Abstract
Given the poor prognosis of MYC-overexpressing diffuse large B cell lymphoma (DLBCL) and B cell lymphoma unclassifiable with features intermediate between DLBCL and Burkitt lymphoma/high grade B cell lymphoma (BCLU/HGBL), and preclinical data suggesting that MYC may regulate the antitumor immune response, we sought to characterize expression of immune checkpoint proteins on tumor tissue from patients diagnosed with these lymphomas. Immunohistochemical staining for immune checkpoint protein expression was applied to 56 cases of MYC-overexpressing DLBCL and BCLU/HGBL, 35 of which also harbored MYC rearrangement (MYC-R). Analysis revealed both frequent overexpression of immune checkpoint proteins as well as differences in overexpression patterns based upon MYC-R status, with MYC-R cases more likely to overexpress PD-L1 and PD-1 in the tumor microenvironment (50 vs. 15%, p = 0.02 and 32 vs. 5%, p = 0.02, respectively) but less likely to overexpress CTLA-4 and CD80 on tumor cells (34 vs. 71%, p = 0.01 and 34 vs. 81%, p = 0.001, respectively), as compared to cases without MYC-R. These data may suggest a biologic rationale for investigation of the effect of checkpoint inhibitor therapies in these subgroups of MYC-overexpressing DLBCL and BCLU/HGBL.
Keywords: MYC, Diffuse large B cell lymphoma, High grade B cell lymphoma, PD-1, PD-L1
Introduction
Patients diagnosed with diffuse large B cell lymphoma (DLBCL) and the recently re-classified histology of B cell lymphoma unclassifiable with features intermediate between DLBCL and Burkitt lymphoma/high grade B cell lymphoma (BCLU/HGBL) experience variable outcomes following receipt of the standard front-line immunochemotherapy regimen of rituximab with cyclophosphamide, doxorubicin, vincristine and prednisone (R-CHOP). One of the more well-characterized poor prognosis features of these diseases at initial diagnosis is the presence of MYC rearrangement (MYC-R) [1, 2] as well as overexpression of MYC protein [3, 4], independent of MYC-R status, and testing for these MYC alterations is frequently performed in clinical practice. Given a high likelihood that patients with these MYC-altered lymphomas will develop chemorefractory disease, treatment with non-cytotoxic agents may be beneficial.
Immune checkpoint inhibitors have emerged as effective therapies in solid tumor malignancies as well as classical Hodgkin lymphoma, and has led to approval of inhibitors of programmed cell death protein 1 (PD-1), programmed-death ligand 1 (PD-L1) and CTLA-4 by the United States Food and Drug Administration (US FDA). In DLBCL, PD-L1 overexpression is reported to be present on approximately 10–50% of cases [5, 6], with variations in reported frequency potentially due to overexpression cutoffs, inter-laboratory differences in IHC sensitivity for these markers, patient ethnicity and receipt of prior therapy. PD-L1 expression is associated with both non-germinal center cell of origin (non-GCB COO) and unfavorable prognosis following receipt of first-line R-CHOP in DLBCL patients [6].
Preclinical data have demonstrated that PD-L1 expression on lymphoma cells may be driven by activation of MYC [7], although tumors from patients with previously untreated DLBCL or BCLU/HGBL harboring MYC-R [8] or expressing MYC protein [5] may demonstrate lower levels of PD-L1 expression on tumor cells and tumor microenvironment (TME) cells, respectively, than those without MYC alterations. There are no published data on expression patterns of other immune checkpoint proteins DLBCL and BCLU/HGBL, although it has been hypothesized that MYC may regulate their expression [7].
In light of the above findings, as well as the clinical availability of checkpoint inhibitor therapies, we sought to determine the prevalence of immune checkpoint protein overexpression on cases of DLBCL and BCLU/HGBL with MYC overexpression and correlate this with MYC-R status, as well as clinical outcomes for previously untreated patients following receipt of front-line immunochemotherapy.
