Abstract
Immune checkpoint inhibitors (ICIs) for programmed death-1 (PD-1) and programmed cell death-ligand 1 (PD-L1) have become preferred treatment strategies for several advanced cancers. However, response rates for these treatments are limited, which encourages the search for new ICI candidates. Recent reports have underscored significant roles of B7 homolog 3 protein (B7-H3) in tumor immunity and disease progression. While its multifaceted roles are being elucidated, B7-H3 has already entered clinical trials as a therapeutic target. In this review, we overview the recent results of clinical trials evaluating the antitumor activity and safety of B7-H3 targeting drugs. On this basis, we also discuss the challenges and opportunities arising from the application of these drugs. Finally, we point out current gaps to address in the understanding of B7-H3 function and regulation in order to fully unleash the future clinical utility of B7-H3-based therapies for the treatment of cancer.
Keywords: Immune checkpoint, B7-H3, Clinical trials, Immunotherapy
Introduction
With the discovery of immune checkpoint molecules, related immunotherapies have gradually been pillars of cancer treatments, which are currently shifting to the front-line for advanced non-small-cell lung cancer (NSCLC) and melanoma [1–3]. However, response rates to immune checkpoint inhibitors (ICIs) are still limited to a very small subset of patients [4], encouraging the need for identifying new ICI candidates. In fact, several other immune checkpoints are already under investigation, such as the B7 homolog 3 protein (B7-H3). B7-H3 is a single-pass type I transmembrane protein discovered in 2001 [5, 6], which belongs to the B7 family. While B7-H3 is low or undetectable in normal tissue, it is widely expressed in cancers [7]. Importantly, B7-H3 has been found in solid tumors with low or negative PD-L1 expression like pulmonary invasive mucinous adenocarcinoma [8], prostate cancer [9], and soft tissue sarcoma [10]. Furthermore, it has been found on keratinocytes, fibroblast synovial cells, endothelial cells [11, 12], and tumor-associated vasculature (TAV) [13].
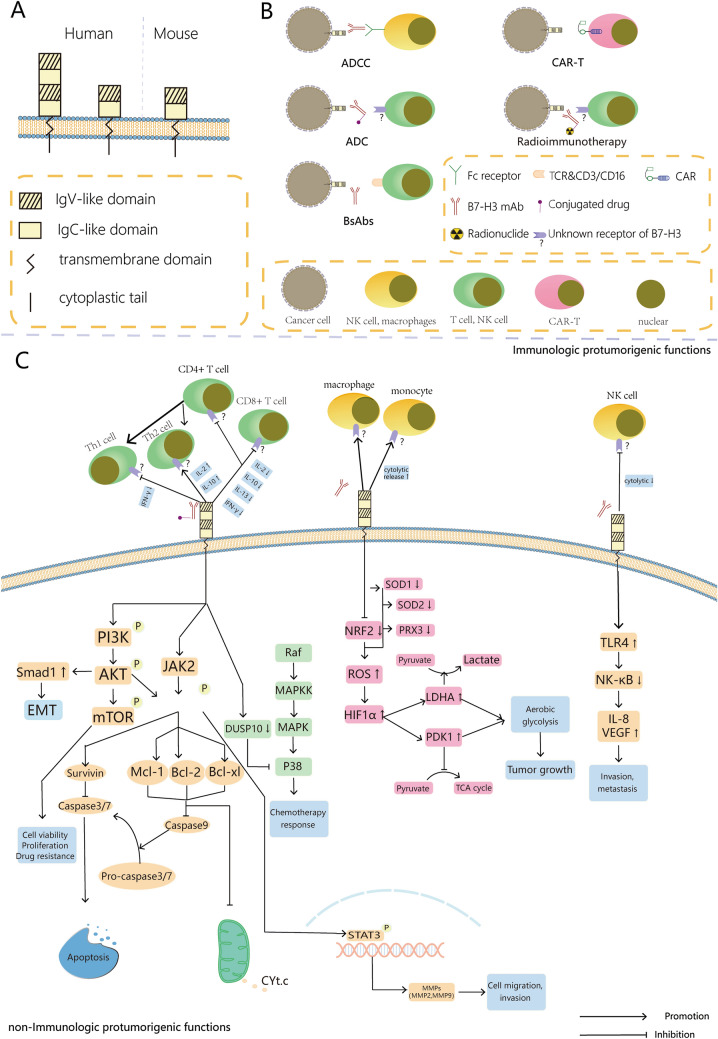
Unlike its well-known homolog B7-H1 (PD-L1), the isoforms of B7-H3 are different between humans and mice [5, 6]. In mice, the extracellular domain contains an immunoglobulin variable (IgV)-like and an immunoglobulin constant (IgC)-like domain. While in humans, it contains one or two pairs of identical domains owning to exon duplication, both of which were encoded by a gene on chromosome 15 [5, 14] (Fig. 1A). Despite B7-H3 has been originally described as a co-stimulatory molecule, a growing number of studies have shed the light on an immune-inhibitory function of B7-H3 in tumors [6, 15] (See Fig. 1C). A detailed review of the multifaceted roles of B7-H3 in tumor immunity has been recently published by Li et al. [16]. Besides, B7-H3 has also been reported to carry non-immunological functions by promoting cancer cell invasion and chemoresistance through a variety of mechanisms, including anti-apoptosis, pro-proliferation, metabolic reprogramming, and pro-angiogenesis [17].
Fig. 1.
Graphical Abstract. (A) B7-H3 is a single-pass type I transmembrane protein with three domains: an extracellular domain, a transmembrane, and an intracellular domain. In mice, there is one (IgV)-like and (IgC)-like domain (2IgB7-H3). In humans, it contains one (2IgB7-H3) or two (4IgB7-H3) pairs of identical domains owning to exon duplication; (B) Overview of therapeutic tools targeting B7-H3 currently under investigation in clinical trial. (C) Roles of B7-H3 in the tumor microenvironment, including immunologic and non-immunologic pro-tumorigenic functions
In recent years, more than 20 clinical trials on B7-H3 have been carried out to evaluate the curative efficacy of B7-H3 targeting therapies. In this review, we provide an overview of these therapies and the latest results of preclinical and clinical studies (Table 1). In addition, we describe the current unknowns on B7-H3 biology and challenges and opportunities of current and future B7-H3 targeting therapies.
Table 1.
Clinical trials with B7-H3-targeting immunotherapies
| Drug(s) | Type of mAb | ID Number | Phase | Recruitment Status | Country | Estimated enrollment | Masking | Dose | Age (years) | Cancer type | Outcomes |
|---|---|---|---|---|---|---|---|---|---|---|---|
| MGC018 | ADC | NCT037295961 | I/II | Recruiting | MacroGenics | 182 | None | Not mentioned | > 18 | Advanced Solid Tumor, Metastatic Castrate Resistant Prostate Cancer, Non-Small Cell Lung Cancer, Triple Negative Breast Cancer, Squamous Cell Carcinoma of Head and Neck, Melanoma, Prostate Cancer | 5/9 metastatic prostate cancer patients with reduction in PSA levels of ≥ 50%, and 3/3 melanoma patients showed partial response with ≥ 24% tumor volume reduction. One patient experienced a grade 4 AE (neutropenia) |
| MGA271 | ADCC | NCT013911433 | I | Terminated | United States | 179 | None | 0.01-15 mg/kg | > 18 | Melanoma, prostate carcinoma and other B7-H3+ tumors | 43.5% of patients (20/46) experienced stable disease (> 12 weeks) and tumor shrinkage (2%-69%), common AEs were observed, including fatigue, nausea, chills, and vomiting |
| MGA271 | ADCC | NCT029231804 | II | Not recruiting | United States | 33 | None | 15 mg/kg (weekly) IV | > 18 | Prostate Cancer | Primary endpoints are safety and tolerability. 3/32 patients showed marked tumor infiltration of CD8 + T cells in MGA271-treated samples |
| 124I-8H9 | Radioimmunotherapy | NCT0150291717 | I | Recruiting | United States | 64 | None | 0.25–12 mCi | 2–21 | Brain Cancer, Brain Stem Glioma | Some patients presented with grade 3 AEs (hemiplegia, skin infection, and anxiety) but no grade 4 or grade 5 AEs was reported suggesting an acceptable safety profile |
| 131I-8H9 | Radioimmunotherapy | NCT0008924518 | I | Not recruiting | United States | 120 | None | 10–100 mCi | All | Brain and Central Nervous System Tumors Neuroblastoma Sarcoma | Nearly 50% of patients were alive at 36 months post-treatment in a setting where overall survival at 36 months is usually less than 10% |
| 131I-8H9 | Radioimmunotherapy | NCT0109964419 | I | Not recruiting | United States | 54 | None | 30–50 mci/m2 | > 1 | Peritoneal Cancer | Three patients (n = 1 each) had transient, self-limiting, possibly therapy-related grade 3 toxicities: neutropenia, hepatic transaminase elevation and thrombocytopenia. 6/7 DSRCT patients treated without evaluable disease remain in remission at a median of 11.1 months post 131I-8H9 |
| DS-7300a | ADC | NCT041456222 | I-II | Recruiting | United States | 160 | None | Stating at 0.8 mg/kg | > 18 | Advanced Solid Tumor, Malignant Solid Tumor | Ongoing |
| MGA271 | ADCC | NCT029829415 | I | Completed | United States | 25 | None | Starting at 10 mg/kg (weekly) IV | 1–35 | Neuroblastoma, Rhabdomyosarcoma, Osteosarcoma, Ewing Sarcoma, Wilms Tumor, Desmoplastic Small Round Cell Tumor | Ongoing |
| MGA271 | ADCC | NCT023813146 | I | Completed | United States | 24 | None | Not mentioned | > 18 |
Melanoma Non-Small Cell Lung Cancer |
Ongoing |
| MGA271 | ADCC | NCT024752137 | I | Not recruiting | MacroGenics | 145 | None | Not mentioned | > 18 |
Melanoma Head and Neck Cancer Non-Small Cell Lung Cancer Urethelial Carcinoma |
Ongoing |
| MGA271 | ADCC | NCT046348258 | II | Recruiting | United States | 80 | None | Not mentioned | > 18 | Head and Neck Cancer | Ongoing |
| DS-5573a | ADCP | NCT021925679 | I | Terminated | Japan | 19 | None | 0.1–30 mg/kg | > 20 | Advanced solid malignant tumor | Ongoing |
| MGD009 | BiAb | NCT0262853510 | I | Terminated | United States | 67 | None | 0.3 ug/kg (every 2 weeks) | > 18 |
Mesothelioma, Bladder Cancer, Melanoma, Squamous Cell Carcinoma of the Head and Neck, Non-Small Cell Lung Cancer, Clear Cell Renal Cell Carcinoma, Ovarian Cancer, Thyroid Cancer, Breast Cancer, Pancreatic Cancer |
Ongoing |
| 4SCAR-276 | CAR-T cell | NCT0443264911 | I-II | Recruiting | China | 100 | None | Not mentioned | 1–75 | Solid Tumor | Ongoing |
| SCRICAR-B7H3(s) | CAR-T cell | NCT0418503812 | I | Recruiting | United States | 70 | None | Not mentioned | 1–26 |
Central Nervous System Tumor, Diffuse Intrinsic Pontine Glioma, Diffuse Midline Glioma, Ependymoma Medulloblastoma, Germ Cell Tumor, Atypical Teratoid/ Rhabdoid Tumor, Primitive Neuroectodermal Tumor, Choroid Plexus Carcinoma, Pineoblastoma, Childhood Glioma |
Ongoing |
| 4-1BBζ B7H3-EGFRt-DHFR | CAR-T cell | NCT0448377813 | I | Recruiting | United States | 68 | None | Not mentioned | < 26 | Advanced/metastatic cancer | Ongoing |
| CAR.B7-H3 | CAR-T cell | NCT0467006814 | I | Recruiting | United States | 21 | None | Not mentioned | > 18 | Epithelial Ovarian Cancer | Ongoing |
| B7-H3 CAR-T | CAR-T cell | NCT0407786615 | I/II | Recruiting | China | 40 | None | Not mentioned | 18–75 | Recurrent Glioblastoma Refractory Glioblastoma | Ongoing |
| B7-H3 CAR-T | CAR-T cell | NCT0438517316 | I | Recruiting | China | 12 | None | Not mentioned | 18–75 | Recurrent Glioblastoma Refractory Glioblastoma | Ongoing |
| 131I-8H9 | Radioimmunotherapy | NCT0327540220 | II-III | Recruiting | Spain | 32 | None | 2 mCi in week 1, 50 mCi in week 2 | < 18 | Neuroblastoma, CNS Metastases, Leptomeningeal Metastases | Ongoing |
| 131I-8H9 | Radioimmunotherapy | NCT0402221321 | II | Recruiting | United States | 55 | None | 80 mCi/m2 | > 1 | Desmoplastic Small Round Cell Tumor, Peritoneal Cancer, Peritoneal Carcinoma | Ongoing |
| 177Lu-DPTA-omburtamab | Radioimmunotherapy | NCT0431524622 | I-II | Not recruiting | United States | 63 | None | Dosimetry dose (5 weeks) ITV | > 18 | Leptomeningeal Metastasis, Solid Tumor | Ongoing |
| 177Lu-DTPA-omburtamab | Radioimmunotherapy | NCT0416761823 | I-II | Recruiting | United States | 40 | None | Not mentioned | 3–19 | Medulloblastoma | Ongoing |
B7-H3 as a tumor-associated antigen targetable with antibody-based therapies
Several therapeutic antibodies targeting B7-H3-expressing cells have been developed in the last decade (Fig. 1B, Table 1). Preclinical studies have been successfully completed leading to multiple dose-escalation phase I/II trials with safety and tolerability as primary endpoints. Few clinical trials have also included antitumor activity as a secondary endpoint. Based on the anticipated completion date, final reports for the majority of clinical trials would be published within the next two or three years.
