Abstract
Neuroblastoma (NBL) accounts for a disproportionate number of deaths among childhood malignancies despite intensive multimodal therapy that includes antibody targeting disialoganglioside GD2, a NBL antigen. Unfortunately, resistance to anti-GD2 immunotherapy is frequent and we aimed to investigate mechanisms of resistance in NBL. GD2 expression was quantified by flow cytometry and anti-GD2 antibody internalization was measured using real-time microscopy in 20 human NBL cell lines. Neutrophil-mediated antibody-dependent cellular cytotoxicity (ADCC) assays were performed on a subset of the cell lines (n = 12), and results were correlated with GD2 expression and antibody internalization. GD2 was expressed on 19 of 20 NBL cell lines at variable levels, and neutrophil-mediated ADCC was observed only in GD2-expressing cell lines. We found no correlation between level of GD2 expression and sensitivity to neutrophil-mediated ADCC, suggesting that GD2 expression of many cell lines was above a threshold required for maximal ADCC, such that expression level could not be used to predict subsequent cytotoxicity. Instead, anti-GD2 antibody internalization, a process that occurred universally but differentially across GD2-expressing NBL cell lines, was inversely correlated with ADCC. Treatment with endocytosis inhibitors EIPA, chlorpromazine, MBCD, and cytochalasin-D showed potential to inhibit antibody internalization; however, only MBCD resulted in significantly increased sensitivity to neutrophil-mediated ADCC in 4 of 4 cell lines in vitro. Our data suggest that antibody internalization may represent a novel mechanism of immunotherapy escape by NBL and provide proof-of-principle that targeting pathways involved in antibody internalization may improve the efficacy of anti-GD2 immunotherapies.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00262-021-02963-y.
Keywords: Neuroblastoma, Immunotherapy, Antibody Internalization, ADCC, Anti-GD2, Disialoganglioside
Introduction
Neuroblastoma (NBL) is the most common extracranial solid tumor of childhood, with 90% of cases occurring in children under age 5 and half of those presenting with high-risk disease. Despite intensive multimodal therapy, nearly 50% of patients with high-risk NBL will experience relapse with associated dismal survival [1]. The introduction of therapeutic antibodies targeting the disialoganglioside GD2, an antigen highly expressed on NBL tumors, has significantly improved survival outcomes of patients with high-risk NBL [2–4]. The addition of anti-GD2 antibody (dinutuximab) to post-consolidation therapy of high-risk NBL improved 2-year event-free survival when compared to patients treated with isotretinoin alone [4]. In relapsed or chemo-refractory NBL patients, the combination of anti-GD2 antibody with chemotherapy resulted in significant disease remission and improved survival [5–7]. Despite these advances, a significant proportion of children will experience persistence of disease or relapse, with the mechanisms underlying resistance remaining poorly understood. Only 10–12% of NBL patients have complete or partial loss of GD2 expression on NBL cells from bone marrow [8], a far lower percentage than the rate of non-response to antibody therapy, and thus, constitutively low or absent GD2 expression can only partially explain anti-GD2 antibody resistance.
As its main anti-tumor mechanism, anti-GD2 antibody engages neutrophils and natural killer (NK) cells to mediate antibody-dependent cellular cytotoxicity (ADCC) [9–11]. Success of ADCC requires anti-GD2 antibody to remain bound and available on the surface of tumor cells and as such, anything that decreases surface-bound antibody may negatively impact the efficacy of antibody therapy and ultimately contribute to therapy resistance. The amount of available membrane-bound monoclonal antibody (mAb) on target cells will be affected by factors that impact antibody distribution including tissue-specific expression, mAb-target binding affinity, accessibility of mAb to the target, and ultimately, the fate of mAb-target complexes once bound including target shedding, internalization, and recycling [12]. In regard to the latter, receptor-mediated endocytosis pathways that mediate routine cell membrane receptor recycling can also mediate internalization of bound mAb resulting in clearance and destruction of antibody with or without subsequent alterations in surface antigen expression.
Antibody internalization has been described for a variety of therapeutic antibodies currently in clinical use [13–15]. Antibody internalization by target cells is proposed to underlie differences in therapeutic efficacy across CD20-targeted antibodies in arthritis and lupus patients [16], and was linked to decreased binding of mAb to solid tumor models by anti-HER2 mAb [15]. Anti-ganglioside antibody internalization has also been shown to be associated with protection of neuronal and glial cells from antibody-dependent complement-mediated nerve injury in a murine model of Guillain–Barre syndrome [17]. However, to our knowledge, internalization of anti-GD2 mAb by NBL cells as a mechanism of immunotherapy resistance has not been demonstrated.
In the current study, we evaluated GD2 expression, sensitivity to neutrophil-mediated ADCC, and anti-GD2 antibody internalization across a large cohort of human NBL cell lines. We assessed relationships between these variables with particular interest in the relationship between internalization of anti-GD2 mAb and NBL cell line sensitivity to neutrophil-mediated ADCC. Finally, we evaluated the effect of endocytosis pathway inhibitors on anti-GD2 internalization and on sensitization of NBL cells to neutrophil-mediated ADCC.
Materials and methods
Cell culture and reagents
Human NBL cell lines CHLA15, CHLA20, CHLA51, CHLA90, CHLA136, CHLA122, CHLA225, and CHLA255 were established at Children’s Hospital Los Angeles (CHLA). Human NBL cell lines Lan1, Lan2, Lan5, Lan6, SMS-KAN, SMS-KANR, SMS-KCN, SMS-KCNR, SMS-SAN, SK-N-BE(1), and SK-N-BE(2), were kind gifts from Dr. Robert Seeger or purchased from ATCC. All cell lines were cultured in IMDM with L-glutamine and 25 mM HEPES (Corning) supplemented with 10% heat-inactivated fetal bovine serum (FBS) (Corning), referred to as complete media. Identity of cell lines was confirmed by short tandem repeat (STR) testing of cell line-derived DNA (University of Arizona Genetics Core; http://uagc.arl.arizona.edu) and all cell lines were routinely tested and found to be mycoplasma-free (Lonza MycoAlert detection kit). Cell line characteristics were compiled from previously reported studies [18, 19] as well as the Childhood Cancer Repository (www.cccells.org) and have been organized in supplementary materials (Supplementary Table 1). Human recombinant GM-CSF (Leukine sargramostim 250 µg/ml) was obtained from CHLA pharmacy and kept at 4 °C until use. Dimethyl sulfoxide (DMSO), EIPA [5-(N-Ethyl-N-isopropyl) amiloride], cytochalasin-D, chlorpromazine hydrochloride, methyl-beta-cyclodextrin (MBCD) were all purchased from MilliporeSigma. Pitstop2™ was purchased from Abcam. Cytochalasin-D came as a ready-made solution, MBCD stock solution was prepared in serum-free IMDM, chlorpromazine stock in PBS while stock solutions of all remaining reagents were prepared in DMSO and stored at -20 °C (exception: MBCD stored at 4 °C per manufacturer recommendations). On day of use, stock solution aliquots were thawed and diluted to 2x−5x working solutions in serum-free IMDM prior to use with a final DMSO concentration of 0.1% or lower in all experiments.
