Abstract
Background
Radioresistance of HNSCCs remains a major challenge for effective tumor control. Combined radiotherapy (RT) and immunotherapy (IT) treatment improved survival for a subset of patients with inflamed tumors or tumors susceptible to RT-induced inflammation. To overcome radioresistance and improve treatment outcomes, an understanding of factors that suppress anti-tumor immunity is necessary. In this regard, regulatory T cells (Tregs) are critical mediators of immune suppression in HNSCCs. In this study, we investigated how radiation modulates Treg infiltration in tumors through the chemokine CCL20. We hypothesized that radiation induces CCL20 secretion resulting in Treg infiltration and suppression of anti-tumor immunity.
Methods
Human and mouse HNSCC cell lines with different immune phenotypes were irradiated at doses of 2 or 10 Gy. Conditioned media, RNA and protein were collected for assessment of CCL20. qPCR was used to determine CCL20 gene expression. In vivo, MOC2 cells were implanted into the buccal cavity of mice and the effect of neutralizing CCL20 antibody was determined alone and in combination with RT. Blood samples were collected before and after RT for analysis of CCL20. Tumor samples were analyzed by flow cytometry to determine immune infiltrates, including CD8 T cells and Tregs. Mass-spectrometry was performed to analyze proteomic changes in the tumor microenvironment after anti-CCL20 treatment.
Results
Cal27 and MOC2 HNSCCs had a gene signature associated with Treg infiltration, whereas SCC9 and MOC1 tumors displayed a gene signature associated with an inflamed TME. In vitro, tumor irradiation at 10 Gy significantly induced CCL20 in Cal27 and MOC2 cells relative to control. The increase in CCL20 was associated with increased Treg migration. Neutralization of CCL20 reversed radiation-induced migration of Treg cells in vitro and decreased intratumoral Tregs in vivo. Furthermore, inhibition of CCL20 resulted in a significant decrease in tumor growth compared to control in MOC2 tumors. This effect was further enhanced after combination with RT compared to either treatment alone.
Conclusion
Our results suggest that radiation promotes CCL20 secretion by tumor cells which is responsible for the attraction of Tregs. Inhibition of the CCR6-CCL20 axis prevents infiltration of Tregs in tumors and suppresses tumor growth resulting in improved response to radiation.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00262-022-03313-2.
Keywords: Head and neck squamous cell carcinomas, Radiotherapy, Radioresistance, Regulatory T cells, CCR6-CCL20 axis
Introduction
Radiotherapy (RT) is a cornerstone in the treatment of head and neck squamous cell carcinoma (HNSCC). Despite the addition of chemotherapy to RT, nearly 30% of patients with locally advanced disease will experience treatment resistance leading to tumor progression or recurrence [1]. There are limited treatment options for patients with recurrent or treatment-resistant tumors. Surgery is the preferred option in most cases, if judged clinically resectable, but often implies technically challenging procedures with significant rates of perioperative complications and postoperative morbidity. Re-irradiation is less frequently warranted, as previous radiation doses to target structures can cause significant locoregional toxicities, with risks such as carotid blowout and radiation-induced myelitis [6–9]. Other therapeutic options include the use of cetuximab (anti-epidermal growth factor receptor) and cisplatin (an antineoplastic agent) as radiosensitizers in HNSCC, but these have provided little benefit for improving overall survival (OS) and progression-free survival (PFS) [2–4]. Immunotherapy in the form of immune checkpoint blockade against the PD-1/PD-L1 axis provided a significant advancement in the treatment of locally advanced HNSCCs. However, response to anti-PD-1/PD-L1 is less than 20% and more than half of patients who respond will develop secondary resistance to immunotherapy [5]. Thus, there is an unmet need to develop rational combinatorial strategies for improving response to RT during the first course of treatment.
Recent advances in the field of immuno-oncology revealed the complexity of the tumor immune microenvironment (TME) in HNSCCs [6]. In virally driven (HPV +) oropharynx HNSCC tumors, the TME is often highly inflamed with a dominance of tumor infiltrating lymphocytes (TILs), antigen-presenting cells (APCs) and natural killer (NK) cells [7, 8]. In contrast, HPV-negative tumors are often poorly inflamed with a dominance of regulatory T cells (Tregs), M2 macrophages and myeloid-derived suppressor cells (MDSCs) [7, 9–11]. HPV status as well the associated inflamed immune phenotype have been linked with improved response to RT and immunotherapy in HNSCC [8, 12–16]. The Danish head and neck cancer group study on the impact of HPV on RT response showed that HPV + oropharynx patients had an 80% loco-regional control rate compared to 52% for HPV-negative patients [13]. While strong causal relationships are lacking, studies suggest that the immune-desert TME of HPV-negative HNSCCs contributes to their increased radioresistance [17].
One strategy to improve response to RT in HNSCCs is through remodelling of the immune TME. This can be done using targeted immunotherapies that suppress the pro-tumoral effects of RT and favor immune-mediated tumor elimination [18]. To that extent, CCR6 is a chemokine receptor that is selectively expressed in Th17 cells and Treg cells and is exclusively activated by the chemokine, CCL20. In a pre-clinical model of HNSCC, CCL20 was shown to correlate positively with the level of intratumoral and circulating Tregs [19]. In addition, the expression of immunosuppressive chemokines such as CCL2 and CCL20 in the TME has been shown to suppress the migration and activation of effector T cells and limit the response to immunotherapy, chemotherapy, and radiotherapy [20–23]. Given the role of Tregs in suppressing anti-tumor immunity and mediating resistance to RT, we sought to investigate whether targeting the CCR6-CCL20 axis can limit Treg migration to the tumor and allow radiation to mount an effective anti-tumor immune response.
Materials and methods
Cell culture
The human HNSCC cell lines, Cal27 and SCC9 and the murine cell lines, MOC1 and MOC2 were kindly obtained from the laboratory of Dr Lynn Heasley (University of Colorado Denver). Cells were maintained at 37 °C and 5% CO2 in a humidified incubator. Cal27 and SCC9 cells were cultured in DMEM and DMEM/F-12 media, respectively. Media was supplemented with 10% fetal bovine serum (FBS), 500 I.U./mL penicillin, 50,000 (μg/mL) streptomycin and 2 mM l-glutamine. Murine MOC cells were cultured in IMDM media mixed with F-12 media and supplemented with 5% FBS, 1% penicillin, 1% streptomycin, 50 μg/mL hydrocortisone, 100 ng/mL epidermal growth factor and 10 mg/mL insulin.
qPCR
RNeasy Mini Kit (Qiagen) was used to extract total RNA from cells according to the manufacturer’s protocol. RNA concentration and quality was determined with BioDrop™ µLITE (Montreal Biotech). The first complementary strand was made from 1 μg total RNA using the Superscript Reverse Transcription kit (Life Technologies Inc) according to manufacturer’s protocol. TaqMan™ Fast Advanced Master Mix (Life Technologies Inc) was used to amplify and detect the DNA products. Gene expression analysis was performed using the QuantStudio™ 3 Real-Time PCR System (ThermoFisher Scientific). CCL20 and GAPDH were probed using TaqMan assays (Life Technologies Inc). GAPDH was used as the internal reference for other genes. All primers showed more than 90% efficiency with a single melting curve. Expression levels of the housekeeping gene (GAPDH) were used to calculate fold induction of the specific genes modulated by the absence or presence of CCL20. Ct values of the genes were used to determine relative fold change through the 2−ΔΔCt method.