Antibody–drug conjugates
Mechanism of action
Antibody–drug conjugates (ADCs) are emerging as promising anticancer therapies with the recent FDA approval of several ADCs for the treatment of metastatic solid tumors [18–20]. ADCs are formed from three different components: a humanized antibody, a linker and a drug payload. Their primary function is to target a tumor-associated surface protein and deliver the cytotoxic payload upon internalization of the antibody-antigen complex. Based on these mechanisms, ADCs have the potential to efficiently eradicate tumors while minimizing off-target toxicities. Nevertheless, the efficacy and safety of ADCs rely on critical parameters including antibody affinity to its target, high expression of the target in tumors and low/no expression in normal tissue, target internalization and payload cytotoxic potency. Tubulin inhibitors monomethyl auristatins (MMAE, MMAF) have been commonly used for ADC development [21]. Duocarmycins represent a novel class of ADC payloads, leading to DNA alkylation and cell death [22]. They are attractive tools because they can act on dividing and non-dividing cancer cells which make hypoxic, chemoresistant and cancer stem-like tumor cells sensitive to these ADC payloads [23]. In addition, duocarmycins are cell-permeant and upon cell death, and they can diffuse through membranes of neighboring tumor cells resulting in bystander killing. Unlike MMAE, duocarmycins are not substrates of multidrug resistance proteins (MDR) which cause resistance to ADC through drug efflux outside the cell. To date, two ADCs targeting B7-H3, namely MGC018 and DS-7300a, are currently under investigation in clinical trials. MGC018 is a humanized IgG1 monoclonal antibody against B7-H3 developed by Macrogenics that contains a cleavable linker-duocarmycin payload [24]. DS7300a (Daiichi Sankyo Inc) is a humanized IgG1 anti-B7-H3 conjugated with a cleavable linker and an exatecan derivative (DXd) that inhibits topoisomerase I and DNA replication in dividing cells [25].
MGC018 (Macrogenics)
Preclinical studies with MGC018 in several patient-derived xenograft models (breast cancer, prostate cancer, head and neck cancer) showed strong antitumor activity with > 95% tumor reduction [24]. No in vivo data with MGC018 have been conducted to demonstrate bystander killing, drug intracellular uptake or off-tumor on-target toxicity. MGC018 does not cross-react with mouse B7-H3 precluding assessment of potential toxicity in normal tissue. Bystander killing activity of MGC018 has only been observed in vitro with co-culture of B7-H3-positive and B7-H3-negative tumor cells. Interestingly, TAV has been shown to upregulate B7-H3 expression, while no expression was detected in normal vasculature [26]. In a follow-up study, Seaman et al. generated an ADC (named m276 conjugated to MMAE) targeting both mouse and human B7-H3 [27]. Using a mouse tumor xenograft model, they show that this ADC was effective against tumor cells as well as TAV supporting a dual therapeutic activity. MGC018 is currently tested in phase I/II clinical study (NCT037295961) with patients presenting advanced solid cancers, including metastatic castrate resistant prostate cancer, NSCLC, triple negative breast cancer, squamous cell carcinoma of head and neck and melanoma [28]. Interim analysis with 29 patients enrolled in the study was presented at the ASCO annual meeting 2021. Preliminary results showed antitumor activity in metastatic prostate cancer patients (5/9 patients with reduction in PSA levels of ≥ 50%) and melanoma patients (3/3 patients showed partial response with ≥ 24% tumor volume reduction). MGC018 showed an acceptable safety profile with manageable hematologic and dermal toxicity. One patient experienced a grade 4 adverse event (neutropenia). Primary outcome measures for this study are expected to be completed by March 2022.
DS-7300a (Daiichi Sankyo Inc)
Another B7-H3 ADC (DS-7300a) is under investigation in a dose-escalation phase I/II trial without reported results yet (NCT041456222). Few data related to DS-7300a are available but a poster presentation at the ENA 2020 symposium reported its antitumor activity in preclinical tumor models [29].
Challenges and opportunities with B7-H3 ADCs
Since FDA approval of the first ADC in 2009 (gemtuzumab ozogamicin), 11 ADCs have been approved for clinical application and more than 80 other ones are under evaluation in clinical studies. To date, no duocarmycin-based ADCs have been approved for oncological practice yet. A HER2-targeting ADC conjugated with duocarmycin (Trastuzumab) has shown promising clinical efficacy in a phase I trial leading the FDA to grant Fast Track Designation status [30]. However, the study also reported that 71% of patients had ocular adverse events causing discontinuation for 10% of treated patients [30]. Despite considerable progress, these clinical data underscore several hurdles that remain to be overcome in ADC development including off-target toxicity, drug penetration, limited drug retention and stability of payload in circulation [31]. In addition, regulation and trafficking of the target need to be well understood as several mechanisms of resistance to ADC have been revealed in prior studies [32], including defective internalization of the target and reduced target expression in ADC-treated tumors [33, 34]. Such knowledge is critical to design rationalized ADC therapies and develop biomarkers that can identify patients who will benefit the most from this type of therapy. While targeting B7-H3 has already entered clinical trials, its regulation and trafficking in normal and tumor tissues has not been fully elucidated. B7-H3 expression has been detected in normal tissue and on the surface of immune cells but it is unclear if ADCs can cause life-threatening off-tumor on-target toxicity [35, 36]. In addition, B7-H3 can be shed from the cell surface as soluble protein or bound to extracellular vesicles [37–39]. It is currently unknown if soluble and EV-bound B7-H3 can act as decoys and impair tumor penetration and therapeutic efficacy of ADCs [40, 41].
Antibody-dependent cell-mediated cytotoxicity
Mechanism of action
Antibody-dependent cellular cytotoxicity (ADCC) represents another promising therapeutic strategy for many cancer types. While ADCs directly induce cell death through the delivery of cytotoxic payload, ADCC efficacy relies on the engagement of the host immune system to eradicate tumor cells [42]. It is one of the defense mechanisms the immune system used to contain an infection. Pathogen-infected target cells are coated by naturally produced antibodies, and the Fc region of the antibody is recognized by NK cells through affinity with Fc receptors. Upon binding, NK cells release cytotoxic molecules (perforin, granzyme B) causing infected target cell death. This natural immune mechanism has been recently exploited to develop synthetic therapeutic antibodies that can induce ADCC upon binding of tumor-associated surface antigens [43, 44]. Besides inherent barriers to antibody-based therapies (tumor penetration, soluble antigens, sensitive and specific recognition of tumor-associated surface antigens), the therapeutic efficacy of ADCC antibodies is also dependent on the binding affinity of the Fc receptor, NK cell tumor infiltration and degree of immune cell activation. Site-directed mutagenesis, glycoengineering of Fc portions of antibodies are currently used to enhance the potency of therapeutic ADCC antibodies [45]. Currently, there are 11 ADCC antibodies approved by the FDA or in clinical trials [45]. Two ADCC antibodies against B7-H3 are currently under investigation, namely MGA271 (Macrogenics) and DS-5573a (Daiichi Sankyo Inc).
MGA271 (Macrogenics)
Enoblituzumab (MGA271) is a Fc-optimized humanized IgG1 antibody against B7-H3, in which the Fc domain contains 5 amino acid changes to increase the affinity for activating FcγR (CD16A) and decrease the affinity for inhibitory FcγR (CD32B) [46]. A preclinical study using renal cell carcinoma and bladder cancer xenograft tumor models showed strong antitumor activity of MGA271 [46]. Following successful preclinical studies, MGA271 safety profile and efficacy have been evaluated in three clinical studies on patients with pediatric and adult solid tumors (NCT013911433, NCT029231804, NCT029829415) (Table 1). The first two studies have been completed in 2019, but final results have not been reported yet. Interim results of NCT013911433 showed that 43.5% of patients (20/46) experienced stable disease (> 12 weeks) and tumor shrinkage (2–69%) [47]. Although no dose-limiting toxicity and no drug-related treatment discontinuation were observed in the interim study, common adverse events (AEs) were observed, including fatigue, nausea, chills, and vomiting [47]. In another trial (NCT029231804), primary endpoints are safety and tolerability and secondary endpoints include analysis of immune infiltration and tumor cell death in prostatectomy specimens. Preliminary results on 13/32 patients showed marked tumor infiltration of CD8 + T cells in MGA271-treated patients samples compared to age- and stage-matched untreated prostatectomy controls suggesting an antitumor immune response induced by treatment with MGA271 [48].
Meanwhile, the combination of MGA271 with other ICIs is currently investigated (NCT023813146, NCT024752137, NCT046348258) (Table 1). In the trial of NCT024752137 [49], the patients with squamous cell carcinoma of the head and neck, NSCLC, and urethelial carcinoma showed clinical remission with the combination therapy.
DS-5573a (Daiichi Sankyo Inc)
DS-5573a is an afucosylated humanized anti-B7H3 IgG1 antibody binding to IgC1 and IgC2 immunoglobulin-like domains of human B7-H3 (Daiichi Sankyo Inc) [50], which has the potency of B7-H3-dependent ADCC and antibody-dependent cellular phagocytosis (ADCP) activity. In tumor xenograft mouse models with poorly differentiated breast adenocarcinoma, DS-5573a showed significant antitumor efficacy at doses of 0.003–3 mg/kg in vivo [50]. The only clinical trial on DS-5573a is an open-label phase I study (NCT021925679) initiated in 2014 to evaluate the safety and pharmacokinetics of DS-5573a in patients with advanced solid tumors, but the study was terminated on the business decision in 2017. A derivative of DS-5573a conjugated with a radioligand (Zirconium-89) has been developed to serve as a molecular imaging probe in clinical studies to measure B7-H3 expression in different tumors [51].