Materials and methods
Cases were identified via review of an internal database of DLBCL and BCLU/HGBL specimens which were reviewed at the University of Pennsylvania from 2009–2018. Those reported to demonstrate increased expression of MYC protein by IHC (≥ 40%) by clinical hematopathology review were selected from the database and tumor tissue was obtained from these cases when available. Cases of primary central nervous system lymphoma, post-transplant lymphoproliferative disorder and from patients with human immunodeficiency virus positivity were excluded. Cutoffs for overexpression of MYC (≥ 40%) and BCL2 (≥ 50%) [9], PD-L1 on tumor cells (≥ 30%) [6], PD-L1 on TME cells (≥ 20%) [6] and PD-1 on TME cells (≥ 30%) [10] were as previously published for DLBCL; a cutoff of ≥ 30% to denote overexpression was used for all other proteins. The presence or absence of MYC-R was detected by fluorescence in situ hybridization with MYC breakapart probe. Cases were reviewed by two expert hematopathologists (GCC and MAW). Relapse free survival (RFS) was defined as time from the start of therapy to disease progression or last follow-up in remission. Overall survival (OS) was defined as time from the start of therapy to death from any cause or last follow-up while alive. Disease progression was defined by clinical radiology report as per the Revised Response Criteria for Malignant Lymphoma [11]. Categorical data were analyzed by Fisher’s exact test. Survival analysis was performed with the log-rank test and the impact of categorical variables on both survival and achievement of response was analyzed using Cox proportional-hazard regressions. Statistical significance was defined as a two-tailed p value of < 0.05 for all analyses. Data were censored on November 1, 2019. This protocol was approved by the Institutional Review Board of the University of Pennsylvania.
Results
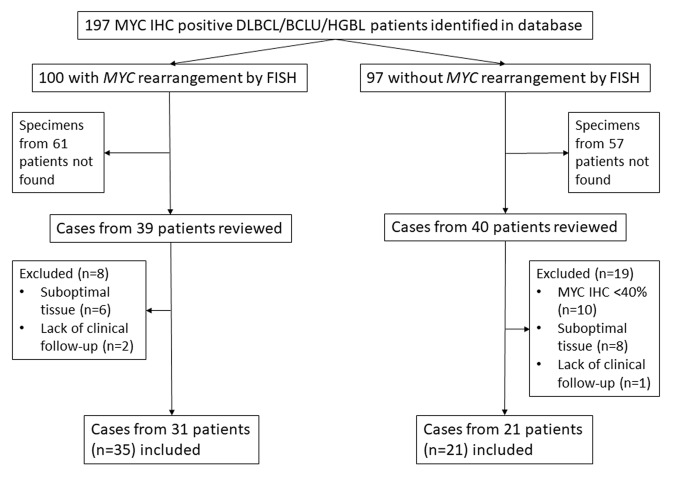
Database query yielded 197 cases of DLBCL or BCLU/HGBL with MYC overexpression. Further stratification and inclusion/exclusion of cases is depicted in Fig. 1. In total, tissue was available for analysis from 56/197 (40%) cases and was similar for patients with (39/100, 39%) and without (40/97, 41%) MYC-R. Ultimately 35 cases from 31 patients with MYC-R and 21 cases from 21 patients without MYC-R were included in the analysis. The proportion of overexpression of immune checkpoint proteins from all cases, as well as the subset from previously untreated patients, are listed in Table 1. Overexpression of both PD-L1 and PD-1 in the TME was more frequently seen in cases with MYC-R than those without (50 vs. 15%, p = 0.02 and 32 vs. 5%, p = 0.02, respectively) and overexpression of CTLA-4 and CD80 on tumor cells less frequently seen in cases with MYC-R than those without (34 vs. 71%, p = 0.01 and 34 vs. 81%, p = 0.001, respectively). In addition, analysis for concurrent overexpression of proteins relevant to the PD-1 and CTLA-4 pathways demonstrated statistically significant co-overexpression of PD-L1 on tumor cells and pSTAT3 (p = 0.001), PD-L1 and PD-1 both on TME cells (p < 0.001) as well as CD80 and CTLA-4 (p = 0.009). While a significantly higher percentage of MYC-R specimens were classified as GCB COO by Hans algorithm [12] (80 vs. 52%, p = 0.04), there were no statistically significant differences in immune checkpoint protein overexpression patterns when comparing cohorts by COO. Immune checkpoint protein overexpression patterns were similar when restricting this analysis to cases from previously untreated patients.
Fig. 1.
Case inclusion/exclusion
Table 1.
