Abstract
Objective
While the incidence and type of blood malignancies are well documented amid primary Sjögren’s syndrome patients (pSS), data focusing on solid neoplasms are more conflicting. We aimed to describe clinical, pathological, and immunological characteristics of pSS patients with cancers, along with the chronological interplay between the two conditions.
Methods
Outcomes concerning both pSS and cancer were retrospectively collected from Montpellier University Hospital (tertiary center) between 2019 and 2020. pSS characteristics were compared to a control group of pSS patients without cancer.
Results
A total of 165 patients with pSS were included: 55 patients with cancer (52 female, mean age 58.4 ± 10.4 years at pSS diagnosis; mean follow-up 10.5 ± 10.1 years, 12 patients had multiple cancers) and 110 controls without cancer. Characteristics of pSS patients with cancers were different from controls mostly for lymphoma prognosis factors. Among the 70 cancers, we recorded 55 solid neoplasms (whom 27 breast cancers and 8 lung cancers, and 82% of adenocarcinomas), with no evidence of disease at the end of follow-up in 85% of them. Among the 15 recorded blood malignancies, ten were lymphomas with an excellent prognosis. Regarding chronological interplay between cancer and pSS, most cancers (43%) were diagnosed close (± 5 years) to pSS diagnosis. Breast cancers were diagnosed before or close to pSS diagnosis (mean delay − 1.8 ± 13.0 years), at an early stage, with only two relapses (no cancer-related death), while lung cancers were diagnosed late after.
Conclusions
The tight chronological interplay between breast cancer and pSS and the intriguing pathological and immunological pattern of pSS in these patients suggest a hypothesis of immune control of cancer.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00262-023-03565-6.
Keywords: Sjögren’s syndrome, Cancer, Breast cancer, Cancer–immune system interactions, Onco-immunology
Introduction
Primary Sjögren’s syndrome (pSS) is a systemic autoimmune disease characterized by sicca syndrome, arthromyalgia, and fatigue. The classical epidemiological distribution of the disease concerns women (9:1) between 40 and 50 years of age [1, 2]. This is also the classical time distribution for cancers, especially breast cancer, in general population (French national data on cancer available on InCA website: https://www.santepubliquefrance.fr/content/download/190588/document_file/172287_spf00000892.pdf). That raises question on the potential bidirectional interactions between the two diseases. The increased occurrence of blood-related malignancies (specifically non-Hodgkin’s lymphoma (NHL) and especially marginal zone lymphoma) is well recognized in primary Sjögren’s syndrome (pSS), but the epidemiology of solid tumors remains a subject of debate [3–9]. The existing literature on the subject exhibits contrasting results. While some studies did not find differences in incidence rates for certain cancers, two reported a higher standardized incidence ratio (SIR) in pSS for lung cancer [3, 5]. A previously reported lower incidence of colon cancer and an increased incidence of thyroid cancer in pSS were noted [6]. Regarding breast cancer, some studies found a reduced risk in pSS patients, while others reported no difference or even a higher incidence [3, 7, 9, 10]. Our team recently described the comparative incidence of cancers between a French population of hospitalized pSS patients and matched controls. Apart from a classical over-incidence of blood malignancies (especially B lymphomas) and thyroid cancers, we observed a decreased incidence of breast cancers among pSS patients, while the incidence of lung and colon cancers was similar [10]. Pulling together these results with those concerning the tight chronological interplay between connective tissue disorder and cancer [11–13], we chose to further explore the clinical relationship between cancer and pSS, delving into a more detailed and clinical analysis of this association. Indeed, the primary limitation of epidemiological studies lies in the absence of a precise description of the biological, histopathological, and clinical characteristics of patients. The aim of the present study was therefore to answer clinical questions that could not be addressed in the epidemiological study, and thus to describe the characteristics of patients followed up at our center and presenting with the association between pSS and cancer.
Methods
Study population
Patients diagnosed with pSS and cancer followed in Montpellier University Hospital from 1990 were included. Patients were selected from three sources: (i) doctor’s reporting of pSS patients with cancer among their patients (Rheumatology and Internal medicine departments); (ii) pSS patients with cancer identified through the hospital claim database (Programme de Médicalisation des Systèmes d’Information, PMSI) at Montpellier University Hospital over the 2009–2018 period, identified through the International Classification of diseases (ICD-10); (iii) pSS with cancer, identified from the control population of pSS and reassigned after complete reviewing of their medical charts (Supplementary Figure S1).
Patients with pSS fulfilled ACR/EULAR 2016 criteria [14, 15], after a comprehensive expertise of their medical charts. The diagnosis was validated by the patient’s referring specialist, and two experts (RG and PG). Patients with secondary Sjögren’s syndrome (i.e., concomitant or later diagnosis of associated systemic lupus, systemic sclerosis, inflammatory myositis, rheumatoid arthritis, mixed or undifferentiated connective tissue disorder), or with inoperable medical files, were excluded.
Cancer diagnoses were assessed by complete reviewing of patients’ medical charts, including past mentioned medical history, pathological results, and radiological findings.
Control population: a random population of pSS patients was identified through the Montpellier University Hospital database, according to ICD-10 codes of Sjögren’s syndrome, and after a complete review of their medical charts (secondary SS excluded and pSS with cancers reassigned).
Outcomes
For each pSS patient, we recorded the following items: gender, past medical history (smoking or alcohol abuse, autoimmune diseases, cardiovascular diseases, personal and familiar history of cancer), age at pSS diagnosis, immunological status (anti-SSa/SSb, rheumatoid factor, monoclonal gammopathy, cryoglobulinemia, complement consumption), and minor salivary gland biopsy (MSGB) results; clinical and biological characteristics of the disease included in EULAR Sjögren’s syndrome disease activity index (ESSDAI) score (15) were recorded; maximum ESSDAI (maxESSDAI) over the available studied period was assessed. Past and actual immunomodulatory treatments were collected. Living status at last follow-up was recorded.
Regarding cancer, we recorded: anatomical site; age at cancer diagnosis, and delay between cancer and pSS diagnosis; pathological features; cancer stage; cancer treatment strategy (surgery, chemotherapy, radiotherapy, hormonal therapy, targeted therapy, palliative care), number of treatment lines; cancer prognosis. We recorded the presence of necrosis, fibrosis, and lymphocytic infiltrates within the cancer tissue. At the end of the follow-up, patients were classified as “no evidence of disease” when: (i) a complete response was observed after a medical treatment; (ii) there was no remaining disease after surgical treatment.
Concerning the analysis of the temporal relationship, we studied each occurrence of cancer independently: metachronous cancer events within a single patient were considered as two cancer occurrences; conversely, synchronous cancers at the same anatomical site in a single patient were considered and analyzed as one single cancer occurrence.
Clinical, laboratory, and radiological features were collected from Montpellier hospital medical software (DxCare®), and from medical charts of Montpellier Cancer Institute.
Statistical analysis
Between-group comparisons of quantitative variables were performed using the Student’s t test or the Mann–Whitney U test when appropriate. Chi-square test or Fisher test was used to compare categorical variables when appropriate. p values < 0.05 were considered as significant. Data and statistical analyses were performed using SAS software (SAS® 9.4; SAS institute inc., Cary, NC, USA).
Ethical approval
Our study was conducted in accordance with the local ethical committee of the University Hospital center of Montpellier. According to French law, this retrospective study does not require written consent from each patient. French law requires a favorable opinion from the local ethics committee, which was obtained: Institutional review board 2019_IRB-MTP_05-22.
Results
General characteristics of patients
Fifty-five pSS patients exhibiting 70 cancers (Supplementary Fig. 1) and followed in Montpellier University Hospital were included. These 55 patients were women in 94.5%, with a mean age at pSS diagnosis of 58.4 years (± 10.4). The main characteristics of pSS are listed in Table 1: MSGB positivity (86%), anti-SSa/SSb positivity (38%), pSS-related lung involvement (20%); 29% had a tobacco exposure, and 11% were heavy drinkers. Sixty percent had been exposed to hydroxychloroquine (HCQ), 16% had received antiCD20 monoclonal antibody, and 4% other immunosuppressive drugs. The mean delay between pSS diagnosis and the first diagnosed cancer was + 1.0 year (± 11.4). Three (5.5%) patients died (two because of cancer, one because of infection; none died because of pSS). The mean time of follow-up was 10.5 years (± 10.1).
