Abstract
Metformin has been widely used as the treatment of type II diabetes mellitus for its anti-hyperglycemic effect. In recent years, it has also been extensively studied for its anti-cancer effect as it diminishes immune exhaustion of CD8 + tumor-infiltrating lymphocytes (TILs). It decreases apoptosis of CD8 + TILs, thereby enhancing T cell-mediated immune response to tumor cells. Here, we present a unique case of a patient with small cell lung cancer (SCLC) who exhibited an overall partial response as per Response Evaluation Criteria in Solid Tumors, version 1.1 (RECIST 1.1) since starting metformin in combination with nivolumab therapy. Our patient had been treated with nivolumab monotherapy for 2 years until she had progression of disease. After she was started on metformin along with nivolumab therapy, she has shown a significant durable response for over 6 months. Many patients develop resistance to immunotherapy such as antibodies against cytotoxic T lymphocyte-associated protein 4 (CTLA-4), programmed cell death 1 (PD-1), and programmed cell death ligand 1 (PD-L1). Tumor hypoxia is one of the resistance factors. Signals activated by hypoxic environments in tumors are associated with decreased sensitivity to the PD-1 blockade. Metformin inhibits oxygen consumption in tumor cells in vitro and in vivo, reducing intratumoral hypoxia. These data suggest that metformin can improve susceptibility to anti-PD-1 treatment. To the best of our knowledge, our case is the first reported example demonstrating a proof-of-concept that metformin can contribute to overcoming acquired resistance to PD-1 inhibitors.
Keywords: Metformin, Small cell lung cancer, Immunotherapy, Acquired resistance, Durable response
Introduction
In recent years, immune checkpoint inhibitors (ICIs) have shown promising efficacy in treating various cancer types. However, little is known about the mechanism of resistance to immunotherapy agents. Research efforts are now focusing on identifying new combinatorial approaches to increase tumor immunogenicity and overcome resistance to ICIs.
Metformin is one of the most widely prescribed oral anti-hyperglycemic agents for type II diabetes mellitus. Numerous retrospective and preclinical studies have demonstrated anti-cancer properties of metformin and possible risk reduction of cancer development [1]. Preclinical experiments have revealed that metformin prevents immunologic exhaustion of CD8 + lymphocytes and thus amplifies the immune response to cancer cells [2].
We have previously reported a case of a patient with non-small cell lung cancer (NSCLC) that showed reversal of disease progression with the addition of metformin to nivolumab regimen [3]. Here, we describe a case of a patient with small cell lung cancer (SCLC) with a significant durable response to metformin in combination with nivolumab therapy for over 6 months. The patient had been treated with nivolumab monotherapy for 2 years until she had progression of disease. After she was started on metformin along with nivolumab, she exhibited an overall partial response as per the Response Evaluation Criteria in Solid Tumors, version 1.1 (RECIST 1.1) [4].
Case presentation
A 64-year-old woman with no known past medical history initially presented to the emergency department with a complaint of acute dyspnea. Diagnostic computed tomography (CT) scan demonstrated a large mediastinal mass measuring 5.6 cm and multiple enlarged lymph nodes in the mediastinum and supraclavicular area. CT-guided biopsy of the mediastinal mass revealed stage III-B poorly differentiated small cell carcinoma. Immunohistochemistry of the mediastinal mass was positive for cytokeratin AE1/AE3, chromogranin, CD56. Next-generation sequencing (NGS) of circulating tumor DNA (ctDNA) was performed and it showed 21% of variant allele frequency (VAF) in breast cancer gene (BRCA1 E1017K) and 1.6% of VAF in anaplastic lymphoma kinase gene (ALK T1312T). The patient was started on concurrent chemoradiation therapy. She received radiation therapy (54 Gy) for 5 weeks with cisplatin 60 mg/m2 on day 1 and etoposide 120 mg/m2 on day 1, 2, 3 every 3 weeks. The size of the mediastinal mass decreased from 5.6 to 0.7 cm after she completed four cycles of cisplatin and etoposide.
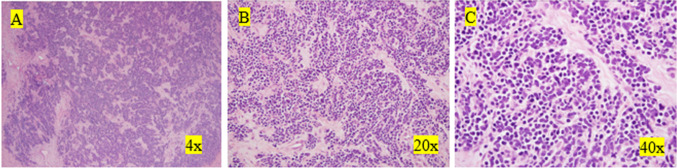
One year after the completion of chemotherapy, a follow-up CT scan was performed. The scan revealed an enlarged cystic and solid mass in the left ovary with enhancing nodular components and septations within the lesion, which was highly suspicious for malignancy. Hence, laparoscopic left salpingo-oophorectomy was performed. A 10.5 × 9.0 × 3.5-cm-sized left adnexa lesion was resected. Pathology findings in the resected specimen were consistent with metastatic small cell carcinoma with extensive necrosis (Fig. 1). A repeat CT scan obtained 2 months after the surgery showed a new soft tissue nodule measuring 1.8 cm and a cystic lesion measuring 5.6 cm in the left para-aortic chain. The patient subsequently received intravenous nivolumab 3 mg/kg every 2 weeks and radiation therapy (55 Gy) to the para-aortic lesions for 5 weeks. After completing radiation therapy, the nodular para-aortic lesion had resolved. Her disease remained stable over 2 years with continued shrinkage of the cystic lesion to 1.5 cm with nivolumab monotherapy.
