Abstract
Although a number of studies have revealed the important roles of miR-34a in cancer, the regulatory roles of miR-34a in cancer immune response remain largely unknown. Our present study demonstrated a mechanism underlying miR-34a-mediated cancer immune evasion via a SIRT1/NF-κB/B7-H3/TNF-α axis. miR-34a upregulated B7-H3, an important immune checkpoint molecule, through direct inhibition of SIRT1 and consequent acetylation of NF-κB subunit p65 (a-p65), which promoted B7-H3 transcription by direct binding to its promoter. The elevated B7-H3 induced production of pro-inflammatory cytokines including TNF-α. This was further confirmed in the colon of Mir34a-deficient mice, where Sirt1 expression was boosted, and the expressions of a-p65, B7h3, and Tnf were repressed. Consequently, the in vivo inhibitory activity of miR-34a on colorectal cancer (CRC) was eradicated by the reinforced B7-H3 and TNF-α. In conclusion, our study uncovered an etiological mechanism underlying miR-34a-mediated CRC immune evasion through inhibition of SIRT1 and promotion of NF-κB/B7-H3/TNF-α axis.
Supplementary Information
The online version contains supplementary material available at(10.1007/s00262-021-02862-2)
Keywords: B7-H3, Colorectal cancer, Immune evasion, miR-34a
Introduction
microRNAs (miRNAs) are endogenous noncoding RNA molecules playing essential roles in a wide range of physiological and pathological processes [1]. In cancer, miRNAs exert both pro- and antitumorigenic effects [2]. Dysregulation of miRNAs has been reported in most cancer types [3, 4]. miR-34a is a pivotal miRNA whose expression is transcriptionally controlled by the tumor suppressor p53 in physiological conditions [5, 6] and is epigenetically regulated in various human cancers [7]. Many researchers have proposed augmenting miR-34a expression as a candidate therapeutic strategy [8–11]. The primary advantage of miR-34a-based therapy is the simultaneous and multifaceted repression of targets implicated in cellular proliferation (CDK4/6, CCND1, MYC, NOTCH1, and MDMX), apoptosis (BCL2, SIRT1, and BIRC5), migration (SNAI1, MET, and AXIN2), senescence (E2F3), cancer stem-like cell phenotype (CD44, NANOG, and SOX2), and/or immune evasion (PD-L1, CCL22, DGKζ, and AXL) [11–15].
As an upstream regulator of PD-L1, CCL22, and AXL, miR-34a has been identified as a critical regulator of immune response in cancers [10, 16, 17]. miR-34a also acts as an ULBP2 repressors in human malignant melanoma, thereby diminishing tumor cell recognition by NK cells and leading to tumor immune surveillance [18]. Another connection between miR-34a and immune response is the SIRT1/NF-κB signaling pathway [19]. SIRT1 normally inhibits NF-κB signaling by deacetylating NF-κB subunit p65 (lysine 310) [20]. miR-34a directly targets SIRT1 [21]. Thus, miR-34a stimulates NF-κB-induced immune responses by inhibiting SIRT1 [22].
T-cell-mediated immune responses play crucial roles in tumor immunity. T-cell receptor (TCR)-mediated antigen-specific stimulation is essential for initiating T-cell activation. TCR signals are modulated by co-signaling molecules including co-stimulatory and co-inhibitory molecules, which positively and negatively control T-cell differentiation and function, respectively. One of the most prominent co-signals is provided by the interactions between B7-CD28 family ligands and receptors. The B7-CD28 family can be phylogenetically divided into three groups: (I) B7-1/B7-2/CD28/CTLA4 and B7h/ICOS; (II) PD-L1/PD-L2/PD-1; (III) B7-H3 (CD276), B7x (B7-H4/B7S1) and HHLA2 (B7H7/B7-H5)/TMIGD2 (IGPR-1/CD28H) [23]. Similar to PD-L1, a direct target of miR-34a [11], B7-H3 is thought to dampen peripheral immune responses via co-inhibition. B7-H3 has been identified as an important co-inhibitory regulator in immune responses [24]. Uniformly overexpressed B7-H3 has been detected in cancers including CRC [25–27]. Intensified expression of B7-H3 is associated with a poor outcome in patients with CRC [27]. These intriguing findings drew our attention and motivated us to explore the regulatory roles of miR-34a in B7-H3 expression.
Our present study demonstrates that miR-34a acts as a driver of immune evasion through inhibiting SIRT1 and thus derepressing a NF-κB/B7-H3/TNF-α axis in CRC. The miR-34a-induced B7-H3 and TNF-α in the tumor microenvironment lead to eradication of antitumor immunity and consequent enhancement of tumor cell growth. Thus, we have uncovered a novel etiological mechanism underlying miR-34a-induced immune evasion in CRC.
Materials and methods
Tissues
All donors were genetically unrelated ethnic Han Chinese and were born and raised in Suzhou, China. The study protocol was approved by the Institutional Review Board of Soochow University. All donors provided signed informed consent. The experiments have been carried out in accordance with the Code of Ethics of the World Medical Association. As regards evaluation of B7-H3 expression, frozen specimens including intestinal normal (> 5 cm away from the tumor margin) and carcinoma tissues were acquired from donors. As regards evaluation of B7-H3 expression in paraffin embedded CRC tissues, samples were acquired from patients at the First Affiliated Hospital of Soochow University (Suzhou, China). Samples were histologically confirmed by two pathologists. None of the patients underwent radiotherapy or chemotherapy before surgery.
Cell lines
Cell lines HCT-8, HCT-116, and CHO were purchased from the American Type Culture Collection (Manassas, VA) and cultured under the conditions recommended by the provider. For the construction of B7-H3 silenced (sh-B7-H3) and overexpressed (oe-B7-H3) cell lines, B7-H3 shRNA lentiviral vectors and B7-H3/pcDNA3.1 vectors were transfected into HCT-116 cells, respectively. The stable clones were established by neomycin selection and evaluated by flow cytometry and western blotting (WB) assays. The primers (GeneWiz, Suzhou, China) used for constrction of vectors are listed in Table S1.
Transfection
To investigate the regulatory role of mimics, inhibitors, siRNAs, and expression vectors in gene expression, 50 nM of the synthetic RNAs (mimics, inhibitor, siRNA, mimics control, inhibitor control, or siRNA control; GenePharma, Shanghai, China; see Table S2), or 1 μg expression vectors (SIRT1/pEX-2, p65/pEX-2, B7-H3/pcDNA3.1, and empty vectors) were transfected into HCT-116 cells using lipofectamine 2000 (Invitrogen) for 48 h before RNA extraction or 72 h before protein extraction. SIRT1/pEX-2 vector and p65/pEX-2 vector were synthesized by GeneWiz (Suzhou, China).
