Abstract
Background
Colorectal cancer is a disease of unmet medical need. Although extracellular vesicles (EVs) have been implicated in anti-tumor responses, discrepancies were observed among studies. We analyzed the role of tumor-derived EVs (TEVs) in tumor progression in vivo by focusing on regulatory T (Treg) cells, which play essential roles in tumor development and progression.
Methods
A mouse model of colorectal cancer lung metastasis was generated using BALB/c mice by tail vein injection of the BALB/c colon adenocarcinoma cell line Colon-26. TEVs derived from Colon-26 and BALB/c lung squamous cell carcinoma ASB-XIV were retrieved from the culture media supernatants. A TEV equivalent to 10 µg protein was injected every other day for 2 weeks.
Results
Histology and immunohistochemistry studies revealed that lung tumors reduced in the Colon-26-EV group when compared to the phosphate-buffered saline (PBS) group. The population of CD4 + FoxP3 + cells in the lung was upregulated in the PBS group mice when compared to the healthy mice (P < 0.001), but was significantly downregulated in the Colon-26-EV group mice when compared to the PBS group mice (P < 0.01). Programmed cell death protein 1, glucocorticoid-induced TNFR-related protein, and CD69 expression in lung Treg cells were markedly upregulated in the PBS group when compared to the healthy mice, but downregulated in the Colon-26-EV group when compared to the PBS group. The changes in expression were dose-dependent for Colon-26-EVs. ASB-EVs also led to significantly downregulated Treg cell expression, although non-cancer line 3T3-derived EVs did not.
Conclusion
Our study suggests that TEVs possess components for tumor suppression.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00262-023-03517-0.
Keywords: Extracellular vesicle, Colorectal cancer, Regulatory T cell, Tumor-derived extracellular vesicles, Tumor progression
Introduction
Colorectal cancer (CRC) still has a high incidence and mortality among malignant neoplasms worldwide [1]. In the last half-century, the mortality of CRC has been declining, owing to improvements in treatment and screening methods [2]. Although advances in metastatic or unresectable CRC treatments have improved the survival rate, the prognosis is still unfavorable [3]. Immune checkpoint inhibitors (ICIs), such as nivolumab, ipilimumab, and pembrolizumab, are emerging in colorectal cancer chemotherapy as immunotherapy agents [4], suggesting that regulatory markers on T cells could be therapeutic targets. Moreover, FoxP3 + regulatory T (Treg) cells play a role in the immune suppression of T cell responses to neoplasms [5]. FoxP3 + Treg cell infiltration into the tumor site negatively correlates with overall survival [6]. However, high microsatellite instability or DNA mismatch repair deficiencies restrict the indication and efficacy of ICI.
Extracellular vesicles (EVs) are lipid-bound nano- to micro-sized particles secreted from a myriad of cell populations and engage in intercellular communication by conveying cytosolic proteins, lipids, and nucleic acids to recipient cells [7]. EVs maintain homeostasis under normal physiological conditions, such as tissue repair and immune cell regulation [8, 9], and are involved in the pathogenesis of various diseases, including malignancies. In the last two decades, tumor-derived EVs (TEVs) have been shown to play a pivotal role in cancer progression, including cell proliferation, angiogenesis, invasion, extravasation, premetastatic niche formation, and host immune evasion [10–15]. However, TEVs possess the potential to promote anti-cancer immunity by providing tumor antigens to dendritic cells (DCs), thus leading to anti-tumor immunity by cytotoxic T cells [16]. EVs derived from malignant tumor cells promote antigen-specific anti-tumor immune responses [17]. This antithetical behavior of TEVs in tumor immunity prompted us to analyze the role of TEVs in tumor progression in vivo.
Since controlling Treg cells leads to CRC treatment, it is crucial to investigate the phenotypic changes in Treg cells caused by TEVs. Although intradermal injection of tumor cells, such as in a xenograft model, is a commonly used experimental technique to determine the therapeutic effect of anti-cancer agents in vivo, it may not reflect the tumor environment because cutaneous metastasis is not a common manifestation in late-stage CRC [18].
Therefore, we generated experimental metastatic lung tumors in CRC mice and analyzed whether TEVs affect tumor progression in vivo in terms of phenotypic changes in lung Treg cells.
Materials and methods
Animals
BALB/c.Cg-Foxp3tm2Tch/J mice were purchased from Jackson Laboratory (Bar Harbor, Marine, USA). All mice were maintained under specific pathogen-free conditions at 22 °C under a 12 h light/dark cycle and received food and water ad libitum in the animal facility at Kansai Medical University (Osaka, Japan). All experiments were performed according to the Guidelines for the Care and Use of Laboratory Animals of the National Institutes of Health, following approval from the Animal Care and Use Committee of Kansai Medical University (#18–115).
Cell lines
BALB/c murine colon carcinoma Colon-26 and lung squamous cell carcinoma ASB-XIV lines were purchased from the Cell Line Service GmbH (Eppelheim, Germany). BALB/c murine fibroblast cells from the BALB/3T3 clone A31 were purchased from ATCC (Manassas, VA, USA). Colon-26 cells were cultured in Roswell Park Memorial Institute-1640 medium. ASB-XIV and 3T3 cells were cultured in Dulbecco’s Modified Eagle’s Medium supplemented with 10% exosome-depleted fetal bovine serum (FBS) and penicillin–streptomycin. All products for cell culture were purchased from Thermo Fisher Scientific (Waltham, MA, USA). Cell lines were cultured in a humidified incubator with 5% CO2 at 37 °C. FBS was deprived of bovine extracellular vesicles by ultracentrifugation at 1,00,000 × g for 70 min. All cell lines were regularly tested for mycoplasma using a mycoplasma detection assay (Lonza, Basel, Switzerland) and were found to be negative.
Extracellular vesicles isolation and quantification
Cell culture supernatants were retrieved at 48 h after reaching 80% confluency, centrifuged at 500 × g for 10 min at 4 °C to remove cell debris, and then pooled via filtration using a 0.2 µm pore filter (Thermo Fisher Scientific). Samples were centrifuged for 20 min at 12,000 × g at 4 °C to remove non-EV components. Next, EVs were retrieved as a pellet by ultracentrifugation at 1,00,000 × g for 70 min, followed by washing with phosphate-buffered saline (PBS) and another round of ultracentrifugation. These procedures were performed as per the work of Hoshino et al. [13]. EVs were concentrated using a nanomembrane concentrator Vivaspin20 100,000 MWCO polyether sulfone (Sartorius, Göttingen, Germany), and EV protein was quantified using a BCA protein assay kit (Thermo Fisher Scientific). Samples were characterized by a nanoparticle tracking analysis (NTA) assay using NanoSight LM10 (Malvern Panalytical, Worcestershire, UK), western blotting using anti-CD9 (ab92726; Abcam, Cambridge, UK), -CD63 (ab217345; Abcam), -CD81 antibody (ab109201; Abcam), anti-TSG1 (bs-1365R), anti-apolipoprotein A1 (66,206-1-Ig, Proteintech, IL, USA), and anti-GRP94 (bs-0194R, MA, USA), and transmission electron microscopy (TEM) using a HITACHI H-7600 (Hitachi, Tokyo, Japan). The primary and secondary antibodies for western blots [anti-CD9 (1:500), anti-CD63 (1:500), anti-CD81 (1:500), anti-tumor susceptibility gene 101 (TSG101) (1:500), anti-apolipoprotein A1 (1:1000), anti-GRP94 (1:500), anti-rabbit IgG (1:2000), and anti-mouse IgG (1:2000)] were diluted with 2% skim milk in 0.1% Tween/PBS. The procedures for isolation, quantification, and qualification of extracellular vesicles complied with the minimal information for studies of extracellular vesicles 2018 guideline [19].