ELISA and multiplex secretome assays
Mouse plasma samples were analyzed for CCL20 levels using ELISA (Eve Technologies, Calgary AB). Conditioned media samples from cell lines were analyzed using multiplex immune-assays designed for human and mouse cell lines (Human Cytokine 71-Plex Discovery assay and Mouse Cytokine 44-Plex Discovery assay) from Eve Technologies (Calgary, AB). Conditioned media was collected 24 and 72 h after irradiation.
Mass spectrometry
For digestion of total extracts, tissues were dissolved in a solution of 10 mM HEPES–KOH pH 7.5, 8 M urea. The protein concentration was determined by BCA Assay. 75 μg of proteins were reduced in 50 μl of 10 mM HEPES–KOH pH 7.5, 8 M urea by adding Dithiothreitol (DTT) to a final concentration of 5 mM and by heating at 95 °C for 2 min. Alkylation of proteins was carried out by adding chloroacetamide (ClAA) (Sigma-Aldrich, Saint-Louis) to a final concentration of 7.5 mM. Urea concentration was diluted to a final concentration of 2 M by adding 50 mM ammonium bicarbonate (NH4HCO3) (Sigma-Aldrich, Saint-Louis). Proteins were digested by adding 1 μg of Pierce MS-grade trypsin (Thermo Fisher Scientific, Waltham). Peptides were purified with micropipette tips containing a C18 column (EMD Millipore, Burlington, VT), concentrated by centrifugal evaporator and then resuspended in FA buffer. Peptides were assessed using a NanoDrop spectrophotometer (Thermo Fisher Scientific, Waltham, MA).
For DIA LC–MS analysis, 250 ng of peptides from each sample were injected into an HPLC (nanoElute, Bruker Daltonics) and loaded onto a trap column with a constant flow of 4 µl/min (Acclaim PepMap100 C18 column, 0.3 mm id × 5 mm, Dionex Corporation) then eluted onto an analytical C18 Column (1.9 µm beads size, 75 µm × 25 cm, PepSep) heated at 50 °C. Peptides were eluted over a 2-h gradient of ACN (5–37%) in 0.1% FA at 400 nL/min while being injected into a TimsTOF Pro ion mobility mass spectrometer equipped with a Captive Spray nano electrospray source (Bruker Daltonics). Data were acquired using diaPASEF mode. Briefly, for each single TIMS (100 ms) in diaPASEF mode, we used 1 mobility window consisting of 27 mass steps (m/z between 114 and 1414 with a mass width of 50 Da) per cycle (1.27 s duty cycle). These steps cover the diagonal scan line for + 2 and + 3 charged peptides in the m/z-ion mobility plane. The raw files were analyzed using MaxQuant (version 2.0.3.0) and the Uniprot mouse proteome database (07/03/2021, 55,315 entries), with uploading in silico generated mouse library files (available at http://annotations.perseus-framework.org/) to run Maxquant in DIA discovery mode. The settings used for the analysis (with TIMS-MaxDIA type in group-specific parameters) were: 1 miscleavage was allowed; fixed modification was carbamidomethylation on cysteine; enzymes were Trypsin (K/R not before P); variable modifications included in the analysis were methionine oxidation and protein N-terminal. A mass tolerance of 20 ppm was used for both precursor and fragment ions. Identification values "PSM FDR", "Protein FDR" and "Site decoy fraction" were set to 0.05. Minimum peptide count was set to 1. Both the "Second peptides" and "Match between runs" options were also allowed. MaxQuant was run with a transfer q value of 0.3.
Irradiation
For in vitro irradiation, cells were irradiated at room temperature using a Gamma Cell 3000 irradiator. Doses of 2, or 10 Gy were given as a single dose at a dose rate of 9.18 Gy/min. For in vivo irradiation of mouse tumors, external beam gamma radiation was performed by the Leskell Gamma Knife (GK) Perfexion (Elekta Instruments AB). The methods for adapting the gamma knife for mouse irradiation were previously published [24]. Briefly, treatment planning was performed using 4-mm collimators delivered at predetermined coordinates targeting the tumor. Radiation dose of 10 Gy was applied to the tumor located on the right side of the mouse’s oral buccal cavity. The mice were anesthetized using 1.5L/min oxygen containing isoflurane and positioned on a custom-designed stereotactic frame that is compatible with the couch of the GK. Tumor growth was measured after treatment twice a week for 3 weeks.
Migration assay
Migration was assessed using the migration transwell assay with 5 μm pore size in 12-well culture plates. Spleens were removed from mice and homogenized into a single-cell suspension using RPMI 1640 medium. Red blood cells were lysed by resuspension in Red Blood Cell (RBC) lysis buffer (Sigma, R7757). Single-cell suspensions from individual spleens were pooled, filtered through a 70 µm cell strainer (Sarstedt, Germany), and counted. CD4+CD25+ cells were isolated using a magnetic cell-sorting kit by negative selection (EasySep Mouse CD4+CD25+ Regulatory T Cell Isolation Kit, Stem Cell Technologies) according to manufacturer’s instructions. CD4+CD25+ cells were suspended in RPMI 1640 supplemented with 20 ng/µL IL-2 (Biolegend, San Diego, CA), 2% FBS and cultured in the upper chamber of a transwell insert. The lower chamber contained irradiated MOC2 cells in RPMI 1640 media supplemented with 2% FBS and 20 ng/µL IL-2. Irradiated MOC2 cells were placed in the lower chamber. After 24 h, migrated cells in the lower chamber were pipetted into a 96-well plate for labeling by monoclonal antibodies and then counted by flow cytometry.
MTT proliferation assay
MOC2 and Cal27 cells were seeded in 96-well plates in triplicate wells at the density of 2000 and 4000 cells/well at 37 °C and 5% CO2. Cells were incubated with 500 ng/mL PBS, 50, and 500 ng/mL of anti-CCL20 (R&D Systems, Minneapolis, MN) or PBS for 24 h. Cell viability assay was performed using the methylthiazol tetrazolium (MTT) based Cell Growth Determination Kit (Sigma, St. Louis, MO) according to manufacturer’s instructions. Absorbance was measured using a microplate reader at 595 nm wavelength.
Flow cytometry
Tumors were finely cut and placed in HBSS solution containing 0.45 U/mL of Collagenase B. Tumor pieces were passed through a nylon mesh and the resulting cell suspension was centrifuged and resuspended in RBC lysis buffer. Draining lymph nodes (DLNs) and spleens were collected and processed into single-cell suspensions through mechanical separation. For flow cytometric analysis, 1 × 106 live cells were plated in 96-well plates and cultured in the presence of monensin to prevent the release of cytokines. Cell viability was assessed using DAPI. After 24 h, cells were plated in a 96-well plate and blocked with anti-CD16/32 (1:100 dilution) antibody (Life Technologies Inc.). For analysis of immune cells, the following conjugated antibodies were used at a dilution of 1:100 unless otherwise specified: PE-Cyanine7-CD8 (53–6.7, Biolegend), APC-eFluor780-CD44 (Clone IM7, Invitrogen, Waltham, MA), AlexaFluor700-CD45 (Clone 30-F11, Invitrogen, Waltham), Brilliant Violet711-CD25 (Clone PC61, BioLegend), PerCP-Cy5.5-CD4 (Clone GK1.5, Biolegend), PE-FOXP3 (Clone 150D, Biolegend), FITC-CCR6 (R6H1, eBioscience), APC-RORγ (Clone B2D, Invitrogen, Waltham). For proper compensation of flow cytometry channels, beads and single-stain samples were used. For gating, isotype controls and fluorescence minus-one (FMO) controls were applied. Both mean fluorescence intensity (MFI) and proportion of positively stained cells were analyzed. Stained cells were run on the Beckman Coulter CytoFlex (Beckman Coulter). Data were analyzed using FlowJo software version 10.5.1 (Ashland, OR).
