Abstract
Background
In patients with advanced clear cell renal cell carcinoma, despite the undoubted benefits from immune checkpoint inhibitor (ICI)-based therapies over monotherapies of angiogenic/mTOR inhibitors in the intention-to-treat population, approximately a quarter of the patients can scarcely gain advantage from ICIs, prompting the search for predictive biomarkers for patient selection.
Methods
Clinical and multi-omic data of 2428 ccRCC patients were obtained from The Cancer Genome Atlas (TCGA, n = 537), JAVELIN Renal 101 (avelumab plus axitinib vs. sunitinib, n = 885), and CheckMate-009/010/025 (nivolumab vs. everolimus, n = 1006).
Results
BAP1 mutations were associated with large progression-free survival (PFS) benefits from ICI-based immunotherapies over sunitinib/everolimus (pooled estimate of interaction HR = 0.71, 95% CI 0.51–0.99, P = 0.045). Using the top 20 BAP1 mutation-associated differentially expressed genes (DEGs) generated from the TCGA cohort, we developed the BAP1-score, negatively correlated with angiogenesis and positively correlated with multiple immune-related signatures concerning immune cell infiltration, antigen presentation, B/T cell receptor, interleukin, programmed death-1, and interferon. A high BAP1-score indicated remarkable PFS benefits from ICI-based immunotherapies over angiogenic/mTOR inhibitors (avelumab plus axitinib vs. sunitinib: HR = 0.55, 95% CI 0.43–0.70, P < 0.001; nivolumab vs. everolimus: HR = 0.72, 95% CI 0.52–1.00, P = 0.045), while these benefits were negligible in the low BAP1-score subgroup (HR = 1.16 and 1.02, respectively).
Conclusion
In advanced ccRCCs, the BAP1-score is a biologically and clinically significant predictor of immune microenvironment and the clinical benefits from ICI-based immunotherapies over angiogenic/mTOR inhibitors, demonstrating its potential utility in optimizing the personalized therapeutic strategies in patients with advanced ccRCC.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00262-023-03424-4.
Keywords: Clear cell renal cell carcinoma, Immunotherapy, The BAP1-score, Predictive biomarker
Background
Immune checkpoint inhibitors (ICIs) including monoclonal antibodies binding to programmed cell death-1 (PD-1), programmed death-ligand 1 (PD-L1), and cytotoxic T-cell lymphocyte-4 (CTLA-4) have revolutionized the treatment landscape of advanced/metastatic clear cell renal cell carcinoma (ccRCC). Compared to the monotherapies of tyrosine kinase inhibitors (TKIs) targeting vascular endothelial growth factor receptor (VEGFR) and mTOR inhibitors, ICI-based combination therapies prolong survival in the intention-to-treat population [1–8]. However, approximately a quarter of the patients can scarcely gain an advantage from the additional ICI [1–8], prompting the search for predictive biomarkers for patient selection.
In contrast to other tumor types that respond well to immunotherapy, PD-L1 and tumor mutational burden (TMB) exhibited little predictive utility in ccRCCs [4, 5, 9–18]. Their biggest drawbacks as predictive biomarkers are the variety of testing methods and different definitions of positivity [19, 20]. Previous studies have demonstrated the associations of better response to ICIs with the mutations of PBRM1 [10, 16, 21] and DNA damage repair (DDR) genes [11, 12]. PBRM1-mutant ccRCCs have a more angiogenic and less immunogenic phenotype [10, 22, 23]. Although multiple retrospective analyses have consistently shown that PBRM1 mutations might be associated with immunotherapy response in the post-VEGFR TKI treatment setting [13, 14, 24], their predictive power remains to be further determined. As for DDR mutations, unlike other solid tumors with a positive correlation between DDR mutations and immune activation, they were associated with an immunosuppressive microenvironment in ccRCCs [25], and their predictive utility has not been validated in a randomized study involving ICIs and other control agents.
Currently, no validated predictive biomarker was used to guide treatment selection for patients with ccRCC. To demonstrate that a biomarker is predictive of treatment benefits, the study requires biomarker status on all patients as well as patients who were treated with the agent of interest and patients not so treated, preferably in the context of a randomized study. JAVELIN Renal 101 (n = 885) and CheckMate-009/010/025 (n = 1006) with a large sample size and available multi-omics data are robust enough to yield insight.
In the original articles concerning the biomarker analysis of these trials, researchers identified “qualitative” predictive biomarkers that were significantly linked with survival in the immunotherapy arm but not in the control arm [10, 14]. However, the difference in the association of a biomarker with survival across treatment arms is the essential proof of its predictive utility [26, 27]. For example, if a biomarker is non-significantly associated with better survival in the immunotherapy arm and poorer survival in the control arm while the difference between these two associations is significant (assessed by interaction), it will be determined as a “quantitative” rather than “qualitative” predictive biomarker [27]. We aimed to re-analyze these two trials in search of “quantitative” biomarkers predicting the benefits from ICI-based therapies to VEGFR/mTOR inhibitors in ccRCCs.
Methods
Study design and data acquisition
In total, 2428 ccRCC patients from The Cancer Genome Atlas-Kidney Renal Clear Cell Carcinoma (TCGA-KIRC, n = 537) and four clinical trials including the JAVELIN Renal 101 (abbreviated as “JAVELIN”, phase III, avelumab + axitinib vs. sunitinib, n = 885), the CheckMate-009 (CM-009, phase I, nivolumab, n = 35), the CheckMate-010 (CM-010, phase II, nivolumab, n = 168), and the CheckMate-025 (CM-025, phase III, nivolumab vs. everolimus, n = 803) were analyzed in the present study. The source of data, clinical setting, regimen, number of patients, treatment lines, outcomes, PD-L1 immunohistochemical (IHC) staining, and next-generation sequencing testing (including whole-exome sequencing [WES] and RNA-seq) of each cohort are shown in Supplementary Table 1. The detailed methods of trial design and IHC/WES/RNA-seq testing of the analyzed trials were described in ClinicalTrials.gov and the original manuscripts [8, 10, 14, 18]. The TCGA-KIRC dataset and the results of immunogenomics analysis from Thorsson et al. [28] were analyzed to explore immune-related mechanisms. Outcomes, mutational analysis, and definition of pathways are shown in Supplementary Methods. The RNA-seq data analyzed in our study were processed (transcripts per million, TPM) for each cohort. The predictive power of the mutations in VHL, MTOR, PBRM1, SETD2, and BAP1 in the CheckMate-214 trial (CM-214, phase III, nivolumab plus ipilimumab vs. sunitinib) were estimated by measuring the hazard ratio (HR) and 95% confidence interval (CI) in the original paper [21].
Our study is following the principles of the Declaration of Helsinki and was approved by the Institution Review Board of Chinese PLA General Hospital (ChiCTR2000030405). This study analyzed the data of public cohorts and therefore informed consents are unnecessary. This report follows the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Calculation of the BAP1-score and other pathway scores
By comparing the BAP1-mutant and the BAP1-WT ccRCCs without receiving neoadjuvant therapy in the TCGA cohort, 45 transcripts with a log ratio above 2 or below -2 and an adjusted p value below 0.05 were identified. Of these, we selected ten upregulated (MOCOS, KY, IL20RB, PPP1R1A, CYP2C9, SPTBN2, SAA1, F10, GJB4, INHBE) and ten downregulated (TRHDE, TRHDE-AS1, SNAP25, ASXL3, CYP39A1, ALPL, CHST9, MASP1, SERPINA6, MGAM) differentially expressed genes (DEGs) with the highest significance and calculated the BAP1-score by subtracting the single sample gene set enrichment analysis (ssGSEA) score of the ten downregulated DEGs from the one of the ten upregulated DEGs [29, 30].
Based on the transcriptomic data of the JAVELIN, CM-009/010/025, and TCGA-KIRC cohorts, ssGSEA was introduced to estimate (1) the infiltration of 28 immune cell types [31], (2) the enrichment of 186 KEGG and 1603 Reactome signatures, and (3) previously published signatures for predicting immunotherapy efficacy in ccRCCs, including JAVELIN Immuno, JAVELIN Angio [14], IMmotion Myeloid, Immotion Teff, and Immotion Angio [24].
Statistical analysis
To assess the between-group difference, we performed (1) Fisher exact test and Chi-square test for categorical variables, (2) Mann–Whitney test for continuous variables, and (3) Kaplan–Meier (KM) method, Log-rank method, and Cox regression (HR and 95% CI) for survival variables. Unsupervised clustering was performed using the R package “ConsensusClusterPlus” with the “hc” method. The variables with a p value below 0.10 in the univariable analysis were included in the following multivariable model. Comparisons of hazard ratios for calculating interactions were performed as previously described [26]. Meta-analysis was performed using the inverse-variance model (fixed effect).
All statistical analyses mentioned above were performed using IBM SPSS Statistics 22 or R 4.1.0. The nominal level of significance was set as 5%, and all 95% Cis were 2-sided. Adjusted P value (Q value) was calculated using the Benjamini and Hochberg method.