Table 1.
General characteristics of patients with primary Sjögren’s syndrome with and without associated cancer
| pSS Patients with solid cancer and/or hemopathy n = 55 | pSS patients with solid cancer without hemopathy n = 40 | pSS patients with pSS and hemopathy only n = 8 | pSS patients with more than one cancer and/or hemopathy n = 12 | pSS patients without history of solid cancer and/or hemopathy n = 110 | |||||
|---|---|---|---|---|---|---|---|---|---|
| n (%) | p value* | n | p value* | n (%) | p value* | n (%) | p value* | n (%) | |
| Female (n, %) | 52 (94.5%) | 1.000 | 37 (92.5%) | 1.000 | 8 (100,0%) | 0.456 | 11 (91.7%) | 0.588 | 102 (92.7%) |
| Age at pSS diagnosis (mean ± SD) | 58.4 (± 10.4) | 0.005 | 59.3 (± 9.36) | 0.004 | 50,6 (± 10,4) | 0.028 | 58 (± 17.5) | 0.364 | 52.3 (± 13.9) |
| Follow-up (mean ± SD) (years) | 10.5 (± 10.1) | 0.178 | 9.9 (± 8.4) | 0.170 | 16.5 (± 15.9) | 0.148 | 10.3 (± 9.7) | 0.663 | 7.9 (± 8.3) |
| MSGB positivity (n, %) | 43 (86.0%) | 0.427 | 30 (75.0%) | 0.352 | 8 (100,0%) | 0.361 | 8 (66.7%) | 0.287 | 93 (84.5%) |
| Anti-Ro/SSa and/or Anti La/SSb positivity (n, %) | 21 (38.2%) | 0.284 | 15 (37.5%) | 0.301 | 4 (50,0%) | 1.000 | 8 (66.7%) | 0.363 | 50 (45.5%) |
| Sicca syndrome (n, %) | 42 (91.3%) | 0.006 | 33 (82.5%) | 0.011 | 5 (62,5%) | 0.574 | 10 (83.3%) | 0.280 | 80 (72.7%) |
| Constitutional symptoms (n, %) | 11 (20.0%) | 0.611 | 4 (10.0%) | 0.321 | 4 (50,0%) | 0.043 | 3 (25.0%) | 0.440 | 15 (13.6%) |
| Joint involvement (n, %) | 40 (72.7%) | 0.690 | 32 (80.0%) | 0.213 | 5 (62,5%) | 0.700 | 7 (58.3%) | 0.514 | 76 (69.1%) |
| Lymphadenopathy (n, %) | 15 (27.3%) | < 0.001 | 4 (10.0%) | 0.454 | 5 (62,5%) | < 0.001 | 6 (50.0%) | < 0.001 | 5 (4.5%) |
| Cutaneous activity (n, %) | 15 (27.3%) | 0.020 | 9 (22.5%) | 0.127 | 4 (50,0%) | 0.017 | 3 (25.0%) | 0.208 | 15 (13.6%) |
| Pulmonary activity (n, %) | 11 (20%) | 0.133 | 5 (12.5%) | 0.802 | 3 (37,5%) | 0.068 | 4 (33.3%) | 0.056 | 11 (10.0%) |
| Renal activity (n, %) | 4 (7.3%) | 0.067 | 1 (2.5%) | 0.519 | 1 (12,5%) | 0.156 | 3 (25.0%) | 0.005 | 2 (1.8%) |
| Peripheral neurological system (n, %) | 9 (16.4%) | 0.159 | 7 (17.5%) | 0.143 | 0 (0,0%) | 0.384 | 3 (25.0%) | 0.114 | 10 (9.1%) |
| Autoimmune cytopenia (n, %) | 4 (7.3%) | 1. 000 | 2 (5.0%) | 1.000 | 0 (0,0%) | 0.454 | 2 (16.7%) | 0.234 | 7 (6.3%) |
| Hypocomplementemia (n, %) | 7 (14.6%) | 0.048 | 2 (5.0%) | 0.649 | 2 (25,0%) | 0.057 | 3 (25.0%) | 0.027 | 4 (3.6%) |
| Cryoglobulinemia (n, %) | 7 (16.3%) | 0.026 | 2 (5.0%) | 0.581 | 2 (25,0%) | 0.041 | 3 (25.0%) | 0.010 | 2 (1.8%) |
| Hypergammaglobulinemia (n, %) | 20 (36.4%) | 0.511 | 12 (30.0%) | 0.907 | 4 (50,0%) | 0.431 | 4 (33.3%) | 1.000 | 31 (28.2%) |
| Monoclonal gammopathy (n, %) | 14 (25.5%) | 0.031 | 5 (12.5%) | 0.870 | 4 (50,0%) | 0.015 | 6 (50.0%) | 0.004 | 11 (10.0%) |
| RF + (n, %) | 22 (40.0%) | 0.003 | 12 (30.0%) | 0.110 | 6 (75,0%) | 0.001 | 4 (33.3%) | 0.242 | 23 (20.9%) |
| ANA + (n, %) | 44 (80.0%) | 0.197 | 32 (80.0%) | 0.249 | 7 (87,5%) | 0.435 | 3 (25.0%) | 1.000 | 78 (70.9%) |
| ESSDAI max (mean ± SD) | 12.0 (± 13.0) | < 0.001 | 7.4 (± 7.1) | 0.136 | 20.6 (± 10.8) | < 0.001 | 20.2 (± 19.9) | 0.002 | 5.5 (± 5.0) |
| Corticosteroids (n, %) | 26 (47.3%) | 0.361 | 17 (42.5%) | 0.752 | 5 (62,5%) | 0.27 | 7 (58.3%) | 0.215 | 42 (38.2%) |
| Hydroxychloroquine (n, %) | 33 (60.0%) | 0.550 | 26 (65.0%) | 0.094 | 5 (62,5%) | 0.717 | 6 (50.0%) | 0.976 | 54 (49.1%) |
| Methotrexate (n, %) | 13 (23.6%) | 0.628 | 9 (22.5%) | 0.769 | 2 (25,0%) | 0.667 | 5 (41.7%) | 0.138 | 19 (17.3%) |
| Rituximab (n, %) | 9 (16.4%) | 0.167 | 3 (7.5%) | 1.000 | 4 (50,0%) | 0.007 | 3 (25.0%) | 0.117 | 8 (7.2%) |
| Immunosuppressive therapy (CYC or AZA) (n, %) | 2 (3.6%) | 0.710 | 2 (5.0%) | 1.000 | 0 (0,0%) | 0.454 | 0 (0%) | 1.000 | 7 (6.3%) |
| Hypertension (n, %) | 28 (50.9%) | 0.082 | 19 (47.5%) | 0.226 | 4 (50,0%) | 0.467 | 9 (75.0%) | 0.012 | 41 (37.3%) |
| Cardiopathy (n, %) | 12 (21.8%) | 0.001 | 6 (15.0%) | 0.067 | 3 (37,5%) | 0.011 | 3 (25.0%) | 0.033 | 8 (7.3%) |
| Diabetes (n, %) | 10 (18.2%) | 0.056 | 8 (20.0%) | 0.042 | 1 (12,5%) | 0.504 | 4 (33.3%) | 0.023 | 9 (8.2%) |
| Autoimmune disease (n, %) | 13 (23.6%) | 0.685 | 10 (25.0%) | 0.842 | 1 (12,5%) | 0.675 | 4 (33.3%) | 0.732 | 31 (28.2%) |
| Family history of breast cancer (n, %) | 7 (20.6%) | 0.026 | 6 (15.0%) | 0.022 | 0 (0,0%) | 1.000 | 1 (8.3%) | 0.284 | 3 (2.7%) |
| Smoking exposure (n, %) | 12 (28.6%) | 0.616 | 11 (27.5%) | 0.433 | 1 (12,5%) | 1.000 | 2 (16.7%) | 0.637 | 24 (21.8%) |
| Excessive alcohol consumption (n, %) | 4 (10.8%) | 0.091 | 3 (7.5%) | 0.131 | 0 (0.0%) | 1.000 | 1 (8.3%) | 0.209 | 3 (2.7%) |
MSBG, minor salivary gland; RF, rheumatoid factor; ANA, antinuclear antibodies; ESSDAI, EULAR Sjögren’s syndrome disease activity index; CYC, cyclophosphamide; AZA; azathioprine
% were adjusted according to available data.
