Abstract
CAP-100 is a novel therapeutic antibody directed against the ligand binding site of human CCR7. This chemokine receptor is overexpressed in chronic lymphocytic leukemia (CLL) and orchestrates the homing of CLL cells into the lymph node. Previous studies, on a very limited number of samples, hypothesized that the Bruton’s tyrosine kinase inhibitor (BTKi) ibrutinib might induce loss of surface CCR7 levels in CLL cells. CAP-100 will be evaluated in clinical trials as a therapy for relapse/refractory CLL patients, who have received at least two systemic therapies (NCT04704323). As nowadays many relapse/refractory CLL patients will have received ibrutinib as a prior therapy, we aimed to investigate in a large cohort of CLL patients the impact of this BTKi on CCR7 expression and functionality as well as on the therapeutic activity of CAP-100. Our data confirm that ibrutinib moderately down-regulates the very high expression of CCR7 in CLL cells but has no apparent effect on CCR7-induced chemotaxis. Moreover, CLL cells are perfectly targetable by CAP-100 which led to a complete inhibition of CCR7-mediated migration and induced strong target cell killing through antibody-dependent cell-mediated cytotoxicity, irrespective of previous or contemporary ibrutinib administration. Together, these results validate the therapeutic utility of CAP-100 as a next-line single-agent therapy for CLL patients who failed to ibrutinib and confirm that CAP-100 and ibrutinib have complementary non-overlapping mechanisms of action, potentially allowing for combination therapy.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00262-021-03014-2.
Keywords: CAP-100, CCR7, CLL, Immunotherapy, Lymph node, Ibrutinib
Introduction
The chemokine receptor CCR7 is G-protein-coupled receptor that controls migration of certain immune cell subsets to the lymph nodes (LNs), where it assists in immune cells organization and activation [1, 2]. CCR7 selectively binds CCL19 and CCL21, two chemokines constitutively secreted in secondary lymphoid tissues [1–3]. In CLL, CCR7 is consistently found overexpressed and has been correlated with bulky lymphadenopathy and aggressive disease [4–9]. Accordingly, CCR7 drives CLL cells homing into LN, guides interstitial migration, rescues cells from spontaneous death, and prolongs CLL cells residency within this tissue [4, 9–16]. Together, this compelling evidence suggests CCR7 as an interesting therapeutic target in CLL. With this purpose, we developed CAP-100, a novel humanized IgG1 blocking monoclonal antibody (mAb) directed against the ligand binding site of hCCR7 which, in addition, induces strong cell killing via antibody-dependent cell-mediated cytotoxicity (ADCC) [17, 18].
Bruton’s tyrosine kinase (BTK), a member of the Tec family of cytoplasmic tyrosine kinases, is abundantly expressed in CLL cells and intimately involved in multiple signal-transduction pathways regulating survival and proliferation in CLL, mainly after B-cell receptor (BCR) activation [19]. Consequently, BTK has emerged as an important molecular target for treatment of this disease and ibrutinib, the first BTK inhibitor (BTKi) to be approved [20], has taken center in the treatment of CLL as both front-line therapy and rescue therapy in relapsed/refractory CLL [21–23]. However, in the last years the number of patients that discontinue ibrutinib and/or progress to ibrutinib relapsed/refractory CLL is increasing and this number is expected to grow more with extended follow-up [24, 25]. In other words, nowadays most relapsed/refractory CLL patients to be enrolled in the first-in-human clinical studies of CAP-100 (NCT04704323) will have received ibrutinib which, in previous studies conducted in a low number of samples, was shown to significantly down-regulate surface CCR7 and its functions [9, 26]. In the present study, we aimed to document, in a larger cohort of patients, the impact of ibrutinib on CCR7 expression and functionality (migration) in CLL cells. In addition, we aimed to address the potential implications of prior or contemporary treatment with the BTKi on the efficacy of CAP-100, including the evaluation of its blocking and killing abilities against CLL cells.