Animal studies
Animal studies were approved by the institutional ethical committee of Université de Sherbrooke, (Québec, Canada) and complies with regulations of the Canadian Council on Animal Care (protocol # 2019–2333). Six- to 8-week-old female C57BL/6 mice (Charles River Laboratories) were used. Mice were inoculated in the buccal cavity as previously reported [25]. Tumors were measured using calipers and tumor volume was determined using the formula (long dimension × short dimension2 × 0.5). Neutralizing CCL20 antibody (R&D Systems) was administered intraperitoneally twice per week (2.5 mg/kg) alone and in combination with RT 7 days post tumor inoculation. Blood samples were collected before and after RT for analysis of serum levels of CCL20.
Gene expression data
Cancer cell gene expression data were downloaded from the Cancer Cell Line Encyclopedia (CCLE) for all available HNSCC cell lines (33 cell lines in total, identified as upper aerodigestive tract cancers). Twelve Treg chemokines were identified based on the literature and their gene expression was analyzed across cell lines. The gene names for the chemokines are: CCL1, CCL2, CCL4, CCL5, CCL17, CCL19, CCL20, CCL21, CCL22, CCL28, CXCL12, CXCL13. For analysis of CCR6 expression in immune cells, the DICE (database of immune cell expression) was used. DICE provides gene data expression for 13 types of immune cells.
Statistical analysis
Statistical analysis was performed using GraphPad Prism 9 (Graphpad Software, Inc., La Jolla, CA). All experiments were performed in triplicates and repeated two to three times. Quantitative analyses were performed using Student’s t test (for two groups) or two-way ANOVA (for groups of more than two). A p value of < 0.05 was considered significant.
Results
Gene expression of Treg chemokines in HNSCC cell lines
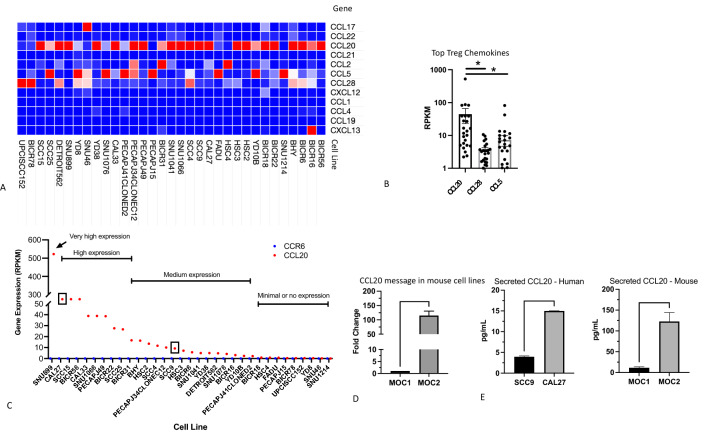
Gene expression analysis was performed on all known Treg chemokines in HNSCC cell lines from the CCLE database. This approach allowed us to determine the chemokines secreted exclusively by cancer cells, in contrast to tumor tissue where chemokines can be secreted by multiple cell types. Data were available for 33 HNSCC cell lines. Based on previous studies, 12 Treg chemokines were identified, including the well characterized CCL17, CCL20 and CCL22 chemokines. Less potent Treg chemokines, predominately known for attraction of myeloid immunosuppressive cells (CCL2, CCL5), were also included in the analysis. In 19/33 (57.6%) of the cell lines, CCL20 was the most highly expressed Treg chemokine (Fig. 1a). When analyzing any expression of all Treg chemokines per cell line, CCL20 and CCL28 were expressed in 24/33 cell lines, followed by CCL5 (21/33). Expression of CCL20 (median = 12.58) was significantly higher than CCL28 (median = 3.30) and CCL5 (median = 2.61) (Fig. 1b). Analysis of CCL20 across cell lines showed a wide range of expression values (RPKM range: 0.1–520) (Fig. 1c). We divided CCL20 expression into 4 levels: Minimal to no expression with RPKM < 1; Medium expression with RPKM 1–10; High expression ranging from RPKM 20–90; Very high expression which only occurred in 1 cell line (SNU899) with RPKM of 520. Based on these criteria, we selected the SCC9 (medium expression) and Cal27 (high expression) cell lines for further analysis (Fig. 1c). In addition to the human HNSCC cell lines, we analyzed the expression of CCL20 by qPCR in 2 murine oral cavity cell lines: MOC1 and MOC2. Consistent with a previously published report regarding the immunogenicity of MOC1 and MOC2 tumors [26], our data showed that the MOC1 Treg-poor cell line has significantly lower expression of CCL20 compared to the MOC2 Treg-rich cell line (Fig. 1D). To determine whether CCL20 gene expression translates to secreted protein, we performed secretome analysis by ELISA on SCC9, Cal27 human cell lines and MOC1, MOC2 murine cell lines. Consistent with gene expression data, secreted CCL20 was significantly higher in the Cal27 cell line compared to SCC9 (~ threefold higher) and similarly was higher in MOC2 compared to MOC1 cell lines (~ 11-fold higher) (Fig. 1e).
Fig. 1.
Analysis of Treg chemokines in HNSCC cell lines. a Heat map of Treg chemokines in 33 HNSCC cell lines analyzed from the CCLE. b Statistical analysis of the top 3 most frequently expressed Treg chemokines in human HNSCC cell lines. Two-way ANOVA was used to assess statistical significance. c Categorization of HNSCC cell lines based on levels of CCL20 expression (red dots). None of the cell lines expressed the CCL20 receptor, CCR6 (blue dots). d qPCR analysis of transcript levels of CCL20 in MOC1 and MOC2 mouse HNSCCs. Expression was normalized to MOC1. e Analysis of secreted CCL20 in human and mouse cell lines. Error bars represent SEM of 4–5 independent experiments. Asterisks show statistical significance
Absence of autonomous CCR6-CCL20 signaling in HNSCC cell lines
We analyzed the expression of CCR6 in immune cells based on the DICE database which provides gene expression data for 13 different immune cell populations. Tregs and Th17 cells showed the highest level of expression compared to all other immune cells (Supplementary Fig. 1). In contrast, none of the 33 HNSCC cell lines expressed the CCR6 receptor (Fig. 1c). Since a previous study showed cancer cell autonomous signalling of CCR6-CCL20 [27], we used an anti-CCL20 antibody to test whether increasing concentrations can reduce or inhibit cancer cell proliferation. Using the MTT proliferation assay, our results showed that anti-CCL20 does not affect the proliferation of any of the cell lines tested (MOC1, MOC2, Cal 27, SCC9) at low (50 μg/µL) and high (500 μg/µL) anti-CCL20 concentrations (Supplementary Fig. 2). These data show the absence of autonomous CCR6-CCL20 signaling in HNSCC cell lines.