Monoclonal antibodies
Murine-derived anti-GD2 antibody, 14G2a, was purified from culture supernatants of 14G2a-producing hybridoma cells (kind gift from Dr. Paul Sondel and originally established by Dr. Ralph Reisfeld) using a HiTrap Protein G HP column (GE Healthcare BioSciences) as per manufacturer’s protocol. SDS-PAGE Coomassie blue gels were used to confirm protein isolation within eluted fractions. Dialysis was then used to exchange the antibody-containing buffer solution to PBS followed by 0.22 µM filter sterilization. Final protein concentration was determined using a Nanodrop Nd-1000 spectrophotometer and aliquots were stored at −20 °C until use. Unituxin™ (chimeric 14.18 or dinutuximab), a human-mouse chimeric anti-GD2 antibody, stock [3.5 mg/ml] was obtained from CHLA Pharmacy and kept at 4 °C per manufacturer’s recommendations until time of use.
Flow cytometry
Cells were detached using trypsin (0.05%) with EDTA, washed in complete media and pelleted by centrifugation. They were counted and 105 cells were washed once in cold FACS buffer (1 × PBS supplemented with 2% heat-inactivated FBS and 2 mM EDTA but not the endocytosis inhibitor NaN3) followed by incubation with anti-GD2-PE antibody (Clone 14G2a, Isotype mouse IgG2a, BD Biosciences) or isotype-matched control mAb of irrelevant (non-biologic) specificity (murine IgG2a-PE; BioLegend) for 45 min in a 4 °C ice water bath in the dark. Cells were then washed twice in FACS buffer and transferred to filter top tubes with addition of DAPI live/dead stain (final concentration 1 µg/ml; Sigma-Aldrich). Data were acquired using a BD LSR II flow cytometer, acquiring 20,000 events from the live (DAPI negative) singlet gate for each cell line. Exported FCS files were then analyzed using FlowJo_v10 software and geometric mean fluorescence intensity determined for every sample. BD PE QuantiBrite Beads (BD Bioscience) were analyzed per the manufacturer’s protocol with every run. This was used to generate a standard curve in Prism GraphPad from which the PE geometric mean for each sample was converted into the number of antibodies bound per cell (ABC) representing surface antigen expression. Mean and standard deviation were calculated from a minimum of 3 replicate values for each cell line.
ADCC assay
Venous blood was collected from healthy donors into EDTA-coated tubes immediately before use in each assay. Donor informed consent was obtained in accordance with institutional review board policies. Whole blood was then processed to obtain highly enriched neutrophils (typically > 95%) using MACSxpress human neutrophil isolation kit (Miltenyi Biotec) per the manufacturer’s protocol with addition of red blood cell lysis as a final step. Neutrophils were resuspended in IMDM, and cell count and viability were determined using a ViCell Cell Counter prior to use.
A digital image microscopy scanning (DIMSCAN) [20] and an Incucyte S3 live-image fluorescent microscopy system were used for assessment of ADCC. ADCC assays utilizing small molecule inhibitors of pinocytosis were carried out on the Incucyte S3 system. For DIMSCAN ADCC assays, NBL cells were labeled with calcein-AM (ThermoFisher Scientific) to a final concentration of 5 µg/ml per 106 cells for 30 min in the dark at 37 °C followed by four washes to remove residual calcein-AM, plated at 104 cells/well in 96-well clear bottom black plates and placed in an incubator to settle prior to start of ADCC experiments. For Incucyte S3 ADCC assays, GFP-expressing LAN1 cells (Lan1GFP) were used at 104 cells/well in clear 96-well plate and allowed to settle prior to start of ADCC experiments and imaged in the Incucyte S3 system housed in a cell culture incubator. ADCC experiments: Dinutuximab and human recombinant GM-CSF stocks were diluted in IMDM prior to addition to wells. Final concentrations per well in a final well volume of 200 µl were as follows: dinutuximab 5 µg/ml, GM-CSF 100 ng/ml, human neutrophils 105 cells/well for an effector to target (E:T) ratio of 10:1. Untreated tumor cells, antibody only, and neutrophil only conditions were included as controls in every assay. For DIMSCAN assays, following a 6-h incubation in a 5% CO2 incubator at 37 °C, plates were scanned to measure retained total fluorescence per well representative of total viable cell count. Cytotoxicity was calculated as a change in viable cell count and reported as cytotoxicity % = 100–[(fluorescent intensity of target wells / fluorescent intensity of untreated control wells) × 100]. Mean cytotoxicity was calculated from 6 replicate wells for each condition and then averaged across triplicate experiments. In ADCC experiments utilizing inhibitors, stock solutions of each drug (EIPA, chlorpromazine, MBCD, Pitstop2, Cytochalasin-D or DMSO) were thawed and adjusted to desired concentration in IMDM. Experimental schemas in Fig. 5 show the duration of pre-treatment with inhibitors, timing of addition of antibody, and/or neutrophils. The value of integrated green fluorescence intensity per well generated from Lan1GFP cells was averaged across 3–6 well replicates per experiment as measured by Incucyte. Cytotoxicity was calculated from values collected 6 h after addition of neutrophils in each assay and reported as cytotoxicity % = 100–[(fluorescent intensity of target wells at hour 6 / fluorescent intensity of untreated control wells at hour 6) × 100]. To allow for comparison across inhibitor-treated experimental replicates, cytotoxicity values for each experiment were normalized by setting the cytotoxicity value of untreated cells at 100% and converting each raw cytotoxicity value to a percent of untreated control.
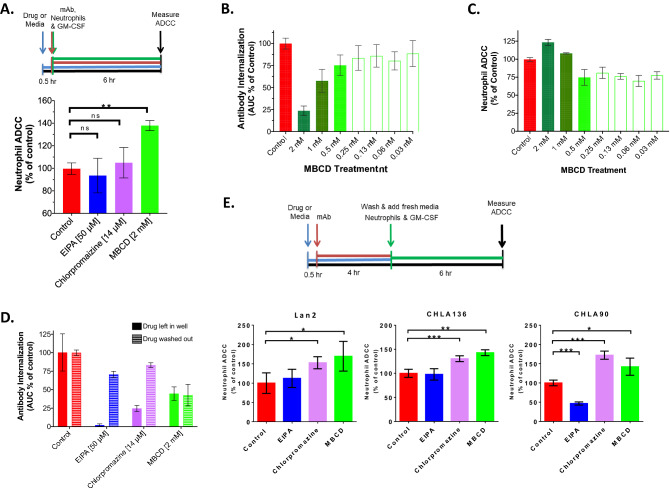
Fig. 5.
