Abstract
Background
Immune checkpoint inhibitor (ICI) has an emerging role in several types of cancer. However, the mechanisms of acquired resistance (AR) to ICI have not been elucidated yet. To identify these mechanisms, we analyzed the pre- and post-ICI paired tumor samples in patients with AR.
Methods
Six patients with renal cell carcinoma, urothelial cell carcinoma, or head and neck cancer, who showed an initial response to ICI followed by progression and had available paired tissue samples, were retrospectively analyzed. Whole exome sequencing, RNA sequencing, and multiplex immunohistochemistry were performed on pre-treatment and resistant tumor samples.
Results
The median time to AR was 370 days (range, 210 to 739). Increased expression of alternative immune checkpoints including TIM3, LAG3, and PD-1 as well as increased CD8+ tumor-infiltrating lymphocytes were observed in post-treatment tumor than in pre-treatment tumor of a renal cell carcinoma patient. In contrast, CD8+ T cells and immunosuppressive markers were all decreased at AR in another patient with human papillomavirus-positive head and neck squamous cell carcinoma. This patient had an evident APOBEC-associated signature, and the tumor mutation burden increased at AR. Resistant tumor tissue of this patient harbored a missense mutation (E542K) in PIK3CA. No significant aberrations of antigen-presenting machinery or IFN-γ pathway were detected in any patient.
Conclusions
Our study findings suggest that the observed increase in immunosuppressive markers after ICI might contribute to AR. Moreover, APOBEC-mediated PIK3CA mutagenesis might be an AR mechanism. To validate these mechanisms of AR, further studies with enough sample size are required.
Electronic supplementary material
The online version of this article (10.1007/s00262-020-02799-y) contains supplementary material, which is available to authorized users.
Keywords: Immunotherapy, Programmed cell death 1 receptor, Tumor escape, Tumor microenvironment
Introduction
Immune checkpoint inhibitors (ICI) have emerged as a new treatment for immunogenic tumors such as lung cancer and melanoma. However, the response rate to ICI alone still falls short of 20%, despite numerous attempts to increase it. In addition to biomarkers for optimal selection of patients such as programmed death-ligand 1 (PD-L1) expression or tumor mutational burden (TMB), the mechanisms of primary resistance to ICI including transcriptional signature [1] and loss of PTEN [2] have also been explored. However, little is known about the mechanisms of acquired resistance (AR), which is defined as progression after initial response to ICI [3]. Strategies for optimal patient management after the onset of AR are not yet established, and there is an unmet need for guidelines in clinical practice.
To date, some mechanisms of AR have been observed in previous studies. Loss-of-function mutation of JAK1 and JAK2 [4], defects in antigen-presenting machinery such as loss of heterozygosity of B2M [4–6], and changes in neo-antigens during ICI [7] can all give rise to AR in clinical practice. Furthermore, upregulation of other immune checkpoint molecules is thought to be an additional AR mechanism [8, 9]. However, these AR mechanisms and were observed only in some patients, and therefore, might not fully represent the mechanisms of AR in all patients who initially respond to ICI. Consequently, the potential mechanisms of AR need further exploration. Moreover, elucidating the changes in immune-related biomarkers at the time of AR might be beneficial for shaping rational combination treatment strategies [10]. In particular, after the onset of AR, this may help clinicians in choosing the optimal sequence of therapeutic agents such as alternative ICIs, conventional chemotherapy, or immuno-modulatory agents.
This study aims to explore the mechanisms of AR to ICI through multi-omics profiling of paired tumor samples that were obtained pre-treatment and post-treatment.
Materials and methods
Patient population
We retrospectively reviewed medical records of patients with renal cell carcinoma, urothelial cell carcinoma, or head and neck cancer who were treated with ICI (programmed cell death protein 1 (PD-1)/PD-L1 blockade, single or in combination) at Seoul National University Hospital between December 2013 and June 2017. Among them, we included patients (1) 19 years old, (2) who demonstrated AR, defined as an initial response to ICI (complete response, partial response, or stable disease > 6 months, assessed by the Response Evaluation Criteria in Solid Tumors [RECIST] guidelines, version 1.1) [11] followed by progression, and 3) who had pre-treatment and post-treatment tumor tissue samples available along with matched peripheral blood mononuclear cells. Six patients were enrolled (Fig. 1a). Baseline patient characteristics (including age, sex, histologic differentiation, and location of tumor) and treatment details were retrospectively obtained from medical records.
Fig. 1.
Study design and characteristics of the patients showing acquired resistance to immune checkpoint inhibitors. a Study design and analytic process. b Swimmer’s plot indicating cancer type, progression-free survival, best response, and the time to acquisition of tissue at acquired resistance to ICI. (c) Clinical course with radiologic images and tissue collection timepoints for whole-exome sequencing, RNA sequencing, and multiplex immunohistochemistry in a patient with renal cell carcinoma (patient #2). At baseline, a 7.5 cm size heterogeneously enhancing mass (circled) in the right kidney was observed at diagnosis. After right nephrectomy, a small nodule in the right upper lobe of the lung (circled) suggesting lung metastasis newly appeared. After 10 months, tiny nodules in bilateral lower lobes of the lung (circled) were noted. After 20 months from the right middle and lower lobe metastasectomy, multiple growing lung nodules (circled) suspicious of lung metastases were located in left upper lobe and lower lobe base. CT image of the chest taken before ICI treatment demonstrated multiple nodules in both lung and right pleura-based nodule (circled). Tumor response maintained for 15 months, followed by disease relapse with an increase in the mass lesions adjacent to the seventh rib and newly noted multiple lung nodules. PD-1 programmed death-1, PD-L1 programmed death-ligand 1, PBMC peripheral blood mononuclear cell, ICI immune checkpoint inhibitor, FFPE formalin-fixed, paraffin-embedded, HNSCC head and neck squamous cell carcinoma, RCC renal cell carcinoma, NPx nasopharyngeal carcinoma, UCC urothelial cell carcinoma of the bladder
Tissue preparation and multiplex immunohistochemistry (IHC)
Tissue specimens from the enrolled patients were formalin-fixed and paraffin-embedded (FFPE) and were obtained from the archive. Two pathologists (SHK and JMK) reviewed the specimens, designated representative tumor regions identified from hematoxylin and eosin-stained sections and marked them on the electronic image file. Quantitative multiplex immunohistochemical staining was conducted using PerkinElmer Opal kit (Perkin-Elmer, Waltham, MA, USA). Rotation microtome was used to cut 4 μm FFPE tissue sections. Subsequently, the sections were heated for at least 1 h in a dry oven at 60 °C, followed by deparaffinization with 100% xylene and then rehydration. Antigen retrieval was performed with Bond Epitope Retrieval 2 (#AR9640, Leica Biosystems, Newcastle, UK) in a pH 9.0 solution for 30 min. A blocking solution of 3% H2O2 followed by Dako antibody diluent was used for blocking. Multiplex immunofluorescence staining was performed with a Leica Bond Rx™ Automated Stainer (Leica Biosystems, Newcastle, UK). We constructed two panels for antibodies as follows: cytokeratin (CK), PD-L1, CD3, PD-1, Lymphocyte-activation gene 3 (LAG3), and T-cell immunoglobulin and mucin-domain containing-3 (TIM3) for panel 1, and CK, CD4, CD8, Human Leukocyte Antigen (HLA) class I, CD86, and CD163 for panel 2. The primary antibody for a certain marker was incubated for one hour in a humidified chamber at room temperature, and the OpalTM Polymer HRP Ms + Rb kit (ARH1001EA, Perkin-Elmer, MA, USA) was used for detection. Thereafter, visualization of the marker was accomplished using tyramide signal amplification, after which the slide was treated with Bond Epitope Retrieval 1 ((#AR9961, Leica Biosystems, Newcastle, UK) for 20 min to remove the bound antibodies before performing the next step in the sequence. Detailed information about the primary antibodies for the markers and the tyramide signal amplification substrates are described in Supplementary Table 1. Nuclei were subsequently visualized with nuclear spectral elements (4′,6-diamidino-2-phenylindole, DAPI) to identify each cell, and the section was subsequently covered with a coverslip using HIGHDEF® IHC fluoromount (ADI-950-260-0025, Enzo, USA). The PerkinElmer Vectra 3.0 Automated Quantitative Pathology Imaging System (Perkin-Elmer, MA, USA) was used for scanning the slides, and the images were analyzed using inForm 2.2 software and TIBCO Spotfire™ (Perkin-Elmer, MA, USA). Using the cell segmentation tool, the entire immune cell population in each panel was quantified and designated as positive or negative for each antibody. The numbers of CK, CD3, PD-L1, PD-1, LAG3, TIM3, CD4, CD8, HLA class I, CD86, and CD163 positive cells were counted in each slide. We also analyzed the data for CD3, CD4, CD8, PD-1, LAG3, and TIM3 in cells with lymphocyte-range of diameter (5 to 15 µm), and the data for CD86 and CD163 in cells with macrophage-range of diameter (15 to 25 µm). To characterize the expression of the markers on tumor cells, PD-L1 and HLA class I on CK-positive cells were used for analysis.