Results
BAP1 mutations predict the benefits from ICI-based therapies over VEGFR/mTOR inhibitors
As for the common mutations (frequency ≥ 3% in the TCGA-KIRC dataset) and the mutational alterations in key pathways (e.g., DNA damage repair, hypoxia, chromatin remodeling, etc.), their associations with the PFS benefits from ICI-based therapies over sunitinib/everolimus in the JAVELIN and the CM-009/010/025 cohorts were analyzed using patient-level data [10, 14]. In the JAVELIN cohort, the PFS benefit from avelumab plus axitinib over sunitinib was significantly larger in the BAP1-mutant (interaction HR = 0.56, P = 0.049) or FAT1-4-mutant patients (interaction HR = 0.64, P = 0.040) and smaller in the PTEN-mutant ones (interaction HR = 2.33, P = 0.018, Fig. 1A). Of these three mutational events, only BAP1 mutations exhibited the similar trend in the CM-009/010/025 cohort (interaction HR = 0.85, P = 0.53, Fig. 1A) as it did in the JAVELIN cohort. The treatment effects in the BAP1-mutant and BAP1-wildtype groups of these two cohorts are illustrated in Fig. 1B. The benefits from ICI-based therapies over sunitinib/everolimus were higher in the BAP1-mutant group (HR = 0.61, 95% CI 0.43–0.86, P = 0.004) compared with the BAP1-wildtype group (HR = 0.80, 95% CI 0.68–0.93, P = 0.005).
Fig. 1.
BAP1 mutations predict favorable benefit from ICI-based therapies over VEGFR/mTOR inhibitors. A The interaction effects between mutational events and treatment choices in the JAVELIN, the CM-009/010/025, and the CM-214 cohorts, and their pooled estimates. B Kaplan–Meier curves illustrating the PFS benefits from ICI-based therapies over VEGFR/mTOR inhibitors in the BAP1-mutant and the BAP1-wildtype subgroups. C Meta-analysis of the interaction HRs of BAP1 mutations in the three cohorts
For further validation, we assessed the predictive power of BAP1 mutations in the CM-214 trial by measuring the HR and 95% CI in the published article concerning biomarker analysis (interaction HR = 0.71, P = 0.35) [21], consistent with the results in the previous two cohorts. Furthermore, the three interaction HRs in the JAVELIN, the CM-009/010/025, and the CM-214 cohorts were combined by meta-analysis, and the pooled interaction HR was 0.71 (95% CI 0.51–0.99, P = 0.045, Fig. 1C). The heterogeneity among three datasets was not significant, suggesting that the three interaction effects were comparable.
Despite that BAP1 mutations can predict larger benefits from ICI-based therapies over VEGFR/mTOR inhibitors in ccRCCs, limited but significant benefits in the BAP1-wildtype patients were observed. We sought to further identify the BAP1 mutation-associated DEGs and then to develop a gene expression score (GES) for recognizing the BAP1-mutant-like ccRCCs from the BAP1-wildtype subpopulation. We anticipated that this score can effectively distinguish between those who can benefit from ICI-based therapies over VEGFR/mTOR inhibitors and those who cannot.
BAP1 mutation-associated signature defines immune contexture in both early-stage and late-stage ccRCCs
BAP1, as a chromatin regulator, was associated with an altered expression pattern of a large number of genes [32]. By comparison of BAP1-mutant and -wildtype stage I-IV ccRCC samples in the TCGA-KIRC cohort, 45 transcripts with a log ratio above 2 or below -2 and an adjusted p value below 0.05 were identified (Fig. 2A). Of these, we selected ten upregulated and ten downregulated DEGs with the highest significance (marked by the blue and pink rectangles in Fig. 2A, Supplementary Table 2) for further analysis. Although the TCGA dataset is dominated by stage I-III ccRCCs, these 20 DEGs were expressed differently between the BAP1-mutant and -wildtype samples in both stage I-III and stage IV ccRCCs (P < 0.05, Fig. 2B), suggesting its applicability for both the early- and late-stage ccRCCs.
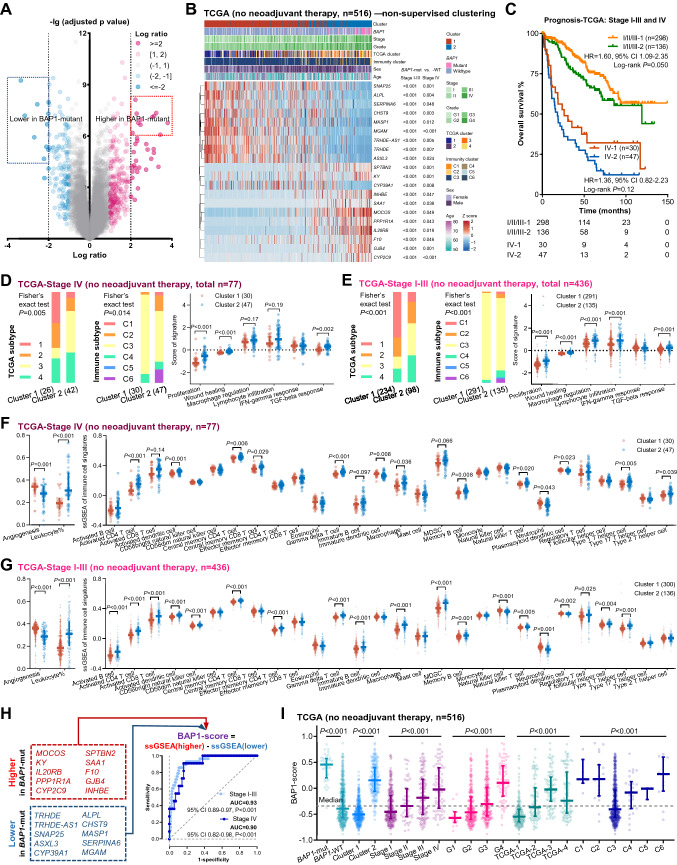
Fig. 2.
Association between BAP1-associated genes and immune characteristics in the TCGA cohort. A Volcano plot illustrating association between gene expression and BAP1 mutations. Ten most significant upregulated genes and ten most significant downregulated genes with a log ratio above 2 or below -2 are highlighted by a pink box and a blue box, respectively. B Heatmap illustrating the results of non-supervised clustering based on the twenty BAP1 mutation-associated differentially expressed genes. C Kaplan–Meier curves illustrating the overall survival of the ccRCC patients grouped by TNM stage and the cluster. D, E Associations of the cluster with TCGA subtype, immune subtype, and six immune subtype-related signatures in the stage IV (D) and stage I-III (E) ccRCCs without receiving neoadjuvant therapy. F, G Associations of the cluster with a angiogenesis signature and immune cell infiltration in the stage IV (F) and stage I-III (G) ccRCCs. H The formula of the BAP1-score and the ROC curves indicating the associations of BAP1 mutations with the BAP1-score and BAP1 mRNA. I The associations of the BAP1-score with BAP1 mutations, the cluster, TNM stage, histological grade, TCGA subtype, and immune subtype in the ccRCCs without receiving neoadjuvant therapy. Abbreviations: ccRCC = clear cell renal cell carcinoma, ROC = receiver operating characteristic, ssGSEA = single sample gene set enrichment analysis
Non-supervised clustering based on the 20 DEGs dichotomized the ccRCCs in the TCGA-KIRC cohort without receiving neoadjuvant therapy (Fig. 2B). BAP1 mutations were enriched in the cluster 2 (P < 0.001), and the OS of the cluster 2 trended poorer in both early-stage and late-stage ccRCCs (Fig. 2C). The cluster 2 was associated with (1) lower proportions of the m1 cluster in the TCGA Research Network study [32] and (2) a lower proportion of the C3 group in the Thorsson’s study (Fig. 2D, E) [33]. BAP1 mutations were enriched in the m2 (10.0%), m3 (10.7%), and m4 (18.1%) clusters compared with the m1 cluster (3.9%). The C3 group in the Thorsson’s study was characterized by better survival, elevated Th1 and Th17 genes, and less immune editing [33]. Of the six key signatures supporting the classification in the Thorsson’s study, the cluster 2 was associated with increased levels of all signatures except the one related to interferon-gamma response (Fig. 2D, E). In addition, the cluster 2 exhibited a lower level of angiogenesis signature and a higher level of leukocyte infiltration, including activated B, CD4, CD8, and dendritic cells (Fig. 2F, G). All the associations described above were consistent in early-stage and late-stage ccRCCs.