Challenges and opportunities with B7-H3 ADCC
The ability of monoclonal antibodies to kill tumor cells largely depends on ADCC and ADCP. In addition to MGA271, some drugs depending on this mechanism are approved for use in several tumors, such as avelumab (anti-PD-L1) for the treatment of metastatic urothelial carcinoma [52], trastuzumab (anti-HER2) for the treatment of metastatic breast cancer [53] and cetuximab (anti-EGFR) for metastatic colorectal cancer [54]. Just like EGFR and HER2, B7-H3 is widely expressed in different tumors [55], but monoclonal antibodies for B7-H3 can be safer owing to the limited-expression level of B7-H3 protein in normal tissues. Additionally, according to the present results of NCT029231804 and NCT029829415, MGA271 not only promotes NK-mediated tumor cell killing directly but also increases tumor infiltration of CD8 + T cells. Combined with previous studies [56, 57], it supports the therapeutic potential of ADCC-related drugs and serves as a basis for future applications.
Bispecific antibodies
Mechanism of action
Bispecific antibodies are a class of therapeutic antibodies characterized by the ability to recognize two different antigens simultaneously. There are several types of bispecific antibodies based on their mechanism of action. ADC-derived bispecific antibodies can bind two tumor-associated surface antigens increasing specificity for tumor cells. Bispecific antibodies can target two immune checkpoint molecules (i.e., cytotoxic T lymphocyte-associated antigen-4 (CTLA-4) and PD-1) to increase antitumor immune response. Bispecific T-cell (BiTE) and NK-cell engagers (BiKE) act as a bridge between cancer cells and T-cells or NK cells, respectively. For instance, blinatumomab, used for the treatment of chronic lymphocytic leukemia, binds CD19 on tumor cells and CD3 on T-cells [58, 59]. The binding of BiTE to CD3 induces TCR-mediated signaling and antitumor immune response similar to antigen presentation. To date, only one bispecific antibody (Blinatumomab) has been approved for clinical use, but recent progress in antibody engineering has paved the way for more than 200 clinical trials testing bispecific antibodies for cancer treatment [60].
MGD009 (B7-H3 × CD3)
MGD009 is a bispecific anti-CD3 × anti-B7-H3 (B7-H3 Bi-Ab) antibody with one arm of the bispecific antibody binding to the CD3 component of the TCR complex on T cells, and the other arm recognizing B7-H3 on the surface of tumor cells. Following successful preclinical studies, a multicenter open-label trial was launched to study safety, pharmacokinetics, pharmacodynamics, and preliminary antitumor activity of MGD009 in advanced cancers (NCT0262853510) [61]. However, the study has been terminated on business decision and no further studies are anticipated with MGD009.
Challenges and opportunities with B7-H3 Bi-Ab
Despite no clinical studies are ongoing with B7-H3 Bi-Ab, several groups have demonstrated the therapeutic potential of B7-H3 Bi-Ab in different malignancies including bladder cancer, melanoma, hematological cancer, and head and neck cancer [62–65]. Through a series of mouse model experiments, it found that B7-H3 Bi-Ab effectively inhibited tumor growth, thereby improving the overall survival of tumor-bearing mice [63, 64]. In 2021, You et al. reported the efficacy of a bispecific antibody (B7-H3 × 4-1BB) to reduce tumor growth in an immunocompetent mouse model of colorectal cancer [66]. 4-1BB (CD137) is a co-stimulatory receptor, which is widely expressed in immune cells, including activated T cells, NK cells, and DCs. In this study, B7-H3 × 4-1BB elicited CD8 T cell mediated antitumor immune response. In addition, they found that B7-H3 × 4-1BB showed synergistic activity with PD-1 blockade. Altogether, these preclinical data support the future development of clinical trials investigating B7-H3 Bi-Ab for the treatment of advanced cancers.
Chimeric antigen receptor (CAR) therapy
Mechanism of action
Chimeric antigen receptor T cell and NK cell (CAR-T/NK) therapy is a form of immunotherapy that holds a lot of potential to treat cancers known as poorly immunogenic. CAR-T therapy consists of injecting patients’ T cells that are genetically modified to specifically recognize a tumor-associated antigen. The chimeric antigen receptor is a fusion protein composed of the epitope binding site of a monoclonal antibody targeting the tumor antigen linked to costimulatory and signaling domains of T-cell receptor. It has been a stepping stone toward the treatment of hematological malignancies [67]. Recent progress made in genetic modification of CAR-T models and molecular profiling of tumors has opened new hopes for introducing CAR-T therapy in the armamentarium of oncologists for the treatment of advanced solid tumors [68]. In the last two years, several groups have designed and tested CAR-T cells targeting B7-H3 in preclinical tumor xenograft models. A B7-H3 CAR was generated with a single-chain variable fragment (scFv) derived from the anti-B7H3 antibodies, and the most common ones include MGA271 (Macrogenics) mAb 376.96 and 8H9. It also includes a costimulatory motif (from 4-1BB, CD28, and CD8 molecules) and a signaling domain (CD3ζ) [69–71]. The antitumor immune activity of B7-H3 CAR-T therapy has been confirmed in a series of hematologic and solid tumors [35, 72].
Clinical trials
Following the positive outcome of preclinical studies, seven ongoing clinical trials have been initiated to evaluate the safety and efficacy of B7-H3 CAR-T therapy in advanced pediatric and adult solid cancers (NC0443264911, NCT0418503812, NCT0448377813, NCT0467006814, NCT0407786615, NCT0438517316, NCT03198052). A first-in-human study was conducted on a 49-year-old woman with multiple recurrent anaplastic meningiomas [73]. MRI imaging showed reduced tumor growth in the treated tumor compared to untreated tumor lesions. An increase in inflammatory cytokines was observed in the cerebrospinal fluid after the last infusion, suggesting a therapeutic response to CAR-T. During the whole treatment, there were no AEs of grade 3 or higher.
Challenges and opportunities with B7-H3 CART
Altogether, preclinical results underscore the promising future for using B7-H3 as a tumor target for CAR-T therapy. Importantly, B7-H3 overexpression has been found in several pediatric cancers which are refractory to current immunotherapies. B7-H3 CAR offers new hopes for treating solid tumors with low immunogenicity. Despite B7-H3 represents one of the most promising cancer targets, inherent limitations of CAR-T therapy including treatment-related toxicities, tumor infiltration and persistence remain to be overcome. Recently, Lei et al. showed that B7-H3 CAR antitumor activity was enhanced in presence of SAHA (vorinostat), a deacetylase inhibitor used for the treatment of advanced cutaneous T-cell lymphoma trials [74]. SAHA mediated upregulation of B7-H3 on tumor cells and B7-H3 CAR on transduced T-cells. An increase in T-cell surface expression of B7-H3 CAR was associated with downregulation of immunosuppressive genes CTLA-4 and TET2 resulting in improved cytotoxic activity. While the molecular mechanisms of SAHA-mediated CAR-T function remain unclear, it represents a promising strategy to improve the therapeutic efficacy of CAR-T toward B7-H3-expressing tumors.
Radioimmunotherapy
Mechanism of action
Radioimmunotherapy (RIT) uses an antibody labeled with a radionuclide to deliver cytotoxic radiation to a target cell. It is a form of unsealed source radiotherapy. In cancer therapy, an antibody with specificity for a tumor-associated antigen is used to deliver a lethal dose of radiation to the tumor cells. The ability for the antibody to specifically bind to a tumor-associated antigen increases the dose delivered to the tumor cells while decreasing the dose to normal tissues. By its nature, RIT requires a tumor cell to express an antigen that is unique to the neoplasm or is not accessible in normal cells. Omburtamab (8H9) is a murine IgG1 monoclonal antibody produced by splenic lymphocytes of BALB/c mice immunized with neuroblastoma cells [75]. In 2001, Modak et al. first showed a high affinity of 8H9 for human brain tumors, childhood sarcoma, and neuroblastoma, while no signal was observed for normal human tissues. A few years later, B7-H3 was reported to be the target for 8H9 antibody [76] and a humanized version of 8H9 has demonstrated antitumor activity through ADCC [77]. Based on in vitro and preclinical studies, the 8H9 antibody has been evaluated as a radioimmunotherapy agent. To date, two different antibodies are tested in clinical trials, iodine-131 labeled Omburtamab and lutetium-177 labeled Omburtamab.
Iodine-131 labeled omburtamab
The earliest clinical study was designed by Kramer et al. for the treatment of neuroblastoma [78]. They found that while 131I-8H9 prolong overall survival (median OS 33 months) compared to previously published studies (median OS < 13 months), it is also associated with AEs in some patients, including self-limited fever, nausea, headache, transient grade 1 creatinine elevation, and grade 1 and 3 transient elevated serum transaminase. Self-limited myelosuppression was observed when the dose of 131I-8H9 injections ≥ 40 mCi. In a phase I trial (NCT0150291717), some patients presented with grade 3 AEs (hemiplegia, skin infection, and anxiety), but no grade 4 or grade 5 AEs were reported suggesting an acceptable safety profile [79, 80]. In NCT0008924518 [78, 81], nearly 50% of patients were alive at 36 months post-treatment in a setting where overall survival at 36 months is usually less than 10%. The same conclusion was observed in the trial of NCT0109964419 for peritoneal cancer [82]. Currently, two clinical trials are focusing on the effect of 131I-8H9 in other cancer types, including leptomeningeal metastasis of adult solid tumors, medulloblastoma and peritoneal cancer (NCT0327540220, NCT0402221321) (Table 1), with no results released.
Lutetium-177 labeled omburtamab
Lutetium-177 is an ideal isotope for radiopharmaceutical therapy. Its half-life makes it easier to combine with biologically active compounds and extends the treatment time [83]. Studies have shown that Lutetium-177 can emit low-energy gamma rays and can be used to image tumors in real-time during treatment. In order to promote rapid radioactive clearance, the monoclonal antibody 8H9 was combined with the chelating agent diethylenetriamine pentaacetate and radiolabeled with Lutetium-177 [84]. In two clinical studies (NCT0431524622 and NCT0416761823), possible adverse reactions and efficacy of lutetium-177 labeled Omburtamab in solid tumors were studied (Table 1), while the results are not published yet.
Challenges and opportunities with omburtamab
Modak et al. confirmed that 8H9 labeled with 131I can provide a therapeutic dose of radiation to solid tumors and inhibit tumor cell growth through established xenografts [85]. In 2010, in patients with recurrence of neuroblastoma (NB), they further confirmed that 8H9 can improve survival, indicating that both radioimmunotherapies can inhibit the growth of CNS NS through the blood–brain barrier. But the similarities and differences between the two need to be further studied and compared. As 8H9 is an anti-B7-H3 antibody, whether it affects the central immune system after labeling with 131I requires more research and discussion. In addition, because 131I -8H9 is radioactive, its value in tumor imaging diagnosis should also be further explored. Since the current research on 8H9 is limited to brain tumors, its efficacy and mechanism would be studied in other solid tumors.
