Abstract
Purpose
This study was designed to investigate the correlation between immune-related adverse events (irAEs) of immune checkpoint inhibitors (ICIs) and corresponding efficacy, and to explore the potential of predicting the efficacy of ICIs via irAEs.
Methods
Electronic databases including PubMed, Embase, Cochrane Library, CNKI and Wanfang were applied to search for relevant studies. The primary endpoint was overall survival (OS) or progression-free survival (PFS), and the secondary endpoint was objective response rate (ORR). Stratification analyses were conducted according to the type of irAEs and ICIs, region of studies and primary tumors. Furthermore, statistical analyses were realized by means of RevMan 5.3 software.
Results
Altogether, 40 studies with 8,641 participants were enrolled, among which the incidence of irAEs ranged from 15.34 to 85.23% and the major sites reached out to skin, endocrine organ, gastrointestinal tract, liver and lung. The ORR, OS and PFS in irAE group were significantly higher than those in non-irAE group as per pooled analyses and stratification analyses. Importantly, patients with irAEs in skin, endocrine organ or gastrointestinal tract rather than in liver and lung were found to obtain survival benefits (p < 0.05).
Conclusion
IrAEs, especially in skin, endocrine organ or gastrointestinal tract, triggered by ICIs indicate significant survival benefits.
Keywords: Immune-related adverse events (irAEs), Immune checkpoint inhibitors (ICIs), Efficacy
Introduction
Immune checkpoint inhibitors (ICIs) have initiated a major revolution with epoch-making significance in the history of tumor therapy. Currently, ICIs have played a key role in treating advanced malignancies, such as melanoma, non-small cell lung cancer (NSCLC), renal cell carcinoma (RCC), urothelial cancer (UC), Hodgkin lymphoma (HL) and so on [1–10]. However, the efficacy of ICIs remains to be fully exerted, bearing overall effective rate of 10%–30% only [11]. Consequently, it is critical to identify dominant population and prognostic indicators of ICIs.
At present, some recommended predictive indexes for efficacy include PD-L1 expression, tumor mutation burden (TMB), and microsatellite instability-high (MSI-H) [12, 13]. However, these indexes are incompetent to fully identify all candidate population for ICIs.
Notably, mounting evidence demonstrated that irAEs triggered by ICIs were compelling enough to predict the efficacy. ICIs activate the immune system, up-regulate the immune response, and trigger a storm of inflammatory cytokines that attack normal organs, thus resulting in a variety of toxic and side effects, which are generally termed as immune-related adverse events (irAEs) [14]. Some studies on melanoma revealed a positive correlation between irAEs and efficacy of ICIs [15–17], but others on NSCLC indicated that pneumonia mediated by ICIs predicted a poor prognosis [18]. Up to date, the correlation between irAEs and efficacy of ICIs remains to be fully elucidated. Accordingly, a comprehensive analysis on 40 studies incorporating 8,641 cases was carried out to explore whether irAEs could served as a predictor of ICI efficacy and to determine candidate population for ICIs.
Materials and methods
Literature search
This study was conducted conforming to Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) Guidelines [19] and Meta-Analysis of Observational Studies in Epidemiology (MOOSE) [20]. As of 31 March 2020, electronic databases such as PubMed, Embase, Cochrane Library, CNKI and Wanfang were applied in search of relevant trials without language restriction by entering the following keywords: immune-related adverse events, immune checkpoint inhibitors, immune efficacy, efficacy of cancer immunotherapy, efficacy of immune checkpoint inhibitors, nivolumab, pembrolizumab, atezolizumab and ipilimumab. General reviews on this topic were scrutinized and excluded. In addition, references of the included studies were manually reviewed to screen additional articles. Meanwhile, letters, comments, expert opinions, reviews without original data, and case reports were excluded.
Selection of studies
Initially, two researchers independently performed a rapid screening of titles and abstracts, and then proceeded with the full-text searches to hunt for relevant studies.
Inclusion criteria
The following criteria are essential for eligible trials: (1) malignant tumor had been administrated with ICIs; (2) the analyses included overall survival (OS) or progression-free survival (PFS) of irAE group and non-irAE group; (3) response rate was determined by the Response Evaluation Criteria in Solid Tumors (RECIST 1.1 Standards); (4) adverse events were assessed in accordance with the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) (version 3.0/4.0/5.0) and the diagnosis and severity of irAEs were determined based on clinical examinations, biological and imaging data; (5) the included study was prospective or retrospective; (6) hazard ratio (HR) with 95% confidence interval (CI) of survival data was available.
Data extraction
Two researchers extracted data from each trial independently and disagreements were addressed by consensus. The following information was abstracted from each of the included studies: first author name, year of publication, region of original trial, type of trial, ICI type, number of patients, gender, median age, Eastern Cooperative Oncology Group (ECOG), previous therapy, irAE type, interventions and outcomes. PFS and OS were defined as the primary endpoint to assess the efficacy of irAE group and non-irAE group through HR. Objective response rate (ORR) was defined as the second endpoint. If provided indirectly, HR with 95% CI was calculated from survival curves by means of Tierney’s methods [21].
Quality assessment
Two researchers independently assessed quality items and possible difference thereof. Those studies (directly or indirectly) concluded the HR for PFS or OS after the treatment of ICIs were deemed to be qualified studies.
Statistical methods
Statistical analyses were performed via the RevMan 5.3 software. Chi-square and I-square tests were adopted to verify the heterogeneity of involved trials. If P > 0.1 and I2 < 50%, the studies were defined as low heterogeneity and fixed effect model was applied, otherwise defined as high heterogeneity and random effect model was adopted accordingly. We also conducted stratification analyses in accordance with the type of irAEs and ICIs, region of studies, and primary tumors.
Subsequently, data analysis generally comprised of pooled risk ratio (RR) for dichotomous endpoints (ORR) resorting to Mantel–Haenszel method [22]. OS and PFS were calculated using effect variables and expressed as the HR. The 95% CIs were calculated and presented in forest plots. Besides, publication bias was evaluated via funnel plots.
Results
Study selection
First, we collected 1,503 relevant studies from the aforementioned databases. Second, 532 duplicates were excluded. Then 891 unrelated ones were further excluded after the title and abstract review. Furthermore, 40 studies failing to meet the inclusion criteria were excluded. Finally, 40 remaining studies [14, 16, 17, 23–59] were included in this meta-analysis. The retrieval process was portrayed by a flowchart (Fig. 1).
Fig. 1.
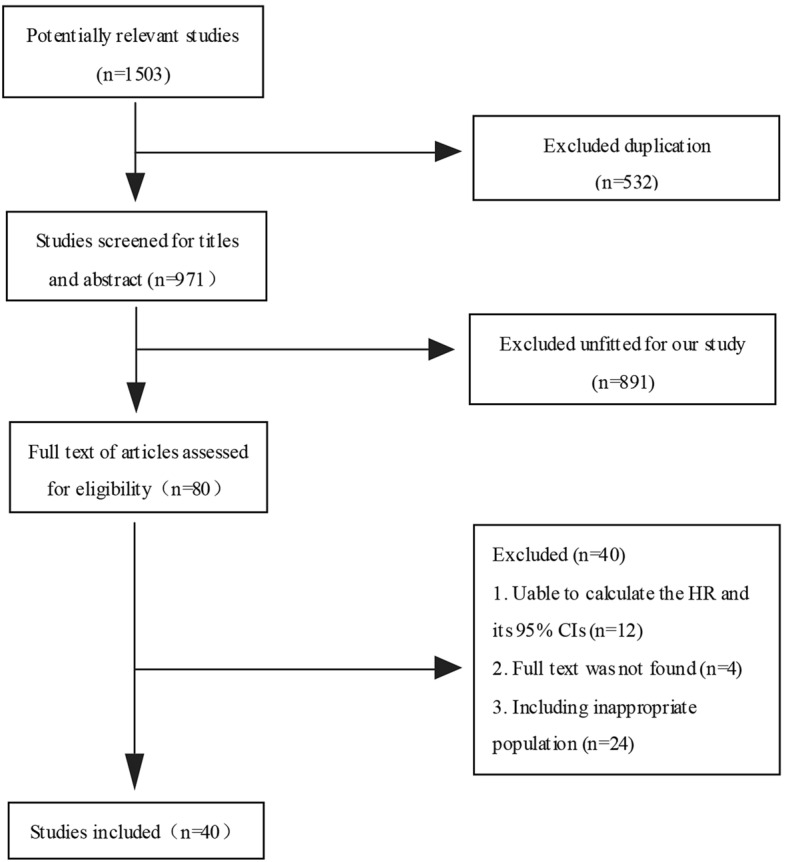
Flow diagram of included studies
Study characteristics
General characteristics of these enrolled studies are presented in Table 1, among which 9 were prospective and 31 retrospective. Fifteen trials were conducted in Asia, 12 in Europe, and 11 in America. Clinical interventions adopted were as follows: anti-PD-1 therapy was used in 29 studies, anti-CTLA-4 in 3, and anti-PD-L1 in 1. In summary, 8,641 individuals were included, and 3,018 complicated with irAEs.