Given this, we developed the BAP1-score by calculating the difference between the ssGSEA score of ten highly expressed genes and the ssGSEA score of ten poorly expressed genes (Fig. 2H). The BAP1-score showed great utility in identifying BAP1 mutations in both stage I-III (AUC = 0.93, P < 0.001) and stage IV (AUC = 0.90, P < 0.001, Fig. 2H) ccRCCs. The ccRCCs with BAP1 mutations or of the cluster 2 exhibited a higher BAP1-score (P < 0.001, Fig. 2I). As suggested by previous studies and our previous results, BAP1 mutations were associated with higher TNM stage, poorer histological grade, and the m3 and m4 TCGA clusters [32, 34, 35]. The BAP1-score was also higher in these BAP1 mutation-enriched subgroups (Fig. 2I), indicating that this score could efficiently reflect the features of BAP1 mutations in ccRCCs.
The BAP1-score predicts the benefit from avelumab plus axitinib over sunitinib in the JAVELIN cohort
First, we validated the utility of the 20 DEGs to identify BAP1 mutations in the JAVELIN cohort. The mRNA expression levels of the 20 DEGs and the BAP1-score were significantly different between BAP1-wildtype and BAP1-mutant samples (P < 0.05, Fig. 3A). The BAP1-score could effectively identify BAP1-mutant samples (AUC = 0.85, P < 0.001).
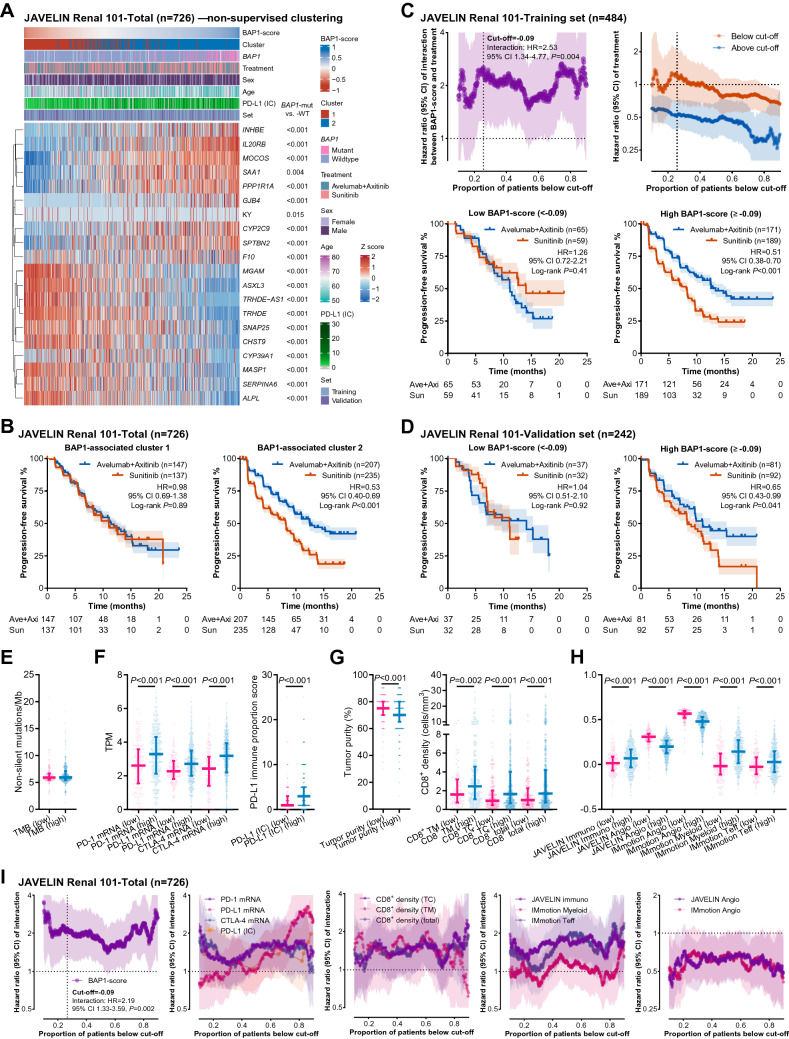
Fig. 3.
BAP1-score as a predictor of favorable benefit from avelumab plus axitinib over sunitinib in the JAVELIN cohort. A Heatmap illustrating the results of non-supervised clustering based on the twenty BAP1 mutation-associated differentially expressed genes. B Kaplan–Meier curves illustrating the PFS benefit from avelumab plus axitinib over sunitinib in the patients classified by the cluster. C This panel shows the results of the training set of the JAVELIN Renal 101 cohort (n = 484). The interaction effect between the BAP1-score and treatment choice at every cut-off (upper left). The PFS benefit in the patients with a BAP1-score above or below every available cut-off (upper right), below the optimal cut-off (-0.09, lower left), and above the optimal cut-off (lower right). D The PFS benefits in the patients of the validation set (n = 242) with a BAP1-score below -0.09 (left) or above -0.09 (right). E–H Associations of the BAP1-score with TMB (E), mRNA expression of PD-1 (PDCD1), PD-L1 (CD274), and CTLA-4, PD-L1 protein expression on immune cells (F), tumor purity, infiltration of CD8-positive cells (G), published gene signatures (H) in the total set (n = 726). I The interaction curves of the BAP1-score, checkpoint expression, CD8-positive cell infiltration, and published gene signatures in the total set (n = 726). Abbreviations: CTLA-4 = cytotoxic T-cell lymphocyte-4, PD-1 = programmed death 1, PD-L1 = programmed death-ligand 1, TMB = tumor mutational burden
The 726 ccRCC patients with available RNA-seq data were dissected into two subgroups by non-supervised clustering using the 20 DEGs (Fig. 3A). BAP1 mutations were enriched in the cluster 2 (cluster 1 vs. cluster 2: 3.7% vs. 24.4%, P < 0.001), where avelumab plus axitinib showed a larger benefit over sunitinib (HR = 0.53, 95% CI 0.40–0.69, P < 0.001) compared to the cluster 1 (HR = 0.98, 95% CI 0.69–1.38, P = 0.89, Fig. 3B).
Next, we randomly separated the 726 patients into a training set (n = 484) and a validation set (n = 242). In the training set, we calculated the interaction effect at each cut-off value between 10 and 90th percentile. The curve of interaction HR (abbreviated as “interaction curve” hereinafter) and the curves of treatment effect in the “below cut-off” and “above cut-off” subgroups are shown in Fig. 3C. The interaction curve looked like a bimodal curve showing two optional cut-offs ([1] cut-off = -0.09, 25.6 percentile; [2] cut-off = 0.25, 74.2 percentile) with remarkable interaction effects. The first cut-off could distinguish the patients (< 1st cut-off) who barely acquired benefits from immunotherapy, while the second cut-off could identify the ones (> 2nd cut-off) who had extraordinary immunotherapy benefits. Currently, the ICI-based combination therapies are the most effective choices in advanced ccRCCs and a large proportion of patients can acquire benefits from immunotherapy over VEGFR/mTOR inhibitors [1–8]. It might be more important to recognize the ones with hardly any ICI benefit compared to extraordinary ICI responders. Given this, we selected the first cut-off (-0.09) for the following analyses.
Based on this cut-off (-0.09), the patients in the validation set were classified into low and high BAP1-score subgroups. A significant benefit from avelumab plus axitinib over sunitinib was observed in the patients with a high BAP-score (HR = 0.65, 95% CI 0.43–0.99, P = 0.041) rather than a low BAP1-score (HR = 1.04, 95% CI 0.51–2.10, P = 0.92, Fig. 3D). Comparable results were obtained in the multivariable analyses (Supplementary Table 3), indicating the potential of the BAP1-score as a predictive biomarker.
A high BAP1-score was associated with (1) BAP1 mutations (low vs. high: 1.6% vs. 21.6%, P < 0.001) rather than a high TMB (Fig. 3E), (2) higher mRNA levels of PDCD1 (PD-1), CD274 (PD-L1), and CTLA-4, and the PD-L1 expression on immune cells (Fig. 3F), (3) low tumor purity accompanied by high CD8+ T cell infiltration in both tumor-center and -margin areas (Fig. 3G), (4) low angiogenesis signatures (JAVELIN Angio and Immotion 150 Angio) and high immune signatures (JAVELIN Immuno, Immotion 150 myeloid inflamed, and Immotion 150 Teff, Fig. 3H). We further plotted the interaction curves of all these immune correlates in the JAVELIN cohort (Fig. 3I). By comparison, the BAP1-score outperformed its correlates due to the stronger predictive effect and the statistical significance at almost every available cut-off (Fig. 3I).
The BAP1-score predicts the benefit from nivolumab over everolimus in the CM-009/010/025 cohort
We first validated the utility of the 20 DEGs to identify BAP1 mutations in the CM-009/010/025 cohort. Although the CM-009/010 trials were single-arm (nivolumab monotherapy), different from the CM-025 trial (randomized controlled trial, nivolumab vs. everolimus), the clinical features, the mRNA expression of CD274, PDCD1, and CTLA-4, the BAP1-score (Supplemental Table 4), and the treatment response in the patients undergoing nivolumab monotherapy in the CM-009/010 and the CM-025 trials were consistent (Supplemental Fig. 1). This consistency indicates the homogeneity of the patients undergoing nivolumab monotherapy in the CM-009/010 and the CM-025 trials and the rationality for the combined analysis.
