Abstract
Introduction
Neurologic immune-related adverse events (nirAEs) are uncommon but potentially lethal complications of immune checkpoint inhibitor (ICI) treatment. However, the incidence, radiographic features and prognostic significance of brain magnetic resonance imaging (MRI) changes after ICI treatment remain largely unknown.
Methods
Consecutive patients with advanced non-small cell lung cancer (NSCLC) at three participating institutions receiving anti-PD-1/PD-L1 therapy from June 2017 to September 2020 were screened, and those who received brain MRI within 6 weeks before ICI initiation and at least one follow-up brain MRI after ICI treatment were included. Serial brain MRI images were independently reviewed by two experienced radiologists.
Results
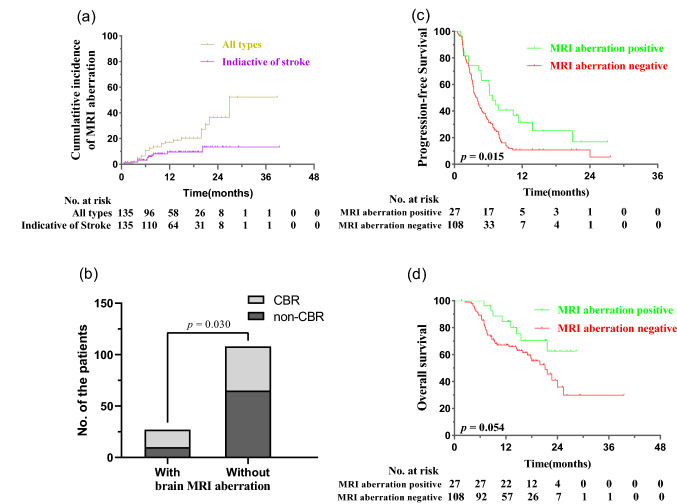
With a median follow-up of 13.2 months, 27 (20.0%) of the 135 enrolled patients developed certain kind of brain MRI aberration. The 1-, 2- and 3-year cumulative incidence of brain MRI aberration was 17.1%, 36.3% and 52.2%, respectively. Brain MRI aberration indicative of stroke, mimicking typical white matter lesions and presenting as T2-hyperintensity suggestive of CNS vasculitis or encephalitis, was documented in 11, 9 and 4 patients, respectively. Patients with brain MRI aberration had higher clinical benefit rate (p = 0.030), longer progression-free survival (p = 0.015) and a tendency of improved overall survival (p = 0.054).
Conclusions
Brain MRI aberrations developed after ICI treatment are not uncommon, and their manifestations vary a lot. Patients developing brain MRI aberrations tended to have better prognosis, which needed to be further investigated.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00262-021-03070-8.
Keywords: Neurologic immune-related adverse events (nirAEs), Non-small cell lung cancer (NSCLC), Brain magnetic resonance imaging (MRI)
Introduction
Neurologic immune-related adverse events (nirAEs) are uncommon but potentially lethal complications of immune checkpoint inhibitor (ICI) treatment, with the reported incidence ranging from 0.3 to 14.0% [1]. The presentations of central nervous system (CNS) involvement of nirAEs vary from non-specific encephalopathy/meningo-encephalopathy to localized involvement of certain CNS regions, which could occur during or up to one year after ICI treatment. Serious nirAEs are documented in less than 1% of patients [2], but cases of mild neurotoxicity are likely underreported, since some patients may only have radiographic or laboratory abnormalities [3]. In order to early detect possible nirAEs, it is of great importance to explore the dynamic changes of brain magnetic resonance imaging (MRI) after ICI treatment and to analyze their prognostic significance.
Material and methods
Patients
Advanced non-small cell lung cancer (NSCLC) patients receiving anti-PD-1/PD-L1 therapy from June 2017 to September 2020 at three participating institutions were screened. Those receiving brain MRI within 6 weeks before ICI initiation and at least one follow-up brain MRI after ICI treatment were included. The clinicopathological characteristics of each patient were obtained, and tumor response was evaluated according to Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 [4]. The data were cutoff on March 30, 2021. This study was approved by the institutional review board at each participating institution.