Table 1.
Baseline of patients characteristics
| Study | Nation | Tumors | ICI type | Patients (male/female) | Median age (years) | ECOG 0–1/ ≥ 2 | Previous therapy 0/ ≥ 1 | IrAE type (n) | irAE (n) | Non-irAE (n) |
|---|---|---|---|---|---|---|---|---|---|---|
| Ascierto 2014 [23] | Italy | Melanoma | Ipilimumab | 855 (460/395) | 61 (16–88) | 830/25 | 0/855 | Pruritus (58), rash (64), diarrhea (60), nausea (47), vomiting (15), abdominal pain (11), constipation (7), endocrine (7), liver toxicity (19), fatigue/asthenia (70) | 286 | 569 |
| Cortellini 2019 [24] | Italy | NSCLC |
Pembrolizumab or nivolumab |
559 (379/180) | 69 (24–88) | 485/74 | 116/443 | Endocrine (78), gastrointestinal (51), skin (59), pneumological (23), hepatic (10), others (46) | 231 | 328 |
| Elias 2019 [25] | USA | RCC | ICIs | 90 | NA | NA | NA | Fatigue (25), nausea (9), decreased appetite (8) | 38 | 52 |
| Freeman-Keller 2015 [14] | USA | Melanoma | Nivolumab | 148 (87/61) | NA | NA | NA | Rash (67), diarrhea/colitis/enteritis (48), vitiligo (19), hypothyroidism (16), elevated amylase/lipase (7), mucositis (9), pneumonitis (3), hyperthyroidism (2), hypophysitis (1), elevated ALT/AST (1) | 101 | 47 |
| Grangeon 2018 [26] | France | NSCLC | Anti-PD-L1 or anti-PD-1 | 270 (177/93) | 61 (32–84) | 233/17 | 16/254 | Thyroiditis (53), rashes (19), colitis (11), hepatitis (8), endocrinopathy (8), pneumonitis (6), nephritis (1), pruritus (9), arthralgia (5), mof (1), myocarditis (1), myocardial ischemia (1), psoriasis (1), irritability (1) | 124 | 146 |
| Horvat 2015 [28] | USA | Melanoma | Ipilimumab | 298 (182/116) | 65 (21–93) | 291/7 | 193/105 | Hepatotoxicity (197), dermatitis (123), diarrhea (87), hypophysitis (17), uveitis (8), other (15) | 254 | 44 |
| Study | Nation | Tumors | ICI type | Patients (male/female) | Median age (years) | ECOG 0–1/ ≥ 2 | Previous therapy 0/ ≥ 1 | IrAE type (n) | irAE (n) | Non-irAE(n) |
| Haratani 2017 [27] | Japan | NSCLC | Nivolumab | 134 (90/44) | 68 (33–85) | 116/12 | 8/126 | Rash (33), pruritus (16), vitiligo (2), pneumonitis (6), thyroiditis/hypothyroidism (10), hypophysitis (1), mucositis (3), diarrhea/colitis (10), hepatitis (5), cholangitis (2), fatigue (7), appetite loss (4), polyarthritis (1), myasthenia gravis (1) | 69 | 65 |
| Indini 2018 [29] | Germany | Melanoma | Pembrolizumab or nivolumab | 173 (107/66) | 62 (18–85) | 166/6 | 74/99 | Rash (12), pruritus (11), lichen (1), vitiligo (8), fatigue (34), fever (4), infusion-related reaction (2), decreased appetite (6), dysgeusia (2), dry mouth (2), oral mucositis (2), nausea (13), vomiting (1), gastritis (2), colitis (1), diarrhea (10), ast, alt increase (19), γgt increase (1), amylase increased (2), lipase increased (3), hyperglycemia (1), serum creatinine increase (2), acute renal failure (1), cough (5), interstitial pneumonitis (2), arthralgias (12), hypothyroidism (14), hypophysitis (2), anemia (6), thrombocytopenia (4), myasthenia with myositis (1), headache (2), paresthesia (2), peripheric sensory neuropathy (8), peripheric motor neuropathy (1), uveitis (1), conjunctivitis (1), photophobia (2) | 102 | 71 |
| Study | Nation | Tumors | ICI type | Patients (male/female) | Median age (yeaars) | ECOG0–1/ ≥ 2 | Previous therapy/ ≥ 1 | IrAE type (n) |
irAE (n) |
Non-irAE(n) |
| Kawai 2019 [30] | Japan | UC | Pembrolizumab | 30 (25/5) | 70 (26–85) | NA | 0/30 | NA | 18 | 12 |
| Masuda 2019 [31] | Japan | Gastric cancer | Nivolumab | 65 (51/14) | 66 (35–83) | 59/6 | NA | Diarrhea/colitis (5), hyperglycemia (2), pruritus (2), rash (2), type 1 dm (2), adrenal insufficiency (1), alt increased (1), ast increased (1), appetite loss (1), appetite loss (1), dry skin (1), edema limbs (1), myalgia (1), peripheral motor neuropathy (1), pneumonitis (1), QTc interval prolonged (1) | 14 | 51 |
| Okada 2018 [32] | Japan | Melanoma | Nivolumab | 15 (4/11) | NA | NA | NA | Rash (6), hypothyroidism (4), diarrhea (2), liver dysfunction (1) | 8 | 7 |
| Okamoto 2019 [33] | Japan | HNC | Nivolumab | 100 (79/21) | 65(23–81) | 95/5 | 0/100 | Dermatitis (3), interstitial lung disease (11), hypothyroidism (8), hyperthyroidism (1), adrenal insufficiency (1), liver dysfunction (4), myositis (1), rheumatoid arthritis (1), eye disorders (1), upper gastrointestinal hemorrhage (1), diarrhea (1), weight loss (1), infusion reaction (1), anemia (1), increased creatinine (1) | 30 | 70 |
| Sato 2018 [36] | Japan | NSCLC | Nivolumab | 38 (28/10) | 68.5 (49–86) | 33/5 | NA | TSH elevation (1), hypothyroidism (3), pneumonitis (5), hyperthyroidism (1), rash (1), liver dysfunction (1), hypopituitarism (1) | 11 | 27 |
| Study | Nation | Tumors | ICI type | Patients (male/female) | Median age (years) | ECOG 0–1/ ≥ 2 | Previous therapy 0/ ≥ 1 | IrAE type (n) | irAE (n) | Non-irAE(n) |
| Ricciuti 2018 [34] | Italy | NSCLC | Nivolumab | 195 (128/67) | 63(30–84) | 160/35 | 0/195 | Rash (18), psoriasis (3), pruritus (2), dry skin (2), skin desquamation (1), paronychia (1), pneumonitis (16), hyper/hypothyroidism (39), hyperprolactinemia (16), ACTH elevation (4), colitis (21), amylase increase (15), lipase increase (8), nausea/vomiting (8), constipation (1), abdominal pain (2), xerostomia (1), γ-GT (18), ALT (16), AST (16), alkaline phosphatase (16), conjunctivitis (2), uveitis (1), fatigue (38), arthritis (7), polymyalgia rheumatica (1), dermatomyositis (1), anorexia (3), anemia (1), thrombocytopenia (3), Neutropenia (1) | 85 | 110 |
| Rogado 2019 [35] | Spain | Lung cancer melanoma, HL HCC, HNC,UC RC,MCC,GBAC | Nivolumab or pembrolizumab | 106 (76/30) | 69(32–86) | 73/33 | 21/85 | Hypothyroidism (22), hyperthyroidism (3), nephritis (7), rash (3), pneumonitis (5), colitis (1), hepatitis (3), arthritis (3), hypophysitis (1), panhypopituitarism (2), suprarenal insufficiency (2), hyperthyroidism (2), ketoacidotic diabetes (1), encephalitis (1), myositis (1) | 40 | 66 |
| Shafqat 2018 [37] | USA | NSCLC, RCC, melanoma, UC HNC, Other | Nivolumab or pembrolizumab or atezolizumab | 157 (100/57) | 65 | NA | NA | Colitis (4), pneumonitis (5), hepatitis (1), endocrinopathy (21), skin toxicity (4), other (1), arthralgia/arthritis (9) | 42 | 114 |
| Study | Nation | Tumors | ICI type | Patients (male/female) | Median age(y) | ECOG 0–1/ ≥ 2 | Previous therapy 0/ ≥ 1 | IrAE type (n) | irAE (n) | Non-irAE (n) |
| Teraoka 2017 [38] | Japan | NSCLC | Nivolumab | 43 (27/16) | 70 (50–82) | 39/4 | 0/43 | Rash (12), pyrexia (6), diarrhea (4), elevated hepatic enzyme levels (1) | 19 | 24 |