The mRNA expression levels of the 20 DEGs and the BAP1-score were significantly different between BAP1-wildtype and BAP1-mutant samples (P < 0.05, Fig. 4A). The BAP1-score could effectively identify BAP1-mutant samples (AUC = 0.83, P < 0.001). By non-supervised clustering using the 20 DEGs, the 311 ccRCC patients were dissected into two subgroups with different frequencies of BAP1 mutations (cluster 1 vs. cluster 2: 6.1% vs. 32.0%, P < 0.001) and benefit from nivolumab over everolimus (Fig. 4B). Nivolumab delivered a significant PFS benefit over everolimus in the patients with a high BAP1-score (cut-off = −0.09, HR = 0.72, 95% CI 0.52–1.00, P = 0.045) rather than a low BAP1-score (HR = 1.02, 95% CI 0.71–1.48, P = 0.91, Fig. 4C).
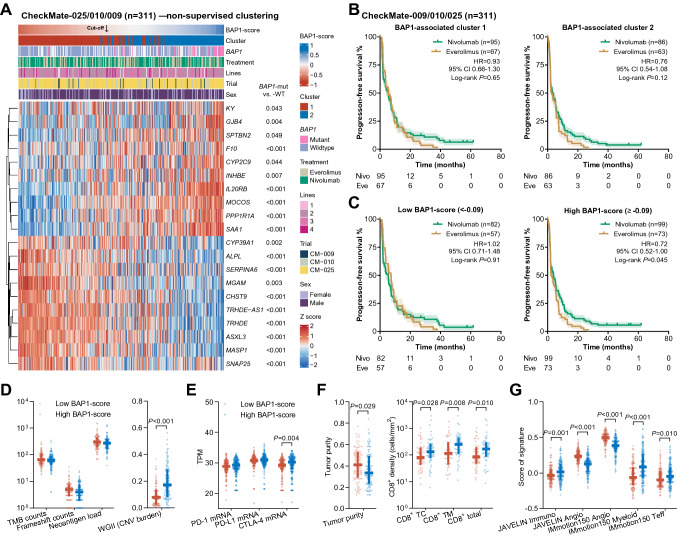
Fig. 4.
BAP1-score as a predictor of favorable benefit from nivolumab over everolimus in the CM-009/010/025 cohort. A Heatmap illustrating the results of non-supervised clustering based on the twenty BAP1 mutation-associated differentially expressed genes. B Kaplan–Meier curves illustrating the PFS benefit from nivolumab over everolimus in the patients classified by the cluster. C The PFS benefits in the patients with a BAP1-score below -0.09 (left) or above -0.09 (right). D-G Associations of the BAP1-score with TMB, frameshift count, neoantigen load (D), mRNA expression of PD-1 (PDCD1), PD-L1 (CD274), and CTLA-4 (E), tumor purity, infiltration of CD8-positive cells (F), and published gene signatures (G). Abbreviations: CTLA-4 = cytotoxic T-cell lymphocyte-4, PD-1 = programmed death 1, PD-L1 = programmed death-ligand 1, PFS = progression-free survival, TMB = tumor mutational burden
A high BAP1-score was associated with (1) BAP1 mutations (low vs. high: 2.1% vs. 31.7%, P < 0.001), (2) a high level of CNV burden rather than TMB, frameshift burden, and neoantigen load (Fig. 4D), (3) high transcriptional levels of CTLA-4, but not PDCD1 (PD-1) and CD274 (PD-L1, Fig. 4E), (4) CD8+ T cell infiltration (Fig. 4F), and (5) immune and angiogenesis signatures (Fig. 4G). The results concerning CNV burden, and PDCD1 and CD274 mRNA were inconsistent with those in the JAVELIN cohort. Given this, we further investigated these associations in the stage IV ccRCCs of the TCGA-KIRC cohort. In the high BAP1-score subgroup, we observed an increased aneuploidy score (P = 0.057, Supplementary Fig. 2A), and higher mRNA expression of CTLA-4 (P = 0.082) instead of PDCD1 and CD274 (P > 0.10, Supplementary Fig. 2B).
Associations of high BAP1-score with poor prognosis and large benefits from immunotherapy
As for the prognostic effect, we first analyzed the association between the BAP1-score and OS in the stage IV ccRCCs without receiving neoadjuvant therapy of the TCGA-KIRC dataset. A high BAP1-score was associated with short OS and the HR was 0.52 (low vs. high: 95% CI 0.30–0.90, P < 0.001, Fig. 5A). Moreover, in the early-stage (I-III) ccRCCs with no history of neoadjuvant therapy, the high BAP1-score subgroup had poorer DFS and OS (Supplementary Fig. 2C, D). These results indicate the association between a high BAP1-score and a poor prognosis in both early-stage and late-stage ccRCCs.
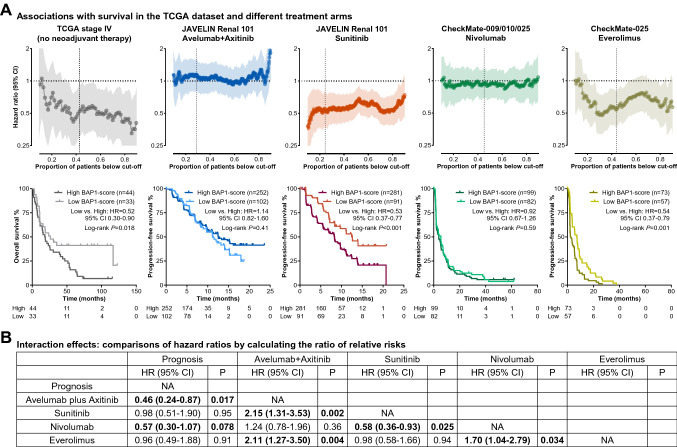
Fig. 5.
Associations of high BAP1-score with poor prognosis and large benefit from immunotherapy. A The associations of the BAP1-score with the OS in the stage IV ccRCC of the TCGA-KIRC dataset, the PFS on avelumab plus axitinib and sunitinib in the JAVELIN cohort, and the PFS on nivolumab and everolimus in the CheckMate-009/010/025 cohort. B Comparisons of the five hazard ratios in the panel A. Abbreviations: OS = overall survival, PD = progressive disease, PFS = progression-free survival
Among the treatment arms, a similar HR to prognostic effect was revealed in the patients treated with sunitinib (HR = 0.53, 95% CI 0.37–0.77, P < 0.001) and everolimus (HR = 0.54, 95% CI 0.37–0.79, P = 0.001) rather than avelumab plus axitinib (HR = 1.14, 95% CI 0.82–1.60, P = 0.41) and nivolumab (HR = 0.92, 95% CI 0.67–1.26, P = 0.59, Fig. 5A), further corroborated by multivariable analyses (Supplementary Table 5). These results indicate that the patients with a high BAP1-score had a poor prognosis compared with those with a low BAP1-score, while the high BAP1-score subgroup can acquire longer survival, equivalent to that of the low BAP1-score subgroup, when treated with avelumab plus axitinib or nivolumab rather than sunitinib or everolimus. From these results, it can be inferred that the patients with a high BAP1-score should be recommended to receive immunotherapy.
The Interaction between a biomarker and treatment effect is the essential proof of its predictive utility. We compared the hazard ratios in different scenarios shown in Fig. 5A by calculating the ratio of relative risks (RRR), similar to the approach in network meta-analysis. As shown in Fig. 5B, the RRRs between VEGFR/mTOR inhibitors were close to 1.00, suggesting that the BAP1-score might not discern the efficacy of VEGFR and mTOR inhibitors. Of note, the RRRs between ICI-based immunotherapies and VEGFR/mTOR inhibitors were significant (P < 0.05, Fig. 5B), indicating that the ccRCCs with a high BAP1-score can acquire large benefits from ICI-based immunotherapies over VEGFR/mTOR inhibitors.