Targeting B7-H3 function for next-generation cancer immunotherapy
Immune checkpoint blockade
Considering the recent success of immune checkpoint blockade in advanced cancers, targeting the inhibitory function of B7-H3 is regarded as one of the most promising strategies for next-generation cancer immunotherapy. However, no ICI has been developed yet. Unlike PD-L1, the receptor of B7-H3 and the downstream events of B7-H3 binding to recipient cells remain poorly elucidated. The protein TREML2 (TLT-2) expressed on the surface of T-cells has been reported as the first identified murine B7-H3 receptor but one following study contradicted the initial finding [86, 87]. Given that mouse B7-H3 sequence and structure differs from its human counterpart, it is reasonable to think they may act via two different receptors [14, 86]. A second study used a high-throughput interaction screening platform and identified IL20RA as a B7-H3 receptor, but no functional outcome has been identified yet. Given that IL20RA [88, 89] has been mostly found on the surface of tumor cells, the nature (cis or trans) of IL20RA: B7-H3 interaction remains unclear. The limited success in discovering B7-H3 receptor(s) may also reflect a more complex mechanism involving additional interacting partners on the same cell surface (cis-interaction). To mediate its inhibitory activity, PD-L1 directly interacts with PD-1 trans [90]. A recent study revealed that PD-L1 can cis-interact with PD-1 on tumor cells and antigen-presenting cells [91]. Cis-interaction of PD-L1 and PD-1 inhibits the ability of PD-L1 to bind with T-cell PD-1 in trans resulting in improved T cell response. CD80 (B7-1) [92–94] has been identified as another PD-L1 cis-ligand and the heterodimer CD80: PD-L1 on the surface of antigen-presenting cells was a critical mediator of the antitumor immune response. Therefore, further studies are needed to uncover the B7-H3 interactome on tumor cells and immune cells and identify protein interactions that mediate tumor immunity. Ultimately, this work will pave the way for developing new therapeutic strategies including immune checkpoint blockade.
Antibody-mediated degradation
While immune checkpoint blockade seems the most prevalent strategy to impair with B7-H3 function, intracellular degradation of the protein may also be a potent and durable therapeutic approach [95]. Other immune checkpoint molecules such as PD-L1 and CTLA-4 are subjected to antibody-mediated degradation [96, 97]. For instance, Ipilimumab (FDA-approved anti-CTLA-4) can induce lysosomal degradation of CTLA-4 by preventing interaction of intracellular CTLA-4 with LRBA, a regulator of CTLA-4 recycling from endosomes to cell surface. In the case of PD-L1, Tu et al. developed a novel antibody that prevents the binding of PD-L1 with CMTM6 resulting in PD-L1 destabilization at the plasma membrane and lysosomal degradation [96, 98, 99]. In a recent study, Durlanik et al. investigated the role of B7-H3 in the crosstalk between macrophages and cancer cells. They observed B7-H3 upregulation on the surface of macrophages when co-cultured with tumor spheroids. Strikingly, selective downregulation of B7-H3 expression on the surface of macrophages (~ 35%) was observed in the presence of anti-B7-H3 (clone EPNCIR122), while no effect was observed on tumor cells. Based on these findings, antibody-induced loss of B7-H3 in macrophages with EPNCIR122 clone may follow a similar mechanism to PD-L1 and CTLA-4 where anti-B7-H3 inhibits interaction with adaptor proteins responsible for its stability or trafficking at the cell surface. Since B7-H3 on tumor cells was unaffected by anti-B7-H3, it also points toward different regulatory mechanisms of cell-surface expression of B7-H3 in tumor and immune cells. Further studies are needed to elucidate the underpinnings of B7-H3 trafficking and leverage this knowledge to develop therapeutic antibodies that can inhibit B7-H3 function through intracellular degradation.
Regulation of B7-H3 expression and its impact on therapy development
Targeting B7-H3 glycosylation as anticancer therapy
In the past years, blocking the PD-1/PD-L1 axis has revolutionized cancer therapy. However, only a minority of patients (~ 30%) truly benefit from PD-1/PD-L1 inhibition [100]. More importantly, tumor PD-L1 expression is not a robust predictor of treatment response which represents a challenging scenario for patients and clinicians [101]. Recent progress in the understanding of PD-L1 expression and post-transcriptional regulation has revealed important regulatory mechanisms that can help design better therapeutic approaches and predictive tools [102–104]. For instance, glycosylation of PD-L1 has been shown to mediate its stability at the plasma membrane and its inhibitory activity through PD-1 binding [103, 104]. Targeting PD-L1 with specific antibodies against the glycosylated form promoted PD-L1 internalization and degradation resulting in enhanced T-cell cytotoxic activity [103]. Important lessons must be learned from heavily studied immune checkpoint molecules such as PD-L1 in order to establish rationalized studies investigating post-transcriptional regulation of B7-H3 and its impact on B7-H3 subcellular trafficking and function. While PD-L1 contains four glycosylation sites in its extracellular domain, B7-H3 has a total of eight glycosylation sites [86, 105, 106] because of the genomic duplication of its two immunoglobulin-like domains. Comprehensive analysis of B7-H3 glycosylation using mass spectrometry shows tumor-specific glycoforms with high levels of fucosylation in oral cancer [105]. A follow-up study identified the fucosyltransferase FUT8 as a critical mediator of B7-H3 glycosylation [106]. FUT8 enzymatic activity was necessary for B7-H3 stability at the plasma membrane and its inhibitory function on cytotoxic T-cells. Further studies are warranted to fully elucidate post-translational mechanisms of B7-H3 glycosylation and their role in its functional activity. Targeting B7-H3 glycosylation may be a promising therapeutic avenue to promote B7-H3 degradation and prevent its immunosuppressive activity.
Alternative splicing of B7-H3
Originally, two variants of B7-H3 have been identified. A full-length B7-H3 variant with 4Ig-like domains expressed in humans and a short variant with 2Ig-like domains expressed in mice [86]. A splicing variant of B7-H3 with 2Ig-like domains has also been reported in human cancer cell lines [86, 107, 108] and synovial monocytes, but it appears to remain a minor transcript compared to the full-length B7-H3. A recent study has revealed the first insights of B7-H3 splicing mediated by the regulator SRSF3, but it remains unknown functional roles of B7-H3 splicing in normal and malignant tissue [109]. Additionally, the functional differences between the short and full-length human B7-H3 isoforms remain controversial. In rheumatoid arthritis, 2Ig-B7-H3 was not found on the cell surface but in cytoplasmic compartments [108]. Using human T-cells, Sun et al. showed that 2Ig-B7-H3 recombinant protein induced T-cell proliferation and release of IFNγ and IL-2 [110]. In contrast, 4Ig-B7-H3 reduced T-cell proliferation suggesting two distinct immune functions for B7-H3. Crystallography analysis showed 34% identity between the two isoforms, but they were both able to bind on the surface of activated peripheral blood mononuclear cells (PBMCs). The 2Ig-B7-H3 isoform can induce the release of TNFα and IL-6 from LPS-activated monocytes, while no effect was observed with the long isoform. While further studies are warranted to fully elucidate the specific roles and regulation of B7-H3 isoforms, it remains possible that human B7-H3 isoforms act differently on immune cell subsets and via two putative receptors.
“Soluble” B7-H3
To add to the complexity of B7-H3 regulation, additional forms of B7-H3 have been found in the extracellular compartment [38, 111, 112]. In 2008, Zhang et al. showed the presence of a soluble and functionally active form of B7-H3 in culture media of immune cells and tumor cells [111]. The release of soluble B7-H3 was inhibited by a pan-metalloprotease inhibitor suggesting proteolytic cleavage of B7-H3. This discovery was followed by several studies reporting high levels of soluble B7-H3 in the serum of cancer patients [37, 38, 113–116]. Besides proteolytic cleavage, soluble B7-H3 can be produced by alternative splicing resulting in an isoform lacking its transmembrane domain [38]. Most studies have used ELISA to measure levels of soluble B7-H3 in cell culture medium and patient serum. However, this method cannot discriminate between membrane-free B7-H3 and membrane-bound B7-H3. In line with this, B7-H3 has also been found on the surface of extracellular vesicles released by cancer cells suggesting that multiple forms of soluble B7-H3 can be found in culture medium and patient’s biofluids [39, 117, 118]. To separate B7-H3-positive extracellular vesicles from unbound B7-H3, EV isolation/enrichment methods with size-exclusion chromatography, ultrafiltration or ultracentrifugation can be employed. In addition, advanced technologies such as nanoscale flow cytometry and single-particle interferometry are suitable to only detect B7-H3-positive extracellular vesicles. The recent groundbreaking findings on the immunoregulatory function of PD-L1-positive extracellular vesicles in local and systemic tumor immunity support the need to elucidate the regulatory mechanisms and functions of soluble B7-H3 [119, 120].
Conclusion
Targeting B7-H3 represents a promising therapeutic strategy at the dawn of the second revolution of cancer immunotherapy revolution. B7-H3 is highly expressed by several pediatric and adult cancers including cancers refractory to PD-1/PD-L1 therapies. Therefore, B7-H3 appears as a suitable tumor-associated antigen for the development of antibody-based therapies. While the first clinical trials to evaluate the safety and antitumor activity of B7-H3-targeting therapies are ongoing, B7-H3 function and regulation remain to be characterized, starting with the identification of the B7-H3 receptor. Further mechanistic studies are warranted to support the development of novel immunotherapeutic strategies, thereby opening new horizons for more efficacious treatments in human cancers.
Authors' contributions
TW and FL conceptualized the study, CL and KX performed the literature search, CL, GZ, YK, RR, FL and TW drafted the work. CL, YK, KX and RR, generated the figure and the table. All authors read and approved the final manuscript.
Funding
This study was funded by the National Natural Science Foundation of China (No. 31770963).
Availability of data and material
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
Code availability
Not applicable.
Declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Footnotes
Chuan Liu and Guangwei Zhang have contributed equally to this work.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Fabrice Lucien, Email: Lucien-Matteoni.Fabrice@mayo.edu.
Ti Wen, Email: wenti@cmu.edu.cn.
