Abstract
Background
Immune checkpoint inhibitors have transformed the treatment landscape of cancer treatment, but only a fraction of patients responds to treatment, leading to an increasing effort to repurpose clinically approved medications to augment ICI therapy. Metformin has been associated with improved survival outcomes in patients undergoing conventional chemotherapy. However, whether metformin provides survival benefits in patients receiving immune checkpoint inhibitors (ICIs) is unknown.
Methods
We performed a retrospective cohort study at two tertiary referral centers in Taiwan. All adult diabetes mellitus patients who were treated with ICIs between January 2015 and December 2021 were included. The primary and secondary outcomes were overall survival (OS) and progression-free survival (PFS), respectively.
Results
In total, 878 patients were enrolled in our study, of which 86 patients used metformin and 78 patients used non-metformin diabetes medications. Compared with non-users, metformin users had a longer median OS (15.4 [IQR 5.6–not reached] vs. 6.1 [IQR, 0.8–21.0] months, P = 0.003) and PFS (5.1 [IQR 2.0—14.3] vs. 1.9 [IQR 0.7—8.6] months, P = 0.041). In a univariate Cox proportional hazard analysis, the use of metformin was associated with a reduction in the risk of mortality (HR: 0.53 [95% confidence interval: 0.35—0.81], P = 0.004) and disease progression (HR: 0.69 [95% CI 0.49—0.99], P = 0.042). The use of metformin remained associated with a lower risk of mortality after adjusting for baseline variables such as age, cancer stage, and underlying comorbidities (OS, HR: 0.55 [95% CI 0.34–0.87], P = 0.011). Similarly, the use of metformin was associated with a lower risk of disease progression. Importantly, the use of metformin before ICI initiation was not associated with a reduction in mortality (HR: 0.61 [95% CI 0.27—1.42], P = 0.25) or disease progression (HR: 0.69 [95% CI 0.33—1.43], P = 0.32).
Conclusion
The use of metformin is associated with survival benefits in patients undergoing immunotherapy. Prospective clinical trials are warranted to define the role of metformin in augmenting immunotherapy.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00262-022-03363-6.
Keywords: Immune checkpoint inhibitors, Immunotherapy, Metformin, Drug re-purposing, Survival
Introduction
Immune checkpoint inhibitors (ICIs) are antibodies that target programmed cell death protein-1 (PD-1) or its ligand (PD-L1), and cytotoxic T-lymphocyte-associated-4 (CLTA-4), thereby activating the immune response against tumor cells [1, 2]. The development of ICI has substantially improved the outcomes of cancer patients and brought about a paradigm shift in the landscape of cancer therapy [3, 4]. However, most of the patients do not benefit from ICI therapy, representing an unmet need to improve patient response to treatment [5, 6].
One potential adjuvant drug theorized to improve patients’ response to ICI therapy is metformin, a biguanide class of antidiabetic drugs commonly used in the treatment of type 2 diabetes mellitus [7]. Metformin decreases serum glucose levels by increasing insulin sensitivity, which results in increased glucose uptake and decreased gluconeogenesis [7]. Other than its antidiabetic effects, metformin also affects cell growth and proliferation by lowering insulin levels in the body [8]. In both cell lines and mouse models, metformin has been demonstrated to inhibit cancer cell proliferation and delay tumor progression [8]. In clinical studies, metformin is associated with improved patient outcomes in patients undergoing conventional chemotherapy across different tumor types [9–11]. These observations have led to efforts to investigate whether metformin may be repurposed to augment and improve response to immunotherapy.