CCL20 is induced in response to high dose, but not low dose radiation
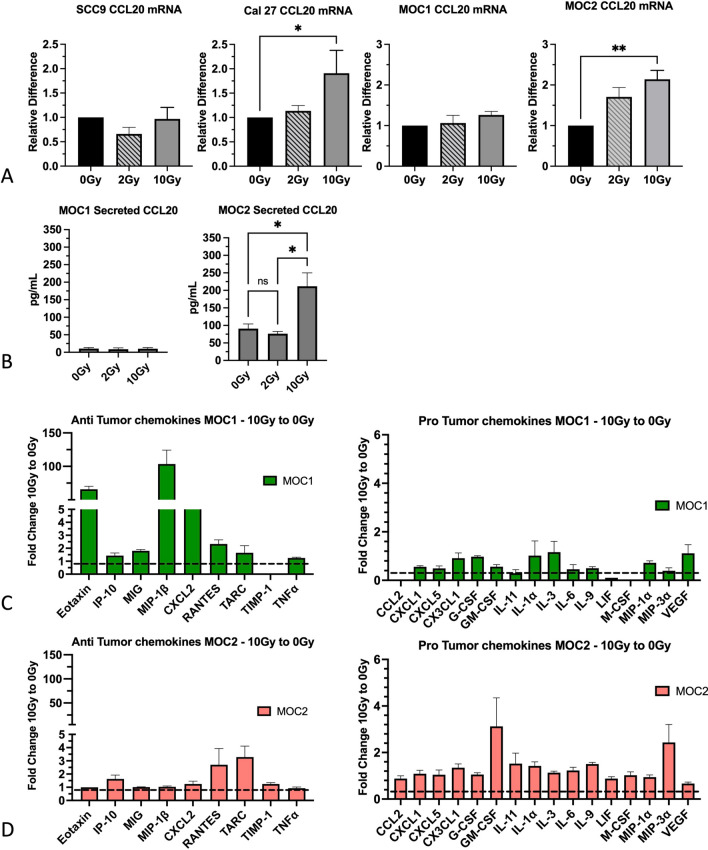
To determine whether CCL20 is induced in response to radiation across cell lines with different basal CCL20 expression, we irradiated the murine MOC1 and MOC2 cell lines and the human Cal27 and SCC9 cell lines at low (2 Gy) and high (10 Gy) doses of radiation. RNA and conditioned media were analyzed for intracellular and secreted CCL20, respectively. Our data show that CCL20 was significantly induced in Cal 27 and MOC2 cells in response to 10 Gy but not 2 Gy relative to non-irradiated control (Fig. 2a). No significant change in CCL20 was observed after radiation in MOC1 and SCC9 cells (Fig. 2a).
Fig. 2.
Induction of CCL20 in response to high dose, but not low dose radiation and associated changes in the secretome. a Analysis of gene expression of CCL20 in human (Cal 27, SCC9) and murine (MOC1, MOC2) HNSCC cell lines after exposure to low (2 Gy) and high (10 Gy) doses of radiation. b Analysis of secreted CCL20 in MOC1 and MOC2 cell lines after radiation. Two-way ANOVA was performed to assess statistical significance. c Analysis of the pro-tumoral chemokines induced in response to 10 Gy radiation in MOC1 and MOC2 cells. d Analysis of anti-tumoral chemokines induced in response to 10 Gy radiation in MOC1 and MOC2 cells. Data for 10 Gy were normalized to 0 Gy. Dashed line represents the normalization value (equal to 1) for each chemokine. A value of 1 indicates no change in the respective chemokine. *P value < 0.05; **P value < 0.01
For subsequent experiments we focused on the murine cell lines. We analyzed secreted CCL20 from conditioned media of irradiated MOC1 and MOC2 cells. In MOC1 cells, no change in CCL20 secretion was observed after 2 or 10 Gy irradiation (Fig. 2b). CCL20 levels remained relatively low in all conditions (< 20 pg/mL). In contrast, CCL20 secretion in MOC2 cells was significantly increased after 10 Gy irradiation (from 90 to 211 pg/mL) (Fig. 2b). To further elucidate the impact of 10 Gy irradiation on cytokines related to CCL20, a multiplex secretome analysis of a panel of 16 cytokines was performed. Our data show that MOC2 cells have increased secretion of the cytokines CXCL5, GM-CSF and IL-11 which are involved in Treg activation and differentiation as well as MDSC differentiation [25–28]. In contrast, MOC1 cells show increased secretion of T cell chemoattractants including CXCL9 (MIG), CXCL10 (IP-10), MIP-1β (CCL4), and RANTES (Fig. 2c–d, Supplementary Table 1).
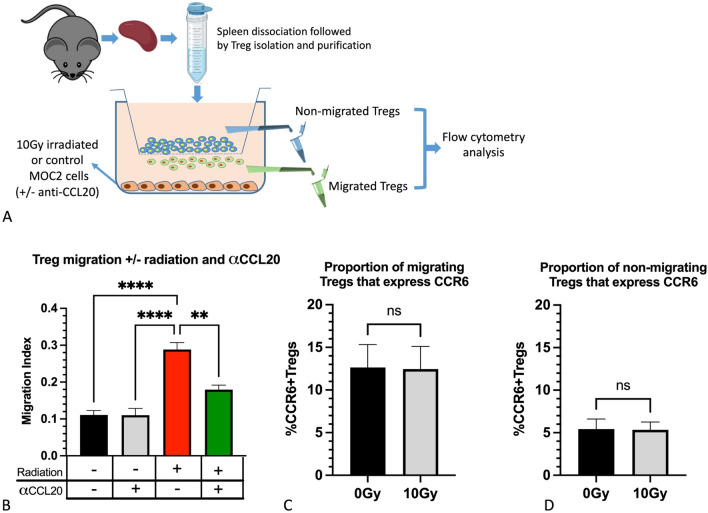
Irradiated tumor cells promote Treg migration
The transwell migration assay was used to assess whether irradiated MOC2 cells promote the migration of Treg cells. Freshly purified Tregs were placed inside migration chambers and exposed to irradiated (10 Gy) or non-irradiated MOC2 tumor cells for 24 h (Fig. 3a). A significant increase in the number of migrating Treg cells that were exposed to irradiated MOC2 cells was observed compared to non-irradiated cells (Fig. 3b). Analysis of CCR6 expression on Tregs showed that nearly 13% of migrating Treg cells were CCR6 positive (Fig. 3c), irrespective of irradiation. This is in contrast to the non-migrating cells where < 5% were CCR6 positive (Fig. 3d). To determine whether CCL20 secreted by the MOC2 tumor cells was responsible for the increased migration of Treg cells, the experiment was repeated in the presence of neutralizing anti-CCL20 antibody. In the presence of anti-CCL20, the effect of radiation on the migration of Treg cells was reversed (Fig. 3b). Collectively, these data show that CCL20 secreted by tumor cells increases the migration of Treg cells and this effect can be reversed with neutralizing anti-CCL20 antibody.
Fig. 3.