Metastatic lung cancer model
The metastatic lung cancer model was induced by injecting 2 × 105 Colon-26 cells diluted in 200 µL PBS into the lateral tail vein of 6–8-week-old mice before or after injecting TEVs every other day. Fourteen days after Colon-26 injection, the mice were euthanized, and lung specimens were extracted for further experiments (n = 7 each).
Antibodies and reagents
Fluorescein isothiocyanate (FITC)-, phycoerythrin (PE)-, PerCP-Cyanide5.5-, or allophycocyanin (APC)-conjugated antibodies specific to CD3 (17A2), CD4 (RM4-5), CD11b (M1/70), CD11c (N418), B220 (RA3-6B2), CD152 (UC10-4B9), programmed cell death protein 1 (PD-1; RMP1-390), and glucocorticoid-induced TNFR-related protein (GITR; DTA) were purchased from eBiosciences (San Diego, CA, USA). CD8 (53–6.7) was purchased from BD Pharmingen (Franklin Lakes, New Jersey, USA) and CD69 (H1.2F3) was purchased from Biolegend (San Diego, CA, USA). Fluorescent dye PKH26 and carboxyfluorescein succinimidyl ester (CFSE) were procured from Sigma-Aldrich (St. Louis, MO, USA) and Dojindo (Kumamoto, Japan), respectively. For EV labeling, Colon-26-EVs in PBS was added to an equal volume of 4 μM PKH-26, incubated for 5 min at 24 ℃ and washed four times with PBS using Vivaspin20 100,000 MWCO. Flow cytometry was performed using BD FACSCalibur (BD Biosciences).
Cell preparation
Lung mononuclear cells for flow cytometry were isolated according to previous reports [20, 21] with slight modifications. Briefly, lung specimens were minced into 1 mm3 pieces and digested using 1 mg/mL of collagenase IV (Sigma-Aldrich, St. Louis, MA, USA), followed by red blood cell lysis (Abcam). Treg cells and CD11b + cells were isolated using the CD4 + CD25 + Regulatory T Cell Isolation Kit and CD11b MicroBeads (Miltenyi Biotec, Bergisch Gladbach, Germany), respectively, according to the manufacturer’s instructions.
Images
Lung specimens were fixed with 10% formalin, embedded in paraffin, sectioned, and stained with hematoxylin and eosin (H&E). Digital images of the lungs were acquired using an Olympus BX53 microscope with the cellSends standard software (Olympus, Tokyo, Japan). The tumor area of the H&E slides was manually measured using inFom Cell Analysis (Kikotech, Osaka, Japan). The embedded specimens were deparaffinized and rehydrated in xylene and ethanol. Primary antibodies, anti-mouse CD3 (ab11089, abcam) (1:50), CD4 (ab183685, abcam) (1:100), CD8 (ab203035, abcam), Foxp3 (ab215206, abcam) or (14-5773-82, Thermo fisher scientific) (1:50), B220 (14-0452-81, Thermo fisher scientific) (1:200), CD11b (ab1333357, abcam) (1:4000), and CD11c (M100-6, MBL International, MA, USA) (1:100), were diluted in tris-buffered saline with 0.1% Tween-20 and incubated at 4 °C in a humidified chamber. The appropriate species-specific AlexaFluor (488 or 568)-conjugated antibodies (Thermo Fisher Scientific) (1:200) were used as the secondary antibodies. Lung images were captured using an Olympus BX53 fluorescence microscope (Olympus).
CFSE-labeled CD11b + cells from metastatic lungs were incubated with PKH26-labeled Colon-26-EVs in a humidified incubator with 5% CO2 at 37 °C for 24 h. Images of Colon-26-EV uptake by CD11b + cells were captured using Dragonfly (Andor Technologies, UK).
In-solution digestion of EVs and identification of peptides using LC-MALDI-MS and -MS/MS
Isolated EVs (20 μg) in reaction buffer (0.1% SDS, 50 mM Tris–HCl (pH 8.0), and 5 mM DTT) were treated with 15 mM iodoacetamide for alkylation for 30 min at room temperature in the dark, and then mixed with dithiothreitol (final concentration = 10 mM) to stop the reaction. The samples were digested with 10 μg/mL Trypsin Gold (Promega Wisconsin, USA) and 10 μg/mL Lysyl Endopeptidase (FUJIFILM Wako Pure Chemical, Osaka, Japan) overnight at 37 °C. The digested peptide solution was treated using a Detergent Removal Spin Column (Thermo Scientific Pierce Protein Biology, Massachusetts, USA) to remove detergent and a Bond Elut C18 cartridge (Agilent Technology, California, USA) to remove salts. The elute was dried using vacuum centrifugation and dissolved in a solution containing 5% acetonitrile and 0.1% trifluoroacetic acid. LC-MALDI-MS and -MS/MS analyses were performed as previously described [22] with slight modifications. Briefly, the digested peptides were separated and spotted onto a 384-well MALDI plate using a nano LC system Dina (TECHNOALPHA, Tokyo, Japan) and analyzed using a TOF/TOF 5800 instrument with the LC-MALDI setting (AB SCIEX, Tokyo, Japan) and ProteinPilot software (AB SCIEX) to identify proteins contained in EVs.
In vitro culture of splenic Treg cells
Lung CD11b + cells were cultured in 1 mL of cRPMI with or without 2 μg of Colon-26-EV in a humidified incubator with 5% CO2 at 37 °C for 24 h. Culture media were retrieved and applied to Vivaspin20 100,000 MWCO to remove Colon-26 EVs. Splenic Treg cells were cultured in 1 mL of cRPMI with PBS, 2 μg of Colon-26-EVs, and 1 mL of culture media from CD11b for 24 h. Treg cell phenotypes were analyzed using Attune NxT Flow Cytometer (Thermo Fisher Scientific).
Statistical analysis
One-way analysis of variance, Mann–Whitney U test, and linear regression analysis were used for data analysis, and P values < 0.05 were considered statistically significant. All calculations were performed using EZR version 1.61 (Jichi Medical University, Saitama, Japan).