Preclinical studies have shown that metformin enhances the response to immunotherapy, potentially by decreasing the levels of PD-L1 expression and increasing tumor-infiltrating lymphocyte levels [12–14]. However, clinical observation studies have not been consistent on whether metformin is associated with survival benefits in patients treated with ICIs [15–19]. Of note, many of these cohort studies used patients without type 2 diabetes as control patients. This would represent a selection bias as patients who received metformin are expected to have type 2 diabetes mellitus. The presence of diabetes is a poor prognostic factor for cancer outcomes and is likely to have reduced the survival benefit of metformin in previous analyses [20]. Thus, whether metformin may improve the survival outcomes of patients undergoing immunotherapy remains inconclusive. In this study, we aimed to investigate the impact of metformin in patients receiving ICI therapy on clinical outcomes.
Methods
Study design
This retrospective cohort study was conducted at two tertiary referral centers in Taiwan. The ethics committee of both hospitals approved this study (Chung Shan Medical University Hospital CS2-21,183, Taipei Tzu Chi Hospital 11-X-035). We included all patients who were administered at least 2 cycles of immunotherapy between January 2015 and September 2021. We excluded patients with incomplete data, less than 20 years old, who received only 1 cycle of immunotherapy, and who did not have diabetes mellitus. We further excluded patients who did not receive metformin or other diabetes medications within 30 days of receiving ICI therapy. We collected data on patient characteristics and clinicopathological features, including age, sex, Eastern Cooperative Oncology Group (ECOG) Performance Status (ECOG-PS), cancer stage, cancer pathology, ICI type and treatment cycle, and underlying comorbidities. We also collected data on the type of diabetes medications prescribed (Supplemental Table 1).
In the primary analysis, patients who received metformin were compared to patients who received non-metformin diabetes medications. In the secondary analysis, patients who received metformin were compared to patients who did not receive metformin, regardless of the presence of diabetes. The non-metformin diabetes medications included the following: insulin, dipeptidyl-peptidase 4 (DPP-4) inhibitors, sulfonylureas, alpha-glucosidase inhibitors, and sodium-glucose co-transporter 2 (SGLT2) inhibitors (Supplemental Table 1).
Outcome definitions
The primary endpoint was overall survival (OS), and the secondary endpoint was progression-free survival (PFS). We adjudicated the tumor-based endpoints using a clinician and radiological-based validated approach because the Response Evaluation Criteria in Solid Tumors (RECIST) criteria were not feasible in a retrospective study based on electronic health record-derived data [21].
Statistical analysis
We evaluated the OS and PFS between metformin and non-metformin users using Kaplan–Meier analysis and Cox proportional hazard model analysis. The variables in the multivariate Cox model were selected a priori and included the following variables: age, gender, performance status, cancer stage, cancer type, class of ICI, hypertension, hyperlipidemia, chronic kidney disease, chronic obstructive pulmonary disease, ischemic heart disease, stroke, heart failure, surgery, and HbA1c. Missing data for HbA1c were addressed using multiple imputations [22]. We carried out two sensitivity analyses. First, we evaluated the relationship between the use of metformin before ICI initiation and oncological outcomes. Second, we investigated if the use of metformin is associated with improved survival benefits when compared with non-diabetes patients. A P value less than 0.05 for a two-sided test indicates statistical significance. All analyses were conducted using Stata version 16.0 (StataCorp LLC, College Station, TX).