RNA sequencing (RNA-seq)
The RNA-seq assays were performed by Shanghai Oebiotech Co., Ltd. Briefly, the total RNA was extracted using the mirVana miRNA Isolation Kit (Ambion). RNA integrity was evaluated using the Agilent 2100 bioanalyzer (Agilent). The libraries were constructed using TruSeq Stranded mRNA LTSample Prep Kit (Illumina). Then, the libraries were sequenced on HiSeq™ 2500 sequencing platform (Illumina) and 125 bp/150 bp paired-end reads were generated.
Quantitative real-time PCR (qPCR)
Total RNA was isolated from tissues or cultured cells using TRIzol reagent (Takara). Then, the total RNA was reverse transcribed into cDNA using NxGen M-MuLV reverse transcriptase (MBI). qPCR was performed using quantitative RT-PCR master mix (Bio-Rad) on CFX96 Touch™ real-time PCR system (Bio-Rad). RNA expression levels were normalized to those of GAPDH or U6. Primers are listed in Table S1.
WB
The colon tissues were collected from two wild-type DAB1/J mice (one male and one female) and Mir34a−/− knockout DAB1/J mice (one male and one female; kindly provided by Prof. Qi-Xiang Shao, Jiangsu University) [28]. Total protein from tissues or cells was extracted using RIPA lysis buffer (Beyotime, China). Protein concentrations were determined using the Pierce BCA Protein Assay Kit (Thermo). Twenty µg of protein was separated on a 10% SDS-PAGE gel and electrotransferred onto a PVDF membrane. After blocking using 5% skim milk, the membrane was incubated with primary antibodies at 4 ℃ overnight and subsequently with the corresponding secondary antibodies (Santa Cruz Biotech, USA) for 1 h at room temperature. The membrane was then developed using Clarity Western ECL substrates (Merck Millipore) and visualized with a ChemiDoc™ MP Imaging System (Bio-Rad). Protein expression was normalized to GAPDH expression. Antibodies against B7-H3 / B7h3 (#376,769), GAPDH (#47,724), and β-actin (#47,778) were purchased from Santa Cruz Biotech (USA). Antibodies against SIRT1/Sirt1 (#9475), acetyl-p65 (#3045), p65 (#4764), and TNF-α/Tnf (#6945) were purchased from Cell Signaling Technology (USA).
Dual-luciferase reporter (DLR) assay
This assay was performed as previously described [29]. Briefly, the pGL3-control vector (Promega) containing the promoter of B7-H3 gene was synthesized by GeneWiz (Suzhou, China). CHO cells were co-transfected with 0.2 μg of pGL3 constructs and 0.5 μg p65/pEX-2 vectors or 50 nM of p65 siRNA (GenePharma, Suzhou, China) using lipofectamine 2000 (Invitrogen). Luciferase activity was measured after 24 h using the DLR assay system (Promega).
Human peripheral blood mononuclear cells (PBMCs) and T cell culture
Human PBMCs were isolated from the buffy coats (leukocyte concentrates) of healthy donors by Ficoll density gradient centrifugation. T cells were enriched by magnetic bead separation using Pan T Cell Kit II, Human (Miltenyi) and seeded in T cell medium containing 100 IU/mL IL-2 (PeproTech, USA). ImmunoCult™ Human CD3/CD28 T Cell Activator (STEMCELL Technologies, Canada) was used to activate T cells.
Flow cytometry
To investigate the effects of B7-H3 on the production of cytokines, the wt-B7-H3 and sh-B7-H3 HCT-116 cells were cultured alone or co-cultured with human T lymphocytes at a ratio of 1:10 for 16 h [30], and then the levels of IL-2, IL-4, IL-6, IL-10, IFN-γ, and TNF-α in the culture media were detected by flow cytometry (Beckman, USA) using Cytometric Bead Array Human Th1/Th2 Cytokine Kit (BD Bioscience). To investigate the effects of miR-34a on the production of cytokines, the wt-B7-H3 cells and sh-B7-H3 cells were transfected with 50 nM miR-34a mimics for 48 h and then were cultured alone or co-cultured with human T lymphocytes at a ratio of 1:10 for 16 h. The levels of IL-2, IL-4, IL-6, IL-10, IFN-γ, and TNF-α in the culture media were detected by flow cytometry assays.
Pull-down assay
B7-H3 promoter was amplified using 5′biotin-CAC ATG CAG AGA GAC GCA CAT G-3′ and 5′biotin-CTG CTG ACC GAG GCC TGA GCC AG-3′. The biotinylated DNA was incubated with total protein from HCT-116 cells on ice for 20 min. One hundred μl streptavidin-agarose G beads (Thermo) were precipitated and resuspended in the mixture of DNA and protein. The mixture was incubated in a tumble blender (Thermo) for 1 h at room temperature. Then, the beads were precipitated and resuspended in loading buffer (Beyotime, China) for WB assays.
Xenografts
Animal protocols were approved by the Institutional Animal Care and Use Committee at Soochow University. All animal experiments were comply with the ARRIVE guidelines and were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 8023, revised 1978). Male NOD-SCID mice were purchased from SLAC int. (Shanghai, China). All mice were kept in specific-pathogen-free conditions in the Animal Resource Center at Soochow University. Tumor cell inoculation and treatment were performed as previously reported [26]. To evaluate the in vivo antitumor activity of miR-34a, 5 × 106 HCT-116 cells were subcutaneously inoculated in the mice lower right flank. When tumors reached a volume of 150–200 mm3, 2 nmol miR-34a agomir was intravenously administered once every 2 d. To investigate the effect of human T cells on the antitumor activity of miR-34a agomir, 2 × 107 T cells were intravenously injected into the mice tail once every 4 d from 1 d before the first agomir treatment. To investigate the effect of human PBMCs on the antitumor activity of miR-34a agomir, 2 × 107 PBMCs were intravenously injected into the mice tail once every 4 d from 1 d before the first agomir treatment. Tumor volumes were measured every 3 d.