Results
Characteristics of TEVs
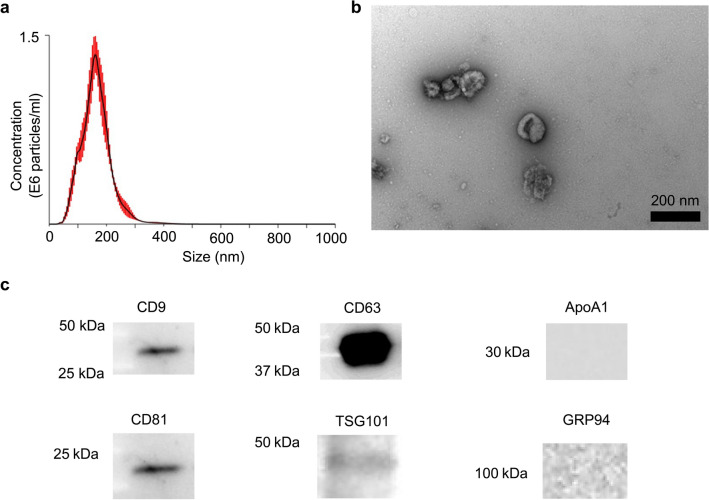
EVs derived from Colon-26 cell lines (Colon-26-EVs) were isolated from the cell culture supernatant and characterized using NTA, TEM, and western blotting (Fig. 1a). NTA and TEM revealed that Colon-26-EVs were small particles in the range of 163 ± 49 nm (Fig. 1a) with oval-shaped morphology (Fig. 1b). Western blotting showed that Colon-26-EVs expressed CD9, CD63, CD81, and TSG101, and did not express apolipoprotein A1 and GRP94 (Fig. 1c).
Fig. 1.
Characteristics of the tumor-derived EVs isolated from the cell culture supernatant of the Colon-26 cell lines. Colon-26-EVs were characterized using NTA to quantify the size of particles (a), TEM (b), and (c) western blot for detecting the expression of CD9, CD63, CD81, and TSG101, and the absence of ApoA1 and GRP94. The black bar indicates the 200 µm scale in (b). Figures are representative of five independent isolations of EVs
Colon-26 EVs reduced metastatic lung tumors
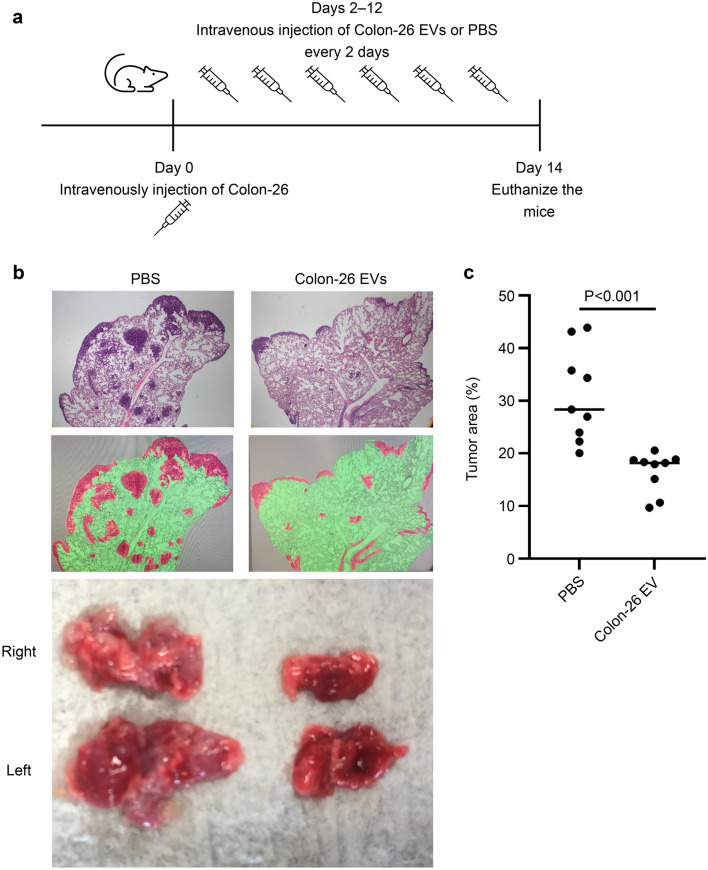
To determine how robustly TEVs function in tumor immunity in vivo, BALB/c mice were injected with Colon-26 cells via the tail vein to promote lung metastasis, injected with Colon-26-EVs equivalent to 10 µg protein every other day for 2 weeks, and then euthanized (Fig. 2a). We confirmed that the Colon-26 cell line was engrafted in the lungs by intravenous injection, thus forming a mass throughout the lungs (Fig. 2b). In contrast, the growth of these lung tumors was suppressed by Colon-26-EVs (Fig. 2b, c).
Fig. 2.
Impact of robust and repeated administration of Colon-26-EVs on lung tumor growth. (a) Experimental design of TEV-injection into metastatic lung tumor mice. Mice were injected with Colon-26 cells via the tail vein to promote lung metastasis, followed by Colon-26-EVs equivalent to 10 µg protein or PBS injections every other day for 2 weeks, and then euthanized. (b) Pathological findings were compared between the PBS (left) and Colon-26-EVs (right)-treated groups. The upper panels are the representative pathological images of each group. The lower panels visualize the tumor sites (red) and normal lungs (green). (c) Tumor sites are compared with the ratio of red/green in (b). Data were derived from nine randomized views of three independent experiments in each group
Colon-26 reduced EVs regulatory T cells in metastatic lung tumors
Given that tumor cells can evade tumor immunity, including the recruitment of Treg cells in the tumor microenvironment (TME) [23], we evaluated the localization of Treg cells in metastatic lung tissue. Immunohistochemistry revealed the recruitment of T cells and a small proportion of Treg cells in the tumor sites (Fig. 3a). In addition to Treg cells, various cell types, including CD4 + , CD8 + , B220 + , CD11b + , and CD11c + cells, were also present and active within the TME (Fig. 3a). In this mouse model, Colon-26-EVs reduced the proportion of Treg cells (Fig. 3b).
Fig. 3.
Identification of Treg cells in metastatic lung tumors. a Immunofluorescence staining reveals the presence of FoxP3 + Treg cells (green) and CD3 + , CD4 + , CD8 + , B220 + , CD11b + , and CD11c + (red) at the tumor sites of the lung (PBS group) (× 200). The right panel shows the highlight of T cells in the area surrounded by the dashed line in (a). b Immunofluorescence staining was performed to compare FoxP3 + Treg cells between the PBS and Colon-26-EVs group. Arrowheads indicate FoxP3 + Treg cells. The bar graph illustrates the ratio of FoxP3+ Treg cells to CD4 + T cells in lung tumor sites. Data were derived from nine randomized views of three independent experiments in each group
Colon-26-EVs attenuate the Treg phenotype in the TME
Flow cytometry also revealed that Colon-26-EVs reduced the Treg cell proportion in the metastatic lung (Fig. 4a). We then estimated the ability of Colon-26-EVs to alter the lung Tregs phenotype in TME, including regulatory molecules CTLA-4, PD-1, and GITR, and the expression of the activation marker CD69 in Treg cells. Although TME augmented the expression of CTLA-4, PD1, GITR, and CD69 in Treg cells, Colon-26-EVs inhibited this upregulation (Fig. 4b). Moreover, the downregulation of these markers and the reduction of lung weight indicated that the tumor volume changed based on the dose of Colon-26-EVs (Fig. 4c). These effects of regulatory phenotype downregulation by Colon-26-EVs were absent in normal mice (Supplementary Fig. 1). These data indicated that colon cancer cells altered TME with regulatory T cell augmentation that Colon-26-EVs could attenuate.