Fig. 1.
Images of metastatic SCLC to the ovary with hematoxylin and eosin stain. A Nests, cords, and trabeculae of small blue cells with associated desmoplastic stroma. B Nuclear size variation, nuclear molding, and nested architecture. Intervening stroma shows desmoplastic response. C Frequent mitotic figures, nuclear molding, and speckled "salt and pepper" chromatin pattern
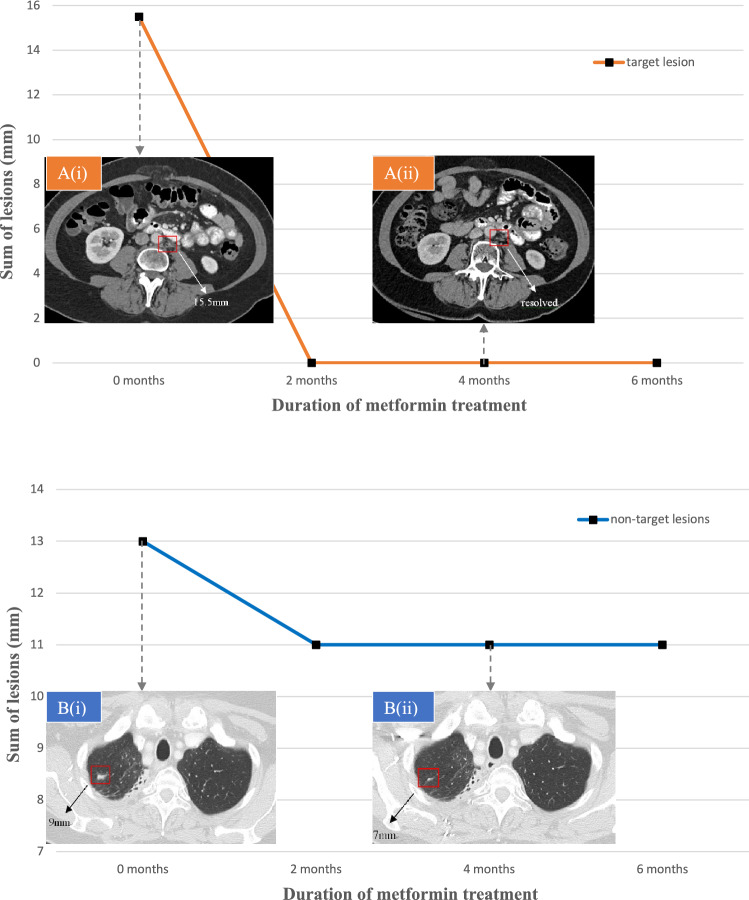
However, a repeat CT scan showed two new small scattered nodules measuring 0.9 cm and 0.4 cm, respectively, in lungs. As an experimental approach, the decision was made to start metformin in combination with nivolumab 3 mg/kg every 2 weeks. Metformin was administered at 1000 mg/day during the first week, 1500 mg/day during the second week, then 2000 mg/day thereafter, in divided doses. A follow-up CT scan at 2 months of combination therapy of metformin and nivolumab demonstrated resolution of a 15.5-mm-sized cystic lesion in the para-aortic area, as well as a decrease in the sum of the lung nodules from 13 to 11 mm. This indicates complete response (CR) of the target lesion in the left para-aortic chain and stable disease (SD) of the non-target lesions in lungs; overall partial response according to RECIST 1.1 (Fig. 2). These measurements and evaluations were carried out by radiologists. The patient subsequently exhibited a significant durable response to metformin and nivolumab combination therapy without any side effects for over 6 months.
Fig. 2.
A target lesion and non-target lesions showing overall partial response. CT scans demonstrating two representative tumor lesions at 0 months (i) and 4 months (ii) of treatment with metformin and nivolumab. A A para-aortic cystic lesion B A nodule in the right upper lobe
Results
To the best of our knowledge, this is the first reported case that exhibited an overall partial response for more than 6 months after starting metformin on a SCLC patient with acquired resistance to nivolumab. Prior to the addition of metformin, the course of her disease showed multiple progressions on multiple modalities including surgery, chemoradiation therapy, and immunotherapy such as nivolumab. The addition of metformin reversed disease progression on treatment with nivolumab monotherapy. Our case suggests that metformin possibly overcomes acquired resistance to programmed cell death 1(PD-1) inhibitors. This is a proof-of-concept demonstrating that metformin can contribute to overcoming acquired resistance to PD-1 inhibitors.