Table 1.
Baseline Clinical Characteristics of Patients
| Patient No. | Sex | Diagnosis | ECOG PS at baseline | Tumor burdena | No. of prior systemic therapy | Type of ICIs | Response | PFS | OS | Treatment duration |
|---|---|---|---|---|---|---|---|---|---|---|
| #1 | Male | Tonsillar cancer (HPV-positive) | 1 | 15 mm | 1 | α-PD-1 + α-CTLA-4 | SD | 15.9mo | 23.1mob | 15.9mo |
| #2 | Male | Clear cell renal cell carcinoma | 0 | 39 mm | 0 | α-PD-L1 | SD | 15.2mo | 36.9mob | 15.2mo |
| #3 | Male | Nasopharyngeal cancer | 1 | 60 mm | 1 | α-PD-1 | PR | 24.6mo | 60.2mo | 23.6mo |
| #4 | Male | Clear cell renal cell carcinoma | 1 | 17 mm | 0 | α-PD-L1 + α-VEGF | PR | 9.3mo | 22.2mo | 19.4mo |
| #5 | Male | Urothelial cell carcinoma of bladder | 1 | 40 mm | 0 | α-PD-L1 | SD | 9.5mob÷ | 28.7mob | 28.7mo |
| #6 | Male | Clear cell renal cell carcinoma | 1 | 73 mm | 3 | α-PD-L1 + MEK inhibitor | SD | 7mo | 20.1mob | 6.1mo |
No. number, ECOG Eastern Cooperative Oncology Group, PS performance status, ICI immune checkpoint inhibitor, PFS progression-free survival, OS overall survival, HPV human papillomavirus, α- anti-, PD-1 programmed cell death-1, PD-L1 programmed death-ligand 1, CTLA-4 cytotoxic T-lymphocyte–associated antigen 4, VEGF vascular endothelial growth factor, MEK mitogen-activated protein kinase kinase, SD stable disease, PR partial response
aTumor burden was defined as baseline tumor size, which was calculated by summation of the largest diameter of the target lesions per RECIST version 1.1 (33)
bOngoing at census
For exploring the activation of PI3K-Akt pathway, IHC was performed for phosphor-AKT (p-AKT) and phospho-S6 (p-S6) using following primary antibodies: p-AKT Ser473 (#9271, Cell Signaling) and p-S6 Ribosomal Protein Ser240/244 (#2215, Cell Signaling). Immunostaining was carried out using the general procedures [12], using Ventana BenchMark XT autostainer (Ventana Medical Systems, Tucson, AZ, USA).
DNA and RNA extraction process
gDNA and RNA were isolated from a 10 µm thick section of FFPE tumor tissue using a Maxwell® RSC DNA/RNA FFPE kit (Promega, Madison, WI, state USA). gDNA from peripheral blood was extracted using the Maxwell® RSC Blood DNA kit (Promega). gDNA and RNA concentration and purity were measured using an EON (BioTek., Winooski, VT, USA) and a Qubit 2.0 Fluorometer (Life Technologies Inc., Carlsbad, CA, USA).
Whole Exome Sequencing (WES) and data analysis
Sequencing libraries were constructed using the SureSelect XT Human All Exon 50 Mb and SureSelect XT reagent kit, HSQ (Agilent) according to the manufacturer’s protocol. The libraries were sequenced by the Illumina HiSeq 2500 platform (paired-end 101 bp reads). We planned a sequencing depth of 200 × for tumor tissue and 80 × for peripheral blood mononuclear cells. Sequencing characteristics and information are described in Supplementary Table 2.
The GRCh37 human reference genome was used to align the sequencing reads via the Burrows-Wheeler Aligner (BWA)-0.7.10 [13]. Read sorting and PCR duplicate removal was performed by Picard-tools-1.124 (http://broadinstitute.github.io/picard/). Genome Analysis Tools Kit (GATK)-3.7 [14] was used for performing data pre-processing including local realignment around small insertions and deletions (indels) and base quality score recalibration. We used MuTect2 to detect somatic single nucleotide polymorphisms and indels using tumor and matched normal blood. ANNOVAR [15] was used to annotate variants. The following criteria were used to reduce false-positive variants: (1) MuTect2 filter = PASS. (2) Significant single nucleotide polymorphism was defined as Alt allele depth ≥ 4 and Alt allele frequency ≥ 0.03, and significant indel as Alt allele depth ≥ 4 and Alt allele frequency ≥ 0.05. Moreover, we only retained exonic and splicing variants. TMB was measured by the number of somatic single nucleotide variants and indel mutations that passed the set criteria per megabase in the coding region. We included the synonymous as well as nonsynonymous mutations [16]. Signature analysis of mutational processes was carried out using deconstructSigs R package [17].
To identify more significant variants in the somatic mutation profile, we removed synonymous variants and common variants with allele frequency > 1% in 1000G, ExAC, and ESP databases. Additionally, we excluded variants that predicted to be functionally neutral by CADD (Phred score < 15) or by both SIFT (pred = “T”) and PolyPhen-2 (pred = “B”) according to ANNOVAR annotation. For identifying AR-associated somatic mutations, which were present only in post-treatment samples, we followed a re-checking process, which entailed examining whether the somatic mutations found in a post-treatment sample were present in MuTect2 raw result without additional filtering of the pre-treatment sample. We generated a mutation diagram (“lollipop plot”) of PIK3CA variant with MutationMapper [18, 19] on cBioPortal. Copy number variations were identified by EXCAVATOR2 [20] and CNVkit [21].
RNA sequencing and data analysis
Libraries for RNA sequencing (RNA-seq) were constructed using a TruSeq RNA Exome (Illumina) according to the manufacturer’s protocol. Sequencing of the RNA libraries was carried out using the 100-bp paired-end mode of the TruSeq RNA Exome (Illumina).