Fig. 4.
Reduced regulatory marker expression of Treg cells in metastatic lung tumors derived from Colon-26 cells by Colon-26-EVs. The regulatory phenotypes of Treg cells were compared among healthy controls and metastatic lung tumor mice with and without the administration of 10 µg Colon-26-EVs every 2 days. a Proportion of lung FoxP3 + Treg cells and b expression of CTLA-4, PD-1, GITR, and CD69 on Treg cells were measured using flow cytometry. The dot plot in (a) represents the percentage of FoxP3 + Treg cells/CD4 + T cells in Colon-26 injected mice. The bar graphs (lower) of (a) and (b) represent the percentage of FoxP3 + Treg cells/CD4 + T cells and the mean fluorescence intensity with SD, respectively (n = 7 per group). FoxP3 + Treg cell proportion and expression of PD-1, GITR, and CD69 were markedly downregulated by the administration of Colon-26-EVs. c Lung weight, FoxP3 + Treg cell proportion, and expression of CTLA-4, PD-1, GITR, and CD69 on Treg cells among 0, 0.4, 2, and 10 µg of Colon-26-EVs were compared using flow cytometry (n = 7 per group). MFI; mean fluorescence intensity
Attenuation of the Treg phenotype by heterogenous TEVs
To determine if these effects were caused by TEVs derived from allogeneic cancer cells, mice with metastatic lung tumors of colorectal cancer were treated with EVs derived from squamous cell lung carcinoma cell line ASB-XIV (ASB-EVs). Notably, ASB-EVs also suppressed tumor progression and regulatory phenotypes of Tregs (Fig. 5), whereas EVs derived from the NIH/3T3 non-cancer fibroblast cell line could not (Supplementary Fig. 2).
Fig. 5.
Reduced regulatory markers expression of Treg cells in metastatic lung tumors derived from Colon-26 cells by AXB-EVs. Lung weight, FoxP3 + Treg cell proportion, and the expression of CTLA-4, PD-1, GITR, and CD69 on Treg cells among healthy controls, with and without every 2-day administration of 10 µg AXB-EVs were compared (n = 7 each). PD-1, GITR, and CD69 expression, lung weight, and Treg cell populations were also markedly downregulated by the administration of Colon-26 EVs. MFI; mean fluorescence intensity
Uptake of TEVs by the CD11b + CD11c + cell population in the lung
To identify which cell populations take up TEVs that affect immune modulation, thus leading to tumor suppression, we inoculated fluorescent dye-labeled Colon-26-EVs into metastatic lung tumor mice. CD11b + cells, especially CD11b + CD11c + cell populations, significantly took up TEVs when compared to B220 + , CD4 + , and CD8 + cells (P < 0.05) (Fig. 6a). In vitro coculture assay revealed that CD11b + cells take up Colon-26 into their cytosol (Fig. 6b). Therefore, to clarify whether the attenuation of Treg cell phenotypes was because of a direct or indirect effect by CD11b + cells, we monitored the expression of CTLA-4, PD-1, and GITR in Treg cells with or without TEVs or Treg cells plus the culture media of CD11b + cells with or without TEVs in vitro. Lung Treg cells from metastatic lungs did not tolerate long-term culture (data not shown). Alternatively, we performed this experiment using splenic Treg cells; TEVs did not alter the phenotypes of splenic Treg cells. However, PD-1 expression on Treg cells was downregulated by co-culture media of lung CD11b + cells and Colon-26-EVs (Supplementary Fig. 3).
Fig. 6.
Cell populations taking up TEV in the lung. a After intravenous administration of Colon-26 cells, Colon-26-EVs were intravenously injected every 2 days. PKH26-labeled Colon-26-EVs were injected 2 days before euthanasia. The uptake of TEVs by lung mononuclear cells was validated as PKH + by flow cytometry (n = 3). b Lung CD11b cells (5 × 105 cells) were isolated from metastatic lungs, stained with CFSE, and cocultured with 2 μg of PKH26-labeled PBS or Colon-26-EVs for 24 h
Comparison of protein patterns among Colon-26, ASB, and 3T3 EVs
To identify proteins that can emphasize host cancer immunity, the expression of proteins derived from Colon-26-, ASB-, and 3T3-EVs were compared by mass spectrometry. Table 1 summarizes the unique proteins overlapping in Colon-26- and ASB-EVs, but not in 3T3-EVs.
Table 1.
Overlapping proteins expressed in Colon-26- and ASB-EVs, but not 3T3-EVs
| Heat shock cognate 71 kDa protein | |
| Fibronectin | |
| Trinucleotide repeat-containing gene 6A protein | |
| Cystic fibrosis transmembrane conductance regulator | |
| Histone H2B type 3-A |
Discussion
EVs are secreted intracellular microparticles that modulate the characteristics of the recipient cells. EVs could be positive or negative inflammation regulators, depending on the individual immunological condition and attributes of secretory cells [24, 25]. In malignancies, cancer cells possess molecules that are advantageous for the survival of cancer cells in TEV and transfer them onto the surrounding cells, utilizing EVs secreted by surrounding cells, thereby leading to cancer progression [10–15, 26].
Our data emphasized that TEVs can suppress tumor growth. We demonstrated that intravenously administered TEVs absorbed by immune cells dominated lung CD11b + /CD11c + cell populations, characterized as CD11b + DCs, interstitial macrophages, or both [27]. Moreover, TEVs can potentially reduce tumor growth in metastatic lungs, and the TEV components decreasing tumor growth should be validated.
However, considering the mass spectrometry results, our study implies that the heat shock protein 70 (HSP70) family and heat shock cognate 71 kDa protein (known as HSC70s or HSPA8) are critical elements in TEV-induced tumor reduction in TME of CRC. HSP70s are highly expressed in most cancers and promote tumor growth and survival [28]. HSP70s constitute a HSP family that engage in cytoprotection by acting as protein chaperones, including properly folding newly synthesized proteins, intracellular polypeptide transfer, protein complex prevention, and protein activity regulation [29]. HSP70s can potentially suppress tumor growth in various ways, such as regulating metastasis and invasion, because silencing HSP70s drives the metastatic ability, resulting in the loss of interactions with neighboring cells [30].
Additionally, HSP70s can also modulate the immune system. In the innate immune system, HSP70s activate DCs and macrophages, thus leading to the induction of proinflammatory cytokines, such as tumor necrosis factor-α, interleukin-1 (IL-1), IL-6, and IL-12, and consequently stimulating adaptive immune cells [31]. HSP70s promote the cytotoxicity and proliferation of natural killer cells [32]. In the adaptive immune system, HSP70s elicit antigen-specific CD8 + cytotoxicity and CD4 + helper T cell responses [33, 34]. TEVs derived from melanoma and colon cancer cell lines that were enriched in HSP70, inhibited tumor growth in a subcutaneous tumor formation model [35, 36], which is related to our present study. However, whether EVs with HSP70s were the only component that could suppress metastatic lung tumor growth was not determined because TEVs possess various tumor cell-derived components. Additionally, since lung Treg cells were unsuitable for long-term culture, the potential impact of downregulating Treg phenotype through TEV administration on enhancing anti-tumor cytotoxicity in this mouse model was not elucidated using the killing assay (data not shown); this represents a limitation of our study.