Discussion
SCLC is one of the most aggressive types of cancer, with a median survival time of less than a year after the diagnosis [5]. There are a few FDA-approved immunotherapy drugs for SCLC. Durvalumab and atezolizumab are approved as first-line treatment for extensive-disease (ED) SCLC in combination with platinum-based systemic chemotherapy (NCT03043872, NCT02763579) [6, 7]. Nivolumab and pembrolizumab are approved as a third-line and beyond therapy (NCT01928394) [8]. However, a phase III study of atezolizumab or durvalumab added to platinum-based systemic chemotherapy as first-line treatment for patients with ED-SCLC showed a gain in median overall survival of only 2 or 3 months [6, 9]. Treatment with nivolumab also did not improve response or survival rates over standard chemotherapy in patients with SCLC who relapsed after the first-line treatment [10]. As for maintenance treatment, either monotherapy with nivolumab or dual therapy with nivolumab and ipilimumab did not prolong the overall survival (NCT02538666) [11]. In the recent clinical trial, the combination of pembrolizumab and chemotherapy did not show a statistically significant difference in overall survival compared with chemotherapy alone (NCT03066778) [12]. Overall, immunotherapy has shown marginal benefit for SCLC [13].
We have previously reported a case of a NSCLC patient who responded to a combination of metformin and nivolumab. The patient’s disease previously progressed on nivolumab monotherapy but showed stabilization with the addition of metformin to nivolumab regimen for over 20 months [3]. In addition, we are conducting a Phase II study to find the benefits of combining nivolumab with metformin in advanced NSCLC with and without prior treatment with immunotherapy (NCT03048500). There is another ongoing clinical trial investigating the efficacy and safety of metformin in combination with sintilimab, a PD-1 inhibitor, in SCLC patients resistant to or relapsed after standard chemotherapy without exposure to prior immunotherapy (NCT03994744).
Many patients do not benefit from immunotherapy such as antibodies against cytotoxic T lymphocyte-associated protein 4 (CTLA-4), PD-1, and programmed cell death ligand 1 (PD-L1). They either exhibit primary resistance to these immunotherapy agents or develop acquired resistance after initially responding to them [14]. Tumor hypoxia is one of the resistance factors. Signals activated by hypoxic environments in tumors are associated with decreased sensitivity to the PD-1 blockade. Metformin inhibits oxygen consumption in tumor cells in vitro and in vivo, reducing intratumoral hypoxia. These data suggest that metformin can improve susceptibility to anti-PD-1 treatment [15].
Recent data have revealed an immunomodulatory effect of metformin on cancer cells. CD8 + TILs are defined as lymphocytes with the capacity to recognize, infiltrate, and attack the tumor cells. Metformin prevents immune exhaustion of CD8 + TILs. It decreases apoptosis of CD8 + TILs and also increases phenotype switching of CD8 + TILs to effector memory T cells. Thus, metformin amplifies T cell-mediated immune response to tumor cells [2].
Metformin is also well known for a good safety profile. Its most common side effects are gastrointestinal symptoms. Moreover, metformin showed a low incidence of dose-limiting toxicity (DLT) in combination with a variety of anti-cancer systemic regimens [16]. Thus, metformin will likely be a tolerable addition to immunotherapy regimens.
Conclusions
Our report strongly suggests metformin as a means to overcome acquired resistance to anti-PD-1/PD-L1 drugs such as nivolumab. Further clinical trials are needed to confirm the anti-cancer effects of metformin in combination with immunotherapy agents in SCLC. Predicting metformin efficacy in anti-cancer treatment response remains a challenge [17]. Further studies exploring predictive biomarkers associated with anti-tumor effects of metformin are warranted in the setting of immuno-oncology.
Acknowledgements
Andrew Alexander is acknowledged for providing pictures of Fig. 1 and writing captions.
Abbreviations
- NSCLC
Non-small cell lung cancer
- CT
Computed tomography
- ED
Extensive-disease
- ICIs
Immune checkpoint inhibitors
- PD-1
Programmed cell death 1
- PD-L1
Programmed cell death ligand 1
- RECIST 1.1
Response Evaluation Criteria in Solid Tumors, version 1.1
- SCLC
Small cell lung cancer
- TILs
Tumor-infiltrating lymphocytes
Author contributions
YK: writing and revising the manuscript content. YKC: supervision and validation. Others: review and editing. All authors read and approved the final version of the manuscript.