References
- 1.Berger KN, Pu JJ. PD-1 pathway and its clinical application: a 20year journey after discovery of the complete human PD-1 gene. Gene. 2018;638:20–25. doi: 10.1016/j.gene.2017.09.050. [DOI] [PubMed] [Google Scholar]
- 2.Kelly RJ, Ajani JA, Kuzdzal J, Zander T, Van Cutsem E, Piessen G, Mendez G, Feliciano J, Motoyama S, Lièvre A, Uronis H, Elimova E, Grootscholten C, Geboes K, Zafar S, Snow S, Ko AH, Feeney K, Schenker M, Kocon P, Zhang J, Zhu L, Lei M, Singh P, Kondo K, Cleary JM, Moehler M. Adjuvant nivolumab in resected esophageal or gastroesophageal junction cancer. N Engl J Med. 2021;384(13):1191–1203. doi: 10.1056/NEJMoa2032125. [DOI] [PubMed] [Google Scholar]
- 3.Tawbi HA, Forsyth PA, Algazi A, Hamid O, Hodi FS, Moschos SJ, Khushalani NI, Lewis K, Lao CD, Postow MA, Atkins MB, Ernstoff MS, Reardon DA, Puzanov I, Kudchadkar RR, Thomas RP, Tarhini A, Pavlick AC, Jiang J, Avila A, Demelo S, Margolin K. Combined nivolumab and ipilimumab in melanoma metastatic to the brain. N Engl J Med. 2018;379(8):722–730. doi: 10.1056/NEJMoa1805453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, Wolchok JD, Hersey P, Joseph RW, Weber JS, Dronca R, Gangadhar TC, Patnaik A, Zarour H, Joshua AM, Gergich K, Elassaiss-Schaap J, Algazi A, Mateus C, Boasberg P, Tumeh PC, Chmielowski B, Ebbinghaus SW, Li XN, Kang SP, Ribas A. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369(2):134–144. doi: 10.1056/NEJMoa1305133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Steinberger P, Majdic O, Derdak SV, Pfistershammer K, Kirchberger S, Klauser C, Zlabinger G, Pickl WF, Stockl J, Knapp W. Molecular characterization of human 4Ig-B7-H3, a member of the B7 family with four Ig-like domains. J Immunol. 2004;172(4):2352–2359. doi: 10.4049/jimmunol.172.4.2352. [DOI] [PubMed] [Google Scholar]
- 6.Chapoval AI, Ni J, Lau JS, Wilcox RA, Flies DB, Liu D, Dong H, Sica GL, Zhu G, Tamada K, Chen L. B7–H3: a costimulatory molecule for T cell activation and IFN-gamma production. Nat Immunol. 2001;2(3):269–274. doi: 10.1038/85339. [DOI] [PubMed] [Google Scholar]
- 7.Kontos F, Michelakos T, Kurokawa T, Sadagopan A, Schwab JH, Ferrone CR, Ferrone S. B7–H3: an attractive target for antibody-based immunotherapy. Clin Cancer Res. 2020 doi: 10.1158/1078-0432.ccr-20-2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nakagomi T, Goto T, Hirotsu Y, Shikata D, Yokoyama Y, Higuchi R, Otake S, Amemiya K, Oyama T, Mochizuki H, Omata M. Genomic characteristics of invasive mucinous adenocarcinomas of the lung and potential therapeutic targets of B7–H3. Cancers. 2018 doi: 10.3390/cancers10120478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roth TJ, Sheinin Y, Lohse CM, Kuntz SM, Frigola X, Inman BA, Krambeck AE, McKenney ME, Karnes RJ, Blute ML, Cheville JC, Sebo TJ, Kwon ED. B7–H3 ligand expression by prostate cancer: a novel marker of prognosis and potential target for therapy. Cancer Res. 2007;67(16):7893–7900. doi: 10.1158/0008-5472.can-07-1068. [DOI] [PubMed] [Google Scholar]
- 10.Majzner RG, Simon JS, Grosso JF, Martinez D, Pawel BR, Santi M, Merchant MS, Geoerger B, Hezam I, Marty V, Vielh P, Daugaard M, Sorensen PH, Mackall CL, Maris JM. Assessment of programmed death-ligand 1 expression and tumor-associated immune cells in pediatric cancer tissues. Cancer. 2017;123(19):3807–3815. doi: 10.1002/cncr.30724. [DOI] [PubMed] [Google Scholar]
- 11.Lai H, Sun Z, Yang J, Wu P, Guo Y, Sun J. Correction to: B7–H3 modulates endothelial cell angiogenesis through the VEGF cytokine. Immunol Res. 2020;68(3):177. doi: 10.1007/s12026-020-09134-8. [DOI] [PubMed] [Google Scholar]
- 12.Quandt D, Fiedler E, Müller A, Marsch WC, Seliger B. High constitutive B7–H3 expression on human keratinocytes supports T cell immunity. J Dermatol Sci. 2017;87(1):82–85. doi: 10.1016/j.jdermsci.2017.02.287. [DOI] [PubMed] [Google Scholar]
- 13.Ingebrigtsen VA, Boye K, Tekle C, Nesland JM, Flatmark K, Fodstad O. B7–H3 expression in colorectal cancer: nuclear localization strongly predicts poor outcome in colon cancer. Int J Cancer. 2012;131(11):2528–2536. doi: 10.1002/ijc.27566. [DOI] [PubMed] [Google Scholar]
- 14.Sun M, Richards S, Prasad DV, Mai XM, Rudensky A, Dong C. Characterization of mouse and human B7–H3 genes. J Immunol. 2002;168(12):6294–6297. doi: 10.4049/jimmunol.168.12.6294. [DOI] [PubMed] [Google Scholar]
- 15.Yonesaka K, Haratani K, Takamura S, Sakai H, Kato R, Takegawa N, Takahama T, Tanaka K, Hayashi H, Takeda M, Kato S, Maenishi O, Sakai K, Chiba Y, Okabe T, Kudo K, Hasegawa Y, Kaneda H, Yamato M, Hirotani K, Miyazawa M, Nishio K, Nakagawa K. B7–H3 negatively modulates CTL-mediated cancer immunity. Clin Cancer Res. 2018;24(11):2653–2664. doi: 10.1158/1078-0432.CCR-17-2852. [DOI] [PubMed] [Google Scholar]
- 16.Li G, Quan Y, Che F, Wang L. B7–H3 in tumors: friend or foe for tumor immunity? Cancer Chemother Pharmacol. 2018;81(2):245–253. doi: 10.1007/s00280-017-3508-1. [DOI] [PubMed] [Google Scholar]
- 17.Zhou X, Ouyang S, Li J, Huang X, Ai X, Zeng Y, Lv Y, Cai M. The novel non-immunological role and underlying mechanisms of B7–H3 in tumorigenesis. J Cell Physiol. 2019;234(12):21785–21795. doi: 10.1002/jcp.28936. [DOI] [PubMed] [Google Scholar]
- 18.Bardia A, Hurvitz SA, Tolaney SM, Loirat D, Punie K, Oliveira M, Brufsky A, Sardesai SD, Kalinsky K, Zelnak AB, Weaver R, Traina T, Dalenc F, Aftimos P, Lynce F, Diab S, Cortés J, O'Shaughnessy J, Diéras V, Ferrario C, Schmid P, Carey LA, Gianni L, Piccart MJ, Loibl S, Goldenberg DM, Hong Q, Olivo MS, Itri LM, Rugo HS. Sacituzumab govitecan in metastatic triple-negative breast cancer. N Engl J Med. 2021;384(16):1529–1541. doi: 10.1056/NEJMoa2028485. [DOI] [PubMed] [Google Scholar]
- 19.Powles T, Rosenberg JE, Sonpavde GP, Loriot Y, Durán I, Lee JL, Matsubara N, Vulsteke C, Castellano D, Wu C, Campbell M, Matsangou M, Petrylak DP. Enfortumab vedotin in previously treated advanced urothelial carcinoma. N Engl J Med. 2021;384(12):1125–1135. doi: 10.1056/NEJMoa2035807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shitara K, Bang YJ, Iwasa S, Sugimoto N, Ryu MH, Sakai D, Chung HC, Kawakami H, Yabusaki H, Lee J, Saito K, Kawaguchi Y, Kamio T, Kojima A, Sugihara M, Yamaguchi K. Trastuzumab deruxtecan in previously treated HER2-positive gastric cancer. N Engl J Med. 2020;382(25):2419–2430. doi: 10.1056/NEJMoa2004413. [DOI] [PubMed] [Google Scholar]
- 21.Birrer MJ, Moore KN, Betella I, Bates RC. Antibody-drug conjugate-based therapeutics: state of the science. J Natl Cancer Inst. 2019;111(6):538–549. doi: 10.1093/jnci/djz035. [DOI] [PubMed] [Google Scholar]
- 22.Tercel M, McManaway SP, Leung E, Liyanage HD, Lu GL, Pruijn FB. The cytotoxicity of duocarmycin analogues is mediated through alkylation of DNA, not aldehyde dehydrogenase 1: a comment. Angew Chem Int Ed Engl. 2013;52(21):5442–5446. doi: 10.1002/anie.201208373. [DOI] [PubMed] [Google Scholar]
- 23.Yao HP, Zhao H, Hudson R, Tong XM, Wang MH. Duocarmycin-based antibody-drug conjugates as an emerging biotherapeutic entity for targeted cancer therapy: pharmaceutical strategy and clinical progress. Drug Discov Today. 2021 doi: 10.1016/j.drudis.2021.06.012. [DOI] [PubMed] [Google Scholar]
- 24.Scribner JA, Brown JG, Son T, Chiechi M, Li P, Sharma S, Li H, De Costa A, Li Y, Chen Y, Easton A, Yee-Toy NC, Chen FZ, Gorlatov S, Barat B, Huang L, Wolff CR, Hooley J, Hotaling TE, Gaynutdinov T, Ciccarone V, Tamura J, Koenig S, Moore PA, Bonvini E, Loo D. Preclinical development of MGC018, a duocarmycin-based antibody-drug conjugate targeting B7–H3 for solid cancer. Mol Cancer Ther. 2020 doi: 10.1158/1535-7163.mct-20-0116. [DOI] [PubMed] [Google Scholar]
- 25.Bendell JC, Doi T, Patel MR, Piha-Paul SA, Sen S, Shimizu T, Cheng B, Mekan SF, Myobatake Y, Okuda Y, Serbest G, Johnson ML (2020) A phase I/II, two-part, multicenter, first-in-human study of DS-7300a in patients with advanced solid malignant tumors. J Clin Oncol 38(15_suppl):TPS3646-TPS3646. 10.1200/JCO.2020.38.15_suppl.TPS3646
- 26.Seaman S, Stevens J, Yang MY, Logsdon D, Graff-Cherry C, St Croix B. Genes that distinguish physiological and pathological angiogenesis. Cancer Cell. 2007;11(6):539–554. doi: 10.1016/j.ccr.2007.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seaman S, Zhu Z, Saha S, Zhang XM, Yang MY, Hilton MB, Morris K, Szot C, Morris H, Swing DA, Tessarollo L, Smith SW, Degrado S, Borkin D, Jain N, Scheiermann J, Feng Y, Wang Y, Li J, Welsch D, DeCrescenzo G, Chaudhary A, Zudaire E, Klarmann KD, Keller JR, Dimitrov DS, St Croix B. Eradication of tumors through simultaneous ablation of CD276/B7-H3-positive tumor cells and tumor vasculature. Cancer Cell. 2017;31(4):501–515.e508. doi: 10.1016/j.ccell.2017.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carolina BioOncology H, NC; Inova Schar Cancer Institute Fairfax V, MacroGenics I, Rockville, MD; Virginia Cancer Specialists F, VA; START Midwest GR, MI (2020) Preliminary dose escalation results from a phase 1/2, first-in-human study of MGC018 (Anti-B7-H3 antibody-drug conjugate) in patients with advanced solid tumors. ASCO20 Virtual Scientific Program