Results
miR-34a induced B7-H3 expression in CRC
To understand the immune-regulatory role of miR-34a, we tested the effect of miR-34a on the expression of genes participated in the regulation of T cell-mediated cytokine production (Table S3), which were provided by The Gene Ontology Resource (http://geneontology.org/). Due to high-expression of miR-34a (Fig. S1) and B7-H3 [31] in HCT-116 and HCT-8 cells, we employed these cell lines for the following studies. We transcfected HCT-116 cells with miR-34a mimics and determined RNA expression using the RNA-seq method. Interestingly, far from the inhibitory role of miR-34a in PD-L1 expression, miR-34a remarkedly augmented B7-H3 expression in HCT-116 cells (Fig. 1a). Then, we reevaluated the influence of miR-34a on B7-H3 mRNA and protein expression in HCT-116 cells using qPCR and WB methods, respectively. We found that both B7-H3 mRNA and protein were significantly elevated by miR-34a in a dose-dependent manner (Fig. 1b, c). We also found that B7-H3 protein was augmented by miR-34a mimics in HCT-8 cells (Fig. 1d). We detected miR-34a expression in 87 pairs of frozen tissues of colorectal normal and carcinoma by qPCR method. We found that miR-34a was significantly overexpressed in the carcinoma tissues as compared with the normal tissues (Fig. 1e). We have recently detected the expression B7-H3 protein in these CRC tissues by IHC method. B7-H3 positive stains were mainly distributed in the cytoplasm of tumor cells [31]. Moreover, we found that miR-34a expression was significantly correlated with B7-H3 expression in this cohort of cancer tissues (Fig. 1f). Since miR-34a is transcriptionally controlled by p53 [5, 6], we analyzed the expression of miR-34a and B7-H3 (CD276), as well as the TP53 mutation status in the TCGA samples. We found that both miR-34a and B7-H3 were significantly highly expressed in colon adenocarcinoma (COAD) and rectum adenocarcinoma (READ) samples (Fig. S2A). Moreover, the expression of miR-34a in the TP53 non-mutant COAD samples was apparently higher than the TP53 mutant COAD samples (Fig. S2B). However, the expression of miR-34a in the READ samples and B7-H3 in the COAD and READ samples was not correlated to the TP53 mutation status (Fig. S2B).
Fig. 1.
miR-34a upregulated B7-H3 in CRC. a miR-34a-mediated regulation of genes in HCT-116 cells. HCT-116 cells were transfected with 50 nM miR-34a mimics for 48 h. Total RNAs were sequenced by Illumina HiSeqTM 2500. b miR-34a mimics significantly increased B7-H3 mRNA expression in HCT-116 cells in a dose-dependent manner. c miR-34a mimics significantly increased B7-H3 protein expression in HCT-116 cells in a dose-dependent manner. B7-H3 protein expression was measured by WB method. d miR-34a mimics (50 nM) significantly increased B7-H3 protein expression in HCT-8 cells. e The expression of miR-34a in the CRC tissues was significantly higher than the normal tissues. f B7-H3 protein expression was positively correlated with miR-34a in the CRC tissues. Each experiment was performed in duplicate. Data represent mean ± SD. Significance was assessed by t-test. ***p < 0.001; **p < 0.01; *p < 0.05
miR-34a upregulated B7-H3 via SIRT1/NF-κB pathway
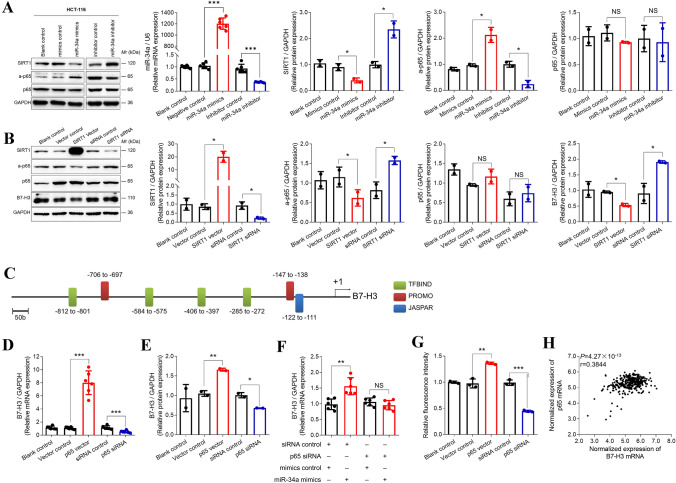
Since SIRT1 is a direct target of miR-34a and is capable of inhibiting NF-κB signaling by deacetylating NF-κB subunit p65 [22, 32], we hypothesized that SIRT1/NF-κB pathway would be involved in miR-34a-mediated upregulation of B7-H3. To test this, we transfected HCT-116 cells with miR-34a mimics or inhibitor and we evaluated the expression of SIRT1, p65, and a-p65. Consistent with our postulation, following successful increase or decrease in the levels of miR-34a, SIRT1 expression was evidently inhibited by miR-34a mimics but was elevated by miR-34a inhibitor (Fig. 2a). Consequently, a-p65 expression was rescued by miR-34a mimics but was suppressed by the miR-34a inhibitor (Fig. 2a). The influences of miR-34a mimics and inhibitor on the expression of SIRT1, a-p65, p65, and B7-H3 were also confirmed in HCT-8 cells (Fig. S3). Then, we, respectively, overexpressed and silenced SIRT1 in HCT-116 ells. Consistently, after the levels of SIRT1 were upregulated by SIRT1-overexpression or downregulated by SIRT1-silence, the expression levels of a-p65 and B7-H3 were accordingly decreased or increased (Fig. 2b).
Fig. 2.