Material and methods
Samples and reagents
Malignant or healthy peripheral blood mononuclear cells (PBMCs) were obtained from freshly donated PB or adult blood buffy coats. Patients and healthy donors signed an informed consent in accordance with the Declaration of Helsinki. Experimental procedures were approved by the Institutional Review Board of Hospital de La Princesa. Whole PB samples were used for determination of CCR7 and CD20 expression in flow cytometry assays. For other in vitro approaches (migration and cytotoxicity assays), PBMCs from freshly donated samples, and containing ≥ 85% malignant CLL cells, were isolated by gradient centrifugation. No further purification was conducted to prevent lymphocyte activation. CLL cells were kept for short-term culture in RPMI-1640 medium supplemented with either 1% bovine serum albumin (BSA) or 10% fetal bovine serum (FBS). Three types of patient samples (n = 215) were included in this study (Table 1): treatment naïve patients, patients on treatment with ibrutinib (or other BTKi), and ibrutinib relapsed/refractory patients. A list of reagents can be found in the supplementary material.
Table 1.
Patients´ characteristics
| Ibrutinib-naïve (n = 144) | Ibrutinib-OT (n = 52) | Ibrutinib-R/R (n = 19) | |
|---|---|---|---|
| Age: mean [range] | 73.9 [42–90] | 71.9 [50–86] | 70.4 [44–85] |
| Sex: male/female; n (%) | 90/54 (62.5/37.5) | 40/12 (76.9/23.1) | 8/11 (42.1/57.9) |
| Rai Stage: n (%) | |||
| O | 110 (76.38) | 39 (75) | 2 (10.5) |
| I | 19 (13.19) | 2 (3.8) | 0 (0) |
| II | 1 (0.7) | 5 (9.6) | 5 (26.3) |
| III | 1 (0.7) | 0 (0) | 1 (5.3) |
| IV | 13 (9.02) | 6 (11.6) | 11 (57.9) |
| WBC (103/µL): mean ± SEM | 29.6 ± 3.1 | 34.7 ± 5.4 | 17.6 ± 2.3 |
| LC (103/µL): mean ± SEM | 23.9 ± 3.0 | 29.3 ± 5.3 | 10.0 ± 1.6 |
| CLL counts (103/µL): mean ± SEM | 18.1 ± 2.6 | 21.5 ± 4.9 | 5.8 ± 1.2 |
| % CLL within LC: mean ± SEM | 68.4 ± 1.6 | 70.1 ± 3.1 | 51.9 ± 3.2 |
| UM IgHV: n (%) | 85 (59) | 43 (82.6) | 6 (31.5) |
| Cytogenetics: n (%) | |||
| na | 42 (29.2) | 3 (5.8) | 12 |
| normal | 38 (26.4) | 34 (65.4) | 1 |
| aberrant | 64 (44.4) | 15 (28.8) | 9 |
| del11q22* | 5 (4.9) | 10 (20.4) | 4 (44.4) |
| Tri12* | 19 (18.6) | 7 (14.3) | 1 (11.2) |
| del13q14* | 51 (50.0) | 13 (26.5) | 0 |
| del17p13/TP53 + * | 19 (18.6) | 23 (46.9) | 4 (44.4) |
| Previous treatment: n (%) | – | 26 (50) | 19 (100) |
| ≥ 2 lines | – | 19 (73.1) | 19 (100) |
| Ibrutinib treatment time (d): mean ± SEM | – | 410.9 ± 46.3 | – |
| Time after ibrutinib ceasing (d): mean ± SEM | – | – | 194.3 ± 34.47 |
d days; LC lymphocyte counts; na not available; OT on treatment; R/R relapsed/refractory; UM un-mutated; WBC white blood cell counts; *, proportion relative to those patients with available cytogenetic study (normal + aberrant)
CCR7 expression
Flow cytometry analysis of CCR7 and CD20 expression in CLL cells was performed with a four-color panel of mAbs: CD19-APC-H7, CD3-FITC, and CD5-APC (all purchased from BD Biosciences, San Jose, CA) and either CCR7-PE (clone 150503) or CD20-PE (both from R&D Systems, Minneapolis, MN). For comparative analysis in expression profiles, anti-CCR7-PE and anti-CD20-PE antibodies were used at a final concentration of 10 µg/ml. Studies were conducted on the CD19+CD3−CD5+CLL population. An appropriate matched isotype control (IC) conjugated to PE was included (R&D Systems). To determine receptor expression, 106 cells were incubated with the antibodies for 15–20 min at room temperature. Red cells were lysed with BD FACS™ Lysing Solution (BD Biosciences) following the manufacturer´s protocol. Data acquisition was performed on a BD FACSCanto™ II Flow Cytometer (BD Biosciences). For all groups, a total of 10.000 CLL cells were acquired and analyzed using the BD FACSDiva™ software. Results are expressed as both a percentage of positive cells [test-control] and relative median of fluorescence intensity (RMFI) of CCR7 and CD20 expression compared to the IC [MFI(test)/MFI(control)].