Proportion of cases with increased immune checkpoint protein expression by immunohistochemical staining from (A) all patients regardless of treatment status and (B) previously untreated patients only
| A | All cases (n = 56) | No MYC-R (n = 21) | MYC-R (n = 35) | p |
|---|---|---|---|---|
| PD-L1 | 6 (11%) | 2 (10%) | 4 (11%) | 1 |
| PD-L1 tumor microenvironment | 20 (37%) | 3 (15%) | 17 (50%) | 0.018 |
| PD-1 tumor microenvironment | 12 (22%) | 1 (5%) | 11 (32%) | 0.019 |
| pSTAT3 | 3 (5%) | 1 (5%) | 2 (6%) | 1 |
| pSTAT5 | 41 (75%) | 12 (60%) | 29 (83%) | 0.11 |
| CD80 | 29 (52%) | 17 (81%) | 12 (34%) | 0.001 |
| CTLA-4 | 27 (48%) | 15 (71%) | 12 (34%) | 0.012 |
| CD86 | 31 (56%) | 8 (40%) | 23 (66%) | 0.091 |
| pERK | 2 (4%) | 0 | 2 (6%) | 0.52 |
| B | All cases (n = 37) | No MYC-R (n = 13) | MYC-R (n = 24) | p |
|---|---|---|---|---|
| PD-L1 | 0 | 0 | 0 | – |
| PD-L1 tumor microenvironment | 14 (38%) | 2 (15%) | 12 (50%) | 0.07 |
| PD-1 tumor microenvironment | 9 (25%) | 1 (7%) | 8 (35%) | 0.11 |
| pSTAT3 | 0 | 0 | 0 | – |
| pSTAT5 | 27 (75%) | 8 (67%) | 19 (79%) | 0.44 |
| CD80 | 16 (43%) | 10 (77%) | 6 (25%) | 0.05 |
| CTLA-4 | 17 (46%) | 9 (69%) | 8 (33%) | 0.047 |
| CD86 | 17 (47%) | 3 (25%) | 14 (58%) | 0.08 |
| pERK | 1 (3%) | 0 | 1 (4%) | 1 |
All proteins were detected on tumor cells unless otherwise noted
Analysis of clinical outcomes was performed for previously untreated patients (n = 37). Relevant clinicopathologic baseline characteristics reported included female sex in n = 14 (38%), age > 60 years in n = 19 (51%), elevated LDH in n = 31 (91%), stage 3–4 disease in n = 28 (76%), Eastern Cooperative Oncology Group (ECOG) performance status ≥ 2 in n = 4 (11%), extranodal disease in n = 25 (68%), Revised International Prognostic Index (R-IPI) score ≥ 3 in n = 20 (59%), GCB COO in n = 30 (70%) and Ki67 ≥ 90% in n = 16 (46%) patients. Of MYC-R patients (n = 24), n = 12 (50%) were classified as double hit lymphoma (7 with MYC/BCL2, 2 with MYC/BCL6 and 3 with MYC/BCL2/BCL6 rearrangements) and n = 19 (83%) of non-double hit lymphoma patients (including those with sole MYC-R) were classified as double expressor lymphoma (overexpression of both MYC and BCL2 proteins).
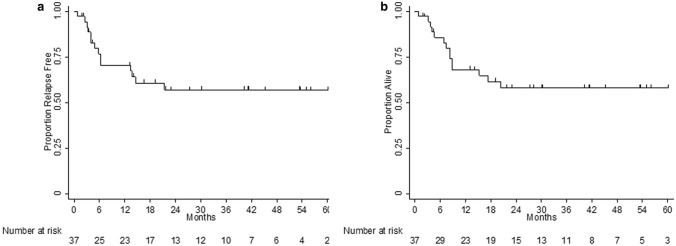
Front-line therapy was R-CHOP in n = 13 (35%), intensive immunochemotherapy in n = 25 (65%) with nearly all of these patients receiving dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, etoposide with rituximab (DA-EPOCH-R), and other regimens in n = 3 (10%) cases. With a median length of follow-up of 40.1 months, 2 year RFS was 57% (95% confidence interval [CI] 38–72%) and 2 year OS 58% (95% CI 40–73%) as shown in Fig. 2. Accurate univariate and/or multivariate analysis for survival based upon baseline clinicopathologic characteristics could not be performed due to the small sample size of patients.
Fig. 2.
a Relapse free survival and b overall survival of previously untreated patients
Discussion
Our data show relatively high rates of immune checkpoint protein overexpression in cases of MYC-overexpressing DLBCL and BCLU/HGBL, with statistically significant differences based upon MYC-R status, with MYC-R specimens more likely to overexpress both PD-L1 and PD-1 in the TME and non-MYC-R specimens CD80 and CTLA-4. RFS and OS at 2 years following receipt of front-line immunochemotherapy were as expected based upon prior analyses of patients with MYC-overexpressing DLBCL and BCLU/HGBL with or without MYC-R treated with intensive front-line immunochemotherapy in the majority of cases [13], suggesting that our cohort is representative of the larger population of these patients encountered in clinical practice.