| Toi 2018 [39] | Japan | NSCLC | Nivolumab or pembrolizumab | 137 (105/32) | 68 (36–88) | 134/3 | 18/119 | Skin reaction (42), pneumonitis (14), hypothyroidism (6), hyperthyroidism (1), hepatitis (6), myositis or peripheral neuropathy (5) | 66 | 71 |
| Verzoni 2019 [40] | Italy | RCC | Nivolumab | 389 (291/98) | 65 (34–85) | 350/24 | 2/387 | Cutaneous (30), endocrine (17), hepatic (7), gastro-intestinal (19), pulmonary (4) | 76 | 313 |
| VonPawel 2017 [41] | Global | NSCLC | Atezolizumab | 850 | NA | NA | NA | NA | 264 | 586 |
| Bjornhart 2019 [42] | Denmark | NSCLC | Nivolumab or pembrolizumab | 118 (55/63) | 66 (59–71) | 106/12 | 46/72 | Pneumonitis, colitis, hypophysitis, diarrhea, arthritis, uveitis, myositis, primary AI, thyroiditis, hepatitis, allergic reaction | 32 | 86 |
| Otsuka 2020 [43] | Japan | Melanoma | Nivolumab | 27 (9/18) | 69 (31–87) | NA | 23/4 | Dermatological (11), gastrointestinal (2), endocrine (4), pulmonary (4), renal (1), infusion-related reaction (1) | 16 | 12 |
| Dick 2016 [44] | Germany | Melanoma | Ipilimumab | 86 (48/38) | 59 (14–83) | NA | 22/64 | Predominantly diarrhea, autoimmune colitis, skin toxicity, infrequently hypophysitis, autoimmune hepatitis, autoimmune pancreatitis, alopecia areata | 36 | 50 |
| Study | Nation | Tumors | ICI type | Patients (male/female) | Median age(y) | ECOG 0–1/ ≥ 2 | Previous therapy 0/ ≥ 1 | IrAE type (n) | irAE (n) | Non-irAE (n) |
| Judd 2017 [45] | USA | RCC, HNSCC, UC, NSCLC, other | Nivolumab or pembrolizumab | 160 (101/59) | 65 | NA | NA | Endocrinopathy, colitis, dermatitis | 64 | 96 |
| Kim 2018 [46] | South Korea | NSCLC | Nivolumab or pembrolizumab | 58 (43/15) | 63.1 (49–68) | NA | NA | Thyroid dysfunction (19) | 19 | 39 |
| Ksienski 2018 [47] | Canada | NSCLC | Nivolumab or pembrolizumab | 271 (137/134) | NA | 187/54 | 21/250 | Hypothyroid (32), dermatitis (35), colitis (18), hyperthyroid (10), hepatitis (12), arthralgias (13), pneumonitis (17), nephritis (8), adrenal insufficiency (3), diabetes (3), hypophysitis (2), pancreatitis (1), neurologic (3), cholangitis (2), myopathy (2), myositis (1), mucositis (1), palmar plantar erythrodysesthesia (1), polymyalgia rheumatica (1), vasculitis (1), idiopathic thrombocytopenic purpra (1), myocarditis (1) | 116 | 155 |
| Lisberg 2018 [49] | USA | NSCLC | Pembrolizumab | 97 (50/47) | 65 (32–83) | NA | 13/84 | Rash (22), fatigue (9), hypothyroidism (6), fever/chills (5), anorexia (4), pneumonitis (6), pruritus (3), abdominal pain (2), worsening dyspnea (2), joint pain (2), edema (2) | 39 | 58 |
| Sugano 2020 [56] | Japan | NSCLC | Nivolumab or atezolizumab or pembrolizumab | 130 (98/32) | NA | 99/31 | NA | Interstitial lung disease (16), hypothyroidism (9), skin reaction (5), nephrotoxicity (3), encephalitis (2) | 39 | 91 |
| Study | Nation | Tumors | ICI type | Patients (male/female) | Median age (years) | ECOG 0–1/ ≥ 2 | Previous therapy 0/ ≥ 1 | IrAE type (n) | irAE (n) | Non-irAE (n) |
| Maher 2019 [50] | Global | UC | Nivolumab or atezolizumab or durvalumab or avelumab or pembrolizumab | 1747 (1328/419) | 68 (29–94) | 1572/175 | NA | NA | 268 | 1479 |
| Hua 2016 [16] | France | Melanoma | Pembrolizumab | 67 (38/29) | 57 (28–72) | NA | NA | Vitiligo (17) | 17 | 50 |
| Nakamura 2017 [17] | Japan | Melanoma | Nivolumab | 35 (18/17) | NA | NA | NA | Vitiligo (9) | 9 | 26 |
| Nakamura 2016 [52] | Japan | Melanoma | Nivolumab | 98 (52/46) | 66.5 (17–93) | 92/6 | 28/70 | Vitiligo (13), hypothyroidism (11), pruritus (10), rash (7), malaise (5) | 51 | 57 |
| Lesueur 2018 [48] | France | NSCLC | Nivolumab | 104 (67/37) | 60.3 (54.5–67.1) | 69/35 | NA | Pulmonary (4), gastrointestinal (21), dermatological (13), endocrinological (10), rheumatological (6), asthenia (19), hematological (1), others (16) | 62 | 42 |
| Minlee 2018 [51] | USA | Lung cancer, HL cutaneous cancer HNC, GC, UC reproductive cancer | Nivolumab or atezolizumab or pembrolizumab | 114 (62/52) | NA | NA | NA | Dermatitis (20) | 20 | 94 |
| Osorio 2016 [53] | USA | NSCLC | Pembrolizumab | 51 (21/30) | NA | NA | NA | Immune-related thyroid dysfunction (10) | 10 | 41 |
| Study | Nation | Tumors | ICI type | Patients (male/female) | Median age (years) | ECOG 0–1/ ≥ 2 | Previous therapy 0/ ≥ 1 | IrAE type (n) | irAE (n) | Non-irAE (n) |
| Owen 2018 [54] | USA | NSCLC | Nivolumab or pembrolizumab or atezolizumab | 91 (39/52) | 67/22 | NA | NA | Pneumonitis (9), dermatologic (6), endocrine (7), colitis (3), hepatitis (1), pancreatic insufficiency (1) | 27 | 64 |
| Yamazaki 2017 [59] | Japan | Melanoma | Nivolumab | 24 (14/10) | 63 (26–81) | 24/0 | 16/8 | Endocrine disorders (7), gastrointestinal toxicity (2), hepatotoxicity (1), pulmonary toxicity (1), skin toxicity (11) | 13 | 10 |
|
Sanlorenzo 2015 [55] |
USA | Melanoma, lung cancer, MCC, prostate cancer | Pembrolizumab | 83 (52/31) | NA | NA | NA | Macular papular eruption (24), pruritus (10), hypopigmentation (7), xerosis (2), keratosis (2), facial erythema (1) | 35 | 48 |
| Suh 2018 [57] | South Korea | NSCLC | Nivolumab/pembrolizumab | 54 (42/12) | NA | 54/0 | 0/54 | 12 | 42 | |
| Weber 2017 [58] | USA | Melanoma | Nivolumab | 576 (349/227) | 61 | 571/0 | NA | Pruritus (99), rash (73), vitiligo (45), rash maculopapular (26), diarrhea (73), colitis (6), hypothyroidism (24), hyperthyroidism (12), hypophysitis (1), AST increased (16), ALT increased (11), γ-glutamyltransferase increased (1), hepatitis (1), liver function test abnormal (1), pneumonitis (10), blood creatinine increased (3), renal failure acute (1), tubulointerstitial nephritis (1) | 255 | 321 |
NSCLC non-small cell lung cancer, UC urothelial cancer, RCC renal cell carcinoma, HL Hodgkin lymphoma, HNC head and neck carcinoma, HCC hepatocellular carcinoma, GC gastrointestinal cancer, GBAC gallbladder adenocarcinoma, MCC Merkel cell carcinoma, irAE immune-related adverse events, NA not available
Data analysis
Incidence of irAEs
The incidence of irAEs in eligible studies ranged from 15.34% to 85.23%, which frequently occurs in the skin (2.56%–56.08%), endocrine organ (0.82%–30.43%), gastrointestinal tract (0%–33.78%), liver (0.64%–16.41%) and lung (0%–4.81%). Furthermore, the correlation between the incidence of irAEs and patients treated with ICIs was as follows: anti-CTLA-4 (46.49%), anti-PD-1 (41.71%), and anti-PD-L1 (31.06%).