miR-34a induced B7-H3 expression through SIRT1/NF-κB axis. a miR-34a mimics (50 nM) significantly increased miR-34a, inhibited SIRT1, and upregulated a-p65 in HCT-116 cells. miR-34a inhibitor (50 nM) significantly reduced miR-34a, rescued SIRT1, and downregulated a-p65 in HCT-116 cells. Neither miR-34a mimics nor inhibitor showed effects on the expression of p65 protein. b The expressions of a-p65 and B7-H3 were inhibited by SIRT1-overexpression, but were enhanced by SIRT1-silence. The expression of p65 protein was not impacted by SIRT1. c Seven binding sites of NF-κB on B7-H3 promoter were predicted by JASPAR, PROMO, and TFBIND. d The expression of B7-H3 mRNA was significantly elevated by p65-overexpression and was reduced by p65-silence in HCT-116 cells. e The expression of B7-H3 protein was significantly elevated by p65-overexpression and was reduced by p65-silence in HCT-116 cells. f miR-34a-mediated upregulation of B7-H3 mRNA was reversed by p65-silence. g The results from DLR assays demonstrated that the expression of B7-H3-promoter/pGL-3 constructs was significantly enhanced by p65-overexpression, but it was reduced by p65-silence. h The expression of B7-H3 mRNA was positively correlated with p65 mRNA in the TCGA colon cancer tissues. Each experiment was performed in triplicate or duplicate. Data represent mean ± SD. Significance compared to negative control was assessed by t-test. ***p < 0.001; **p < 0.01; *p < 0.05; NS, no significance
Next, we evaluated whether B7-H3 transcription is directly promoted by NF-κB. We employed softwares TFBIND, PROMO, and JASPAR to predict NF-κB binding sites in the B7-H3 promoter. As shown in Fig. 2c, seven NF-κB binding sites were identified in the promoter region of B7-H3. Then, we reinforced or silenced p65 expression in HCT-116 cells (Fig. S4). Both B7-H3 protein and mRNA expression were elevated by p65-overexpression but were attenuated by p65-silence (Fig. 2d, e). Further investigation revealed that p65-silence repressed miR-34a-induced B7-H3 mRNA (Fig. 2f). Moreover, DLR assays were carried out to verify the direct binding of p65 to the B7-H3 promoter. The results showed that the fluorescence intensity from the pGL-3 control vector containing the B7-H3 promoter was significantly increased by p65-overexpression, but it was decreased by p65-silence (Fig. 2g). Chromatin immunoprecipitation experiments were also performed. WB of the pull-down substances demonstrated that p65 directly interacted with the B7-H3 promoter (Fig. S5). In addition, B7-H3 expression was positively correlated with p65 expression in the TCGA samples from 331 CRC patients (Fig. 2h).
miR-34a stimulated the production of cytokines in a B7-H3-dependent manner
B7-H3 has been identified as an important regulator in immune responses [24]. Hence, we first investigated the immunoregulatory role of B7-H3 in CRC. Since B7-H3 is highly expressed in CRC cells, we silenced B7-H3 in HCT-116 cells using shRNA lentiviral vectors (sh-B7-H3), and then we examined the levels of Th1/Th2 cytokines including IL-2, IL-4, IL-6, IL-10, IFN-γ, and TNF-α in the culture media. We found that the levels of TNF-α, IL-2, IL-4, and IFN-γ were significantly reduced (Fig. 3a and Fig. S6A). When we co-cultured the sh-B7-H3 HCT-116 cells with the activated human T lymphocytes, the levels of TNF-α, IL-2, IL-4, IL-6, and IL-10 were further decreased (Fig. 3a and Fig. S6B). These findings demonstrate that B7-H3 is critical for the production of Th1/Th2 cytokines in the tumor microenvironment.
Fig. 3.
miR-34a induced TNF-α through B7-H3. a The level of TNF-α in the culture media of sh-B7-H3 cells was significantly lower than that of wt-B7-H3 cells. The cells were cultured alone or co-cultured with human T lymphocytes at a ratio of 1:10 for 16 h. b miR-34a significantly increased TNF-α level in the culture media of HCT-116 cells. The cells were transfected with 50 nM miR-34a mimics for 48 h and then were cultured alone or co-cultured with human T lymphocytes at a ratio of 1:10 for 16 h. c miR-34a showed no effects on TNF-α level in the culture media for sh-B7-H3 cells. The cells were transfected with 50 nM miR-34a mimics for 48 h and then were cultured alone or co-cultured with human T lymphocytes at a ratio of 1:10 for 16 h. d The level of TNF-α was remarkedly lower in the sh-B7-H3 cells than the wt-B7-H3 cells. Each experiment was performed in triplicate. Data represent mean ± SD. Significance was assessed by t-test. **p < 0.01; *p < 0.05; NS, no significance
Next, we investigated the influence of miR-34a on the production of cytokines in CRC. We transfected HCT-116 cells with miR-34a mimics and examined the levels of IL-2, IL-4, IL-6, IL-10, IFN-γ, and TNF-α in the culture media. Consistently, we found that TNF-α was significantly induced by the miR-34a mimics (Fig. 3b). When we transfected HCT-116 cells with miR-34a mimics for 48 h and then co-cultured the cells with the activated human T lymphocytes, the levels of TNF-α, IL-6, IL-10, and IFN-γ were also significantly enhanced (Fig. 3b, Fig. S6C and S6D). We also tested the effects of these cytokines on the expression of B7-H3. TGF-β1 was used as a positive control [26]. We found that TNF-α evidently enhanced the expression of B7-H3 in a dose-dependent manner (Fig. S7). However, when we transfected sh-B7-H3 HCT-116 cells with miR-34a mimics, the levels of IL-2, IL-4, IL-6, IL-10, IFN-γ, and TNF-α were not altered, whether the cells were cultured alone or co-cultured with the human T lymphocytes (Fig. 3c, Fig. S6E and S6F). Moreover, we found that the expression of intracellular TNF-α protein was significantly decreased in the sh-B7-H3 HCT-116 cells as compared with the wt-B7-H3 HCT-116 cells (Fig. 3d). These findings suggest that miR-34a stimulates the production of TNF-α in a B7-H3-dependent manner.
miR-34a induced intracellular TNF-α via a SIRT1/NF-κB/B7-H3 axis
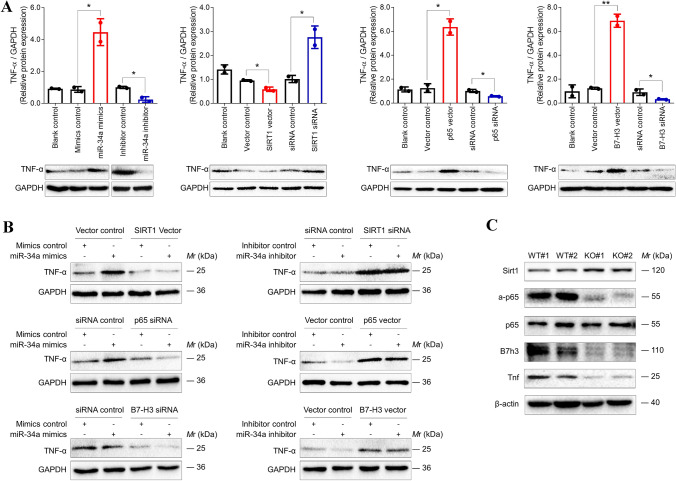
The pro-inflammatory cytokine TNF-α is one of the principal mediators of immune response. Studies have demonstrated that TNF-α promotes tumor growth by inducing cell survival, proliferation, angiogenesis, and epithelial-to-mesenchymal transition via NF-κB activation [33–35]. To elucidate the mechanism that miR-34a induced the production of TNF-α through a SIRT1/NF-κB/B7-H3 axis, we individually modulated the expression of miR-34a, SIRT1, NF-κB, and B7-H3 in HCT-116 cells and monitored intracellular TNF-α expression. We found that TNF-α expression was significantly elevated by the forced-expression of miR-34a, p65 or B7-H3, or silence of SIRT1, but it was decreased by the inhibition of miR-34a, p65 or B7-H3, or overexpression of SIRT1 (Fig. 4a and Fig. S8A). To further confirm the involvement of the SIRT1/NF-κB/B7-H3 axis in miR-34a-induced production of TNF-α, we, respectively, transfected miR-34a mimics or inhibitor into HCT-116 cells together with each of the expression vectors and siRNAs of the downstream SIRT1, p65, and B7-H3. We found that miR-34a mimics-induced TNF-α was reversed by SIRT1-overexpression, p65-silence, or B7-H3-silence (Fig. 4b). Moreover, TNF-α was slightly inhibited by miR-34a inhibitor, but it was markedly elevated by SIRT1-inhibition, p65-overexpression, or B7-H3-overexpression whether there is miR-34a inhibitor or not (Fig. 4b). In addition, positive relationships between TNF-α and B7-H3 or p65 were also observed from the TCGA database (Fig. S8B). Notably, we found that the expression of Sirt1 was boosted, but the expressions of a-p65, B7h3, and Tnf were reduced in the colon of the Mir34a−/− knockout mice (Fig. 4c).