Analyses of brain MRI aberration
For patients with baseline brain metastasis (BM), brain MRI was generally performed every 8–12 weeks and it was at the discretion of the treating physicians for patients without baseline BM. Detailed information about the processes of brain MRI was reported previously [5]. Brain MRI aberration was defined as newly emergent abnormality other than BM after initiation of anti-PD-1/PD-L1 therapy, which was evaluated by two independent, experienced radiologists. Several patients with ambiguous brain MRI findings were excluded.
Statistical analysis
Frequencies and descriptive statistics were calculated of clinicopathological variables. Pearson’s Chi-squared test was used to explore the relation between brain MRI aberration and common clinicopathological parameters as well as treatment efficacy. Clinical benefit rate (CBR) was defined as the percentage of patients who had objective response and those who had stable disease lasting ≥ 6 months. Progression-free survival (PFS) and overall survival (OS) were calculated from the time of ICI initiation, estimated using the Kaplan–Meier method and compared using the log-rank test. Time-dependent Cox regression model was used to correct the guarantee-time bias.
Results
Patient characteristics
A total of 135 patients were enrolled, including 68 patients with baseline BMs and 67 without (Supplementary Fig. 1). Detailed baseline patient’s characteristics are listed in Table 1. Of note, the median times of follow-up brain MRI were 4 (range 1–8).
Table 1.
Baseline disease characteristics
| Clinicopathological characteristics | Number of patients | % |
|---|---|---|
| Age | ||
| < 65 | 71 | 52.6 |
| ≥ 65 | 64 | 47.4 |
| Sex | ||
| Female | 27 | 20.0 |
| Male | 108 | 80.0 |
| ECOG PS | ||
| 0–1 | 104 | 77.0 |
| 2–3 | 31 | 23.0 |
| Smoking history | ||
| Ever smoker | 79 | 58.5 |
| Never smoker | 56 | 41.5 |
| Drinking history | ||
| Ever drinker | 40 | 29.6 |
| Never drinker | 95 | 70.4 |
| Past medical history | ||
| History of myocardial or cerebral infraction | 4 | 3.0 |
| Hypertension | 29 | 21.5 |
| Coronary heart disease | 5 | 3.7 |
| Diabetes | 8 | 5.9 |
| Hyperlipidemia | 2 | 1.5 |
| Pathology | ||
| Squamous carcinoma | 31 | 23.0 |
| Non-squamous carcinoma | 104 | 77.0 |
| Baseline brain metastasis | ||
| Positive | 68 | 50.4 |
| Negative | 67 | 49.6 |
| Treatment modality | ||
| ICI monotherapy | 57 | 42.2 |
| Combined therapy | 78 | 57.8 |
| Line of ICI treatment | ||
| 1 | 56 | 41.5 |
| ≥ 2 | 79 | 58.5 |
ECOG Eastern Cooperative Oncology Group, PS performance status, ICI immune checkpoint inhibitor
Incidence of brain MRI aberration
With a median follow-up of 13.2 months (range, 1.6 months–39.5 months), 27 (20%) patients had developed brain MRI aberration. The 1-, 2- and 3-year cumulative incidence of brain MRI aberration was 17.1%, 36.3% and 52.2% for all types and 9.4%, 13.3% and 13.3% for those indicative of stroke, respectively (Fig. 1a). The median time from anti-PD-1/PD-L1 therapy initiation to the documentation of brain MRI aberration was 6.8 months (range 0.7 months–21.7 months). 15 (55.6%) patients developed brain MRI aberration during the anti-PD-1/PD-L1 therapy, and the remaining 12 (44.4%) developed brain MRI aberration within 4.5 months after anti-PD-1/PD-L1 therapy discontinuation. No significant association was found between clinicopathological parameters and occurrence of brain MRI aberration.
Fig. 1.