The paired-end sequencing reads were aligned to the hg38 reference genome using STAR aligner-2.6.0 [22] and gene expression values were quantified by RNA-Seq by Expectation–Maximization (RSEM)-1.3.1 [23]. Tumor purity was calculated using Estimation of STromal and Immune cells in MAlignant Tumor tissues using Expression data (ESTIMATE) [24]. The fractions of immune-associated cell types were calculated by CIBERSORT [25] using the RNA-seq expression profiles.
We considered genes with average fragments per kilobase million (FPKM) of two samples ≥ 5 and log2 (fold change of FPKM) ≥ 2 to be differentially expressed genes (DEGs) between pre-treatment and post-treatment sample for each case. We utilized DAVID bioinformatics resources (DAVID, https://david.ncifcrf.gov/) [26] for performing pathway enrichment analysis using only genes with an average FPKM of two samples ≥ 20 as a differentially expressed gene for input.
Acquisition of gene sets for profile identification
We regarded known cancer genes from the Cancer Gene Census (CGC) [27] as cancer-associated genes. We further inferred oncogenic variants in cancer-associated genes based on COSMIC (Occurrence ≥ 3) [27], ClinVar (CLNSIG = “Pathogenic”) [28], and OncoKB [29]. We examined the profiles of multiple gene sets likely to be involved in AR mechanism such as immune checkpoint (HisgAtlas, http://biokb.ncpsb.org/HisgAtlas/index.php/Home/Browse/) [30], cytolytic activity [31], antigen-presenting machinery (KEGG, https://www.genome.jp/dbget-bin/www_bget?hsa04612) [5, 32], IFN-γ signaling pathway [33], and IFN-γ signature [34].
Statistical analysis
Overall survival was defined as the time from initiating ICI until death, and the progression-free survival was defined as the time from the initiating ICI to disease progression or any cause of death.
Results
Baseline clinical characteristics
The baseline clinical characteristics of the study population are shown in Table 1 and Fig. 1b. The median time to AR was 370 days (range, 210 to 739 days). The patients comprised a case of head and neck squamous cell carcinoma (HNSCC) (#1), a case of nasopharyngeal carcinoma (#3), three cases of renal cell carcinoma (#2, #4, and #6), and a case of invasive urothelial carcinoma of the bladder (#5). Three patients received single ICI and three patients received combination treatment. Clinical course with radiologic images and tissue collection timepoints in six patients were depicted in Fig. 1c and Supplementary Fig. 1a–e. Tumor samples from patient #1, #4, and #5 were obtained just before the initiation of ICI, whereas those from patient #2, #3, and #6 were obtained before an earlier course of therapy. Clinical characteristics of tumor samples are described in Supplementary Table 2.
Immune infiltrates, immune-related markers, tumor microenvironment and their changes during immune checkpoint inhibitor treatment
To explore the immune infiltrates, immune-related markers, and the changes in them before and after ICI, we evaluated the pathologic changes in FFPE samples and performed RNA-seq.
Patient #2, a patient who diagnosed with clear cell renal cell carcinoma underwent right nephrectomy at primary diagnosis followed by right lung metastasectomy after 2 years when there was a disease metastasis in the lung. Since he had very slowly progressive disease, he was started on anti-PD-L1 monotherapy 14 months after an additional resection for lung metastases without preceding chemotherapy. He achieved stable disease after 3 cycles of the treatment. However, nearly 15 months after the treatment, there was an increase in the mass lesions adjacent to the seventh rib. Wedge resection of right upper and lower lobes and video-assisted thoracoscopic rib excision were performed (Fig. 1c). The baseline biopsy sample (#2-pre 1) showed clear cell renal cell carcinoma with minimal tumor-infiltrating lymphocytes (TILs) at the invasive margin (Fig. 2a, Supplementary Fig. 2a–e). PD-L1 expression on the tumor cells was scarce (Fig. 2b). However, multiplex IHC after the onset of AR revealed that intratumoral lymphocytic infiltrates, especially positive for CD8, were markedly increased, whereas PD-L1 expression on tumor cells still remained negative.
Fig. 2.
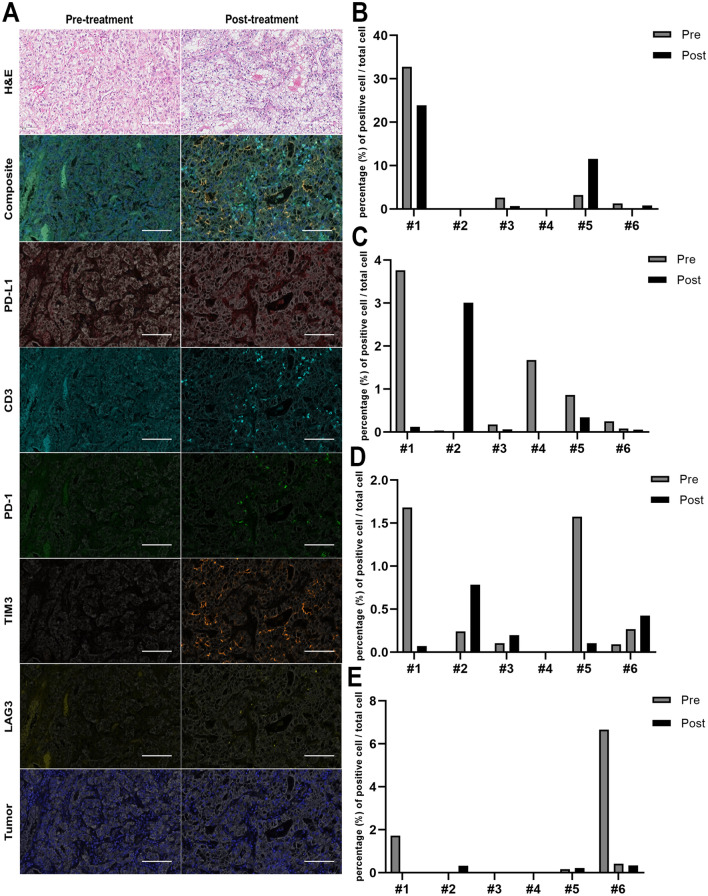
Pathologic evaluation of immune infiltrates, immune-related markers and their changes between pre-treatment and post-treatment. a Hematoxylin and eosin (H&E), PD-L1, CD3, PD-1, LAG3, and TIM3 immunohistochemical staining of the tumors before and after immune checkpoint inhibitor treatment (scale bar, 100 μm). b The percentage of PD-L1-positive cells per total cells in CK-positive cells, c The percentage of PD-1-positive cells, d LAG3-positive cells, and e TIM3-positive cells per total cells in CK-negative, lymphocyte-size-matched cells by multiplex immunohistochemistry. PD-1 programmed death-1, PD-L1 programmed death-ligand 1, CK cytokeratin, LAG3 lymphocyte-activation gene 3, TIM3 T-cell immunoglobulin and mucin-domain containing-3
PD-L1 expression on tumor cells in patient #1 and #3 markedly decreased at the time of AR compared to the baseline, while PD-L1 expression in patient #5 increased 3.5-fold (Fig. 2b). The levels of three TIL markers (CD3, CD4, and CD8) in patient #1 showed a rapid decline from the baseline to the time of AR, which was consistently demonstrated in the RNA-seq data (Supplementary Fig. 2). The proportion of CD3, CD4, and CD8-positive cells were all increased in post-treatment samples compared to pre-treatment samples in patient #3. However, in the other patients (#4, #5, and #6) there was variability in the direction of the change in TILs.