Inhibition of antibody internalization results in increased ADCC. a (Above) ADCC experimental design schema. (Below) Average (± SD) neutrophil-mediated ADCC against Lan1GFP cells (% of control) measured by Incucyte 6 h after addition of neutrophils (E:T of 10:1) with dinutuximab (5 µg/ml) and GM-CSF (100 ng/ml). Prior to addition, cells were pretreated with EIPA, chlorpromazine, MBCD, or media control for 30 min (4 replicate wells each over 3 individual experiments). b Mean (± SEM) normalized 24 h AUC of 14G2a internalization for Lan1 cells pretreated with twofold dilutions of MBCD as noted or media only (mean of 3 individual replicates). c Average (± SEM) neutrophil-mediated ADCC against Lan1 cells (% of control) measured 6 h after addition of neutrophils (E:T of 10:1), dinutuximab (5 ug/ml) and GM-CSF (100 ng/ml) following the design schema shown in (A). Prior to addition, cells were pretreated with MBCD at twofold dilutions as noted for 30 min. (mean of 3 individual replicates). d Mean (± SD) 24-h AUC (expressed as % of control) of 14G2a internalization for Lan1 cells following a 4-h pre-treatment period with indicated endocytosis inhibitors or media only followed by addition of 14G2a-pHrodo (10 ug/ml) in the continued presence of inhibitor (solid bars) or immediately following drug removal (striped bars). e A modified ADCC experimental design schema is on top. Below, Average (± SD) neutrophil-mediated ADCC against LAN2, CHLA90, and CHLA136 neuroblastoma cells (expressed as % of control) measured 6 h after addition of neutrophils (E:T of 10:1), dinutuximab (1 ug/ml) and GM-CSF (100 ng/ml) is shown. Prior to addition, cells were pretreated with EIPA, chlorpromazine, MBCD or media for 30 min followed by addition of dinutuximab in the continued presence of drug or media for 4 h and then washing the cells 3x to remove drug and dinutuximab. This allowed to evaluate ADCC with remaining cell-bound dinutuximab and evaluate effect of inhibitors. (**p < 0.01, ns = non-significant)
Antibody internalization
Purified 14G2a or dinutuximab was adjusted to 1 mg/ml in PBS followed by addition of pHrodo (ThermoFisher Scientific) in 100% DMSO using a molar ratio of 10 and incubated in the dark at room temperature for 45 min as per manufacturer’s protocol. Unused pHrodo-conjugated mAb was stored at 4 °C. To assess internalization, 105 tumor cells were plated per well in 96 well clear plates, allowed to settle overnight in a 37 °C/5% CO2 humidified incubator followed by addition of pHrodo-labeled 14G2a (14G2a-pHrodo) for a final concentration of 10 µg/ml per well. Plates were placed in an Incucyte S3 live-cell imaging system where phase contrast and red fluorescent images were captured every 30 min for 24 h. The GD2-negative cell line, Lan6, and the highest internalizing cell line, Lan1, were run with each experimental replicate as negative and positive controls, respectively. Data were analyzed using Incucyte S3 software with top-hat background subtraction and an analysis definition customized to the unique morphology of each cell line and applied to all replicate experiments. A measure of ‘red area percent’ was calculated for every timepoint by normalizing red fluorescent area per well to cell confluence (phase area) per well to control for differences in cell plating densities and cell proliferation rates across cell lines and across experimental replicates (Supplementary Fig. 1a). Data were exported from the Incucyte system and, using Prism GraphPad, the red area percent values over 24 h were converted into an area under the curve (AUC) of the internalization values. AUC values of each experimental replicate were then normalized by setting the Lan1 AUC value for that replicate to 1000 and adjusting the other cell line AUCs accordingly to account for differences in pHrodo degree of antibody labeling that may occur across experimental days and contribute to differences in red fluorescence across replicates. AUCs were then averaged across 3–6 wells per experimental replicate and across a minimum of 3 experiments performed on different days for each cell line. All images included in figures were exported using the same image settings and are free of alteration or modification.
For internalization assays in the presence of endocytosis inhibitors, drug dose-finding was first determined by measuring cell viability of Lan1GFP cells over a 24-h incubation with each drug at concentrations commonly reported, along with additional concentrations both above and below. A dose resulting in less than 10% cell death at 24 h was selected for subsequent use. For internalization, 104 Lan1 cells/well were plated in 96-well plates as above and allowed to attach overnight followed by addition of inhibitor of interest the following morning. After 30 min of drug pretreatment, 14G2a-pHrodo was added to wells to a final concentration of 10 µg/ml, the plate was replaced inside the Incucyte and images were captured every 30 min for 24 h as above with corresponding Lan1-specific analysis definition subsequently applied. Internalization AUCs were calculated, converted to percent of the untreated Lan1 AUC for each replicate, and then averaged across 3–6 wells per experiment with a minimum of three replicate experiments. Finally, for drug wash-out assays, 105 Lan1 cells were pretreated with each drug in a 15 ml conical tube incubated at 37 °C for 4 h with manual agitation hourly followed by two washes in IMDM to remove drug, after which cells were plated and internalization assay run as above.
Statistical analysis
Statistical analysis was performed with Prism GraphPad (v.8.4.1). Comparison of values between treatment groups was carried out using unpaired two-tailed t-test with significance set at P < 0.05. Comparison of multiple groups was performed using analysis of variance (ANOVA), one-way for single variable and two-way for analysis of two variables. Pearson correlation coefficient was used to calculate the r value when comparing two independently measured normally distributed numerical variables (i.e., GD2 ABC and internalization AUC).
Results
Level of GD2 expression and sensitivity to neutrophil-mediated ADCC are not correlated
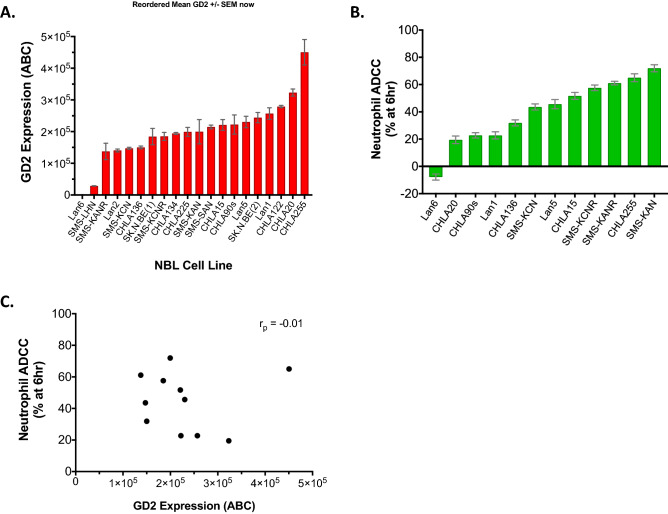
We quantified GD2 expression on the surface of NBL cells using BD Quantibrite beads for 20 human NBL cell lines. This cohort of cell lines was composed of MYCN non-amplified (n = 6) and MYCN amplified (n = 14) cell lines. Thirteen cell lines were established from tissue obtained at time of relapse or disease progression while seven were established from diagnostic tissue samples (Supplementary Table 1). We found that 19 of 20 cell lines expressed GD2, and 18 of the 19 GD2-expressing cell lines expressed > 105 GD2 molecules per cell surface. There was significant heterogeneity in the level of GD2 expression, ranging from around 1 × 105 to 4 × 105 molecules of GD2 per cell on the 18 of 20 cell lines that had high GD2 (p < 0.001) (Fig. 1a). Lan6 was confirmed to be a GD2-negative cell line. GD2 expression also did not significantly differ by MYCN status or disease status at time of cell line establishment (Supplementary Fig. 3). We then examined cell line sensitivity to human neutrophil-mediated ADCC in the presence of anti-GD2 antibody (dinutuximab) for eleven of the GD2-expressing NBL cell lines and the GD2-negative Lan6. Using percent cytotoxicity at 6 h as a measure of cell line sensitivity to neutrophil-mediated ADCC, we observed significant variation across cell lines (range −7.8–72%, p < 0.001) (Fig. 1b). Of the GD2-expressing lines tested, SMS-KAN demonstrated the highest sensitivity to ADCC (72 ± 3% cytotoxicity), while CHLA20 demonstrated the lowest (19.5 ± 3%). The GD2-negative cell line, Lan6, demonstrated no cytotoxicity (−7.8 ± 2%) and this lack of sensitivity to ADCC for Lan6 is consistent with a requirement for GD2 expression for neutrophils to mediate ADCC against NBL cells. To further assess whether the level of GD2 expression is related to degree of resistance to ADCC, we examined these two variables for the cohort of 11 GD2-expressing cell lines for which ADCC was measured and found no significant correlation between GD2 expression level (ABC) and ADCC (r = −0.01, (Fig. 1c), suggesting that differences in sensitivity to neutrophil-mediated ADCC are not solely explained by differences in GD2 expression among GD2-expressing cell lines.