The incidence and prognostic significance of brain MRI aberration. a Cumulative incidence of brain MRI aberration. b–d Comparison of the CBR (b), PFS (c), and OS (d) among patients with and without brain MRI aberration
Radiographic features of brain MRI aberration
Hyperintense signals on T2-weighted images and T2-fluid-attenuated inversion recovery (FLAIR) indicative of anterior/posterior circulation stroke were seen in 11 patients (Fig. 2a). White matter lesions with T2-FLAIR hyperintensity frequently involving the lateral ventricle developed in 9 patients (Fig. 2b). Other types of T2-hyperintensity suggestive of CNS vasculitis or encephalitis were seen in 4 patients (Fig. 2c). The remaining 3 patients developed other MRI aberrations, including 1 cystic lesion, 1 hyperintensity on diffusion-weighted imaging and 1 calcific lesion.
Fig. 2.
Radiographic features of brain MRI aberration in three patients. Hyperintensity on T2-weighted fast spin-echo (FSE) sequence in bilateral frontal and parietal lobes that developed 5 months after anti-PD-1 monotherapy in Patient 1. Three continuous layers were displayed (a1-3). a Hyperintensity on T2-weighted fluid-attenuated inversion recovery (FLAIR) sequence in bilateral ventricle that developed 3 months after anti-PD-1 monotherapy in Patient 2. Three continuous layers were displayed (b1-3). b Abnormal signal foci in the right temporal occipital lobe that developed 15 months after anti-PD-1 monotherapy in patient 3. Baseline T2-weighted image one month before the initiation of anti-PD-1 monotherapy (c1). Newly developed brain MRI aberration on T2-weighted images (c2), T2-FLAIR (c3), diffusion-weighted imaging (DWI) (b value 50, 800 mm2/s) (c4) and apparent diffusion coefficient (ADC) (c5) sequence. The brain MRI aberration shrunk 17 months after anti-PD-1 monotherapy without discontinuation of anti-PD-1 therapy (c6)
Prognostic significance of brain MRI aberration
Compared with those without brain MRI aberration, patients with brain MRI aberration had a significantly higher CBR (p = 0.030), longer PFS (p = 0.015) and tended to have an improved OS (p = 0.054, Fig. 1b, c and d). The prolonged PFS associated with brain MRI aberration remained statistically significant (HR = 0.725; 95% CI, 0.534–0.984; p = 0.039) after adjustment by time-dependent Cox regression analyses.
Discussion
To the best of our knowledge, this the first study to investigate the incidence, radiographic feature and prognostic significance of brain MRI aberration after ICI treatment in advanced NSCLC. Although the brain MRI aberration documented in the current study was not necessarily related to nirAEs, they were found to be related with treatment efficacy and patient’s survival, indicating that at least some of them were triggered or aggravated by ICI treatment [6, 7].
Ischemic stroke frequently developed in elder patients. However, the cumulative incidence of brain MRI aberration indicative of stroke in our study was numerically higher than historical controls, which collaborated with previous studies [8–10]. In fact, accumulating data suggest that ICIs may accelerate atherogenesis and increase the risk of cardiovascular complications [11–13]. Various white matter lesions have been noted after ICI treatment [14–16], and other types of T2-hyperintensity suggestive of CNS vasculitis and encephalitis in our study were also reported previously [17–19]. Mechanically, PD-1/PD-L1 inhibitors may exacerbrate pro-coagulant activity and damage the primary vascular immune-privilege by stimulating T cell activation and effector functions [12, 20]. These results unmasked the potential roles of PD-1/PD-L1 inhibitors in nirAEs, which collaborate with our findings.
Due to the retrospective nature of the study, it was hard for us to collect sufficient evidence to identify definite cases of nirAEs. Therefore, we could not completely attribute the brain MRI aberration to the use of PD-1/PD-L1 inhibitors. Given the consistency and similarity of the incidence and brain MRI manifestation from our study and historic data of nirAEs, it can be postulated that PD-1/PD-L1 inhibitors may play certain roles in the occurrence of brain MRI aberrations. Since the majority of symptomatic nirAEs are severe and the manifestations of brain MRI aberrations after ICI treatment vary significantly, active surveillance of brain MRI among these patients is to be encouraged. Moreover, future prospective studies with larger sample size investigating the functional correlations between the brain MRI aberrations and nirAEs are warranted.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contributions
Jianjiao Ni was involved in conceptualization, formal analysis, methodology and writing—original draft. Yue Zhou was involved in conceptualization, formal analysis and writing—original draft. Shengping Wang was involved in conceptualization, formal analysis, methodology and writing—review & editing. Tiantian Guo was involved in data curation and formal analysis. Jie Hu was involved in data curation and writing—review & editing. Qian Chu was involved in data curation and writing—review & editing. Xi Yang was involved in data curation. Li Chu was involved in data curation. Xiao Chu was involved in data curation. Yida Li was involved in data curation. Zhengfei Zhu was involved in funding acquisition, supervision and writing—review & editing.