Results
Patient characteristics
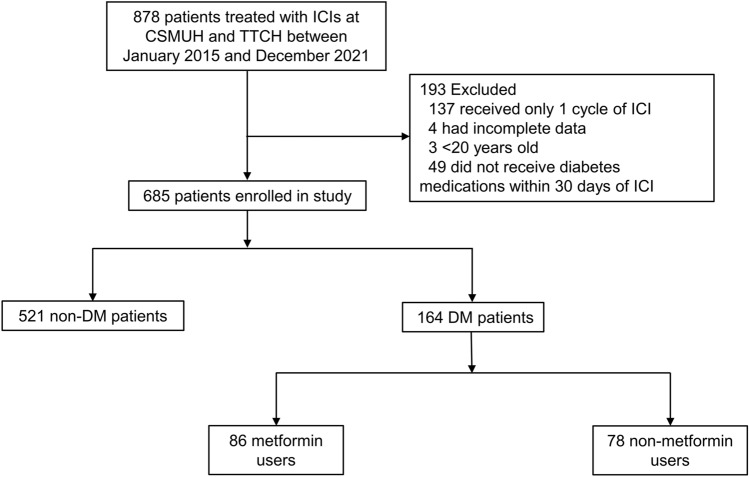
We identified 878 patients who met the inclusive criteria. We excluded patients with missing data, patients under the age of 20, and those who received only one cycle of ICI. We further excluded patients if they had no medical history of diabetes mellitus or if they did not receive diabetes medications within 30 days of ICI therapy (Fig. 1). A total of 685 patients (86 metformin users, 78 non-metformin users, and 521 non-diabetes patients) were subsequently enrolled in this study. The median ages of metformin users, non-metformin users, and non-diabetes patients were 66 (Interquartile range (IQR), 59–70), 66 (Interquartile range (IQR), 59–75), and 62 (Interquartile range (IQR), 54–69), respectively. The median HbA1c for the metformin and non-metformin users was 7 (6–8) and 7 (6–10), respectively. The most common cancer type was lung cancer (46%) and hepatobiliary cancer (18%). The baseline characteristics of all included patients are summarized in Supplemental Table 2.
Fig. 1.
Flowchart of patient enrolment
Survival outcomes
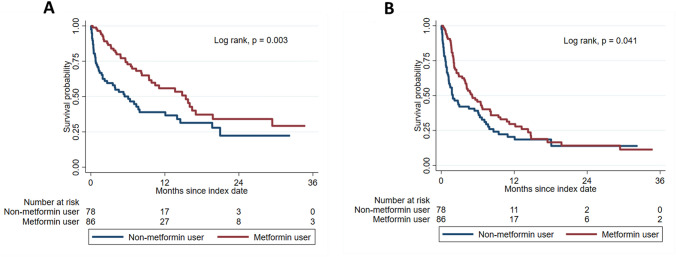
Compared with non-metformin users, metformin users had a longer median OS (15.4 [IQR, 5.6—not reached] vs. 6.1 [IQR, 0.8–21.0] months; P = 0.003) and PFS (5.1 [IQR, 2.0–14.3] vs. 1.9 [IQR, 0.7–8.6] months; P = 0.041) (Fig. 2 and Supplemental Table 3). In Cox proportional hazard model analysis, the use of metformin was associated with a 47% lower risk of all-cause mortality (univariate HR, 0.53 [95% CI 0.35–0.81, P = 0.004) and 31% lower risk of disease progression or death (univariate HR, 0.69 [95% CI 0.49–0.99, P = 0.042) (Table 1). These trends were also observed in multivariate Cox proportional hazard analyses adjusting for underlying characteristics and comorbidities (All-cause mortality, multivariate HR, 0.55 [95% CI 0.34–0.87, P = 0.011; disease progression or death, multivariate HR, 0.66 [95% CI 0.44–0.99, P = 0.045). Similarly, the use of metformin was associated with a lower risk of death and disease progression after adjusting for HbA1c levels (Table 1).
Fig. 2.
Survival outcomes of patients treated with and without metformin. A Overall survival, B Progression-free survival
Table 1.