Analysis of Treg cell migration in the presence of irradiated MOC2 cells and anti-CCL20 treatment. a Schematic showing the experimental design. b Analysis of Treg cell migration 24 h after exposure to irradiated MOC2 cells and anti-CCL20 treatment. Control groups included no radiation + IgG and radiation (10 Gy) + IgG. Data were analyzed using the migration index which represents the #number of cells migrated per total # of cells seeded. Statistical analysis was performed using Two-way ANOVA. c–d Assessment of the percentage of Tregs that express CCR6 in the upper chamber (non-migrating Treg cells) and the lower chamber (migrating Treg cells). *P value < 0.05; **P value < 0.05; ***P value < 0.001
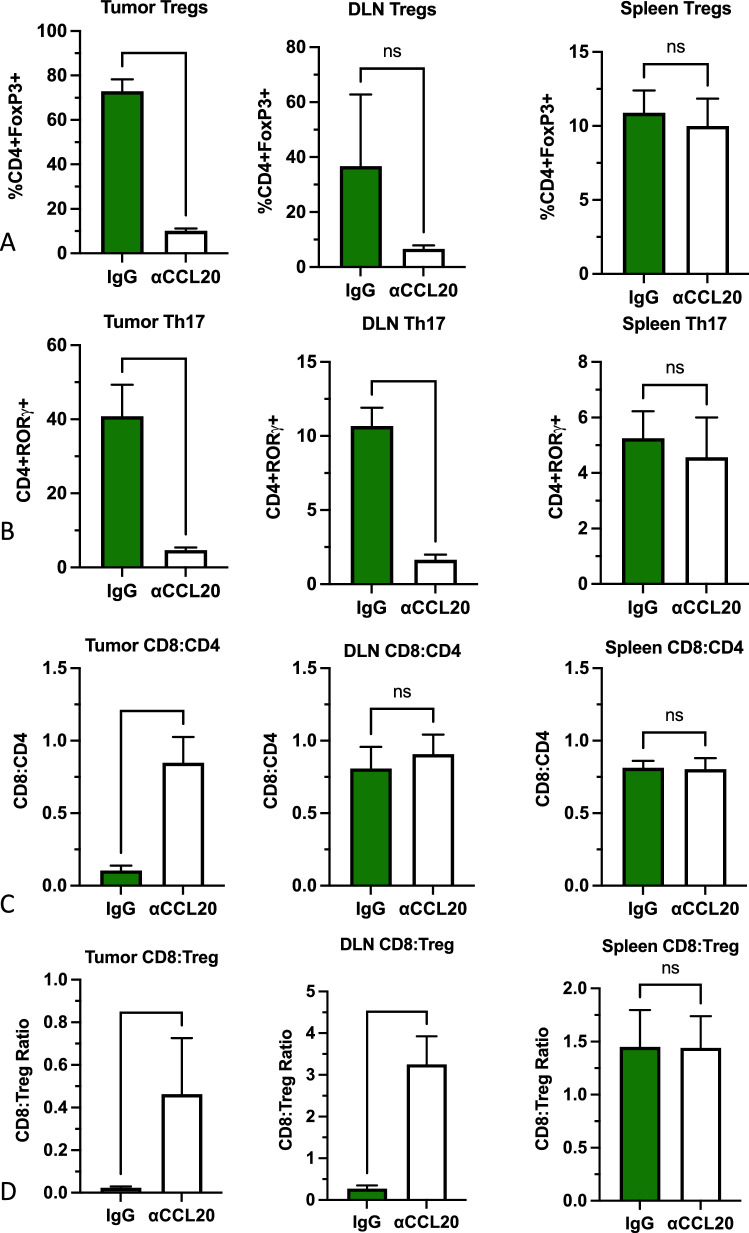
Inhibition of CCL20 reduces infiltration of Treg and Th17 cells into tumors and draining lymph nodes (DLNs)
We studied the effect of CCL20 inhibition on tumor immune infiltrates with particular emphasis on the CCR6-expressing Treg and Th17 cells. Administration of anti-CCL20 was started 2 days before inoculation of mice with MOC2 tumor cells in the buccal mucosa. Mice were euthanized after 3 doses of anti-CCL20 (10 days post inoculation), and tumors were processed for flow cytometry analysis. The proportion of Treg cells decreased by 7.2-fold and the proportion of Th17 cells decreased by 8.7-fold in mice treated with anti-CCL20 compared to those treated with control anti-IgG (Fig. 4a–b). A significant decrease in Th17 cells was observed in DLNs from mice treated with anti-CCL20 compared to anti-IgG (Fig. 4b). A similar decrease was observed for Treg cells, but it did not reach significance (Fig. 4a). Analysis of Treg and Th17 cells from spleens of mice in both groups did not show a significant change (Fig. 4a–b). In addition to the decrease in Treg and Th17 cells in tumors, we observed a significant increase in the CD8:CD4 ratio (Fig. 4c) and a significant increase in the CD8:Treg ratio in tumors and DLNs (Fig. 4d).
Fig. 4.
Analysis of T cell populations in tumors, draining lymph nodes (DLN) and spleens of mice treated with anti-CCL20 or IgG. a Analysis of CD4 + CD25 + FoxP3 + Tregs. b Analysis of CD4 + RORgt + Th17 cells and c analysis of the CD8:CD4 ratio and d CD8:Treg ratio. Two-tailed student’s t-test was performed to assess statistical significance between the groups (n = 4 mice per group). *P value < 0.05; **P value < 0.01
Inhibition of CCL20 reduces tumor growth and enhances radiotherapy response
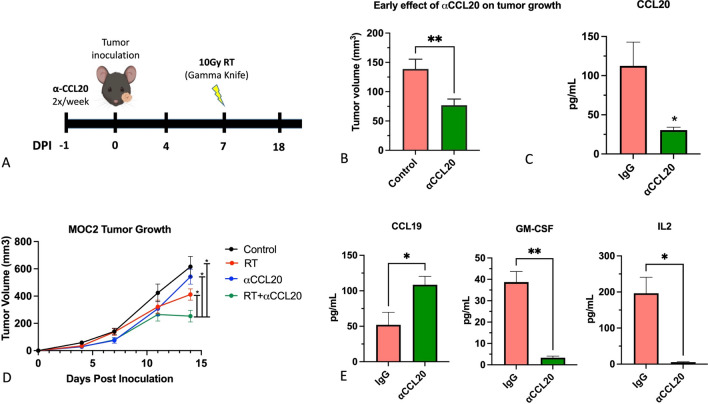
We tested the effect of anti-CCL20 alone and in combination with RT on tumor growth in an orthotopic HNSCC mouse model (Fig. 5a). The poorly immunogenic MOC2 cell line was used for these studies.
Fig. 5.
Effect of combination RT + anti-CCL20 on tumor growth in orthotopic HNSCCs. a Timeline of the experiment. b Validation of anti-CCL20 target specificity by assessment of plasma levels of CCL20 using ELISA. c Tumor volumes prior to irradiation in the IgG and anti-CCL20 groups (n = 16 per group). d Tumor growth assessment in the RT + anti-CCL20 group compared to IgG control, anti-CCL20 and RT + IgG. Two-way ANOVA was performed to assess significance between the groups on the last day of the experiment. e Analysis of CCL19, GM-CSF and IL-2 in plasma from anti-CCL20 and IgG treated mice. *P value < 0.05; **P value < 0.01
Inhibition of CCL20 alone resulted in a significant decrease in tumor growth compared to control in MOC2 tumors (Fig. 5b). Reduction of serum levels of CCL20 was observed in mice treated with the anti-CCL20 antibody (Fig. 5c). On day 7 when only anti-CCL20 or anti-IgG treatment was applied (n = 10 per group), average tumor growth in the anti-IgG group was 138.8 mm3 ± 16.7 compared to 77.0 ± 10.5 mm3 in the anti-CCL20 group (Fig. 5b). The combination of RT and anti-CCL20 showed a significant decrease in tumor volume compared to all other groups starting on day 4 after exposure to 10 Gy RT (Fig. 5d). Average tumor volume for the RT + anti-CCL20 group on day 14 was 252.7 ± 42.9 mm3 compared to 386.3 ± mm3 for the RT group, 439.1 mm3 ± 6.3 for the anti-CCL20 only group and 615.5 ± 75.3 mm3 for the anti-IgG control group. Analysis of circulating chemokines showed that treatment with anti-CCL20 significantly increased the T cell chemokine CCL19 and reduced the immunosuppressive chemokine, GM-CSF (Fig. 5e). In addition, IL-2 which supports Treg cell differentiation was significantly reduced in anti-CCL20-treated mice (Fig. 5e).