*univariate analyses were performed against the control group of 110 pSS patients without cancer, p value < 0.05
In parallel, we constituted a control group of 110 verified pSS patients: 93% of women, mean age at pSS diagnosis of 52.3 (± 13.9) years, mean time of follow-up of 7.9 (± 8.3) years. Their characteristics are listed in Table 1: MSGB positivity (84.5%), anti-SSa/SSb positivity (45.5%), pSS-related lung involvement (10%); 22% had a tobacco exposure, and 3% were heavy drinkers; 49% had been exposed to HCQ, 7% to antiCD20 monoclonal antibody, and 6% to other immunosuppressive drugs.
The characteristics of pSS patients with and without cancer were similar except the following ones (all more prevalent in pSS patients with cancer): mean age at diagnosis, sicca syndrome, prognosis factors for lymphoma (lymph node enlargement, hypocomplementemia, monoclonal gammopathy, presence of rheumatoid factor or cryoglobulinemia), familial history of breast cancer, and past cardiopathy (Table 1).
The overall mean survival rate of patients with pSS and cancer (n = 55) was 9.2 years [6, 10]. 2/3 patients died because of cancer.
General characteristics of malignancies
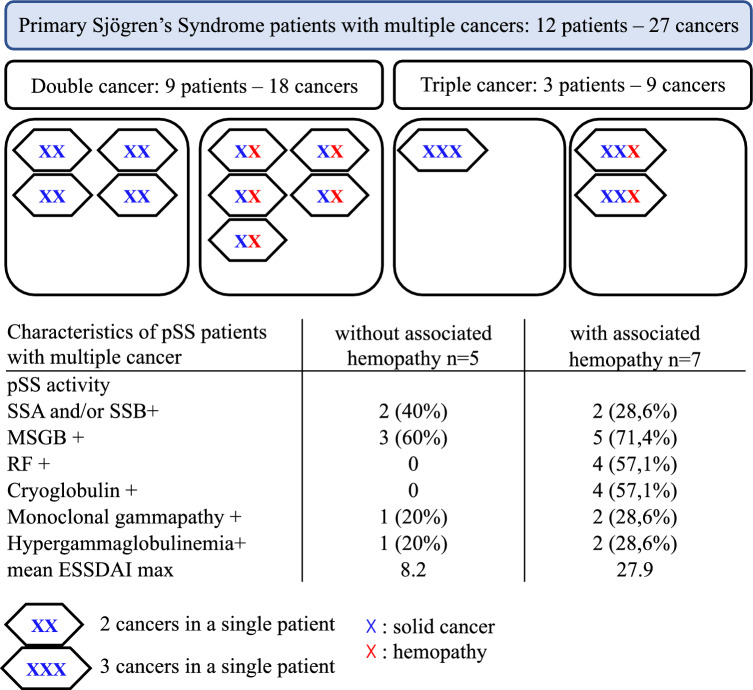
Among these 55 pSS patients, 70 cancers were identified: 55 solid neoplasms and 15 blood malignancies (Fig. 1). Twelve pSS patients experienced multiple cancers (nine patients with two cancers, and three patients with three cancers) (Fig. 2). The mean age for diagnosis of the first cancer was 59.1 years (± 11.9).
Fig. 1.
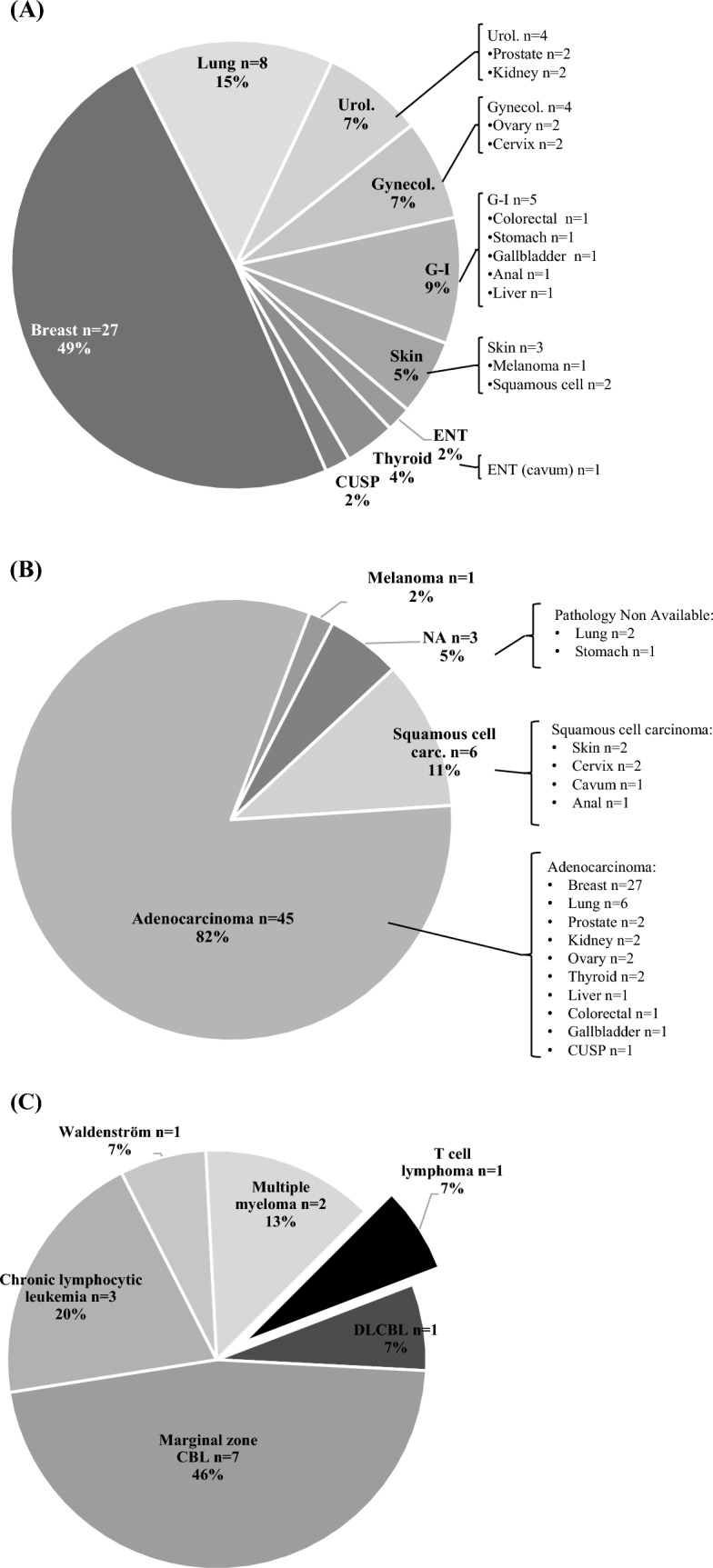
Distribution of 70 cancers, and their pathological characteristics, among 55 patients with primary Sjögren’s syndrome. A Distribution of 55 solid neoplasms. B Pathological classification of 55 solid neoplasms. C Subtypes of 15 blood malignancies. Urol., urological; Gynecol.; gynecological; G–I, gastrointestinal; ENT, ear–nose–throat; CUSP, carcinoma of unknown origin; NA, non-available; carc., carcinoma; CBL, B cell lymphoma; DLCBL, diffuse large B cell lymphoma
Fig. 2.
Distribution and characteristics of primary Sjögren’s syndrome patients with multiple cancers. pSS, primary Sjögren’s syndrome patients; SSa/SSb, Sjögren’s syndrome antigen; MSBG, minor salivary gland; RF, rheumatoid factor; ESSDAI, EULAR Sjögren’s syndrome disease activity index. 7 Blood malignancies: four lymphomas (one diffuse large B cell lymphoma and three marginal zone lymphomas), two chronic lymphocytic leukemia, and one light chain multiple myeloma. 20 solid cancers: three squamous cell carcinomas (one anal canal carcinoma and two cervical cancers, all three potentially induced by human papillomavirus (HPV); 17 adenocarcinomas (eight breast cancers, five lung cancers; one prostate, colon, ovarian, and thyroid cancers
Solid neoplasms
Among the 55 solid neoplasms, 27 (49%) concerned breast cancer, and 8 (15%) concerned lung cancer. Gastrointestinal cancers were the third group, followed by gynecological cancers, and urological cancers (two prostate cancers and two clear cell renal cell carcinomas) (Fig. 1A). There were one ear–nose–throat cancer, two thyroid cancers (one of which is papillary thyroid carcinoma, the second being of unknown histology), three skin cancers, and one cancer of unknown origin.