Response rate
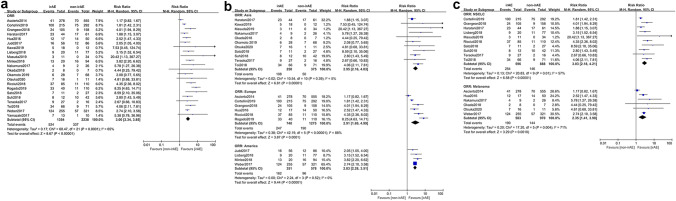
A total of 22 studies [16, 17, 23, 24, 26, 27, 30–36, 38, 39, 43, 45, 49, 51, 57–59] concluded response rates of irAE group and non-irAE group. Due to the high heterogeneity (p < 0.0001, I2 = 65%), a random effect model was utilized for the meta-analysis. With regard to ORR, a pooled analysis on outcomes displayed a significant difference between irAE group and non-irAE group (RR = 3.00, 95% CI [2.34–3.85], p < 0.00001) (Fig. 2a). In stratification analysis, patients with irAEs had higher ORR than those without irAEs in Asia (RR = 2.95, 95% CI [2.16–4.03], p < 0.00001), Europe (RR = 2.91, 95% CI [1.69–4.99], p = 0.0001) and America (RR = 2.83, 95% CI [2.28–3.51], p < 0.00001) (Fig. 2b). Analogously, the irAE group still had higher ORR in NSCLC (RR = 3.03, 95% CI [2.18–4.21], p < 0.00001) and melanoma (RR = 2.35, 95% CI [1.41–3.90], p = 0.0010) (Fig. 2c). Collectively, individuals with irAEs had higher ORR than those without irAEs.
Fig. 2.
Analyses of irAEs for ORR. a ORR of the study; b stratification analysis of trial regions; c stratification analysis of tumor
Overall survival
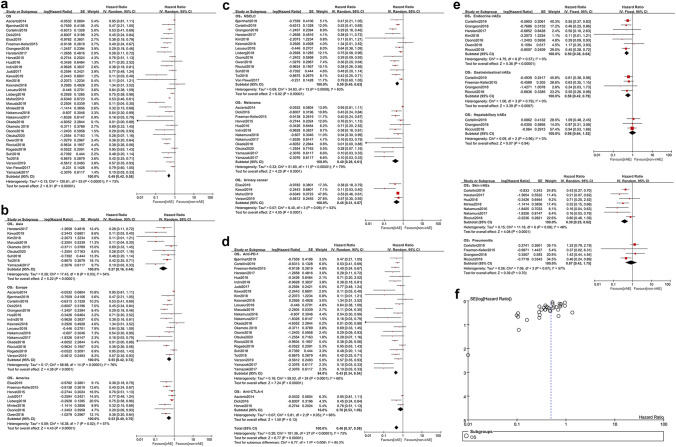
Altogether, 34 studies [14, 16, 17, 23–35, 39–54, 57, 59] contributed to the OS hereof. On account of the heterogeneity (p < 0.00001, I2 = 73%), a random effect model was applied for the meta-analysis. The results showed that compared with non-irAE group, irAE group had a significantly longer OS (HR = 0.49, 95% CI [0.42–0.58], p < 0.00001) (Fig. 3a). In the region stratification analysis, a better survival occurred in irAE group of Asia (HR = 0.27, 95% CI [0.16–0.44], p < 0.00001), Europe (HR = 0.55, 95% CI [0.42–0.72], p < 0.0001), and America (HR = 0.53, 95% CI [0.40–0.70], p < 0.00001) (Fig. 3b). Additionally, the irAEs correlated with longer OS, regardless of NSCLC (HR = 0.50, 95% CI [0.40–0.63], p < 0.00001), melanoma (HR = 0.40, 95% CI [0.26–0.61], p < 0.0001) or urinary cancer (HR = 0.45, 95% CI [0.31–0.67], p < 0.0001) (Fig. 3c). Participants treated with anti-PD-1 had a longer OS when irAEs emerged (HR = 0.43, 95% CI [0.34–0.54], p < 0.00001) (Fig. 3d). On the contrary, OS was not correlated with irAEs in anti-CTLA-4 subgroup (HR = 0.76, 95% CI [0.53–1.09], p = 0.13) (Fig. 3d). Stratification analysis on common irAEs (Fig. 3e) indicated that evident survival benefits existed in endocrine irAEs (HR = 0.50, 95% CI [0.38–0.64], p < 0.00001), skin irAEs (HR = 0.39, 95% CI [0.25–0.62], p < 0.0001) and gastrointestinal irAEs (HR = 0.58, 95% CI [0.42–0.79], p = 0.0007) while no favorable OS was observed in pulmonary irAEs (HR = 0.87, 95% CI [0.43–1.75], P = 0.70) and hepatobiliary irAEs (HR = 0.98, 95% CI [0.64–1.52], p = 0.94). No significant publication bias for OS was found by funnel plot (Fig. 3f).
Fig. 3.
Analyses of irAEs for OS. a OS of the study; b stratification analysis of trial regions; c stratification analysis of tumors; d stratification analysis of ICI types; e stratification analysis of common irAEs; f funnel plot
Progression-free survival
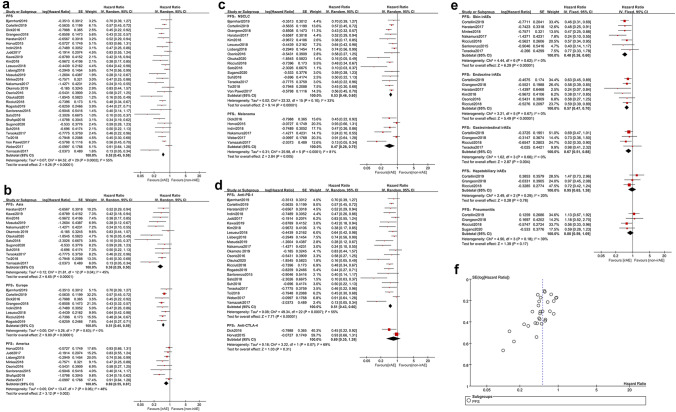
In total, 30 studies [17, 24, 26, 27, 29–39, 42–46, 48–51, 53, 55–59] documented PFS and a random effect model was applied owing to heterogeneity (p = 0.0002, I2 = 55%). Compared with non-irAE group, irAE group presented a prolonged PFS (HR = 0.52, 95% CI [0.45–0.59], p < 0.00001) (Fig. 4a). Stratification analysis on the region showed that compared with non-irAE group, irAE group gained a longer PFS in Asia (HR = 0.38, 95% CI [0.29–0.50], p < 0.00001), Europe (HR = 0.51, 95% CI [0.45–0.59], p < 0.00001) and America (HR = 0.69, 95% CI [0.55–0.87], p = 0.002) (Fig. 4b). Furthermore, the irAEs were positively associated with PFS in NSCLC (HR = 0.53, 95% CI [0.46–0.60], p < 0.00001) and melanoma (HR = 0.47, 95% CI [0.28–0.79], p = 0.005) (Fig. 4c). Participants treated with anti-PD-1 had a longer PFS when irAEs emerged (HR = 0.51, 95% CI [0.43–0.60], p < 0.00001) (Fig. 4d). However, PFS was not correlated with irAEs in anti-CTLA-4 subgroup (HR = 0.69, 95% CI [0.35–1.39], p = 0.31) (Fig. 4d). Stratification analysis on common irAEs (Fig. 4e) indicated that evident survival benefits existed in endocrine irAEs (HR = 0.57, 95% CI [0.47–0.70], p < 0.00001), skin irAEs (HR = 0.48, 95% CI [0.38–0.60], p < 0.00001) and gastrointestinal irAEs (HR = 0.67, 95% CI [0.51–0.88], p = 0.004), whereas no favorable PFS was observed in pulmonary irAEs (HR = 0.80, 95% CI [0.59–1.09], p = 0.17) and hepatobiliary irAEs (HR = 0.95, 95% CI [0.65–1.38], p = 0.78). No significant publication bias for PFS was found by funnel plot (Fig. 4f).