Fig. 4.
miR-34a stimulated intracellular TNF-α via SIRT1/NF-κB/B7-H3 axis. a The expression of intracellular TNF-α was significantly enhanced by SIRT1-silence and reinforced miR-34a, p65, or B7-H3, but it was inhibited by SIRT1-overexpression and suppression of miR-34a, p65, or B7-H3. Data represent mean ± SD. Significance was assessed by t-test. **p < 0.01; *p < 0.05. b miR-34a-induced TNF-α was resuppressed by SIRT1-overexpression and silence of p65 or B7-H3. On the contrary, miR-34a inhibitor-mediated downregulation of TNF-α was reversed by SIRT1-silence and reinforced p65 or B7-H3. c Immunoblots showed that Sirt1 expression was boosted and the expressions of a-p65, B7h3, and Tnf were repressed in the colon of the Mir34a−/− knockout mice. WT, wild-type; KO, knockout. Each experiment was performed in duplicate
miR-34a promoted cancer immune escaping via B7-H3/TNF-α
miR-34a has been identified as a potent tumor suppressor in vitro and in vivo [12, 36–38]. We subcutaneously grafted HCT-116 cells into the lower back of NOD-SCID mice. After palpable tumors had formed, the tumor-bearing mice were intravenously injected with 1 mg/kg/day of miR-34a agomir. As shown in Fig. 5a, miR-34a agomir significantly inhibited tumor growth. The expression of B7-H3 and TNF-α in tumors was not influenced by miR-34a (Fig. 5b). However, when freshly separated human T lymphocytes were intravenously injected into tumor-bearing NOD-SCID mice before adminstration of miR-34a agomir, the inhibitory activity of miR-34a in tumor growth was sharply degraded (Fig. 5c). Most notably, one mouse died after 5 treatments of miR-34a agomir, although miR-34a agomir had no influence on the body weights of the mice (Fig. S9). At the endpoint, both B7-H3 and TNF-α expression in the tumors were slightly elevated by miR-34a (Fig. 5d). Furthermore, in experiments in which freshly separated PBMCs were intravenously injected into tumor-bearing NOD-SCID mice before adminstration of miR-34a agomir, the anticancer activity of miR-34a totally vanished (Fig. 5e), and both B7-H3 and TNF-α expression were significantly enhanced by miR-34a (Fig. 5f). These findings demonstrated that miR-34a promoted tumor growth within the immune microenvironment by reinforcing B7-H3 and TNF-α expression.
Fig. 5.
miR-34a promoted tumor growth by inducing B7-H3 and TNF-α. a miR-34a agomir significantly inhibited tumor growth in NOD-SCID mice (n = 6). b miR-34a agomir had no effects on B7-H3 and TNF-α expression in xenografts. c miR-34a agomir slightly inhibited tumor growth in NOD-SCID mice (n = 4) engrafted with human T lymphocytes. d miR-34a agomir slightly induced B7-H3 and TNF-α expression in tumors in NOD-SCID mice (n = 4) engrafted with human T lymphocytes. e miR-34a agomir had no effects on tumor growth in NOD-SCID mice (n = 6) engrafted with human PBMCs. f miR-34a agomir significantly induced B7-H3 and TNF-α expression in tumors in NOD-SCID mice (n = 6) engrafted with human PBMCs. Data represent mean ± SD. Significance was assessed by t-test. ***p < 0.001; **p < 0.01; *p < 0.05
Discussion
In this study, we demonstrated that miR-34a-induced B7-H3 overexpression leading to immunosuppression in CRC through inhibiting SIRT1 and consequently triggering a NF-κB/B7-H3/TNF-α axis. Furthermore, we presented evidence to show that the in vivo inhibitory activity of miR-34a on CRC cell growth was eradicated by a concomitant elevation of B7-H3 and TNF-α in an immune environment. These findings led us to re-understand the roles of miR-34a in the tumor microenvironment.
B7-H3 is an important immune checkpoint [24] and is uniformly overexpressed in CRC [25–27]. Regulators such as miR-29c [39] and miR-143 [26] are involved in B7-H3 regulation. However, the regulatory mechanisms underlying B7-H3 overexpression in tumors are still largely unclear. In addition to overexpression of B7-H3 in CRC [25–27], miR-34a overexpression in CRC has also been identified in several previous studies [40–42]. Our present study provided several lines of evidence to demonstrate that B7-H3 was upregulated by miR-34a. First, both B7-H3 and miR-34a were overexpressed in CRC tissues. Second, B7-H3 expression was positively correlated with miR-34a expression in CRC tissues and TCGA smaples. Third, miR-34a mimics robustly enhanced the expression of B7-H3 mRNA and protein in a dose-dependent manner in HCT-116 cells. Furthermore, B7-H3 expression in HCT-116-cell xenografts was clearly induced by miR-34a agomir in the in vivo tumor microenvironment.