Cox proportional hazards regression of all-cause mortality or disease progression
| Analysis | Outcome | Univariate model HR (95% CI) | P value | Multivariate model 1 HR (95% CI) | P value | Multivariate model 2 HR (95% CI) | P value |
|---|---|---|---|---|---|---|---|
| Metformin versus non-metformin user | All-cause mortality | 0.53 (0.35–0.81) | 0.004 | 0.55 (0.34–0.87) | 0.011 | 0.46 (0.30–0.69) | < 0.001 |
| Disease progression or mortality | 0.69 (0.49–0.99) | 0.042 | 0.66 (0.44–0.99) | 0.045 | 0.57 (0.40–0.82) | 0.002 | |
| Metformin before ICI initiation versus non-metformin before ICI initiation | All-cause mortality | 0.61 (0.27–1.42) | 0.25 | 0.49 (0.19–1.26) | 0.14 | 0.39 (0.15–1.03) | 0.057 |
| Disease progression or mortality | 0.69 (0.33–1.43) | 0.32 | 0.61 (0.27–1.40) | 0.24 | 0.57 (0.24–1.32) | 0.19 | |
| Metformin versus non-diabetes | All-cause mortality | 1.06 (0.76–1.49) | 0.72 | 1.01 (0.69–1.46) | 0.98 | – | – |
| Disease progression or mortality | 1.06 (0.76–1.49) | 0.96 | 0.96 (0.71–1.29) | 0.77 | – | – |
HR hazard ratio; ICI immune checkpoint inhibitor
In multivariate model 1, the following variables were used: age, gender, cancer stage, cancer type, Eastern Cooperative Oncology Group Performance Status, class of immune checkpoint inhibitors, surgery, underlying comorbidities that include hypertension, hyperlipidemia, chronic obstructive pulmonary disease, chronic kidney disease, ischemic heart disease, stroke, and heart failure
In multivariate model 2, the following variables were used: age, gender, cancer stage, cancer type, Eastern Cooperative Oncology Group Performance Status, class of immune checkpoint inhibitors, surgery, underlying comorbidities that include hypertension, hyperlipidemia, chronic obstructive pulmonary disease, chronic kidney disease, ischemic heart disease, stroke, and heart failure, and HbA1c. Missing values for HbA1c were addressed using multiple imputation
Sensitivity analysis
In a sensitivity analysis, patients who used metformin before ICI initiation had a similar median OS (3.9 [IQR, 1.7–not reached] vs. 1.8 [IQR, 0.3–19.7] months; P = 0.25) and PFS (3.0 [IQR, 1.3–7.1] versus 1.1 [IQR, 0.2–7.4] months; P = 0.31) compared with those who did not use metformin before ICI initiation (Supplemental Fig. 1 and Supplemental Table 3). The use of metformin before ICI initiation was not associated with a decrease in the risk of all-cause mortality and disease progression or death in both univariate and multivariate Cox regression analyses (Table 1).
In another sensitivity analysis, diabetes patients who were treated with metformin had a similar median OS (16.6 [IQR, 7.5–not reached] vs. 25.6 [IQR, 6.1–58.4] months; P = 0.72) and PFS (6.4 [IQR, 3.5–15.2] vs. 6.5 [IQR, 2.3–24.0] months; P = 0.96) compared with patients who did not have diabetes mellitus (Supplemental Fig. 2 and Supplemental Table 3). Compared with non-diabetes patients, the use of metformin was not associated with better survival outcomes (Table 1).
Discussion
In this cohort study, we report two important findings. First, patients who received metformin experienced a better survival outcome than patients who received other types of diabetes medications. Second, the use of metformin prior to the initiation of ICI was not associated with a better clinical benefit. Overall, our results suggest that metformin may potentially be used to augment and improve response to immunotherapy.
Most of the previous clinical studies have not found an association between the use of metformin and improved clinical outcomes among patients treated with ICIs [15–19]. Only one study conducted by Afzal et al. found a trend toward improved clinical benefits in patients receiving metformin [15]. An explanation for this is that the controls selected for comparison against metformin users were mostly patients without diabetes [15–19]. Patients who do not have diabetes mellitus generally have a better survival outcome than those who have diabetes mellitus [23]. Thus, the potential survival benefits associated with metformin might have been confounded or masked by the presence of diabetes in the metformin group. In line with this theory, our analysis found that metformin users had a similar OS and PFS when compared with non-diabetes patients, but a longer OS and PFS when compared with diabetes patients treated with non-metformin diabetes medications. We also adjusted for the baseline differences in HbA1c levels before the initiation of ICI. This was important because metformin is commonly used as first-line therapy for diabetes and patients who received metformin would tend to have better glycemic control than those who received other diabetes medications [24, 25]. Thus, our study provides robust data to support metformin’s role in augmenting and improving the response to immunotherapy.