We also investigated the expression of immunosuppressive markers by multiplex IHC (PD-1, LAG3, and TIM3, Fig. 2c–e) and RNA-seq (22 genes) (Fig. 3a). In patient #2, T cell exhaustion markers including PD-1, LAG3, and TIM3 were hardly expressed at baseline whereas they were all elevated in post-treatment samples. Consistent with the multiplex IHC data, similar changes were seen in the RNA-seq data, although elevated expressions of immune activation markers such as cytolytic activity and IFN-γ signature were also observed at the time of AR in patient #2 (Fig. 3a). On the other hand, both immunosuppressive markers as well as immune activation markers were decreased in the post-treatment samples of patient #1.
Fig. 3.
Changes in immune-related genes and components within the tumor microenvironment. FPKM Fragments Per Kilobase of transcripts per Million mapped reads. a Heatmap comparison of the expression of genes related to immune suppression (immune checkpoints) and immune activation, cytolytic activity and IFN-γ-associated signature between pre-treatment and post-treatment samples by RNA sequencing. b Comparison of the absolute infiltration scores of the immune cell population estimated by CIBERSORT between pre-treatment and post-treatment samples
The components of the tumor microenvironment and the changes in it before and after ICI were evaluated using CIBERSORT (Fig. 3b and Supplementary Fig. 3 for absolute mode and relative mode of the CIBERSORT, respectively). Although regulatory T cells are known to play a role in the downregulation of immune responses [35, 36], we did not observe the presence of regulatory T cell in our samples. In contrast, a difference in the abundance of macrophages between pre-treatment and post-treatment samples was observed in some patients. For example, the absolute numbers of M1 and M2 macrophages decreased in the post-treatment sample of patient #1 (Fig. 3b). We also performed multiplex IHC for CD86 (representative of M1 macrophage) and CD163 (representative of M2 macrophage) (Supplementary Fig. 4). According to multiplex IHC analysis, the percentage of CD86-positive cells and CD163-positive cells per total number of cells both decreased in the post-treatment sample of patient #1. From multiplex IHC results, we observed that both M1 and M2 macrophages increased in the post-treatment sample of patient #5, whereas only M2 macrophages increased in the post-treatment sample of patient #2. These findings were inconsistent with the results of CIBERSORT-applied RNAseq data.
Fig. 4.
The genomic landscape of acquired resistance to immune checkpoint inhibitors. a The number of somatic tumor mutations. b Somatic mutation number per megabase (Mb). c The frequency of the mutation spectrum. d Mutation signature. e Acquired resistance-associated somatic mutation profiles (post-treatment only) of cancer genes. Variants in genes marked with bold font mean oncogenic variants inferred from COSMIC, ClinVar, and OncoKB
The genomic landscape of patients with acquired resistance
We analyzed the TMB and mutational signature from the processed WES data. The median numbers of tumor purity before and after ICI were 0.63 and 0.61, respectively (range, 0.47 to 0.73; 0.48 to 0.79, respectively) (Supplementary Table 2). The median somatic TMB in the pre-treatment samples was 3.31 (range 0.74 to 5.5) (Fig. 4a, b). Compared to the TMB in the same cancer types from previously reported data [16], the somatic TMB in our pre-treatment samples was low, except for patient #1 and #3. We also analyzed the change in somatic TMB between pre-treatment and post-treatment samples. In patient #2 and #3, the somatic TMB decreased after the acquisition of resistance. We found increased TMB only in the post-treatment sample of patient #1.
Analysis of the mutation spectrum of the pre-treatment samples showed prominent C > T transitions, as has been demonstrated in many solid cancers (Fig. 4c). The predominant mutational signatures of the pre-treatment samples were mostly retained after ICI, with the exception of two paired cases (patient #3 and #4) (Fig. 4d). Patient #1 exhibited a mutational signature (signature 13) rich in both C > T transitions and C > G transversions at TC dinucleotides, suggestive of being associated with apolipoprotein B mRNA editing catalytic polypeptide-like (APOBEC) mutations [35].
Since we were interested in AR-associated somatic mutations, we identified only the somatic mutations present in post-treatment samples (Fig. 4e and Supplementary Fig. 5). None of the AR-associated somatic mutations showed an overlap between patients. Patient #1 displayed the most numerous missense mutations in genes including PIK3CA and MAP2K1. In the post-treatment sample from patient #1, we identified an E542K missense mutation that lies within the PIK helical domain of the Pik3ca protein (variant allele frequency, 34.1%) (Fig. 5a). This mutation has been known as a hotspot mutation, which is commonly found in breast cancer and colorectal cancer [37, 38]. It has been reported that PIK3CA mutagenesis occurs more commonly in APOBEC-high tumors than in APOBEC-low tumors [39]. An Integrative Genomics Viewer plot displayed that the E542K mutation was observed at a higher frequency in the post-resistance sample than in the pre-treatment sample (Fig. 5b). By pathway enrichment analysis, we found that the PI3K-Akt signaling pathway was enriched in the post-treatment sample of #1 (Fig. 5c). From the IHC analysis for p-AKT and p-S6 in patient #1, both p-AKT and p-S6 increased in post-treatment sample compared to pre-treatment sample (Fig. 5d).
Fig. 5.
Acquired gain-of-function mutation in the PIK3CA gene at the time of resistance and activation of PI3K-Akt signaling pathway. a Integrative genomics viewer plots showing that a PIK3CA E542K missense mutation is found at acquired resistance. b Lollipop plot of PIK3CA E542K mutation located in the helical domain. c The pathway enrichment analysis result showing that PI3K-Akt signaling pathway was enriched in the post-treatment sample of patient #1. d p-AKT (upper) and p-S6 (lower) immunohistochemical staining of the tumors before treatment and at acquired resistance to immune checkpoint inhibitor treatment. Scale bars, 250 μm
Patient #3 had a frameshift deletion mutation in the gene encoding PHOX2B and a stop-gain mutation in the gene encoding AXIN2. Patient #4 had a frameshift deletion mutation in the gene encoding TET2.
From KEGG pathway analysis (Supplementary Fig. 6), we found that the downregulated DEGs were enriched in ‘Alanine, aspartate, glutamate metabolism’ in the post-treatment sample of patient #2. The upregulated DEGs were highly associated with ‘Complement and coagulation cascades’ in patient #3 and were enriched in ‘Rap1 signaling pathway’ in patient #4. Otherwise, significant copy number amplifications and deletions explaining immune escape as a cause of AR were not detected in any patients.
Fig. 6.
Hypothetical diagram showing the development of PIK3CA-mutant clones mediated by APOBEC-signature in HPV-infected head and neck squamous cell carcinoma patient. ICI immune checkpoint inhibitor, APOBEC the apolipoprotein B mRNA editing catalytic polypeptide-like, HPV human papillomavirus
Evaluation of previously known mechanisms of acquired resistance
We aimed to evaluate whether AR in our cohort developed by previously known mechanisms such as defective IFN-γ-related signaling which stimulates JAK-STAT pathway and impaired immuno-recognition due to defective antigen presentation. First, except for a few missense mutations detected in post-treatment samples of patient #1, #4, and #5, no mutations conferring defects in the IFN-γ pathway were detected (Supplementary Fig. 7). No significant trend in changes in the IFN-γ-related gene expression was found. Next, to identify a defect in the antigen-presenting machinery, we analyzed the expression status of HLA class I in CK-positive cells. None of the samples demonstrated the loss of HLA class I by multiplex IHC in post-treatment samples compared to pre-treatment samples (Supplementary Fig. 8a). Moreover, the expression of antigen processing machinery-associated genes including HLA-A, HLA-B, HLA-C, B2M, TAP1, TAP2, and TAPBP was not significantly altered during ICI (Supplementary Fig. 8b). The WES data did not reveal any mutation related to antigen-presenting machinery except for a nonsense mutation in NFYC.