Funding
This work was supported by the Chinese Society of Clinical Oncology (CSCO) foundation [grant numbers Y-BMS2019082].
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Conflict of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Consent for participate
Informed consent was waived by the institutional review board because this study was retrospective.
Consent for publication
Informed consent was waived by the institutional review board because this study was retrospective.
Ethical approval
This study was approved by the institutional review board at each participating institution.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jianjiao Ni, Yue Zhou and Shengping Wang have contributed equally.
References
- 1.Sechi E, Zekeridou A. Neurologic complications of immune checkpoint inhibitors in thoracic malignancies. J Thorac Oncol: Off Publ Int Assoc Study Lung Cancer. 2021;16:381–394. doi: 10.1016/j.jtho.2020.11.005. [DOI] [PubMed] [Google Scholar]
- 2.Wick W, Hertenstein A, Platten M. Neurological sequelae of cancer immunotherapies and targeted therapies. Lancet Oncol. 2016;17:e529–e541. doi: 10.1016/S1470-2045(16)30571-X. [DOI] [PubMed] [Google Scholar]
- 3.Larkin J, Chmielowski B, Lao CD, Hodi FS, Sharfman W, Weber J, Suijkerbuijk KPM, Azevedo S, Li H, Reshef D, Avila A, Reardon DA. Neurologic serious adverse events associated with nivolumab plus ipilimumab or nivolumab alone in advanced melanoma, including a case series of encephalitis. Oncologist. 2017;22:709–718. doi: 10.1634/theoncologist.2016-0487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eisenhauer EA, Verweij J. 11 New response evaluation criteria in solid tumors: Recist guideline version 1.1. Eur J Cancer Suppl. 2009;7:5. doi: 10.1016/S1359-6349(09)70018-7. [DOI] [PubMed] [Google Scholar]
- 5.Chu L, Ni J, Yang X, Tong T, Wang J, Yin F, Li R, Li Y, Zou L, Li Y, Xie C, Li G, Zhu Z. Radiographic features of metastatic brain tumors from ALK-rearranged non-small cell lung cancer: implications for optimal treatment modalities. J Cancer. 2019;10:6660–6665. doi: 10.7150/jca.30091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haratani K, Hayashi H, Chiba Y, Kudo K, Yonesaka K, Kato R, Kaneda H, Hasegawa Y, Tanaka K, Takeda M, Nakagawa K. Association of immune-related adverse events with nivolumab efficacy in non-small-cell lung cancer. JAMA Oncol. 2018;4:374–378. doi: 10.1001/jamaoncol.2017.2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Naqash AR, Ricciuti B, Owen DH, Florou V, Toi Y, Cherry C, Hafiz M, De Giglio A, Muzaffar M, Patel SH, Sugawara S, Burkart J, Park W, Chiari R, Sugisaka J, Otterson GA, de Lima LG, Walker PR. Outcomes associated with immune-related adverse events in metastatic non-small cell lung cancer treated with nivolumab: a pooled exploratory analysis from a global cohort. Cancer Immunol Immunother: CII. 2020;69:1177–1187. doi: 10.1007/s00262-020-02536-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ando Y, Hayashi T, Sugimoto R, Nishibe S, Ito K, Kawada K, Ikeda Y, Yamada S, Imaizumi K. Risk factors for cancer-associated thrombosis in patients undergoing treatment with immune checkpoint inhibitors. Invest New Drugs. 2020;38:1200–1206. doi: 10.1007/s10637-019-00881-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bar J, Markel G, Gottfried T, Percik R, Leibowitz-Amit R, Berger R, Golan T, Daher S, Taliansky A, Dudnik E, Shulman K, Urban D, Onn A. Acute vascular events as a possibly related adverse event of immunotherapy: a single-institute retrospective study. Eur J Cancer (Oxford, England: 1990) 2019;120:122–131. doi: 10.1016/j.ejca.2019.06.021. [DOI] [PubMed] [Google Scholar]