Migration assays
Chemotaxis toward CCL19 or CCL21 (1 µg/ml, PeproTech, Rocky Hills, NJ) was conducted in transwell chambers (6.5 mm diameter, 10 µm thickness, 5-µm diameter pore size, Corning-Costar, Tewksbury, MA) following published protocols [16, 27]. To this end, 5 × 105 CLL cells suspended in RPM1-1640 medium supplemented with 1% BSA were loaded in the upper chamber. Only samples with viability ≥ 90%, as determined by 7-AAD (BD Biosciences) staining by flow cytometry, were used for migration assays. When indicated, and prior to the chemotaxis assay, cells were pre-incubated for 3 h with ibrutinib (provided by HULP Pharmacy Department) at different final concentrations [0, 0.01, 0.1, 1, 10 µM], or for 30 min with anti-CCR7 mAb (CAP-100) in the following dose-range: 0, 0.01, 0.1, 1, 10, and 100 µg/ml. To discard that ibrutinib in vitro treatment could be reducing CLL cells viability and hence overall results on CCR7-induced migration, cell viability was checked up upon conclusion of migration assays by means of 7-AAD staining by flow cytometry. Migration was allowed to proceed for 4 h at 37 °C in 5% CO2 atmosphere. The percentage of migrated cells (% of input) was calculated according to the formula: 100 x (number of cells in the lower chamber/number of cells loaded in the upper chamber). The % of inhibition was calculated with [(% input without mAb—% input with mAb) × 100]/[% input without mAb].
Antibody-dependent cell-mediated cytotoxicity (ADCC)
Target tumor cells were incubated at 37 °C for 30 min with media alone (RPMI + 10% FBS) or in the presence of IC, rituximab, or CAP-100 antibodies, all at the following final concentrations: 0, 0.01, 0.1, 1, 10, and 100 µg/ml. The cells were plated at 105 cells/well in p96 U-bottom plates. Human PBMCs from healthy donors were used as effector cells. For the assay, effector cells were labeled with calcein-UV Cell Tracker (Invitrogen, Carlsbad, CA) according to the manufacturer´s protocol and stimulated with recombinant human IL-2 (500 UI/ml, StemCell Technologies, Vancouver, Canada). By default, an effector/target (E/T) ratio of 10:1 was used. After 6 h, cells were stained with 7-AAD (BD Biosciences), CD3-PE, and CD5-APC and analyzed by flow cytometry. The percentage of specific lysis in Cell-Tracker−CD5+CD3− CLL cells was determined by incorporation of 7-AAD and calculated according to the following formula: % Specific Lysis = 100 × (ER-SR)/(MR-SR). ER, SR, and MR represent experimental, spontaneous, and maximum cell death.
Statistical analysis
Unless otherwise stated, quantitative variables are expressed as measures of central tendency (mean) and dispersion (SD, SEM). Parametric variables were analyzed using t-test or one-factor ANOVA. For heteroscedasticity, unpaired samples were tested with Mann–Whitney U or Kruskal–Wallis and paired samples with Wilcoxon or Friedman tests. Significance was set at values of < 0.05(*), < 0.01(**) or < 0.001(***).