- 29.Yamato M, Hasegawa J, Hattori C, Maejima T, Shibutani T, Deguchi T, Izumi N, Watanabe A, Nishiya Y, Nakada T, Abe Y, Agatsuma T. 28 Poster discussion-DS-7300a, a novel B7–H3-targeting antibody-drug conjugate with a novel DNA topoisomerase I inhibitor DXd, exhibits potent anti-tumor effects in nonclinical models. Eur J Cancer. 2020;138:S14–S15. doi: 10.1016/S0959-8049(20)31102-3. [DOI] [Google Scholar]
- 30.Banerji U, van Herpen CML, Saura C, Thistlethwaite F, Lord S, Moreno V, Macpherson IR, Boni V, Rolfo C, de Vries EGE, Rottey S, Geenen J, Eskens F, Gil-Martin M, Mommers EC, Koper NP, Aftimos P. Trastuzumab duocarmazine in locally advanced and metastatic solid tumours and HER2-expressing breast cancer: a phase 1 dose-escalation and dose-expansion study. Lancet Oncol. 2019;20(8):1124–1135. doi: 10.1016/s1470-2045(19)30328-6. [DOI] [PubMed] [Google Scholar]
- 31.Joubert N, Beck A, Dumontet C, Denevault-Sabourin C. Antibody-drug conjugates: the last decade. Pharmaceuticals (Basel) 2020 doi: 10.3390/ph13090245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.García-Alonso S, Ocaña A, Pandiella A. Resistance to antibody-drug conjugates. Cancer Res. 2018;78(9):2159–2165. doi: 10.1158/0008-5472.can-17-3671. [DOI] [PubMed] [Google Scholar]
- 33.Kollmannsberger C, Choueiri TK, Heng DYC, George S, Jie F, Croitoru R, Poondru S, Thompson JA. A randomized phase II study of AGS-16C3F versus axitinib in previously treated patients with metastatic renal cell carcinoma. Oncologist. 2021;26(3):182–e361. doi: 10.1002/onco.13628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Loganzo F, Tan X, Sung M, Jin G, Myers JS, Melamud E, Wang F, Diesl V, Follettie MT, Musto S, Lam MH, Hu W, Charati MB, Khandke K, Kim KS, Cinque M, Lucas J, Graziani E, Maderna A, O'Donnell CJ, Arndt KT, Gerber HP. Tumor cells chronically treated with a trastuzumab-maytansinoid antibody-drug conjugate develop varied resistance mechanisms but respond to alternate treatments. Mol Cancer Ther. 2015;14(4):952–963. doi: 10.1158/1535-7163.mct-14-0862. [DOI] [PubMed] [Google Scholar]
- 35.Zhang Z, Jiang C, Liu Z, Yang M, Tang X, Wang Y, Zheng M, Huang J, Zhong K, Zhao S, Tang M, Zhou T, Yang H, Guo G, Zhou L, Xu J, Tong A. B7–H3-targeted CAR-T cells exhibit potent antitumor effects on hematologic and solid tumors. Mol Ther Oncolytics. 2020;17:180–189. doi: 10.1016/j.omto.2020.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yim J, Koh J, Kim S, Song SG, Ahn HK, Kim YA, Jeon YK, Chung DH. Effects of B7–H3 expression on tumour-infiltrating immune cells and clinicopathological characteristics in non-small-cell lung cancer. Eur J Cancer. 2020;133:74–85. doi: 10.1016/j.ejca.2020.03.033. [DOI] [PubMed] [Google Scholar]
- 37.Wang L, Kang FB, Zhang GC, Wang J, Xie MF, Zhang YZ. Clinical significance of serum soluble B7–H3 in patients with osteosarcoma. Cancer Cell Int. 2018;18:115. doi: 10.1186/s12935-018-0614-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen W, Liu P, Wang Y, Nie W, Li Z, Xu W, Li F, Zhou Z, Zhao M, Liu H. Characterization of a soluble B7–H3 (sB7-H3) spliced from the intron and analysis of sB7-H3 in the sera of patients with hepatocellular carcinoma. PLoS ONE. 2013;8(10):e76965. doi: 10.1371/journal.pone.0076965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim Y, Lavoie RR, Dong H, Park S, Lucien-Matteoni F. Abstract 675: radiotherapy inhibits the antitumor immune response through release of immunosuppressive tumor-derived extracellular vesicles in prostate cancer. Can Res. 2021;81(13 Supplement):675–675. doi: 10.1158/1538-7445.am2021-675. [DOI] [Google Scholar]
- 40.Barok M, Puhka M, Yazdi N, Joensuu H. Extracellular vesicles as modifiers of antibody-drug conjugate efficacy. J Extracell Vesicles. 2021;10(4):e12070. doi: 10.1002/jev2.12070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Samineni D, Girish S, Li C. Impact of shed/soluble targets on the PK/PD of approved therapeutic monoclonal antibodies. Expert Rev Clin Pharmacol. 2016;9(12):1557–1569. doi: 10.1080/17512433.2016.1243055. [DOI] [PubMed] [Google Scholar]
- 42.Wang W, Erbe AK, Hank JA, Morris ZS, Sondel PM. NK Cell-mediated antibody-dependent cellular cytotoxicity in cancer immunotherapy. Front Immunol. 2015;6:368. doi: 10.3389/fimmu.2015.00368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Clynes RA, Towers TL, Presta LG, Ravetch JV. Inhibitory Fc receptors modulate in vivo cytotoxicity against tumor targets. Nat Med. 2000;6(4):443–446. doi: 10.1038/74704. [DOI] [PubMed] [Google Scholar]
- 44.Hubert P, Heitzmann A, Viel S, Nicolas A, Sastre-Garau X, Oppezzo P, Pritsch O, Osinaga E, Amigorena S. Antibody-dependent cell cytotoxicity synapses form in mice during tumor-specific antibody immunotherapy. Cancer Res. 2011;71(15):5134–5143. doi: 10.1158/0008-5472.CAN-10-4222. [DOI] [PubMed] [Google Scholar]
- 45.Zahavi D, AlDeghaither D, O'Connell A, Weiner LM. Enhancing antibody-dependent cell-mediated cytotoxicity: a strategy for improving antibody-based immunotherapy. Antib Ther. 2018;1(1):7–12. doi: 10.1093/abt/tby002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Loo D, Alderson RF, Chen FZ, Huang L, Zhang W, Gorlatov S, Burke S, Ciccarone V, Li H, Yang Y, Son T, Chen Y, Easton AN, Li JC, Rillema JR, Licea M, Fieger C, Liang TW, Mather JP, Koenig S, Stewart SJ, Johnson S, Bonvini E, Moore PA. Development of an Fc-enhanced anti-B7-H3 monoclonal antibody with potent antitumor activity. Clin Cancer Res. 2012;18(14):3834–3845. doi: 10.1158/1078-0432.CCR-12-0715. [DOI] [PubMed] [Google Scholar]
- 47.Powderly J, Cote G, Flaherty K, Szmulewitz RZ, Ribas A, Weber J, Loo D, Baughman J, Chen F, Moore P, Bonvini E, Vasselli J, Wigginton J, Cohen R, Burris H, Chmielowski B. Interim results of an ongoing Phase I, dose escalation study of MGA271 (Fc-optimized humanized anti-B7-H3 monoclonal antibody) in patients with refractory B7–H3-expressing neoplasms or neoplasms whose vasculature expresses B7–H3. J Immunother Cancer. 2015 doi: 10.1186/2051-1426-3-s2-o8. [DOI] [Google Scholar]
- 48.Shenderov E, Demarzo A, Boudadi K, Allaf M, Wang H, Chapman C, Pavlovich C, Bivalacqua T, O'Neal TS, Harb R. Phase II neoadjuvant and immunologic study of B7–H3 targeting with enoblituzumab in localized intermediate-and high-risk prostate cancer. J Clin Oncol. 2018;36:TPS5099. doi: 10.1200/JCO.2018.36.15_suppl.TPS5099. [DOI] [Google Scholar]
- 49.Villarroel-Espindola F, Sanmamed M, Patsenker J, Lin YW, Henick B, Yu J. Spatially resolved and multiplexed immunoprofiling of NSCLC using imaging mass cytometry reveals distinct functional profile of CD4 and CD8 TILs associated with response to immune checkpoint blockers. J Immunother Cancer. 2018;6(Suppl 1):114. doi: 10.1186/s40425-018-0422-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nagase-Zembutsu A, Hirotani K, Yamato M, Yamaguchi J, Takata T, Yoshida M, Fukuchi K, Yazawa M, Takahashi S, Agatsuma T. Development of DS-5573a: a novel afucosylated mAb directed at B7–H3 with potent antitumor activity. Cancer Sci. 2016;107(5):674–681. doi: 10.1111/cas.12915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Burvenich IJG, Parakh S, Lee F-T, Guo N, Liu Z, Gan HK, Rigopoulos A, O'Keefe GJ, Gong SJ, Goh YW. Molecular imaging of T cell co-regulator factor B7–H3 with 89Zr-DS-5573a. Theranostics. 2018;8(15):4199. doi: 10.7150/thno.25575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Powles T, Park SH, Voog E, Caserta C, Valderrama BP, Gurney H, Kalofonos H, Radulovic S, Demey W, Ullen A, Loriot Y, Sridhar SS, Tsuchiya N, Kopyltsov E, Sternberg CN, Bellmunt J, Aragon-Ching JB, Petrylak DP, Laliberte R, Wang J, Huang B, Davis C, Fowst C, Costa N, Blake-Haskins JA, di Pietro A, Grivas P. Avelumab maintenance therapy for advanced or metastatic urothelial carcinoma. N Engl J Med. 2020;383(13):1218–1230. doi: 10.1056/NEJMoa2002788. [DOI] [PubMed] [Google Scholar]
- 53.Musolino A, Naldi N, Bortesi B, Pezzuolo D, Capelletti M, Missale G, Laccabue D, Zerbini A, Camisa R, Bisagni G, Neri TM, Ardizzoni A. Immunoglobulin G fragment C receptor polymorphisms and clinical efficacy of trastuzumab-based therapy in patients with HER-2/neu-positive metastatic breast cancer. J Clin Oncol. 2008;26(11):1789–1796. doi: 10.1200/jco.2007.14.8957. [DOI] [PubMed] [Google Scholar]
- 54.Bibeau F, Lopez-Crapez E, Di Fiore F, Thezenas S, Ychou M, Blanchard F, Lamy A, Penault-Llorca F, Frébourg T, Michel P, Sabourin JC, Boissière-Michot F. Impact of Fc{gamma}RIIa-Fc{gamma}RIIIa polymorphisms and KRAS mutations on the clinical outcome of patients with metastatic colorectal cancer treated with cetuximab plus irinotecan. J Clin Oncol. 2009;27(7):1122–1129. doi: 10.1200/jco.2008.18.0463. [DOI] [PubMed] [Google Scholar]