Fig. 1.
Human neuroblastoma cell lines vary significantly in GD2 expression and in sensitivity to neutrophil-mediated ADCC. a Mean (± SEM) GD2 surface expression (ABC = antibodies bound per cell) as measured by flow cytometry and quantified with BD Quantibrite beads (minimum 3 replicates per cell line). b Average (± SEM) neutrophil-mediated ADCC (%) against NBL cell lines measured by digital image microscopy 6 h (hr) after addition of neutrophils (E:T of 10:1) with dinutuximab (5 µg/ml) and GM-CSF (100 ng/ml) (6 replicates per experiment and three independent experiments). c Scatter plot of GD2 expression (mean ABC) versus average neutrophil-mediated ADCC (cytotoxicity % at 6 h) for the 11 GD2-expressing cells lines shown in (B) (r = −0.01)
Differential anti-GD2 antibody internalization inversely correlates with ADCC
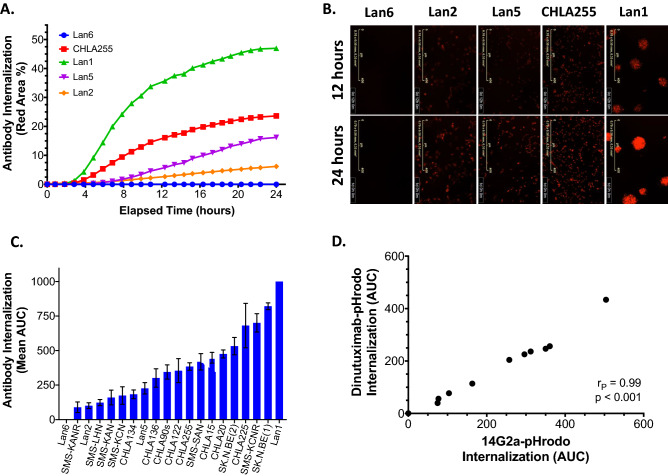
All 19 of the NBL cell lines that expressed GD2 demonstrated internalization of anti-GD2 antibody (14G2a-pHrodo) over 24 h as measured on an Incucyte S3 live-cell imaging system (Supplementary Fig. 1b). The internalization time course data quantified as 24-h AUC demonstrated significant heterogeneity across the 19 GD2-expressing neuroblastoma cell lines (normalized AUC range: 89.8–1000, p < 0.001) (Fig. 2a-c, Supplementary Fig. 1c). As expected, no anti-GD2 antibody internalization was measured for the GD2-negative Lan6 cells (AUC 1.2 ± 0.4). Lan1 cells demonstrated the highest and SMS-KANR cells the lowest antibody internalization rate among GD2-expressing cell lines (normalized AUC of 1000 and 89.9, respectively). The relationship between GD2 expression level and extent of antibody internalization was not directly linear; for example, CHLA255 cells had the highest level of GD2 expression but only the fourth highest rate of antibody internalization (Supplementary Fig. 1), possibly reflecting complex mechanisms involved in the receptor-mediated endocytosis pathway.
Fig. 2.
Human neuroblastoma cell lines differentially internalize anti-GD2 antibody. a Real-time measurement of 14G2a-pHrodo internalization as measured by normalized red fluorescent area (%) over 24 h for four representative GD2-expressing NBL cell lines (Lan1, CHLA255, Lan5, Lan2) and one GD2-negative cell line (Lan6). b Representative red fluorescent images (magnification 10x) corresponding to cell lines depicted in (A) at 12 and 24 h of incubation with 14G2a-pHrodo. c Mean (± SEM) normalized 24 h area under the curve (AUC) of 14G2a antibody internalization for all 20 NBL cell lines tested (3–6 replicate wells per experiment and minimum 3 individual experiments). d Scatter plot of mean internalization AUC for 14G2a-pHrodo versus dinutuximab-pHrodo for ten GD2-expressing NBL cell lines (SMS-KANR, SMS-KCN, SMS-KAN, Lan5, CHLA20, CHLA255, SK.N.BE(2), CHLA15, SK.N.BE(1), Lan1)
With our initial studies using 14G2a, we next aimed to determine if dinutuximab, the only FDA-approved chimeric anti-GD2 antibody for clinical use, was internalized by NBL cells in a similar manner to the fully murine 14G2a antibody, given that the two mAbs share the same antigen-binding fragment specificity. We simultaneously tested internalization of each antibody in ten GD2-expressing NBL cell lines along with GD2-negative Lan6 cells and found a near perfect correlation (r = 0.99) (Fig. 2d, Supplementary Fig. 3a-b).
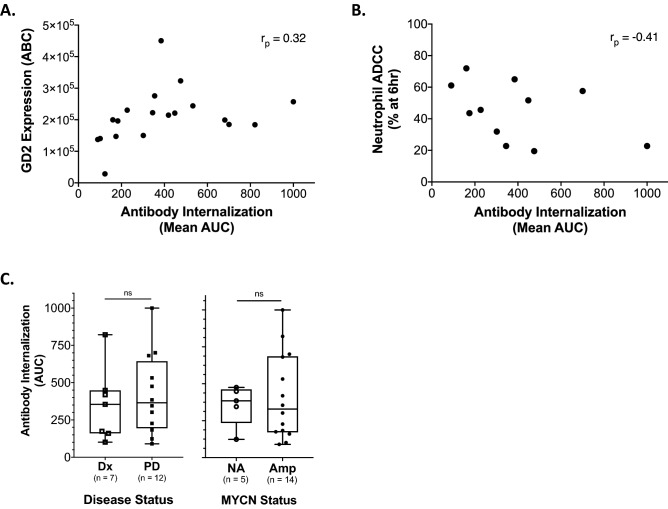
We next evaluated the relationship between GD2 expression, as quantified by the Quantibrite ABC method, and internalization of anti-GD2 antibody over 24 h (mean AUC) and found a weak positive correlation (r = 0.32) (Fig. 3a). We then evaluated for relationship between anti-GD2 antibody internalization over 24 h and cell line sensitivity to neutrophil-mediated ADCC and found a moderate inverse correlation (r = −0.41) (Fig. 3b). There were no significant differences when examining these AUC values in the context of MYCN amplification status or between cell lines established from diagnostic tissue and those established from relapse or progressive disease tissue (Fig. 3c).
