Abstract
Background
The International Society for Human and Animal Mycology (ISHAM) working group proposed recommendations for managing allergic bronchopulmonary aspergillosis (ABPA) a decade ago. There is a need to update these recommendations due to advances in diagnostics and therapeutics.
Methods
An international expert group was convened to develop guidelines for managing ABPA (caused by Aspergillus spp.) and allergic bronchopulmonary mycosis (ABPM; caused by fungi other than Aspergillus spp.) in adults and children using a modified Delphi method (two online rounds and one in-person meeting). We defined consensus as ≥70% agreement or disagreement. The terms “recommend” and “suggest” are used when the consensus was ≥70% and <70%, respectively.
Results
We recommend screening for A. fumigatus sensitisation using fungus-specific IgE in all newly diagnosed asthmatic adults at tertiary care but only difficult-to-treat asthmatic children. We recommend diagnosing ABPA in those with predisposing conditions or compatible clinico-radiological presentation, with a mandatory demonstration of fungal sensitisation and serum total IgE ≥500 IU·mL−1 and two of the following: fungal-specific IgG, peripheral blood eosinophilia or suggestive imaging. ABPM is considered in those with an ABPA-like presentation but normal A. fumigatus-IgE. Additionally, diagnosing ABPM requires repeated growth of the causative fungus from sputum. We do not routinely recommend treating asymptomatic ABPA patients. We recommend oral prednisolone or itraconazole monotherapy for treating acute ABPA (newly diagnosed or exacerbation), with prednisolone and itraconazole combination only for treating recurrent ABPA exacerbations. We have devised an objective multidimensional criterion to assess treatment response.
Conclusion
We have framed consensus guidelines for diagnosing, classifying and treating ABPA/M for patient care and research.
Shareable abstract
The International Society for Human and Animal Mycology working group convened an international expert group that has framed simple guidelines for diagnosing, classifying and treating ABPA/M for patient care and research using a modified Delphi method https://bit.ly/3I2KIGj
Introduction
Allergic bronchopulmonary mycoses are complex pulmonary disorders caused by immune reactions mounted against fungi, most often Aspergillus fumigatus, which colonise the airways of patients with chronic lung disease, most commonly asthma or cystic fibrosis (CF) [1–5]. Allergic bronchopulmonary aspergillosis (ABPA) may occasionally occur in the absence of any predisposing condition and other chronic lung conditions, including bronchiectasis and COPD [6–9]. Conventionally, the term ABPA is used when the causative pathogen is A. fumigatus. In contrast, allergic bronchopulmonary mycosis (ABPM) is an ABPA-like syndrome caused by fungi other than A. fumigatus [10]. Among the allergens involved in asthma, no other allergen generates as much interest as A. fumigatus because the fungus is growing in the airways. Also, ABPA responds exceptionally well to a specific form of therapy. Accordingly, ABPA is considered an asthma endotype [11] and a treatable trait in CF and non-CF bronchiectasis [12]. Early identification and treatment of ABPA is crucial to prevent the progression of bronchiectasis.
The diagnostic criteria proposed by the International Society for Human and Animal Mycology (ISHAM)-ABPA working group (AWG) in 2013 are widely used for diagnosing ABPA [13, 14]. Since the inception of these guidelines a decade ago, newer evidence has emerged concerning diagnostic test performance for ABPA. For instance, the skin test was found inferior to serum A. fumigatus-specific IgE assay [15, 16], serum A. fumigatus-specific IgG detection by enzyme immunoassay proved superior to immunoprecipitation [17, 18], a lateral flow assay is now available for A. fumigatus-IgG [19–21] and the minimal diagnostic level of 500 IU·mL−1 is more sensitive than 1000 IU·mL−1 for serum total IgE [16]. Also, several randomised controlled trials (RCTs) in ABPA therapy have been published in the last decade [22–30]. In light of the above evidence, several international groups have proposed modifications to the ISHAM-AWG criteria [31, 32]. The diagnosis of ABPA in CF and non-CF bronchiectasis is even more challenging as manifestations such as bronchiectasis, Aspergillus bronchitis and mucus plugging are seen in these entities independently of ABPA [33, 34].
Given the emergence of novel yet occasionally conflicting findings and the lack of evidence in certain areas, new guidelines are needed to assist clinicians and researchers in managing ABPA. With this end in view, an expert group was constituted to develop a statement on diagnosing and treating allergic bronchopulmonary mycoses for clinical practice and research in adults and children.
Methods
As a first step, a core committee was formed with five authors of this statement (R. Agarwal, I.S. Sehgal, V. Muthu, D.W. Denning and A. Chakrabarti). Two authors (R. Agarwal and V. Muthu) performed a systematic literature review of the PubMed and Embase databases (to 15 March 2023) to support the guidelines statement and identify the current gaps in managing ABPA. The following search terms were used: “allergic bronchopulmonary aspergillosis” OR “abpa” OR “allergic bronchopulmonary mycosis” OR “fungal sensit*” OR “fungal allerg*” OR “mould allerg*” OR “mold allerg*” OR “mould sensit*” OR “mold sensit*” OR “fungal asthma” OR “aspergillus sensit*” OR “aspergillus hypersensitivity” OR aspergillosis, allergic bronchopulmonary [MeSH]. The core committee then framed the questions for the first round of the Delphi consensus and searched the Scopus researcher discovery database (www.scopus.com/search/form.uri#researcher-discovery) to identify participants for the Delphi expert consensus group (DECG). The DECG included specialists from adult and paediatric pulmonary medicine, infectious diseases, clinical mycology, and radiodiagnosis who were actively involved in the clinical or laboratory aspects of managing ABPA (supplementary table S1).
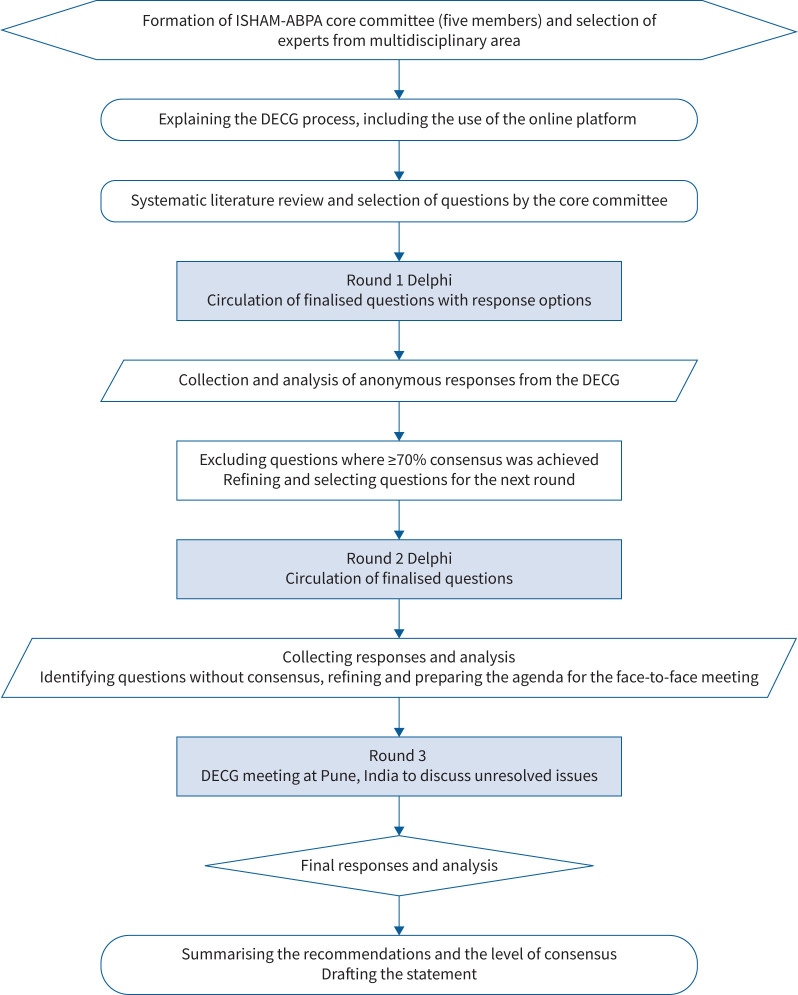
We followed a modified Delphi method (figure 1) [35]. The experts were briefed about the objectives and methodology of the Delphi process. The questions were initially circulated to the experts by e-mail and additional (or modification of) questions were invited. The questions were modified after receiving opinions from the expert group. The first-round questionnaire contained topics spanning various domains (individual diagnostic tests, optimal cut-offs, diagnostic and classification criteria, and treatment options). The questionnaire was circulated online using the commercially available Delphi platform (www.edelphi.org) and anonymous responses were obtained from the participants. We refined and recirculated the questions for the second round. Reminders were sent by e-mail before concluding each Delphi round to ensure participation. We defined consensus as ≥70% of experts agreeing or disagreeing on a statement.
FIGURE 1.
Decision flowchart explaining the various steps of the Delphi methodology used for the revised International Society for Human and Animal Mycology (ISHAM)-allergic bronchopulmonary aspergillosis (ABPA) working group guidelines. DECG: Delphi expert consensus group.
The statements and questions that did not achieve consensus online were discussed in a hybrid meeting of all experts (face-to-face or virtual participation; 7 September 2023, Pune, India). The answers to the entire set of questions were also refined wherever required. The guidelines were formulated by the responses and comments received during the two online rounds and the subsequent in-person discussion. The draft was then circulated among the experts for further comments and suggestions. We used the terms “recommend” and “suggest” where the consensus was ≥70% and <70%, respectively. Finally, we provided the level of consensus (LoC) for important summary statements based on rounds 1 and 2 (supplementary table S2). We used the LoC achieved during the final round for statements not achieving consensus in rounds 1 and 2.
Results
The online surveys were conducted between 15 June and 15 August 2023. We sent invitations to 43 experts, of whom 39 participated. The 39 experts represented 13 countries across six continents (supplementary table S1). Most experts had managed asthma with ABPA for at least 5 years and 51.3% also reported caring for patients with CF-ABPA. Adult (49%) and paediatric (5%) pulmonologists accounted for over half of the experts. The results of the Delphi process are presented in supplementary table S2.
Nomenclature of allergic bronchopulmonary mycoses
We first deliberated on the nomenclature of ABPA and ABPM. The most common form of allergic airway mycoses is ABPA [13], while ABPM is far less common [36]. Given the considerable overlap of the antigen repertoire of the Aspergillus species, the DECG recommended using the term ABPA when allergic mycoses are caused by any Aspergillus spp. (not A. fumigatus only) and ABPM when attributable to fungi other than Aspergillus spp. The most common fungi responsible for ABPM include Bipolaris spp., Schizophyllum commune and Curvularia spp. [10]. Candida albicans has been implicated in several cases of ABPM [36]; however, its pathogenicity remains uncertain.
Diagnosis of fungal sensitisation
As sensitisation represents the first diagnostic step in allergic mycoses [1], we discussed a few questions regarding fungal sensitisation. However, we do not provide detailed guidance on fungal asthma without ABPA, which can be found elsewhere [37–44].
A. fumigatus is the most common fungus associated with allergic sensitisation and ABPA [13]. In a recent meta-analysis, the pooled prevalence of A. fumigatus sensitisation in asthmatic adults was 25% in tertiary care. Of the Aspergillus-sensitised individuals, nearly 37% could develop ABPA [13]. The prevalence of Aspergillus sensitisation was high (16–17%) even in population-based studies [45, 46]. While most patients with ABPA have moderate-to-severe asthma, some have mild asthma and thus screening solely based on symptoms or asthma control may miss several cases [15, 16]. Given the high prevalence of A. fumigatus sensitisation (and ABPA in A. fumigatus sensitisation), all asthmatic adults seeking tertiary care should be evaluated for sensitisation against A. fumigatus. Screening is essential since ABPA can occur even in mild asthmatic subjects and there is a high risk of progression to bronchiectasis if ABPA is undetected. Other fungi (other Aspergillus spp., Candida, Penicillium, Alternaria, Cladosporium and Trichophyton) are also implicated in allergic sensitisation; however, they rarely cause allergic airway mycoses [10, 47–50]. Thus, evaluation for sensitisation to other fungi may be reserved for difficult-to-treat asthma patients who do not have A. fumigatus sensitisation. The literature on fungal sensitisation in children is predominantly for A. fumigatus [51] and data on other fungi are scarce [52]. The experts agreed that among children, only those with difficult-to-treat asthma require screening for A. fumigatus sensitisation rather than all asthmatic children [53].
The IgE immunoassay (cut-off 0.35 kUA·L−1, fluorescent enzyme immunoassay (FEIA)) is the most widely used test to diagnose Aspergillus sensitisation [13]. The DECG accepted A. fumigatus-specific IgE as the preferred screening tool for Aspergillus sensitisation (and ABPA), given its higher sensitivity (99–100%) than the Aspergillus skin test (88–94%) [15, 16]. Also, A. fumigatus-IgE can detect sensitisation against other Aspergillus spp., especially Aspergillus flavus [54]. A skin prick test may be performed additionally or if fungus-specific IgE is unavailable. In asthmatic subjects without known A. fumigatus sensitisation, sensitisation may be re-evaluated if there is unexplained deterioration in asthma control. While a few studies have investigated repeated evaluation for sensitisation [55, 56], more evidence is required on the frequency of periodic evaluation in those with previously negative A. fumigatus-specific IgE and uncontrolled asthma.
Recommendations
We recommend evaluation for A. fumigatus sensitisation (LoC: 94.9%) rather than all fungi. Assessment of sensitisation to other fungi is suggested in difficult-to-treat asthmatic subjects with negative A. fumigatus sensitisation (LoC: 61.5%).
We recommend fungus-specific IgE in preference to a skin prick test for documenting fungal sensitisation in asthmatic subjects (LoC: 76.5%).
We recommend evaluating Aspergillus sensitisation in all newly diagnosed asthmatic adults in tertiary care settings (LoC: 71.4%).
For children, we recommend evaluating Aspergillus sensitisation only in those with difficult-to-treat asthma (LoC: 73.0%).
We are unable to recommend the periodicity of screening for A. fumigatus sensitisation in those with a negative test at the first screening.
Investigations for ABPA/M and the diagnostic cut-offs
Asthmatic subjects with A. fumigatus sensitisation need further evaluation to exclude ABPA [57]. Notably, the methodology of performing the various immunological tests and the different cut-offs are important sources of variation in practice across different centres [58–60], with the cut-off values varying with the method used.
There was consensus for performing the following immunological tests in suspected ABPA: A. fumigatus-specific IgE and IgG, serum total IgE, and peripheral blood eosinophil count. We could not reach a consensus for recommending the Aspergillus skin test and serum precipitins against Aspergillus, partly because access to these different test formats varies widely, and they have varying diagnostic accuracy. However, these tests can be used when automated immunoassays are unavailable. Serum total IgE is a non-specific marker of immunological activity, with a broad differential diagnosis when elevated [44]. However, it reflects disease activity and is an essential monitoring tool in ABPA [61–63]. The serum total IgE values decrease during treatment and the last recorded value during clinical stability is termed the “new baseline” [61]. An increase of ≥50% of this new baseline serum total IgE is used for diagnosing exacerbation. A value ≥500 IU·mL−1 (by enzyme immunoassay) was recommended as the IgE cut-off to diagnose ABPA. This recommendation deviates from the previous ISHAM-AWG guidelines (≥1000 IU·mL−1) [14], as the lower cut-off offers higher sensitivity (98% versus 91%) [16, 31, 32].
Immunoassay and immunoprecipitation (precipitins) are standard methods to detect IgG against A. fumigatus. A recent meta-analysis found the pooled sensitivity of immunoassays better than immunoprecipitation [17]. Automated immunoassays are easier to implement and more sensitive than immunoprecipitation. On the other hand, immunoprecipitation allows in-house methods to vary the antigens tested and is useful in diagnosing ABPM [64]. The cut-off of A. fumigatus-IgG for automated immunoassays differs from the manufacturer's recommendation and between assays and different populations [65, 66]. For instance, the cut-off values for A. fumigatus-IgG used in India (≥27 mgA·L−1) and Japan (≥60 mgA·L−1) differ from the UK cut-off (≥40 mgA·L−1; manufacturer's recommendation) [18, 67, 68]. The experts stressed the need for data on the optimal cut-offs for A. fumigatus-IgG in different populations and using different immunoassays. Until such data are available, other population-specific cut-offs or the manufacturer's recommendation should be used.
Eosinophils primarily drive ABPA pathogenesis; thus, lung or blood eosinophilia is a common feature of ABPA [69–71]. However, eosinophilia may also be present in asthma, fungal-sensitised asthma and several other disorders [70]. Also, overlap between different eosinophilia-associated diseases is frequent and contributes to higher levels of eosinophilia [52]. Despite a modest diagnostic performance for differentiating ABPA from asthma [69, 72], blood eosinophilia can guide therapy, such as initiating anti-type 2 biological agents or a need for combination therapy (with prednisolone and itraconazole) [28]. The DECG thus recommended blood eosinophil count to evaluate ABPA (cut-off 500 cells·µL−1).
Sputum eosinophilia may be a more accurate marker of eosinophilic inflammation and can guide therapy [73, 74], although the experts felt that in many practice settings it may be difficult to obtain quality sputum differential cell counts. The experts acknowledged the underutilised potential of sputum eosinophil count and identified this as an unmet research need in ABPA [75]. Sputum differential cell counts could also guide therapy in patients with ABPA exacerbations. One suggested algorithm that needs further research is provided in supplementary figure S1.