Discussion
Although there has been a rapid increase in the number of studies focusing on the efficacy of immunotherapy, little is known about the mechanism of AR to ICI so far [3]. Majority of the studies exploring the AR mechanisms found that defects in the antigen-presenting machinery [4, 5, 40], loss of neo-antigens [7], or loss-of-function mutations in the IFN-γ signaling pathway [4, 41] may result in the acquisition of AR. Despite these studies, the reason why the majority of patients acquire AR is still unclear. Using WES, RNA-seq, and multiplex IHC in our study, we showed that previously known mechanisms were partly seen in one patient, but the rest showed the different possible mechanism of AR.
We found that the expression levels of immunosuppressive molecules such as TIM3, LAG3, CTLA4, or PD-1 were elevated during the acquisition of resistance against ICI in patient #2. This is consistent with the results in previous literature in which upregulation of TIM3 was observed in the mouse model showing AR to ICI [9]. Although TILs, especially positive for CD8, increased at the time of onset of resistance, immunosuppressive signals via upregulation of alternate immune checkpoints may interact with TILs and impair their function to kill tumor cells [3, 42]. In the above patient, no significant mutations or copy number alterations were detected, so these transcriptional changes in alternative immune checkpoint molecules may be associated with AR. Sequential treatment for inhibiting these immunosuppressive molecules might be a strategy for overcoming AR [9, 43].
An interesting finding in our study was that the tumor clone with PIK3CA E542K mutation expanded significantly during the onset of resistance in the human papillomavirus (HPV)-positive HNSCC patient who had predominant APOBEC features before ICI. APOBEC3 appears to play a role in HPV-associated carcinogenesis by generating somatic mutations on the basis of viral oncoprotein (E6 and E7) expression [44]. In addition, APOBEC-signature, which is characterized by C > T transitions and C > G transversions at TpC dinucleotides [35, 37, 45] is also widely found in HNSCC, especially in HPV-positive HNSCC due to reduced exposure to exogenous carcinogens such as smoking [39]. Among mutations of the PI3K pathway, which are frequently found in HNSCC patients [46, 47], E542K (c.1624G > A) mutation in the helical domain of the PIK3CA gene has been reported to be caused by APOBEC-mediated TCW mutation in HPV-positive HNSCC tumors [39]. It can be explained by the observation that PIK3CA DNA is deaminated by APOBEC3B in vitro [39]. To our knowledge, this is the first study to discover an APOBEC-mediated PIK3CA mutation in a patient who was treated with immunotherapy and showed AR. APOBEC-signature has been known to be associated with a better response to immunotherapy [48, 49]; therefore, APOBEC-high features in patient #1 before ICI could be associated with the initial response to ICI. Our hypothesis was that the newly detected E542K mutation in PIK3CA helical domain may result from APOBEC activity and lead to immune escape at the time of AR by decreasing the immune infiltrates (Fig. 6). As supported by the evidence that PI3K pathway decreases the survival of immune cells [50, 51] and that inhibition of PI3K pathway can increase immune infiltration [35], we observed a significant decrease in the immune cell population in the post-treatment sample of patient #1. Moreover, the post-treatment tumor of patient #1 that harbored a PIK3CA mutation showed an increased rate of TMB, which is consistent with the findings from The Cancer Genome Atlas HNSCC cohort [46]. We propose that PI3K inhibition in addition to ICI may be beneficial to overcome AR to ICI in patient #1.
It has suggested that disruption of antigen presentation could play a key role in immune evasion of patients who developed AR to ICI by the tumor even if the level of cytotoxic CD8-positive T cells remains elevated [4–6, 40]. However, we detected no defects in genes involved in antigen presentation in our samples suggesting that immuno-recognition may be intact at the time of resistance and alternative mechanisms may induce AR. Moreover, any clear evidence of a defect that can lead to decreased sensitivity to IFN-γ, which might have caused AR, was also not detected in our samples. Although we identified three potential pathways - amino acid metabolism in patient #2, complement and coagulation cascades in patient #3, and Rap1 signaling pathway in patient #4 – which might be related to AR to ICI, these had only weak supporting evidence that generates hypothesis. Thus, no evidence of aberrations in these pathways thus implies that different immune escape mechanisms may be present in patient #3, #4, #5, and #6.
This study has several limitations. Firstly, the number of patients included in this study was small due to the unavailability of pre-treatment and post-treatment tissues for WES, RNA-seq, and multiplex IHC. Further prospective studies with sufficient patients who display AR are needed. Secondly, whether PIK3CA mutagenesis can lead to AR or is just a coincident event with tumor progression should be validated in an animal model or in another patient cohort. Third, the discrepancies were found between RNAseq data and multiplex IHC results in some patients (patient #5 and #6). Since we performed bulk-seq in this study, we tried to supplement the limitation of interpreting mRNA expression level obtained by bulk-seq by using the CIBERSORT algorithm. However, single-cell sequencing would be considered in further study to estimate the accurate number of specific immune cells. Fourth, intratumor or intermetastatic tumor heterogeneity may veil possible positive findings associated with AR to ICI. Although methods such as multiple sample acquisitions through serial biopsy or biopsy from different sites could be helpful for considering the tumor heterogeneity issue, they are very limited in a clinical setting. In this study, we have attempted not to underestimate the impact of this heterogeneity on the interpretation of the results. Finally, mutations in tumor samples with relatively low purity might not be detected and missed as false negatives.
Our study demonstrated that the increase in immunosuppressive markers seen at the time of acquisition of resistance might have contributed to AR in a renal cell carcinoma patient in our study. In another HPV-positive HNSCC patient, we found a hotspot mutation in the gene encoding PIK3CA, which may be mediated by an APOBEC-associated signature, and this mechanism might have contributed to AR in this patient. However, the previously reported mechanisms of AR to ICI – defects in antigen presentation or defects of IFN-γ signaling were not detected. To validate the AR mechanisms discovered in our study and to generalize the findings, additional cases with AR to ICI should be evaluated.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This study was supported by a grant of the Korea Health Technology R&D Project “Strategic Center of Cell and Bio Therapy for Heart, Diabetes & Cancer” through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare (MHW), Republic of Korea (grant number : HI17C2085). This study was also supported by Seoul National University Hospital Research Fund (No. 03-2015-0380).
Abbreviations
- ICI
Immune checkpoint inhibitor
- AR
Acquired resistance
- PD-L1
Programmed death-ligand 1
- TMB
Tumor mutational burden
- PD-1
Programmed cell death protein 1
- FFPE
Formalin-fixed and paraffin-embedded
- LAG3
Lymphocyte-activation gene 3
- TIM3
T cell immunoglobulin and mucin-domain containing-3
- HLA
Human Leukocyte Antigen
- DAPI
4′,6-diamidino-2-phenylindole
- WES
Whole Exome Sequencing
- RNA-seq
RNA sequencing
- FPKM
Fragments per kilobase million
- DEG
Differentially expressed genes
- HNSCC
Head and neck squamous cell carcinoma
- TIL
Tumor-infiltrating lymphocyte
- APOBEC
Apolipoprotein B mRNA editing catalytic polypeptide-like
- HPV
Human papillomavirus
Author contributions
Conception and design: SHY, BK, CYO; Development of methodology: SHY, JY, BK, CYO, JIK; Acquisition of data: BK, MK, TMK, DWK, DSH; Analysis and interpretation of data: SHY, JY, SPH, BK, CYO, JK, SK; Administrative, technical, or material support: BK, YKJ, KCJ, JIK; Study supervision: BK, DSH; All authors read and approved the final manuscript.