Fig. 4.
Analyses of irAEs for PFS. a PFS of the study; b stratification analysis of trial regions; c stratification analysis of tumors; d stratification analysis of ICI types; e stratification analysis of common irAEs; f funnel plot
Minor or chronic irAEs
Altogether, 23 studies [16, 17, 23, 24, 26–31, 33–35, 37, 39, 42, 47–50, 56, 58, 59] reported some relatively minor or chronic irAEs, such as connective tissue diseases and neurological irAEs. However, only the outcomes of patients with these irAEs were presented, by which we found patients with connective tissue diseases tended to generate better survival statistics (Table 2). However, such severe irAEs may require systemic glucocorticoid therapy, which then become chronic disease and also partially lead to the cessation of ICIs. Unfortunately, no survival data were available for neuromuscular irAEs. Hence, further studies are required to clarify the correlation.
Table 2.
Outcome of minor or chronic irAEs
| Study | Rheumatic or neuromuscular irAEs (n) | Outcome | Treatment of irAEs | IrAE response to treatment |
|---|---|---|---|---|
| Ascierto 2014 [23] | Vasculitis (1) | Therapy discontinued (1) | Therapy discontinued (1) | NA |
| Cortellini 2019 [24] | Rheumatologic, neuromuscular |
OS HR 0.61 [0.38–0.97], P = 0.04 PFS HR 0.84 [0.57–1.23], P = 0.37 |
NA | NA |
| Grangeon 2018 [26] | Arthralgia (5), psoriasis (1) | NA | NA | NA |
| Horvat 2015 [28] | Uveitis (8) | Therapy discontinued (1) |
Systemic corticosteroids (1), therapy discontinued (1) |
Improved |
| Neurotoxicity (1) | Therapy discontinued (1) | Therapy discontinued (1) | NA | |
| Arthritis (1) | NA | Systemic corticosteroids (1) | Improved | |
| Haratani 2017 [27] | Polyarthritis (1), myasthenia gravis (1) | NA | NA | NA |
|
Indini 2018 [29] |
Arthralgias (11), myasthenia with myositis (1), headache (2), paresthesia (2), peripheral sensory neuropathy (8), peripheral motor neuropathy (1), uveitis (1) | NA | NA | NA |
| Kawai 2019 [30] | Myasthenia gravis (1) | NA | NA | NA |
| Masuda 2019 [31] | Myalgia (1), peripheral motor neuropathy (1) | NA | NA | NA |
| Okamoto 2019 [33] | Myositis (1), rheumatoid arthritis (1) | NA | NA | NA |
| Ricciuti 2018 [34] | Uveitis (1), arthritis (7), polymyalgia rheumatica (1), dermatomyositis (1) | NA | NA | NA |
| Rogado 2019 [35] | Arthritis (3) | Therapy discontinued (1) | Therapy discontinued (1) | NA |
| Myositis (1) | NA | NA | NA | |
| Shafqat 2018 [37] | Arthralgia/arthritis (9) | NA | Prednisone (5) | NA |
| Study | Rheumatic or neuromuscular irAEs (n) | Outcome | Treatment of irAEs | IrAE response to treatment |
| Toi 2018 [39] | Myositis or peripheral neuropathy (5) | PR (4), SD (1) | NA | NA |
| Rheumatoid factor positive |
PFS HR 0.61 [0.38–0.97], P = 0.04 |
NA | NA | |
| Bjornhart 2019 [42] | Arthritis (24) |
OS HR 0.35 [0.11–1.12], P = 0.08 PFS HR 0.51 [0.22–1.17], P = 0.11 |
NA | NA |
| Uveitis, myositis | NA | NA | NA | |
| Ksienski 2018 [47] | Arthralgias (8) (caused by nivolumab) |
OS (month) NR (8.27-NR) vs 10.1 (8.3–14.2), P = 0.44 |
NA | NA |
| Arthralgias (5) (caused by pembrolizumab) |
OS (month) NR (NR-NR) vs 13.5 (10.6-NR), P = 0.42 |
NA | NA | |
| Neurologic (3), myopathy (2), myositis (1), polymyalgia rheumatica (1), vasculitis (1) | NA | NA | NA | |
| Lisberg 2018 [49] | Joint pain (2) | NA | NA | NA |
| Sugano 2020 [56] | Ocular myasthenia gravis (1) | NA | NA | NA |
| Maher 2019 [50] | Musculoskeletal pain, rhabdomyolysis, Muscle spasms | NA | NA | NA |
| Hua 2016 [16] | Arthralgia (11) | NA | NA | NA |
| Lesueur 2018 [48] | Rheumatological (6) | NA | NA | NA |
| Nakamura 2017 [17] | Psoriasis (1) | Therapy discontinued (1) | NA | NA |
| Weber 2017 [58] | Arthralgia (39) | NA | NA | NA |
| Yamazaki 2017 [59] | Arthralgia (3) | NA | NA | NA |
NA not available, NR not reached
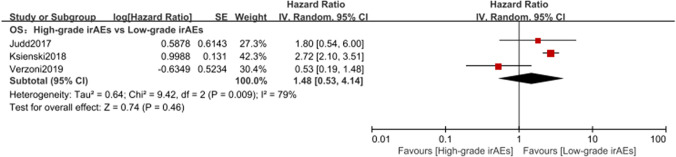
Severity of irAEs
A total of three studies [40, 45, 47] illustrated the relationship between the severity of irAEs and efficacy. On account of the heterogeneity (p = 0.009, I2 = 79%), a random effect model was applied for the meta-analysis. The results showed that compared with high-grade irAE group, low-grade irAE group tended to harbor a longer OS (HR = 1.48, 95% CI [0.53–4.14], p = 0.46) (Fig. 5). It is possible that severe irAEs on the body damage offset the immune efficacy.
Fig. 5.
Analysis on the severity of irAEs for OS
Discussion
At present, the correlation between irAEs and efficacy remains controversial. To our best knowledge, this is a comprehensive study on the correlation between efficacy and irAEs of ICIs. This study revealed that irAE group enjoyed a better survival benefit than non-irAE group. Regarding to the types of irAEs, the survival benefit for patients with irAEs was observed in patients presenting skin, endocrine organ or gastrointestinal tract irAEs. With respect to the severity of irAEs, low-grade irAEs tended to be actively associated with efficacy. The occurrence of irAEs was significantly associated with a favorable efficacy of PD-1 inhibitors rather than CTLA-4 inhibitors.
Currently, the mechanism of irAEs is not completely elucidated. ICIs activate immune system against tumor, and provoke inflammatory side effects termed irAEs [60]. It is evidenced that irAEs may be triggered by an antigen common to both tumor and normal tissue, and then the release of T cells would attack both tissues, generating both response and toxicity [61]. Consequently, on the basis of the antigen mimicry theory, it is logical that more severe irAEs are, the better the prognosis is. However, this study indicated that the severity of irAEs was not associated with efficacy. This may be attributed to the increasing risk of death, which may counteract the immune efficacy. Therefore, further studies are required to illustrate the mechanism behind irAEs.
With regard to skin irAEs of ICIs, this study demonstrated that patients with skin irAEs enjoyed a significantly prolonged survival. The mechanism behind irAEs is that T cell infiltration triggers an inflammatory side effect, and also provokes an anti-tumor effect [62]. A typical instance is vitiligo and its skin pigment loss is positively associated with the efficacy of ICIs [15–17, 63]. Meanwhile, vitiligo is a unique side effect of melanoma. Unfortunately, due to data deficiency, this study did not explore any correlation between different irAEs with certain cancer.
Gastrointestinal irAEs were also positively associated with efficacy of ICIs in this study. But some studies pointed out that there were no evident survival benefits from colitis or diarrhea [14–16, 38]. Nevertheless, it was documented that gastrointestinal irAEs predicted improved OS and PFS [63]. The discrepancy may attribute to the diversity of baseline intestinal flora [64, 65].