Airway colonisation by Aspergillus spp. (or other fungi in ABPM) is crucial in initiating and sustaining immunological responses against the fungi [76]. Unfortunately, the sensitivity and specificity of sputum fungal culture are low in diagnosing ABPA. Further, it is difficult to assign causality to the isolated fungi in ABPA, and dissociation between colonising and sensitising fungi is known [54, 77]. Thus, the DECG did not recommend sputum fungal culture for diagnosing ABPA but recommended its use in ABPM. Unlike ABPA, repeated isolation of a fungus is crucial for diagnosing ABPM [78]. Sputum fungal cultures are essential to assess for azole resistance and could be obtained before starting antifungal treatment and later to characterise treatment failures better [79]. Galactomannan is a vital component of the Aspergillus cell wall and detecting serum galactomannan antigen has been approved to diagnose invasive pulmonary aspergillosis. However, given the poor accuracy of serum galactomannan testing in ABPA [80], the DECG recommended against its use for diagnosing ABPA.
Immunological tests for ABPA currently utilise crude A. fumigatus extracts [81]. Several A. fumigatus-specific antigens (f1, f2, f3, f4 and f6) are commercially available through recombinant technology [82, 83]. Recombinant A. fumigatus (rAsp) antigens can identify true A. fumigatus sensitisation [84, 85]. IgE against rAsp antigens (f1, f2 and f4) was found specific for ABPA in two different studies [86, 87], and is particularly helpful in cases where there is a mismatch between the colonising and sensitising fungi [39]. While the elevation of blood eosinophil count, serum total IgE and A. fumigatus-specific IgG can have several other causes, the IgE against rAsp antigens is highly specific [39, 83–87]. Despite these advantages, the group recommended against the routine use of rAsp antigens for diagnosing ABPA as they are not widely available [59, 83]. However, the experts suggested that IgE against rAsp f1, f2 and f4 may be used for specific purposes, such as differentiating ABPA from ABPM and clinical research [84]. IgE against rAsp f6 has been found helpful in diagnosing ABPA in systematic reviews [83, 88]; however, it lacks specificity and can be falsely positive in subjects with atopic dermatitis, possibly due to Malassezia cross-sensitisation [39, 85, 87]. Thus, the expert guidance based on prospective studies suggests that only IgE against rAsp f1 and f2 (followed by f4) consistently differentiates asthmatic subjects with and without ABPA. The manufacturer-recommended cut-offs may be suboptimal and appropriate cut-offs should be derived for different populations [86].
Imaging the lungs is critical in diagnosing ABPA and the DECG recommended using thin-section computed tomography (CT) (1.25–1.5 mm) [89, 90]. We have provided the technical details of the CT acquisition protocol for ABPA in supplementary table S3. The higher sensitivity, identification of the type and distribution of bronchiectasis, and recognition of mucus plugs are advantages of CT over a chest radiograph [91, 92]. High-attenuation mucus (HAM), i.e. mucus visually denser than the paraspinal muscles on non-contrast thorax CT, is a pathognomonic feature found in a subset of patients with ABPA [93]. The sensitivity and specificity of HAM are 35% and 100%, respectively [16]. The DECG recommended performing chest CT at baseline for diagnosis, assessment of bronchiectasis and prognostication. A chest radiograph, not a chest CT, should be used during follow-up. While magnetic resonance imaging is radiation-free, the DECG did not routinely recommend its use as it has no significant diagnostic advantage over the readily available chest CT [94–96].
Flexible bronchoscopy is used to obtain respiratory samples for fungal culture [32]. However, considering the invasive nature of the procedure, most experts did not recommend the routine use of bronchoscopy in diagnosing ABPA. Instead, the DECG suggested performing bronchoscopy in suspected ABPA/M patients in the following situations: 1) uncertain diagnosis, 2) in those with suspected ABPM where sputum cultures are uninformative or cannot be obtained, 3) unexplained haemoptysis, or 4) in patients with suspicion of chronic infection (tuberculous or non-tuberculous mycobacterial infection) before initiating systemic glucocorticoids. Infrequently, therapeutic bronchoscopy is required in ABPA patients to remove mucus plugs in the setting of respiratory failure or recalcitrant mucus plugs despite systemic therapy [97–99].
Recommendations
In asthmatic subjects with Aspergillus sensitisation, we recommend performing serum total IgE (LoC: 89.7%), A. fumigatus-specific IgG (LoC: 82.1%) and peripheral blood eosinophil count (LoC: 87.2%).
We recommend using population-specific cut-offs to interpret Aspergillus-specific IgG. When data are unavailable, we recommend using manufacturer-recommended cut-offs (LoC: 82.8%).
We recommend the following cut-offs: serum total IgE ≥500 IU·mL−1 (LoC: 71.8%) and blood eosinophil count ≥500 cells·µL−1 for diagnosing ABPA (LoC: 73.0%).
We do not recommend using serum galactomannan for diagnosing ABPA (LoC: 92.3%).
Sputum fungal culture is suggested during the evaluation of ABPA and may help identify the species or guide therapy (LoC: 61.5%).
Sputum fungal culture is recommended during the evaluation of ABPM (LoC: 100%).
We recommend a thin-section chest CT at baseline to identify and characterise bronchiectasis, mucus plugging, HAM and other abnormalities in patients with suspected ABPA (LoC: 92.3%).
We suggest using a chest radiograph to assess treatment response in ABPA (LoC: 62.3%).
Bronchoscopy is not routinely recommended for diagnosing ABPA (LoC: 86.1%).
Diagnostic criteria
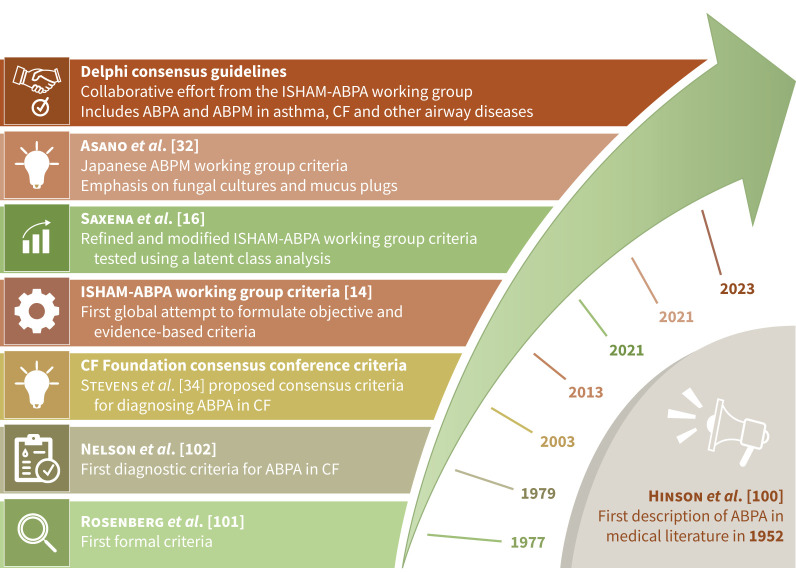
ABPA was first described in 1952 by Hinson et al. [100]. However, the first attempt to formulate diagnostic criteria was made in 1977 by Rosenberg et al. [101]. Subsequently, several criteria have been proposed, including the 2013 ISHAM-AWG criteria (figure 2) [14, 16, 32, 102, 103]. The group suggested modifying the existing ISHAM-AWG criteria. In both rounds, consensus could not be reached (LoC: 48.7% and 53.8%). Most experts felt that the criteria must be simple and allow identification and differentiation of ABPA and ABPM. Finally, after achieving consensus, we recommend separate criteria for diagnosing ABPA and ABPM (tables 1 and 2).
FIGURE 2.
The evolution of various criteria for diagnosing allergic bronchopulmonary aspergillosis (ABPA). ISHAM: International Society for Human and Animal Mycology; ABPM: allergic bronchopulmonary mycosis; CF: cystic fibrosis.
TABLE 1.
Revised International Society for Human and Animal Mycology (ISHAM)-ABPA working group consensus criteria for diagnosing allergic bronchopulmonary aspergillosis (ABPA)
| Predisposing conditions (asthma, cystic fibrosis, chronic obstructive lung disease, bronchiectasis) or acompatible clinico-radiological presentation |
| Essential components |
| bA. fumigatus-specific IgE ≥0.35 kUA·L−1 |
| cSerum total IgE ≥500 IU·mL−1 |
| Other components (any two) |
| dPositive IgG against A. fumigatus |
| Blood eosinophil count ≥500 cells·µL−1 (could be historical) |
| Thin-section chest computed tomography consistent with ABPA (bronchiectasis, mucus plugging and ehigh-attenuation mucus) or fleeting opacities on chest radiograph consistent with ABPA |
| Important considerations |
| aExpectoration of mucus plugs, finger-in-glove and fleeting opacities on chest radiograph, lung collapse, and others. |
| bA positive type 1 skin test is acceptable when Aspergillus-IgE is unavailable. |
| cSerum total IgE <500 IU·mL−1 may be acceptable if all other criteria are fulfilled. |
| dA. fumigatus-specific IgG can be detected using lateral flow assays or enzyme immunoassays. The cut-offs for A. fumigatus-specific IgG must be developed for specific populations (e.g. ≥27, ≥60 and ≥40 mgA·L−1 for India, Japan and the UK, respectively). In the absence of population-specific cut-offs, we suggest using manufacturer recommendations. |
| eHigh-attenuation mucus is pathognomonic of ABPA and confirms ABPA diagnosis even if all other criteria are not fulfilled. |
| Elevated IgE against rAsp f1, f2 and f4 supports the diagnosis of ABPA and could be used as another component for diagnosing ABPA. |
A. fumigatus: Aspergillus fumigatus; rAsp: recombinant A. fumigatus.
TABLE 2.
Revised International Society for Human and Animal Mycology (ISHAM)-ABPA working group consensus criteria for diagnosing allergic bronchopulmonary mycosis (ABPM)
| Predisposing conditions (asthma, cystic fibrosis, chronic obstructive lung disease, bronchiectasis) or acompatible clinico-radiological presentation |
| bEssential components |
| cElevated fungus-specific IgE |
| dSerum total IgE ≥500 IU·mL−1 |
| Other components (any two) |
| ePositive fungus-specific IgG |
| Blood eosinophil count ≥500 cells·µL−1 (could be historical) |
| Two sputum (or one bronchoalveolar lavage fluid) fungal cultures growing the causative fungus |
| Thin-section chest computed tomography consistent with ABPA (bronchiectasis, mucus plugging and fhigh-attenuation mucus) or fleeting opacities on chest radiograph |
| Important considerations |
| aExpectoration of mucus plugs, finger-in-glove and fleeting opacities on chest radiograph, lung collapse, and others. |
| bA. fumigatus-IgE should be <0.35 kUA·L−1. |
| cA positive type 1 skin test is acceptable. |
| dSerum total IgE <500 IU·mL−1 may be acceptable if all other criteria are fulfilled. |
| eFungus-specific IgG can be detected using enzyme immunoassays or double diffusion methods using in-house/commercial antigens. The cut-offs for fungus-specific IgG must be developed for specific populations. In the absence of population-specific cut-offs, we suggest using manufacturer recommendations. |
| fHigh-attenuation mucus is pathognomonic of ABPM and confirms ABPM diagnosis even if all other criteria are not fulfilled. |
| The absence of elevated IgE against rAsp f1, f2 and f4 excludes ABPA and strongly supports the diagnosis of ABPM. |
ABPA: allergic bronchopulmonary aspergillosis; A. fumigatus: Aspergillus fumigatus; rAsp: recombinant A. fumigatus.
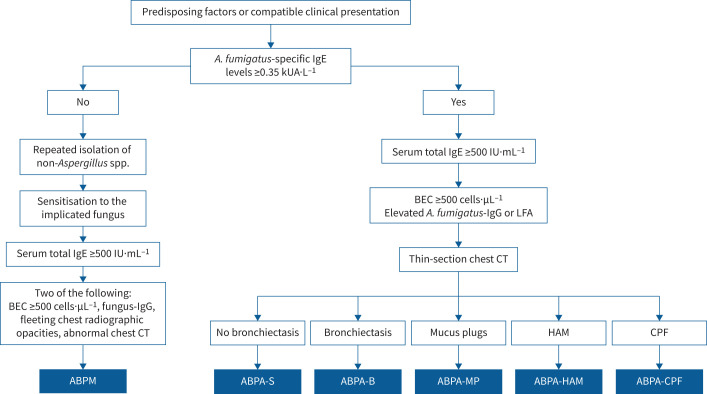
The diagnosis of ABPA/M should be suspected in patients with predisposing conditions or a compatible clinico-radiological presentation (expectoration of mucus plugs, fleeting opacities on chest imaging, finger-in-glove opacities and lung collapse). Thus, the revised criteria include a compatible presentation to enable diagnoses of ABPA/M in those without predisposing conditions [7]. Additionally, two components are essential to make a diagnosis. The first is to document sensitisation against the implicated fungus (using fungus-specific IgE), while the other is to demonstrate immunological activity (raised serum total IgE). However, these two tests can also be positive in patients with fungal sensitisation without ABPA. Here, besides the essential components, the presence of other features, including fungal-specific IgG, peripheral blood eosinophilia and consistent imaging, confirms the diagnosis of ABPA/M. Importantly, the presence of HAM on chest CT is pathognomonic and diagnoses ABPA/M, even when a few other criteria components are missing [6, 104]. We have added another radiological finding, namely “fleeting opacities consistent with ABPA” on chest radiographs, given its high specificity for diagnosing ABPA [89]. In most ABPA/M patients, serum total IgE is ≥500 IU·mL−1. Uncommonly, serum total IgE could be <500 IU·mL−1 despite the presence of all other components. Low serum total IgE can be seen in those with prior glucocorticoid treatment [105], the elderly [6] or when the patient has constitutively low IgE before developing ABPA [106, 107]. Also, any range only covers 95% of the population and all individuals will not meet a specific cut-off [108]. If available, IgE against rAsp antigens (f1, f2 and f4) may be used to diagnose ABPA.
While investigating a patient for ABPA, we recommend performing A. fumigatus-specific IgE (figure 3). If the value is ≥0.35 kUA·L−1, serum total IgE levels should be measured. If the value is ≥500 IU·mL−1, other tests for ABPA, including A. fumigatus-specific IgG, peripheral blood eosinophil count, chest CT and lung function tests, should be done to characterise the disease (table 1). The basic framework for diagnosing ABPM is similar to ABPA, with a few differences (table 2). ABPM should be considered in patients with possible ABPA, but A. fumigatus-specific IgE is <0.35 kUA·L−1. ABPM can be suspected when a causative fungus is isolated in at least two sputum culture specimens or bronchoalveolar lavage fluid culture. ABPM is then confirmed by demonstrating allergic sensitisation (skin test or fungus-specific IgE), combined with a raised serum total IgE and consistent radiological features [109]. Unfortunately, commercial assays for detecting IgE and IgG against fungi other than Aspergillus spp. are available only for a few species (Alternaria, Cladosporium, Candida, Mucor, Trichophyton and Penicillium). For other fungi, including S. commune, Bipolaris and others, in-house assays are required for detecting IgE and IgG. A skin test or immunoprecipitation would be required when fungus-specific IgE or IgG is unavailable. There is also a high probability of misclassifying ABPA as ABPM if IgE and IgG against Aspergillus spp. are performed using non-standardised assays. The rest of the workup for ABPM is similar to ABPA (table 2). Notably, the absence of elevated IgE against rAsp f1, f2 and f4 strongly supports the diagnosis of ABPM over ABPA in a patient with allergic pulmonary mycoses [84]. In settings where fungus-specific serology is not available, ABPM may be pragmatically diagnosed if there is repeated and consistent culture growth, serum total IgE ≥500 IU·mL−1, peripheral blood eosinophilia and radiological features of ABPM, provided the Aspergillus-specific serology is negative.
FIGURE 3.
Diagnostic algorithm for allergic bronchopulmonary aspergillosis/mycosis (ABPA/M). Occasionally, patients may present with imaging features of consolidation, centrilobular nodules (with a tree-in-bud appearance), atelectasis and mosaic attenuation. A. fumigatus: Aspergillus fumigatus; LFA: lateral flow assay; CT: computed tomography; BEC: blood eosinophil count; ABPA-S: serological ABPA; ABPA-B: ABPA with bronchiectasis; ABPA-MP: ABPA with mucus plugging; ABPA-HAM: ABPA with high-attenuation mucus; ABPA-CPF: ABPA with chronic pleuropulmonary fibrosis.
The differential diagnosis of ABPA/M is broad and caution is advised in making the diagnosis in patients without either asthma or CF. A. fumigatus-specific IgE and IgG can be elevated in COPD, pulmonary tuberculosis and bronchiectasis, and some of these patients can develop ABPA. Patients with chronic pulmonary aspergillosis may have raised serum A. fumigatus-IgE and total IgE in addition to A. fumigatus-IgG [109, 110]. Aspergillus (and fungal) bronchitis is associated with at least two positive respiratory samples yielding the same fungus and may be associated with a raised A. fumigatus-IgG and bronchiectasis, but without fulfilling the diagnostic criteria for ABPA/M. Patients with severe asthma may be sensitised to A. fumigatus or multiple other fungi with a raised total IgE. They are classified as severe asthma with fungal sensitisation under the umbrella of fungal asthma when they do not fulfil the ABPA/M criteria. Patients with ABPA may also have an additional underlying aetiology for bronchiectasis [111]. Therefore, a search for other causes of bronchiectasis (immunodeficiencies, ciliary disorders and mycobacterial infection) is prudent [112]. The diagnostic workup of bronchiectasis includes complete blood count, A. fumigatus-specific IgE, sweat chloride test, immunoglobulin levels and mycobacterial cultures from sputum [9, 113]. If the initial workup is negative, whole-exome sequencing can be performed (to identify aetiologies such as primary ciliary dyskinesias, primary immunodeficiency and atypical CF), especially in those with extensive bronchiectasis and recurrent infections since childhood.