Fig. 3.
Internalization of anti-GD2 antibody by human neuroblastoma cell lines is inversely correlated with sensitivity to neutrophil-mediated ADCC in vitro. a Scatter plot of mean GD2 expression (ABC) for cell lines shown in Fig. 1a and corresponding 14G2a-pHrodo internalization 24 h mean normalized AUC (r = 0.32). b Scatter plot of 24 h anti-GD2 internalization AUC (normalized mean) versus average cytotoxicity (% at 6 h) to neutrophil-mediated ADCC (measured on an Incucyte) for the 11 cell lines with both data points assessed (SMS-KANR, SMS-KAN, SMS-KCN, LAN5, CHLA15, CHLA255, CHLA20, CHLA90s, SMS-KCNR, LAN1) (r = −0.41). c Mean 24 h normalized AUC of 14G2a internalization for all tested NBL cell lines sorted by disease status at time of cell line initiation (Dx = diagnostic, PD = progressive Disease; left panel) or MYCN status (NA = non-amplified, Amp = amplified; right panel) (ns = p value non-significant)
Inhibitors of pinocytosis effectively inhibit antibody internalization
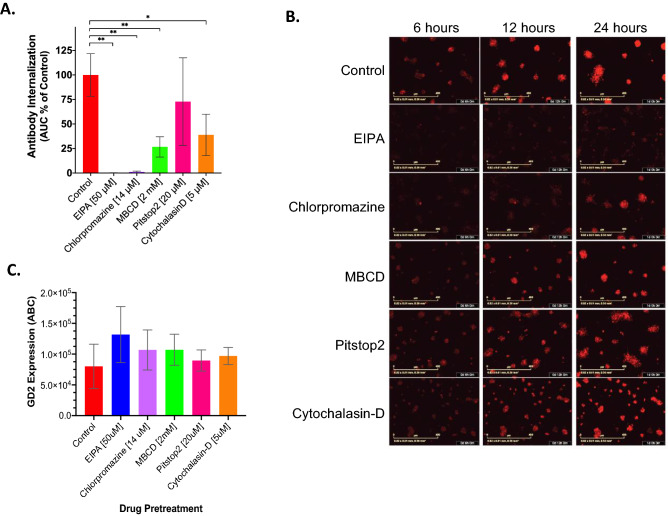
Antigen recycling occurs through various endocytosis pathways which can include pinocytosis and/or phagocytosis with pinocytosis encompassing clathrin-mediated endocytosis (CME), clathrin-independent endocytosis (CIE), and macropinocytosis (21). Given that the specific pathway involved in recycling of GD2 in NBL cells remains unknown, we investigated a select group of endocytosis inhibitors, EIPA, MBCD, chlorpromazine, Pitstop2, and cytochalasin-D, for their effect on internalization of anti-GD2 mAb in an effort to deduce which major endocytosis pathways are involved in antibody internalization by NBL.
We found that antibody internalization by Lan1 cells was significantly inhibited by 4 of the 5 compounds when compared to untreated Lan1 cells. EIPA (99.8% inhibition relative to control, p = 0.001) and chlorpromazine (98.8% inhibition, p = 0.009) treatment resulted in near complete inhibition of antibody internalization. MBCD treatment inhibited internalization by 73.3% compared to control (p = 0.006), and cytochalasin-D treatment inhibited internalization by 61.1% (p = 0.03). Treatment with Pitstop2 did not significantly inhibit internalization (28.2% inhibition, p = 0.40) (Fig. 4a-b). We next investigated the effect of each drug on GD2 expression to ensure that inhibition of internalization seen with drug treatment was not simply due to alterations in surface GD2. Following drug pretreatment at the concentrations shown to inhibit antibody internalization and for the same treatment duration, we quantified surface GD2 expression and found no significant differences in surface expression of GD2 (ABC) when compared to untreated Lan1 cells (p > 0.05) (Fig. 4c).
Fig. 4.
Treatment with endocytosis inhibitors decreases anti-GD2 antibody internalization. a Mean (± SD) 24 h AUC (expressed as % of control) of 14G2a internalization measured by Incucyte for Lan1GFP cells, a high rate antibody-internalizing cell line, following a 30-min pretreatment with endocytosis inhibitors at noted concentrations or media-only control, (*p < 0.05, **p < 0.01). b Representative red fluorescent images of 14G2a internalization by Lan1 subjected to each inhibitor treatment as shown in (A) at 6, 12, and 24 h. c Average (± SD) GD2 surface expression of Lan1 cells by flow cytometry quantified by BD Quantibrite beads following a 30 min treatment with endocytosis inhibitors (EIPA, MBCD, chlorpromazine, Pitstop2) or control (average of 3 replicates; p > 0.05 for all)
Inhibition of antibody internalization results in increased ADCC
We next tested the three most effective inhibitors of internalization, EIPA, chlorpromazine, and MBCD for their ability to affect Lan1 sensitivity to ADCC. In initial ADCC assays, Lan1 cells were pre-treated with drug for 30 min followed by simultaneous addition of anti-GD2 antibody and human neutrophils (Fig. 5a schema). We found that MBCD significantly sensitized Lan1 to ADCC (37.9% increase cytotoxicity over control; p < 0.001) whereas EIPA and chlorpromazine did not (Fig. 5a). The effect of MBCD on both internalization of anti-GD2 antibody and ADCC for Lan1 was also dose-dependent, with decreased effect observed as the concentration of MBCD was decreased (Fig. 5b-c).
We next investigated possible reasons why MBCD, EIPA, and chlorpromazine had differential effects on sensitization of Lan1 to ADCC. To test if the drugs were adversely affecting neutrophil function and thus negatively impacting our ADCC assay, we assessed if removal of drug following a pretreatment course would result in similar inhibition of internalization as when drug remained in wells. We found that the inhibitory effects on antibody internalization were significantly diminished for EIPA and chlorpromazine after drug removal but not for MBCD, despite an extended (4 h) pre-treatment period (Fig. 5d). Thus, we concluded that continuous EIPA and chlorpromazine exposure were needed to ensure inhibition of antibody internalization during the ADCC assay.