Results
3.1. Surface expression of CCR7 is maintained high in CLL cells from patients treated with BTK inhibitors
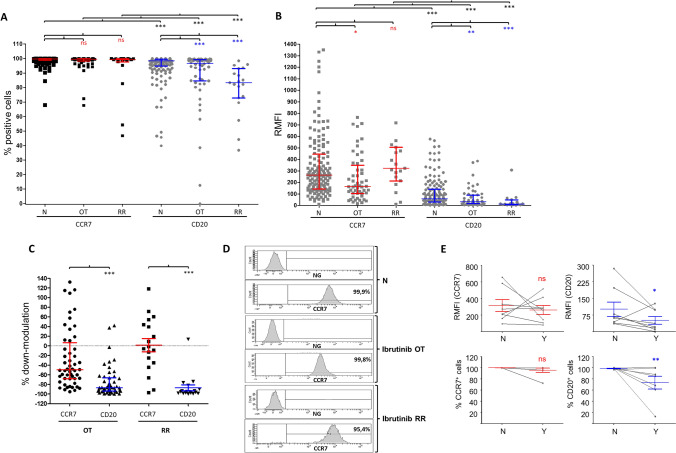
Since a previous study reported a substantial loss of surface CCR7 in CLL cells from few patients treated with ibrutinib [9], our first aim was to validate these results in a larger cohort of patients. To this end, we measured by flow cytometry the surface expression of CCR7 in samples from patients on ibrutinib treatment (OT) at the time of the determination (n = 52) and in samples obtained from patients who stopped ibrutinib treatment because they had developed relapsed/refractory disease (n = 19), and compared it to that of samples obtained from naïve, untreated patients (n = 144). In terms of proportion of CLL cells expressing the receptor, we determined that approximately 100% of leukemic cells expressed CCR7 irrespective of current or previous treatment with ibrutinib (Fig. 1a). In terms of RMFI, in our cohort, CCR7 surface expression was down-modulated ~ 30% in patients receiving ibrutinib as the current treatment when compared to untreated, naïve controls (Fig. 1b–d). Despite this reduction, CLL cells kept high surface levels since RMFI was still over 240 arbitrary units. In other words, expression was still 240 times higher than a negative irrelevant IC. Regarding the impact of other BTKi, no changes were seen in the few samples of patients receiving acalabrutinib or zanubrutinib (Supplementary Fig. 1-A-B), suggesting specific roles for ibrutinib in CCR7 down-modulation in CLL cells. Accordingly, when RMFI was analyzed in CLL cells from ibrutinib relapsed/refractory patients who had stopped ibrutinib, we observed that CCR7 surface levels were similar to those of naïve patients (Fig. 1a–d). From our relapsed/refractory cohort, we could study in a limited number of patients (n = 5) the relation between CCR7 surface levels and time after discontinuation of ibrutinib (Supplementary Fig. 1-C). In this follow-up, CCR7 seemed to remain relatively stable during the first month after discontinuation, time after which CCR7 surface levels tended to grow to reach double or triple RMFI values after 1.5–5 months. Although further work is still needed to validate this recovery kinetics, these preliminary results suggest ibrutinib to transiently reduce surface CCR7.
Fig. 1.
CCR7 expression in CLL cells remains high in patients treated with BTK inhibitors. a–b Expression of surface CCR7 (or CD20) was analyzed in terms of proportion of malignant cells expressing the receptor or of relative median fluorescence intensity (RMFI, relative to an irrelevant isotype control, arbitrary units). Expression of CCR7 and CD20 was determined in CLL cells from PB samples obtained from naïve patients (N, n = 144), patients on current treatment with ibrutinib (OT, n = 52), patients with relapsed/refractory CLL to ibrutinib (RR, n = 19). In A and B, the graphs show the median ± interquartile range. Man–Whitney U was used to test statistical differences: ns, not significant; *, p < 0.05; **, p < 0.01; ***, p < 0.001. c In OT and RR CLL cells, the surface levels of CD20 decrease to a higher extent than that of CCR7. The graph shows the % of receptor down-modulation in each patient relative to the mean value in the naïve group. Man–Whitney U test was used to test statistical differences in OT while t-test was used in