Clinical classification and treatment response criteria
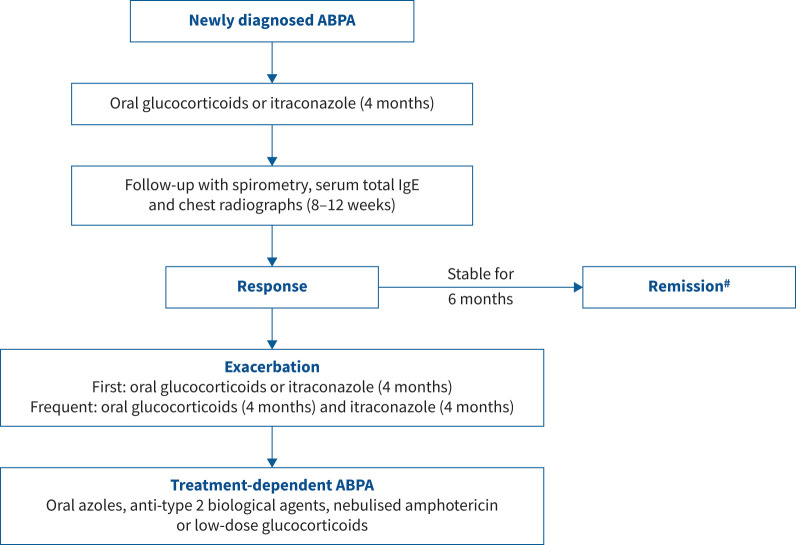
A clinical framework for classifying ABPA is essential due to the chronic relapsing nature of the illness and the propensity for developing severe complications. Also, an objective treatment response criterion is useful for monitoring therapy during routine care and in clinical trials. The first classification attempt categorised ABPA into five stages [63]. As the stages were imprecise, the ISHAM-AWG previously proposed a modified staging with more detailed definitions [14]. However, there were several unresolved issues. Most importantly, the stages were labelled 0–6, but a patient does not necessarily progress from one to another. The previous classification also did not reflect progressive severity since stage 4 (remission) is a more stable clinical state than stage 3 (exacerbation), which is counterintuitive. To overcome these limitations, we proposed modifications that achieved consensus in the second round (LoC: 85.3%).
In the new ABPA/M classification, we have removed the numbered stages and retained five categories: acute ABPA, response, remission, treatment-dependent ABPA and advanced ABPA (table 3). We have removed the asymptomatic stage and glucocorticoid-dependent asthma as they had no clear treatment implications in ABPA. Also, we have included newly diagnosed ABPA and exacerbation together as acute ABPA. To diagnose ABPA exacerbation, asthma or bronchiectasis (infective) exacerbations need to be excluded, and we provide definitions for the two entities in table 3. Remission, as in asthma [114], is diagnosed when the patient has no asthma or ABPA exacerbations, is not dependent on oral glucocorticoid therapy and has the best possible lung function. Remission may be achieved spontaneously after treatment or with antifungal azoles or biological agents. Finally, advanced ABPA is defined in patients with extensive bronchiectasis and type 2 respiratory failure or secondary pulmonary hypertension (table 3).
TABLE 3.
Revised International Society for Human and Animal Mycology (ISHAM)-ABPA working group recommendations for clinical classification and treatment response criteria for allergic bronchopulmonary aspergillosis/mycosis (ABPA/M)
| Acute ABPA | Newly diagnosed: Previously undiagnosed ABPA/M meeting the diagnostic criteria (tables 1 and 2). |
|
Exacerbation: In a patient with diagnosed ABPA/M: • Sustained (>14 days) clinical worsening; or • Radiological worsening; and • Increase in serum total IgE by ≥50% from the last recorded IgE value during clinical stability, along with • Exclusion of other causes of worsening. | |
| Asthma exacerbation: worsening respiratory symptoms for at least 48 h without immunological or radiological deterioration of ABPA/M. | |
| Infective/bronchiectasis exacerbation: clinical deterioration for at least 48 h with an increase in cough, breathlessness, sputum volume or consistency, sputum purulence, fatigue, malaise, fever, or haemoptysis, without immunological or radiological deterioration of ABPA/M. | |
| Response | • Symptomatic improvement by at least 50% (on a Likert or visual analogue scale) after 8 weeks; and • Major radiological improvement (>50% reduction in radiological opacities) or decline in serum total IgE by at least 20% after 8 weeks of treatment. |
| Remission | • Sustained (≥6 months) clinico-radiological improvement, off glucocorticoids; and • Lack of rise in serum total IgE by ≥50% from the last recorded IgE value during clinical stability. Patients on biological agents or long-term antifungal agents may also be considered in remission if they meet the above criteria. |
| Treatment-dependent ABPA | • Two or more consecutive ABPA/M exacerbations, each within 3 months of stopping glucocorticoids. • Worsening respiratory symptoms AND worse imaging or rise in serum total IgE by 50% within 4 weeks of tapering oral steroids on two separate occasions. |
| Advanced ABPA | • Extensive bronchiectasis (≥10 segments) due to ABPA/M on chest imaging; and • Cor pulmonale or chronic type 2 respiratory failure. |
Radiological classification of ABPA
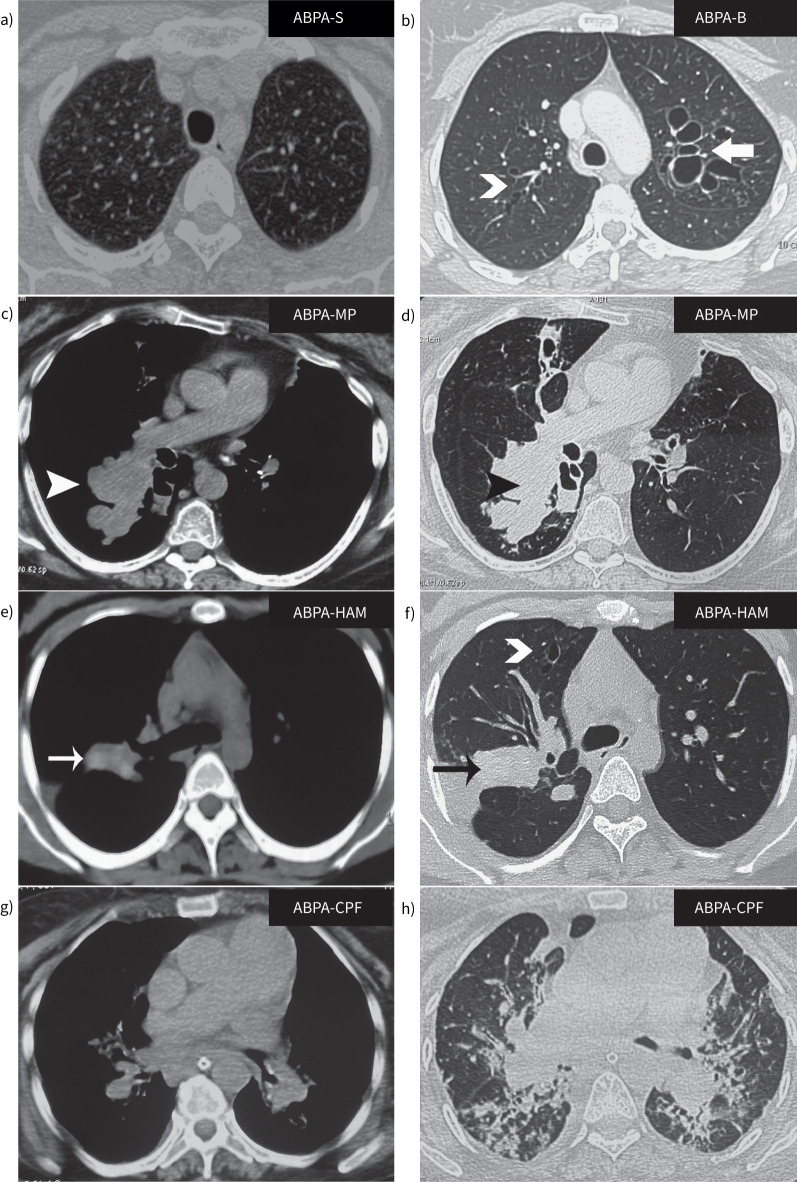
CT of the thorax is crucial in diagnosing ABPA. However, due consideration should be given to the radiation dosage when CT scans are ordered, especially in children. Chest CT is also prognostic. For instance, the extent of bronchiectasis, HAM and any fungal ball are independent predictors of recurrent ABPA exacerbations [91, 93, 115–118]. Central bronchiectasis (usually bilateral) is the predominant pattern seen in ABPA, although it is not uncommon to find both central and peripheral bronchiectasis [90, 119]. Isolated central bronchiectasis is encountered only in a few conditions, including ABPA and tracheobronchomegaly, and is thus a helpful distinguishing feature [120]. Previously, Greenberger's group classified ABPA as ABPA with central bronchiectasis (ABPA-CB) or serological ABPA (ABPA-S) based on the presence or absence of bronchiectasis [121, 122]. Subsequently, Kumar [123] classified ABPA into three groups: ABPA-S, ABPA-CB and ABPA-CB with other radiological findings (ABPA-CB-ORF). In a study involving 234 patients, Agarwal et al. [93] categorised ABPA into ABPA-S (mild), ABPA-CB (moderate) and ABPA-CB-HAM (severe). Based on all the evidence, the ISHAM-AWG had previously classified ABPA radiologically into four categories: ABPA-S, ABPA with bronchiectasis (ABPA-B), ABPA-HAM and ABPA with chronic pleuropulmonary fibrosis (ABPA-CPF) [14]. The term “bronchiectasis” (B) was used instead of “central bronchiectasis” (CB) as bronchiectasis in ABPA can extend to the periphery in up to 40% of the lobes [90, 92, 119, 124]. Mucus plugging without HAM is another common radiological finding in ABPA. Mucus plugs are consistently associated with eosinophilic inflammation and immunologically severe ABPA [71, 125].
The DECG discussed several radiological classifications. Finally, the scheme presented in table 4 achieved consensus (LoC: 88.2%). The new classification includes five classes: ABPA-S, ABPA-B, ABPA with mucus plugging (ABPA-MP), ABPA-HAM and ABPA-CPF (figure 4). ABPA-S refers to patients of ABPA without bronchiectasis, while ABPA-B includes patients with bronchiectasis. ABPA-HAM has been retained, as HAM is an independent and pathognomonic diagnostic feature of ABPA [126]. ABPA-MP includes patients with non-hyperattenuating mucus plugs. Patients with bronchiectasis and mucus plugging are labelled as ABPA-MP, given the greater immunological severity in those with mucus plugging. Other radiological findings frequently observed in ABPA include centrilobular nodules (with a tree-in-bud appearance), atelectasis, mosaic attenuation and consolidation. These findings can be seen in isolation or with ABPA-B, ABPA-MP and ABPA-HAM. In patients with ABPA-CPF, a vital consideration is the exclusion of chronic pulmonary aspergillosis [109, 110, 127, 128].
TABLE 4.
Revised International Society for Human and Animal Mycology (ISHAM)-ABPA working group recommendations for radiological classification for allergic bronchopulmonary aspergillosis/mycosis (ABPA/M)
| Serological ABPA (ABPA-S) | ABPA with no bronchiectasis |
| ABPA with bronchiectasis (ABPA-B) | ABPA with radiological evidence of bronchiectasis |
| ABPA with mucus plugging (ABPA-MP) | ABPA with mucus plugging but without high-attenuation mucus; patients with both bronchiectasis and mucus plugging will be classified as ABPA-MP |
| ABPA with high-attenuation mucus (ABPA-HAM) | ABPA with high-attenuation mucus |
| ABPA with chronic pleuropulmonary fibrosis (ABPA-CPF) | ABPA with two or more of the following: pulmonary fibrosis, fibro-cavitary lesions, fungal ball and pleural thickening |
Consolidation, centrilobular nodules (with a tree-in-bud appearance), atelectasis and mosaic attenuation are other common radiological findings seen in ABPA that can occur in isolation or in the presence of ABPA-B, ABPA-MP and ABPA-HAM. In patients with ABPA-CPF, chronic pulmonary aspergillosis complicating ABPA should be excluded.
FIGURE 4.
Computed tomography showing various radiological categories in allergic bronchopulmonary aspergillosis (ABPA). The scans are from different patients with ABPA. Lung windows for patients with a) serological ABPA (ABPA-S) and b) ABPA with bronchiectasis (ABPA-B). Both lung and mediastinal windows for patients with c, d) ABPA and mucus plugging (ABPA-MP), e, f) ABPA with high-attenuation mucus (ABPA-HAM) and g, h) ABPA with chronic pleuropulmonary fibrosis (ABPA-CPF). Chevron arrows: cylindrical bronchiectasis; bold arrow: cystic bronchiectasis; arrowheads: mucus plugs; thin arrows: high-attenuation mucus plugs (the attenuation of mucus plugs should be observed in the mediastinal windows).
Treatment of ABPA
The principles of treating ABPA involve using anti-inflammatory agents (glucocorticoids or biological agents targeting type 2 immune response) to control immune responses or antifungal agents to decrease airway fungal colonisation. The treatment goals are symptom relief, improving asthma control, preventing asthma and ABPA exacerbations, abrogating bronchiectasis progression, and minimising therapy-related adverse events. The treatment principles of ABPM are like ABPA, except that the implicated fungus guides the choice of antifungal drugs. The DECG reviewed the RCTs and the therapies available for treating ABPA patients (tables 5 and 6) [22–29, 34, 42, 43, 92, 129–144].
TABLE 5.
Treatment of allergic bronchopulmonary aspergillosis (ABPA): key studies with the level of evidence
| Summary statements | Level of evidence | References |
| High-dose inhaled steroids do not achieve symptom control or reduce ABPA exacerbations when used as a primary therapy for acute ABPA | II | [129, 130] |
| Oral glucocorticoids are the most rapid acting treatment for acute ABPA | I | [23, 27, 92, 131] |
| Oral itraconazole or voriconazole have similar efficacy as systemic glucocorticoids in acute ABPA, with fewer adverse events | II | [25, 26, 28, 43, 133] |
| Oral itraconazole has steroid-sparing action in treatment-dependent ABPA | II | [34, 42, 132] |
| Oral posaconazole achieves clinical remission in ABPA patients intolerant to other azoles | III | [133] |
| Omalizumab reduces asthma exacerbations and has steroid-sparing action in treatment-dependent ABPA | II | [22, 134] |
| Other biological agents (mepolizumab, benralizumab, dupilumab and tezepelumab) reduce asthma exacerbations and have steroid-sparing action in treatment-dependent ABPA | III | [135–142, 163] |
| Nebulised amphotericin B may reduce the number of ABPA exacerbations and increase the time-to-first ABPA exacerbation when used as a maintenance therapy in stable ABPA | II | [24, 29, 143] |
Level of evidence I: well-conducted randomised controlled trial (RCT) or meta-analysis of well-conducted studies. Level of evidence II: well-conducted prospective observational studies, RCTs with small sample size or high risk of bias. Level of evidence III: small case series or case reports, observational studies with a high risk of bias, case–control or retrospective studies with a high risk of bias
TABLE 6.
Summary of various drugs available for treating allergic bronchopulmonary aspergillosis/mycosis
| Dose | Adverse events | Other comments | |
| Systemic glucocorticoids | |||
| Prednisolone | 0.5 mg·kg−1·day−1 for 2 weeks, followed by 0.5 mg·kg−1·day−1 for alternate days for 8 weeks; then taper by 5 mg every 2 weeks and discontinue over 3–5 months OR 0.5, 0.25 and 0.125 mg·kg−1·day−1, for 4 weeks each; then taper by 5 mg every 2 weeks till discontinuation |
Hyperglycaemia, hirsutism, acne, osteoporosis, hypertension, cushingoid habitus, weight gain, opportunistic infections, psychosis, depression, cataracts, gastritis, HPA suppression, etc. | |
| Methylprednisolone | 0.4, 0.2 and 0.1 mg·kg−1·day −1, each, for 4 weeks; then taper by 4 mg·week−1 to complete 4 months | Same as prednisolone | Itraconazole increases the plasma levels of methylprednisolone but not prednisolone |
| Deflazacort | 0.75 mg·kg−1·day−1 for 4 weeks; decrease by half every 4 weeks for the next 2 months; then taper by 6 mg every 2 weeks and discontinue to complete 4 months | Lesser metabolic side-effects; an ongoing trial is comparing prednisolone with deflazacort (ClinicalTrials.gov: NCT04227483) | |
| Antifungal agents | |||
| Conventional itraconazole capsule | 400 mg·day−1 in two divided doses for 4 months; maximum dose 600 mg·day−1; it should be administered with meals to improve absorption | Headache, gastritis, nausea, vomiting, liver toxicity, pedal oedema, heart failure, tingling or numbness, hypertension, palpitations, etc. | TDM should be performed (trough itraconazole levels ≥0.5 mg·L−1) |
| Super bioavailable itraconazole capsule | 260 mg·day−1 in two divided doses for 4 months; maximum dose 390 mg·day−1; it should be given on an empty stomach | Same as conventional itraconazole | PPIs or antacids do not affect serum levels |
| Voriconazole capsule | 400 mg·day−1 in two divided doses; maximum dose 600 mg·day−1; it should be given on an empty stomach | Headache, hallucinations, anxiety, insomnia, rash, photosensitivity, mucositis, liver toxicity, prolonged QTc, tingling, numbness, skin cancer, etc. | TDM should be performed (trough itraconazole levels ≥1 mg·L−1) |
| Posaconazole (delayed-release tablet form or suspension) | 800 mg·day−1 (oral suspension) in two divided doses; 300 mg·day−1 (delayed-release tablet) once daily; better absorption with meals (tablet form) or fatty meals (suspension) | GI intolerance, ankle oedema, rash, fatigue, etc. | Routine TDM is not required; for better outcomes, target trough level ≥1 mg·L−1 |
| Isavuconazole | 200 mg·day−1 once daily; no relation to food intake | GI intolerance, ankle oedema, rash, fatigue, etc. | Routine TDM is not required; for better outcomes, target trough level ≥1 mg·L−1 |
| Nebulised amphotericin B deoxycholate | 10 mg twice daily 3–6 times per week | Bronchospasm, cough, fever, headache, sinusitis, etc. | Reconstituted solution can be stored for 1 week at 2–8°C; safe in pregnancy |
| Nebulised liposomal amphotericin B | 25–50 mg once or twice weekly | Bronchospasm, cough, fever, sinusitis, etc. | Reconstituted solution can be stored for 48 h at 2–8°C; safe in pregnancy |
| Biological agents | |||
| Omalizumab | Dose is based on body weight and serum total IgE values not exceeding 375 mg s.c. injection twice a month | Local reaction, headache, peripheral oedema, abdominal pain, arthralgia, serum sickness, anaphylaxis and TIA | |
| Mepolizumab | 100 mg s.c. injection monthly | Local reaction, eczema, headache, diarrhoea, fatigue, arthralgia, herpes zoster, angioedema and anaphylaxis | |
| Benralizumab | 30 mg s.c. injection 4-weekly for three doses, after that every 8 weeks | Headache, fever, angioedema, local site reaction and anaphylaxis | |
| Dupilumab | 600 mg s.c. injection followed by 300 mg every 2 weeks | Diarrhoea, parasitic infections, dizziness, insomnia, arthralgia, thrombosis, skin rash, local reaction, anaphylaxis, etc. | |
| Tezepelumab | 210 mg s.c. injection every 4 weeks | Arthralgia, local reaction, anaphylaxis and URTIs |
HPA: hypothalamic–pituitary axis; TDM: therapeutic drug monitoring; PPI: proton pump inhibitor; QTc: corrected QT interval; GI: gastrointestinal; s.c.: subcutaneous; TIA: transient ischaemic attack; URTI: upper respiratory tract infection.