Likewise, TEVs can modulate regulatory molecule expression on lung T cells in metastatic lungs. TEVs contain elements that suppress tumor progression by reducing the regulatory phenotypes of T cells in vivo. Notably, TEVs substantially suppressed the expression of regulatory markers, such as CTLA-4, PD-1, and GITR, and the activation marker CD69 on Treg cells. However, when a comparative analysis was performed with normal mice, TEVs could not modulate these markers on Treg cells. These data suggest that TEVs can attenuate immune evasion by malignant tumors. Moreover, culture media of lung CD11b + cells plus EVs, but not TEVs themselves, downregulated PD-1 expression on Treg cells in vitro. Previous research demonstrated that CD69, human leukocyte antigen-DR, and PD-1 expression on T cells showed greater upregulation in tumor lesions than the periphery and draining lymph nodes [37]. Moreover, tumor-infiltrating Tregs were enriched and upregulated CTLA-4 and PD-1 expression [38]. In our metastatic lung tumor model, the dynamics of these regulatory markers of Treg cells were comparable with those reported in previous studies, and TEVs reduced this upregulation. Originally, TEVs facilitated the inhibition of immune surveillance by modulating immune cell function. For instance, TEVs promote T cell dysfunction by suppressing cell proliferation and cytotoxicity, inhibiting Th1 and Th17 differentiation, and reprogramming T cell metabolism [39]. In Treg cell immunity, TEVs derived from melanoma cell lines induce CD300a internalization of DCs and IFN-β production by DCs, thus leading to Treg cell proliferation and infiltration [40]. TEVs derived from the colon cancer cell line cargo TGF-β upregulate regulatory molecules and cytokines, such as FoxP3, CTLA-4, LAG3, IL-10, Perforin-1, and Granzyme B on T cells [41]. Moreover, HSP70 treatment of Treg cells confers a suppressing function of Treg cells [42]. Our in vitro experiment showed no evidence that TEVs directly downregulate Treg phenotypes. In contrast, HSP70 can activate DCs and promote Th1 polarization, thus contributing to anti-tumor immunities [43]. Moreover, HSP70s elicit the anti-tumor cytotoxicity of NK cells and reduction of arginase-1-positive M2 macrophages in a subcutaneous cancer injection model [35]. Thus, as the effect of HSP70-induced tumor immunity remains controversial, simplifying the HSP70-induced immunological modulation in vivo appears to be challenging. In our metastatic lung cancer model, owing to experimental difficulties, it was impossible to verify whether TEVs induced cytokine changes or chemokines altered the Treg phenotype in the lung TME. The uptake of TEVs was overt in CD11b + DCs, interstitial macrophages, or both, rather than in Treg cells, and induced PD-1 downregulation in vitro, thereby implying that robust TEVs administration worked as tumor vaccination and reduced the Treg phenotype underlying enhanced Th1 polarization in TMEs. Vaccination with TEVs-pulsed DCs suppressed tumor growth in subcutaneous tumor formation in a lung tumor model with CD8 + T cells recruitment in TME. Moreover, TEV can downregulate PD-L1 expression in DCs, and TEV-pulsed DCs suppress Treg cell populations in vitro [44].
EVs are emerging as a new strategy for cancer treatment, owing to their delivery properties to drugs, proteins, nucleic acids, and oncolytic viruses [45]. One issue arises, as it is likely that TEV has a dual effect on tumor immunity that plays a role in tumor progression or suppression. Therefore, components that positively contribute to tumor growth should be eliminated. In contrast, due to the chaperoning effect of HSP70, TEVs could be novel tumor antigen carriers. TEVs surpass tumor lysates in DC cancer vaccination [44]. Therefore, based on both our current work and previous studies, administering TEVs as a new strategy for cancer treatment should be modified and engineered based on its advantages and disadvantages. We demonstrated that high-dose TEVs can decrease tumor growth by reducing regulatory phenotypes of Treg cells in an experimental metastatic lung tumor model. We believe that the present study sheds light on the use of TEVs as a new therapeutic strategy for malignant tumors.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to thank Editage (www.editage.jp) for English language editing.
Abbreviations
- CFSE
Carboxyfluorescein succinimidyl ester
- CRC
Colorectal cancer
- DC
Dendritic cells
- EV
Extracellular vesicle
- FBS
Fetal bovine serum
- H&E
Hematoxylin and eosin
- ICI
Immune checkpoint inhibitor
- NTA
Nanoparticle tracking analysis
- PBS
Phosphate-buffered saline
- Treg
Regulatory T
- TEM
Transmission electron microscopy
- TME
Tumor microenvironment
- TEVs
Tumor-derived EVs
Author contributions
SK and TT designed the study and wrote the initial draft of the manuscript. SK, TT, NK, NN, MM, and YM performed the experiments. YH, TT, TI, TF, KO, and MN contributed to the analysis and interpretation of data and assisted in the preparation of the manuscript. All other authors have contributed to data collection and interpretation and critically reviewed the manuscript.
Funding
This study was funded by (1) Grant-in-Aid for Young Scientists JSPS KAKENHI Grant Number JP20K17066, (2) Grants-in-Aid from the Ministry of Education, Culture, Sports, Science, and Technology of Japan, (3) Grant-in-Aid for Scientific Research (C) of the Ministry of Culture and Science of Japan (20590810, 23591017, 24591020, 12008507, 17877850, 17K09468, 15K09052), (4) the Research Program on Intractable Diseases, from the Ministry of Labor and Welfare of Japan, and (5) grants-in-aid from the Ministry of Education, Culture, Sports, Science and Technology of Japan, (6) the Research Program from the Japan Medical Research and Development (AMED) (17824893).
Data availability
The datasets of this study are available from the corresponding author upon reasonable request.
Declarations
Conflicts of interest
The authors have no competing interests to declare.
Research involving Animals
This research has been approved by Animal Ethics Committee (#18-115(01)) and Recombinant DNA Experiments Committee (#30-012-1) of Kansai Medical University.
Informed consent
Not applicable.