- 10.Sussman TA, Li H, Hobbs B, Funchain P, McCrae KR, Khorana AA. Incidence of thromboembolism in patients with melanoma on immune checkpoint inhibitor therapy and its adverse association with survival. J Immunother cancer. 2021;9:e001719. doi: 10.1136/jitc-2020-001719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Drobni ZD, Alvi RM, Taron J, Zafar A, Murphy SP, Rambarat PK, Mosarla RC, Lee C, Zlotoff DA, Raghu VK, Hartmann SE, Gilman HK, Gong J, Zubiri L, Sullivan RJ, Reynolds KL, Mayrhofer T, Zhang L, Hoffmann U, Neilan TG. Association between immune checkpoint inhibitors with cardiovascular events and atherosclerotic plaque. Circulation. 2020;142:2299–2311. doi: 10.1161/CIRCULATIONAHA.120.049981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sato R, Imamura K, Sakata S, Ikeda T, Horio Y, Iyama S, Akaike K, Hamada S, Jodai T, Nakashima K, Ishizuka S, Sato N, Saruwatari K, Saeki S, Tomita Y, Sakagami T. Disorder of coagulation-fibrinolysis system: an emerging toxicity of anti-PD-1/PD-L1 monoclonal antibodies. J clin Med. 2019;8:762. doi: 10.3390/jcm8060762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cochain C, Chaudhari SM, Koch M, Wiendl H, Eckstein H-H, Zernecke A. Programmed cell death-1 deficiency exacerbates T cell activation and atherogenesis despite expansion of regulatory T cells in atherosclerosis-prone mice. PloS one. 2014;9:e93280. doi: 10.1371/journal.pone.0093280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pillonel V, Dunet V, Hottinger AF, Berthod G, Schiappacasse L, Peters S, Michielin O, Aedo-Lopez V. Multiple nivolumab-induced CNS demyelination with spontaneous resolution in an asymptomatic metastatic melanoma patient. J Immunother Cancer. 2019;7:336. doi: 10.1186/s40425-019-0818-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oliveira MCB, de Brito MH, Simabukuro MM. Central nervous system demyelination associated with immune checkpoint inhibitors: review of the literature. Front Neurol. 2020;11:538695. doi: 10.3389/fneur.2020.538695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garcia CR, Jayswal R, Adams V, Anthony LB, Villano JL. Multiple sclerosis outcomes after cancer immunotherapy. Clin Trans oncol: Off Publ Fed Span Oncol Soc Nat Cancer Inst Mexico. 2019;21:1336–1342. doi: 10.1007/s12094-019-02060-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Daxini A, Cronin K, Sreih AG. Vasculitis associated with immune checkpoint inhibitors-a systematic review. Clin Rheumatol. 2018;37:2579–2584. doi: 10.1007/s10067-018-4177-0. [DOI] [PubMed] [Google Scholar]
- 18.Yshii LM, Hohlfeld R, Liblau RS. Inflammatory CNS disease caused by immune checkpoint inhibitors: status and perspectives. Nat Rev Neurol. 2017;13:755–763. doi: 10.1038/nrneurol.2017.144. [DOI] [PubMed] [Google Scholar]
- 19.Khoja L, Maurice C, Chappell M, MacMillan L, Al-Habeeb AS, Al-Faraidy N, Butler MO, Rogalla P, Mason W, Joshua AM, Hogg D. Eosinophilic Fasciitis and Acute Encephalopathy Toxicity from Pembrolizumab Treatment of a Patient with Metastatic Melanoma. Cancer Immunol Res. 2016;4:175–178. doi: 10.1158/2326-6066.CIR-15-0186. [DOI] [PubMed] [Google Scholar]
- 20.Weyand CM, Berry GJ, Goronzy JJ. The immunoinhibitory PD-1/PD-L1 pathway in inflammatory blood vessel disease. J Leukoc Biol. 2018;103:565–575. doi: 10.1189/jlb.3MA0717-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.