Initiating treatment for newly diagnosed ABPA
Patients with acute ABPA require treatment with systemic therapies. Glucocorticoids are the most effective treatment for acute ABPA [131]. An RCT involving 92 ABPA patients compared two glucocorticoid dosing protocols (low dose (prednisolone 0.5 mg·kg−1·day−1 for 2 weeks, then on alternate days for 8 weeks; then tapered by 5 mg every 2 weeks and discontinued after 3–5 months) versus high dose (prednisolone 0.75 and 0.5 mg·kg−1·day−1 for 6 weeks each; subsequently, tapered by 5 mg every 6 weeks and discontinued after 8–10 months)). The frequency of ABPA exacerbations was similar in the two groups and the lower dose resulted in fewer adverse events. However, there was a lower clinico-radiological and immunological response at 6 weeks with the lower dose [23]. Several centres use doses intermediate between the low and high doses (prednisolone 0.5, 0.25 and 0.125 mg·kg−1·day−1 for 4 weeks each, then tapered by 5 mg every 2 weeks till discontinuation). The DECG recommended using a 4-month course of low-to-moderate dose oral prednisolone (0.5 mg·kg−1·day−1 for 2–4 weeks, tapered and completed over 4 months) for acute ABPA (table 6) [23, 25–28, 131]. Care should be taken while using methylprednisolone because when combined with oral itraconazole, there is a higher risk of exogenous Cushing's syndrome and adrenal insufficiency [145, 146]. Notably, the experts suggested the need for trials with even shorter duration of glucocorticoids, as the 4-month duration was derived from the need to randomise against longer-term azole therapy. Many clinicians administer an initial 2-week course of glucocorticoids in those started on an oral azole, and as symptoms are controlled, transition to high-dose inhaled corticosteroids (ICS). Importantly, a combination of inhaled budesonide or fluticasone and itraconazole can also cause exogenous Cushing's syndrome [147]. While asymptomatic ABPA patients do not routinely require systemic therapy, the treatment decision needs to be individualised. For instance, patients can have well-controlled asthma on high-dose inhaled steroids and may benefit from treatment of underlying ABPA, especially if the chest CT shows bronchiectasis or mucus plugging. Also, asymptomatic patients with prolonged mucus plugging can progress to irreversible bronchiectasis. Thus, optimisation of asthma treatment and close observation with a clinical review, chest radiograph and serum total IgE every 3–6 months is required if a decision is made not to treat patients with asymptomatic ABPA.
Oral antifungal triazoles, especially itraconazole, have similar effects as glucocorticoids but a slower trajectory to improvement and a better safety profile than glucocorticoids [26]. Although the evidence was limited to a single RCT, the DECG recommended using oral itraconazole (for 4 months) as an alternative initial therapy for acute ABPA, given the considerable clinical experience with itraconazole. While voriconazole has similar efficacy as glucocorticoids for treating acute ABPA [25], the experts expressed concerns with its use as first-line therapy due to poorer patient tolerance. Also, prednisolone decreases the plasma concentration of voriconazole in a dose-dependent fashion [148, 149]. A recent RCT of 191 patients found no significant reduction in ABPA exacerbations with a combination of prednisolone and itraconazole compared to prednisolone alone and a propensity for higher adverse events [28]. However, patients with blood eosinophil count ≥1000 cells·µL−1 and extensive bronchiectasis (≥10 segments) had a reduced 1-year exacerbation rate with the combination therapy [28]. Similarly, there is little evidence for posaconazole, isavuconazole or a combination of newer azoles and glucocorticoids as the initial therapy for ABPA.
There are no data supporting the use of biological agents as first-line therapy in ABPA. Adjunctive vitamin D supplementation was not helpful in managing ABPA [27], but vitamin D deficiency should be corrected as it aggravates osteopenia due to long-term glucocorticoid usage. High doses of ICS alone do not achieve immunological control or reduce exacerbations when used as therapy for ABPA [129, 130]. The efficacy of nebulised amphotericin B in acute ABPA is also poor [143]. Notably, most studies on ABPA therapy have included mainly patients with ABPA-B, with little data on ABPA-S [1, 40]. Most experts do not routinely treat ABPA-S with systemic ABPA-specific treatment. Instead, patients with ABPA-S are managed like asthma. However, oral glucocorticoids or azoles may be required in those with poor asthma control or recurrent exacerbations despite optimal asthma management.
Recommendations
We do not recommend treating asymptomatic ABPA patients with systemic therapy (LoC: 85.7%).
ABPA-S should be treated with systemic therapy only if there is poor asthma control (LoC: 79.4%) or recurrent exacerbations despite asthma therapy (LoC: 85.3%).
We recommend a low-to-moderate dose (0.5 mg·kg−1·day−1 for 2–4 weeks, tapered and completed over 4 months) of oral prednisolone (LoC: 78.1%) or oral itraconazole (LoC: 73.5%) for 4 months as the initial therapy for treating acute ABPA.
We recommend oral itraconazole as the initial therapy where systemic glucocorticoids are contraindicated (LoC: 84.6%).
We do not recommend using a combination of itraconazole and glucocorticoids as first-line therapy for acute ABPA (LoC: 71.9%). However, a short course of glucocorticoids (<2 weeks) may be used as initial therapy along with oral itraconazole.
Oral voriconazole, posaconazole and isavuconazole should not be used as first-line agents for treating acute ABPA (LoC: 78.1–96.9%). They may be used if there are contraindications to systemic glucocorticoids and intolerance, failure or resistance to itraconazole therapy (LoC: 12.8–64.1%).
High-dose ICS should not be used as primary therapy for acute ABPA (LoC: 100%).
We do not recommend using biological agents as first-line therapy for acute ABPA (LoC: 96.9%).
Treatment of ABPA exacerbation
After treatment cessation, almost 50% of patients experience ABPA exacerbations [118]. In a patient with ABPA, worsening of respiratory symptoms may occur due to asthma exacerbation, immunologically exacerbated ABPA and infective exacerbation of bronchiectasis, apart from unrelated causes. The three types of exacerbations can usually be differentiated using chest radiographs, serum total IgE and sputum bacterial cultures. Occasionally, there can be an overlap of more than one cause of exacerbation in an individual patient.
ABPA exacerbations are characterised by sustained worsening (≥2 weeks) of clinical symptoms or the appearance of new infiltrates on chest imaging, along with an increase in serum total IgE by ≥50% above the “new baseline” IgE (during clinical stability) [1, 118]. Asthma exacerbations are not associated with increased serum total IgE or new infiltrates on chest imaging and should be managed with a short course of oral glucocorticoids. Bronchiectasis (infective) exacerbations are diagnosed with clinical worsening without elevation in serum total IgE ≥50% compared to baseline. Sputum cultures frequently show bacterial growth in bronchiectasis exacerbations.
There are no RCTs for managing ABPA exacerbations and the options include using prednisolone, itraconazole or their combination [150]. In clinical practice, ABPA exacerbation is managed like newly diagnosed ABPA using either prednisolone or itraconazole. Many experts use a combination of oral prednisolone and oral itraconazole in patients with recurrent exacerbations (≥2 in the last 1–2 years), especially in those with extensive bronchiectasis. Also, nebulised amphotericin B has poor efficacy for treating ABPA exacerbations [143]. Pulse doses of methylprednisolone have been used for ABPA exacerbations refractory to oral glucocorticoids [151].
Recommendations
We recommend treating acute ABPA exacerbations in the same manner as newly diagnosed ABPA (LoC: 100%).
A combination of oral prednisolone and itraconazole should be used for treating recurrent (≥2 in the last 1–2 years) ABPA exacerbations (LoC: 71.4%).
We do not recommend using biological agents (LoC: 94.3%) or nebulised amphotericin B (LoC: 100%) for treating acute ABPA exacerbations.
Monitoring treatment response
After treatment initiation, patients should be monitored for response after 8–12 weeks using clinical symptoms, serum total IgE and chest radiographs (figure 5). Instead of subjective assessment, the DECG suggested that symptom monitoring be done using a semiquantitative Likert scale as no improvement (or worsening), mild (<25% of baseline), moderate (25–50% of baseline) or significant improvement (>50% of baseline) for routine clinical care. The experts suggested using a more quantitative scale like a visual analogue scale (VAS) for clinical trials. A good response is indicated by a significant improvement in symptoms (Likert score or VAS ≥50%) and imaging (figure 6), along with at least a 20% reduction in the serum total IgE levels [118].
FIGURE 5.
Stagewise management of patients with allergic bronchopulmonary aspergillosis/mycosis (ABPA/M). #: remission may be achieved on antifungal azoles or biological agents, or spontaneously after therapy.
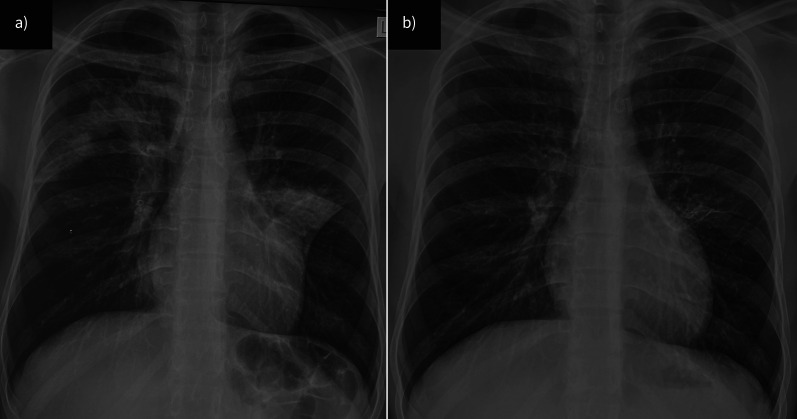
FIGURE 6.
Chest radiographs showing major improvement of pulmonary opacities from a) before to b) after treatment in a patient with allergic bronchopulmonary aspergillosis.
Spirometry may be used to monitor treatment response. A recent study reported the minimal clinically important difference (MCID) of forced expiratory volume in 1 s of 158 mL for treatment response in ABPA [152]. Quality-of-life questionnaires are cumbersome and were discouraged unanimously by experts for routine patient care [153]. However, for clinical trials, quality-of-life questionnaires could be used. An important consideration is that the MCID for the quality-of-life questionnaires could differ in ABPA from asthma. For instance, in one study, the MCID of the St George's Respiratory Questionnaire was 8 points, rather than the 4 points used in asthma [153]. The serum A. fumigatus-specific IgE and IgG levels do not consistently fall following treatment [18, 61]. No studies have evaluated blood eosinophil count or concentrations of IgE/IgG against rAsp antigens for assessing treatment response.
While using oral azoles, therapeutic drug monitoring is recommended initially at 2-week and 3-month intervals or during clinical worsening. Significant variations in the bioavailability of different itraconazole preparations compromise response [154]. Likewise, drug–drug interactions are frequent with itraconazole, with common issues being ICS exposure boosting and undetectable levels of itraconazole with rifampicin [155]. The recommended minimum therapeutic levels are >0.5, >1 and >1 µg·mL−1 for itraconazole, voriconazole and posaconazole, respectively [156]. Monitoring for treatment-related adverse events is paramount. When using systemic glucocorticoids, the DECG recommended monitoring plasma glucose, blood pressure, body weight and mental status at the least. Monitoring liver function is necessary for patients receiving oral azoles (table 6).
Recommendations
We recommend assessing the initial treatment response after 8–12 weeks using a combination of clinical, immunological and imaging findings (LoC: 100%). A good response is indicated by a major improvement in symptoms (Likert score or VAS ≥50%) and chest radiographs, along with at least a 20% reduction in serum total IgE.
A. fumigatus-specific IgE and IgG (LoC: 100%), peripheral blood eosinophil count (LoC: 73.6%), and IgE against recombinant antigens are not recommended for response assessment (LoC: 100%).
We recommend therapeutic drug monitoring while using antifungal azoles (LoC: 91.4%).
Management of treatment-dependent ABPA
Almost 10–25% of ABPA patients become treatment-dependent [92, 121]. Two RCTs (84 patients) have evaluated the efficacy of itraconazole in treatment-dependent ABPA [132, 157]. Itraconazole reduced the oral glucocorticoid dose, sputum eosinophil count and ABPA exacerbations [132, 157]. A significant limitation was that neither study reported outcomes beyond 8 months regarding ABPA exacerbations.
In the last two decades, several trials of monoclonal antibodies against IgE, interleukin (IL)-5, IL-5 receptor (IL-5R), IL-4 receptor α (IL-4Rα) and thymic stromal lymphopoietin (TSLP) have demonstrated clinical benefit in severe eosinophilic asthma [158–161]. Biological agents are likely helpful in stable treatment-dependent ABPA based on case reports and small case series [41, 134, 162]. Omalizumab is a potential therapeutic approach since ABPA is associated with elevated IgE levels. Mepolizumab (anti-IL-5), benralizumab (anti-IL-5R), dupilumab (anti-IL-4Rα) and tezepelumab (anti-TSLP) have also been used in ABPA patients [135, 142, 162–165]. Most experience of biological agents in ABPA is with omalizumab. Using omalizumab in ABPA led to improvement in symptoms, reduction in exacerbations and asthma hospitalisations, improvement in lung function, and reduction in the dose of oral steroids [134, 166]. In the only crossover RCT of 13 patients, omalizumab use (versus placebo) was associated with less frequent exacerbations and decreased basophil reactivity to A. fumigatus [22]. Biological agents should also be considered for maintenance therapy for underlying asthma [167]. Finally, continuous low-dose glucocorticoids should be the last option in managing treatment-dependent ABPA.
Recommendation
Long-term itraconazole (100%), nebulised amphotericin B (LoC: 100%) or biological agents (LoC: 71%) are recommended options for managing treatment-dependent ABPA.
Management of ABPA in remission
During remission (stable disease), ABPA patients should be managed for underlying asthma and bronchiectasis (ICS and long-acting bronchodilators, nebulised saline, antibiotics, and others) per the existing guidelines [112, 167]. Patients should be monitored with clinical review, serum total IgE levels and lung function test every 3–6 months for the first year and then every 6–12 months. Remission can be prolonged by using long-term itraconazole, nebulised amphotericin B (LoC: 100%) and biological agents (LoC: 71%), especially in those with treatment-dependent ABPA.
Two RCTs have also evaluated the role of nebulised amphotericin B as maintenance to prevent future ABPA exacerbations [24, 29]. In both studies, patients with ABPA exacerbation were treated for 4 months with oral glucocorticoids or prednisolone and itraconazole. After that, they were randomised to receive nebulised amphotericin B versus placebo. In the smaller pilot study (21 patients), using nebulised amphotericin B deoxycholate (10 mg twice daily, three times a week) reduced ABPA exacerbations at 1 year [24]. In the larger NEBULAMB study (139 patients), while the primary outcome was not significant, the time-to-first exacerbation was significantly longer with nebulised liposomal amphotericin B (25 mg weekly) compared to the control group [29].
Recommendations
We recommend that patients in remission be managed for underlying asthma and bronchiectasis per the existing guidelines (LoC: 100%).
For patients achieving remission with antifungal azoles or biological agents, we recommend periodic assessments to determine the need for these therapies (LoC: 100%).