Exploration of the molecular correlates of the BAP1-score in ccRCC
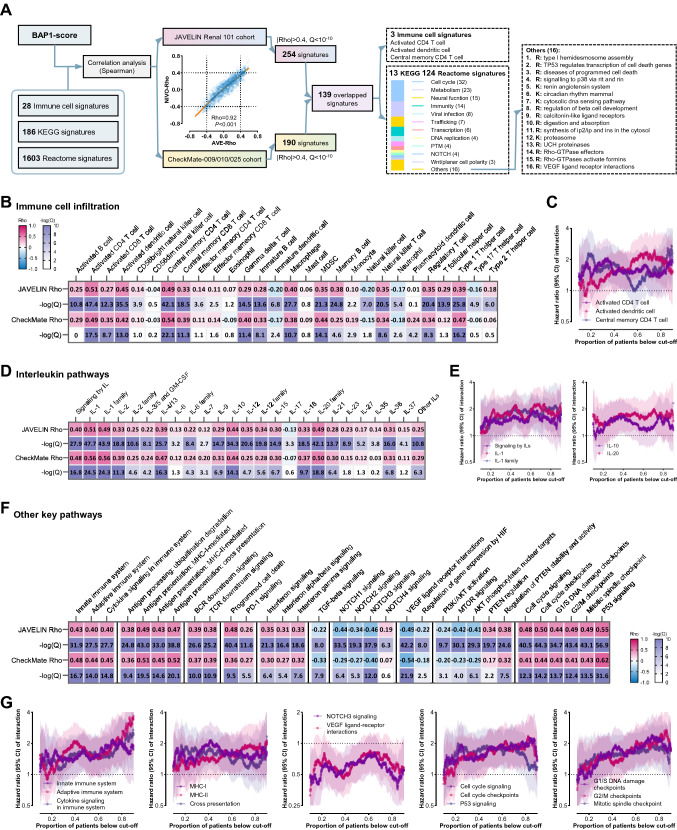
As illustrated in Fig. 6A, we calculated the correlations of the BAP1-score with the ssGSEA scores of 28 immune cell signatures, 186 KEGG signatures, and 1603 Reactome signatures in the JAVELIN cohort and the CM-009/010/025 cohort. The two Rho values of a single signature in the two different cohorts were highly correlated (Rho = 0.92, P < 0.001), demonstrating considerable credibility. We filtered the signatures by two criteria (the absolute value of Rho > 0.4 and P < 10–10 in both cohorts) and identified 139 key overlapped signatures. Of these, three were immune cell signatures and the other 136 signatures (KEGG: n = 13; Reactome, n = 124) were commonly associated with cell cycle (n = 32), metabolism (n = 23), neural function (n = 15), and immunity (n = 14).
Fig. 6.
Associations of BAP1-score with biological functions. A Workflow of the analysis in this figure. Correlation analysis of the Rho values of same signature in the two different cohorts. B Associations of the BAP1-score with immune cell infiltration in the two cohorts. C The interaction curves of the immune cell infiltration with a high Rho value above 0.4 in both cohorts. D Associations of the BAP1-score with the signatures of interleukin pathways in the two cohorts. E The interaction curves of the signatures of interleukin pathways with a high Rho value above 0.4 in both cohorts. F Associations of the BAP1-score with the signatures of other key pathways in the two cohorts. G The interaction curves of the signatures of other key pathways with a high Rho value above 0.4 in both cohorts
The BAP1-score was strongly correlated (Rho > 0.4 in both cohorts) with the signatures concerning (1) activated CD4 T cell, activated dendritic cell, central memory CD4 T cell (Fig. 6B), (2) signaling by iLs, IL-1, IL-1 family, IL-10, and IL-20 (Fig. 6D), (3) immune activation including the upregulation of antigen processing, B/T cell receptor downstream signaling, PD-1 axis, and interferon signaling and the downregulation of transforming growth factor-β pathway (Fig. 6F), and (4) activation of NOTCH3 and cell cycle and inactivation of PI3K/AKT/mTOR and angiogenesis (Fig. 6F and Supplementary Table 6).
These correlates of the BAP1-score may partially explain its predictive power [10, 14, 24, 36–40]. However, none of the signatures exhibited better discriminative effectiveness (Fig. 6C, E, and G) compared to the BAP1-score which showed a stronger predictive effect and statistical significance at nearly every available cut-off (Fig. 3I). These results indicate the superiority of the BAP1-score in predicting the benefits from ICI-based therapies over VEGFR/mTOR inhibitors in ccRCCs.
Discussion
In the original articles of the biomarker analyses of the JAVELIN [14], the CM-009/010/025 [10], and the CM-214 trials [21], researchers identified “qualitative” rather than “quantitative” predictive biomarkers [26, 27]. In our study, we first re-analyzed these three cohorts and identified BAP1 mutations as predictors of large benefits from ICI-based therapies over VEGFR/mTOR inhibitors in ccRCCs. By the 20 BAP1 mutation-associated DEGs, the ccRCC samples of the TCGA-KIRC dataset were dissected into two subgroups with distinct prognosis and immune features. The BAP1 mutation-enriched subgroup had more tumor-infiltrating immune cells and a lower angiogenesis score, consistent with the previous findings by Wang et al. [41]. We used the 20 DEGs to construct the BAP1-score and revealed its utility of predicting the benefits from ICI-based therapies over VEGFR/mTOR inhibitors in the JAVELIN and CM-009/010/025 cohorts.
Although the 20 DEGs were determined by the TCGA-KIRC dataset dominated by early-stage (stage I-III) ccRCCs, these 20 DEGs were expressed differently between the BAP1-mutant and BAP1-wildtype groups in both (1) the early-stage and (2) the late-stage ccRCCs of the TCGA-KIRC dataset, and the advanced ccRCCs in (3) the JAVELIN and (4) the CM-009/010/025 cohorts. In the four above-mentioned scenarios, the BAP1-score can effectively identify BAP1 mutations and the association between the 20 DEGs and immune features was consistent. These results demonstrate (1) the applicability of the 20 DEGs in both the early-stage and late-stage ccRCCs and (2) the robustness of the linkage between immunity and the BAP1-score which may potentially guide systemic therapies in both perioperative and late-stage settings.
BAP1 is a ubiquitin carboxy-terminal hydrolase found both in nucleus and cytoplasm [42], whose activity is critical for DNA synthesis and replication under stress conditions [43, 44], double-strand DNA repair by homologous recombination [45], chromatin modification and transcriptional regulation [45–48], maintaining metabolic homeostasis [49–51], inhibiting apoptosis and ferroptosis [52–54], strengthening NOTCH signaling and cell cycle control [55, 56], etc. As its powerful tumor-suppressing functions, BAP1 loss defines an aggressive class of ccRCCs with short DFS and OS [32, 34, 35, 57–64]. In our study, the molecular correlates and prognostic effect of the BAP1-score were similar to those described above, indicating that our BAP1-score could reflect BAP1 function efficiently.
In the present study, we revealed the negative associations of the BAP1-score with (1) the DFS in stage I-III ccRCCs (HR = 0.52), (2) the OS in stage IV ccRCCs (HR = 0.52), (3) the PFS on first-line sunitinib (HR = 0.53), and (4) the PFS on second-line everolimus (HR = 0.54), consistent with previous studies concerning prognosis [32, 34, 35, 57–64] and survival on VEGFR/mTOR inhibitors [65, 66]. These four above-mentioned HRs were highly comparable and the interaction effects between these HRs were insignificant, indicating that the ccRCC patients with a high BAP1-score may have a poor prognosis that cannot be rescued by VEGFR/mTOR inhibitors. In contrast, the survival outcomes on avelumab plus axitinib or nivolumab were similar in the high BAP1-score group and the low BAP1-score group; therefore, the high BAP1-score group can achieve longer survival, equivalent to that of the low BAP1-score group, when treated with ICI-based therapies, rather than VEGFR/mTOR inhibitors. Our results suggest that ICI-based immunotherapies, rather than VEGFR/mTOR inhibitors, might be recommended for the ccRCCs with a high BAP1-score.
In previous biomarker studies of the trials investigating ICI-based therapies, such as JAVELIN Renal 101 [14], CM-009/010/025 [10], CM-214 [21], and IMmotion151 [23], the identified predictive power of BAP1 mutations are “discordant”, partially due to different definitions of predictive biomarker in these studies. In the biomarker studies of JAVELIN Renal 101 [14], CM-009/010/025 [10], and CM-214 [21], researchers identified “qualitative” predictive biomarkers for immunotherapy that were significantly linked with survival in the immunotherapy arm but not in the control arm. However, the difference in the association of a biomarker with survival across treatment arms (estimated by interaction effect between biomarker and treatment choices) is the essential proof of its predictive utility [26, 27]. In these three trials, BAP1 mutations was non-significantly associated with better survival on ICI-based therapies and poorer survival on sunitinib/everolimus, which were not considered as a “qualitative” predictor. Nonetheless, the pooled estimate of the interaction effects in these three trials was significant, distinguishing BAP1 mutations as “quantitative” predictors for larger benefits from ICI-based therapies over VEGFR/mTOR inhibitors. In the biomarker study of IMmotion151, BAP1 mutations were enriched in the T-eff/proliferative cluster with significant benefit from atezolizumab plus bevacizumab over sunitinib [23], in concordance with our results.
BAP1 germline mutation predisposed to mesothelioma [67], uveal melanoma [68], cutaneous melanoma [69], and less frequently renal cell carcinoma [70, 71]. Several bioinformatic studies have demonstrated the associations of low BAP1 expression with high levels of immune infiltrates, cytokine and interferon signaling, and immune checkpoint activation in peritoneal mesothelioma [72], uveal melanoma [73], and ccRCC [23, 74]. In our study, we also uncovered the remarkable correlations of the BAP1-score with immune cell infiltration and the activation of immune pathways, which might explain its predictive utility for immunotherapeutic benefit. Despite this, none of the immune correlates exhibited better discriminative effectiveness compared to the BAP1-score, suggesting the superiority of the BAP1-score in predicting the benefits from ICI-based therapies over VEGFR/mTOR inhibitors in ccRCCs.