Our data agree with others demonstrating a low frequency of PD-L1 overexpression in previously-untreated MYC-altered DLBCL and BCLU/HGBL [5, 8], which we hypothesize is due to the relative lack of pSTAT3 expression, as this appears to be required for PD-L1 expression in T-cell non-Hodgkin lymphomas [14, 15]. Interestingly, all three cases with pSTAT3 overexpression also demonstrated PD-L1 overexpression. Also of note is the fact that PD-L1 overexpression on tumor cells was only detected in previously-treated cases (n = 6, 31%), including two cases with both pre- and post-treatment biopsies, suggesting that overexpression of PD-L1 on tumor cells could serve as a mechanism of resistance to front-line immunochemotherapy in these lymphomas.
As seen in our cohort, discordance of the degree of PD-L1 overexpression on tumor vs. TME cells has been previously reported in clinical trials evaluating the therapeutic efficacy of PD-L1 inhibitor therapy in non-small cell lung cancer (NSCLC) patients, with responses seen in patients overexpressing PD-L1 on either tumor or TME cells [16]. Overexpression of PD-L1 on TME cells, as demonstrated in approximately 1/3 of cases in our series, may be of clinical relevance given that the PD-L1 inhibitor atezolizumab was approved by the US FDA for treatment of metastatic NSCLC and triple negative breast cancer with increased expression of PD-L1 on tumor-infiltrating immune cells based upon efficacy data for these patient populations [17].
Preclinical data have demonstrated that PD-L1 expression is regulated by MYC gene function in tumor cells [18] and expression of MYC protein is significantly higher in tumors harboring PD-1 positive T cells along with PD-L1 positive, but not PD-L1 negative, tumor cells [19]. However, it is unclear why the subset of cases with MYC-R were more likely to overexpress PD-L1 and PD-1 in the TME as compared to cases without MYC-R in our series. MYC protein expression has been associated with MYC mutations [3], copy number alterations [20] as well as other mechanisms of upregulation [9] independent of MYC-R, and associations between pathways driving MYC protein and immune checkpoint protein expression could be explored further.
Limitations of this analysis include a relatively small sample size, which precludes our ability to determine whether or not survival outcomes were associated with the presence or absence of overexpression of specific immune checkpoint proteins. Additionally, we were unable to obtain tissue for analysis of all cases identified by database query.
In conclusion, these data may support further investigation of immune checkpoint protein regulation/expression as well as the therapeutic efficacy of checkpoint inhibitors in MYC-overexpressing DLBCL and BCLU/HGBL.
Author contributions
DJL, SJS, MAW and GCC contributed to the study conception and design. Material preparation, data collection and analysis were performed by DJL, MAW and GCC. The first draft of the manuscript was written by DJL and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
This research was funded by philanthropic donations to the Lymphoma Molecular Diagnostics Fund of the Abramson Cancer Center.
Availability of data and material
Data may be made available if requested.
Code availability
Not applicable.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
This protocol was approved by the Institutional Review Board of the University of Pennsylvania.
Consent to participate
A waiver of informed consent was granted by the Institutional Review Board of the University of Pennsylvania.