Management of extensive bronchiectasis and advanced ABPA
There are no specific studies on advanced ABPA [168]. Nebulised hypertonic saline (3–7%, 4–5 mL) can reduce sputum viscosity and ease the expectoration of mucus plugs in bronchiectasis patients [112]. Treatment should be preceded by nebulised salbutamol to minimise the risk of bronchospasm. Also, the first dose of nebulised hypertonic saline should be administered under supervision [169, 170]. Nebulised antibiotics and long-term azithromycin therapy can improve outcomes in ABPA patients with bronchiectasis and frequent infective exacerbations [112, 171]. Caution is advised when using azithromycin in those receiving itraconazole, as it can cause QTc prolongation. In some ABPA patients, widespread bronchiectasis can eventually lead to chronic type 2 respiratory failure and pulmonary hypertension [168].
The DECG recommended extrapolating guidance from other pulmonary disorders (chronic obstructive lung disease, interstitial lung disease and bronchiectasis) for long-term oxygen therapy (LTOT), vaccination and lung transplantation [112, 172, 173]. LTOT in those with resting hypoxaemia (arterial oxygen tension (PaO2) ≤55 mmHg) reduces pulmonary hypertension and improves survival in patients with chronic obstructive lung disease. There is no role for LTOT in mild hypoxaemia (PaO2 >55 mmHg at rest) or nocturnal oxygen desaturation [174]. In one study, patients with chronic and allergic aspergillosis responded poorly to the 23-valent pneumococcal polysaccharide vaccine compared to healthy adults [175]. Thus, influenza or pneumococcal vaccine should be administered before initiating glucocorticoid therapy or delayed until the underlying condition is better controlled (e.g. after treatment with antifungals or glucocorticoids). The International Society for Heart and Lung Transplantation criteria should be used to refer and list patients with advanced ABPA [172].
Recommendation
We recommend using standard guidelines from other pulmonary disorders for LTOT, vaccination and lung transplantation (LoC: 100%).
Treatment in special conditions
ABPA during pregnancy
The principles of managing ABPA during pregnancy are to optimise asthma and ABPA control while preventing harm to the fetus. Most ABPA trials have excluded pregnant patients and data are extrapolated. Oral glucocorticoids for the duration and dose required in ABPA are safe in pregnancy [176], while the use of oral itraconazole is associated with a higher risk of premature births and abortions [177]. Experience with biological agents in ABPA during pregnancy is limited. When used with oral glucocorticoids, omalizumab increases the risk of pre-term delivery [178]. In a systematic review of biological agent use during pregnancy with atopic disease, major fetal defects, low birthweight, pre-term delivery and stillbirths were reported [179].
Recommendations
We recommended using systemic glucocorticoids (in the same doses as for non-pregnant) for managing acute-stage or treatment-dependent ABPA in pregnancy (LoC: 73.0%).
We recommend avoiding the use of biological agents (LoC: 86.5%) or oral azoles (LoC: 100%) for managing acute-stage or treatment-dependent ABPA in pregnancy.
ABPA in CF
There are no RCTs of treatment in CF-ABPA [180, 181]. Glucocorticoids or azoles are the preferred initial agents. Glucocorticoids can induce diabetes mellitus, with grave consequences in CF. Monthly doses of intravenous methylprednisolone therapy alone or with azoles have been used to limit the toxicity associated with daily glucocorticoids [182]. The combination of glucocorticoids with azoles is also increasingly being used in CF-ABPA. In one survey, combination therapy was preferred by most respondents for treating newly diagnosed ABPA and first exacerbation [183]. A 3-week course of oral corticosteroids combined with oral itraconazole for 12 months effectively treated CF-ABPA [184]. While using itraconazole capsules, therapeutic drug monitoring is essential as the drug is poorly absorbed in CF. The super bioavailable itraconazole formulation was well-tolerated and achieved therapeutic response in 81% of patients with CF-ABPA [185]. Voriconazole leads to photosensitivity and should be used with caution. Posaconazole achieved therapeutic drug levels and good clinical response in CF-ABPA [144]. While a previous review suggested that omalizumab might benefit patients with ABPA [186], a recent case series found no benefit with omalizumab [187]. Like in asthma, pulmonary exacerbations in a patient with CF-ABPA could be related to ABPA or pulmonary infection [34].
Recommendation
We recommend diagnosing and treating CF-ABPA using the same treatment tenets outlined for ABPA in asthma; however, exercising due caution for issues specific to CF (LoC: 100%).
ABPA in children
Growth retardation with the use of systemic corticosteroids and the erratic bioavailability of azoles due to age-related changes in cytochrome P450 enzymes are of primary concern [188]. There are no randomised trials on the treatment of ABPA in children [189]. Omalizumab and mepolizumab are approved for asthma therapy in the age group ≥6 years [190, 191], while other biological agents can be used in those ≥12 years old. The DECG recommended using systemic glucocorticoids or oral itraconazole for treating acute ABPA in children as in adults. The experts felt more research was needed to address the therapeutic issues affecting ABPA in children.
Recommendation
We recommend treating ABPA in children using the same treatment principles outlined for ABPA in asthmatic adults; however, exercising due caution for issues specific to children (LoC: 100%).
Environmental control
Aspergillus is a common mould found in various environments, including soil, decaying vegetation and indoor environments. Evidence suggests that environmental exposures to Aspergillus spores could drive exacerbations [100, 192–194]. The DECG, therefore, suggested minimising activities that could result in the inhalation of large numbers of Aspergillus conidia [195]. If such activities are unavoidable, surgical masks or the more effective N95 respirators may minimise spore inhalation. Further, regular cleaning and maintenance of heating, ventilation and air conditioning (HVAC) systems may prevent mould growth [196–198]. Identifying and promptly addressing water leaks is essential, as damp environments promote mould growth. Similarly, ensuring proper ventilation in bathrooms, kitchens and other areas prone to moisture accumulation may prevent mould growth [199]. Cleaning and dusting living spaces should be performed regularly using damp cloths to minimise the accumulation and dispersion of spores into the air. Indoor air quality monitoring represents a valuable tool to evaluate the fungal exposome [200].
Limitations
Our guidelines have a few limitations. We used a modified Delphi methodology and the third round was non-anonymised. However, this was by design, as we wanted a consensus on most questions, especially the diagnostic criteria. A consensus might not have been achieved if we followed the conventional Delphi for the third round. We tried to ensure the representation of experts from various disciplines. However, nearly half of the experts were from India. One can argue that the agreement for several questions was due to a significant representation from a few countries (India and the UK). However, this was not the case, as we can note that we did not reach even 50% consensus for nearly half of the questions circulated in the first round of Delphi (supplementary table S2), including the most critical aspects of the guidelines (the diagnostic criteria and therapeutics), thus indicating a significant heterogeneity in practice. Finally, most recommendations were consensus-based rather than evidence-based due to the lack of evidence in many areas. However, the Delphi process is ideally suited for such situations.
Future directions
More studies are needed on the community prevalence of fungal sensitisation and ABPA/M to evaluate the need for screening asthmatic subjects for ABPA in primary or secondary care. The prevalent fungus in ABPM must also be evaluated in various geographic locations. The pathogenesis of ABPA needs to be better understood, such as research on airway mucus biology, genetic predisposition and detailed elucidation of the host–pathogen interaction. In the diagnosis of ABPA, most studies have used the FEIA platform to perform immunological investigations. Hence, more studies using different platforms and their comparisons are needed. Increasingly, newer automated platforms are being introduced and would require fresh performance evaluations.
ABPM is under-recognised because of a lack of standardised commercial assays, which need to be developed. The practical application of component-resolved diagnostics requires the development of diagnostic cut-offs and validation from various countries to enable their use in routine care. While we have proposed guidance for differentiating asthma, bronchiectasis and ABPA exacerbations, this may not always be possible. More research is required to evaluate the role of rAsp antigens, sputum Aspergillus PCR and sputum eosinophil count to differentiate between these entities accurately (supplementary figure S1). The treatment of ABPA/M requires detailed evaluation. For instance, the radiological categorisation of ABPA has no clear therapeutic implication. However, future trials should investigate personalised therapy for ABPA, depending on the specific imaging subgroups. Randomised trials are necessary to define the role of various biological agents (as maintenance therapy for glucocorticoid-dependent ABPA and acute ABPA), inhaled antifungals (for acute ABPA as well as for maintenance therapy) and safer glucocorticoids in different patient populations, including CF, children and others. Future studies must also address indoor air quality control using air purifiers, dehumidifiers and others to reduce fungal airway colonisation and improve ABPA outcomes.
Conclusions
We present Delphi consensus guidelines on diagnosing, classifying and treating allergic bronchopulmonary mycoses from the ISHAM-AWG. These guidelines will help bring uniformity in diagnosis and simplify the management of ABPA patients in both clinical care and research.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERJ-00061-2024.Supplement (389.6KB, pdf)
Shareable PDF
Footnotes
Conflict of interest: R. Agarwal has received grants from Cipla Pharmaceuticals, India for conducting research in ABPA. D.W. Denning and family hold founder shares in F2G Ltd, a University of Manchester spin-out antifungal discovery company, and share options in TFF Pharma. He acts or has recently acted as a consultant to Pulmatrix, Pulmocide, Biosergen, TFF Pharmaceuticals, Rostra Therapeutics, Mucpharma PTY and Lifemine Therapeutics. In the last 3 years, he has been paid for talks on behalf of Mundipharma, Bio-Rad, Basilea, Gilead, Avir and Pfizer. He has been involved in multiple guideline groups, primarily focused on diagnostics and aspergillosis. D. Armstrong-James holds share options in Pulmocide Ltd. K. Asano is partially supported by a research grant on allergic disease and immunology from the Japan Agency for Medical Research and Development under grant number 22ek0410097. S.H. Chotirmall has served on advisory boards for CSL Behring, Pneumagen Ltd and Boehringer Ingelheim, on data monitoring boards for Inovio Pharmaceuticals and Imam Abdulrahman Bin Faisal University, and has received personal fees from AstraZeneca and Chiesi Farmaceutici, all unrelated to this work. H.J.F. Salzer has received honoraria for lectures or consulting fees from Ismed, GlaxoSmithKline, AstraZeneca, Advanz Pharma, MSD and Chiesi, all unrelated to this work. J.D. Chalmers has received research grants from AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Gilead Sciences, Grifols, Novartis, Insmed and Trudell, and received consultancy or speaker fees from Antabio, AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Insmed, Janssen, Novartis, Pfizer, Trudell and Zambon. C. Godet has received speaker fees and travel support from Pfizer and MSD, fees for board memberships from SOS Oxygène and Pulmatrix, and grant support from Ohre Pharma, Pfizer, MSD, SOS Oxygène, ISIS Medical, LVL Médical, Oxyvie, Vivisol, Elivie, CF Santé, Boehringer, Sandoz and AstraZeneca. M. Joest has received honoraria for lectures or grants from ALK-Abelló, AstraZeneca, Bencard, Berlin-Chemie, Boehringer Ingelheim, GlaxoSmithKline and HAL Allergy, all unrelated to this work. P. Nair reports grants and personal fees from AstraZeneca, Teva and Sanofi, personal fees from Equillium, Arrowhead Pharma and GlaxoSmithKline, and grants from Foresee and Cyclomedica, outside the submitted work. C.G. Baxter serves on strategic advisory board for Nob Hill Therapeutics Ltd. W.J. Calhoun is a member of the scientific advisory board of Pulmatrix, and that work is unrelated to this publication. O.A. Cornely reports grants from Cidara, F2G, Gilead, MSD, Mundipharma, Pfizer and Scynexis, consulting fees from AiCuris, Basilea, Cidara, Gilead, IQVIA, Matinas, Pfizer, PSI, Pulmocide and Scynexis, honoraria for lectures from Al-Jazeera, Gilead, Knight, MSD, Pfizer and Shionogi, and payment for expert testimony from Cidara. J.A. Douglass has received honoraria for educational presentations from AstraZeneca, GlaxoSmithKline, Novartis and CSL, has served on advisory boards for Sanofi-Aventis, Novartis, GlaxoSmithKline, AstraZeneca, Immunosis and CSL, and has undertaken contracted or investigator-initiated research on behalf of GlaxoSmithKline, Novartis, Immunosis, AstraZeneca, Sanofi-Aventis, Grifols, CSL, BioCryst and Equillium. R. Moss reports consulting fees from the Cystic Fibrosis Foundation, Astellas Pharma Inc., Nob Hill Therapeutics Inc., Pulmatrix, Mayne Pharma, Zambon Company SpA, Aridis Pharmaceuticals Inc., Regeneron Pharmaceuticals Inc. and 4D Molecular Therapeutics, royalties from UpToDate, Inc., and is a member of the board of directors for the Cystic Fibrosis Research Institute. D. Seidel received speaker fees from Pfizer, outside of the submitted work. R. Sprute has received speaker fees from Hikma and Pfizer, and travel support from Pfizer, all outside of the submitted work. The remaining authors have no potential conflicts of interest to disclose.