Funding
This study was supported by a grant of the Korea Health Technology R&D Project “Strategic Center of Cell and Bio Therapy for Heart, Diabetes & Cancer” through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare (MHW), Republic of Korea (Grant Number: HI17C2085). This study was also supported by Seoul National University Hospital Research Fund (No. 03-2015-0380).
Availability of data and materials
The dataset used and/or analyzed during the current study is provided in the supplemental materials and additional materials are available from the corresponding author on reasonable request.
Compliance with ethical standards
Competing interest
The authors report no conflict of interest.
Ethics approval and consent to participate
The study protocol was reviewed and approved by the Institutional Review Board of Seoul National University Hospital (no. H-1809-144-978 and H-1506-092-681). We conducted the study in accordance with the Principles of the Declaration of Helsinki. Informed consent was obtained from all individual participants included in the study.
Consent for publication
Patients signed informed consent regarding publishing their data and photographs.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Shin Hye Yoo and Jihui Yun have contributed equally to this work.
References
- 1.Hugo W, Zaretsky JM, Sun L, Song C, Moreno BH, Hu-Lieskovan S, Berent-Maoz B, Pang J, Chmielowski B, Cherry G, Seja E, Lomeli S, Kong X, Kelley MC, Sosman JA, Johnson DB, Ribas A, Lo RS. Genomic and transcriptomic features of response to Anti-PD-1 therapy in metastatic melanoma. Cell. 2016;165(1):35–44. doi: 10.1016/j.cell.2016.02.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peng W, Chen JQ, Liu C, Malu S, Creasy C, Tetzlaff MT, Xu C, McKenzie JA, Zhang C, Liang X, Williams LJ, Deng W, Chen G, Mbofung R, Lazar AJ, Torres-Cabala CA, Cooper ZA, Chen PL, Tieu TN, Spranger S, Yu X, Bernatchez C, Forget MA, Haymaker C, Amaria R, McQuade JL, Glitza IC, Cascone T, Li HS, Kwong LN, Heffernan TP, Hu J, Bassett RL, Jr, Bosenberg MW, Woodman SE, Overwijk WW, Lizee G, Roszik J, Gajewski TF, Wargo JA, Gershenwald JE, Radvanyi L, Davies MA, Hwu P. Loss of PTEN promotes resistance to t cell-mediated immunotherapy. Cancer Discov. 2016;6(2):202–216. doi: 10.1158/2159-8290.CD-15-0283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gide TN, Wilmott JS, Scolyer RA, Long GV. Primary and acquired resistance to immune checkpoint inhibitors in metastatic melanoma. Clin Cancer Res. 2018;24(6):1260–1270. doi: 10.1158/1078-0432.CCR-17-2267. [DOI] [PubMed] [Google Scholar]
- 4.Zaretsky JM, Garcia-Diaz A, Shin DS, Escuin-Ordinas H, Hugo W, Hu-Lieskovan S, Torrejon DY, Abril-Rodriguez G, Sandoval S, Barthly L, Saco J, Homet Moreno B, Mezzadra R, Chmielowski B, Ruchalski K, Shintaku IP, Sanchez PJ, Puig-Saus C, Cherry G, Seja E, Kong X, Pang J, Berent-Maoz B, Comin-Anduix B, Graeber TG, Tumeh PC, Schumacher TN, Lo RS, Ribas A. Mutations associated with acquired resistance to pd-1 blockade in melanoma. N Engl J Med. 2016;375(9):819–829. doi: 10.1056/NEJMoa1604958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gettinger S, Choi J, Hastings K, Truini A, Datar I, Sowell R, Wurtz A, Dong W, Cai G, Melnick MA, Du VY, Schlessinger J, Goldberg SB, Chiang A, Sanmamed MF, Melero I, Agorreta J, Montuenga LM, Lifton R, Ferrone S, Kavathas P, Rimm DL, Kaech SM, Schalper K, Herbst RS, Politi K. Impaired HLA class i antigen processing and presentation as a mechanism of acquired resistance to immune checkpoint inhibitors in lung cancer. Cancer Discov. 2017;7(12):1420–1435. doi: 10.1158/2159-8290.CD-17-0593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tran E, Robbins PF, Lu YC, Prickett TD, Gartner JJ, Jia L, Pasetto A, Zheng Z, Ray S, Groh EM, Kriley IR, Rosenberg SA. T-Cell transfer therapy targeting mutant kras in cancer. N Engl J Med. 2016;375(23):2255–2262. doi: 10.1056/NEJMoa1609279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anagnostou V, Smith KN, Forde PM, Niknafs N, Bhattacharya R, White J, Zhang T, Adleff V, Phallen J, Wali N, Hruban C, Guthrie VB, Rodgers K, Naidoo J, Kang H, Sharfman W, Georgiades C, Verde F, Illei P, Li QK, Gabrielson E, Brock MV, Zahnow CA, Baylin SB, Scharpf RB, Brahmer JR, Karchin R, Pardoll DM, Velculescu VE. Evolution of neoantigen landscape during immune checkpoint blockade in non-small cell lung cancer. Cancer Discov. 2017;7(3):264–276. doi: 10.1158/2159-8290.CD-16-0828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Efremova M, Rieder D, Klepsch V, Charoentong P, Finotello F, Hackl H, Hermann-Kleiter N, Lower M, Baier G, Krogsdam A, Trajanoski Z. Targeting immune checkpoints potentiates immunoediting and changes the dynamics of tumor evolution. Nat Commun. 2018;9(1):32. doi: 10.1038/s41467-017-02424-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koyama S, Akbay EA, Li YY, Herter-Sprie GS, Buczkowski KA, Richards WG, Gandhi L, Redig AJ, Rodig SJ, Asahina H, Jones RE, Kulkarni MM, Kuraguchi M, Palakurthi S, Fecci PE, Johnson BE, Janne PA, Engelman JA, Gangadharan SP, Costa DB, Freeman GJ, Bueno R, Hodi FS, Dranoff G, Wong KK, Hammerman PS. Adaptive resistance to therapeutic PD-1 blockade is associated with upregulation of alternative immune checkpoints. Nat Commun. 2016;7:10501. doi: 10.1038/ncomms10501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Syn NL, Teng MWL, Mok TSK, Soo RA. De-novo and acquired resistance to immune checkpoint targeting. Lancet Oncol. 2017;18(12):e731–e741. doi: 10.1016/S1470-2045(17)30607-1. [DOI] [PubMed] [Google Scholar]