Concerning pathological features, 45 (82%) were adenocarcinoma, and 6 (11%) squamous cell carcinoma (Fig. 1B). Concerning the prognosis, 31 (56%) of cancers were at stage < T3 at diagnosis, and only four (7.3%) were metastatic (Table 2).
Table 2.
Primary Sjögren’s syndrome patients characteristics and cancer extension according to malignancies subtypes
| All cancers n = 70† |
All solid cancers n = 55† |
Breast | Lung | Other * | Lymphoma n = 9 | Other Hemopathy n = 6 | |
|---|---|---|---|---|---|---|---|
| n = 27† | n = 8† | n = 20† | |||||
| Female (n, %) | 64 (94.1%) | 49 (92.4%) | 26 (100%) | 6 (85.7%) | 17 (85%) | 9 (100%) | 6 (100%) |
| Age at diagnosis of pSS (mean ± SD) | 58.4 (10.7) | 59.1 (10.0) | 58.6 (11.8) | 53.6 (7.7) | 61.7 (8.4) | 51.8 (7.1) | 62.3 (17.5) |
| Age at diagnosis of cancer (mean ± SD) | 59.1 (11.9) | 59.8 (11.7) | 56.3 (10.5) | 62.0 (5.3) | 63.5 (13.6) | 54.8 (10.5) | 60.2 (15.6) |
| Time between diagnosis of cancer and pSS (years) (mean ± SD) | 1.0 (11.4) | 1.0 (12.4) | − 1.8 (13.0)# | 8.8 (7.8)# | 1.9 (11.9) | 3 (8.0) | − 2.1 (5.8) |
| ESSDAI Max (mean ± SD) | 14.7 (16.1) | 11.9 (14.9) | 9.1 (8.1) | 17.4 (23.0) | 13.8 (15.6) | 30.7 (18.0) | 14.8 (9.7) |
| pSS: immunological and biological characteristics | |||||||
| MSGB positivity (n, %) | 53 (77.9%) | 40 (75.5%) | 20 (74.1%) | 3 (42.9%) | 17 (85.0%) | 7 (77.8%) | 6 (100.0%) |
| Anti-Ro/SSa and/or Anti La/SSb positivity (n, %) | 27 (39.7%) | 20 (37.7%) | 7 (26.9%)# | 6 (85.7%)# | 7 (35.0%) | 6 (66.7%) | 1 (16.7%) |
| RF + (n, %) | 27 (39.7%) | 17 (32.1%) | 7 (26.9%) | 2 (28.6%) | 8 (40.0%) | 8 (88.9%) | 2 (33.3%) |
| Cryoglobulinemia (n, %) | 12 (17.6%) | 7 (13.2%) | 3 (11.5%) | 2 (28.6%) | 2 (10.0%) | 5 (55.6%) | 0 (0.0%) |
| Monoclonal gammopathy (n, %) | 22 (32.4%) | 13 (24.5%) | 6 (23.1%) | 3 (42.9%) | 4 (20.0%) | 6 (66.7%) | 3 (50.0%) |
| Cancer: TNM classification | |||||||
| Tis (n, %) | NA | 8 (14.5%) | 7 (41.2%) | 1 (12.5%) | 0 (0.0%) | ||
| T1-2 (n, %) | NA | 23 (41.8%) | 10 (58.8%) | 6 (75.0%) | 7 (35.0%) | ||
| T3-4 (n, %) | NA | 6 (10.9%) | 0 (0.0%) | 1 (12.5%) | 5 (25.0%) | ||
| Metastasis at diagnosis (n, %) | NA | 4 (7.2%) | 0 (0.0%) | 0 (0.0%) | 4 (20.0%) | ||
| Cancer: tissue samples pathological characteristics ‡ | |||||||
| Fibrosis (n, %) | – | 22 (75.9%) | 12 (92.3%) | 4 (66.7%) | 6 (30.0%) | ||
| Lymphocytic infiltrate (n, %) | – | 13 (44.8%) | 3 (23.1%)# | 5 (83.3%)# | 5 (25.0%) | ||
| Tumoral necrosis (n, %) | – | 9 (31.0%) | 3 (23.1%) | 1 (16.7%) | 5 (25.0%) | ||
| Number of cancer treatment lines | |||||||
| 1 (n, %) | 52 (74.3%) | 45 (81.2%) | 23 (85.2%) | 8 (100%) | 14 (70.0%) | 6 (66.7%) | 1 (16.7%) |
| > 1 (n, %) | 8 (11.4%) | 4 (7.2%) | 2 (7.4%) | 0 (0.0%) | 2 (10.0%) | 3 (33.3%) | 1 (16.7%) |
| Abstention (n, %) | 6 (8.6%) | 2 (3.6%) | 0 (0.0%) | 0 (0.0%) | 2 (10.0%) | 0 (0.0%) | 4 (66.7%) |
| Cancer treatments | |||||||
| Chemotherapy (n, %) | 19 (27.1%) | 12 (21.8%) | 8 (29.6%) | 0 (0.0%) | 4 (20.0%) | 5 (55.6%) | 2 (33.3%) |
| Surgery (n, %) | 50 (71.4%) | 47 (85.5%) | 26 (96.3%) | 8 (100%) | 13 (65.0%) | 3 (33.3%) | 0 (0.0%) |
| Radiotherapy (n, %) | 27 (38.6%) | 25 (45.5%) | 19 (70.4%) | 0 (0.0%) | 6 (30.0%) | 2 (22.2%) | 0 (0.0%) |
| Hormonal therapy (n, %) | 13 (18.6%) | 13 (23.6%) | 12 (44.4%) | 0 (0.0%) | 1 (5.0%) | 0 (0.0%) | 0 (0.0%) |
| Targeted therapy (n, %) | 7 (10.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 6 (66.7%) | 1 (16.7%) |
| Palliative care only (n, %) | 1 (1.4%) | 1 (1.8%) | 0 (0.0%) | 0 (0.0%) | 1 (5.0%) | 0 (0.0%) | 0 (0.0%) |
| Cancer evolution | |||||||
| Complete response (n, %) | 55 (78.6%) | 47 (85.5%) | 26 (96.3%) | 7 (87.5%) | 14 (70.0%) | 8 (88.9%) | 0 (0.0%) |
| Partial response (n, %) | 3 (4.3%) | 2 (3.6%) | 0 (0.0%) | 0 (0.0%) | 2 (10.0%) | 0 (0.0%) | 1 (16.7%) |
| Progression (n, %) | 4 (5.7%) | 2 (3.6%) | 0 (0.0%) | 0 (0.0%) | 2 (10.0%) | 1 (11.1%) | 0 (0.0%) |
| Relapse (n, %) | 6 (8.6%) | 3 (5.4%) | 2 (7.4%) | 0 (0.0%) | 1 (5.0%) | 3 (33.3%) | 0 (0.0%) |
| Stable disease (n, %) | 4 (5.7%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 4 (66.7%) |
| Death (n, %) | 2 (2.9%) | 1 (1.8%) | 0 (0.0%) | 0 (0.0%) | 1 (5.0%) | 1 (11.1%) | 0 (0.0%) |
| Ongoing treatment (n, %) | 1 (1.4%) | 1 (1.8%) | 0 (0.0%) | 0 (0.0%) | 1 (5.0%) | 0 (0.0%) | 0 (0.0%) |
| Lost to follow-up (n, %) | 2 (2.9%) | 2 (3.6%) | 0 (0.0%) | 1 (12.5%) | 1 (5.0%) | 0 (0.0%) | 0 (0.0%) |
pSS, primary Sjögren’s syndrome; MSBG, minor salivary gland biopsy; RF, rheumatoid factor; ANA, Antinuclear antibodies; ESSDAI, EULAR Sjögren’s syndrome disease activity index; CYC, cyclophosphamide; AZA, azathioprine; TNM, tumor, node, metastasis; NA, not available
† = Characteristics of pSS studied after exclusion of synchronous or metachronous cancer in one patient (lung cancer n = 7)
* = Other solid cancer, including: urological (n = 4), gastrointestinal (n = 5), gynecological (n = 4), cutaneous (n = 3), thyroid (n = 2), Ear–nose–throat (n = 1), unknown origin (n = 1)
‡ = percentage calculated after exclusion of missing data (breast n = 13, lung n = 6, other n = 10, all solid cancer n = 29). Available data for pathological characteristics: breast n = 13, lung n = 6, other n = 10, all solid cancer n = 29. Available data for tumor characteristics: breast n = 17, lung n = 8, other n = 15, all solid cancer n = 40
#Significant differences between subgroups
A single line of treatment was required in the majority of cases (81%). Treatment modalities were distributed as follows: 85.5% of patients underwent surgical treatment, 45.5% received radiotherapy, and 21.8% were administered chemotherapy in conjunction with a local treatment. Among those with breast cancer, 44.4% were treated with additional hormonal therapy, and one patient with prostate cancer (50% of the cases) received similar treatment. No patients were treated with checkpoint inhibitor immunotherapy (Table 2). One patient, diagnosed as metastatic, was provided supportive care without any specific treatment. By the end of the follow-up period, a complete response was achieved in 85% of the cases involving solid neoplasms. Only one death related to cancer was recorded.