As predictive biomarkers, GES may outperform mutation in two aspects. First, in the cases where the functional alteration of one gene is underlying the mechanisms other than mutations (e.g., methylation, miRNAs, lncRNAs, and transcriptional regulators), employing the GES may be more efficient than using mutation alone to identify the samples with BAP1 functional alteration. Second, GES scores are continuous variables enabling us to manually select a cut-off for a specific purpose, while mutational biomarkers usually only dichotomize the patients into wildtype and mutant subgroups. The BAP1-score showed significant predictive effects at nearly all available cut-offs. In our analysis, we chose an optimal cut-off from the training set of the JAVELIN cohort which identified 25.6% of patients as low-score patients with hardly any benefit from ICI-based therapies over control therapies. If the aim turned into distinguishing extraordinary responders to ICIs, the cut-off could be increased so that the treatment effect in the high-score group would be magnified. A large proportion of patients with advanced ccRCC can acquire benefits from ICI-based therapies over VEGFR/mTOR inhibitors [1–8]. If a high cut-off was selected to identify the super ICI responders in the high-score group, a mild but significant ICI benefit would also be observed in the low-score group. In this case, this cut-off cannot guide the treatment choice between ICIs and VEGFR/mTOR inhibitors; therefore, we chose a low cut-off in the present study. Moreover, identifying patients who are highly sensitive to ICIs and do not need VEGFR TKIs as part of the combos is of great interest. This topic may be explored in a trial including two arms of patients treated by ICI plus VEGFR TKI or ICI alone in the future.
As for limitation, first, the retrospective setting of our study may introduce biases, however, which can be minimized by the context of large randomized trials and the implementation of multivariable analysis and independent validation. Second, the ICI regimens analyzed are avelumab plus axitinib and nivolumab monotherapy, which may represent anti-PD-(L)1 plus VEGFR TKI and anti-PD-(L)1 monotherapy, respectively. The combination of anti-PD-(L)1 and anti-CTLA-4 was not included in our study due to the lack of patient-level data. Despite this, a positive correlation between the BAP1-score and CTLA-4 mRNA expression was observed in all three cohorts in our analysis, suggesting the potential predictive utility of a high BAP1-score for a large benefit from combination immunotherapy including anti-CTLA-4 over monotherapies of VEGFR/mTOR inhibitors. Third, the optimal cut-off derived from the JAVELIN cohort (avelumab plus axitinib vs. sunitinib) might not be the best for the CheckMate cohort (nivolumab vs. everolimus). However, the sample size of the CheckMate cohort is not sufficient for both training and internal validation. The search for a cut-off value for predicting the benefit from anti-PD-(L)1 over mTOR inhibitors needs further exploration.
Through retrospective analysis of three large datasets including TCGA-KIRC (algorithm development), JAVELIN (training and internal validation), and CM-009/010/025 (external validation), the BAP1-score has been identified as a biologically and clinically significant predictor for inflamed immune microenvironment and benefit from ICI-based therapies over VEGFR/mTOR inhibitors in ccRCCs, which provides insights into the immunomodulatory role of BAP1 and a novel tool consisting of 20 transcripts for the patient selection for immunotherapy. Furthermore, a specific and effective inhibitor of BAP1 enzymatic activity with tolerable toxicity in vivo has been discovered [75]. Our findings may inform personalized therapeutic strategies for patients with ccRCCs and other tumor types, especially peritoneal mesothelioma and uveal/cutaneous melanoma where BAP1 loss is common.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Dizai Shi (Stitch) for his emotional support and the researchers involved in the TCGA-KIRC dataset and the clinical trials (JAVELIN and CM-009/010/025) who shared patient-level data for public use.
Abbreviations
- ccRCC
Clear cell renal cell carcinoma
- CI
Confidence interval
- CM-009/010/025
Checkmate-009/010/025
- CTLA-4
Cytotoxic T-cell lymphocyte-4
- DDR
DNA damage repair
- DEG
Differentially expressed gene
- GES
Gene expression score
- HR
Hazard ratio
- ICI
Immune checkpoint inhibitor
- IHC
Immunohistochemical
- PD-1
Programmed cell death-1
- PD-L1
Programmed death-ligand 1
- TCGA-KIRC
The cancer genome atlas-kidney renal clear cell carcinoma
- TKI
Tyrosine kinase inhibitor
- TMB
Tumor mutational burden
- TPM
Transcripts per million,
- RRR
Ratio of relative risk
- ssGSEA
Single sample gene set enrichment analysis
- STROBE
Strengthening the reporting of observational studies in epidemiology
- VEGFR
Vascular endothelial growth factor receptor
- WES
Whole-exome sequencing
Author contributions
KL, YH, YX, and TS conceptualized and designed this study. KL and YX developed the methodology and acquired the data. KL, YH, YX, and TS analyzed and interpreted the data. All authors contributed to the writing, review, and/or revision of the manuscript. GW, SC, and XZ provided administrative, technical, and/or material support. XZ and TS supervised this study. All authors approved the submitted version of manuscript.
Funding
This work was supported by National Natural Science Foundation of China (81770790 and 81972389 to Xu Zhang) and Hainan Province Science and Technology Special Fund (ZDYF2021SHFZ056 to Taoping Shi).
Data availability
The authors declare that relevant data supporting the findings of this study are available within the paper and its Supplementary files. The references of all the included datasets are shown in Supplementary Table 1. Due to ethical and privacy concerns, we are unable to publish their full data in our study. Readers could contact the corresponding authors for the access of individual patient-level data for non-commercial purposes.
Declarations
Conflict of interest
The authors declare no potential conflicts of interest, except the employment of Yu Xu, Guoqiang Wang, and Shangli Cai in Burning Rock Biotech.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Ethical approval
Our study is following the principles of the Declaration of Helsinki and was approved by the Institution Review Board of Chinese PLA General Hospital (ChiCTR2000030405).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Kan Liu and Yan Huang have contributed equally to this work.
Contributor Information
Xu Zhang, Email: xzhang301@163.com.
Taoping Shi, Email: stopping3721@foxmail.com.
References
- 1.Choueiri TK, Powles T, Burotto M, et al. Nivolumab plus cabozantinib versus sunitinib for advanced renal-cell carcinoma. New Engl J Med. 2021;384:829–841. doi: 10.1056/NEJMoa2026982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Motzer RJ, Rini BI, McDermott DF, et al. Nivolumab plus ipilimumab versus sunitinib in first-line treatment for advanced renal cell carcinoma: extended follow-up of efficacy and safety results from a randomised, controlled, phase 3 trial. Lancet Oncol. 2019;20:1370–1385. doi: 10.1016/s1470-2045(19)30413-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Motzer RJ, Tannir NM, McDermott DF, et al. Nivolumab plus Ipilimumab versus sunitinib in advanced renal-cell carcinoma. New Engl J Med. 2018;378:1277–1290. doi: 10.1056/NEJMoa1712126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Powles T, Plimack ER, Soulières D, et al. Pembrolizumab plus axitinib versus sunitinib monotherapy as first-line treatment of advanced renal cell carcinoma (KEYNOTE-426): extended follow-up from a randomised, open-label, phase 3 trial. Lancet Oncol. 2020;21:1563–1573. doi: 10.1016/s1470-2045(20)30436-8. [DOI] [PubMed] [Google Scholar]
- 5.Motzer R, Alekseev B, Rha SY, et al. Lenvatinib plus pembrolizumab or everolimus for advanced renal cell carcinoma. New Engl J Med. 2021;384:1289–1300. doi: 10.1056/NEJMoa2035716. [DOI] [PubMed] [Google Scholar]
- 6.Rini BI, Plimack ER, Stus V, et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. New Engl J Med. 2019 doi: 10.1056/NEJMoa1816714. [DOI] [PubMed] [Google Scholar]
- 7.Rini BI, Powles T, Atkins MB, et al. Atezolizumab plus bevacizumab versus sunitinib in patients with previously untreated metastatic renal cell carcinoma (IMmotion151): a multicentre, open-label, phase 3, randomised controlled trial. Lancet. 2019;393:2404–2415. doi: 10.1016/S0140-6736(19)30723-8. [DOI] [PubMed] [Google Scholar]