There are several potential mechanisms as to why metformin might lead to improved survival over other diabetes medications. Metformin has been found to possess antitumor effects, possibly through activating the immune system to fight against tumor cells [8, 13]. In mice, metformin was found to increase the number of CD8 + tumor-infiltrating lymphocytes and protect these cells from apoptosis and exhaustion in the tumor microenvironment [8, 13]. Based on this mechanism, metformin might work synergistically with immune checkpoint inhibitors, which would activate and increase the antitumor activity of these T lymphocytes. Metformin also has cationic amphiphilic properties. Medications with these properties can permeabilize the lysosomal membrane and trigger cascades of events, resulting in plasma membrane rupture and cell death [26]. Alternatively, destabilizing the lysosomal membrane may also replenish PD-L1 expression, leading to an enhanced response to immune checkpoint blockade [27]. Consistent with this hypothesis, one previous study showed that patients treated with antihistamines possessing cationic amphiphilic properties experienced survival benefits over patients who were not treated with these antihistamines [27].
This study has several limitations. Because this was a retrospective study, we were unable to utilize the RECIST criteria to adjudicate the tumor-based endpoints. Nevertheless, we did use a validated approach that is appropriate for real-world data [21]. There might be residual confounders that were not included in the multivariate cox models used to adjust for the baseline differences between metformin users and users of other diabetes medications. However, we included most of the important confounders, including HbA1c, the key indicator of glycemic control in our models. The survival benefits associated with metformin were observed for patients with type 2 diabetes mellitus, and further studies would be required to investigate if these benefits are also seen in patients without diabetes mellitus. There was a substantial loss to follow-up in this study, which was inevitable given that this was a cancer population. Finally, this study was derived from an Asian cohort and therefore these observations might not be directly applicable to other populations.
Conclusion
In conclusion, the use of metformin is associated with improved patient survival and response to ICI therapy. Prospective clinical trials are needed to further clarify the role of metformin in augmenting immunotherapy.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank the Department of Hematology and Oncology at Taipei Tzu Chi Hospital for technical assistance.
Abbreviations
- CSMUH
Chung Shan Medical University Hospital
- ICI
Immune checkpoint inhibitor
- TTCH
Taipei Tzu Chi Hospital
Author contributions
CMP and CHC contributed to study concept and design. CHC, YJC, CHC, CYC, YCC, SSW, XYS, CSH, CYP, and YPH contributed to acquisition of data. CHC, YJC, and CHC contributed to analysis of data. CHC, YJC, CHC, CHC, and CMP contributed to drafting of the manuscript. CHC, YJC, CHC, CYC, YCC, SSW, XYS, CSH, CYP, YPH, CMP, and CHC contributed to critical revision of the manuscript for important intellectual content. CMP and CHC contributed to study supervision.
Funding
No funding.
Data availability
The data that support the findings of this study are available on request from the corresponding author, CHu Chiang. The data are not publicly available as their containing information could compromise the privacy of research participants.
Declarations
Conflict of interests
We have no conflicts of interest to declare.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Cho-Han Chiang, Yuan-Jen Chen, Cho-Hsien Chiang: These authors have contributed joint first authors equally.
Contributor Information
Cheng-Ming Peng, Email: jimy5989@gmail.com.
Cho-Hung Chiang, Email: ustwhealth.datascience.group@gmail.com.