Since the SIRT1/NF-κB signaling pathway has been shown to be involved in miR-34a-mediated immune rsponse [22], we hypothesized that miR-34a upregulated B7-H3 through this pathway. Indeed, miR-34a mimics inhibited SIRT1 and consequently enhanced a-p65 expression in HCT-116 cells, while the miR-34a inhibitor restored SIRT1 but repressed a-p65. Reinforced SIRT1 reduced a-p65 and resulted in downregulation of B7-H3. Conversely, SIRT1-silence induced a-p65 and B7-H3 expression in HCT-116 cells. Moreover, miR-34a mimics-mediated upregulation of B7-H3 was repressed by either SIRT1 overexpression or p65 silence, and miR-34a inhibitor-mediated downregulation of B7-H3 was reversed by either SIRT1 silence or p65 overexpression. In addition, we found that the regulatory potency of either SIRT1 or NF-κB on the expression of B7-H3 protein was consistent with that of miR-34a, suggesting the miR-34a/ SIRT1/NF-κB axis is strictly controlled in CRC cells.
The present study also shows that NF-κB is a transcriptional factor of B7-H3. The presence of potential binding sites of NF-κB on B7-H3 promoter were predicted by software. Both B7-H3 mRNA and protein were increased by overexpression of p65 but were decreased by p65 silence. The silence of p65 also reversed miR-34a-mediated upregulation of B7-H3 mRNA and protein. Additionally, B7-H3 mRNA expression was positively correlated with p65 mRNA in TCGA samples. Moreover, ChIP assay results demonstrated direct binding of p65 to B7-H3 promoter. As far as we know, NF-κB is the first transcription factor that has been proven to promote B7-H3 transcription.
B7-H3-induced production of pro-inflammatory cytokines and chemokines has recently been shown to be mediated by phosphorylation of downstream NF-κB p65 and MAPK p38 [43]. Other reseachers also showed that B7-H3 stimulated cytokine production by binding to TLT2 [44]. In the present study, we identified B7-H3 in CRC cells as a critical regulator in the production of cytokines, such as IL-2, IL-6, IL-10, IFN-γ, and TNF-α. B7-H3 deficiency in HCT-116 cells reduced the production of IL-2, IL-4, IL-6, IL-10, IFN-γ, and TNF-α in the culture media. Moreover, the miR-34a-induced production of cytokines was reversed by deletion of B7-H3 in HCT-116 cells. Further investigation showed that forced B7-H3 in HCT-116 cells increased intracellular TNF-α expression, which was reversed by B7-H3 silence. However, the acetylation of NF-κB p65 was not influenced by B7-H3, suggesting that B7-H3 induced intracellular TNF-α expression through pathways other than NF-κB. This possibility warrents further investigation.
Several studies have shown that miR-34a is involved in immune response through AXL regulation [17], c-Fos decrease and TNF-α increase [45], Notch-mediated control of NF-κB signaling [46], SIRT1/NF-κB pathway [22, 33], or IL-6R/STAT3/miR-34a feedback loop [47]. In this study, we provided a rich set of evidence to demonstrate that miR-34a promoted immunosuppression in CRC by inducing production of pro-inflammatory cytokines through the SIRT1/NF-κB pathway and the ensuing upregulation of B7-H3. First, miR-34a apparently stimulated secretion of IL-6, IL-10, IFN-γ, and TNF-α in HCT-116/T cell coculture system. Second, the expression of intracellular TNF-α was significantly enhanced by miR-34a mimics but was reduced by the miR-34a inhibitor. Third, SIRT1 siRNA, p65 expression vector, and B7-H3 expression significantly induced the expression of intracellular TNF-α, which was reversed by SIRT1 expression vector, p65 siRNA, and B7-H3 siRNA, respectively. Even more, the expression of Sirt1 was elevated, but the expression of a-p65, B7h3, and Tnf was attenuated in the colon of the Mir-34a knockout mice.
Apart from the immunemodulatory activity of miR-34a, cell- and animal-based study models have demonstrated the potential ability of miR-34a to suppress CRC cell growth. Ectopic expression of miR-34a in CRC cells significantly inhibited cell growth, migration, invasion and metastasis [48, 49]. The therapeutic potential of miR-34a was also confirmed in subcutaneous xenograft models [48, 49]. In our hands, we also obtained robust inhibition of tumor growth in the CRC mouse model using a chemically synthesized miR-34a, which was well tolerated. However, in experiments to test the antitumor potential of this miR-34a preparation in NOD-SCID nude mice engrafted with human peripheral T cells derived from healthy volunteers, miR-34a produced only a slight suppression of tumor growth. The observation that one mouse died after administration of 5 doses suggests that miR-34a has a potential severe immune-related adverse effect in this cellular environment. Even more notably, the anticancer activity of miR-34a totally vanished in the tumor-bearing NOD-SCID mice which were intravenously injected with freshly separated human PBMCs. Interestingly, the expression levels of B7-H3 and TNF-α in xenografts were inversely correlated with the anticancer activity of miR-34a. For instance, the anticancer activity of miR-34a was the highest in the HCT-116 xenografts, in which neither B7-H3 nor TNF-α was dysregulated. However, in the tumor-bearing mice engrafted with human PBMCs, the anticancer activity of miR-34a was the lowest and both B7-H3 and TNF-α expression were evidently boosted. Although no decrease in tumor growth is observed in the mice engrafted immune cells possibly due to the constitutive expression of B7-H3 on HCT-116 cells, our findings demonstrate that miR-34a-induced B7-H3 and TNF-α promoted tumor growth in immune microenvironment.
In summary, we discovered a novel mechanism explaining miR-34a-mediated B7-H3 overexpression and immunosuppression in CRC. It entails miR-34a inhibiting SIRT1 and triggering a NF-κB/B7-H3/TNF-α axis. The elevated B7-H3 promotes a massive release of pro-inflammatory cytokines resulting in tumor growth. Thus, our findings provide new insights in miR-34a-mediated immunosuppression in CRC and offer the rationale for developing novel cancer immunotherapies by targeting B7-H3.
Supplementary Information
Acknowledgments
We acknowledge Prof. Zengjie Yang and Dr. Christopher Y. Chan for critical revision of the manuscript.
Author contributions
W.C.C. and W.P.W. conceived the idea and directed the study. F.Y.M., M.Y., Y.S.C., W.C.C., and W.P.W. designed the experiments. F.Y.M., M.Y., and Y.S.C. performed experiments, analyzed the data, and generated figures. F.Y.M., M.Y., Y.S.C., W.C.C., and W.P.W. wrote the manuscript.
Funding
This research was supported by the National Natural Science Foundation of China (No. 81773044), Science and Technology Special Project of Clinical Medicine in Jiangsu Province (BL2014046), Social Development Project of Jiangsu Province (BE2019657), Qinglan Project of Jiangsu Province, and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Data availability
All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Additional data related to this paper may be requested from the authors.