Blood malignancies
Among the 70 cancers identified, there were 15 malignant hematologic diseases in 15 different patients, including ten lymphomas, three chronic lymphocytic leukemia (CLL), and two multiple myelomas (one was a light chain myeloma) (Fig. 1C). We did not observe any leukemia or myeloid hemopathy in our cohort. Among the ten lymphomas, there were three distinct diseases: (i) seven cases concerned low-grade lymphomas of the marginal zone lymphoma (MZL) type, and more precisely six were mucosa-associated lymphoid tissue (MALT) lymphomas; notably, four of them were of parotid location, one of lymph node location, and two of unspecified location; (ii) there was only one lymphoplasmacytic lymphoma (Waldenström disease); (iii) two cases were high-grade lymphomas: one diffuse large B cell lymphoma (DLBCL) and one angioimmunoblastic T lymphoma (AIL). Seven (46.7%) of these blood malignancies required only one therapeutic line, after an average follow-up time of 5 years (Table 2). The primary treatment for lymphoma predominantly involved a combination of chemotherapy and the anti-CD20 antibody for four B cell lymphomas and one lymphoplasmacytic lymphoma. One patient was treated solely with chemotherapy (T lymphoma), while another received only the anti-CD20 antibody (MZL). Three patients diagnosed with parotid MALT lymphoma underwent surgical treatment, and two others were treated with radiotherapy. Among the other hematologic disorders, one myeloma case was managed with chemotherapy. The remaining patients (one myeloma, three chronic lymphocytic leukemia) required only ongoing surveillance (Table 2). With the application of these measures, complete remission was observed in eight out of nine lymphoma cases with a mean follow-up of 16.5 years [15.9].
Comparing the baseline characteristics (available data) of eight patients with blood malignancies only to those of our control group, we confirmed that patients with blood malignancies presented a more active disease (general signs, lymph node enlargement, skin symptoms, higher ESSDAI) (Table 1). Regarding the 15 patients with at least one blood malignancy, their characteristics followed the same pattern.
Characteristics according to malignancy types
The immunological and histological characteristics of pSS according to the main anatomical site of cancer are shown in Table 2.
There were 27 breast cancers in 26 women (one patient with two synchronous cancers), exhibiting a mean age at pSS diagnosis of 58.6 years (± 11.8 years), with mostly mucosal and rheumatic pattern of the disease. Nine of them (34.6%) had received an immunosuppressive or immunomodulatory treatment (among whom six received rituximab). The main mode of cancer diagnosis was screening strategy (asymptomatic breast cancers). Regarding breast cancer pathological patterns, among the available data, all cancers were hormone receptor positive/HER2 negative ductal adenocarcinoma. No cases were classified as triple negative nor HER2 positive. Regarding locoregional extension (data available for 17 cancers), there were seven (41%) noninvasive tumors (in situ ductal carcinoma), and ten (59%) cancers of small to moderate size (classified as T1/T2). None of the breast cancers was metastatic at diagnosis (one missing data). We identified only five patients with lymph node involvement. Overall, all the patients were diagnosed with early breast cancers. Thus, 96% of breast cancers were treated by surgery, 70% received additional radiotherapy. Hormone therapy was given to 44% of patients and 30% received additional chemotherapy. The prognosis was excellent (96% of the patients remained without evidence of relapse at last follow-up). Two patients had recurrences, with complete remission in both cases. No patient died from her breast cancer.
Moreover, there were eight lung cancers in six female (one with two metachronous cancers) and one male patients (5/6 were smokers), with a mean age at Sjögren’s diagnosis of 53.6 years (± 7.7 years). Two of them had pSS-specific lung involvement. Four of them (57%) had received an immunosuppressive or immunomodulatory treatment (no rituximab). The main mode of cancer diagnosis was fortuitous (4/6 discovery on imaging). All of them were adenocarcinomas. Out of these eight cancer cases, all underwent surgery with no residual disease left behind. During the follow-up period, no relapse was observed among six of these patients, with one patient unfortunately lost to follow-up. No death occurred because of cancer.
Among the other known immunogenic cancers, we report one patient with melanoma diagnosed 18 years before pSS, which was treated with surgery alone, without recurrence. Interestingly, pSS in this patient seemed immunologically active with anti-Ro/SSa antibodies, cutaneous and articular involvement, and needed systemic treatment (steroids, hydroxychloroquine, and methotrexate).
Comparative chronological, pathological, and immunological characteristics of breast and lung cancers were as follows: (i) the delay between cancer diagnosis and pSS was − 1.8 (± 13.0) years for breast cancers (n = 26) and + 8.8 (± 7.8) years for lung cancers (n = 6) (p = 0.021) (in case of multiple cancers, the first cancer in a single patient was included); (ii) 20/22 (90%) patients with breast cancers exhibited MSGB positivity, versus 3/6 (50%) for lung cancers (p = 0.050); (iii) 7/26 (27%) patients with breast cancer had anti-Ro/SSa antibody positivity versus 6/7 (86%) (p = 0.008) for lung cancers.
We collected detailed histopathological data concerning breast and lung cancers in 19 patients (breast cancer n = 13, lung n = 6). Fibrosis and lymphocytic infiltrates were described in, respectively, 12 (92%) and three (23%) cases (n = 13), within breast tissue, versus, respectively, four (67%) and five (83%) within lung tissue (n = 6) (respective Fisher’s test p values of 0.22 and 0.04) (Table 2).
pSS patients with multiples cancers
Twelve patients had multiple cancers, 27 in total. The repartition and the subtypes of those cancers are depicted in Fig. 2. There were 11 women and one man, of 58 ± 17.5 years old at diagnosis of pSS. They had more lymph node enlargement (p < 0.001) and more renal damage (p = 0.005), than control patients. Biological features are those of pSS patients with blood malignancies (hypocomplementemia, cryoglobulinemia, monoclonal gammopathy), associated to a significant higher maximum ESSDAI than the control group (20.2 ± 19.9 vs. 5.5 ± 5.0 (p = 0.002)) (Table 1).