- 55.Ni L, Dong C. New B7 family checkpoints in human cancers. Mol Cancer Ther. 2017;16(7):1203–1211. doi: 10.1158/1535-7163.mct-16-0761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Srivastava RM, Lee SC, Andrade Filho PA, Lord CA, Jie HB, Davidson HC, López-Albaitero A, Gibson SP, Gooding WE, Ferrone S, Ferris RL. Cetuximab-activated natural killer and dendritic cells collaborate to trigger tumor antigen-specific T-cell immunity in head and neck cancer patients. Clin Cancer Res. 2013;19(7):1858–1872. doi: 10.1158/1078-0432.ccr-12-2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chaganty BK, Lu Y, Qiu S, Somanchi SS, Lee DA, Fan Z. Trastuzumab upregulates expression of HLA-ABC and T cell costimulatory molecules through engagement of natural killer cells and stimulation of IFNγ secretion. Oncoimmunology. 2016;5(4):e1100790. doi: 10.1080/2162402x.2015.1100790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kantarjian H, Stein A, Gokbuget N, Fielding AK, Schuh AC, Ribera JM, Wei A, Dombret H, Foa R, Bassan R, Arslan O, Sanz MA, Bergeron J, Demirkan F, Lech-Maranda E, Rambaldi A, Thomas X, Horst HA, Bruggemann M, Klapper W, Wood BL, Fleishman A, Nagorsen D, Holland C, Zimmerman Z, Topp MS. Blinatumomab versus chemotherapy for advanced acute lymphoblastic leukemia. N Engl J Med. 2017;376(9):836–847. doi: 10.1056/NEJMoa1609783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tian Z, Liu M, Zhang Y, Wang X. Bispecific T cell engagers: an emerging therapy for management of hematologic malignancies. J Hematol Oncol. 2021;14(1):75. doi: 10.1186/s13045-021-01084-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lim SM, Pyo KH, Soo RA, Cho BC. The promise of bispecific antibodies: clinical applications and challenges. Cancer Treat Rev. 2021;99:102240. doi: 10.1016/j.ctrv.2021.102240. [DOI] [PubMed] [Google Scholar]
- 61.Tolcher AW, Alley EW, Chichili G, Baughman JE, Moore PA, Bonvini E, Vasselli JR, Wigginton JM, Powderly JD. Phase 1, first-in-human, open label, dose escalation ctudy of MGD009, a humanized B7–H3 x CD3 dual-affinity re-targeting (DART) protein in patients with B7-H3-expressing neoplasms or B7–H3 expressing tumor vasculature. J Clin Oncol. 2016;34(15):3105–3105. doi: 10.1200/JCO.2016.34.15_suppl.TPS3105. [DOI] [Google Scholar]
- 62.Sun X, Yu Y, Ma L, Xue X, Gao Z, Ma J, Zhang M. T cell cytotoxicity toward hematologic malignancy via B7–H3 targeting. Invest New Drugs. 2019 doi: 10.1007/s10637-019-00819-y. [DOI] [PubMed] [Google Scholar]
- 63.Ma W, Ma J, Ma P, Lei T, Zhao M, Zhang M. Targeting immunotherapy for bladder cancer using anti-CD3× B7–H3 bispecific antibody. Cancer Med. 2018;7(10):5167–5177. doi: 10.1002/cam4.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ma J, Shang T, Ma P, Sun X, Zhao J, Sun X, Zhang M. Bispecific anti-CD3 x anti-B7-H3 antibody mediates T cell cytotoxic ability to human melanoma in vitro and in vivo. Invest New Drugs. 2019;37(5):1036–1043. doi: 10.1007/s10637-018-00719-7. [DOI] [PubMed] [Google Scholar]
- 65.Hu J, Jiang C, Zheng M, Guo Y, Tang X, Ren J, Lu D, Yu L, Gan W, Liu S, Tong A, Yang H. Overexpression of B7–H3 as an opportunity for targeted therapy in head and neck cancers. Am J Transl Res. 2019;11(8):5183–5196. [PMC free article] [PubMed] [Google Scholar]
- 66.You G, Lee Y, Kang YW, Park HW, Park K, Kim H, Kim YM, Kim S, Kim JH, Moon D, Chung H, Son W, Jung UJ, Park E, Lee S, Son YG, Eom J, Won J, Park Y, Jung J, Lee SW. B7–H3×4–1BB bispecific antibody augments antitumor immunity by enhancing terminally differentiated CD8(+) tumor-infiltrating lymphocytes. Sci Adv. 2021 doi: 10.1126/sciadv.aax3160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Singh AK, McGuirk JP. CAR T cells: continuation in a revolution of immunotherapy. Lancet Oncol. 2020;21(3):e168–e178. doi: 10.1016/S1470-2045(19)30823-X. [DOI] [PubMed] [Google Scholar]
- 68.Wagner J, Wickman E, De Renzo C, Gottschalk S (2020) CAR T-cell therapy for solid tumors: bright future or dark reality? Mol Ther [DOI] [PMC free article] [PubMed]
- 69.Majzner RG, Theruvath JL, Nellan A, Heitzeneder S, Cui Y, Mount CW, Rietberg SP, Linde MH, Xu P, Rota C, Sotillo E, Labanieh L, Lee DW, Orentas RJ, Dimitrov DS, Zhu Z, Croix BS, Delaidelli A, Sekunova A, Bonvini E, Mitra SS, Quezado MM, Majeti R, Monje M, Sorensen PHB, Maris JM, Mackall CL. CAR T cells targeting B7–H3, a pan-cancer antigen, demonstrate potent preclinical activity against pediatric solid tumors and brain tumors. Clin Cancer Res. 2019;25(8):2560–2574. doi: 10.1158/1078-0432.CCR-18-0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Du H, Hirabayashi K, Ahn S, Kren NP, Montgomery SA, Wang X, Tiruthani K, Mirlekar B, Michaud D, Greene K, Herrera SG, Xu Y, Sun C, Chen Y, Ma X, Ferrone CR, Pylayeva-Gupta Y, Yeh JJ, Liu R, Savoldo B, Ferrone S, Dotti G. Antitumor responses in the absence of toxicity in solid tumors by targeting B7–H3 via chimeric antigen receptor T cells. Cancer Cell. 2019;35(2):221–237.e228. doi: 10.1016/j.ccell.2019.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lichtman EI, Du H, Shou P, Song F, Suzuki K, Ahn S, Li G, Ferrone S, Su L, Savoldo B, Dotti G. Preclinical evaluation of B7–H3-specific chimeric antigen receptor T cells for the treatment of acute myeloid leukemia. Clin Cancer Res. 2021;27(11):3141–3153. doi: 10.1158/1078-0432.ccr-20-2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Haydar D, Houke H, Chiang J, Yi Z, Odé Z, Caldwell K, Zhu X, Mercer KS, Stripay JL, Shaw TI, Vogel P, DeRenzo C, Baker SJ, Roussel MF, Gottschalk S, Krenciute G. Cell-surface antigen profiling of pediatric brain tumors: B7–H3 is consistently expressed and can be targeted via local or systemic CAR T-cell delivery. Neuro Oncol. 2021;23(6):999–1011. doi: 10.1093/neuonc/noaa278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tang X, Liu F, Liu Z, Cao Y, Zhang Z, Wang Y, Huang J, Fan S, Zhao S, Chen Y, Li G, Wang S, Zheng M, Hu Y, Li H, Jiang C, Yang M, Yang H, Xu J, Guo G, Tong A, Zhou L. Bioactivity and safety of B7–H3-targeted chimeric antigen receptor T cells against anaplastic meningioma. Clin Transl Immunol. 2020;9(6):e1137. doi: 10.1002/cti2.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lei X, Ou Z, Yang Z, Zhong J, Zhu Y, Tian J, Wu J, Deng H, Lin X, Peng Y, Li B, He L, Tu Z, Chen W, Li Q, Liu N, Zhang H, Wang Z, Fang Z, Yamada T, Lv X, Tian T, Pan G, Wu F, Xiao L, Zhang L, Cai T, Wang X, Tannous BA, Li J, Kontos F, Ferrone S, Fan S. A pan-histone deacetylase inhibitor enhances the antitumor activity of B7–H3-specific CAR T cells in solid tumors. Clin Cancer Res. 2021 doi: 10.1158/1078-0432.ccr-20-2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Modak S, Kramer K, Gultekin SH, Guo HF, Cheung NK. Monoclonal antibody 8H9 targets a novel cell surface antigen expressed by a wide spectrum of human solid tumors. Cancer Res. 2001;61(10):4048–4054. [PubMed] [Google Scholar]
- 76.Xu H, Cheung IY, Guo HF, Cheung NK. MicroRNA miR-29 modulates expression of immunoinhibitory molecule B7–H3: potential implications for immune based therapy of human solid tumors. Cancer Res. 2009;69(15):6275–6281. doi: 10.1158/0008-5472.CAN-08-4517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ahmed M, Cheng M, Zhao Q, Goldgur Y, Cheal SM, Guo HF, Larson SM, Cheung NK. Humanized affinity-matured monoclonal antibody 8H9 has potent antitumor activity and binds to FG loop of tumor antigen B7–H3. J Biol Chem. 2015;290(50):30018–30029. doi: 10.1074/jbc.M115.679852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kramer K, Kushner BH, Modak S, Pandit-Taskar N, Smith-Jones P, Zanzonico P, Humm JL, Xu H, Wolden SL, Souweidane MM, Larson SM, Cheung NK. Compartmental intrathecal radioimmunotherapy: results for treatment for metastatic CNS neuroblastoma. J Neurooncol. 2010;97(3):409–418. doi: 10.1007/s11060-009-0038-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Souweidane MM, Kramer K, Pandit-Taskar N, Zanzonico P, Dunkel IJ. A phase I study of convection enhanced delivery (CED) of 124I–8H9 radio-labeled monoclonal antibody in children with diffuse intrinsic pontine glioma (DIPG) J Clin Oncol. 2017;35(15):2010–2010. doi: 10.1200/JCO.2017.35.15_suppl.2010. [DOI] [Google Scholar]
- 80.Souweidane MM, Kramer K, Pandit-Taskar N, Zhou Z, Haque S, Zanzonico P, Carrasquillo JA, Lyashchenko SK, Thakur SB, Donzelli M, Turner RS, Lewis JS, Cheung NV, Larson SM, Dunkel IJ. Convection-enhanced delivery for diffuse intrinsic pontine glioma: a single-centre, dose-escalation, phase 1 trial. Lancet Oncol. 2018;19(8):1040–1050. doi: 10.1016/s1470-2045(18)30322-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kramer K, Kushner BH, Modak S, Pandit-Taskar N, Cheung NKV. A curative approach to central nervous system metastases of neuroblastoma. J Clin Oncol. 2017;35(15):10545–10545. doi: 10.1200/JCO.2017.35.15_suppl.10545. [DOI] [Google Scholar]
- 82.Modak S, Quaglia MPL, Carrasquillo JA, Zanzonico P, Cheung NKV. Intraperitoneal radioimmunotherapy (RIT) for desmoplastic small round cell tumor (DSRCT): initial results from a phase I trial. J Clin Oncol. 2013;31(15):3033–3033. doi: 10.1200/jco.2013.31.15_suppl.3033. [DOI] [Google Scholar]
- 83.Vogel WV, van der Marck SC, Versleijen MWJ. Challenges and future options for the production of lutetium-177. Eur J Nucl Med Mol Imaging. 2021;48(8):2329–2335. doi: 10.1007/s00259-021-05392-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Securities and Exchange Commission. Y-mAbs Therapeutics, Inc. Available from: https://www.sec.gov/Archives/edgar/data/1722964/000104746918005786/a2236551zs-1.htm. 2021.