Inhibition of CCL20 transforms the tumor proteome by promoting apoptosis and downregulating hypoxia and glycolysis pathways
We analyzed global changes to the tumor proteome through time of flight mass spectrometry (TOF–MS). Analysis of differentially expressed proteins showed that 70 proteins were increased by > 1.5-fold while 58 proteins were decreased by > 1.5-fold in anti-CCL20-treated tumors compared to control. Gene set enrichment analysis (GSEA) of differentially expressed proteins revealed important changes to hallmark pathways in anti-CCL20-treated tumors. Pathways that were upregulated included: Interferon gamma (IFNγ) response and Interferon alpha (IFNα) response (Table 1A). Pathways that were downregulated included: oxidative phosphorylation (OXPHOS), glycolysis, and hypoxia (Table 1B). Since OXPHOS was previously shown to mediate resistance to BRAF and MEK inhibitors [28], it is conceivable that anti-CCL20 could be combined with those therapies. Analysis of significantly differentially expressed proteins showed that SerpinB1, a protease inhibitor and neutrophil survival factor responsible for Th17 cell activation was reduced by 1.5-fold after anti-CCL20 treatment. This is consistent with our observation of decreased Th17 cells in anti-CCL20 treated tumors. We also observed a 2.6-fold increase in the tumor rejection antigen, Sart1, which has been shown to induce activation of cytotoxic T cells [29, 30]. Collectively, these data show that anti-CCL20 treatment remodels the tumor microenvironment from an immunosuppressive, Treg dominant to a pro-inflammatory anti-tumoral TME.
Table 1.
Anti-CCL20 treatment remodels the TME in orthotopic murine HNSCC
| (A) Gene-set enrichment analysis (GSEA) of hallmark gene sets after anti-CCL20 treatment | |||
|---|---|---|---|
| Upregulated | P-Value | Downregulated | P-Value |
| Interferon gamma response | 3.83E−06 | Oxidative phosphorylation | 4.40E−30 |
| MTORC1 signalling | 1.67E−04 | Adipogenesis | 2.73E−13 |
| Interferon alpha response | 1.25E−03 | Fatty acid metabolism | 3.18E−06 |
| Glycolysis | 1.93E−04 | ||
| Reactive oxygen species pathway | 2.24E−03 | ||
| Hypoxia | 2.94E−03 | ||
| Myogenesis | 9.70E−03 | ||
| Peroxisome | |||
| (B) Top differentially regulated proteins after anti-CCL20 treatment | |||||
|---|---|---|---|---|---|
| Upregulated | Fold change | P-Value | Downregulated | Fold change | P-Value |
| Ccdc124 | 2.00 | 0.0118 | Mrps25 | 2.01 | 0.0264 |
| Sart1 | 2.61 | 0.0094 | Clip1 | 1.98 | 0.0113 |
| Ipo9 | 1.54 | 0.0242 | Mtor | 1.75 | 0.0439 |
| Frg1 | 1.76 | 0.0483 | Ssbp | 1.72 | 0.0254 |
| Tm9sf2 | 1.54 | 0.0264 | |||
| Prosc | 1.54 | 0.0099 | |||
| Serpinb1a | 1.53 | 0.0103 | |||
| Gpx3 | 1.48 | 0.0190 | |||
| Prkag1 | 1.47 | 0.0298 | |||
| Prelp | 1.46 | 0.0341 | |||
| Sod1 | 1.45 | 0.0287 | |||
| Acyp1 | 1.44 | 0.0291 | |||
Discussion
The tumor’s immune composition can be a decisive factor in the response to RT. In this work, we investigated a novel immune-based approach to enhance RT response. In previous works, we discovered that tumor recurrence after RT is associated with an increase in proliferative Tregs in HNSCC tumors [19, 31]. This finding is important since Treg cells are known to suppress the function of cytotoxic T cells which induce tumor cell death and are frequently deployed in response to RT [32–36]. Our data led us to investigate the mechanism by which Treg cells are recruited to the tumor microenvironment and whether blocking this mechanism could enhance the response to RT.
Cytokines and chemokines are critical contributors to the immune phenotype in tumors. These secreted factors dictate the type and activation status of immune cells that populate the TME [37, 38]. Our analysis of all reported Treg chemokines in 33 HNSCC cell lines showed that CCL20 is the most frequently expressed. It is conceivable that once these cell lines are implanted into recipient hosts, their secretion of chemokines such as CCL20 would dictate their immune phenotype. Immune-inflamed tumors (hot tumors) are known to have high T-cell infiltration while immune-desert tumors (cold tumors) are characterized by their poor T-cell infiltration [39]. In our murine cell lines, this is supported by the observation that MOC2 cells (high CCL20 expression) develop into cold tumors with minimal TILs compared to MOC1 cells (low CCL20 expression), which develop into hot tumors [26].
The sole receptor for CCL20 is CCR6, which is predominantly expressed on Treg cells and Th17 cells. In non-small cell lung cancer (NSCLC) tissue, activation of the CCR6-CCL20 axis is responsible for recruitment of Treg cells into tumors in a stage-dependent manner and correlates with decreased overall survival [36]. Similarly, in breast cancer tissue, CCR6+Foxp3+ Tregs in the tumor mass correlates with impaired CD8 T cell function and poor prognosis [40]. Phenotypic analysis of circulating and intratumoral Tregs from hepatocellular carcinoma (HCC) patients showed that CCR6-positive Tregs selectively migrate to tumors which secrete CCL20 and the prevalence of these Tregs correlated with disease progression and decreased survival [48]. Few studies have investigated the CCR6-CCL20 axis in HNSCCs. Shigeoka M et al. showed that tumors from patients with oral squamous cell carcinoma demonstrate high expression of CCR6 and CCL20. The study identified the source of CCL20 to be tumor cells while CCR6 was correlated with immunosuppressive CD163-positive macrophages. Lee et al. showed that the prevalence of IL-17-producing CD4+Foxp3+ tumor infiltrating lymphocytes is increased in oral squamous cell carcinoma [41]. In a separate study, they characterized the phenotypical and functional differences between CCR6+ and CCR6− Treg cell subsets. They showed that CCR6+ Treg cells have an effector/memory phenotype, whereas CCR6− Treg cells include both memory and naïve Treg cells [42]. The study also showed that activated CCR6-positive Treg cells exhibit stronger suppressive activity than CCR6-negative Treg cells due to enhanced IL-10 production as well as a higher expression of CD25 and HLA-DR. Consistent with these findings, our migration assay results showed that the majority of migrating Treg cells are CCR6-positive, in contrast to the non-migrating cells which are CCR6-negative. These results point to an important role for CCR6+ Tregs in promoting an immunosuppressive TME and mediating radioresistance.
In radioresistant tumors, remodeling of the immune TME to exclude or eliminate immunosuppressive cell populations can lead to tumor sensitization to treatments including radiotherapy, chemotherapy, and immunotherapy. Such TME remodeling can be achieved by targeted manipulation of chemokines that dictate the immune phenotype of tumors. Our study showed that inhibition of CCL20 reduces the prevalence of intratumoral Tregs, Th17 cells and increases CD8+ lymphocytes. This is consistent with a previous study in colorectal cancer which showed that tumor cell-secreted CCL20 recruits Tregs and promotes chemoresistance through Nf-kB signaling [22]. The study showed that CCL20 inhibition suppressed tumor progression and restored tumor sensitivity to 5-FU chemotherapy.