Support statement: This work was partially supported by the International Society for Human and Animal Mycology. Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Agarwal R, Muthu V, Sehgal IS, et al. Allergic bronchopulmonary aspergillosis. Clin Chest Med 2022; 43: 99–125. doi: 10.1016/j.ccm.2021.12.002 [DOI] [PubMed] [Google Scholar]
- 2.Kosmidis C, Denning DW. The clinical spectrum of pulmonary aspergillosis. Thorax 2015; 70: 270–277. doi: 10.1136/thoraxjnl-2014-206291 [DOI] [PubMed] [Google Scholar]
- 3.Denning DW, Pleuvry A, Cole DC. Global burden of allergic bronchopulmonary aspergillosis with asthma and its complication chronic pulmonary aspergillosis in adults. Med Mycol 2013; 51: 361–370. doi: 10.3109/13693786.2012.738312 [DOI] [PubMed] [Google Scholar]
- 4.Agarwal R, Denning DW, Chakrabarti A. Estimation of the burden of chronic and allergic pulmonary aspergillosis in India. PLoS One 2014; 9: e114745. doi: 10.1371/journal.pone.0114745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maturu VN, Agarwal R. Prevalence of Aspergillus sensitization and allergic bronchopulmonary aspergillosis in cystic fibrosis: systematic review and meta-analysis. Clin Exp Allergy 2015; 45: 1765–1778. doi: 10.1111/cea.12595 [DOI] [PubMed] [Google Scholar]
- 6.Oguma T, Taniguchi M, Shimoda T, et al. Allergic bronchopulmonary aspergillosis in Japan: a nationwide survey. Allergol Int 2018; 67: 79–84. doi: 10.1016/j.alit.2017.04.011 [DOI] [PubMed] [Google Scholar]
- 7.Muthu V, Sehgal IS, Prasad KT, et al. Allergic bronchopulmonary aspergillosis (ABPA) sans asthma: a distinct subset of ABPA with a lesser risk of exacerbation. Med Mycol 2020; 58: 260–263. doi: 10.1093/mmy/myz051 [DOI] [PubMed] [Google Scholar]
- 8.Muthu V, Prasad KT, Sehgal IS, et al. Obstructive lung diseases and allergic bronchopulmonary aspergillosis. Curr Opin Pulm Med 2021; 27: 105–112. doi: 10.1097/MCP.0000000000000755 [DOI] [PubMed] [Google Scholar]
- 9.Sehgal IS, Dhooria S, Prasad KT, et al. Sensitization to A fumigatus in subjects with non-cystic fibrosis bronchiectasis. Mycoses 2021; 64: 412–419. doi: 10.1111/myc.13229 [DOI] [PubMed] [Google Scholar]
- 10.Asano K, Kamei K, Hebisawa A. Allergic bronchopulmonary mycosis – pathophysiology, histology, diagnosis, and treatment. Asia Pac Allergy 2018; 8: e24. doi: 10.5415/apallergy.2018.8.e24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lotvall J, Akdis CA, Bacharier LB, et al. Asthma endotypes: a new approach to classification of disease entities within the asthma syndrome. J Allergy Clin Immunol 2011; 127: 355–360. doi: 10.1016/j.jaci.2010.11.037 [DOI] [PubMed] [Google Scholar]
- 12.Boaventura R, Sibila O, Agusti A, et al. Treatable traits in bronchiectasis. Eur Respir J 2018; 52: 1801269. doi: 10.1183/13993003.01269-2018 [DOI] [PubMed] [Google Scholar]
- 13.Agarwal R, Muthu V, Sehgal IS, et al. Prevalence of Aspergillus sensitization and allergic bronchopulmonary aspergillosis in adults with bronchial asthma: a systematic review of global data. J Allergy Clin Immunol Pract 2023; 11: 1734–1751. doi: 10.1016/j.jaip.2023.04.009 [DOI] [PubMed] [Google Scholar]
- 14.Agarwal R, Chakrabarti A, Shah A, et al. Allergic bronchopulmonary aspergillosis: review of literature and proposal of new diagnostic and classification criteria. Clin Exp Allergy 2013; 43: 850–873. doi: 10.1111/cea.12141 [DOI] [PubMed] [Google Scholar]
- 15.Agarwal R, Maskey D, Aggarwal AN, et al. Diagnostic performance of various tests and criteria employed in allergic bronchopulmonary aspergillosis: a latent class analysis. PLoS One 2013; 8: e61105. doi: 10.1371/journal.pone.0061105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saxena P, Choudhary H, Muthu V, et al. Which are the optimal criteria for the diagnosis of allergic bronchopulmonary aspergillosis? A latent class analysis. J Allergy Clin Immunol Pract 2021; 9: 328–335. doi: 10.1016/j.jaip.2020.08.043 [DOI] [PubMed] [Google Scholar]
- 17.Sehgal IS, Dhooria S, Prasad KT, et al. Comparative diagnostic accuracy of immunoprecipitation versus immunoassay methods for detecting Aspergillus fumigatus-specific IgG in allergic bronchopulmonary aspergillosis: a systematic review and meta-analysis. Mycoses 2022; 65: 866–876. doi: 10.1111/myc.13488 [DOI] [PubMed] [Google Scholar]
- 18.Agarwal R, Dua D, Choudhary H, et al. Role of Aspergillus fumigatus-specific IgG in diagnosis and monitoring treatment response in allergic bronchopulmonary aspergillosis. Mycoses 2017; 60: 33–39. doi: 10.1111/myc.12541 [DOI] [PubMed] [Google Scholar]
- 19.Piarroux RP, Romain T, Martin A, et al. Multicenter evaluation of a novel immunochromatographic test for anti-Aspergillus IgG detection. Front Cell Infect Microbiol 2019; 9: 12. doi: 10.3389/fcimb.2019.00012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hunter ES, Page ID, Richardson MD, et al. Evaluation of the LDBio Aspergillus ICT lateral flow assay for serodiagnosis of allergic bronchopulmonary aspergillosis. PLoS One 2020; 15: e0238855. doi: 10.1371/journal.pone.0238855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sehgal IS, Muthu V, Dhooria S, et al. Sensitivity and specificity of LDBio Aspergillus ICT lateral flow assay for diagnosing allergic bronchopulmonary aspergillosis in adult asthmatics. Mycoses 2024; 67: e13700. doi: 10.1111/myc.13700 [DOI] [PubMed] [Google Scholar]
- 22.Voskamp AL, Gillman A, Symons K, et al. Clinical efficacy and immunologic effects of omalizumab in allergic bronchopulmonary aspergillosis. J Allergy Clin Immunol Pract 2015; 3: 192–199. doi: 10.1016/j.jaip.2014.12.008 [DOI] [PubMed] [Google Scholar]
- 23.Agarwal R, Aggarwal AN, Dhooria S, et al. A randomised trial of glucocorticoids in acute-stage allergic bronchopulmonary aspergillosis complicating asthma. Eur Respir J 2016; 47: 490–498. doi: 10.1183/13993003.01475-2015 [DOI] [PubMed] [Google Scholar]
- 24.Ram B, Aggarwal AN, Dhooria S, et al. A pilot randomized trial of nebulized amphotericin in patients with allergic bronchopulmonary aspergillosis. J Asthma 2016; 53: 517–524. doi: 10.3109/02770903.2015.1127935 [DOI] [PubMed] [Google Scholar]
- 25.Agarwal R, Dhooria S, Sehgal IS, et al. A randomised trial of voriconazole and prednisolone monotherapy in acute-stage allergic bronchopulmonary aspergillosis complicating asthma. Eur Respir J 2018; 52: 1801159. doi: 10.1183/13993003.01159-2018 [DOI] [PubMed] [Google Scholar]
- 26.Agarwal R, Dhooria S, Singh Sehgal I, et al. A randomized trial of itraconazole vs prednisolone in acute-stage allergic bronchopulmonary aspergillosis complicating asthma. Chest 2018; 153: 656–664. doi: 10.1016/j.chest.2018.01.005 [DOI] [PubMed] [Google Scholar]
- 27.Dodamani MH, Muthu V, Thakur R, et al. A randomised trial of vitamin D in acute-stage allergic bronchopulmonary aspergillosis complicating asthma. Mycoses 2019; 62: 320–327. doi: 10.1111/myc.12879 [DOI] [PubMed] [Google Scholar]
- 28.Agarwal R, Muthu V, Sehgal IS, et al. A randomised trial of prednisolone versus prednisolone and itraconazole in acute-stage allergic bronchopulmonary aspergillosis complicating asthma. Eur Respir J 2022; 59: 2101787. doi: 10.1183/13993003.01787-2021 [DOI] [PubMed] [Google Scholar]
- 29.Godet C, Couturaud F, Marchand-Adam S, et al. Nebulised liposomal amphotericin-B as maintenance therapy in allergic bronchopulmonary aspergillosis: a randomised, multicentre trial. Eur Respir J 2022; 59: 2102218. doi: 10.1183/13993003.02218-2021 [DOI] [PubMed] [Google Scholar]
- 30.Akinseye C, Crim C, Newlands A, et al. Efficacy and safety of GSK3772847 in participants with moderate-to-severe asthma with allergic fungal airway disease: a phase IIa randomized, multicenter, double-blind, sponsor-open, comparative trial. PLoS One 2023; 18: e0281205. doi: 10.1371/journal.pone.0281205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Agarwal R, Saxena P, Muthu V, et al. Evaluation of simpler criteria for diagnosing allergic bronchopulmonary aspergillosis complicating asthma. Front Cell Infect Microbiol 2022; 12: 861866. doi: 10.3389/fcimb.2022.861866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Asano K, Hebisawa A, Ishiguro T, et al. New clinical diagnostic criteria for allergic bronchopulmonary aspergillosis/mycosis and its validation. J Allergy Clin Immunol 2021; 147: 1261–1268. doi: 10.1016/j.jaci.2020.08.029 [DOI] [PubMed] [Google Scholar]
- 33.Chalmers JD, Chang AB, Chotirmall SH, et al. Bronchiectasis. Nat Rev Dis Primers 2018; 4: 45. doi: 10.1038/s41572-018-0042-3 [DOI] [PubMed] [Google Scholar]
- 34.Stevens DA, Moss RB, Kurup VP, et al. Allergic bronchopulmonary aspergillosis in cystic fibrosis – state of the art: Cystic Fibrosis Foundation Consensus Conference. Clin Infect Dis 2003; 37: Suppl. 3, S225–S264. doi: 10.1086/376525 [DOI] [PubMed] [Google Scholar]
- 35.Shang Z. Use of Delphi in health sciences research: a narrative review. Medicine 2023; 102: e32829. doi: 10.1097/MD.0000000000032829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chowdhary A, Agarwal K, Kathuria S, et al. Allergic bronchopulmonary mycosis due to fungi other than Aspergillus: a global overview. Crit Rev Microbiol 2014; 40: 30–48. doi: 10.3109/1040841X.2012.754401 [DOI] [PubMed] [Google Scholar]
- 37.Agarwal R, Gupta D. Severe asthma and fungi: current evidence. Med Mycol 2011; 49: Suppl. 1, S150–S157. doi: 10.3109/13693786.2010.504752 [DOI] [PubMed] [Google Scholar]
- 38.Denning DW, Pashley C, Hartl D, et al. Fungal allergy in asthma – state of the art and research needs. Clin Transl Allergy 2014; 4: 14. doi: 10.1186/2045-7022-4-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fukutomi Y, Taniguchi M. Sensitization to fungal allergens: resolved and unresolved issues. Allergol Int 2015; 64: 321–331. doi: 10.1016/j.alit.2015.05.007 [DOI] [PubMed] [Google Scholar]
- 40.Agarwal R, Muthu V, Sehgal IS. Relationship between Aspergillus and asthma. Allergol Int 2023; 72: 507–520. doi: 10.1016/j.alit.2023.08.004 [DOI] [PubMed] [Google Scholar]
- 41.Moss RB. Severe fungal asthma: a role for biologics and inhaled antifungals. J Fungi 2023; 9: 85. doi: 10.3390/jof9010085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Denning DW, O'Driscoll BR, Powell G, et al. Randomized controlled trial of oral antifungal treatment for severe asthma with fungal sensitization: the Fungal Asthma Sensitization Trial (FAST) study. Am J Respir Crit Care Med 2009; 179: 11–18. doi: 10.1164/rccm.200805-737OC [DOI] [PubMed] [Google Scholar]
- 43.Agbetile J, Bourne M, Fairs A, et al. Effectiveness of voriconazole in the treatment of Aspergillus fumigatus-associated asthma (EVITA3 study). J Allergy Clin Immunol 2014; 134: 33–39. doi: 10.1016/j.jaci.2013.09.050 [DOI] [PubMed] [Google Scholar]
- 44.Denning DW, Pfavayi LT. Poorly controlled asthma – easy wins and future prospects for addressing fungal allergy. Allergol Int 2023; 72: 493–506. doi: 10.1016/j.alit.2023.07.003 [DOI] [PubMed] [Google Scholar]
- 45.Arroyave WD, Rabito FA, Carlson JC. The relationship between a specific IgE level and asthma outcomes: results from the 2005–2006 National Health and Nutrition Examination Survey. J Allergy Clin Immunol Pract 2013; 1: 501–508. doi: 10.1016/j.jaip.2013.06.013 [DOI] [PubMed] [Google Scholar]
- 46.Soundappan K, Muthu V, Dhooria S, et al. Population prevalence of allergic bronchopulmonary aspergillosis in asthma: an epidemiological study of 43,261 participants from North India. Clin Exp Allergy 2023; 53: 777–780. doi: 10.1111/cea.14299 [DOI] [PubMed] [Google Scholar]
- 47.Bavbek S, Erkekol FO, Ceter T, et al. Sensitization to Alternaria and Cladosporium in patients with respiratory allergy and outdoor counts of mold spores in Ankara atmosphere, Turkey. J Asthma 2006; 43: 421–426. doi: 10.1080/02770900600710706 [DOI] [PubMed] [Google Scholar]
- 48.Reijula K, Leino M, Mussalo-Rauhamaa H, et al. IgE-mediated allergy to fungal allergens in Finland with special reference to Alternaria alternata and Cladosporium herbarum. Ann Allergy Asthma Immunol 2003; 91: 280–287. doi: 10.1016/S1081-1206(10)63531-4 [DOI] [PubMed] [Google Scholar]
- 49.Kespohl S, Raulf M. Mold sensitization in asthmatic and non-asthmatic subjects diagnosed with extract-based versus component-based allergens. Adv Exp Med Biol 2019; 1153: 79–89. doi: 10.1007/5584_2019_342 [DOI] [PubMed] [Google Scholar]
- 50.Hayes D Jr, Jhaveri MA, Mannino DM, et al. The effect of mold sensitization and humidity upon allergic asthma. Clin Respir J 2013; 7: 135–144. doi: 10.1111/j.1752-699X.2012.00294.x [DOI] [PubMed] [Google Scholar]
- 51.Agarwal R, Muthu V, Sehgal IS, et al. Aspergillus sensitization and allergic bronchopulmonary aspergillosis in asthmatic children: a systematic review and meta-analysis. Diagnostics 2023; 13: 922. doi: 10.3390/diagnostics13050922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Byeon JH, Ri S, Amarsaikhan O, et al. Association between sensitization to mold and impaired pulmonary function in children with asthma. Allergy Asthma Immunol Res 2017; 9: 509–516. doi: 10.4168/aair.2017.9.6.509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gray-Ffrench M, Fernandes RM, Sinha IP, et al. Allergen management in children with type 2-high asthma. J Asthma Allergy 2022; 15: 381–394. doi: 10.2147/JAA.S276994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sehgal IS, Choudhary H, Dhooria S, et al. Prevalence of sensitization to Aspergillus flavus in patients with allergic bronchopulmonary aspergillosis. Med Mycol 2019; 57: 270–276. doi: 10.1093/mmy/myy012 [DOI] [PubMed] [Google Scholar]
- 55.Yoshida Y, Shirai T, Mikamo M, et al. Development of allergic bronchopulmonary aspergillosis with central bronchiectasis over a 10-year period: the need to recheck allergen sensitization. Intern Med 2013; 52: 2135–2138. doi: 10.2169/internalmedicine.52.0069 [DOI] [PubMed] [Google Scholar]
- 56.Watai K, Fukutomi Y, Hayashi H, et al. De novo sensitization to Aspergillus fumigatus in adult asthma over a 10-year observation period. Allergy 2018; 73: 2385–2388. doi: 10.1111/all.13566 [DOI] [PubMed] [Google Scholar]
- 57.Dhooria S, Agarwal R. Diagnosis of allergic bronchopulmonary aspergillosis: a case-based approach. Future Microbiol 2014; 9: 1195–1208. doi: 10.2217/fmb.14.74 [DOI] [PubMed] [Google Scholar]
- 58.Asano K. Allergic bronchopulmonary aspergillosis: pathogenesis, diagnosis, and management. Curr Pulmonol Rep 2023; 12: 91–96. doi: 10.1007/s13665-023-00316-x [DOI] [Google Scholar]
- 59.Agarwal R, Sehgal IS, Muthu V, et al. Allergic bronchopulmonary aspergillosis in India. Clin Exp Allergy 2023; 53: 751–764. doi: 10.1111/cea.14319 [DOI] [PubMed] [Google Scholar]
- 60.Greenberger PA, Bush RK, Demain JG, et al. Allergic bronchopulmonary aspergillosis. J Allergy Clin Immunol Pract 2014; 2: 703–708. doi: 10.1016/j.jaip.2014.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Agarwal R, Aggarwal AN, Sehgal IS, et al. Utility of IgE (total and Aspergillus fumigatus specific) in monitoring for response and exacerbations in allergic bronchopulmonary aspergillosis. Mycoses 2016; 59: 1–6. doi: 10.1111/myc.12423 [DOI] [PubMed] [Google Scholar]
- 62.Patterson R, Wang JLF, Roberts M, et al. Allergic bronchopulmonary aspergillosis: diverse effects of acute phase illness and prednisone on total and specific serum immunoglobins. J Clin Lab Immunol 1979; 2: 199–203. [Google Scholar]
- 63.Patterson R, Greenberger PA, Radin RC, et al. Allergic bronchopulmonary aspergillosis: staging as an aid to management. Ann Intern Med 1982; 96: 286–291. doi: 10.7326/0003-4819-96-3-286 [DOI] [PubMed] [Google Scholar]
- 64.Persat F, Hennequin C, Gangneux JP. Aspergillus antibody detection: diagnostic strategy and technical considerations from the Société Française de Mycologie Médicale (French Society for Medical Mycology) expert committee. Med Mycol 2017; 55: 302–307. doi: 10.1093/mmy/myw078 [DOI] [PubMed] [Google Scholar]
- 65.Richardson MD, Page ID. Aspergillus serology: have we arrived yet? Med Mycol 2017; 55: 48–55. doi: 10.1093/mmy/myw116 [DOI] [PubMed] [Google Scholar]
- 66.Page ID, Richardson MD, Denning DW. Comparison of six Aspergillus-specific IgG assays for the diagnosis of chronic pulmonary aspergillosis (CPA). J Infect 2016; 72: 240–249. doi: 10.1016/j.jinf.2015.11.003 [DOI] [PubMed] [Google Scholar]