References
- 1.Das S, Johnson DB. Immune-related adverse events and anti-tumor efficacy of immune checkpoint inhibitors. J Immunother Cancer. 2019;7(1):306. doi: 10.1186/s40425-019-0805-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chiang CH, Chiang CH, Ma KS, Hsia YP, Lee YW, Wu HR, et al. The incidence and risk of cardiovascular events associated with immune checkpoint inhibitors in Asian populations. Jpn J Clin Oncol. 2022;52(12):1389–1398. doi: 10.1093/jjco/hyac150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dudnik E, Kareff S, Moskovitz M, Kim C, Liu SV, Lobachov A, et al. Real-world survival outcomes with immune checkpoint inhibitors in large-cell neuroendocrine tumors of lung. J Immunother Cancer. 2021;9(2):e001999. doi: 10.1136/jitc-2020-001999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Correction: efficacy of immune checkpoint inhibitors for in-transit melanoma. J ImmunoTherapy Cancer. 2020;8 (2): e000440corr1 [DOI] [PMC free article] [PubMed]
- 5.Wolchok JD, Chiarion-Sileni V, Gonzalez R, Rutkowski P, Grob JJ, Cowey CL, et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. 2017;377(14):1345–1356. doi: 10.1056/NEJMoa1709684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372(4):320–330. doi: 10.1056/NEJMoa1412082. [DOI] [PubMed] [Google Scholar]
- 7.Rojas LBA, Gomes MB. Metformin: an old but still the best treatment for type 2 diabetes. Diabetol Metab Syndr. 2013;5(1):6. doi: 10.1186/1758-5996-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eikawa S, Nishida M, Mizukami S, Yamazaki C, Nakayama E, Udono H. Immune-mediated antitumor effect by type 2 diabetes drug, metformin. Proc Natl Acad Sci U S A. 2015;112(6):1809–1814. doi: 10.1073/pnas.1417636112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Landman GW, Kleefstra N, van Hateren KJ, Groenier KH, Gans RO, Bilo HJ. Metformin associated with lower cancer mortality in type 2 diabetes: ZODIAC-16. Diabetes Care. 2010;33(2):322–326. doi: 10.2337/dc09-1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sadeghi N, Abbruzzese JL, Yeung SC, Hassan M, Li D. Metformin use is associated with better survival of diabetic patients with pancreatic cancer. Clin Cancer Res. 2012;18(10):2905–2912. doi: 10.1158/1078-0432.CCR-11-2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meng F, Song L, Wang W. Metformin improves overall survival of colorectal cancer patients with diabetes: a meta-analysis. J Diabetes Res. 2017;2017:5063239. doi: 10.1155/2017/5063239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Munoz LE, Huang L, Bommireddy R, Sharma R, Monterroza L, Guin RN, et al. Metformin reduces PD-L1 on tumor cells and enhances the anti-tumor immune response generated by vaccine immunotherapy. J Immunother Cancer. 2021;9(11):e002614. doi: 10.1136/jitc-2021-002614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Veeramachaneni R, Yu W, Newton JM, Kemnade JO, Skinner HD, Sikora AG, et al. Metformin generates profound alterations in systemic and tumor immunity with associated antitumor effects. J Immunother Cancer. 2021;9(7):e002773. doi: 10.1136/jitc-2021-002773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu W, Wang Y, Luo J, Liu M, Luo Z. Pleiotropic Effects of Metformin on the Antitumor Efficiency of Immune Checkpoint Inhibitors. Front Immunol. 2020;11:586760. doi: 10.3389/fimmu.2020.586760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Afzal MZ, Mercado RR, Shirai K. Efficacy of metformin in combination with immune checkpoint inhibitors (anti-PD-1/anti-CTLA-4) in metastatic malignant melanoma. J Immunother Cancer. 2018;6(1):64. doi: 10.1186/s40425-018-0375-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buti S, Bersanelli M, Perrone F, Tiseo M, Tucci M, Adamo V, et al. Effect of concomitant medications with immune-modulatory properties on the outcomes of patients with advanced cancer treated with immune checkpoint inhibitors: development and validation of a novel prognostic index. Eur J Cancer. 2021;142:18–28. doi: 10.1016/j.ejca.2020.09.033. [DOI] [PubMed] [Google Scholar]