- 8.Motzer RJ, Penkov K, Haanen J, et al. Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. New Engl J Med. 2019 doi: 10.1056/NEJMoa1816047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rini BI, Huseni M, Atkins MB, et al. Molecular correlates differentiate response to atezolizumab (atezo) + bevacizumab (bev) vs sunitinib (sun): Results from a phase III study (IMmotion151) in untreated metastatic renal cell carcinoma (mRCC) Ann Oncol. 2018;29:viii724–viii725. doi: 10.1093/annonc/mdy424.037. [DOI] [Google Scholar]
- 10.Braun DA, Hou Y, Bakouny Z, et al. Interplay of somatic alterations and immune infiltration modulates response to PD-1 blockade in advanced clear cell renal cell carcinoma. Nat Med. 2020;26:909–918. doi: 10.1038/s41591-020-0839-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ged Y, Chaim JL, DiNatale RG, et al. DNA damage repair pathway alterations in metastatic clear cell renal cell carcinoma and implications on systemic therapy. J Immunother Cancer. 2020 doi: 10.1136/jitc-2019-000230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Labriola MK, Zhu J, Gupta RT, et al. Characterization of tumor mutation burden, PD-L1 and DNA repair genes to assess relationship to immune checkpoint inhibitors response in metastatic renal cell carcinoma. J Immunother Cancer. 2020 doi: 10.1136/jitc-2019-000319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Motzer RJ, Choueiri TK, McDermott DF, et al. Biomarker analyses from the phase III CheckMate 214 trial of nivolumab plus ipilimumab (N+I) or sunitinib (S) in advanced renal cell carcinoma (aRCC) J Clin Oncol. 2020;38:5009. doi: 10.1200/JCO.2020.38.15_suppl.5009. [DOI] [Google Scholar]
- 14.Motzer RJ, Robbins PB, Powles T, et al. Avelumab plus axitinib versus sunitinib in advanced renal cell carcinoma: biomarker analysis of the phase 3 JAVELIN Renal 101 trial. Nat Med. 2020;26:1733–1741. doi: 10.1038/s41591-020-1044-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ross-Macdonald P, Walsh AM, Chasalow SD, Ammar R, Papillon-Cavanagh S, Szabo PM, Choueiri TK, Sznol M, Wind-Rotolo M. Molecular correlates of response to nivolumab at baseline and on treatment in patients with RCC. J Immunother Cancer. 2021 doi: 10.1136/jitc-2020-001506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miao D, Margolis CA, Gao W, et al. Genomic correlates of response to immune checkpoint therapies in clear cell renal cell carcinoma. Science. 2018;359:801–806. doi: 10.1126/science.aan5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dizman N, Lyou Y, Salgia N, et al. Correlates of clinical benefit from immunotherapy and targeted therapy in metastatic renal cell carcinoma: comprehensive genomic and transcriptomic analysis. J Immunother Cancer. 2020 doi: 10.1136/jitc-2020-000953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. New Engl J Med. 2015;373:1803–1813. doi: 10.1056/NEJMoa1510665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tucker MD, Rini BI. Predicting response to immunotherapy in metastatic renal cell carcinoma. Cancers (Basel) 2020 doi: 10.3390/cancers12092662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guida A, Sabbatini R, Gibellini L, De Biasi S, Cossarizza A, Porta C. Finding predictive factors for immunotherapy in metastatic renal-cell carcinoma: what are we looking for? Cancer Treat Rev. 2021;94:102157. doi: 10.1016/j.ctrv.2021.102157. [DOI] [PubMed] [Google Scholar]
- 21.Motzer RJ, Choueiri TK, McDermott DF, et al. Biomarker analysis from CheckMate 214: nivolumab plus ipilimumab versus sunitinib in renal cell carcinoma. J Immunother Cancer. 2022 doi: 10.1136/jitc-2021-004316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu XD, Kong W, Peterson CB, et al. PBRM1 loss defines a nonimmunogenic tumor phenotype associated with checkpoint inhibitor resistance in renal carcinoma. Nat Commun. 2020;11:2135. doi: 10.1038/s41467-020-15959-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Motzer RJ, Banchereau R, Hamidi H, et al. Molecular subsets in renal cancer determine outcome to checkpoint and angiogenesis blockade. Cancer Cell. 2020;38:803–817. doi: 10.1016/j.ccell.2020.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McDermott DF, Huseni MA, Atkins MB, et al. Clinical activity and molecular correlates of response to atezolizumab alone or in combination with bevacizumab versus sunitinib in renal cell carcinoma. Nat Med. 2018;24:749–757. doi: 10.1038/s41591-018-0053-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Z, Zhao J, Wang G, et al. Comutations in DNA damage response pathways serve as potential biomarkers for immune checkpoint blockade. Cancer Res. 2018;78:6486–6496. doi: 10.1158/0008-5472.CAN-18-1814. [DOI] [PubMed] [Google Scholar]
- 26.Altman DG, Bland JM. Interaction revisited: the difference between two estimates. BMJ. 2003;326:219. doi: 10.1136/bmj.326.7382.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ballman KV. Biomarker: predictive or prognostic? J Clin Oncol. 2015;33:3968–3971. doi: 10.1200/JCO.2015.63.3651. [DOI] [PubMed] [Google Scholar]
- 28.Newman AM, Liu CL, Green MR, Gentles AJ, Feng W, Xu Y, Hoang CD, Diehn M, Alizadeh AA. Robust enumeration of cell subsets from tissue expression profiles. Nat Methods. 2015;12:453–457. doi: 10.1038/nmeth.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barbie DA, Tamayo P, Boehm JS, et al. Systematic RNA interference reveals that oncogenic KRAS-driven cancers require TBK1. Nature. 2009;462:108–112. doi: 10.1038/nature08460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Subramanian A, Tamayo P, Mootha VK, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Charoentong P, Finotello F, Angelova M, Mayer C, Efremova M, Rieder D, Hackl H, Trajanoski Z. Pan-cancer immunogenomic analyses reveal genotype-immunophenotype relationships and predictors of response to checkpoint blockade. Cell Rep. 2017;18:248–262. doi: 10.1016/j.celrep.2016.12.019. [DOI] [PubMed] [Google Scholar]
- 32.Cancer Genome Atlas Research N Comprehensive molecular characterization of clear cell renal cell carcinoma. Nature. 2013;499:43–49. doi: 10.1038/nature12222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thorsson V, Gibbs DL, Brown SD, et al. The immune landscape of cancer. Immunity. 2018;48:812–830. doi: 10.1016/j.immuni.2018.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pena-Llopis S, Vega-Rubin-de-Celis S, Liao A, et al. BAP1 loss defines a new class of renal cell carcinoma. Nat Genet. 2012;44:751–759. doi: 10.1038/ng.2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hakimi AA, Ostrovnaya I, Reva B, et al. Adverse outcomes in clear cell renal cell carcinoma with mutations of 3p21 epigenetic regulators BAP1 and SETD2: a report by MSKCC and the KIRC TCGA research network. Clin Cancer Res. 2013;19:3259–3267. doi: 10.1158/1078-0432.CCR-12-3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang Z, Wang X, Xu Y, et al. Mutations of PI3K-AKT-mTOR pathway as predictors for immune cell infiltration and immunotherapy efficacy in dMMR/MSI-H gastric adenocarcinoma. BMC Med. 2022 doi: 10.1186/s12916-022-02327-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Loi S, Michiels S, Baselga J, et al. PIK3CA genotype and a PIK3CA mutation-related gene signature and response to everolimus and letrozole in estrogen receptor positive breast cancer. PLoS ONE. 2013;8:e53292. doi: 10.1371/journal.pone.0053292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang K, Hong X, Song Z, et al. Identification of deleterious NOTCH mutation as novel predictor to efficacious immunotherapy in NSCLC. Clin Cancer Res. 2020 doi: 10.1158/1078-0432.CCR-19-3976. [DOI] [PubMed] [Google Scholar]
- 39.Zhang F, Wang J, Xu Y, Cai S, Li T, Wang G, Li C, Zhao L, Hu Y. Co-occurring genomic alterations and immunotherapy efficacy in NSCLC. NPJ Precis Oncol. 2022;6:4. doi: 10.1038/s41698-021-00243-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bai H, Duan J, Li C, et al. EPHA mutation as a predictor of immunotherapeutic efficacy in lung adenocarcinoma. J Immunother Cancer. 2020 doi: 10.1136/jitc-2020-001315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang T, Lu R, Kapur P, et al. An empirical approach leveraging tumorgrafts to dissect the tumor microenvironment in renal cell carcinoma identifies missing link to prognostic inflammatory factors. Cancer Discov. 2018;8:1142–1155. doi: 10.1158/2159-8290.CD-17-1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bononi A, Giorgi C, Patergnani S, et al. BAP1 regulates IP3R3-mediated Ca(2+) flux to mitochondria suppressing cell transformation. Nature. 2017;546:549–553. doi: 10.1038/nature22798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee HS, Seo HR, Lee SA, Choi S, Kang D, Kwon J. BAP1 promotes stalled fork restart and cell survival via INO80 in response to replication stress. Biochem J. 2019;476:3053–3066. doi: 10.1042/BCJ20190622. [DOI] [PubMed] [Google Scholar]