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Edwards BK, Ward E, Kohler BA, Eheman C, Zauber AG, Anderson RN, Jemal A, Schymura MJ, Lansdorp-Vogelaar I, Seeff LC, van Ballegooijen M, Goede SL, Ries LA. Annual report to the nation on the status of cancer, 1975–2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer. 2010;116:544–573. doi: 10.1002/cncr.24760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xie YH, Chen YX, Fang JY. Comprehensive review of targeted therapy for colorectal cancer. Signal Transduct Target Ther. 2020;5:22. doi: 10.1038/s41392-020-0116-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ganesh K, Stadler ZK, Cercek A, Mendelsohn RB, Shia J, Segal NH, Diaz LA. Immunotherapy in colorectal cancer: rationale, challenges and potential. Nat Rev Gastroenterol Hepatol. 2019;16:361–375. doi: 10.1038/s41575-019-0126-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 6.Shang B, Liu Y, Jiang SJ, Liu Y. Prognostic value of tumor-infiltrating FoxP3+ regulatory T cells in cancers: a systematic review and meta-analysis. Sci Rep. 2015;5:15179. doi: 10.1038/srep15179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Siljander PR, Andreu Z, Zavec AB, Borràs FE, Buzas EI, Buzas K, Casal E, Cappello F, Carvalho J, Colás E, Cordeiro-da Silva A, Fais S, Falcon-Perez JM, Ghobrial IM, Giebel B, Gimona M, Graner M, Gursel I, Gursel M, Heegaard NH, Hendrix A, Kierulf P, Kokubun K, Kosanovic M, Kralj-Iglic V, Krämer-Albers EM, Laitinen S, Lässer C, Lener T, Ligeti E, Linē A, Lipps G, Llorente A, Lötvall J, Manček-Keber M, Marcilla A, Mittelbrunn M, Nazarenko I, Nolte-’t Hoen EN, Nyman TA, O’Driscoll L, Olivan M, Oliveira C, Pállinger E, Del Portillo HA, Reventós J, Rigau M, Sammar M, Sánchez-Madrid F, Santarém N, Schallmoser K, Ostenfeld MS, Stoorvogel W, Stukelj R, Van der Grein SG, Vasconcelos MH, Wauben MH, De Wever O. Biological properties of extracellular vesicles and their physiological functions Yáñez-Mó M. J Extracell Vesicles. 2015;4:27066. doi: 10.3402/jev.v4.27066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roefs MT, Sluijter JPG, Vader P. Extracellular vesicle-associated proteins in tissue repair. Trends Cell Biol. 2020;30:990–1013. doi: 10.1016/j.tcb.2020.09.009. [DOI] [PubMed] [Google Scholar]
- 9.Zhou X, Xie F, Wang L, Zhang L, Zhang S, Fang M, Zhou F. The function and clinical application of extracellular vesicles in innate immune regulation. Cell Mol Immunol. 2020;17:323–334. doi: 10.1038/s41423-020-0391-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ko SY, Lee W, Kenny HA, Dang LH, Ellis LM, Jonasch E, Lengyel E, Naora H. Cancer-derived small extracellular vesicles promote angiogenesis by heparin-bound, bevacizumab-insensitive VEGF, independent of vesicle uptake. Commun Biol. 2019;2:386. doi: 10.1038/s42003-019-0609-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cui Y, Wang D, Xie M (2021) Tumor-derived extracellular vesicles promote activation of carcinoma-associated fibroblasts and facilitate invasion and metastasis of ovarian cancer by carrying miR-630. Front Cell Dev Biol 9:652322. 10.3389/fcell.2021.652322 [DOI] [PMC free article] [PubMed] [Retracted]
- 12.Jung T, Castellana D, Klingbeil P, Cuesta Hernández I, Vitacolonna M, Orlicky DJ, Roffler SR, Brodt P, Zöller M. CD44v6 dependence of premetastatic niche preparation by exosomes. Neoplasia. 2009;11:1093–1105. doi: 10.1593/neo.09822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoshino A, Costa-Silva B, Shen TL, Rodrigues G, Hashimoto A, Tesic Mark M, Molina H, Kohsaka S, Di Giannatale A, Ceder S, Singh S, Williams C, Soplop N, Uryu K, Pharmer L, King T, Bojmar L, Davies AE, Ararso Y, Zhang T, Zhang H, Hernandez J, Weiss JM, Dumont-Cole VD, Kramer K, Wexler LH, Narendran A, Schwartz GK, Healey JH, Sandstrom P, Labori KJ, Kure EH, Grandgenett PM, Hollingsworth MA, de Sousa M, Kaur S, Jain M, Mallya K, Batra SK, Jarnagin WR, Brady MS, Fodstad O, Muller V, Pantel K, Minn AJ, Bissell MJ, Garcia BA, Kang Y, Rajasekhar VK, Ghajar CM, Matei I, Peinado H, Bromberg J, Lyden D. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527:329–335. doi: 10.1038/nature15756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bardi GT, Smith MA, Smith HJLGT, Bardi Melanoma exosomes promote mixed M1 and M2 macrophage polarization. Cytokine. 2018;105:63–72. doi: 10.1016/j.cyto.2018.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu J, Wu S, Zheng X, Zheng P, Fu Y, Wu C, Lu B, Ju J, Jiang J. Immune suppressed tumor microenvironment by exosomes derived from gastric cancer cells via modulating immune functions. Sci Rep. 2020;10:14749. doi: 10.1038/s41598-020-71573-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wolfers J, Lozier A, Raposo G, Regnault A, Théry C, Masurier C, Flament C, Pouzieux S, Faure F, Tursz T, Angevin E, Amigorena S, Zitvogel L. Tumor-derived exosomes are a source of shared tumor rejection antigens for CTL cross-priming. Nat Med. 2001;7:297–303. doi: 10.1038/85438. [DOI] [PubMed] [Google Scholar]
- 17.Altieri SL, Khan AN, Tomasi TB. Exosomes from plasmacytoma cells as a tumor vaccine. J Immunother. 2004;27:282–288. doi: 10.1097/00002371-200407000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Wang DY, Ye F, Lin JJ, Xu X. Cutaneous metastasis: a rare phenomenon of colorectal cancer. Ann Surg Treat Res. 2017;93:277–280. doi: 10.4174/astr.2017.93.5.