Consent for publication
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Savage KJ, Johnson NA, Ben-Neriah S, Connors JM, Sehn LH, Farinha P, Horsman DE, Gascoyne RD. MYC gene rearrangements are associated with a poor prognosis in diffuse large B-cell lymphoma patients treated with R-CHOP chemotherapy. Blood. 2009;114:3533–3537. doi: 10.1182/blood-2009-05-220095. [DOI] [PubMed] [Google Scholar]
- 2.Barrans S, Crouch S, Smith A, Turner K, Owen R, Patmore R, Roman E, Jack A. Rearrangement of MYC is associated with poor prognosis in patients with diffuse large B-cell lymphoma treated in the era of rituximab. J Clin Oncol. 2010;28:3360–3365. doi: 10.1200/JCO.2009.26.3947. [DOI] [PubMed] [Google Scholar]
- 3.Xu-Monette ZY, Deng Q, Manyam GC, et al. Clinical and biologic significance of MYC genetic mutations in de novo diffuse large B-cell lymphoma. Clin Cancer Res. 2016;22:3593–3605. doi: 10.1158/1078-0432.CCR-15-2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kluk MJ, Chapuy B, Sinha P, et al. Immunohistochemical detection of MYC-driven diffuse large B-cell lymphomas. PLoS ONE. 2012;7:e33813. doi: 10.1371/journal.pone.0033813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hu LY, Xu XL, Rao HL, et al. Expression and clinical value of programmed cell death-ligand 1 (PD-L1) in diffuse large B cell lymphoma: a retrospective study. Chin J Cancer. 2017;36:94. doi: 10.1186/s40880-017-0262-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kiyasu J, Miyoshi H, Hirata A, et al. Expression of programmed cell death ligand 1 is associated with poor overall survival in patients with diffuse large B-cell lymphoma. Blood. 2015;126:2193–2201. doi: 10.1182/blood-2015-02-629600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Casey SC, Baylot V, Felsher DW. The MYC oncogene is a global regulator of the immune response. Blood. 2018;131:2007–2015. doi: 10.1182/blood-2017-11-742577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elbaek MV, Pedersen MO, Breinholt MF, et al. PD-L1 expression is low in large B-cell lymphoma with MYC or double-hit translocation. Hematol Oncol. 2019;37:375–382. doi: 10.1002/hon.2664. [DOI] [PubMed] [Google Scholar]
- 9.Johnson NA, Slack GW, Savage KJ, et al. Concurrent expression of MYC and BCL2 in diffuse large B-cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J Clin Oncol. 2012;30:3452–3459. doi: 10.1200/JCO.2011.41.0985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kwon D, Kim S, Kim PJ, et al. Clinicopathological analysis of programmed cell death 1 and programmed cell death ligand 1 expression in the tumour microenvironments of diffuse large B cell lymphomas. Histopathology. 2016;68:1079–1089. doi: 10.1111/his.12882. [DOI] [PubMed] [Google Scholar]
- 11.Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25:579–586. doi: 10.1200/JCO.2006.09.2403. [DOI] [PubMed] [Google Scholar]
- 12.Hans CP, Weisenburger DD, Greiner TC, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004;103:275–282. doi: 10.1182/blood-2003-05-1545. [DOI] [PubMed] [Google Scholar]
- 13.Landsburg DJ. Management of patients with MYC-altered lymphomas. Curr Hematol Malig Rep. 2016;11:208–217. doi: 10.1007/s11899-016-0320-7. [DOI] [PubMed] [Google Scholar]
- 14.Marzec M, Zhang Q, Goradia A, et al. Oncogenic kinase NPM/ALK induces through STAT3 expression of immunosuppressive protein CD274 (PD-L1, B7-H1) Proc Natl Acad Sci USA. 2008;105:20852–20857. doi: 10.1073/pnas.0810958105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Atsaves V, Tsesmetzis N, Chioureas D, et al. PD-L1 is commonly expressed and transcriptionally regulated by STAT3 and MYC in ALK-negative anaplastic large-cell lymphoma. Leukemia. 2017;31:1633–1637. doi: 10.1038/leu.2017.103. [DOI] [PubMed] [Google Scholar]
- 16.Kowanetz M, Zou W, Gettinger SN, et al. Differential regulation of PD-L1 expression by immune and tumor cells in NSCLC and the response to treatment with atezolizumab (anti-PD-L1) Proc Natl Acad Sci USA. 2018;115:E10119–E10126. doi: 10.1073/pnas.1802166115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davis AA, Patel VG. The role of PD-L1 expression as a predictive biomarker: an analysis of all US Food and Drug Administration (FDA) approvals of immune checkpoint inhibitors. J Immunother Cancer. 2019;7:278. doi: 10.1186/s40425-019-0768-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Casey SC, Tong L, Li Y, et al. MYC regulates the antitumor immune response through CD47 and PD-L1. Science. 2016;352:227–231. doi: 10.1126/science.aac9935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li L, Sun R, Miao Y, et al. PD-1/PD-L1 expression and interaction by automated quantitative immunofluorescent analysis show adverse prognostic impact in patients with diffuse large B-cell lymphoma having T-cell infiltration: a study from the International DLBCL Consortium Program. Mod Pathol. 2019;32:741–754. doi: 10.1038/s41379-018-0193-5. [DOI] [PubMed] [Google Scholar]
- 20.Valera A, Lopez-Guillermo A, Cardesa-Salzmann T, et al. MYC protein expression and genetic alterations have prognostic impact in patients with diffuse large B-cell lymphoma treated with immunochemotherapy. Haematologica. 2013;98:1554–1562. doi: 10.3324/haematol.2013.086173. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data may be made available if requested.
Not applicable.