IASLC expert panel and recommendations. J Thorac Oncol. 2020;15(6):914–947. doi: 10.1016/j.jtho.2020.03. [DOI] [PubMed] [Google Scholar]
- 2.Xie C, Wan X, Quan H, Zheng M, Fu L, Li Y, Lou L. Preclinical characterization of anlotinib, a highly potent and selective vascular endothelial growth factor receptor-2 inhibitor. Cancer Sci. 2018;109(4):1207–1219. doi: 10.1111/cas.13536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Han B, Li K, Wang Q, Zhang L, Shi J, Wang Z, Cheng Y, He J, Shi Y, Zhao Y, Yu H, Zhao Y, Chen W, Luo Y, Wu L, Wang X, Pirker R, Nan K, Jin F, Dong J, Li B, Sun Y. Effect of anlotinib as a third-line or further treatment on overall survival of patients with advanced non-small cell lung cancer: the ALTER 0303 phase 3 randomized clinical trial. JAMA Oncol. 2018;4(11):1569–1575. doi: 10.1001/jamaoncol.2018.3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tine B, Chawla S, Trent J, Wilky B, Chugh R, Chmielowski B, Kummar S, Mallick A, Somaiah N, Cranmer L, Agulnik M, Keedy V, Stacchiotti S, Vincenzi B, Badalamenti G, Siontis B, Attia S. A phase III study (APROMISS) of AL3818 (Catequentinib, Anlotinib) hydrochloride monotherapy in subjects with metastatic or advanced synovial sarcoma. J Clin Oncol. 2021;39:11505. doi: 10.1200/JCO.2021.39.15_suppl.11505. [DOI] [Google Scholar]
- 5.Chu T, Zhong R, Zhong H, Zhang B, Zhang W, Shi C, Qian J, Zhang Y, Chang Q, Zhang X, Dong Y, Teng J, Gao Z, Qiang H, Nie W, Zhao Y, Han Y, Chen Y, Han B. Phase 1b study of sintilimab plus anlotinib as first-line therapy in patients with advanced NSCLC. J Thorac Oncol. 2021;16(4):643–652. doi: 10.1016/j.jtho.2020.11.026. [DOI] [PubMed] [Google Scholar]
- 6.Zhang X, Zeng L, Li Y, Xu Q, Yang H, Lizaso A, Mao X, Jin R, Zeng Y, Li Q, Wang J, Li Y, Zhang Y, Yang N. Anlotinib combined with PD-1 blockade for the treatment of lung cancer: a real-world retrospective study in China. Cancer Immunol Immunother. 2021;70(9):2517–2528. doi: 10.1007/s00262-021-02869-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang P, Fang X, Yin T, Tian H, Yu J, Teng F. Efficacy and safety of anti-PD-1 plus anlotinib in patients with advanced non-small-cell lung cancer after previous systemic treatment failure-a retrospective study. Front Oncol. 2021;11:628124. doi: 10.3389/fonc.2021.628124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fukumura D, Kloepper J, Amoozgar Z, Duda D, Jain R. Enhancing cancer immunotherapy using antiangiogenics: opportunities and challenges. Nat Rev Clin Oncol. 2018;15(5):325–340. doi: 10.1038/nrclinonc.2018.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang Y, Yuan J, Righi E, Kamoun W, Ancukiewicz M, Nezivar J, Santosuosso M, Martin J, Martin M, Vianello F, Leblanc P, Munn L, Huang P, Duda D, Fukumura D, Jain R, Poznansky M. Vascular normalizing doses of antiangiogenic treatment reprogram the immunosuppressive tumor microenvironment and enhance immunotherapy. Proc Natl Acad Sci U S A. 2012;109(43):17561–17566. doi: 10.1073/pnas.1215397109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Veldt A, Lubberink M, Bahce I, Walraven M, Boer M, Greuter H, Hendrikse N, Eriksson J, Windhorst A, Postmus P, Verheul H, Serné E, Lammertsma A, Smit E. Rapid decrease in delivery of chemotherapy to tumors after anti-VEGF therapy: implications for scheduling of anti-angiogenic drugs. Cancer Cell. 2012;21(1):82–91. doi: 10.1016/j.ccr.2011.11.023. [DOI] [PubMed] [Google Scholar]
- 11.Huang Y, Goel S, Duda D, Fukumura D, Jain R. Vascular normalization as an emerging strategy to enhance cancer immunotherapy. Cancer Res. 2013;73(10):2943–2948. doi: 10.1158/0008-5472.CAN-12-4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao S, Ren S, Jiang T, Zhu B, Li X, Zhao C, Jia Y, Shi J, Zhang L, Liu X, Qiao M, Chen X, Su C, Yu H, Zhou C, Zhang J, Camidge D, Hirsch F. Low-dose apatinib optimizes tumor microenvironment and potentiates antitumor effect of PD-1/PD-L1 blockade in lung cancer. Cancer Immunol Res. 2019;7(4):630–643. doi: 10.1158/2326-6066.CIR-17-0640. [DOI] [PubMed] [Google Scholar]