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Théry C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R, Antoniou A, Arab T, Archer F, Atkin-Smith GK, Ayre DC, Bach JM, Bachurski D, Baharvand H, Balaj L, Baldacchino S, Bauer NN, Baxter AA, Bebawy M, Beckham C, Bedina Zavec A, Benmoussa A, Berardi AC, Bergese P, Bielska E, Blenkiron C, Bobis-Wozowicz S, Boilard E, Boireau W, Bongiovanni A, Borràs FE, Bosch S, Boulanger CM, Breakefield X, Breglio AM, Brennan M, Brigstock DR, Brisson A, Broekman ML, Bromberg JF, Bryl-Górecka P, Buch S, Buck AH, Burger D, Busatto S, Buschmann D, Bussolati B, Buzás EI, Byrd JB, Camussi G, Carter DR, Caruso S, Chamley LW, Chang YT, Chen C, Chen S, Cheng L, Chin AR, Clayton A, Clerici SP, Cocks A, Cocucci E, Coffey RJ, Cordeiro-da-Silva A, Couch Y, Coumans FA, Coyle B, Crescitelli R, Criado MF, D’Souza-Schorey C, Das S, Datta Chaudhuri A, de Candia P, De Santana EF, De Wever O, Del Portillo HA, Demaret T, Deville S, Devitt A, Dhondt B, Di Vizio D, Dieterich LC, Dolo V, Dominguez Rubio AP, Dominici M, Dourado MR, Driedonks TA, Duarte FV, Duncan HM, Eichenberger RM, Ekström K, El Andaloussi S, Elie-Caille C, Erdbrügger U, Falcón-Pérez JM, Fatima F, Fish JE, Flores-Bellver M, Försönits A, Frelet-Barrand A, Fricke F, Fuhrmann G, Gabrielsson S, Gámez-Valero A, Gardiner C, Gärtner K, Gaudin R, Gho YS, Giebel B, Gilbert C, Gimona M, Giusti I, Goberdhan DC, Görgens A, Gorski SM, Greening DW, Gross JC, Gualerzi A, Gupta GN, Gustafson D, Handberg A, Haraszti RA, Harrison P, Hegyesi H, Hendrix A, Hill AF, Hochberg FH, Hoffmann KF, Holder B, Holthofer H, Hosseinkhani B, Hu G, Huang Y, Huber V, Hunt S, Ibrahim AG, Ikezu T, Inal JM, Isin M, Ivanova A, Jackson HK, Jacobsen S, Jay SM, Jayachandran M, Jenster G, Jiang L, Johnson SM, Jones JC, Jong A, Jovanovic-Talisman T, Jung S, Kalluri R, Kano SI, Kaur S, Kawamura Y, Keller ET, Khamari D, Khomyakova E, Khvorova A, Kierulf P, Kim KP, Kislinger T, Klingeborn M, Klinke DJ, Kornek M, Kosanović MM, Kovács Á, Krämer-Albers EM, Krasemann S, Krause M, Kurochkin IV, Kusuma GD, Kuypers S, Laitinen S, Langevin SM, Languino LR, Lannigan J, Lässer C, Laurent LC, Lavieu G, Lázaro-Ibáñez E, Le Lay S, Lee MS, Lee YXF, Lemos DS, Lenassi M, Leszczynska A, Li IT, Liao K, Libregts SF, Ligeti E, Lim R, Lim SK, Linē A, Linnemannstöns K, Llorente A, Lombard CA, Lorenowicz MJ, Lörincz Á, Lötvall J, Lovett J, Lowry MC, Loyer X, Lu Q, Lukomska B, Lunavat TR, Maas SL, Malhi H, Marcilla A, Mariani J, Mariscal J, Martens-Uzunova ES, Martin-Jaular L, Martinez MC, Martins VR, Mathieu M, Mathivanan S, Maugeri M, McGinnis LK, McVey MJ, Meckes DG, Meehan KL, Mertens I, Minciacchi VR, Möller A, Møller Jørgensen M, Morales-Kastresana A, Morhayim J, Mullier F, Muraca M, Musante L, Mussack V, Muth DC, Myburgh KH, Najrana T, Nawaz M, Nazarenko I, Nejsum P, Neri C, Neri T, Nieuwland R, Nimrichter L, Nolan JP, Nolte-’t Hoen EN, Noren Hooten N, O’Driscoll L, O’Grady T, O’Loghlen A, Ochiya T, Olivier M, Ortiz A, Ortiz LA, Osteikoetxea X, Østergaard O, Ostrowski M, Park J, Pegtel DM, Peinado H, Perut F, Pfaffl MW, Phinney DG, Pieters BC, Pink RC, Pisetsky DS, Pogge von Strandmann E, Polakovicova I, Poon IK, Powell BH, Prada I, Pulliam L, Quesenberry P, Radeghieri A, Raffai RL, Raimondo S, Rak J, Ramirez MI, Raposo G, Rayyan MS, Regev-Rudzki N, Ricklefs FL, Robbins PD, Roberts DD, Rodrigues SC, Rohde E, Rome S, Rouschop KM, Rughetti A, Russell AE, Saá P, Sahoo S, Salas-Huenuleo E, Sánchez C, Saugstad JA, Saul MJ, Schiffelers RM, Schneider R, Schøyen TH, Scott A, Shahaj E, Sharma S, Shatnyeva O, Shekari F, Shelke GV, Shetty AK, Shiba K, Siljander PR, Silva AM, Skowronek A, Snyder OL, Soares RP, Sódar BW, Soekmadji C, Sotillo J, Stahl PD, Stoorvogel W, Stott SL, Strasser EF, Swift S, Tahara H, Tewari M, Timms K, Tiwari S, Tixeira R, Tkach M, Toh WS, Tomasini R, Torrecilhas AC, Tosar JP, Toxavidis V, Urbanelli L, Vader P, van Balkom BW, van der Grein SG, Van Deun J, van Herwijnen MJ, Van Keuren-Jensen K, van Niel G, van Royen ME, van Wijnen AJ, Vasconcelos MH, Vechetti IJ, Veit TD, Vella LJ, Velot É, Verweij FJ, Vestad B, Viñas JL, Visnovitz T, Vukman KV, Wahlgren J, Watson DC, Wauben MH, Weaver A, Webber JP, Weber V, Wehman AM, Weiss DJ, Welsh JA, Wendt S, Wheelock AM, Wiener Z, Witte L, Wolfram J, Xagorari A, Xander P, Xu J, Yan X, Yáñez-Mó M, Yin H, Yuana Y, Zappulli V, Zarubova J, Žėkas V, Zhang JY, Zhao Z, Zheng L, Zheutlin AR, Zickler AM, Zimmermann P, Zivkovic AM, Zocco D, Zuba-Surma EK (2018) Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles 7:1535750. 10.1080/20013078.2018.1535750 [DOI] [PMC free article] [PubMed]
- 20.Rayamajhi M, Redente EF, Condon TV, Gonzalez-Juarrero M, Riches DW, Lenz LL (2011) Non-surgical intratracheal instillation of mice with analysis of lungs and lung draining lymph nodes by flow cytometry. J Vis Exp 51. 10.3791/2702 [DOI] [PMC free article] [PubMed]
- 21.Gao X, Zhu Y, Li G, Huang H, Zhang G, Wang F, Sun J, Yang Q, Zhang X, Lu B (2012) TIM-3 expression characterizes regulatory T cells in tumor tissues and is associated with lung cancer progression. PLOS ONE 7:e30676. 10.1371/journal.pone.0030676 [DOI] [PMC free article] [PubMed]
- 22.Katano T, Fukuda M, Furue H, Yamazaki M, Abe M, Watanabe M, Nishida K, Yao I, Yamada A, Hata Y, Okumura N, Nakazawa T, Yamamoto T, Sakimura K, Takao T, Ito S (2016) Involvement of brain-enriched guanylate kinase-associated protein (BEGAIN) in chronic pain after peripheral nerve injury. eNeuro 3. 10.1523/ENEURO.0110-16.2016 [DOI] [PMC free article] [PubMed]
- 23.Saleh R, Elkord E. FoxP3+ T regulatory cells in cancer: prognostic biomarkers and therapeutic targets. Cancer Lett. 2020;490:174–185. doi: 10.1016/j.canlet.2020.07.022. [DOI] [PubMed] [Google Scholar]
- 24.Glémain A, Néel M, Néel A, André-Grégoire G, Gavard J, Martinet B, Le Bloas R, Riquin K, Hamidou M, Fakhouri F, Bruneau S (2022) Neutrophil-derived extracellular vesicles induce endothelial inflammation and damage through the transfer of miRNAs. J Autoimmun 129:102826. 10.1016/j.jaut.2022.102826 [DOI] [PubMed]
- 25.Zhou H, Shen X, Yan C, Xiong W, Ma Z, Tan Z, Wang J, Li Y, Liu J, Duan A, Liu F. Extracellular vesicles derived from human umbilical cord mesenchymal stem cells alleviate osteoarthritis of the knee in mice model by interacting with METTL3 to reduce m6A of NLRP3 in macrophage. Stem Cell Res Ther. 2022;13:322. doi: 10.1186/s13287-022-03005-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ono M, Kosaka N, Tominaga N, Yoshioka Y, Takeshita F, Takahashi RU, Yoshida M, Tsuda H, Tamura K, Ochiya T (2014) Exosomes from bone marrow mesenchymal stem cells contain a microRNA that promotes dormancy in metastatic breast cancer cells. Sci Signal 7:ra63. 10.1126/scisignal.2005231 [DOI] [PubMed]