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the ethics committee of Soochow University (No.IRB-29-20120512H) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Fanyi Meng, Man Yang and Yinshuang Chen contributed equally to this work.
Contributor Information
Weichang Chen, Email: weichangchen@126.com.
Weipeng Wang, Email: wangweipeng@suda.edu.cn.
References
- 1.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/S0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 2.Esquela-Kerscher A, Slack FJ. Oncomirs-microRNAs with a role in cancer. Nat Rev Cancer. 2006;6:259–269. doi: 10.1038/nrc1840. [DOI] [PubMed] [Google Scholar]
- 3.Lu J, Getz G, Miska EA, et al. MicroRNA expression profiles classify human cancers. Nature. 2005;435:834–838. doi: 10.1038/nature03702. [DOI] [PubMed] [Google Scholar]
- 4.Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer. 2006;6:857–866. doi: 10.1038/nrc1997. [DOI] [PubMed] [Google Scholar]
- 5.Chang TC, Wentzel EA, Kent OA, et al. Transactivation of miR-34a by p53 broadly influences gene expression and promotes apoptosis. Mol Cell. 2007;26:745–752. doi: 10.1016/j.molcel.2007.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.He L, He X, Lim LP, et al. A microRNA component of the p53 tumour suppressor network. Nature. 2007;447:1130–1134. doi: 10.1038/nature05939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lodygin D, Tarasov V, Epanchintsev A, Berking C, Knyazeva T, Korner H, Knyazev P, Diebold J, Hermeking H. Inactivation of miR-34a by aberrant CpG methylation in multiple types of cancer. Cell Cycle. 2008;7:2591–2600. doi: 10.4161/cc.7.16.6533. [DOI] [PubMed] [Google Scholar]
- 8.Bader AG. miR-34-a microRNA replacement therapy is headed to the clinic. Frontiers in genetics. 2012;3:120. doi: 10.3389/fgene.2012.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang WP, Ho PY, Chen QX, et al. Bioengineering novel chimeric microRNA-34a for prodrug cancer therapy: high-yield expression and purification, and structural and functional characterization. J Pharmacol Exp Ther. 2015;354:131–141. doi: 10.1124/jpet.115.225631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang X, Li J, Dong K, et al. Tumor suppressor miR-34a targets PD-L1 and functions as a potential immunotherapeutic target in acute myeloid leukemia. Cell Signal. 2015;27:443–452. doi: 10.1016/j.cellsig.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 11.Cortez MA, Ivan C, Valdecanas D, et al. PDL1 regulation by p53 via miR-34. J Natl Cancer Inst. 2016 doi: 10.1093/jnci/djv303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Misso G, Di Martino MT, De Rosa G, et al. Mir-34: a new weapon against cancer? Molecular therapy Nucleic acids. 2014;3:e194. doi: 10.1038/mtna.2014.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ito Y, Inoue A, Seers T, Hato Y, Igarashi A, Toyama T, Taganov KD, Boldin MP, Asahara H. Identification of targets of tumor suppressor microRNA-34a using a reporter library system. Proc Natl Acad Sci USA. 2017;114:3927–3932. doi: 10.1073/pnas.1620019114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hermeking H. The miR-34 family in cancer and apoptosis. Cell Death Differ. 2010;17:193–199. doi: 10.1038/cdd.2009.56. [DOI] [PubMed] [Google Scholar]
- 15.Kong J, Wang W. A systemic review on the regulatory roles of miR-34a in gastrointestinal cancer. Onco Targets Ther. 2020;13:2855–2872. doi: 10.2147/OTT.S234549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang P, Li QJ, Feng Y, et al. TGF-beta-miR-34a-CCL22 signaling-induced Treg cell recruitment promotes venous metastases of HBV-positive hepatocellular carcinoma. Cancer Cell. 2012;22:291–303. doi: 10.1016/j.ccr.2012.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kurowska-Stolarska M, Alivernini S, Melchor EG, et al. MicroRNA-34a dependent regulation of AXL controls the activation of dendritic cells in inflammatory arthritis. Nature commun. 2017;8:15877. doi: 10.1038/ncomms15877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heinemann A, Zhao F, Pechlivanis S, Eberle J, Steinle A, Diederichs S, Schadendorf D, Paschen A. Tumor suppressive microRNAs miR-34a/c control cancer cell expression of ULBP2, a stress-induced ligand of the natural killer cell receptor NKG2D. Can Res. 2012;72:460–471. doi: 10.1158/0008-5472.CAN-11-1977. [DOI] [PubMed] [Google Scholar]
- 19.Hart M, Walch-Ruckheim B, Friedmann KS, et al. miR-34a: a new player in the regulation of T cell function by modulation of NF-kappaB signaling. Cell death dis. 2019;10:46. doi: 10.1038/s41419-018-1295-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yeung F, Hoberg JE, Ramsey CS, Keller MD, Jones DR, Frye RA, Mayo MW. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. The EMBO J. 2004;23:2369–2380. doi: 10.1038/sj.emboj.7600244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamakuchi M, Ferlito M, Lowenstein CJ. miR-34a repression of SIRT1 regulates apoptosis. Proc Natl Acad Sci USA. 2008;105:13421–13426. doi: 10.1073/pnas.0801613105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mohan M, Kumar V, Lackner AA, Alvarez X. Dysregulated miR-34a-SIRT1-acetyl p65 axis is a potential mediator of immune activation in the colon during chronic simian immunodeficiency virus infection of rhesus macaques. J immunol. 2015;194:291–306. doi: 10.4049/jimmunol.1401447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Janakiram M, Shah UA, Liu W, Zhao A, Schoenberg MP, Zang X. The third group of the B7-CD28 immune checkpoint family: HHLA2, TMIGD2, B7x, and B7–H3. Immunol Rev. 2017;276:26–39. doi: 10.1111/imr.12521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chapoval AI, Ni J, Lau JS, et al. B7–H3: a costimulatory molecule for T cell activation and IFN-gamma production. Nat Immunol. 2001;2:269–274. doi: 10.1038/85339. [DOI] [PubMed] [Google Scholar]