With this in mind, and in an effort to allow for internalization of the antibody to occur or to be inhibited in drug-treated cells, we further modified the ADCC assay to separate the addition of antibody from the addition of effector cells by 4 h (see schema in Fig. 5e). When assessed in this modified assay, we were able to measure a statistically significant effect on sensitization of Lan1 to ADCC for EIPA treated cells (2.3-fold increase in cytotoxicity compared to control, p = 0.002) and MBCD treated cells (3.2-fold increase in cytotoxicity compared to control, p = 0.007) (Fig. 5e). Inclusion of three additional cell lines (Lan2, CHLA90, CHLA136) revealed that only MBCD increased ADCC in all 4 of 4 cell lines. After a 4.5-h pre-treatment with MBCD and overlapping 4-h pre-treatment with 14G2a, a washing away of MBCD and mAb immediately prior to addition of neutrophils and GM-CSF had no statistically significant effect on the level of neutrophil-mediated ADCC against 3 of 4 cell lines tested, suggesting a relatively long-lasting effect of MBCD. Overall, our data suggest that inhibition of macropinocytosis with an agent such as MBCD can suppress anti-GD2 antibody internalization and enhance neutrophil-mediated ADCC.
Discussion
While the addition of anti-GD2 antibody to the treatment of children with high-risk NBL has improved survival outcomes, significant challenges remain in controlling the disease in nearly half of patients. Potential mechanisms of resistance to antibody therapy are likely multifactorial and remain poorly understood but in general, can be thought of as paralleling the same factors that affect antibody efficacy. In the case of anti-GD2 antibody, the presence of neutralizing antibodies [22] along with FcγR polymorphisms and associated differences in effector cell function [23–25] can underlie resistance in some but are insufficient to explain all anti-GD2 antibody treatment resistance seen in NBL patients. Our findings provide novel evidence that antibody internalization by NBL cells is an important additional contributing factor mediating resistance to anti-GD2 therapy.
We demonstrate that anti-GD2 antibody internalization occurs in a heterogeneous manner across a large cohort of NBL cell lines. We also demonstrated that NBL cell lines differ in GD2 surface expression and sensitivity to neutrophil-mediated ADCC in vitro but that these two variables are not correlated, indicating that factors other than GD2 expression level are at play in mediating resistance to ADCC. In this regard, we investigated and showed that internalization of anti-GD2 antibody by NBL cell lines is inversely correlated with their sensitivity to neutrophil-mediated ADCC. Finally, we identified an endocytosis-targeting agent, MBCD, with the ability to inhibit anti-GD2 internalization and sensitize 4 of 4 cell lines to neutrophil-mediated ADCC. Cumulatively, the results of these inhibitor studies suggest that anti-GD2 internalization occurs through macropinocytosis rather than clathrin-mediated endocytosis or clathrin-independent endocytosis.
Antibody internalization has been described for other therapeutic antibodies including anti-HER2 and anti-CD20 [14, 15, 26]. An early study of anti-GD2 antibody conjugated to an immunotoxin found its internalization to be associated with cytotoxicity of a GD2-expressing melanoma and carcinoma cell line [27]. Our findings in 20 NBL cell lines now conclusively demonstrate that anti-GD2 antibody internalization occurs in GD2-expressing NBL cell lines but at varying rates and represents the first description that internalization of anti-GD2 mAb by NBL may act as a mechanism of resistance as indicated by its inverse correlation with cell line sensitivity to neutrophil-mediated ADCC. It is worth noting that none of the NBL cell lines evaluated were derived from tumors with prior exposure to anti-GD2 therapy and thus the impact of anti-GD2 antibody exposure on NBL internalization kinetics, if any, remains unknown. Neutrophils and NK cells are both considered important effectors of ADCC against cancer cells. When engaging in ADCC, neutrophils kill by ingestion of parts of antibody-opsonized target cells independently of granule release, in what amounts to a lytic or necrotic process. In contrast, NK cells kill via granule exocytosis that releases perforin and granzymes into the synapse between NK cell and their targets; thus, these two effector cell types kill through non-redundant mechanisms. By inhibiting apoptotic mechanisms, some cancer cells can achieve a degree of resistance to granzymes secreted from NK cells, but these same cells may remain susceptible to engulfment by neutrophils. We focused on neutrophils which exhibit no intrinsic cytotoxicity against NBL cells and which are unaffected by mismatch between killer immunoglobulin receptors on donor immune cells and MHC class I molecules on tumor cells, allowing us to evaluate this important arm of antibody-mediated cytotoxicity mechanistically in relation to GD2 expression and internalization. Future studies are warranted to determine if GD2 internalization affects neutrophil-mediated ADCC in vivo, and to determine if GD2 internalization affects assessment of antigen density and organization within the membrane, an issue separate from expression level, and outside the scope of the present study [28].
There are a number of factors impacting antibody internalization, and targeting these mechanisms is of great interest. However, the variability in endocytosis processes involved in antigen recycling across cell types, along with the promiscuity of inhibitors available to target these pathways, makes their study challenging [21]. Compounding this complexity is the potential for many inhibitors to have unintended effects on other cellular processes in both target and effector cells which creates an added challenge when evaluating inhibitors in coculture ADCC assays. Our data, demonstrating significant inhibition of anti-GD2 antibody internalization by EIPA, MBCD, and cytochalasin-D but not by Pitstop2 (primarily a CME inhibitor), suggest that macropinocytosis most likely is involved in anti-GD2 internalization. The inhibitory effect of chlorpromazine along with MBCD suggests a possible concurrent role for CIE as well. Thus, it is possible that two separate processes, dependent on ligand binding, underlie antibody internalization within a single NBL cell. This has been reported for CD46 on the surface of non-lymphoid cells in which CME predominates for routine antigen recycling but is switched to internalization via macropinocytosis following antibody binding and resultant antigen crosslinking [29].
In examining the effect of three of these drugs on ADCC sensitivity of Lan1, our initial in vitro ADCC assay (Fig. 5a) was only able to detect a significant sensitizing effect by MBCD. When Lan1 cells were instead preincubated with antibody for an extended time to allow for internalization to occur in control cells (less antibody present for ADCC) or in the presence of an antibody internalization inhibitor (more antibody present for ADCC) prior to addition of neutrophils, sensitization of Lan1 to ADCC was observed for both EIPA and MBCD. These data support the role of antibody internalization as a mechanism of resistance to ADCC since macropinocytosis agents found to slow internalization also resulted in greater ADCC effect. However, we did observe a discrepancy between the degree of antibody internalization inhibition and ADCC sensitization by EIPA and chlorpromazine. While not tested directly in the present study, both EIPA and chlorpromazine have been documented to negatively affect neutrophil cytotoxic potential, EIPA through decreasing neutrophil production of reactive oxygen species [30] and chlorpromazine through its inhibitory effects on neutrophil maturity [31, 32] as well as impairing neutrophil priming and respiratory burst activity [33]. The need to have EIPA and chlorpromazine continuously in the media to inhibit anti-GD2 internalization precludes us from decoupling their desired effect on antibody internalization from their possible detrimental effect on neutrophils. Nevertheless, the results seen with MBCD treatment in enhancing ADCC against 4 of 4 cell lines, despite the potential side effects of hemolysis and renal toxicity from β-cyclodextrins such as MBCD, provide proof-of-principle for using endocytosis inhibition to enhance ADCC.