An exceptional evidence is that no increasing efficacy coupled with pulmonary and hepatobiliary irAEs in our study. This may be attributed to the importance of involved organ. The reasons may be as follows: first, pneumonia and hepatobiliary irAEs are generally severe, even fatal, which counteracts the efficacy of ICIs [66]; second, the major agent was anti-PD-1 in this study which had the highest incidence of pneumonia and hepatobiliary irAEs, thus abating the survival benefits [67]; finally, the majority of the individuals were advanced NSCLC who accompanied with basic lung disease, which would attenuate the therapeutic effect to some extent.
Concerning the correlation between the irAEs of CTLA-4 inhibitor and efficacy, this study failed to discover the positive outcome. CTLA-4 inhibitor activates T cells at an earlier stage of their development and might thus directly disrupt central tolerance without affecting the tumor immune response, while PD-1/PD-L1 inhibits activate T cells in the effect stage [68, 69]. Thus, patients treated with CTLA-4 inhibitor developed more severe irAEs. We make an attempt to interpret this phenomenon as follows: one is that anti-CTLA-4 therapy mediated the higher severity and mortality of irAEs than those of PD-1/PD-L1 inhibitor [66, 67], which compromised the effect to some degree; the other is that the therapeutic course of CTLA-4 was only within 4 cycles (12 weeks) in included studies and its efficacy was not as good as expected [70].
We analyzed the common irAEs, but some chronic or rare irAEs cannot be ignored. In the case of chronic irAEs, because of its chronic nature, these irAEs may affect patients' quality of life. In our study, although patients with rheumatic irAEs tend to have better survival benefits, such severe irAEs may require systemic glucocorticoid therapy, which then become chronic disease and also partially lead to the cessation of ICIs. A study [71] showed that a majority of patients experienced long courses of immunotherapy, but only a minority of them needed the discontinuation of ICIs. Therefore, more studies are expected to focus on the quality of life in patients with rheumatic irAEs.
Comprehensive analysis was accomplished in this study as for the correlation between irAEs and immune efficacy. In addition, stratification analyses were performed based on the region of studies, type of tumors, ICIs and irAEs as well as severity of irAEs. If ICI efficacy is positively correlated with side effects, irAEs could be defined as a simple, economical and easily observed predictor of efficacy.
There have been previous studies similar to ours. A systematic review and meta-analysis reported by Xiaoxiang Zhou et al. [72] analyzed the association between irAEs and ICI efficacy. However, they did not conduct the analyses on the correlation between irAEs and efficacy for ORR. Additionally, they did not conduct stratified analysis of tumor types and regions. However, in our study, more participants and trials were included, and more comprehensive stratified analyses were conducted.
Limitations
Although this study involved comprehensive data (included 40 studies encompassing 8,641 participants), it was confronted with the following two limitations: only 9 prospective studies were available and just 4 studies with monotherapy of anti-CTLA-4/anti-PD-L1 were included. Thereby, more large-scaled prospective studies are recommended.
Conclusion
IrAEs, especially in skin, endocrine organ or gastrointestinal tract, triggered by ICIs implied significant survival benefits.
Author contributions
Conceptualization: XX and LZ; methodology: LZ and JL; software: LZ; validation: all authors; formal analysis: LZ, FC and XX; data curation: LZ; writing—original draft preparation: LZ, QW and XX; writing—review and editing: all authors; visualization: LZ; supervision: XX.
Funding
This work was supported by the Natural Science Foundation of Fujian Province (2019J01457).
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372(4):320–330. doi: 10.1056/NEJMoa1412082. [DOI] [PubMed] [Google Scholar]
- 2.Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. 2015;372(26):2521–2532. doi: 10.1056/NEJMoa1503093. [DOI] [PubMed] [Google Scholar]
- 3.Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ, Srinivas S, et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373(19):1803–1813. doi: 10.1056/NEJMoa1510665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bellmunt J, de Wit R, Vaughn DJ, Fradet Y, Lee JL, Fong L, et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med. 2017;376(11):1015–1026. doi: 10.1056/NEJMoa1613683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferris RL, Blumenschein G, Jr, Fayette J, Guigay J, Colevas AD, Licitra L, et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. 2016;375(19):1856–1867. doi: 10.1056/NEJMoa1602252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, et al. Pembrolizumab versus chemotherapy for PD-L1 positive non-small-cell lung Cancer. N Engl J Med. 2016;375(19):1823–1833. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 7.Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357(6349):409–413. doi: 10.1126/science.aan6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Overman MJ, McDermott R, Leach JL, Lonardi S, Lenz HJ, Morse MA, et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. Lancet Oncol. 2017;18(9):1182–1191. doi: 10.1016/S1470-2045(17)30422-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.El-Khoueiry AB, Sangro B, Yau T, Crocenzio TS, Kudo M, Hsu C, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389(10088):2492–2502. doi: 10.1016/S0140-6736(17)31046-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fuchs CS, Doi T, Jang RW, Muro K, Satoh T, Machado M, et al. Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: phase 2 clinical KEYNOTE-059 trial. JAMA Oncol. 2018;4(5):e180013. doi: 10.1001/jamaoncol.2018.0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ito A, Kondo S, Tada K, Kitano S. Clinical development of immune checkpoint inhibitors. Biomed Res Int. 2015;2015:605478. doi: 10.1155/2015/605478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu YY, Wan B, Chen X, Zhan P, Zhao Y, Zhang T, et al. The association of PD-L1 expression with the efficacy of anti-PD-1/PD-L1 immunotherapy and survival of non-small cell lung cancer patients: a meta-analysis of randomized controlled trials. Transl Lung Cancer Res. 2019;8(4):413–428. doi: 10.21037/tlcr.2019.08.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cao DD, Xu HL, Xu XM, Guo T, Ge W. High tumor mutation burden predicts better efficacy of immunotherapy: a pooled analysis of 103078 cancer patients. Onconimmunology. 2019;8(9):e1629258. doi: 10.1080/2162402X.2019.1629258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Freeman-Keller M, Kim Y, Cronin H, Richards A, Gibney G, Weber JS. Nivolumab in resected and unresectable metastatic melanoma: characteristics of immune-related adverse events and association with outcomes. Clin Cancer Res. 2016;22(4):886–894. doi: 10.1158/1078-0432.CCR-15-1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Teulings HE, Limpens J, Jansen SN, Zwinderman AH, Reitsma JB, Spuls PI, et al. Vitiligo-like depigmentation in patients with stage III-IV melanoma receiving immunotherapy and its association with survival: a systematic review and meta-analysis. J Clin Oncol. 2015;33(7):773–781. doi: 10.1200/JCO.2014.57.4756. [DOI] [PubMed] [Google Scholar]
- 16.Hua C, Boussemart L, Mateus C, Routier E, Boutros C, Cazenave H, et al. Association of vitiligo with tumor response in patients with metastatic melanoma treated with pembrolizumab. JAMA Dermatol. 2016;152(1):45–51. doi: 10.1001/jamadermatol.2015.2707. [DOI] [PubMed] [Google Scholar]
- 17.Nakamura Y, Tanaka R, Asami Y, Teramoto Y, Imamura T, Sato S, et al. Correlation between vitiligo occurrence and clinical benefit in advanced melanoma patients treated with nivolumab: a multi-institutional retrospective study. J Dermatol. 2017;44(2):117–122. doi: 10.1111/1346-8138.13520. [DOI] [PubMed] [Google Scholar]