- 67.Shinfuku K, Suzuki J, Takeda K, et al. Validity of Platelia Aspergillus IgG and Aspergillus precipitin test to distinguish pulmonary aspergillosis from colonization. Microbiol Spectr 2023; 11: e0343522. doi: 10.1128/spectrum.03435-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hamada Y, Fukutomi Y, Nakatani E, et al. Optimal Aspergillus fumigatus and Asp f 1 serum IgG cut-offs for the diagnosis of allergic bronchopulmonary aspergillosis. Allergol Int 2021; 70: 74–80. doi: 10.1016/j.alit.2020.07.006 [DOI] [PubMed] [Google Scholar]
- 69.Agarwal R, Khan A, Aggarwal AN, et al. Clinical relevance of peripheral blood eosinophil count in allergic bronchopulmonary aspergillosis. J Infect Public Health 2011; 4: 235–243. doi: 10.1016/j.jiph.2011.08.006 [DOI] [PubMed] [Google Scholar]
- 70.Asano K, Ueki S, Tamari M, et al. Adult-onset eosinophilic airway diseases. Allergy 2020; 75: 3087–3099. doi: 10.1111/all.14620 [DOI] [PubMed] [Google Scholar]
- 71.Muniz VS, Silva JC, Braga YAV, et al. Eosinophils release extracellular DNA traps in response to Aspergillus fumigatus. J Allergy Clin Immunol 2018; 141: 571–585. doi: 10.1016/j.jaci.2017.07.048 [DOI] [PubMed] [Google Scholar]
- 72.Agarwal R, Aggarwal AN, Garg M, et al. Cut-off values of serum IgE (total and A. fumigatus-specific) and eosinophil count in differentiating allergic bronchopulmonary aspergillosis from asthma. Mycoses 2014; 57: 659–663. doi: 10.1111/myc.12214 [DOI] [PubMed] [Google Scholar]
- 73.Aziz-Ur-Rehman A, Dasgupta A, Kjarsgaard M, et al. Sputum cell counts to manage prednisone-dependent asthma: effects on FEV1 and eosinophilic exacerbations. Allergy Asthma Clin Immunol 2017; 13: 17. doi: 10.1186/s13223-017-0190-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hargreave FE, Nair P. Point: is measuring sputum eosinophils useful in the management of severe asthma? Yes. Chest 2011; 139: 1270–1273. doi: 10.1378/chest.11-0618 [DOI] [PubMed] [Google Scholar]
- 75.Wark PA, Saltos N, Simpson J, et al. Induced sputum eosinophils and neutrophils and bronchiectasis severity in allergic bronchopulmonary aspergillosis. Eur Respir J 2000; 16: 1095–1101. doi: 10.1034/j.1399-3003.2000.16f13.x [DOI] [PubMed] [Google Scholar]
- 76.Welsh KG, Holden KA, Wardlaw AJ, et al. Fungal sensitization and positive fungal culture from sputum in children with asthma are associated with reduced lung function and acute asthma attacks respectively. Clin Exp Allergy 2021; 51: 790–800. doi: 10.1111/cea.13799 [DOI] [PubMed] [Google Scholar]
- 77.Matsuse H, Tsuchida T, Fukahori S, et al. Dissociation between sensitizing and colonizing fungi in patients with allergic bronchopulmonary aspergillosis. Ann Allergy Asthma Immunol 2013; 111: 190–193. doi: 10.1016/j.anai.2013.06.022 [DOI] [PubMed] [Google Scholar]
- 78.Oguma T, Ishiguro T, Kamei K, et al. Clinical characteristics of allergic bronchopulmonary mycosis caused by Schizophyllum commune. Clin Transl Allergy 2024; 14: e12327. doi: 10.1002/clt2.12327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Meis JF, Chowdhary A, Rhodes JL, et al. Clinical implications of globally emerging azole resistance in Aspergillus fumigatus. Philos Trans R Soc Lond B Biol Sci 2016; 371: 20150460. doi: 10.1098/rstb.2015.0460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Agarwal R, Aggarwal AN, Sehgal IS, et al. Performance of serum galactomannan in patients with allergic bronchopulmonary aspergillosis. Mycoses 2015; 58: 408–412. doi: 10.1111/myc.12334 [DOI] [PubMed] [Google Scholar]
- 81.Sarma UP, Kurup VP, Madan T. Immunodiagnosis of ABPA. Front Biosci 2003; 8: s1187–s1198. doi: 10.2741/991 [DOI] [PubMed] [Google Scholar]
- 82.Crameri R. Recombinant Aspergillus fumigatus allergens: from the nucleotide sequences to clinical applications. Int Arch Allergy Immunol 1998; 115: 99–114. doi: 10.1159/000023889 [DOI] [PubMed] [Google Scholar]
- 83.Muthu V, Sehgal IS, Dhooria S, et al. Utility of recombinant Aspergillus fumigatus antigens in the diagnosis of allergic bronchopulmonary aspergillosis: a systematic review and diagnostic test accuracy meta-analysis. Clin Exp Allergy 2018; 48: 1107–1136. doi: 10.1111/cea.13216 [DOI] [PubMed] [Google Scholar]
- 84.Muthu V, Singh P, Choudhary H, et al. Role of recombinant Aspergillus fumigatus antigens in diagnosing Aspergillus sensitisation among asthmatics. Mycoses 2020; 63: 928–936. doi: 10.1111/myc.13124 [DOI] [PubMed] [Google Scholar]
- 85.Fukutomi Y, Tanimoto H, Yasueda H, et al. Serological diagnosis of allergic bronchopulmonary mycosis: progress and challenges. Allergol Int 2016; 65: 30–36. doi: 10.1016/j.alit.2015.08.004 [DOI] [PubMed] [Google Scholar]
- 86.Muthu V, Singh P, Choudhary H, et al. Diagnostic cutoffs and clinical utility of recombinant Aspergillus fumigatus antigens in the diagnosis of allergic bronchopulmonary aspergillosis. J Allergy Clin Immunol Pract 2020; 8: 579–587. doi: 10.1016/j.jaip.2019.08.041 [DOI] [PubMed] [Google Scholar]
- 87.Tanimoto H, Fukutomi Y, Yasueda H, et al. Molecular-based allergy diagnosis of allergic bronchopulmonary aspergillosis in Aspergillus fumigatus-sensitized Japanese patients. Clin Exp Allergy 2015; 45: 1790–1800. doi: 10.1111/cea.12590 [DOI] [PubMed] [Google Scholar]
- 88.Li BCM, Huh SM, Prieto MD, et al. Biomarkers for the diagnosis of allergic bronchopulmonary aspergillosis in cystic fibrosis: a systematic review and meta-analysis. J Allergy Clin Immunol Pract 2021; 9: 1909–1930. doi: 10.1016/j.jaip.2020.12.064 [DOI] [PubMed] [Google Scholar]
- 89.Agarwal R, Khan A, Garg M, et al. Pictorial essay: allergic bronchopulmonary aspergillosis. Indian J Radiol Imaging 2011; 21: 242–252. doi: 10.4103/0971-3026.90680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Agarwal R, Khan A, Garg M, et al. Chest radiographic and computed tomographic manifestations in allergic bronchopulmonary aspergillosis. World J Radiol 2012; 4: 141–150. doi: 10.4329/wjr.v4.i4.141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Agarwal R, Gupta D, Aggarwal AN, et al. Clinical significance of hyperattenuating mucoid impaction in allergic bronchopulmonary aspergillosis: an analysis of 155 patients. Chest 2007; 132: 1183–1190. doi: 10.1378/chest.07-0808 [DOI] [PubMed] [Google Scholar]
- 92.Agarwal R, Gupta D, Aggarwal AN, et al. Allergic bronchopulmonary aspergillosis: lessons from 126 patients attending a chest clinic in north India. Chest 2006; 130: 442–448. doi: 10.1378/chest.130.2.442 [DOI] [PubMed] [Google Scholar]
- 93.Agarwal R, Khan A, Gupta D, et al. An alternate method of classifying allergic bronchopulmonary aspergillosis based on high-attenuation mucus. PLoS One 2010; 5: e15346. doi: 10.1371/journal.pone.0015346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Benlala I, Klaar R, Gaass T, et al. Non-contrast-enhanced functional lung MRI to evaluate treatment response of allergic bronchopulmonary aspergillosis in patients with cystic fibrosis: a pilot study. J Magn Reson Imaging 2024; 59: 909–919. doi: 10.1002/jmri.28844 [DOI] [PubMed] [Google Scholar]
- 95.Sodhi KS, Gupta P, Shrivastav A, et al. Evaluation of 3 T lung magnetic resonance imaging in children with allergic bronchopulmonary aspergillosis: pilot study. Eur J Radiol 2019; 111: 88–92. doi: 10.1016/j.ejrad.2018.12.021 [DOI] [PubMed] [Google Scholar]
- 96.Dournes G, Berger P, Refait J, et al. Allergic bronchopulmonary aspergillosis in cystic fibrosis: MR imaging of airway mucus contrasts as a tool for diagnosis. Radiology 2017; 285: 261–269. doi: 10.1148/radiol.2017162350 [DOI] [PubMed] [Google Scholar]
- 97.Cosio BG, Shafiek H, Mosteiro M, et al. Redefining the role of bronchoscopy in the workup of severe uncontrolled asthma in the era of biologics: a prospective study. Chest 2023; 164: 837–845. doi: 10.1016/j.chest.2023.03.012 [DOI] [PubMed] [Google Scholar]
- 98.Kodaka N, Nakano C, Oshio T, et al. Effectiveness of mucus plug removal by bronchoscopy for typical high-attenuation mucus with allergic bronchopulmonary mycosis. Allergol Int 2022; 71: 150–152. doi: 10.1016/j.alit.2021.07.001 [DOI] [PubMed] [Google Scholar]
- 99.Agarwal R, Srinivas R, Agarwal AN, et al. Pulmonary masses in allergic bronchopulmonary aspergillosis: mechanistic explanations. Respir Care 2008; 53: 1744–1748. [PubMed] [Google Scholar]
- 100.Hinson KF, Moon AJ, Plummer NS. Broncho-pulmonary aspergillosis; a review and a report of eight new cases. Thorax 1952; 7: 317–333. doi: 10.1136/thx.7.4.317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rosenberg M, Patterson R, Mintzer R, et al. Clinical and immunologic criteria for the diagnosis of allergic bronchopulmonary aspergillosis. Ann Intern Med 1977; 86: 405–414. doi: 10.7326/0003-4819-86-4-405 [DOI] [PubMed] [Google Scholar]
- 102.Nelson LA, Callerame ML, Schwartz RH. Aspergillosis and atopy in cystic fibrosis. Am Rev Respir Dis 1979; 120: 863–873. doi: 10.1164/arrd.1979.120.4.863 [DOI] [PubMed] [Google Scholar]
- 103.Stevens DA, Kan VL, Judson MA, et al. Practice guidelines for diseases caused by Aspergillus. Clin Infect Dis 2000; 30: 696–709. doi: 10.1086/313756 [DOI] [PubMed] [Google Scholar]
- 104.Agarwal R. High attenuation mucoid impaction in allergic bronchopulmonary aspergillosis. World J Radiol 2010; 2: 41–43. doi: 10.4329/wjr.v2.i1.41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Dorsaneo D, Borowitz D, Sharp J, et al. Allergic bronchopulmonary aspergillosis with normal serum IgE in a child with cystic fibrosis. Pediatr Asthma Allergy Immunol 2004; 17: 146–150. doi: 10.1089/0883187041269922 [DOI] [Google Scholar]
- 106.Schwartz RH, Hollick GE. Allergic bronchopulmonary aspergillosis with low serum immunoglobulin E. J Allergy Clin Immunol 1981; 68: 290–294. doi: 10.1016/0091-6749(81)90154-8 [DOI] [PubMed] [Google Scholar]
- 107.Hamburger RN. Allergic bronchopulmonary aspergillosis with normal serum IgE in a child with cystic fibrosis. Pediatr Asthma Allerg Immunol 2005; 18: 46–47. doi: 10.1089/pai.2005.18.46 [DOI] [Google Scholar]
- 108.Agarwal R, Sehgal IS, Dhooria S, et al. Challenging cases in fungal asthma. Med Mycol 2019; 57: Suppl. 2, S110–S117. doi: 10.1093/mmy/myy063 [DOI] [PubMed] [Google Scholar]
- 109.Sehgal IS, Choudhary H, Dhooria S, et al. Is there an overlap in immune response between allergic bronchopulmonary and chronic pulmonary aspergillosis? J Allergy Clin Immunol Pract 2019; 7: 969–974. doi: 10.1016/j.jaip.2018.08.034 [DOI] [PubMed] [Google Scholar]
- 110.Sehgal IS, Dhooria S, Muthu V, et al. Identification of distinct immunophenotypes in chronic pulmonary aspergillosis using cluster analysis. Mycoses 2023; 66: 299–303. doi: 10.1111/myc.13553 [DOI] [PubMed] [Google Scholar]
- 111.Sehgal IS, Dhooria S, Bal A, et al. Allergic bronchopulmonary aspergillosis in an adult with Kartagener syndrome. BMJ Case Rep 2015; 2015: bcr2015211493. doi: 10.1136/bcr-2015-211493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Polverino E, Goeminne PC, McDonnell MJ, et al. European Respiratory Society guidelines for the management of adult bronchiectasis. Eur Respir J 2017; 50: 1700629. doi: 10.1183/13993003.00629-2017 [DOI] [PubMed] [Google Scholar]
- 113.Sehgal IS, Dhooria S, Bal A, et al. A young girl with bronchiectasis and elevated sweat chloride. Chest 2021; 159: e155–e158. doi: 10.1016/j.chest.2020.09.276 [DOI] [PubMed] [Google Scholar]
- 114.Menzies-Gow A, Hoyte FL, Price DB, et al. Clinical remission in severe asthma: a pooled post hoc analysis of the patient journey with benralizumab. Adv Ther 2022; 39: 2065–2084. doi: 10.1007/s12325-022-02098-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Agarwal R, Gupta D, Aggarwal AN, et al. Clinical significance of decline in serum IgE levels in allergic bronchopulmonary aspergillosis. Respir Med 2010; 104: 204–210. doi: 10.1016/j.rmed.2009.09.005 [DOI] [PubMed] [Google Scholar]
- 116.Agarwal R, Aggarwal AN, Garg M, et al. Allergic bronchopulmonary aspergillosis with aspergilloma: an immunologically severe disease with poor outcome. Mycopathologia 2012; 174: 193–201. doi: 10.1007/s11046-012-9535-x [DOI] [PubMed] [Google Scholar]
- 117.Muthu V, Sehgal IS, Prasad KT, et al. Epidemiology and outcomes of allergic bronchopulmonary aspergillosis in the elderly. Mycoses 2022; 65: 71–78. doi: 10.1111/myc.13388 [DOI] [PubMed] [Google Scholar]
- 118.Agarwal R, Sehgal IS, Muthu V, et al. Long-term follow-up of allergic bronchopulmonary aspergillosis treated with glucocorticoids: a study of 182 subjects. Mycoses 2023; 66: 953–959. doi: 10.1111/myc.13640 [DOI] [PubMed] [Google Scholar]
- 119.Reiff DB, Wells AU, Carr DH, et al. CT findings in bronchiectasis: limited value in distinguishing between idiopathic and specific types. AJR Am J Roentgenol 1995; 165: 261–267. doi: 10.2214/ajr.165.2.7618537 [DOI] [PubMed] [Google Scholar]
- 120.Pakzad A, Jacob J. Radiology of bronchiectasis. Clin Chest Med 2022; 43: 47–60. doi: 10.1016/j.ccm.2021.11.004 [DOI] [PubMed] [Google Scholar]
- 121.Patterson R, Greenberger PA, Halwig JM, et al. Allergic bronchopulmonary aspergillosis. Natural history and classification of early disease by serologic and roentgenographic studies. Arch Intern Med 1986; 146: 916–918. doi: 10.1001/archinte.1986.00360170130020 [DOI] [PubMed] [Google Scholar]
- 122.Agarwal R, Garg M, Aggarwal AN, et al. Serologic allergic bronchopulmonary aspergillosis (ABPA-S): long-term outcomes. Respir Med 2012; 106: 942–947. doi: 10.1016/j.rmed.2012.03.001 [DOI] [PubMed] [Google Scholar]
- 123.Kumar R. Mild, moderate, and severe forms of allergic bronchopulmonary aspergillosis: a clinical and serologic evaluation. Chest 2003; 124: 890–892. doi: 10.1378/chest.124.3.890 [DOI] [PubMed] [Google Scholar]
- 124.Mitchell TA, Hamilos DL, Lynch DA, et al. Distribution and severity of bronchiectasis in allergic bronchopulmonary aspergillosis (ABPA). J Asthma 2000; 37: 65–72. doi: 10.3109/02770900009055429 [DOI] [PubMed] [Google Scholar]
- 125.Okada N, Yamamoto Y, Oguma T, et al. Allergic bronchopulmonary aspergillosis with atopic, nonatopic, and sans asthma – factor analysis. Allergy 2023; 78: 2933–2943. doi: 10.1111/all.15820 [DOI] [PubMed] [Google Scholar]
- 126.Phuyal S, Garg MK, Agarwal R, et al. High-attenuation mucus impaction in patients with allergic bronchopulmonary aspergillosis: objective criteria on high-resolution computed tomography and correlation with serologic parameters. Curr Probl Diagn Radiol 2016; 45: 168–173. doi: 10.1067/j.cpradiol.2015.07.006 [DOI] [PubMed] [Google Scholar]
- 127.Denning DW, Cadranel J, Beigelman-Aubry C, et al. Chronic pulmonary aspergillosis: rationale and clinical guidelines for diagnosis and management. Eur Respir J 2016; 47: 45–68. doi: 10.1183/13993003.00583-2015 [DOI] [PubMed] [Google Scholar]
- 128.Lowes D, Chishimba L, Greaves M, et al. Development of chronic pulmonary aspergillosis in adult asthmatics with ABPA. Respir Med 2015; 109: 1509–1515. doi: 10.1016/j.rmed.2015.09.007 [DOI] [PubMed] [Google Scholar]
- 129.Report to the Research Committee of the British Thoracic Association . Inhaled beclomethasone dipropionate in allergic bronchopulmonary aspergillosis. Br J Dis Chest 1979: 73: 349–356. doi: 10.1016/0007-0971(79)90073-1 [DOI] [PubMed] [Google Scholar]
- 130.Agarwal R, Khan A, Aggarwal AN, et al. Role of inhaled corticosteroids in the management of serological allergic bronchopulmonary aspergillosis (ABPA). Intern Med 2011; 50: 855–860. doi: 10.2169/internalmedicine.50.4665 [DOI] [PubMed] [Google Scholar]
- 131.Rosenberg M, Patterson R, Roberts M, et al. The assessment of immunologic and clinical changes occurring during corticosteroid therapy for allergic bronchopulmonary aspergillosis. Am J Med 1978; 64: 599–606. doi: 10.1016/0002-9343(78)90579-X [DOI] [PubMed] [Google Scholar]
- 132.Wark PA, Hensley MJ, Saltos N, et al. Anti-inflammatory effect of itraconazole in stable allergic bronchopulmonary aspergillosis: a randomized controlled trial. J Allergy Clin Immunol 2003; 111: 952–957. doi: 10.1067/mai.2003.1388 [DOI] [PubMed] [Google Scholar]
- 133.Chishimba L, Niven RM, Cooley J, et al. Voriconazole and posaconazole improve asthma severity in allergic bronchopulmonary aspergillosis and severe asthma with fungal sensitization. J Asthma 2012; 49: 423–433. doi: 10.3109/02770903.2012.662568 [DOI] [PubMed] [Google Scholar]
- 134.Jin M, Douglass JA, Elborn JS, et al. Omalizumab in allergic bronchopulmonary aspergillosis: a systematic review and meta-analysis. J Allergy Clin Immunol Pract 2023; 11: 896–905. doi: 10.1016/j.jaip.2022.12.012 [DOI] [PubMed] [Google Scholar]