- 11.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 11) Eur J Cancer. 2009;45(2):228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 12.Kim S, Jang JY, Koh J, Kwon D, Kim YA, Paeng JC, Ock CY, Keam B, Kim M, Kim TM, Heo DS, Chung DH, Jeon YK. Programmed cell death ligand-1-mediated enhancement of hexokinase 2 expression is inversely related to T-cell effector gene expression in non-small-cell lung cancer. J Exp Clin Cancer Res. 2019;38(1):462. doi: 10.1186/s13046-019-1407-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li H, Durbin R. Fast and accurate long-read alignment with Burrows-Wheeler transform. Bioinformatics. 2010;26(5):589–595. doi: 10.1093/bioinformatics/btp698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McKenna A, Hanna M, Banks E, Sivachenko A, Cibulskis K, Kernytsky A, Garimella K, Altshuler D, Gabriel S, Daly M, DePristo MA. The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010;20(9):1297–1303. doi: 10.1101/gr.107524.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang K, Li M, Hakonarson H. ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res. 2010;38(16):e164. doi: 10.1093/nar/gkq603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chalmers ZR, Connelly CF, Fabrizio D, Gay L, Ali SM, Ennis R, Schrock A, Campbell B, Shlien A, Chmielecki J, Huang F, He Y, Sun J, Tabori U, Kennedy M, Lieber DS, Roels S, White J, Otto GA, Ross JS, Garraway L, Miller VA, Stephens PJ, Frampton GM. Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Med. 2017;9(1):34. doi: 10.1186/s13073-017-0424-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosenthal R, McGranahan N, Herrero J, Taylor BS, Swanton C. DeconstructSigs: delineating mutational processes in single tumors distinguishes DNA repair deficiencies and patterns of carcinoma evolution. Genome Biol. 2016;17:31. doi: 10.1186/s13059-016-0893-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, Sun Y, Jacobsen A, Sinha R, Larsson E, Cerami E, Sander C, Schultz N. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6(269):pl1. doi: 10.1126/scisignal.2004088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E, Antipin Y, Reva B, Goldberg AP, Sander C, Schultz N. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2(5):401–404. doi: 10.1158/2159-8290.CD-12-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.D’Aurizio R, Pippucci T, Tattini L, Giusti B, Pellegrini M, Magi A. Enhanced copy number variants detection from whole-exome sequencing data using EXCAVATOR2. Nucleic Acids Res. 2016;44(20):e154. doi: 10.1093/nar/gkw695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Talevich E, Shain AH, Botton T, Bastian BC. CNVkit: genome-wide copy number detection and visualization from targeted DNA sequencing. PLoS Comput Biol. 2016;12(4):e1004873. doi: 10.1371/journal.pcbi.1004873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, Batut P, Chaisson M, Gingeras TR. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29(1):15–21. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li B, Dewey CN. RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinformatics. 2011;12:323. doi: 10.1186/1471-2105-12-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yoshihara K, Shahmoradgoli M, Martinez E, Vegesna R, Kim H, Torres-Garcia W, Trevino V, Shen H, Laird PW, Levine DA, Carter SL, Getz G, Stemke-Hale K, Mills GB, Verhaak RG. Inferring tumour purity and stromal and immune cell admixture from expression data. Nat Commun. 2013;4:2612. doi: 10.1038/ncomms3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Newman AM, Liu CL, Green MR, Gentles AJ, Feng W, Xu Y, Hoang CD, Diehn M, Alizadeh AA. Robust enumeration of cell subsets from tissue expression profiles. Nat Methods. 2015;12(5):453–457. doi: 10.1038/nmeth.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.da Huang W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4(1):44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 27.Tate JG, Bamford S, Jubb HC, Sondka Z, Beare DM, Bindal N, Boutselakis H, Cole CG, Creatore C, Dawson E, Fish P, Harsha B, Hathaway C, Jupe SC, Kok CY, Noble K, Ponting L, Ramshaw CC, Rye CE, Speedy HE, Stefancsik R, Thompson SL, Wang S, Ward S, Campbell PJ, Forbes SA. COSMIC: the Catalogue Of Somatic Mutations In Cancer. Nucleic Acids Res. 2019;47(D1):D941–D947. doi: 10.1093/nar/gky1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Landrum MJ, Lee JM, Benson M, Brown G, Chao C, Chitipiralla S, Gu B, Hart J, Hoffman D, Hoover J, Jang W, Katz K, Ovetsky M, Riley G, Sethi A, Tully R, Villamarin-Salomon R, Rubinstein W, Maglott DR. ClinVar: public archive of interpretations of clinically relevant variants. Nucleic Acids Res. 2016;44(D1):D862–868. doi: 10.1093/nar/gkv1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chakravarty D, Gao J, Phillips SM, Kundra R, Zhang H, Wang J, Rudolph JE, Yaeger R, Soumerai T, Nissan MH, Chang MT, Chandarlapaty S, Traina TA, Paik PK, Ho AL, Hantash FM, Grupe A, Baxi SS, Callahan MK, Snyder A, Chi P, Danila D, Gounder M, Harding JJ, Hellmann MD, Iyer G, Janjigian Y, Kaley T, Levine DA, Lowery M, Omuro A, Postow MA, Rathkopf D, Shoushtari AN, Shukla N, Voss M, Paraiso E, Zehir A, Berger MF, Taylor BS, Saltz LB, Riely GJ, Ladanyi M, Hyman DM, Baselga J, Sabbatini P, Solit DB, Schultz N. OncoKB: a precision oncology knowledge base. JCO Precis Oncol. 2017 doi: 10.1200/PO.17.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu Y, He M, Wang D, Diao L, Liu J, Tang L, Guo S, He F, Li D. HisgAtlas 1.0: a human immunosuppression gene database. Database (Oxford) 2017 doi: 10.1093/database/bax094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rooney MS, Shukla SA, Wu CJ, Getz G, Hacohen N. Molecular and genetic properties of tumors associated with local immune cytolytic activity. Cell. 2015;160(1–2):48–61. doi: 10.1016/j.cell.2014.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kanehisa M, Goto S. KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000;28(1):27–30. doi: 10.1093/nar/28.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gao J, Shi LZ, Zhao H, Chen J, Xiong L, He Q, Chen T, Roszik J, Bernatchez C, Woodman SE, Chen PL, Hwu P, Allison JP, Futreal A, Wargo JA, Sharma P. Loss of IFN-gamma pathway genes in tumor cells as a mechanism of resistance to anti-CTLA-4 therapy. Cell. 2016;167(2):397–404. doi: 10.1016/j.cell.2016.08.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ayers M, Lunceford J, Nebozhyn M, Murphy E, Loboda A, Kaufman DR, Albright A, Cheng JD, Kang SP, Shankaran V, Piha-Paul SA, Yearley J, Seiwert TY, Ribas A, McClanahan TK. IFN-gamma-related mRNA profile predicts clinical response to PD-1 blockade. J Clin Invest. 2017;127(8):2930–2940. doi: 10.1172/JCI91190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alexandrov LB, Nik-Zainal S, Wedge DC, Aparicio SA, Behjati S, Biankin AV, Bignell GR, Bolli N, Borg A, Borresen-Dale AL, Boyault S, Burkhardt B, Butler AP, Caldas C, Davies HR, Desmedt C, Eils R, Eyfjord JE, Foekens JA, Greaves M, Hosoda F, Hutter B, Ilicic T, Imbeaud S, Imielinski M, Jager N, Jones DT, Jones D, Knappskog S, Kool M, Lakhani SR, Lopez-Otin C, Martin S, Munshi NC, Nakamura H, Northcott PA, Pajic M, Papaemmanuil E, Paradiso A, Pearson JV, Puente XS, Raine K, Ramakrishna M, Richardson AL, Richter J, Rosenstiel P, Schlesner M, Schumacher TN, Span PN, Teague JW, Totoki Y, Tutt AN, Valdes-Mas R, van Buuren MM, van’t Veer L, Vincent-Salomon A, Waddell N, Yates LR, Australian Pancreatic Cancer Genome I, Consortium IBC, Consortium IM-S. PedBrain I, Zucman-Rossi J, Futreal PA, McDermott U, Lichter P, Meyerson M, Grimmond SM, Siebert R, Campo E, Shibata T, Pfister SM, Campbell PJ, Stratton MR. Signatures of mutational processes in human cancer. Nature. 