- 25.Sun J, Chen LJ, Zhang GB, Jiang JT, Zhu M, Tan Y, Wang HT, Lu BF, Zhang XG. Clinical significance and regulation of the costimulatory molecule B7–H3 in human colorectal carcinoma. Cancer immunol, immunother : CII. 2010;59:1163–1171. doi: 10.1007/s00262-010-0841-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou X, Mao Y, Zhu J, et al. TGF-beta1 promotes colorectal cancer immune escape by elevating B7–H3 and B7–H4 via the miR-155/miR-143 axis. Oncotarget. 2016;7:67196–67211. doi: 10.18632/oncotarget.11950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang B, Pan X, Cobb GP, Anderson TA. microRNAs as oncogenes and tumor suppressors. Dev Biol. 2007;302:1–12. doi: 10.1016/j.ydbio.2006.08.028. [DOI] [PubMed] [Google Scholar]
- 28.Qiu SL, Liu X, Wang ZT, et al. Construction of miRNA-34a knockout DBA1/J mice. J Jiangsu Univ (Med Ed) 2019;29:461–466. [Google Scholar]
- 29.Tao LH, Zhou XR, Li FC, et al. A polymorphism in the promoter region of PD-L1 serves as a binding-site for SP1 and is associated with PD-L1 overexpression and increased occurrence of gastric cancer. Cancer immunol, immunother : CII. 2017;66:309–318. doi: 10.1007/s00262-016-1936-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu S, Tao Z, Hai B, et al. miR-424(322) reverses chemoresistance via T-cell immune response activation by blocking the PD-L1 immune checkpoint. Nat commun. 2016;7:11406. doi: 10.1038/ncomms11406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang C, Chen Y, Li F, Yang M, Meng F, Zhang Y, Chen W, Wang W. B7–H3 is spliced by SRSF3 in colorectal cancer. Cancer immunol, immunother: CII. 2020 doi: 10.1007/s00262-020-02683-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kauppinen A, Suuronen T, Ojala J, Kaarniranta K, Salminen A. Antagonistic crosstalk between NF-kappaB and SIRT1 in the regulation of inflammation and metabolic disorders. Cell Signal. 2013;25:1939–1948. doi: 10.1016/j.cellsig.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 33.Li CW, Xia W, Huo L, et al. Epithelial-mesenchymal transition induced by TNF-alpha requires NF-kappaB-mediated transcriptional upregulation of Twist1. Can Res. 2012;72:1290–1300. doi: 10.1158/0008-5472.CAN-11-3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee DF, Kuo HP, Chen CT, et al. IKK beta suppression of TSC1 links inflammation and tumor angiogenesis via the mTOR pathway. Cell. 2007;130:440–455. doi: 10.1016/j.cell.2007.05.058. [DOI] [PubMed] [Google Scholar]
- 35.Hu MCT, Lee DF, Xia WY, et al. I kappa B kinase promotes tumorigenesis through inhibition of forkhead FOXO3a. Cell. 2004;117:225–237. doi: 10.1016/S0092-8674(04)00302-2. [DOI] [PubMed] [Google Scholar]
- 36.Agostini M, Knight RA. miR-34: from bench to bedside. Oncotarget. 2014;5:872–881. doi: 10.18632/oncotarget.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scognamiglio I, Di Martino MT, Campani V, et al. Transferrin-conjugated SNALPs encapsulating 2'-O-methylated miR-34a for the treatment of multiple myeloma. Biomed Res Int. 2014;2014:217365. doi: 10.1155/2014/217365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Di Martino MT, Campani V, Misso G, et al. In vivo activity of miR-34a mimics delivered by stable nucleic acid lipid particles (SNALPs) against multiple myeloma. PLoS ONE. 2014;9:e90005. doi: 10.1371/journal.pone.0090005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu H, Cheung IY, Guo HF, Cheung NK. MicroRNA miR-29 modulates expression of immunoinhibitory molecule B7–H3: potential implications for immune based therapy of human solid tumors. Can Res. 2009;69:6275–6281. doi: 10.1158/0008-5472.CAN-08-4517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schetter AJ, Leung SY, Sohn JJ, et al. MicroRNA expression profiles associated with prognosis and therapeutic outcome in colon adenocarcinoma. JAMA. 2008;299:425–436. doi: 10.1001/jama.299.4.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Monzo M, Navarro A, Bandres E, et al. Overlapping expression of microRNAs in human embryonic colon and colorectal cancer. Cell Res. 2008;18:823–833. doi: 10.1038/cr.2008.81. [DOI] [PubMed] [Google Scholar]
- 42.Bandres E, Cubedo E, Agirre X, et al. Identification by Real-time PCR of 13 mature microRNAs differentially expressed in colorectal cancer and non-tumoral tissues. Mol cancer. 2006;5:29. doi: 10.1186/1476-4598-5-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen X, Quinn EM, Ni H, Wang J, Blankson S, Redmond HP, Wang JH, Feng X. B7–H3 participates in the development of experimental pneumococcal meningitis by augmentation of the inflammatory response via a TLR2-dependent mechanism. J Immunol. 2012;189:347–355. doi: 10.4049/jimmunol.1103715. [DOI] [PubMed] [Google Scholar]
- 44.Hashiguchi M, Kobori H, Ritprajak P, Kamimura Y, Kozono H, Azuma M. Triggering receptor expressed on myeloid cell-like transcript 2 (TLT-2) is a counter-receptor for B7–H3 and enhances T cell responses. Proc Natl Acad Sci USA. 2008;105:10495–10500. doi: 10.1073/pnas.0802423105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shikama Y, Cao M, Ono T, et al. Reduction of c-Fos via overexpression of miR-34a results in enhancement of TNF- production by LPS in neutrophils from myelodysplastic syndrome patients. PLoS ONE. 2016;11:e0158527. doi: 10.1371/journal.pone.0158527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ventura S, Aryee DN, Felicetti F, et al. CD99 regulates neural differentiation of Ewing sarcoma cells through miR-34a-notch-mediated control of NF-kappaB signaling. Oncogene. 2016;35:3944–3954. doi: 10.1038/onc.2015.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Misso G, Zarone MR, Lombardi A, et al. miR-125b upregulates miR-34a and sequentially activates stress adaption and cell death mechanisms in multiple myeloma. Mol Ther Nucleic Acids. 2019;16:391–406. doi: 10.1016/j.omtn.2019.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gao J, Li N, Dong Y, et al. miR-34a-5p suppresses colorectal cancer metastasis and predicts recurrence in patients with stage II/III colorectal cancer. Oncogene. 2015;34:4142–4152. doi: 10.1038/onc.2014.348. [DOI] [PubMed] [Google Scholar]
- 49.Wang Y, Ding Q, Yen CJ, et al. The crosstalk of mTOR/S6K1 and Hedgehog pathways. Cancer Cell. 2012;21:374–387. doi: 10.1016/j.ccr.2011.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Additional data related to this paper may be requested from the authors.