- 13.Xiong Q, Qin B, Xin L, Yang B, Song Q, Wang Y, Zhang S, Hu Y. Real-world efficacy and safety of anlotinib with and without immunotherapy in advanced non-small cell lung cancer. Front Oncol. 2021;11:659380. doi: 10.3389/fonc.2021.659380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun Y, Niu W, Du F, Du C, Li S, Wang J, Li L, Wang F, Hao Y, Li C, Chi Y. Safety, pharmacokinetics, and antitumor properties of anlotinib, an oral multi-target tyrosine kinase inhibitor, in patients with advanced refractory solid tumors. J Hematol Oncol. 2016;9(1):105. doi: 10.1186/s13045-016-0332-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Detterbeck F, Boffa D, Kim A, Tanoue L. The eighth edition lung cancer stage classification. Chest. 2017;151(1):193–203. doi: 10.1016/j.chest.2016.10.010. [DOI] [PubMed] [Google Scholar]
- 16.Brahmer J, Reckamp K, Baas P, Crinò L, Eberhardt W, Poddubskaya E, Antonia S, Pluzanski A, Vokes E, Holgado E, Waterhouse D, Ready N, Gainor J, Frontera O, Havel L, Steins M, Garassino M, Aerts J, Domine M, Paz-Ares L, Reck M, Baudelet C, Harbison C, Lestini B, Spigel D. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373(2):123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Borghaei H, Paz-Ares L, Horn L, Spigel D, Steins M, Ready N, Chow L, Vokes E, Felip E, Holgado E, Barlesi F, Kohlhäufl M, Arrieta O, Burgio M, Fayette J, Lena H, Poddubskaya E, Gerber D, Gettinger S, Rudin C, Rizvi N, Crinò L, Blumenschein G, Antonia S, Dorange C, Harbison C, Finckenstein F, Brahmer J. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373(17):1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aggarwal C, Gubens M, Horn L, Carcereny E, Ahn M, Felip E, Lee J, Hellmann M, Hamid O, Goldman J, Soria J, Dolled-Filhart M, Rutledge R, Zhang J, Lunceford J, Rangwala R, Lubiniecki G, Roach C, Emancipator K, Gandhi L. KEYNOTE-001 Investigators Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372(21):2018–2028. doi: 10.1056/NEJMoa1501824. [DOI] [PubMed] [Google Scholar]
- 19.Wu Y, Lu S, Cheng Y, Zhou C, Wang J, Mok T, Zhang L, Tu H, Wu L, Feng J, Zhang J, Luft A, Zhou J, Ma Z, Lu Y, Hu C, Shi Y, Baudelet C, Cai J, Chang J. Nivolumab versus docetaxel in a predominantly Chinese patient population with previously treated advanced NSCLC: checkMate 078 randomized phase III clinical trial. J Thorac Oncol. 2019;14(5):867–875. doi: 10.1016/j.jtho.2019.01.006. [DOI] [PubMed] [Google Scholar]
- 20.Lu S, Wang J, Cheng Y, Mok T, Chang J, Zhang L, Feng J, Tu H, Wu L, Zhang Y, Luft A, Zhou J, Ma Z, Lu Y, Hu C, Shi Y, Ying K, Zhong H, Poddubskaya E, Soo R, Chia Y, Li A, Li A, Wu Y. Nivolumab versus docetaxel in a predominantly Chinese patient population with previously treated advanced non-small cell lung cancer: 2-year follow-up from a randomized, open-label, phase 3 study (CheckMate 078) Lung Cancer. 2021;152:7–14. doi: 10.1016/j.lungcan.2020.11.013. [DOI] [PubMed] [Google Scholar]
- 21.Yuan M, Zhu Z, Mao W, Wang H, Qian H, Wu J, Guo X, Xu Q. Anlotinib combined with anti-PD-1 antibodies therapy in patients with advanced refractory solid tumors: a single-center, observational, prospective study. Front Oncol. 2021;11:683502. doi: 10.3389/fonc.2021.683502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhai C, Zhang X, Ren L, You L, Pan Q, Pan H, Han W. The Efficacy and safety of anlotinib combined with PD-1 antibody for third-line or further-line treatment of patients with advanced non-small-cell lung cancer. Front Oncol. 2021;10:619010. doi: 10.3389/fonc.2020.619010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Su Y, Luo B, Lu Y, Wang D, Yan J, Zheng J, Xiao J, Wang Y, Xue Z, Yin J, Chen P, Li L, Zhao Q. Anlotinib induces a T cell-inflamed tumor microenvironment by facilitating vessel normalization and enhances the efficacy of PD1 checkpoint blockade in neuroblastoma. Clin Cancer Res. 2022;28(4):793–809. doi: 10.1158/1078-0432.CCR-21-2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang Y, Li L, Jiang Z, Wang B, Pan Z. Anlotinib optimizes anti-tumor innate immunity to potentiate the therapeutic effect of PD-1 blockade in lung cancer. Cancer Immunol Immunother. 2020;69(12):2523–2532. doi: 10.1007/s00262-020-02641-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.