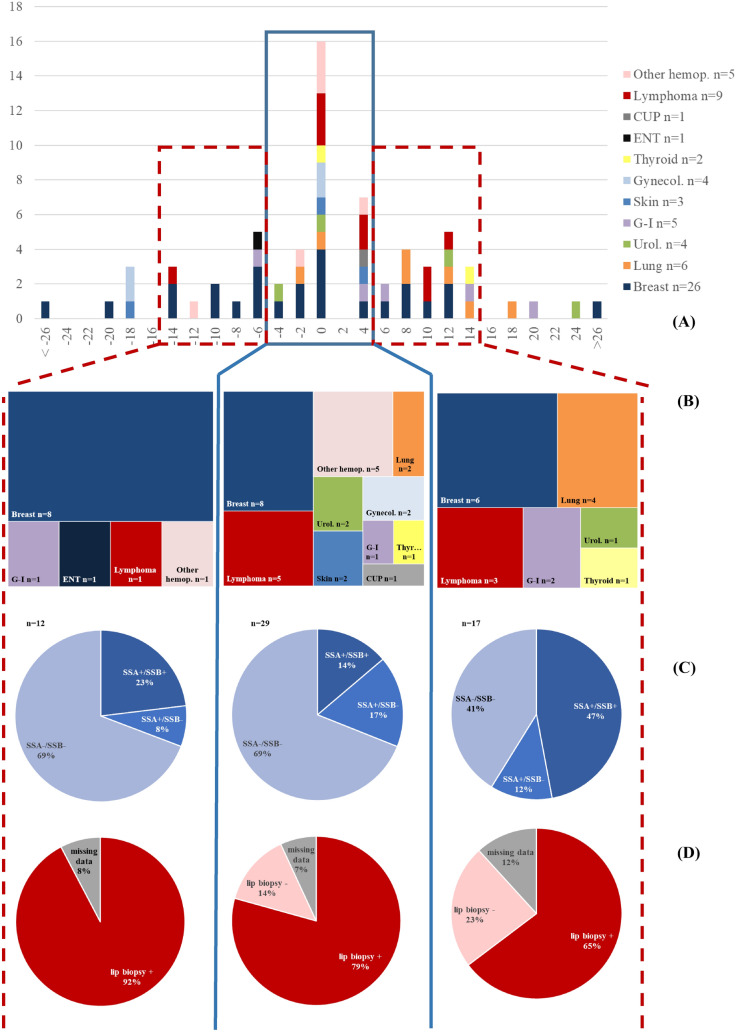
Temporal relationship between pSS and cancer
Considering the temporal relationship between cancer and pSS diagnosis (Fig. 3), we observed that most of cancers (n = 30, 43%) were diagnosed close (± 5 years) to the diagnosis of pSS. More precisely, we observed one peak of occurrence of cancer in the same year of pSS diagnosis (n = 16, 23%). In addition, the cardinal features of pSS were different according to the chronological sequence between pSS and cancer. Notably, patients with late-onset cancers (> 5 years after pSS diagnosis) had more antibody (SSA/SSb) positivity (57%, 13/23 versus 31%, 14/45, Chi-square p = 0.043) and less MSGB positivity than early onset (< 5 years after pSS diagnosis) cancers (75%, 15/20 versus 90%, 37/41, Fisher p = 0.039).
Fig. 3.
Cancer subtypes, patterns of Sjögren’s syndrome (pSS), according to temporal relationship between cancer and pSS diagnosis. A Cancer subtypes occurrence according to delay between cancer diagnosis and diagnosis of pSS diagnosis (years). B Cancer subtypes according to delay between cancer and pSS (< 5 years; [ − 5; + 5] years; > 5 years). C Immunological status and D Minor salivary gland biopsy (MSGB) results according to the same delay. Other hemop. other hemopathy; CUP, cancer of unknown primitive; ENT, ear–nose–throat; Gynecol., gynecological; G-I., gastrointestinal; Urol., urological; NA, not available. Data on a total of n = 68 cancers (in case of multiple cancers in same localization: first cancer only)
Discussion
Our study involving 55 pSS patients confirms the increased prevalence of blood malignancies in pSS patients, already well described in literature [3–5, 7, 8, 16], along with their classical prognosis markers [17–22]. We also provide original data on solid neoplasms, and observed a high frequency of adenocarcinomas among lung cancers in pSS patients. The high proportion of breast cancer is consistent with epidemiological data among this population of female patients ≥ 50 years old (French national data on cancer available on InCA website: https://www.santepubliquefrance.fr/content/download/190588/document_file/172287_spf00000892.pdf), but contrasts with the low incidence of breast cancer that we observed in hospitalized pSS patients on the French hospitalization database (2.57 breast cancers per 1000 person-years in pSS patients) [10]. Otherwise, consistently with these previous results on epidemiological data, we observed here a low proportion of colon cancer (only one case) and a high proportion of multiple cancers (12/55 patients), one of them being a blood malignancy in many cases.
The present study also provides information on the factors associated with cancers. Regarding potential carcinogens, the prevalence of tobacco consumption and alcohol abuse was similar in pSS patients with and without cancer. There was no difference in immunosuppressants’ exposure between the groups. However, among the six patients who received immunosuppressant (cyclophosphamide, azathioprine, rituximab) before the cancer onset, two cancers could have been favored by these exposures (one skin cancer and one anal carcinoma induced by HPV). Finally, we mainly observed a higher proportion of familial history of breast cancer in pSS patients with cancer than controls, which is easily explained by the high proportion of breast cancers in the population from our study and consistent with the findings in general population.
In this retrospective study that encompassed a wide variety of cancers, presenting homogeneous results regarding treatments was unreachable. Certainly, treatments for tumors were tailored based on the type of cancer. Given the significant presence of localized cancers, the predominance of surgical treatments was consistent. Considering the extensive period over which cancers were diagnosed (from 1966 to 2019, Fig. 3), treatment recommendations likely evolved, so we refrained from detailing specific chemotherapy types. Targeted therapy, mainly anti-CD20 antibodies like rituximab, was used solely for hematologic malignancies, either as a standalone treatment or in combination with chemotherapy. Of course, this treatment has immunosuppressive effects, which may have improved systemic manifestations pSS to some extent. None of the patients received immune checkpoint inhibitors, either due to the treatment’s unavailability at the time of diagnosis or a lack of indication. Of note, tolerance of treatments was similar to that observed in the general population, and no atypical side effect was noted. Although we were unable to accurately gather information regarding the tolerance to hormonal therapy, it is noteworthy that breast cancer was most often diagnosed either prior to or “simultaneously” with the detection of pSS. Thus, hormonal therapy, known to cause arthromyalgia, might have amplified latent symptoms, indirectly improving the capacity for diagnosing pSS in some patients.
Some results concerning breast cancers raise a number of comments and questions. Our results from this clinical series have to be studied in parallel of our epidemiological previous study involving hospitalized pSS patients in comparison with controls [10], suggesting a “protective effect” of pSS against incidental breast cancers (adjusted HR of 0.60 [0.49–0.74]). Chen and coll [23] observed a lower incidence of pSS in a population of patients with breast cancer. Such a “protective effect” might reflect an immune control of breast cancer in pSS. Interestingly, pSS-specific immune response toward salivary glands may be also directed against breast tissue, as illustrated by our previous study of pSS breast involvement in pSS (including patients with or without breast cancer) [24]. Thus, one could hypothesize that pSS immune response targeting glandular tissue may protect from the development or the spreading of breast cancers, through targeting neoplastic epithelial antigens localized within the mammary glandular epithelium. In some specific cases with accumulating oncogenic events, the immune control system would be overwhelmed, and the cancer would become “clinical.” According to these latter statements, the present study on pSS-associated cancers could be considered as a photographic negative of these epidemiological studies reporting a protective effect of pSS, describing herein the cancers escaping from immune control.
Although this scenario remains hypothetical, our results do not deny the existence of an anti-tumor immune response within mammary glands. Firstly, mammary tissue exhibited a high proportion of fibrosis and less lymphocytic infiltrates in patients with breast cancer compared to lung tissue, suggesting eventually a more prolonged immune reaction. Secondly, breast cancers were mostly diagnosed before or at the same time as pSS. Thus, there was a limited progression of breast cancer in all cases, without any metastatic cancer, and with an overall excellent prognosis. Interestingly, we observed previously a lower incidence of hospitalized death among pSS patients with breast cancers in our epidemiological study [10]. In this latter work, a reduced risk of incidental breast cancer was observed among pSS patients, including all stages of cancer, except in situ cancers. In other terms, the incidence rate of in situ breast cancer was not different between pSS and matched controls, suggesting that the reduced incidence of progressive breast cancers was not linked to an earlier diagnosis through screening strategy.