- 18.Tone M, Izumo T, Awano N, Kuse N, Inomata M, Jo T, et al. High mortality and poor treatment efficacy of immune checkpoint inhibitors in patients with severe grade checkpoint inhibitor pneumonitis in non-small cell lung cancer. Thorac Cancer. 2019;10(10):2006–2012. doi: 10.1111/1759-7714.13187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Panic N, Leoncini E, de Belvis G, Ricciardi W, Boccia S. Evaluation of the endorsement of the preferred reporting items for systematic reviews and meta-analysis (PRISMA) statement on the quality of published systematic review and meta-analyses. PLoS ONE. 2013;8(12):e83138. doi: 10.1371/journal.pone.0083138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA. 2000;283(15):2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 21.Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR, et al. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. doi: 10.1186/1745-6215-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719–748. [PubMed] [Google Scholar]
- 23.Ascierto PA, Simeone E, Sileni VC, Pigozzo J, Maio M, Altomonte M, et al. Clinical experience with ipilimumab 3 mg/kg: real-world efficacy and safety data from an expanded access programme cohort. J Transl Med. 2014;12:116. doi: 10.1186/1479-5876-12-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cortellini A, Chiari R, Ricciuti B, Metro G, Perrone F, Tiseo M, et al. Correlations between the immune-related adverse events spectrum and efficacy of anti-PD1 immunotherapy in NSCLC patients. Clin Lung Cancer. 2019;20(4):237–247. doi: 10.1016/j.cllc.2019.02.006. [DOI] [PubMed] [Google Scholar]
- 25.Elias R, Yan F, Singla N, Levonyack N, Formella J, Christie A, et al. Immune-related adverse events are associated with improved outcomes in ICI-treated renal cell carcinoma patients. J Clin Oncol. 2019;37(7):S645. doi: 10.1200/JCO.2019.37.7_suppl.645. [DOI] [Google Scholar]
- 26.Grangeon M, Tomasini P, Chaleat S, Jeanson A, Souquet-Bressand M, Khobta N, et al. Association between immune-related adverse events and efficacy of immune checkpoint inhibitors in non-small-cell lung cancer. Clin Lung Cancer. 2019;20(3):201–207. doi: 10.1016/j.cllc.2018.10.002. [DOI] [PubMed] [Google Scholar]
- 27.Haratani K, Hayashi H, Chiba Y, Kudo K, Yonesaka K, Kato R, et al. Association of immune-related adverse events with nivolumab efficacy in non-small-cell lung cancer. JAMA Oncol. 2018;4(3):374–378. doi: 10.1001/jamaoncol.2017.2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Horvat TZ, Adel NG, Dang TO, Momtaz P, Postow MA, Callahan MK, et al. Immune-related adverse events, need for systemic immunosuppression, and effects on survival and time to treatment failure in patients with melanoma treated with ipilimumab at Memorial Sloan Kettering Cancer Center. J Clin Oncol. 2015;33(28):3193–3198. doi: 10.1200/JCO.2015.60.8448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Indini A, Guardo LD, Cimminiello C, Prisciandaro M, Randon G, Braud FD, et al. Immune-related adverse events correlate with improved survival in patients undergoing anti-PD1 immunotherapy for metastatic melanoma. J Cancer Res Clin Oncol. 2019;145(2):511–521. doi: 10.1007/s00432-018-2819-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kawai T, Sato Y, Makino K, Yamada Y, Nomiya A, Nakamura M, et al. Immune-related adverse events predict the therapeutic efficacy of pembrolizumab in urothelial cancer patients. Eur J Cancer. 2019;116:114–115. doi: 10.1016/j.ejca.2019.05.017. [DOI] [PubMed] [Google Scholar]
- 31.Masuda K, Shoji H, Nagashima K, Yamamoto S, Ishikawa M, Imazeki H, et al. Correlation between immune-related adverse events and prognosis in patients with gastric cancer treated with nivolumab. BMC Cancer. 2019;19(1):974. doi: 10.1186/s12885-019-6150-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Okada N, Kawazoe H, Takechi K, Matsudate Y, Utsunomiya R, Zamami Y, et al. Association between immune-related adverse events and clinical efficacy in patients with melanoma treated with nivolumab: a multicenter retrospective study. Clin Ther. 2019;41(1):59–67. doi: 10.1016/j.clinthera.2018.11.004. [DOI] [PubMed] [Google Scholar]
- 33.Okamoto I, Sato H, Kondo T, Koyama N, Fushimi C, Okada T, et al. Efficacy and safety of nivolumab in 100 patients with recurrent or metastatic head and neck cancer - a retrospective multicentre study. Acta Otolaryngol. 2019;139(10):918–925. doi: 10.1080/00016489.2019.1648867. [DOI] [PubMed] [Google Scholar]
- 34.Ricciuti B, Genova C, Giglio AD, Bassanelli M, Giovanna MDB, Metro G, et al. Impact of immune-related adverse events on survival in patients with advanced non-small cell lung cancer treated with nivolumab: long-term outcomes from a multi-institutional analysis. J Cancer Res Clin Oncol. 2019;145(2):479–485. doi: 10.1007/s00432-018-2805-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rogado J, Sánchez-Torres JM, Romero-Laorden N, Ballesteros AI, Pacheco-Barcia V, Ramos-Leví A, et al. Immune-related adverse events predict the therapeutic efficacy of anti-PD-1 antibodies in cancer patients. Eur J Cancer. 2019;109:21–27. doi: 10.1016/j.ejca.2018.10.014. [DOI] [PubMed] [Google Scholar]
- 36.Sato K, Akamatsu H, Murakami E, Sasaki S, Kanai K, Hayata A, et al. Correlation between immune-related adverse events and efficacy in non-small cell lung cancer treated with nivolumab. Lung Cancer. 2018;115:71–74. doi: 10.1016/j.lungcan.2017.11.019. [DOI] [PubMed] [Google Scholar]
- 37.Shafqat H, Gourdin T, Sion A. Immune-related adverse events are linked with improved progression-free survival in patients receiving anti-PD-1/PD-L1 therapy. Semin Oncol. 2018;45(3):156–163. doi: 10.1053/j.seminoncol.2018.07.003. [DOI] [PubMed] [Google Scholar]
- 38.Teraoka S, Fujimoto D, Morimoto T, Kawachi H, Ito M, Sato Y, et al. Early immune-related adverse events and association with outcome in advanced non-small cell lung cancer patients treated with nivolumab: a prospective cohort study. J Thorac Oncol. 2017;12(12):1798–1805. doi: 10.1016/j.jtho.2017.08.022. [DOI] [PubMed] [Google Scholar]
- 39.Toi Y, Sugawara S, Sugisaka J, Ono H, Kawashima Y, Aiba T, et al. Profiling preexisting antibodies in patients treated with anti-PD-1 therapy for advanced non-small cell lung cancer. JAMA Oncol. 2019;5(3):376–383. doi: 10.1001/jamaoncol.2018.5860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Verzoni E, Cartenì G, Cortes E, Giannarelli D, Giglio AD, Sabbatin R, et al. Real-world efficacy and safety of nivolumab in previously-treated metastatic renal cell carcinoma, and association between immune-related adverse events and survival: the Italian expanded access program. J Immunother Cancer. 2019;7(1):99. doi: 10.1186/s40425-019-0579-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Von Pawel J, Syrigos K, Mazieres J, Cortinovis D, Dziadziuszko R, Gandara DR, et al (2017) Association between immune-related adverse events (irAEs) and atezolizumab efficacy in advanced NSCLC: analyses from the phase III study OAK. Ann Oncol 28(suppl 5): mdx380.017-mdx380.017
- 42.Bjørnhart B, Hansen KH, Jørgensen TL, Herrstedt J, Schytte T. Efficacy and safety of immune checkpoint inhibitors in a Danish real life non-small cell lung cancer population: a retrospective cohort study. Acta Oncol. 2019;58(7):953–961. doi: 10.1080/0284186X.2019.1615636. [DOI] [PubMed] [Google Scholar]
- 43.Otsuka M, Sugihara S, Mori S, Hamada K, Sasaki Y, Yoshikawa S, et al. Immune-related adverse events correlate with improved survival in patients with advanced mucosal melanoma treated with nivolumab: a single-center retrospective study in Japan. J Dermatol. 2020;47(4):356–362. doi: 10.1111/1346-8138.15246. [DOI] [PubMed] [Google Scholar]
- 44.Dick J, Lang N, Slynko A, Kopp-Schneider A, Schulz C, Dimitrakopoulo-strauss A, et al. Use of LDH and autoimmune side effects to predict response to ipilimumab treatment. Immunotherapy. 2016;8(9):1033–1044. doi: 10.2217/imt-2016-0083. [DOI] [PubMed] [Google Scholar]