2013;500(7463):415–421. doi: 10.1038/nature12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arce Vargas F, Furness AJS, Solomon I, Joshi K, Mekkaoui L, Lesko MH, Miranda Rota E, Dahan R, Georgiou A, Sledzinska A, Ben Aissa A, Franz D, Werner Sunderland M, Wong YNS, Henry JY, O’Brien T, Nicol D, Challacombe B, Beers SA, Melanoma TC, Renal TC, Lung TC, Turajlic S, Gore M, Larkin J, Swanton C, Chester KA, Pule M, Ravetch JV, Marafioti T, Peggs KS, Quezada SA. Fc-optimized anti-CD25 depletes tumor-infiltrating regulatory t cells and synergizes with PD-1 blockade to eradicate established tumors. Immunity. 2017;46(4):577–586. doi: 10.1016/j.immuni.2017.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roberts SA, Lawrence MS, Klimczak LJ, Grimm SA, Fargo D, Stojanov P, Kiezun A, Kryukov GV, Carter SL, Saksena G, Harris S, Shah RR, Resnick MA, Getz G, Gordenin DA. An APOBEC cytidine deaminase mutagenesis pattern is widespread in human cancers. Nat Genet. 2013;45(9):970–976. doi: 10.1038/ng.2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nik-Zainal S, Alexandrov LB, Wedge DC, Van Loo P, Greenman CD, Raine K, Jones D, Hinton J, Marshall J, Stebbings LA, Menzies A, Martin S, Leung K, Chen L, Leroy C, Ramakrishna M, Rance R, Lau KW, Mudie LJ, Varela I, McBride DJ, Bignell GR, Cooke SL, Shlien A, Gamble J, Whitmore I, Maddison M, Tarpey PS, Davies HR, Papaemmanuil E, Stephens PJ, McLaren S, Butler AP, Teague JW, Jonsson G, Garber JE, Silver D, Miron P, Fatima A, Boyault S, Langerod A, Tutt A, Martens JW, Aparicio SA, Borg A, Salomon AV, Thomas G, Borresen-Dale AL, Richardson AL, Neuberger MS, Futreal PA, Campbell PJ, Stratton MR, Breast Cancer Working Group of the International Cancer Genome C Mutational processes molding the genomes of 21 breast cancers. Cell. 2012;149(5):979–993. doi: 10.1016/j.cell.2012.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Henderson S, Chakravarthy A, Su X, Boshoff C, Fenton TR. APOBEC-mediated cytosine deamination links PIK3CA helical domain mutations to human papillomavirus-driven tumor development. Cell Rep. 2014;7(6):1833–1841. doi: 10.1016/j.celrep.2014.05.012. [DOI] [PubMed] [Google Scholar]
- 40.Paulson KG, Voillet V, McAfee MS, Hunter DS, Wagener FD, Perdicchio M, Valente WJ, Koelle SJ, Church CD, Vandeven N, Thomas H, Colunga AG, Iyer JG, Yee C, Kulikauskas R, Koelle DM, Pierce RH, Bielas JH, Greenberg PD, Bhatia S, Gottardo R, Nghiem P, Chapuis AG. Acquired cancer resistance to combination immunotherapy from transcriptional loss of class I HLA. Nat Commun. 2018;9(1):3868. doi: 10.1038/s41467-018-06300-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Benci JL, Xu B, Qiu Y, Wu TJ, Dada H, Twyman-Saint Victor C, Cucolo L, Lee DSM, Pauken KE, Huang AC, Gangadhar TC, Amaravadi RK, Schuchter LM, Feldman MD, Ishwaran H, Vonderheide RH, Maity A, Wherry EJ, Minn AJ. Tumor interferon signaling regulates a multigenic resistance program to immune checkpoint blockade. Cell. 2016;167(6):1540–1554. doi: 10.1016/j.cell.2016.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sharma P, Hu-Lieskovan S, Wargo JA, Ribas A. Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell. 2017;168(4):707–723. doi: 10.1016/j.cell.2017.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Taube JM, Young GD, McMiller TL, Chen S, Salas JT, Pritchard TS, Xu H, Meeker AK, Fan J, Cheadle C, Berger AE, Pardoll DM, Topalian SL. Differential expression of immune-regulatory genes associated with PD-L1 display in melanoma: implications for PD-1 pathway blockade. Clin Cancer Res. 2015;21(17):3969–3976. doi: 10.1158/1078-0432.CCR-15-0244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smith NJ, Fenton TR. The APOBEC3 genes and their role in cancer: insights from human papillomavirus. J Mol Endocrinol. 2019 doi: 10.1530/JME-19-0011. [DOI] [PubMed] [Google Scholar]
- 45.Alexandrov LB, Nik-Zainal S, Wedge DC, Campbell PJ, Stratton MR. Deciphering signatures of mutational processes operative in human cancer. Cell Rep. 2013;3(1):246–259. doi: 10.1016/j.celrep.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lui VW, Hedberg ML, Li H, Vangara BS, Pendleton K, Zeng Y, Lu Y, Zhang Q, Du Y, Gilbert BR, Freilino M, Sauerwein S, Peyser ND, Xiao D, Diergaarde B, Wang L, Chiosea S, Seethala R, Johnson JT, Kim S, Duvvuri U, Ferris RL, Romkes M, Nukui T, Kwok-Shing Ng P, Garraway LA, Hammerman PS, Mills GB, Grandis JR. Frequent mutation of the PI3K pathway in head and neck cancer defines predictive biomarkers. Cancer Discov. 2013;3(7):761–769. doi: 10.1158/2159-8290.CD-13-0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lechner M, Frampton GM, Fenton T, Feber A, Palmer G, Jay A, Pillay N, Forster M, Cronin MT, Lipson D, Miller VA, Brennan TA, Henderson S, Vaz F, O’Flynn P, Kalavrezos N, Yelensky R, Beck S, Stephens PJ, Boshoff C. Targeted next-generation sequencing of head and neck squamous cell carcinoma identifies novel genetic alterations in HPV + and HPV- tumors. Genome Med. 2013;5(5):49. doi: 10.1186/gm453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Boichard A, Pham TV, Yeerna H, Goodman A, Tamayo P, Lippman S, Frampton GM, Tsigelny IF, Kurzrock R. APOBEC-related mutagenesis and neo-peptide hydrophobicity: implications for response to immunotherapy. Oncoimmunology. 2019;8(3):1550341. doi: 10.1080/2162402X.2018.1550341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang S, Jia M, He Z, Liu XS. APOBEC3B and APOBEC mutational signature as potential predictive markers for immunotherapy response in non-small cell lung cancer. Oncogene. 2018;37(29):3924–3936. doi: 10.1038/s41388-018-0245-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Camacho M, Leon X, Fernandez-Figueras MT, Quer M, Vila L. Prostaglandin E(2) pathway in head and neck squamous cell carcinoma. Head Neck. 2008;30(9):1175–1181. doi: 10.1002/hed.20850. [DOI] [PubMed] [Google Scholar]
- 51.Whiteside TL, Jackson EK. Adenosine and prostaglandin e2 production by human inducible regulatory T cells in health and disease. Front Immunol. 2013;4:212. doi: 10.3389/fimmu.2013.00212. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The dataset used and/or analyzed during the current study is provided in the supplemental materials and additional materials are available from the corresponding author on reasonable request.