Funding
Not applicable.
Code availability
Not applicable.
Compliance with ethical standards
Conflict of interests
Young Kwang Chae—Research Grant: Abbvie, BMS, Biodesix, Lexent Bio, and Freenome; Honoraria/Advisory Boards: Roche/Genentech, AstraZeneca, Foundation Medicine, Counsyl, Neogenomics, Guardant Health, Boehringher Ingelheim, Biodesix, Immuneoncia, Lilly Oncology, Merck, Takeda, Pfizer, and Tempus.
Ethical approval
We have obtained written informed consent from the patient for the publication of the case report.
Availability of data and material
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
Consent for publication
We have obtained written informed consent from the patient for the publication of the case report.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Chae YK, et al. Repurposing metformin for cancer treatment: current clinical studies. Oncotarget. 2016;7(26):40767–40780. doi: 10.18632/oncotarget.8194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scharping NE, et al. Efficacy of PD-1 blockade is potentiated by metformin-induced reduction of tumor hypoxia. Cancer Immunol Res. 2017;5(1):9–16. doi: 10.1158/2326-6066.CIR-16-0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Viveiros P, et al. EP1.04-12 response to combination of metformin and nivolumab in a NSCLC patient whose disease previously progressed on nivolumab. J Thor Oncol. 2019;14(10):S976. doi: 10.1016/j.jtho.2019.08.2141. [DOI] [Google Scholar]
- 4.Eisenhauer EA, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45(2):228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 5.Wang S, et al. Survival changes in patients with small cell lung cancer and disparities between different sexes, socioeconomic statuses and ages. Sci Rep. 2017;7(1):1339. doi: 10.1038/s41598-017-01571-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paz-Ares L, et al. Durvalumab plus platinum–etoposide versus platinum–etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. Lancet. 2019;394(10212):1929–1939. doi: 10.1016/S0140-6736(19)32222-6. [DOI] [PubMed] [Google Scholar]
- 7.Horn L, et al. First-Line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. New Engl J Med. 2018;379(23):2220–2229. doi: 10.1056/NEJMoa1809064. [DOI] [PubMed] [Google Scholar]
- 8.Antonia SJ, et al. Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): a multicentre, open-label, phase 1/2 trial. Lancet Oncol. 2016;17(7):883–895. doi: 10.1016/S1470-2045(16)30098-5. [DOI] [PubMed] [Google Scholar]
- 9.Horn L, et al. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med. 2018;379(23):2220–2229. doi: 10.1056/NEJMoa1809064. [DOI] [PubMed] [Google Scholar]
- 10.Reck M, et al. Efficacy and safety of nivolumab (nivo) monotherapy versus chemotherapy (chemo) in recurrent small cell lung cancer (SCLC): results from CheckMate 331. Ann Oncol. 2018;1:29. [Google Scholar]
- 11.Owonikoko TK, et al. Nivolumab (nivo) plus ipilimumab (ipi), nivo, or placebo (pbo) as maintenance therapy in patients (pts) with extensive disease small cell lung cancer (ED-SCLC) after first-line (1L) platinum-based chemotherapy (chemo): results from the double-blind, randomized phase III CheckMate 451 study. Ann Oncol. 2019;30:77. doi: 10.1093/annonc/mdz094. [DOI] [Google Scholar]
- 12.Tucker N. Pembrolizumab plus chemotherapy phase III study shows mixed results in sclc, in target oncology. Oncology. 2020;3:6. [Google Scholar]
- 13.Pavan A, et al. Immunotherapy in small-cell lung cancer: from molecular promises to clinical challenges. J ImmunoThera Cancer. 2019;7(1):205. doi: 10.1186/s40425-019-0690-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chae YK, Oh MS, Giles FJ. Molecular biomarkers of primary and acquired resistance to T-cell-mediated immunotherapy in cancer: landscape, clinical implications, and future directions. Oncol. 2018;23(4):410–421. doi: 10.1634/theoncologist.2017-0354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou X, et al. Metformin suppresses hypoxia-induced stabilization of HIF-1 alpha through reprogramming of oxygen metabolism in hepatocellular carcinoma. Oncotarget. 2016;7(1):873–884. doi: 10.18632/oncotarget.6418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Joshua AM, et al. A pilot ‘window of opportunity’ neoadjuvant study of metformin in localised prostate cancer. Prostate Cancer Prostatic Dis. 2014;17(3):252–258. doi: 10.1038/pcan.2014.20. [DOI] [PubMed] [Google Scholar]
- 17.Camacho L, Dasgupta A, Jiralerspong S. Metformin in breast cancer-an evolving mystery. Breast Cancer Res. 2015;17(1):88. doi: 10.1186/s13058-015-0598-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.