Our findings showed that the inhibition of CCL20 causes in changes in other chemokines. We observed that GM-CSF and IL-2 were decreased while CCL19 was increased. Previous studies have shown that GM-CSF contributes to an increased polarization of macrophages to the pro-tumoral M2 phenotype and IL-2 drives Treg cell proliferation, differentiation, and function whereas CCL19 was shown to induce chemotaxis of M1 macrophages [43–45]. The increase in GM-CSF observed after radiation could explain the higher number of migrating Treg cells in the radiation + anti-CCL20 group in migration assays. Future studies investigating combined blockade of GM-CSF in combination with anti-CCL20 could further limit Treg migration and improve anti-tumor immunity after RT.
A limitation of our study is that we did not analyze the impact of CCR6-CCL20 inhibition on myeloid cell populations such as tumor-associated macrophages. These populations have been shown to play a role in treatment resistance of HNSCCs [46] and can express the CCR6 receptor [47, 48]. In addition, CCR6 is expressed on B cells and has been shown to enhance angiogenesis in hepatocellular carcinomas that express CCL20 [49]. Future studies in our lab will further elucidate the impact of CCR6-CCL20 signaling on B cells and myeloid cells.
Conclusion
Collectively, our data suggest that radiation promotes the induction of CCL20 in tumors with immune-suppressive mechanisms and the inhibition of CCL20 can enhance the response to RT. We suggest that implementing a personalized approach based on the identification and targeting of factors that mediate radioresistance can address the void currently present for treatment-resistant tumors.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
This reseach was funded by the Cancer Research Society and the CIHR-Institute of Musculoskeletal Health and Arthritis (Grant # 837109).
Author’s Contribution
CR and AO designed the studies, performed the experiments and wrote the manuscript. RH, NS and JPO performed experiments. DL and FMB contributed to Table 1 and performed mass-spectrometry experiments. PHF, MB and LF assisted in experimental design and manuscript preparation. GAT, CSW, PD, ER, VHT and BP assisted with design of radiation-based experiments and associated data analysis.
Declarations
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.McDonald MW, et al. ACR appropriateness criteria retreatment of recurrent head and neck cancer after prior definitive radiation expert panel on radiation oncology-head and neck cancer. Int J Radiat Oncol Biol Phys. 2011;80:1292–1298. doi: 10.1016/j.ijrobp.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 2.Bonner JA, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006;354:567–578. doi: 10.1056/NEJMoa053422. [DOI] [PubMed] [Google Scholar]
- 3.Ang KK, et al. Randomized phase III trial of concurrent accelerated radiation plus cisplatin with or without cetuximab for stage III to IV head and neck carcinoma: RTOG 0522. J Clin Oncol. 2014;32:2940–2950. doi: 10.1200/JCO.2013.53.5633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gillison ML, et al. Radiotherapy plus cetuximab or cisplatin in human papillomavirus-positive oropharyngeal cancer (NRG Oncology RTOG 1016): a randomised, multicentre, non-inferiority trial. Lancet. 2019;393:40–50. doi: 10.1016/S0140-6736(18)32779-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mehra R, et al. Efficacy and safety of pembrolizumab in recurrent/metastatic head and neck squamous cell carcinoma: pooled analyses after long-term follow-up in KEYNOTE-012. Br J Cancer. 2018;119:153–159. doi: 10.1038/s41416-018-0131-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qin Y, Zheng X, Gao W, Wang B, Wu Y. Tumor microenvironment and immune-related therapies of head and neck squamous cell carcinoma. Mol Ther Oncolytics. 2021;20:342–351. doi: 10.1016/j.omto.2021.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Partlova S, et al. Distinct patterns of intratumoral immune cell infiltrates in patients with HPV-associated compared to non-virally induced head and neck squamous cell carcinoma. Oncoimmunology. 2015;4:e965570. doi: 10.4161/21624011.2014.965570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang J, et al. HPV-positive status associated with inflamed immune microenvironment and improved response to anti-PD-1 therapy in head and neck squamous cell carcinoma. Sci Rep. 2019;9:13404. doi: 10.1038/s41598-019-49771-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oguejiofor K et al (2017) Distinct patterns of infiltrating CD8+ T cells in HPV+ and CD68 macrophages in HPV- oropharyngeal squamous cell carcinomas are associated with better clinical outcome but PD-L1 expression is not prognostic. Oncotarget. 10.18632/oncotarget.14796. [DOI] [PMC free article] [PubMed]
- 10.Mito I, et al. Comprehensive analysis of immune cell enrichment in the tumor microenvironment of head and neck squamous cell carcinoma. Sci Rep. 2021;11:16134. doi: 10.1038/s41598-021-95718-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mandal R, et al. The head and neck cancer immune landscape and its immunotherapeutic implications. JCI Insight. 2016;1:e89829. doi: 10.1172/jci.insight.89829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lassen P, Overgaard J, Eriksen JG. Expression of EGFR and HPV-associated p16 in oropharyngeal carcinoma: correlation and influence on prognosis after radiotherapy in the randomized DAHANCA 5 and 7 trials. Radiother Oncol. 2013;108:489–494. doi: 10.1016/j.radonc.2013.08.036. [DOI] [PubMed] [Google Scholar]
- 13.Lassen P, et al. Impact of HPV-associated p16-expression on radiotherapy outcome in advanced oropharynx and non-oropharynx cancer. Radiother Oncol. 2014;113:310–316. doi: 10.1016/j.radonc.2014.11.032. [DOI] [PubMed] [Google Scholar]
- 14.Lindel K, Beer KT, Laissue J, Greiner RH, Aebersold DM. Human papillomavirus positive squamous cell carcinoma of the oropharynx: a radiosensitive subgroup of head and neck carcinoma. Cancer. 2001;92:805–813. doi: 10.1002/1097-0142(20010815)92:4<805::aid-cncr1386>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 15.Gottgens EL, Ostheimer C, Span PN, Bussink J, Hammond EM. HPV, hypoxia and radiation response in head and neck cancer. Br J Radiol. 2019;92:20180047. doi: 10.1259/bjr.20180047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Galvis MM, et al. Immunotherapy improves efficacy and safety of patients with HPV positive and negative head and neck cancer: a systematic review and meta-analysis. Crit Rev Oncol Hematol. 2020;150:102966. doi: 10.1016/j.critrevonc.2020.102966. [DOI] [PubMed] [Google Scholar]
- 17.Bol V, Gregoire V. Biological basis for increased sensitivity to radiation therapy in HPV-positive head and neck cancers. Biomed Res Int. 2014;2014:696028. doi: 10.1155/2014/696028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duan Q, Zhang H, Zheng J, Zhang L. Turning cold into hot: firing up the tumor microenvironment. Trends Cancer. 2020;6:605–618. doi: 10.1016/j.trecan.2020.02.022. [DOI] [PubMed] [Google Scholar]