- 44.Lee HS, Lee SA, Hur SK, Seo JW, Kwon J. Stabilization and targeting of INO80 to replication forks by BAP1 during normal DNA synthesis. Nat Commun. 2014;5:5128. doi: 10.1038/ncomms6128. [DOI] [PubMed] [Google Scholar]
- 45.Nishikawa H, Wu W, Koike A, Kojima R, Gomi H, Fukuda M, Ohta T. BRCA1-associated protein 1 interferes with BRCA1/BARD1 RING heterodimer activity. Cancer Res. 2009;69:111–119. doi: 10.1158/0008-5472.CAN-08-3355. [DOI] [PubMed] [Google Scholar]
- 46.Daou S, Barbour H, Ahmed O, et al. Monoubiquitination of ASXLs controls the deubiquitinase activity of the tumor suppressor BAP1. Nat Commun. 2018;9:4385. doi: 10.1038/s41467-018-06854-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Daou S, Hammond-Martel I, Mashtalir N, et al. The BAP1/ASXL2 histone H2A deubiquitinase complex regulates cell proliferation and is disrupted in cancer. J Biol Chem. 2015;290:28643–28663. doi: 10.1074/jbc.M115.661553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ji Z, Mohammed H, Webber A, Ridsdale J, Han N, Carroll JS, Sharrocks AD. The forkhead transcription factor FOXK2 acts as a chromatin targeting factor for the BAP1-containing histone deubiquitinase complex. Nucleic Acids Res. 2014;42:6232–6242. doi: 10.1093/nar/gku274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ruan HB, Han X, Li MD, et al. O-GlcNAc transferase/host cell factor C1 complex regulates gluconeogenesis by modulating PGC-1alpha stability. Cell Metab. 2012;16:226–237. doi: 10.1016/j.cmet.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Baughman JM, Rose CM, Kolumam G, et al. NeuCode proteomics reveals bap1 regulation of metabolism. Cell Rep. 2016;16:583–595. doi: 10.1016/j.celrep.2016.05.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bononi A, Yang H, Giorgi C, et al. Germline BAP1 mutations induce a Warburg effect. Cell Death Differ. 2017;24:1694–1704. doi: 10.1038/cdd.2017.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang Y, Shi J, Liu X, et al. BAP1 links metabolic regulation of ferroptosis to tumour suppression. Nat Cell Biol. 2018;20:1181–1192. doi: 10.1038/s41556-018-0178-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Peng H, Yang F, Hu Q, Sun J, Peng C, Zhao Y, Huang C. The ubiquitin-specific protease USP8 directly deubiquitinates SQSTM1/p62 to suppress its autophagic activity. Autophagy. 2020;16:698–708. doi: 10.1080/15548627.2019.1635381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.He M, Chaurushiya MS, Webster JD, et al. Intrinsic apoptosis shapes the tumor spectrum linked to inactivation of the deubiquitinase BAP1. Science. 2019;364:283–285. doi: 10.1126/science.aav4902. [DOI] [PubMed] [Google Scholar]
- 55.Luo Z, Mu L, Zheng Y, Shen W, Li J, Xu L, Zhong B, Liu Y, Zhou Y. NUMB enhances Notch signaling by repressing ubiquitination of NOTCH1 intracellular domain. J Mol Cell Biol. 2020;12:345–358. doi: 10.1093/jmcb/mjz088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Misaghi S, Ottosen S, Izrael-Tomasevic A, et al. Association of C-terminal ubiquitin hydrolase BRCA1-associated protein 1 with cell cycle regulator host cell factor 1. Mol Cell Biol. 2009;29:2181–2192. doi: 10.1128/MCB.01517-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kapur P, Peña-Llopis S, Christie A, Zhrebker L, Pavía-Jiménez A, Rathmell WK, Xie X-J, Brugarolas J. Effects on survival of BAP1 and PBRM1 mutations in sporadic clear-cell renal-cell carcinoma: a retrospective analysis with independent validation. Lancet Oncol. 2013;14:159–167. doi: 10.1016/s1470-2045(12)70584-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kapur P, Christie A, Raman JD, et al. BAP1 immunohistochemistry predicts outcomes in a multi-institutional cohort with clear cell renal cell carcinoma. J Urol. 2014;191:603–610. doi: 10.1016/j.juro.2013.09.041. [DOI] [PubMed] [Google Scholar]
- 59.Miura Y, Inoshita N, Ikeda M, et al. Loss of BAP1 protein expression in the first metastatic site predicts prognosis in patients with clear cell renal cell carcinoma. Urol Oncol. 2017;35:386–391. doi: 10.1016/j.urolonc.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 60.Tennenbaum DM, Manley BJ, Zabor E, et al. Genomic alterations as predictors of survival among patients within a combined cohort with clear cell renal cell carcinoma undergoing cytoreductive nephrectomy. Urol Oncol. 2017;35(532):e7–e13. doi: 10.1016/j.urolonc.2017.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Oka S, Inoshita N, Miura Y, et al. The loss of BAP1 protein expression predicts poor prognosis in patients with nonmetastatic clear cell renal cell carcinoma with inferior vena cava tumor thrombosis. Urol Oncol. 2018;36(365):e9–e14. doi: 10.1016/j.urolonc.2018.04.015. [DOI] [PubMed] [Google Scholar]
- 62.Ge YZ, Xu LW, Zhou CC, et al. A BAP1 mutation-specific MicroRNA signature predicts clinical outcomes in clear cell renal cell carcinoma patients with wild-type BAP1. J Cancer. 2017;8:2643–2652. doi: 10.7150/jca.20234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Minardi D, Lucarini G, Milanese G, Di Primio R, Montironi R, Muzzonigro G. Loss of nuclear BAP1 protein expression is a marker of poor prognosis in patients with clear cell renal cell carcinoma. Urol Oncol. 2016;34(338):e11–e18. doi: 10.1016/j.urolonc.2016.03.006. [DOI] [PubMed] [Google Scholar]
- 64.da Costa WH, da Cunha IW, Fares AF, et al. Prognostic impact of concomitant loss of PBRM1 and BAP1 protein expression in early stages of clear cell renal cell carcinoma. Urol Oncol. 2018;36(243):e1–e8. doi: 10.1016/j.urolonc.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 65.Hsieh JJ, Chen D, Wang PI, et al. Genomic biomarkers of a randomized trial comparing first-line everolimus and sunitinib in patients with metastatic renal cell carcinoma. Eur Urol. 2017;71:405–414. doi: 10.1016/j.eururo.2016.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Voss MH, Reising A, Cheng Y, et al. Genomically annotated risk model for advanced renal-cell carcinoma: a retrospective cohort study. Lancet Oncol. 2018;19:1688–1698. doi: 10.1016/s1470-2045(18)30648-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Panou V, Gadiraju M, Wolin A, et al. Frequency of germline mutations in cancer susceptibility genes in malignant mesothelioma. J Clin Oncol. 2018;36:2863–2871. doi: 10.1200/JCO.2018.78.5204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Harbour JW, Onken MD, Roberson ED, et al. Frequent mutation of BAP1 in metastasizing uveal melanomas. Science. 2010;330:1410–1413. doi: 10.1126/science.1194472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Njauw CN, Kim I, Piris A, et al. Germline BAP1 inactivation is preferentially associated with metastatic ocular melanoma and cutaneous-ocular melanoma families. PLoS ONE. 2012;7:e35295. doi: 10.1371/journal.pone.0035295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Popova T, Hebert L, Jacquemin V, et al. Germline BAP1 mutations predispose to renal cell carcinomas. Am J Hum Genet. 2013;92:974–980. doi: 10.1016/j.ajhg.2013.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Farley MN, Schmidt LS, Mester JL, et al. A novel germline mutation in BAP1 predisposes to familial clear-cell renal cell carcinoma. Mol Cancer Res. 2013;11:1061–1071. doi: 10.1158/1541-7786.MCR-13-0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shrestha R, Nabavi N, Lin YY, et al. BAP1 haploinsufficiency predicts a distinct immunogenic class of malignant peritoneal mesothelioma. Genome Med. 2019;11:8. doi: 10.1186/s13073-019-0620-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Figueiredo CR, Kalirai H, Sacco JJ, Azevedo RA, Duckworth A, Slupsky JR, Coulson JM, Coupland SE. Loss of BAP1 expression is associated with an immunosuppressive microenvironment in uveal melanoma, with implications for immunotherapy development. J Pathol. 2020;250:420–439. doi: 10.1002/path.5384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhou Q, Qi Y, Wang Z, et al. CCR5 blockade inflames antitumor immunity in BAP1-mutant clear cell renal cell carcinoma. J Immunother Cancer. 2020 doi: 10.1136/jitc-2019-000228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang L, Birch NW, Zhao Z, et al. Epigenetic targeted therapy of stabilized BAP1 in ASXL1 gain-of-function mutated leukemia. Nat Cancer. 2021;2:515–526. doi: 10.1038/s43018-021-00199-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors declare that relevant data supporting the findings of this study are available within the paper and its Supplementary files. The references of all the included datasets are shown in Supplementary Table 1. Due to ethical and privacy concerns, we are unable to publish their full data in our study. Readers could contact the corresponding authors for the access of individual patient-level data for non-commercial purposes.