- 17.Cortellini A, Tucci M, Adamo V, Stucci LS, Russo A, Tanda ET, et al. Integrated analysis of concomitant medications and oncological outcomes from PD-1/PD-L1 checkpoint inhibitors in clinical practice. J Immunother Cancer. 2020;8(2):e001361. doi: 10.1136/jitc-2020-001361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Failing JJ, Finnes HD, Kottschade LA, Allred JB, Markovic SN. Effects of commonly used chronic medications on the outcomes of ipilimumab therapy in patients with metastatic melanoma. Melanoma Res. 2016;26(6):609–615. doi: 10.1097/CMR.0000000000000299. [DOI] [PubMed] [Google Scholar]
- 19.Svaton M, Zemanova M, Zemanova P, Kultan J, Fischer O, Skrickova J, et al. Impact of concomitant medication administered at the time of initiation of nivolumab therapy on outcome in non-small cell lung cancer. Anticancer Res. 2020;40(4):2209–2217. doi: 10.21873/anticanres.14182. [DOI] [PubMed] [Google Scholar]
- 20.Ranc K, Jørgensen ME, Friis S, Carstensen B. Mortality after cancer among patients with diabetes mellitus: effect of diabetes duration and treatment. Diabetologia. 2014;57(5):927–934. doi: 10.1007/s00125-014-3186-z. [DOI] [PubMed] [Google Scholar]
- 21.Griffith SD, Miksad RA, Calkins G, You P, Lipitz NG, Bourla AB, et al. Characterizing the feasibility and performance of real-world tumor progression end points and their association with overall survival in a large advanced non-small-cell lung cancer data set. JCO Clin Cancer Inform. 2019;3:1–13. doi: 10.1200/CCI.19.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abdel-Qadir H, Bobrowski D, Zhou L, Austin PC, Calvillo-Argüelles O, Amir E, et al. Statin exposure and risk of heart failure after anthracycline- or Trastuzumab-based chemotherapy for early breast cancer: a propensity score-matched cohort study. J Am Heart Assoc. 2021;10(2):e018393. doi: 10.1161/JAHA.119.018393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harding JL, Andes LJ, Gregg EW, Cheng YJ, Weir HK, Bullard KM, et al. Trends in cancer mortality among people with vs without diabetes in the USA, 1988–2015. Diabetologia. 2020;63(1):75–84. doi: 10.1007/s00125-019-04991-x. [DOI] [PubMed] [Google Scholar]
- 24.Flory J, Lipska K. Metformin in 2019. JAMA. 2019;321(19):1926–1927. doi: 10.1001/jama.2019.3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chiang CH, Chiang CH, Chiang CH, Ma KS, Peng CY, Hsia YP, et al. Impact of sodium-glucose cotransporter-2 inhibitors on heart failure and mortality in patients with cancer. Heart. 2022 doi: 10.1136/heartjnl-2022-321545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Petersen NH, Olsen OD, Groth-Pedersen L, Ellegaard AM, Bilgin M, Redmer S, et al. Transformation-associated changes in sphingolipid metabolism sensitize cells to lysosomal cell death induced by inhibitors of acid sphingomyelinase. Cancer Cell. 2013;24(3):379–393. doi: 10.1016/j.ccr.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 27.Chiang C-H, Chiang C-H, Peng C-Y, Hsia YP, See XY, Horng C-S, et al. Efficacy of cationic amphiphilic antihistamines on outcomes of patients treated with immune checkpoint inhibitors. Eur J Cancer. 2022;174:1–9. doi: 10.1016/j.ejca.2022.07.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author, CHu Chiang. The data are not publicly available as their containing information could compromise the privacy of research participants.