- 45.Judd J, Ma Z, Handorf E, O'Neill J, Ramamurthy C, Bentota S, et al. Immune-related adverse events as a biomarker in non-melanoma patients treated with programmed cell death 1 inhibitors. Oncologist. 2017;22(10):1232–1237. doi: 10.1634/theoncologist.2017-0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim HI, Kim M, Lee SH, Park SY, Kim YN, Kim H, et al. Development of thyroid dysfunction is associated with clinical response to PD-1 blockade treatment in patients with advanced non-small cell lung cancer. Oncoimmunology. 2017;7(1):e1375642. doi: 10.1080/2162402X.2017.1375642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ksienski D, Wai ES, Croteau N, Fiorino L, Brooks E, Poonja Z, et al. Efficacy of nivolumab and pembrolizumab in patients with advanced nonsmall cell lung cancer needing treatment interruption due to adverse events: aretrospective multicenter analysis. Clin Lung Cancer. 2018;20(1):e97–e106. doi: 10.1016/j.cllc.2018.09.005. [DOI] [PubMed] [Google Scholar]
- 48.Lesueur P, Escande A, Thariat J, Vauléon E, Monnet I, Cortot A, et al. Safety of combined PD-1 pathway inhibition and radiation therapy for non-small-cell lung cancer: A multicentric retrospective study from the GFPC. Cancer Med. 2018;7(11):5505–5513. doi: 10.1002/cam4.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lisberg A, Tucker DA, Goldman JW, Wolf B, Carroll J, Ariana H, et al. Treatment-related adverse events predict improved clinical outcome in NSCLC patients on KEYNOTE-001 at a single center. Cancer Immunol Res. 2018;6(3):288–294. doi: 10.1158/2326-6066.CIR-17-0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Maher VE, Fernandes LL, Weinstock C, Tang S, Agarwal S, Brave M, et al. Analysis of the association between adverse events and outcome in patients receiving a programmed death protein 1 or programmed death ligand 1 antibody. J Clin Oncol. 2019;37(30):2730–2737. doi: 10.1200/JCO.19.00318. [DOI] [PubMed] [Google Scholar]
- 51.Min Lee CK, Li S, Tran DC, Zhu GA, Kim J, Kwong BY, et al. Characterization of dermatitis after PD-1/PD-L1 inhibitor therapy and association with multiple oncologic outcomes: a retrospective case-control study. J Am Acad Dermatol. 2018;79(6):1047–1052. doi: 10.1016/j.jaad.2018.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nakamura Y, Kitano S, Takahashi A, Tsutsumida A, Namikawa K, Tanese K, et al. Nivolumab for advanced melanoma: pretreatment prognostic factors and early outcome markers during therapy. Oncotarget. 2016;7(47):77404–77415. doi: 10.18632/oncotarget.12677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Osorio JC, Ni A, Chaft JE, Pollina R, Kasler MK, Stephens D, et al. Antibody-mediated thyroid dysfunction during T-cell checkpoint blockade in patients with non-small-cell lung cancer. Ann Oncol. 2017;28(3):583–589. doi: 10.1093/annonc/mdw640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Owen DH, Wei L, Bertino EM, Edd T, Villalona-Calero MA, He K, et al. Incidence, risk factors, and effect on survival of immune-related adverse events in patients with non-small-cell lung cancer. Clin Lung Cancer. 2018;19(6):e893–e900. doi: 10.1016/j.cllc.2018.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sanlorenzo M, Vujic I, Daud A, Algazi A, Gubens M, Luna SA, et al. Pembrolizumab cutaneous adverse events and their association with disease progression. JAMA Dermatol. 2015;151(11):1206–1212. doi: 10.1001/jamadermatol.2015.1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sugano T, Seike M, Saito Y, Kashiwada T, Terasaki Y, Takano N, et al. Immune checkpoint inhibitor-associated interstitial lung diseases correlate with better prognosis in patients with advanced non-small-cell lung cancer. Thorac Cancer. 2020;11(4):1052–1060. doi: 10.1111/1759-7714.13364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Suh KJ, Kim SH, Kim YJ, Kim M, Keam B, Kim TM, et al. Post-treatment neutrophil-to-lymphocyte ratio at week 6 is prognostic in patients with advanced non-small cell lung cancers treated with anti-PD-1 antibody. Cancer Immunol Immunother. 2018;67(3):459–470. doi: 10.1007/s00262-017-2092-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Weber JS, Hodi FS, Wolchok JD, Topalian SL, et al. Safety profile of nivolumab monotherapy: apooled analysis of patients with advanced melanoma. J Clin Oncol. 2017;35(7):785–792. doi: 10.1200/JCO.2015.66.1389. [DOI] [PubMed] [Google Scholar]
- 59.Yamazaki N, Kiyohara Y, Uhara H, Uehara J, Fujimoto M, Takenouchi T, et al. Efficacy and safety of nivolumab in Japanese patients with previously untreated advanced melanoma: a Phase II study. Cancer Sci. 2017;108(6):1223–1230. doi: 10.1111/cas.13241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Postow MA, Sidlow R, Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med. 2018;378(2):158–168. doi: 10.1056/NEJMra1703481. [DOI] [PubMed] [Google Scholar]
- 61.Patil PD, Fernandez AP, Velcheti V, Tarhini A, Funchain P, Rini B, et al. Cases from the irAE tumor board: a multidisciplinary approach to a patient treated with immune checkpoint blockade who presented with a new rash. Oncologist. 2019;24(1):4–8. doi: 10.1634/theoncologist.2018-0434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Le Poole IC, Wañkowicz-Kaliñska A, van den Wijngaard RM, Nickoloff BJ, Das PK (2004) Autoimmune aspects of depigmentation in vitiligo. J Investig Dermatol Symp Proc 9(1): 68–72. https://doi.org/10.1111/j.1087-0024.2004.00825.x [DOI] [PubMed]
- 63.Abu-Sbeih H, Ali FS, Qiao W, Lu Y, Patel S, Diab A, et al. Immune checkpoint inhibitor-induced colitis as a predictor of survival in metastatic melanoma. Cancer Immunol Immunother. 2019;68(4):553–561. doi: 10.1007/s00262-019-02303-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chaput N, Lepage P, Coutzac C, Soularue E, Le Roux K, Monot C, et al. Baseline gut microbiota predicts clinical response and colitis in metastatic melanoma patients treated with ipilimumab. Ann Oncol. 2017;28(6):1368–1379. doi: 10.1093/annonc/mdx108. [DOI] [PubMed] [Google Scholar]
- 65.Dubin K, Callahan MK, Ren B, Khanin R, Viale A, Ling L, et al. Intestinal microbiome analyses identify melanoma patients at risk for checkpoint-blockade-induced colitis. Nat Commun. 2016;7:10391. doi: 10.1038/ncomms10391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Osta BE, Hu F, Sadek R, Chintalapally R, Tang SC. Not all immune-checkpoint inhibitors are created equal: meta-analysis and systematic review of immune-related adverse events in cancer trials. Crit Rev Oncol Hematol. 2017;119:1–12. doi: 10.1016/j.critrevonc.2017.09.002. [DOI] [PubMed] [Google Scholar]
- 67.Wang DY, Salem J, Cohen JV, Chandra S, Menzer C, Fi Ye, et al. Fatal toxic effects associated with immune checkpoint inhibitors: a systematic review and meta-analysis. JAMA Oncol. 2018;4(12):1721–1728. doi: 10.1001/jamaoncol.2018.3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Melero I, Hervas-Stubbs S, Glennie M, Pardoll DM, Chen L. Immunostimulatory monoclonal antibodies for cancer therapy. Nat Rev Cancer. 2007;7(2):95–106. doi: 10.1038/nrc2051. [DOI] [PubMed] [Google Scholar]
- 69.Okazaki T, Chikuma S, Iwai Y, Fagarasan S, Honjo T. A rheostat for immune responses: the unique properties of PD-1 and their advantages for clinical application. Nat Immunol. 2013;14(12):1212–1218. doi: 10.1038/ni.2762. [DOI] [PubMed] [Google Scholar]
- 70.Wolchok JD, Neyns B, Linette G, Negrier S, Lutzky J, Thomas L, et al. Ipilimumab monotherapy in patients with pretreated advanced melanoma: a randomised, double-blind, multicentre, phase 2, dose-ranging study. Lancet Oncol. 2020;11(2):155–164. doi: 10.1016/S1470-2045(09)70334-1. [DOI] [PubMed] [Google Scholar]
- 71.Richter MD, Crowson C, Kottschade LA, Finnes HD, Markovic SN, Thanarajasingam U. Rheumatic syndromes associated with immune-checkpoint inhibitors: a single-center cohort of 61 patients. Arthritis Rheumatol. 2019;71(3):468–475. doi: 10.1002/art.40745. [DOI] [PubMed] [Google Scholar]
- 72.Zhou XX, Yao ZR, Yang HX, Liang NX, Zhang X, Zhang FC. Are immune-related adverse events associated with the efficacy of immune checkpoint inhibitors in patients with cancer? A systematic review and meta-analysis. BMC Med. 2020;18(1):87–100. doi: 10.1186/s12916-020-01549-2. [DOI] [PMC free article] [PubMed] [Google Scholar]