The role of anti-GD2 antibody therapy in the treatment of NBL is continuing to evolve with its success, prompting its incorporation into upfront treatment of newly diagnosed children with high-risk NBL in a recent Children’s Oncology Group clinical trial [34]. GD2-targeted antibody therapy is additionally being evaluated for use in GD2-expressing osteosarcoma and small cell lung cancer [35, 36] and has the potential for broader use since other pediatric solid malignancies have been shown to express GD2 [37]. Overall, our results suggest that while GD2 expression is required for ADCC, the level of expression cannot be used to predict subsequent cytotoxicity. Our data suggest that anti-GD2 antibody internalization, found to universally occur in all GD2-expressing NBL cell lines, is likely a mechanism of resistance to anti-GD2 immunotherapy. Further study into mechanisms underlying differential anti-GD2 internalization and identifying clinically relevant inhibitors of antibody internalization may help in designing new multimodal therapies aimed at improving outcomes in children with NBL.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to acknowledge Drs. Yves DeClerck, Nora Heisterkamp, and Chintan Parekh for manuscript review, and Drs. John Li and Alan Wayne for their valuable input.
Author contributions
RT and SA conceived and designed the study. RT, KKY, SM, ML performed the research and also analyzed the data. SM, CJ, TP, and MS contributed reagents and analysis tools. The manuscript was written by RT, SA, and MS. All authors edited and approved the submission of this manuscript.
Funding
This work was supported, in part, by a T32 CA009659 training grant (RT) from the National Cancer Institute (PI Y.A. DeClerck) and by the V Foundation (SA); and in part by the CA170257P1 from the Department of Defense (SA), P01 CA217959 National Cancer Institute (co-I SA, PI R.C. Seeger, J Maris), T.J. Martell Foundation, and Nautica Malibu Triathlon (SA).
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Pinto NR, Applebaum MA, Volchenboum SL, et al. Advances in risk classification and treatment strategies for neuroblastoma. J Clin Oncol Off J Am Soc Clin Oncol. 2015;33:3008–3017. doi: 10.1200/jco.2014.59.4648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheung NK, Cheung IY, Kushner BH, Ostrovnaya I, Chamberlain E, Kramer K, Modak S. Murine anti-GD2 monoclonal antibody 3F8 combined with granulocyte-macrophage colony-stimulating factor and 13-cis-retinoic acid in high-risk patients with stage 4 neuroblastoma in first remission. J Clin Oncol Off J Am Soc Clin Oncol. 2012;30:3264–3270. doi: 10.1200/jco.2011.41.3807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ladenstein R, Pötschger U, Valteau-Couanet D, et al. Interleukin 2 with anti-GD2 antibody ch14.18/CHO (dinutuximab beta) in patients with high-risk neuroblastoma (HR-NBL1/SIOPEN): a multicentre, randomised, phase 3 trial. Lancet Oncol. 2018;19:1617–1629. doi: 10.1016/s1470-2045(18)30578-3. [DOI] [PubMed] [Google Scholar]
- 4.Yu AL, Gilman AL, Ozkaynak MF, et al. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N Engl J Med. 2010;363:1324–1334. doi: 10.1056/NEJMoa0911123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Federico SM, McCarville MB, Shulkin BL, et al. A pilot trial of humanized Anti-GD2 monoclonal antibody (hu14.18K322A) with chemotherapy and natural killer cells in children with recurrent/refractory neuroblastoma. Clin Cancer Res Off J Am Assoc Cancer Res. 2017;23:6441–6449. doi: 10.1158/1078-0432.Ccr-17-0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mody R, Naranjo A, Van Ryn C, et al. Phase II randomized trial of rinotecan/temozolomide (I/T) with temsirolimus (TEM) or dinutuximab plus granulocyte colony stimulating factor (DIN/GMCSF) in children with refractory or relapsed neuroblastoma: A report from the Children's Oncology Group (COG) J Clin Oncol. 2016;34:2. doi: 10.1200/JCO.2016.34.15_suppl.10502. [DOI] [Google Scholar]
- 7.Mody R, Yu AL, Naranjo A et al. (2020) Irinotecan, Temozolomide, and Dinutuximab With GM-CSF in Children With Refractory or Relapsed Neuroblastoma: A Report From the Children's Oncology Group. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 10.1200/jco.20.00203 [DOI] [PMC free article] [PubMed]
- 8.Schumacher-Kuckelkorn R, Volland R, Gradehandt A, Hero B, Simon T, Berthold F. Lack of immunocytological GD2 expression on neuroblastoma cells in bone marrow at diagnosis, during treatment, and at recurrence. Pediatr Blood Cancer. 2017;64:46–56. doi: 10.1002/pbc.26184. [DOI] [PubMed] [Google Scholar]
- 9.Batova A, Kamps A, Gillies SD, Reisfeld RA, Yu AL. The Ch14.18-GM-CSF fusion protein is effective at mediating antibody-dependent cellular cytotoxicity and complement-dependent cytotoxicity in vitro. Clin Cancer Res Off J Am Assoc Cancer Res. 1999;5:4259–4263. [PubMed] [Google Scholar]
- 10.Chen RL, Reynolds CP, Seeger RC. Neutrophils are cytotoxic and growth-inhibiting for neuroblastoma cells with an anti-GD2 antibody but, without cytotoxicity, can be growth-stimulating. Cancer Immunol Immunother CII. 2000;48:603–612. doi: 10.1007/s002620050008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zeng Y, Fest S, Kunert R, Katinger H, Pistoia V, Michon J, Lewis G, Ladenstein R, Lode HN. Anti-neuroblastoma effect of ch14.18 antibody produced in CHO cells is mediated by NK-cells in mice. Mol Immunol. 2005;42:1311–1319. doi: 10.1016/j.molimm.2004.12.018. [DOI] [PubMed] [Google Scholar]
- 12.Thomas VA, Balthasar JP. Understanding inter-individual variability in monoclonal antibody disposition. Antibodies (Basel, Switzerland) 2019 doi: 10.3390/antib8040056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beers SA, French RR, Chan HT, et al. Antigenic modulation limits the efficacy of anti-CD20 antibodies: implications for antibody selection. Blood. 2010;115:5191–5201. doi: 10.1182/blood-2010-01-263533. [DOI] [PubMed] [Google Scholar]
- 14.Lim SH, Vaughan AT, Ashton-Key M, et al. Fc gamma receptor IIb on target B cells promotes rituximab internalization and reduces clinical efficacy. Blood. 2011;118:2530–2540. doi: 10.1182/blood-2011-01-330357. [DOI] [PubMed] [Google Scholar]