- 27.Misharin AV, Morales-Nebreda L, Mutlu GM, Budinger GR, Perlman H. Flow cytometric analysis of macrophages and dendritic cell subsets in the mouse lung. Am J Respir Cell Mol Biol. 2013;49:503–510. doi: 10.1165/rcmb.2013-0086MA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Calderwood SK, Khaleque MA, Sawyer DB, Ciocca DR. Heat shock proteins in cancer: chaperones of tumorigenesis. Trends Biochem Sci. 2006;31:164–172. doi: 10.1016/j.tibs.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 29.Rosenzweig R, Nillegoda NB, Mayer MP, Bukau B. The Hsp70 chaperone network. Nat Rev Mol Cell Biol. 2019;20:665–680. doi: 10.1038/s41580-019-0133-3. [DOI] [PubMed] [Google Scholar]
- 30.Kasioumi P, Vrazeli P, Vezyraki P, Zerikiotis S, Katsouras C, Damalas A, Angelidis C. Hsp70 (HSP70A1A) downregulation enhances the metastatic ability of cancer cells. Int J Oncol. 2019;54:821–832. doi: 10.3892/ijo.2018.4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tsan MF, Gao B. Heat shock protein and innate immunity. Cell Mol Immunol. 2004;1:274–279. [PubMed] [Google Scholar]
- 32.Multhoff G, Mizzen L, Winchester CC, Milner CM, Wenk S, Eissner G, Kampinga HH, Laumbacher B, Johnson J. Heat shock protein 70 (Hsp70) stimulates proliferation and cytolytic activity of natural killer cells. Exp Hematol. 1999;27:1627–1636. doi: 10.1016/s0301-472x(99)00104-6. [DOI] [PubMed] [Google Scholar]
- 33.Blachere NE, Li Z, Chandawarkar RY, Suto R, Jaikaria NS, Basu S, Udono H, Srivastava PK. Heat shock protein-peptide complexes, reconstituted in vitro, elicit peptide-specific cytotoxic T lymphocyte response and tumor immunity. J Exp Med. 1997;186:1315–1322. doi: 10.1084/jem.186.8.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ueda G, Tamura Y, Hirai I, Kamiguchi K, Ichimiya S, Torigoe T, Hiratsuka H, Sunakawa H, Sato N. Tumor-derived heat shock protein 70-pulsed dendritic cells elicit tumor-specific cytotoxic T lymphocytes (CTLs) and tumor immunity. Cancer Sci. 2004;95:248–253. doi: 10.1111/j.1349-7006.2004.tb02211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Komarova EY, Suezov RV, Nikotina AD, Aksenov ND, Garaeva LA, Shtam TA, Zhakhov AV, Martynova MG, Bystrova OA, Istomina MS, Ischenko AM, Margulis BA, Guzhova IV. Hsp70-containing extracellular vesicles are capable of activating of adaptive immunity in models of mouse melanoma and colon carcinoma. Sci Rep. 2021;11:21314. doi: 10.1038/s41598-021-00734-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cho JA, Lee YS, Kim SH, Ko JK, Kim CW. MHC independent anti-tumor immune responses induced by Hsp70-enriched exosomes generate tumor regression in murine models. Cancer Lett. 2009;275:256–265. doi: 10.1016/j.canlet.2008.10.021. [DOI] [PubMed] [Google Scholar]
- 37.Piersiala K, Neves F, da Silva P, Hjalmarsson E, Kolev A, Kågedal Å, Starkhammar M, Elliot A, Marklund L, Margolin G, Munck-Wikland E, Kumlien Georén S, Cardell LO. CD4+ and CD8+ T cells in sentinel nodes exhibit distinct pattern of PD-1, CD69, and HLA-DR expression compared to tumor tissue in oral squamous cell carcinoma. Cancer Sci. 2021;112:1048–1059. doi: 10.1111/cas.14816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim HR, Park HJ, Son J, Lee JG, Chung KY, Cho NH, Shim HS, Park S, Kim G, In Yoon H, Kim HG, Jung YW, Cho BC, Park SY, Rha SY, Ha SJ. Tumor microenvironment dictates regulatory T cell phenotype: upregulated immune checkpoints reinforce suppressive function. J Immunother Cancer. 2019;7:339. doi: 10.1186/s40425-019-0785-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ma F, Vayalil J, Lee G, Wang Y, Peng G (2021) Emerging role of tumor-derived extracellular vesicles in T cell suppression and dysfunction in the tumor microenvironment. J Immunother Cancer 9. 10.1136/jitc-2021-003217 [DOI] [PMC free article] [PubMed]
- 40.Nakazawa Y, Nishiyama N, Koizumi H, Kanemaru K, Nakahashi-Oda C, Shibuya A (2021) Tumor-derived extracellular vesicles regulate tumor-infiltrating regulatory T cells via the inhibitory immunoreceptor CD300a. eLife 10. 10.7554/eLife.61999 [DOI] [PMC free article] [PubMed]
- 41.Yamada N, Kuranaga Y, Kumazaki M, Shinohara H, Taniguchi K, Akao Y (2016) Colorectal cancer cell-derived extracellular vesicles induce phenotypic alteration of T cells into tumor-growth supporting cells with transforming growth factor-β1-mediated suppression. Oncotarget 7:27033–27043. 10.18632/oncotarget.7041 [DOI] [PMC free article] [PubMed]
- 42.Wachstein J, Tischer S, Figueiredo C, Limbourg A, Falk C, Immenschuh S, Blasczyk R, Eiz-Vesper B (2012) HSP70 enhances immunosuppressive function of CD4(+)CD25(+)FoxP3(+) T regulatory cells and cytotoxicity in CD4(+)CD25(-) T cells. PLOS ONE 7:e51747. 10.1371/journal.pone.0051747 [DOI] [PMC free article] [PubMed]
- 43.Fang H, Wu Y, Huang X, Wang W, Ang B, Cao X, Wan T. Toll-like receptor 4 (TLR4) is essential for Hsp70-like protein 1 (HSP70L1) to activate dendritic cells and induce Th1 response. J Biol Chem. 2011;286:30393–30400. doi: 10.1074/jbc.M111.266528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang C, Huang X, Wu Y, Wang J, Li F, Guo G. Tumor cell-associated exosomes robustly elicit anti-tumor immune responses through modulating dendritic cell vaccines in lung tumor. Int J Biol Sci. 2020;16:633–643. doi: 10.7150/ijbs.38414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kugeratski FG, McAndrews KM, Kalluri R (2021) Multifunctional applications of engineered extracellular vesicles in the treatment of cancer. Endocrinology 162. 10.1210/endocr/bqaa250 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets of this study are available from the corresponding author upon reasonable request.