- 135.Soeda S, Kono Y, Tsuzuki R, et al. Allergic bronchopulmonary aspergillosis successfully treated with benralizumab. J Allergy Clin Immunol Pract 2019; 7: 1633–1635. doi: 10.1016/j.jaip.2018.11.024 [DOI] [PubMed] [Google Scholar]
- 136.Schleich F, Vaia ES, Pilette C, et al. Mepolizumab for allergic bronchopulmonary aspergillosis: report of 20 cases from the Belgian Severe Asthma Registry and review of the literature. J Allergy Clin Immunol Pract 2020; 8: 2412–2413. doi: 10.1016/j.jaip.2020.03.023 [DOI] [PubMed] [Google Scholar]
- 137.Tsubouchi H, Tsuchida S, Yanagi S, et al. Successful treatment with mepolizumab in a case of allergic bronchopulmonary aspergillosis complicated with nontuberculous mycobacterial infection. Respir Med Case Rep 2019; 28: 100875. doi: 10.1016/j.rmcr.2019.100875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Tomomatsu K, Yasuba H, Ishiguro T, et al. Real-world efficacy of anti-IL-5 treatment in patients with allergic bronchopulmonary aspergillosis. Sci Rep 2023; 13: 5468. doi: 10.1038/s41598-023-32246-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Tomomatsu K, Sugino Y, Okada N, et al. Rapid clearance of mepolizumab-resistant bronchial mucus plugs in allergic bronchopulmonary aspergillosis with benralizumab treatment. Allergol Int 2020; 69: 636–638. doi: 10.1016/j.alit.2020.03.003 [DOI] [PubMed] [Google Scholar]
- 140.Matsuura H, Fujiwara K, Omori H, et al. Successful treatment with benralizumab for allergic bronchopulmonary aspergillosis that developed after disastrous heavy rainfall in western Japan. Intern Med 2021; 60: 1443–1450. doi: 10.2169/internalmedicine.6217-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Corren J, Sher L, Zhu X, et al. D201 Dupilumab efficacy in patients with uncontrolled, moderate-to-severe asthma and serologic evidence of allergic bronchopulmonary aspergillosis. Ann Allergy Asthma Immunol 2019; 123: 222–224. doi: 10.1016/j.anai.2019.04.028 [DOI] [PubMed] [Google Scholar]
- 142.Ramonell RP, Lee FE, Swenson C, et al. Dupilumab treatment for allergic bronchopulmonary aspergillosis: a case series. J Allergy Clin Immunol Pract 2020; 8: 742–743. doi: 10.1016/j.jaip.2019.11.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Chishimba L, Langridge P, Powell G, et al. Efficacy and safety of nebulised amphotericin B (NAB) in severe asthma with fungal sensitisation (SAFS) and allergic bronchopulmonary aspergillosis (ABPA). J Asthma 2015; 52: 289–295. doi: 10.3109/02770903.2014.958853 [DOI] [PubMed] [Google Scholar]
- 144.Periselneris J, Nwankwo L, Schelenz S, et al. Posaconazole for the treatment of allergic bronchopulmonary aspergillosis in patients with cystic fibrosis. J Antimicrob Chemother 2019; 74: 1701–1703. doi: 10.1093/jac/dkz075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lebrun-Vignes B, Archer VC, Diquet B, et al. Effect of itraconazole on the pharmacokinetics of prednisolone and methylprednisolone and cortisol secretion in healthy subjects. Br J Clin Pharmacol 2001; 51: 443–450. doi: 10.1046/j.1365-2125.2001.01372.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Varis T, Kaukonen KM, Kivisto KT, et al. Plasma concentrations and effects of oral methylprednisolone are considerably increased by itraconazole. Clin Pharmacol Ther 1998; 64: 363–368. doi: 10.1016/S0009-9236(98)90066-2 [DOI] [PubMed] [Google Scholar]
- 147.Garg VK, Tickoo V, Prasad VP, et al. Iatrogenic Cushing's syndrome in a case of allergic bronchopulmonary aspergillosis treated with oral itraconazole and inhaled budesonide. BMJ Case Rep 2023; 16: e256788. doi: 10.1136/bcr-2023-256788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Dolton MJ, Ray JE, Chen SC, et al. Multicenter study of voriconazole pharmacokinetics and therapeutic drug monitoring. Antimicrob Agents Chemother 2012; 56: 4793–4799. doi: 10.1128/AAC.00626-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Imataki O, Yamaguchi K, Uemura M, et al. Voriconazole concentration is inversely correlated with corticosteroid usage in immunocompromised patients. Transpl Infect Dis 2018; 20: e12886. doi: 10.1111/tid.12886 [DOI] [PubMed] [Google Scholar]
- 150.Agarwal R, Muthu V, Sehgal IS. Clinical manifestation and treatment of allergic bronchopulmonary aspergillosis. Semin Respir Crit Care Med 2024; 45: 114–127. doi: 10.1055/s-0043-1776912 [DOI] [PubMed] [Google Scholar]
- 151.Sehgal IS, Agarwal R. Pulse methylprednisolone in allergic bronchopulmonary aspergillosis exacerbations. Eur Respir Rev 2014; 23: 149–152. doi: 10.1183/09059180.00004813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Agarwal R, Sehgal IS, Muthu V, et al. Estimating the minimal important difference in FEV1 for patients with allergic bronchopulmonary aspergillosis. Eur Respir J 2022; 60: 2201242. doi: 10.1183/13993003.01242-2022 [DOI] [PubMed] [Google Scholar]
- 153.Agarwal R, Sehgal IS, Muthu V, et al. Estimating the clinically important change for Saint George's Respiratory Questionnaire in allergic bronchopulmonary aspergillosis. J Allergy Clin Immunol Pract 2022; 10: 2456–2458. doi: 10.1016/j.jaip.2022.05.029 [DOI] [PubMed] [Google Scholar]
- 154.Pasqualotto AC, Denning DW. Generic substitution of itraconazole resulting in sub-therapeutic levels and resistance. Int J Antimicrob Agents 2007; 30: 93–94. doi: 10.1016/j.ijantimicag.2006.11.027 [DOI] [PubMed] [Google Scholar]
- 155.Niazi-Ali S, Atherton GT, Walczak M, et al. Drug–drug interaction database for safe prescribing of systemic antifungal agents. Ther Adv Infect Dis 2021; 8: 20499361211010605. doi: 10.1177/20499361211010605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ashbee HR, Barnes RA, Johnson EM, et al. Therapeutic drug monitoring (TDM) of antifungal agents: guidelines from the British Society for Medical Mycology. J Antimicrob Chemother 2014; 69: 1162–1176. doi: 10.1093/jac/dkt508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Stevens DA, Schwartz HJ, Lee JY, et al. A randomized trial of itraconazole in allergic bronchopulmonary aspergillosis. N Engl J Med 2000; 342: 756–762. doi: 10.1056/NEJM200003163421102 [DOI] [PubMed] [Google Scholar]
- 158.Nair P, Pizzichini MM, Kjarsgaard M, et al. Mepolizumab for prednisone-dependent asthma with sputum eosinophilia. N Engl J Med 2009; 360: 985–993. doi: 10.1056/NEJMoa0805435 [DOI] [PubMed] [Google Scholar]
- 159.Wenzel S, Ford L, Pearlman D, et al. Dupilumab in persistent asthma with elevated eosinophil levels. N Engl J Med 2013; 368: 2455–2466. doi: 10.1056/NEJMoa1304048 [DOI] [PubMed] [Google Scholar]
- 160.Bleecker ER, FitzGerald JM, Chanez P, et al. Efficacy and safety of benralizumab for patients with severe asthma uncontrolled with high-dosage inhaled corticosteroids and long-acting β2-agonists (SIROCCO): a randomised, multicentre, placebo-controlled phase 3 trial. Lancet 2016; 388: 2115–2127. doi: 10.1016/S0140-6736(16)31324-1 [DOI] [PubMed] [Google Scholar]
- 161.Corren J, Parnes JR, Wang L, et al. Tezepelumab in adults with uncontrolled asthma. N Engl J Med 2017; 377: 936–946. doi: 10.1056/NEJMoa1704064 [DOI] [PubMed] [Google Scholar]
- 162.Albogami S. Use of biologic drugs for treatment of allergic bronchopulmonary aspergillosis. Int J Pulmon Respir Sci 2021; 5: 555663. doi: 10.19080/IJOPRS.2021.05.555663 [DOI] [Google Scholar]
- 163.Soeda S, To M, Kono Y, et al. Case series of allergic bronchopulmonary aspergillosis treated successfully and safely with long-term mepolizumab. Allergol Int 2019; 68: 377–379. doi: 10.1016/j.alit.2018.12.008 [DOI] [PubMed] [Google Scholar]
- 164.Mümmler C, Kemmerich B, Behr J, et al. Differential response to biologics in a patient with severe asthma and ABPA: a role for dupilumab? Allergy Asthma Clin Immunol 2020; 16: 55. doi: 10.1186/s13223-020-00454-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Ogata H, Sha K, Kotetsu Y, et al. Tezepelumab treatment for allergic bronchopulmonary aspergillosis. Respirol Case Rep 2023; 11: e01147. doi: 10.1002/rcr2.1147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Koutsokera A, Corriveau S, Sykes J, et al. Omalizumab for asthma and allergic bronchopulmonary aspergillosis in adults with cystic fibrosis. J Cyst Fibros 2020; 19: 119–124. doi: 10.1016/j.jcf.2019.07.011 [DOI] [PubMed] [Google Scholar]
- 167.Reddel HK, Bacharier LB, Bateman ED, et al. Global Initiative for Asthma Strategy 2021: executive summary and rationale for key changes. Eur Respir J 2022; 59: 2102730. doi: 10.1183/13993003.02730-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Agarwal R, Singh N, Gupta D. Pulmonary hypertension as a presenting manifestation of allergic bronchopulmonary aspergillosis. Indian J Chest Dis Allied Sci 2009; 51: 37–40. [PubMed] [Google Scholar]
- 169.Hogan C, Denning DW. Allergic bronchopulmonary aspergillosis and related allergic syndromes. Semin Respir Crit Care Med 2011; 32: 682–692. doi: 10.1055/s-0031-1295716 [DOI] [PubMed] [Google Scholar]
- 170.Knutsen AP, Bush RK, Demain JG, et al. Fungi and allergic lower respiratory tract diseases. J Allergy Clin Immunol 2012; 129: 280–291. doi: 10.1016/j.jaci.2011.12.970 [DOI] [PubMed] [Google Scholar]
- 171.Serisier DJ, Martin ML. Long-term, low-dose erythromycin in bronchiectasis subjects with frequent infective exacerbations. Respir Med 2011; 105: 946–949. doi: 10.1016/j.rmed.2011.01.009 [DOI] [PubMed] [Google Scholar]
- 172.Leard LE, Holm AM, Valapour M, et al. Consensus document for the selection of lung transplant candidates: an update from the International Society for Heart and Lung Transplantation. J Heart Lung Transplant 2021; 40: 1349–1379. doi: 10.1016/j.healun.2021.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Agustí A, Celli BR, Criner GJ, et al. Global Initiative for Chronic Obstructive Lung Disease 2023 report: GOLD executive summary. Eur Respir J 2023; 61: 2300239. doi: 10.1183/13993003.00239-2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Jindal SK, Agarwal R. Long-term oxygen therapy. Expert Rev Respir Med 2012; 6: 639–649. doi: 10.1586/ers.12.69 [DOI] [PubMed] [Google Scholar]
- 175.Kosmidis C, Powell G, Borrow R, et al. Response to pneumococcal polysaccharide vaccination in patients with chronic and allergic aspergillosis. Vaccine 2015; 33: 7271–7275. doi: 10.1016/j.vaccine.2015.10.114 [DOI] [PubMed] [Google Scholar]
- 176.Sehgal IS, Dhooria S, Thurai Prasad K, et al. Pregnancy complicated by allergic bronchopulmonary aspergillosis: a case-control study. Mycoses 2021; 64: 35–41. doi: 10.1111/myc.13180 [DOI] [PubMed] [Google Scholar]
- 177.De Santis M, Di Gianantonio E, Cesari E, et al. First-trimester itraconazole exposure and pregnancy outcome: a prospective cohort study of women contacting teratology information services in Italy. Drug Saf 2009; 32: 239–244. doi: 10.2165/00002018-200932030-00006 [DOI] [PubMed] [Google Scholar]
- 178.Namazy JA, Blais L, Andrews EB, et al. Pregnancy outcomes in the omalizumab pregnancy registry and a disease-matched comparator cohort. J Allergy Clin Immunol 2020; 145: 528–536. doi: 10.1016/j.jaci.2019.05.019 [DOI] [PubMed] [Google Scholar]
- 179.Shakuntulla F, Chiarella SE. Safety of biologics for atopic diseases during pregnancy. J Allergy Clin Immunol Pract 2022; 10: 3149–3155. doi: 10.1016/j.jaip.2022.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Elphick HE, Southern KW. Antifungal therapies for allergic bronchopulmonary aspergillosis in people with cystic fibrosis. Cochrane Database Syst Rev 2016; 11: CD002204. doi: 10.1002/14651858.CD002204.pub5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Jat KR, Walia DK, Khairwa A. Anti-IgE therapy for allergic bronchopulmonary aspergillosis in people with cystic fibrosis. Cochrane Database Syst Rev 2018; 3: CD010288. doi: 10.1002/14651858.CD010288.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Moss RB. Allergic bronchopulmonary aspergillosis and Aspergillus infection in cystic fibrosis. Curr Opin Pulm Med 2010; 16: 598–603. doi: 10.1097/MCP.0b013e32833e24a6 [DOI] [PubMed] [Google Scholar]
- 183.Boyle M, Moore JE, Whitehouse JL, et al. The diagnosis and management of respiratory tract fungal infection in cystic fibrosis: a UK survey of current practice. Med Mycol 2019; 57: 155–160. doi: 10.1093/mmy/myy014 [DOI] [PubMed] [Google Scholar]
- 184.Gothe F, Schmautz A, Häusler K, et al. Treating allergic bronchopulmonary aspergillosis with short-term prednisone and itraconazole in cystic fibrosis. J Allergy Clin Immunol Pract 2020; 8: 2608–2614. doi: 10.1016/j.jaip.2020.02.031 [DOI] [PubMed] [Google Scholar]
- 185.Abbotsford J, Foley DA, Goff Z, et al. Clinical experience with SUBA-itraconazole at a tertiary paediatric hospital. J Antimicrob Chemother 2021; 76: 249–252. doi: 10.1093/jac/dkaa382 [DOI] [PubMed] [Google Scholar]
- 186.Tanou K, Zintzaras E, Kaditis AG. Omalizumab therapy for allergic bronchopulmonary aspergillosis in children with cystic fibrosis: a synthesis of published evidence. Pediatr Pulmonol 2014; 49: 503–507. doi: 10.1002/ppul.22937 [DOI] [PubMed] [Google Scholar]
- 187.Ashkenazi M, Sity S, Sarouk I, et al. Omalizumab in allergic bronchopulmonary aspergillosis in patients with cystic fibrosis. J Asthma Allergy 2018; 11: 101–107. doi: 10.2147/JAA.S156049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Bentley S, Gupta A, Balfour-Lynn IM. Subtherapeutic itraconazole and voriconazole levels in children with cystic fibrosis. J Cyst Fibros 2013; 12: 418–419. doi: 10.1016/j.jcf.2012.09.007 [DOI] [PubMed] [Google Scholar]
- 189.Mathew JL, Kumar K, Agrawal S, et al. Evidence-based guidelines for the management of allergic bronchopulmonary aspergillosis (ABPA) in children and adolescents with asthma. Indian J Pediatr 2023; 90: 708–717. doi: 10.1007/s12098-023-04592-y [DOI] [PubMed] [Google Scholar]
- 190.Licari A, Marseglia A, Caimmi S, et al. Omalizumab in children. Pediatric Drugs 2014; 16: 491–502. doi: 10.1007/s40272-014-0107-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Jackson DJ, Bacharier LB, Gergen PJ, et al. Mepolizumab for urban children with exacerbation-prone eosinophilic asthma in the USA (MUPPITS-2): a randomised, double-blind, placebo-controlled, parallel-group trial. Lancet 2022; 400: 502–511. doi: 10.1016/S0140-6736(22)01198-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Henderson AH. Allergic aspergillosis: review of 32 cases. Thorax 1968; 23: 501–512. doi: 10.1136/thx.23.5.501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Kramer MN, Kurup VP, Fink JN. Allergic bronchopulmonary aspergillosis from a contaminated dump site. Am Rev Respir Dis 1989; 140: 1086–1088. doi: 10.1164/ajrccm/140.4.1086 [DOI] [PubMed] [Google Scholar]
- 194.Allmers H, Huber H, Baur X. Two year follow-up of a garbage collector with allergic bronchopulmonary aspergillosis (ABPA). Am J Ind Med 2000; 37: 438–442. doi: [DOI] [PubMed] [Google Scholar]
- 195.Gangneux JP, Godet C, Denning DW. Allergic diseases and fungal exposome: prevention is better than cure. Allergy 2022; 77: 3182–3184. doi: 10.1111/all.15436 [DOI] [PubMed] [Google Scholar]
- 196.Gangneux JP, Bouvrais M, Frain S, et al. Asthma and indoor environment: usefulness of a global allergen avoidance method on asthma control and exposure to molds. Mycopathologia 2020; 185: 367–371. doi: 10.1007/s11046-019-00417-9 [DOI] [PubMed] [Google Scholar]
- 197.Okada N, Shiraishi Y, Tomomatsu K, et al. Moldy odor from air conditioners in the residences of Japanese participants with and without asthma. Indoor Air 2022; 32: e13156. doi: 10.1111/ina.13156 [DOI] [PubMed] [Google Scholar]
- 198.Shiraishi Y, Harada K, Maeda C, et al. A method to evaluate and eliminate fungal contamination in household air conditioners. Indoor Air 2023; 2023: 8984619. doi: 10.1155/2023/8984619 [DOI] [Google Scholar]
- 199.Le Cann P, Paulus H, Glorennec P, et al. Home environmental interventions for the prevention or control of allergic and respiratory diseases: what really works. J Allergy Clin Immunol Pract 2017; 5: 66–79. doi: 10.1016/j.jaip.2016.07.011 [DOI] [PubMed] [Google Scholar]
- 200.Gangneux JP, Sassi M, Lemire P, et al. Metagenomic characterization of indoor dust bacterial and fungal microbiota in homes of asthma and non-asthma patients using next generation sequencing. Front Microbiol 2020; 11: 1671. doi: 10.3389/fmicb.2020.01671 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERJ-00061-2024.Supplement (389.6KB, pdf)
This one-page PDF can be shared freely online.
Shareable PDF ERJ-00061-2024.Shareable (597.5KB, pdf)