- 19.Oweida AJ, et al. STAT3 modulation of regulatory T cells in response to radiation therapy in head and neck cancer. J Natl Cancer Inst. 2019 doi: 10.1093/jnci/djz036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang B, et al. CCL2/CCR2 pathway mediates recruitment of myeloid suppressor cells to cancers. Cancer Lett. 2007;252:86–92. doi: 10.1016/j.canlet.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 21.Kalbasi A, et al. Tumor-derived CCL2 mediates resistance to radiotherapy in pancreatic ductal adenocarcinoma. Clin Cancer Res. 2017;23:137–148. doi: 10.1158/1078-0432.CCR-16-0870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang D, et al. Colorectal cancer cell-derived CCL20 recruits regulatory T cells to promote chemoresistance via FOXO1/CEBPB/NF-kappaB signaling. J Immunother Cancer. 2019;7:215. doi: 10.1186/s40425-019-0701-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harlin H, et al. Chemokine expression in melanoma metastases associated with CD8+ T-cell recruitment. Cancer Res. 2009;69:3077–3085. doi: 10.1158/0008-5472.CAN-08-2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bouchard G, et al. Pre-irradiation of mouse mammary gland stimulates cancer cell migration and development of lung metastases. Br J Cancer. 2013;109:1829–1838. doi: 10.1038/bjc.2013.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oweida AJ, Bhatia S, Van Court B, Darragh L, Serkova N, Karam SD (2019) Intramucosal inoculation of squamous cell carcinoma cells in mice for tumor immune profiling and treatment response assessment. J Vis Exp. Published online Jan. 2019. [DOI] [PMC free article] [PubMed]
- 26.Judd NP, Allen CT, Winkler AE, Uppaluri R. Comparative analysis of tumor-infiltrating lymphocytes in a syngeneic mouse model of oral cancer. Otolaryngol Head Neck Surg. 2012;147:493–500. doi: 10.1177/0194599812442037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lu E, Su J, Zhou Y, Zhang C, Wang Y. CCL20/CCR6 promotes cell proliferation and metastasis in laryngeal cancer by activating p38 pathway. Biomed Pharmacother. 2017;85:486–492. doi: 10.1016/j.biopha.2016.11.055. [DOI] [PubMed] [Google Scholar]
- 28.Gopal YN, et al. Inhibition of mTORC1/2 overcomes resistance to MAPK pathway inhibitors mediated by PGC1alpha and oxidative phosphorylation in melanoma. Cancer Res. 2014;74:7037–7047. doi: 10.1158/0008-5472.CAN-14-1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Inoue Y, et al. Induction of human leukocyte antigen-A26-restricted and tumor-specific cytotoxic T lymphocytes by a single peptide of the SART1 antigen in patients with cancer with different A26 subtypes. J Immunother. 2000;23:296–303. doi: 10.1097/00002371-200005000-00002. [DOI] [PubMed] [Google Scholar]
- 30.Narita M, et al. Immune responses in patients with esophageal cancer treated with SART1 peptide-pulsed dendritic cell vaccine. Int J Oncol. 2015;46:1699–1709. doi: 10.3892/ijo.2015.2846. [DOI] [PubMed] [Google Scholar]
- 31.Oweida A, Harrah MK, Phan, A, Binder D, Bhatia S, Lennon S, Bukkapatnam S, Vancourt B, Uyanga N, Darragh L, Kim HM, Raben D, Tan AC, Heasley L, Clambey E, Nemenoff R, Karam SD (2018) Resistance to radiotherapy and PD-L1 blockade is mediated by TIM-3 upregulation and regulatory T-cell infiltration. Clin Cancer Res [DOI] [PMC free article] [PubMed]
- 32.Gasparoto TH, et al. Patients with oral squamous cell carcinoma are characterized by increased frequency of suppressive regulatory T cells in the blood and tumor microenvironment. Cancer Immunol Immunother. 2010;59:819–828. doi: 10.1007/s00262-009-0803-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wing K, Sakaguchi S. Regulatory T cells exert checks and balances on self tolerance and autoimmunity. Nat Immunol. 2010;11:7–13. doi: 10.1038/ni.1818. [DOI] [PubMed] [Google Scholar]
- 34.Shevach EM. Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity. 2009;30:636–645. doi: 10.1016/j.immuni.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 35.Moore EC, et al. Enhanced tumor control with combination mTOR and PD-L1 inhibition in syngeneic oral cavity cancers. Cancer Immunol Res. 2016;4:611–620. doi: 10.1158/2326-6066.CIR-15-0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang CY, et al. The role of CCL20/CCR6 axis in recruiting Treg cells to tumor sites of NSCLC patients. Biomed Pharmacother. 2015;69:242–248. doi: 10.1016/j.biopha.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 37.Bromley SK, Mempel TR, Luster AD. Orchestrating the orchestrators: chemokines in control of T cell traffic. Nat Immunol. 2008;9:970–980. doi: 10.1038/ni.f.213. [DOI] [PubMed] [Google Scholar]
- 38.Nagarsheth N, Wicha MS, Zou W. Chemokines in the cancer microenvironment and their relevance in cancer immunotherapy. Nat Rev Immunol. 2017;17:559–572. doi: 10.1038/nri.2017.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gajewski TF, Schreiber H, Fu YX. Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol. 2013;14:1014–1022. doi: 10.1038/ni.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu L, Xu W, Qiu S, Xiong S. Enrichment of CCR6+Foxp3+ regulatory T cells in the tumor mass correlates with impaired CD8+ T cell function and poor prognosis of breast cancer. Clin Immunol. 2010;135:466–475. doi: 10.1016/j.clim.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 41.Lee JJ, et al. Increased prevalence of interleukin-17-producing CD4(+) tumor infiltrating lymphocytes in human oral squamous cell carcinoma. Head Neck. 2011;33:1301–1308. doi: 10.1002/hed.21607. [DOI] [PubMed] [Google Scholar]
- 42.Lee JJ, et al. Enrichment of Human CCR6(+) Regulatory T Cells with Superior Suppressive Activity in Oral Cancer. J Immunol. 2017;199:467–476. doi: 10.4049/jimmunol.1601815. [DOI] [PubMed] [Google Scholar]
- 43.Xuan W, Qu Q, Zheng B, Xiong S, Fan GH. The chemotaxis of M1 and M2 macrophages is regulated by different chemokines. J Leukoc Biol. 2015;97:61–69. doi: 10.1189/jlb.1A0314-170R. [DOI] [PubMed] [Google Scholar]
- 44.Sierra-Filardi E, et al. CCL2 shapes macrophage polarization by GM-CSF and M-CSF: identification of CCL2/CCR2-dependent gene expression profile. J Immunol. 2014;192:3858–3867. doi: 10.4049/jimmunol.1302821. [DOI] [PubMed] [Google Scholar]
- 45.Zorn E, et al. IL-2 regulates FOXP3 expression in human CD4+CD25+ regulatory T cells through a STAT-dependent mechanism and induces the expansion of these cells in vivo. Blood. 2006;108:1571–1579. doi: 10.1182/blood-2006-02-004747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Balermpas P, et al. Head and neck cancer relapse after chemoradiotherapy correlates with CD163+ macrophages in primary tumour and CD11b+ myeloid cells in recurrences. Br J Cancer. 2014;111:1509–1518. doi: 10.1038/bjc.2014.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nandi B, et al. Stromal CCR6 drives tumor growth in a murine transplantable colon cancer through recruitment of tumor-promoting macrophages. Oncoimmunology. 2016;5:e1189052. doi: 10.1080/2162402X.2016.1189052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Boyle ST, Faulkner JW, McColl SR, Kochetkova M. The chemokine receptor CCR6 facilitates the onset of mammary neoplasia in the MMTV-PyMT mouse model via recruitment of tumor-promoting macrophages. Mol Cancer. 2015;14:115. doi: 10.1186/s12943-015-0394-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.He H, et al. CCR6(+) B lymphocytes responding to tumor cell-derived CCL20 support hepatocellular carcinoma progression via enhancing angiogenesis. Am J Cancer Res. 2017;7:1151–1163. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.