Admittedly, the concept of immune surveillance remains to be fully demonstrated in pSS at this stage. These questions arise as new immunological concepts in cancer, ranging from anti-cancer immunotherapies to the relationship between cancer and connective tissue disorders. The most emblematic disease in this area is systemic sclerosis, in which anti-RNA polymerase III antibodies have been directly linked to a tolerance breakdown toward modified tumor antigens (following somatic mutations) [11, 12]. These pathophysiological hypotheses linking mucosal immunosurveillance and carcinogenesis are also consistent with the data coming from the study of immune checkpoint inhibitors (ICIs) use in oncology. Indeed, the spectacular advances brought by the use of ICIs on the prognosis of cancers has shed to light the close relationship between carcinogenesis and immune system, illustrated by the autoimmune toxicities of ICIs. Among them, sicca syndrome and/or Sjögren’s syndrome have been reported with these drugs [25–30]. Actually, these findings in patients treated by ICIs (mainly for lung, skin, and renal cancers) support the concept of a pSS anti-tumor immune response, far beyond the field of breast cancer. In the immune system's fight against cancer, the occurrence of cancer may simply imply that the immune response is insufficient, inappropriate or eventually overwhelmed.
Among the solid cancers to which a pSS-related immune surveillance may apply, lung cancers also merit some comments. Whereas pSS observed during ICIs mainly concern lung neoplasm [28], there are few literature data concerning lung cancers and SS, which do not suggest any clear positive interaction in the absence of ICIs. Epidemiological data are heterogeneous: three studies [31–33] reported a higher standardized incidence ratio (SIR) of about 1.5 in pSS patients for lung cancers incidence; other epidemiological studies [3, 5, 8, 10] did not find any differences in incidence rates between pSS and matched patients. Interestingly, lung cancers mainly belong to the group of “late-onset” cancers, which also include thyroid and ENT cancers. This is consistent with the findings by Yang et al. [34], who reported 83% of adenocarcinoma, with 67% of late-stage cancer, among 18 patients with SS and lung cancers. Notably, we observed a more humoral disease pattern in patients with “late-onset” cancers (lung, thyroid, and ENT cancers), which were associated with negative MSBG in 50% of cases, but frequent antibody positivity, and a lymphocytic infiltrate in 83% of the tumoral tissues (data available on lung tissue). This may reflect some different immune mechanisms in this group of pSS patients with late-onset cancers.
In any case, the relationships between glandular autoimmune inflammation and cancer-induced inflammation remain to be clarified, probably in light of the complete characteristics of each case. Further investigation into the histological and mutational profiles of cancer is of great importance, with particular attention to specific oncogenic drivers, such as EGFR in lung cancer, HER2 in breast cancer or microsatellite instability status in digestive and gynecological cancers. This is particularly true for EGFR mutations, considering their role in activating the EGFR pathway, which has been reported to lead to chronic inflammation in Sjögren’s disease [35]. However, in the present work, we did not gather information regarding driver mutations in cancers due to the comprehensive data available on histology and the complexity arising from the heterogeneity of the cancers and varying times of diagnosis. In addition, the characterization of immune responses within tumors, alongside a comparison with glandular inflammation, represents a crucial objective. It is anticipated that utilizing mass cytometry approaches may shed light on these questions and provide answers in the near future. Since pSS manifests in glandular foci, a form of tertiary lymphoid structures, the examination of tumor-induced lymphocytes (TILs) will be particularly insightful. This analysis will complement the study of PDL1 expression status within tumors, further enriching our understanding.
Finally, our results suggest that the temporal relationship between pSS and cancer is important for the clinical expression of both conditions (cancer types and pSS phenotype). In particular, patients with early onset cancer had more frequently MSGB positivity, whereas patients with late-onset cancer had more frequently antibody positivity. Unfortunately, the low number of patients and heterogeneity of cancer subtypes within chronological groups do not allow us to comment further this discrepancy.
Undoubtedly, our hypotheses remain to be documented in larger studies, with pathological and immunohistochemistry analysis of tissues. The retrospective nature of the work, the small sample size, and the monocentricity are the main limitations of the study. Indeed, our study concerned only a small number of patients with pSS and cancers, in a single center. Despite the classical limitations of such a study, this allowed us to dig into the medical charts and provide some details. Anyway, the other main limitations of the present work include the following points: 1) there are some missing data, especially concerning the immunological and histological patterns of some breast cancers, and regarding their extension; 2) we do not have complete data on survival and response to cancer treatments and we cannot draw definitive conclusions on this point; 3) the building of our control group could not involve any specific matching between case and control (especially concerning age, sex, and potential cancer risk factors). However, our findings probably open the way for hypotheses on immunological links between pSS and cancers. Finally, because of the study period, none of the patients in our study were administered anti-cancer immunotherapy. Consequently, we are unable to provide information on how immunotherapy impacts primary Sjögren’s syndrome (pSS), although earlier research has documented cases of pSS induced by such treatments [28, 36, 37]. It is critical for emerging cancer therapeutic strategies to also consider their potential side effects on pSS patients. New data concerning the safety profile of these therapies in the context of this autoimmune condition are expected to become available in the coming years.
Conclusion
Among our 55 patients with pSS and cancer, we confirm the high prevalence of blood malignancies, and also report the relationships between pSS and solid cancers. Notably, we describe a specific pattern of pSS patients with breast cancers, mainly occurring before or concomitantly to pSS, with MSGB positivity rather than anti-SSa/SSb positivity, fibrosis within tumor tissue samples, and a very good overall prognosis. This intriguing relationship between cancer (mainly adenocarcinomas) and pSS (an autoimmune epithelitis) led us to propose the concept of pSS immune surveillance, which remains to be confirmed in larger clinical and translational studies.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contributions
P.G. and R.G. were responsible for conceptualization, methodology and supervision, P.W.D.V., K.H. and R.G. were responsible for data curation, P.W.D.V. and R.G. did the formal analysis and prepared the figures and tables. Main manuscript was written by P.W.D.V., R.G. and P.G. All authors reviewed the manuscript.
Funding
No specific funding, grants, or other support was received from any bodies in the public, commercial, or not-for-profit sectors to carry out the work described in this article.
Data availability
Anonymized data access is available on reasonable request to the corresponding author.
Declarations
Conflict of interest
The authors declare no conflicts of interest.
Ethical approval
According to French law, this retrospective study does not require written consent from each patient and obtained a favorable opinion from the local ethics committee (Institutional review board 2019_IRB-MTP_05-22).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Radjiv Goulabchand and Philippe Guilpain contributed equally to this work.