- 15.Rudnick SI, Lou J, Shaller CC, Tang Y, Klein-Szanto AJ, Weiner LM, Marks JD, Adams GP. Influence of affinity and antigen internalization on the uptake and penetration of Anti-HER2 antibodies in solid tumors. Cancer Res. 2011;71:2250–2259. doi: 10.1158/0008-5472.Can-10-2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reddy V, Cambridge G, Isenberg DA, Glennie MJ, Cragg MS, Leandro M (2015) Internalization of rituximab and the efficiency of B Cell depletion in rheumatoid arthritis and systemic lupus erythematosus. Arthritis & rheumatology (Hoboken, N.J.). 67: 2046–55. doi: 10.1002/art.39167 [DOI] [PMC free article] [PubMed]
- 17.Fewou SN, Rupp A, Nickolay LE, Carrick K, Greenshields KN, Pediani J, Plomp JJ, Willison HJ. Anti-ganglioside antibody internalization attenuates motor nerve terminal injury in a mouse model of acute motor axonal neuropathy. J Clin Investig. 2012;122:1037–1051. doi: 10.1172/jci59110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keshelava N, Seeger RC, Groshen S, Reynolds CP. Drug resistance patterns of human neuroblastoma cell lines derived from patients at different phases of therapy. Cancer Res. 1998;58:5396–5405. [PubMed] [Google Scholar]
- 19.Keshelava N, Zuo JJ, Chen P, Waidyaratne SN, Luna MC, Gomer CJ, Triche TJ, Reynolds CP. Loss of p53 function confers high-level multidrug resistance in neuroblastoma cell lines. Cancer Res. 2001;61:6185–6193. [PubMed] [Google Scholar]
- 20.Keshelava N, Frgala T, Krejsa J, Kalous O, Reynolds CP. DIMSCAN: a microcomputer fluorescence-based cytotoxicity assay for preclinical testing of combination chemotherapy. Methods Mol Med. 2005;110:139–153. doi: 10.1385/1-59259-869-2:139. [DOI] [PubMed] [Google Scholar]
- 21.Dutta D, Donaldson JG. Search for inhibitors of endocytosis: intended specificity and unintended consequences. Cell Logist. 2012;2:203–208. doi: 10.4161/cl.23967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Siebert N, Troschke-Meurer S, Marx M et al. (2018) Impact of HACA on Immunomodulation and Treatment Toxicity Following ch14.18/CHO Long-Term Infusion with Interleukin-2: Results from a SIOPEN Phase 2 Trial. Cancers. 10. doi: 10.3390/cancers10100387 [DOI] [PMC free article] [PubMed]
- 23.Metelitsa LS, Gillies SD, Super M, Shimada H, Reynolds CP, Seeger RC. Antidisialoganglioside/granulocyte macrophage-colony-stimulating factor fusion protein facilitates neutrophil antibody-dependent cellular cytotoxicity and depends on FcgammaRII (CD32) and Mac-1 (CD11b/CD18) for enhanced effector cell adhesion and azurophil granule exocytosis. Blood. 2002;99:4166–4173. doi: 10.1182/blood.v99.11.4166. [DOI] [PubMed] [Google Scholar]
- 24.Siebert N, Jensen C, Troschke-Meurer S, Zumpe M, Juttner M, Ehlert K, Kietz S, Muller I, Lode HN (2016) Neuroblastoma patients with high-affinity FCGR2A, -3A and stimulatory KIR 2DS2 treated by long-term infusion of anti-GD2 antibody ch14.18/CHO show higher ADCC levels and improved event-free survival. Oncoimmunology. 5: e1235108. doi: 10.1080/2162402x.2016.1235108 [DOI] [PMC free article] [PubMed]
- 25.Wang W, Erbe AK, Hank JA, Morris ZS, Sondel PM. NK cell-mediated antibody-dependent cellular cytotoxicity in cancer immunotherapy. Front Immunol. 2015;6:368. doi: 10.3389/fimmu.2015.00368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fehling-Kaschek M, Peckys DB, Kaschek D, Timmer J, Jonge N. Mathematical modeling of drug-induced receptor internalization in the HER2-positive SKBR3 breast cancer cell-line. Sci Rep. 2019;9:12709. doi: 10.1038/s41598-019-49019-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wargalla UC, Reisfeld RA. Rate of internalization of an immunotoxin correlates with cytotoxic activity against human tumor cells. Proc Natl Acad Sci USA. 1989;86:5146–5150. doi: 10.1073/pnas.86.13.5146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ackerman ME, Pawlowski D, Wittrup KD. Effect of antigen turnover rate and expression level on antibody penetration into tumor spheroids. Mol Cancer Ther. 2008;7:2233–2240. doi: 10.1158/1535-7163.Mct-08-0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Crimeen-Irwin B, Ellis S, Christiansen D, Ludford-Menting MJ, Milland J, Lanteri M, Loveland BE, Gerlier D, Russell SM. Ligand binding determines whether CD46 is internalized by clathrin-coated pits or macropinocytosis. J Biol Chem. 2003;278:46927–46937. doi: 10.1074/jbc.M308261200. [DOI] [PubMed] [Google Scholar]
- 30.Diaz FE, Dantas E, Cabrera M, et al. Fever-range hyperthermia improves the anti-apoptotic effect induced by low pH on human neutrophils promoting a proangiogenic profile. Cell Death Dis. 2016 doi: 10.1038/cddis.2016.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Delieu JM, Badawoud M, Williams MA, Horobin RW, Duguid JK. Antipsychotic drugs result in the formation of immature neutrophil leucocytes in schizophrenic patients. J Psychopharmacol. 2001;15:191–194. doi: 10.1177/026988110101500306. [DOI] [PubMed] [Google Scholar]
- 32.Delieu JM, Horobin RW, Duguid JK. Exploring the relationship of drug-induced neutrophil immaturity & haematological toxicity to drug chemistry using quantitative structure-activity models. Med Chem. 2009;5:7–14. doi: 10.2174/157340609787049307. [DOI] [PubMed] [Google Scholar]
- 33.Creed TM, Tandon S, Ward RA, McLeish KR. Endocytosis is required for exocytosis and priming of respiratory burst activity in human neutrophils. Inflamm Res. 2017;66:891–899. doi: 10.1007/s00011-017-1070-2. [DOI] [PubMed] [Google Scholar]
- 34.Dinutuximab, Sargramostim, and Combination Chemotherapy in Treating Patients With Newly Diagnosed High-Risk Neuroblastoma Undergoing Stem Cell Transplant. https://ClinicalTrials.gov/show/NCT03786783
- 35.Dinutuximab and Irinotecan Versus Irinotecan to Treat Subjects With Relapsed or Refractory Small Cell Lung Cancer. https://ClinicalTrials.gov/show/NCT03098030
- 36.Dinutuximab in Combination With Sargramostim in Treating Patients With Recurrent Osteosarcoma. https://ClinicalTrials.gov/show/NCT02484443.
- 37.Dobrenkov K, Ostrovnaya I, Gu J, Cheung IY, Cheung NK. Oncotargets GD2 and GD3 are highly expressed in sarcomas of children, adolescents, and young adults. Pediatr Blood Cancer. 2016;63:1780–1785. doi: 10.1002/pbc.26097. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.