- 85.Modak S, Guo HF, Humm JL, Smith-Jones PM, Larson SM, Cheung NK. Radioimmunotargeting of human rhabdomyosarcoma using monoclonal antibody 8H9. Cancer Biother Radiopharm. 2005;20(5):534–546. doi: 10.1089/cbr.2005.20.534. [DOI] [PubMed] [Google Scholar]
- 86.Ling V, Wu PW, Spaulding V, Kieleczawa J, Luxenberg D, Carreno BM, Collins M. Duplication of primate and rodent B7–H3 immunoglobulin V- and C-like domains: divergent history of functional redundancy and exon loss. Genomics. 2003;82(3):365–377. doi: 10.1016/s0888-7543(03)00126-5. [DOI] [PubMed] [Google Scholar]
- 87.Vigdorovich V, Ramagopal UA, Lazar-Molnar E, Sylvestre E, Lee JS, Hofmeyer KA, Zang X, Nathenson SG, Almo SC. Structure and T cell inhibition properties of B7 family member, B7–H3. Structure. 2013;21(5):707–717. doi: 10.1016/j.str.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gao W, Wen H, Liang L, Dong X, Du R, Zhou W, Zhang X, Zhang C, Xiang R, Li N. IL20RA signaling enhances stemness and promotes the formation of an immunosuppressive microenvironment in breast cancer. Theranostics. 2021;11(6):2564–2580. doi: 10.7150/thno.45280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yu D, Yang X, Lin J, Cao Z, Lu C, Yang Z, Zheng M, Pan R, Cai W. Super-enhancer induced IL-20RA promotes proliferation/metastasis and immune evasion in colorectal cancer. Front Oncol. 2021;11:724655. doi: 10.3389/fonc.2021.724655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zak KM, Kitel R, Przetocka S, Golik P, Guzik K, Musielak B, Domling A, Dubin G, Holak TA. Structure of the complex of human programmed death 1, PD-1, and its ligand PD-L1. Structure. 2015;23(12):2341–2348. doi: 10.1016/j.str.2015.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhao Y, Harrison DL, Song Y, Ji J, Huang J, Hui E. Antigen-presenting cell-intrinsic PD-1 neutralizes PD-L1 in cis to attenuate PD-1 signaling in T cells. Cell Rep. 2018;24(2):379–390. doi: 10.1016/j.celrep.2018.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chaudhri A, Xiao Y, Klee AN, Wang X, Zhu B, Freeman GJ. PD-L1 binds to B7–1 only in cis on the same cell surface. Cancer Immunol Res. 2018;6(8):921–929. doi: 10.1158/2326-6066.CIR-17-0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sugiura D, Maruhashi T, Okazaki IM, Shimizu K, Maeda TK, Takemoto T, Okazaki T. Restriction of PD-1 function by cis-PD-L1/CD80 interactions is required for optimal T cell responses. Science. 2019;364(6440):558–566. doi: 10.1126/science.aav7062. [DOI] [PubMed] [Google Scholar]
- 94.Zhao Y, Lee CK, Lin CH, Gassen RB, Xu X, Huang Z, Xiao C, Bonorino C, Lu LF, Bui JD, Hui E. PD-L1:CD80 cis-heterodimer triggers the co-stimulatory receptor CD28 while repressing the inhibitory PD-1 and CTLA-4 pathways. Immunity. 2019;51(6):1059–1073. doi: 10.1016/j.immuni.2019.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Xu J, Brosseau JP, Shi H. Targeted degradation of immune checkpoint proteins: emerging strategies for cancer immunotherapy. Oncogene. 2020;39(48):7106–7113. doi: 10.1038/s41388-020-01491-w. [DOI] [PubMed] [Google Scholar]
- 96.Tu X, Qin B, Zhang Y, Zhang C, Kahila M, Nowsheen S, Yin P, Yuan J, Pei H, Li H, Yu J, Song Z, Zhou Q, Zhao F, Liu J, Zhang C, Dong H, Mutter RW, Lou Z. PD-L1 (B7–H1) competes with the RNA exosome to regulate the DNA damage response and can be targeted to sensitize to radiation or chemotherapy. Mol Cell. 2019;74(6):1215–1226. doi: 10.1016/j.molcel.2019.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhang Y, Du X, Liu M, Tang F, Zhang P, Ai C, Fields JK, Sundberg EJ, Latinovic OS, Devenport M, Zheng P, Liu Y. Hijacking antibody-induced CTLA-4 lysosomal degradation for safer and more effective cancer immunotherapy. Cell Res. 2019;29(8):609–627. doi: 10.1038/s41422-019-0184-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Burr ML, Sparbier CE, Chan YC, Williamson JC, Woods K, Beavis PA, Lam EYN, Henderson MA, Bell CC, Stolzenburg S, Gilan O, Bloor S, Noori T, Morgens DW, Bassik MC, Neeson PJ, Behren A, Darcy PK, Dawson SJ, Voskoboinik I, Trapani JA, Cebon J, Lehner PJ, Dawson MA. CMTM6 maintains the expression of PD-L1 and regulates anti-tumour immunity. Nature. 2017;549(7670):101–105. doi: 10.1038/nature23643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mezzadra R, Sun C, Jae LT, Gomez-Eerland R, de Vries E, Wu W, Logtenberg MEW, Slagter M, Rozeman EA, Hofland I, Broeks A, Horlings HM, Wessels LFA, Blank CU, Xiao Y, Heck AJR, Borst J, Brummelkamp TR, Schumacher TNM. Identification of CMTM6 and CMTM4 as PD-L1 protein regulators. Nature. 2017;549(7670):106–110. doi: 10.1038/nature23669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sun L, Zhang L, Yu J, Zhang Y, Pang X, Ma C, Shen M, Ruan S, Wasan HS, Qiu S. Clinical efficacy and safety of anti-PD-1/PD-L1 inhibitors for the treatment of advanced or metastatic cancer: a systematic review and meta-analysis. Sci Rep. 2020;10(1):2083. doi: 10.1038/s41598-020-58674-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Davis AA, Patel VG. The role of PD-L1 expression as a predictive biomarker: an analysis of all US Food and Drug Administration (FDA) approvals of immune checkpoint inhibitors. J Immunother Cancer. 2019;7(1):278. doi: 10.1186/s40425-019-0768-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lee HH, Wang YN, Xia W, Chen CH, Rau KM, Ye L, Wei Y, Chou CK, Wang SC, Yan M, Tu CY, Hsia TC, Chiang SF, Chao KSC, Wistuba II, Hsu JL, Hortobagyi GN, Hung MC. Removal of N-linked glycosylation enhances PD-L1 detection and predicts anti-PD-1/PD-L1 therapeutic efficacy. Cancer Cell. 2019;36(2):168–178. doi: 10.1016/j.ccell.2019.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Li CW, Lim SO, Chung EM, Kim YS, Park AH, Yao J, Cha JH, Xia W, Chan LC, Kim T, Chang SS, Lee HH, Chou CK, Liu YL, Yeh HC, Perillo EP, Dunn AK, Kuo CW, Khoo KH, Hsu JL, Wu Y, Hsu JM, Yamaguchi H, Huang TH, Sahin AA, Hortobagyi GN, Yoo SS, Hung MC. Eradication of triple-negative breast cancer cells by targeting glycosylated PD-L1. Cancer Cell. 2018;33(2):187–201. doi: 10.1016/j.ccell.2018.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Li CW, Lim SO, Xia W, Lee HH, Chan LC, Kuo CW, Khoo KH, Chang SS, Cha JH, Kim T, Hsu JL, Wu Y, Hsu JM, Yamaguchi H, Ding Q, Wang Y, Yao J, Lee CC, Wu HJ, Sahin AA, Allison JP, Yu D, Hortobagyi GN, Hung MC. Glycosylation and stabilization of programmed death ligand-1 suppresses T-cell activity. Nat Commun. 2016;7:12632. doi: 10.1038/ncomms12632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chen JT, Chen CH, Ku KL, Hsiao M, Chiang CP, Hsu TL, Chen MH, Wong CH. Glycoprotein B7–H3 overexpression and aberrant glycosylation in oral cancer and immune response. Proc Natl Acad Sci U S A. 2015;112(42):13057–13062. doi: 10.1073/pnas.1516991112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Huang Y, Zhang HL, Li ZL, Du T, Chen YH, Wang Y, Ni HH, Zhang KM, Mai J, Hu BX, Huang JH, Zhou LH, Yang D, Peng XD, Feng GK, Tang J, Zhu XF, Deng R. FUT8-mediated aberrant N-glycosylation of B7H3 suppresses the immune response in triple-negative breast cancer. Nat Commun. 2021;12(1):2672. doi: 10.1038/s41467-021-22618-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wang Z, Yang J, Zhu Y, Zhu Y, Zhang B, Zhou Y. Differential expression of 2IgB7-H3 and 4IgB7-H3 in cancer cell lines and glioma tissues. Oncol Lett. 2015;10(4):2204–2208. doi: 10.3892/ol.2015.3611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yoon BR, Chung YH, Yoo SJ, Kawara K, Kim J, Yoo IS, Park CG, Kang SW, Lee WW. Preferential induction of the T Cell auxiliary signaling molecule B7–H3 on synovial monocytes in rheumatoid arthritis. J Biol Chem. 2016;291(8):4048–4057. doi: 10.1074/jbc.M115.680298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhang C, Chen Y, Li F, Yang M, Meng F, Zhang Y, Chen W, Wang W. B7–H3 is spliced by SRSF3 in colorectal cancer. Cancer Immunol Immunother. 2021;70(2):311–321. doi: 10.1007/s00262-020-02683-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sun J, Fu F, Gu W, Yan R, Zhang G, Shen Z, Zhou Y, Wang H, Shen B, Zhang X. Origination of new immunological functions in the costimulatory molecule B7–H3: the role of exon duplication in evolution of the immune system. PLoS ONE. 2011;6(9):e24751. doi: 10.1371/journal.pone.0024751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhang G, Hou J, Shi J, Yu G, Lu B, Zhang X. Soluble CD276 (B7–H3) is released from monocytes, dendritic cells and activated T cells and is detectable in normal human serum. Immunology. 2008;123(4):538–546. doi: 10.1111/j.1365-2567.2007.02723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Luan Y, Ju J, Luo L, Zhang Z, Wang J, Zhu DM, Cheng L, Zhang SY, Chen L, Wang FS, Wang S. Potential role of soluble B7–H3 in liver immunopathogenesis during chronic HBV infection. J Viral Hepatitis. 2012;19(1):23–31. doi: 10.1111/j.1365-2893.2010.01421.x. [DOI] [PubMed] [Google Scholar]
- 113.Azuma T, Sato Y, Ohno T, Azuma M, Kume H. Serum soluble B7–H3 is a prognostic marker for patients with non-muscle-invasive bladder cancer. PLoS ONE. 2020;15(12):e0243379. doi: 10.1371/journal.pone.0243379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chen L, Zhang G, Sheng S, Zhou Q, Pan Y, Guan S. Upregulation of soluble B7–H3 in NSCLC-derived malignant pleural effusion: a potential diagnostic biomarker correlated with NSCLC staging. Clin Chim Acta. 2016;457:81–85. doi: 10.1016/j.cca.2016.04.009. [DOI] [PubMed] [Google Scholar]
- 115.Sun J, Chen LJ, Zhang GB, Jiang JT, Zhu M, Tan Y, Wang HT, Lu BF, Zhang XG. Clinical significance and regulation of the costimulatory molecule B7–H3 in human colorectal carcinoma. Cancer Immunol Immunother. 2010;59(8):1163–1171. doi: 10.1007/s00262-010-0841-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Xu F, Yi J, Wang F, Wang W, Wang Z, Xue J, Luan X. Involvement of soluble B7–H3 in combination with the serum inflammatory cytokines interleukin-17, -8 and -6 in the diagnosis of hepatocellular carcinoma. Oncol Lett. 2017;14(6):8138–8143. doi: 10.3892/ol.2017.7215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lehmann BD, Paine MS, Brooks AM, McCubrey JA, Renegar RH, Wang R, Terrian DM. Senescence-associated exosome release from human prostate cancer cells. Cancer Res. 2008;68(19):7864–7871. doi: 10.1158/0008-5472.CAN-07-6538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Purvis IJ, Velpula KK, Guda MR, Nguyen D, Tsung AJ, Asuthkar S. B7–H3 in medulloblastoma-derived exosomes: a novel tumorigenic role. Int J Mol Sci. 2020 doi: 10.3390/ijms21197050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Poggio M, Hu T, Pai CC, Chu B, Belair CD, Chang A, Montabana E, Lang UE, Fu Q, Fong L, Blelloch R. Suppression of exosomal PD-L1 induces systemic anti-tumor immunity and memory. Cell. 2019;177(2):414–427.e413. doi: 10.1016/j.cell.2019.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chen G, Huang AC, Zhang W, Zhang G, Wu M, Xu W, Yu Z, Yang J, Wang B, Sun H, Xia H, Man Q, Zhong W, Antelo LF, Wu B, Xiong X, Liu X, Guan L, Li T, Liu S, Yang R, Lu Y, Dong L, McGettigan S, Somasundaram R, Radhakrishnan R, Mills G, Lu Y, Kim J, Chen YH, Dong H, Zhao Y, Karakousis GC, Mitchell TC, Schuchter LM, Herlyn M, Wherry EJ, Xu X, Guo W. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature. 2018;560(7718):382–386. doi: 10.1038/s41586-018-0392-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
Not applicable.