References
- 1.Bowman S, Ibrahim G, Holmes G, et al. Estimating the prevalence among Caucasian women of primary Sjögren’s syndrome in two general practices in Birmingham, UK. Scand J Rheumatol. 2004;33:39–43. doi: 10.1080/03009740310004676. [DOI] [PubMed] [Google Scholar]
- 2.Shahane A, Patel R. The epidemiology of Sjögren’s syndrome. Clin Epidemiol. 2014;6:247. doi: 10.2147/CLEP.S47399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brom M, Moyano S, Gandino IJ, et al. Incidence of cancer in a cohort of patients with primary Sjögren syndrome in Argentina. Rheumatol Int. 2019;39:1697–1702. doi: 10.1007/s00296-019-04433-2. [DOI] [PubMed] [Google Scholar]
- 4.Wang L-H, Wang W-M, Lin C-Y, et al. Bidirectional relationship between primary Sjögren’s syndrome and Non-Hodgkin’s lymphoma: a nationwide population-based study. J Rheumatol Jrheum. 2020 doi: 10.3899/jrheum.191027. [DOI] [PubMed] [Google Scholar]
- 5.Theander E, Henriksson G, Ljungberg O, et al. Lymphoma and other malignancies in primary Sjögren’s syndrome: a cohort study on cancer incidence and lymphoma predictors. Ann Rheum Dis. 2006;65:796–803. doi: 10.1136/ard.2005.041186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weng M-Y, Huang Y-T, Liu M-F, Lu T-H. Incidence of cancer in a nationwide population cohort of 7852 patients with primary Sjögren’s syndrome in Taiwan. Ann Rheum Dis. 2012;71:524–527. doi: 10.1136/annrheumdis-2011-200402. [DOI] [PubMed] [Google Scholar]
- 7.Liang Y, Yang Z, Qin B, Zhong R. Primary Sjogren’s syndrome and malignancy risk: a systematic review and meta-analysis. Ann Rheum Dis. 2014;73:1151–1156. doi: 10.1136/annrheumdis-2013-203305. [DOI] [PubMed] [Google Scholar]
- 8.Brito-Zerón P, Kostov B, Fraile G, et al. Characterization and risk estimate of cancer in patients with primary Sjögren syndrome. J Hematol Oncol. 2017;10:1–12. doi: 10.1186/s13045-017-0464-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hemminki K, Liu X, Ji J, et al. Effect of autoimmune diseases on risk and survival in female cancers. Gynecol Oncol. 2012;127:180–185. doi: 10.1016/j.ygyno.2012.07.100. [DOI] [PubMed] [Google Scholar]
- 10.Goulabchand R, Malafaye N, Jacot W, et al. Cancer incidence in primary Sjögren’s syndrome: data from the french hospitalization database. Autoimmun Rev. 2021;20:102987. doi: 10.1016/j.autrev.2021.102987. [DOI] [PubMed] [Google Scholar]
- 11.Maria ATJ, Partouche L, Goulabchand R, et al. Intriguing relationships between cancer and systemic sclerosis: role of the immune system and other contributors. Front Immunol. 2019;9:1–14. doi: 10.3389/fimmu.2018.03112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Partouche L, Goulabchand R, Maria ATJ, et al. Biphasic temporal relationship between cancers and systemic sclerosis: a clinical series from montpellier university hospital and review of the literature. J Clin Med. 2020;9:853. doi: 10.3390/jcm9030853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giat E, Ehrenfeld M, Shoenfeld Y. Cancer and autoimmune diseases. Autoimmun Rev. 2017;16:1049–1057. doi: 10.1016/j.autrev.2017.07.022. [DOI] [PubMed] [Google Scholar]
- 14.Shiboski CH, Shiboski SC, Seror R, et al. 2016 American College of Rheumatology/European league against rheumatism classification criteria for primary Sjögren’s syndrome: a consensus and data-driven methodology involving three international patient cohorts. Arthritis Rheumatol. 2017;69:35–45. doi: 10.1002/art.39859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seror R, Bowman SJ, Brito-Zeron P, et al. EULAR Sjögren’s syndrome disease activity index (ESSDAI): a user guide. RMD Open. 2015 doi: 10.1136/rmdopen-2014-000022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ahn JK, Hwang J, Seo GH. Risk of non-Hodgkin’s lymphoma and thyroid cancer in primary Sjögren’s syndrome measured using the Korean Health Insurance Claims Database. Clin Exp Rheumatol. 2020;38:S00. [PubMed] [Google Scholar]
- 17.Vasaitis L, Nordmark G, Theander E, et al. Population-based study of patients with primary Sjögren’s syndrome and lymphoma: lymphoma subtypes, clinical characteristics, and gender differences. Scand J Rheumatol. 2020;49:225–232. doi: 10.1080/03009742.2019.1696403. [DOI] [PubMed] [Google Scholar]
- 18.Nocturne G, Seror R, Fogel O, et al. CXCL13 and CCL11 serum levels and lymphoma and disease activity in primary Sjögren’s syndrome. Arthritis Rheumatol. 2015;67:3226–3233. doi: 10.1002/art.39315. [DOI] [PubMed] [Google Scholar]
- 19.Nocturne G, Pontarini E, Bombardieri M, Mariette X. Lymphomas complicating primary Sjögren’s syndrome: from autoimmunity to lymphoma. Rheumatology. 2021;60:3513–3521. doi: 10.1093/rheumatology/kez052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nocturne G, Mariette X. B cells in the pathogenesis of primary Sjögren syndrome. Nat Rev Rheumatol. 2018;14:133–145. doi: 10.1038/nrrheum.2018.1. [DOI] [PubMed] [Google Scholar]
- 21.Goules AV, Tzioufas AG. Lymphomagenesis in Sjögren’s syndrome: predictive biomarkers towards precision medicine. Autoimmun Rev. 2019;18:137–143. doi: 10.1016/j.autrev.2018.08.007. [DOI] [PubMed] [Google Scholar]
- 22.Retamozo S, Brito-Zerón P, Ramos-Casals M. Prognostic markers of lymphoma development in primary Sjögren syndrome. Lupus. 2019;28:923–936. doi: 10.1177/0961203319857132. [DOI] [PubMed] [Google Scholar]
- 23.Chen HH, Lin CH, Chen DY, et al. Risk of major autoimmune diseases in female breast cancer patients: a nationwide, population-based cohort study. PLoS ONE. 2019;14:1–9. doi: 10.1371/journal.pone.0222860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goulabchand R, Hafidi A, Millet I, et al. Mastitis associated with Sjögren’s syndrome: a series of nine cases. Immunol Res. 2017;65:218–229. doi: 10.1007/s12026-016-8830-x. [DOI] [PubMed] [Google Scholar]
- 25.Calabrese LH, Calabrese C, Cappelli LC. Rheumatic immune-related adverse events from cancer immunotherapy. Nat Rev Rheumatol. 2018;14:569–579. doi: 10.1038/s41584-018-0074-9. [DOI] [PubMed] [Google Scholar]
- 26.ter Borg EJ, Kelder JC. Development of new extra-glandular manifestations or associated auto-immune diseases after establishing the diagnosis of primary Sjögren’s syndrome: a long-term study of the Antonius Nieuwegein Sjögren (ANS) cohort. Rheumatol Int. 2017;37:1153–1158. doi: 10.1007/s00296-017-3715-4. [DOI] [PubMed] [Google Scholar]
- 27.Le Burel S, Champiat S, Mateus C, et al. Prevalence of immune-related systemic adverse events in patients treated with anti-programmed cell death 1/anti-programmed cell death-ligand 1 agents: a single-centre pharmacovigilance database analysis. Eur J Cancer. 2017;82:34–44. doi: 10.1016/j.ejca.2017.05.032. [DOI] [PubMed] [Google Scholar]
- 28.Ramos-Casals M, Maria A, Suárez-Almazor ME, et al. Sicca/Sjögren’s syndrome triggered by PD-1/PD-L1 checkpoint inhibitors. Data from the international immunocancer registry (ICIR) Clin Exp Rheumatol. 2019;37:S114–S122. [PubMed] [Google Scholar]
- 29.Warner BM, Baer AN, Lipson EJ, et al. Sicca syndrome associated with immune checkpoint inhibitor therapy. Oncologist. 2019;24:1259–1269. doi: 10.1634/theoncologist.2018-0823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ortiz Brugués A, Sibaud V, Herbault-Barrés B, et al. Sicca syndrome induced by immune checkpoint inhibitor therapy: optimal management still pending. Oncologist. 2020;25:e391–e395. doi: 10.1634/theoncologist.2019-0467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu Y, Fei Y, Zhong W, et al. The prevalence and clinical characteristics of primary Sjogren’s syndrome patients with lung cancer: an analysis of ten cases in China and literature review. Thorac Cancer. 2015;6:475–479. doi: 10.1111/1759-7714.12216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yu K-H, Kuo C-F, Huang LH, et al. Cancer risk in patients with inflammatory systemic autoimmune rheumatic diseases. Medicine (Baltimore) 2016;95:e3540. doi: 10.1097/MD.0000000000003540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhong H, Liu S, Wang Y, et al. Primary Sjögren’s syndrome is associated with increased risk of malignancies besides lymphoma: a systematic review and meta-analysis. Autoimmun Rev. 2022;21:103084. doi: 10.1016/j.autrev.2022.103084. [DOI] [PubMed] [Google Scholar]
- 34.Yang H, Yao Z, Zhou X, et al. Survival and prognostic factors of lung cancer patients with preexisting connective tissue disease: a retrospective cohort study. Ann Transl Med. 2020;8:1415–1415. doi: 10.21037/atm-20-1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lisi S, Sisto M, Ribatti D, et al. Chronic inflammation enhances NGF-β/TrkA system expression via EGFR/MEK/ERK pathway activation in Sjögren’s syndrome. J Mol Med. 2014;92:523–537. doi: 10.1007/s00109-014-1130-9. [DOI] [PubMed] [Google Scholar]
- 36.Ceccarelli F, Natalucci F, Picciariello L, et al. Rheumatic diseases development in patients treated by anti-PD1 immune checkpoint inhibitors: a single-centre descriptive study. Life. 2023 doi: 10.3390/life13040877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang S, Zhou Z, Wang L, et al. Rheumatic immune-related adverse events associated with immune checkpoint inhibitors compared with placebo in oncologic patients: a systemic review and meta-analysis. Ther Adv Chronic Dis. 2021 doi: 10.1177/2040622320976996. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Anonymized data access is available on reasonable request to the corresponding author.