the RR. ***, p < 0.001. d The graph shows CCR7 expression from one representative naïve (N, untreated) CLL patient, one representative CLL patient receiving ibrutinib (OT), and one representative CLL patient who failed to ibrutinib (RR). In each patient, CCR7 expression is disclosed as frequency histograms compared to a matched irrelevant control (NG). Each histogram shows the intensity of fluorescence for cells labeled with anti-CCR7-PE. The proportion of CCR7-positive cells (within the marker) is also shown. e Changes in the expression of CCR7 and CD20 in eight CLL patients who started treatment with ibrutinib. For each patient, the expression of CCR7 and CD20 (determined as RMFI or proportion of positive cells) before (N, no) and after administration (Y, yes) of ibrutinib is shown
A cornerstone therapeutic target in CLL is CD20. This receptor is known to be strongly down-modulated, or even lost, at both mRNA [28] and protein [29] levels by clinical ibrutinib treatment. A comparative analysis between CD20 and CCR7 (Fig. 1a–c) allowed us to confirm that the ibrutinib-induced down-modulation of CCR7 was significantly lower than that for CD20, which was highly sensitive to down-regulation in patients treated with ibrutinib. In this group, mean relative down-modulation for CCR7 was − 27.6 ± 8.5 SEM (Fig. 1c), a proportion three times lower than that seen for CD20 (− 75.38 ± 4.416). Even, in most cases BTKi led to a complete loss of CD20 in some patients as determined by the reduction in the proportion of CD20-positive cells compared to naïve patients (Fig. 1a, c). Regarding relapsed/refractory patients, CCR7 levels were 1.5 ± 13.5 SEM, while CD20 down-modulation reached a mean of − 86.9 ± 6.2 SEM in the same group (Fig. 1c). These results suggested that, opposite to CCR7, down-modulation of CD20 was not reverted in ibrutinib relapsed/refractory patients, where CD20 expression was found to be even lower than in patients on treatment with ibrutinib (Fig. 1a–c). Accordingly, Fig. 1e shows how ibrutinib treatment (Y) led to a consistent, homogeneous down-regulation of both CD20 RMFI and proportion of CD20-expressing cells in 87.5% (7/8) of patients as compared to their naïve status, before they started ibrutinib treatment (N). In the case of CCR7, RMFI was reduced in 4/8 patients, did not change in 2/8, and increased in 2/8, while the proportion of CCR7-expressing cells remained highly constant (unchanged in 7/8). These results were further confirmed with in vitro approaches where CLL cells obtained from naïve patients were incubated with different concentrations of ibrutinib (0-vehicle; 0.01; 0.1; 1; 10 µM). Neither the proportion of CCR7-positive CLL cells nor the RMFI was significantly impacted by the presence of the BTKi (Supplementary Fig. 1-D).
Ibrutinib does not impair in vitro migration mediated by CCR7 in CLL cells
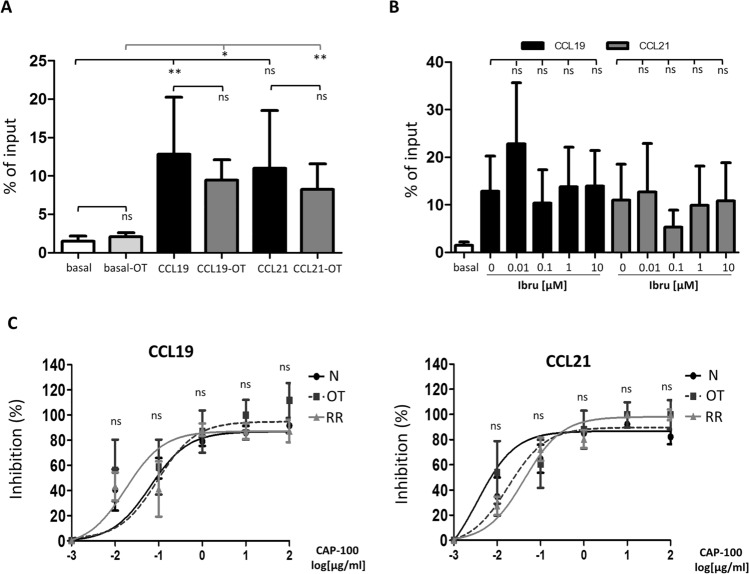
Once it was determined that ibrutinib did only modestly affect CCR7 expression on CLL cells surface, we studied whether this compound could impair migration induced by CCR7 ligands. To this end, we performed a comparative analysis of the in vitro chemotactic response of CLL cells obtained from naïve patients (N) versus patients on the current treatment with ibrutinib (OT) (Fig. 2a). In both groups, basal migration indices were similar and CLL cells migrated in a significant way toward CCR7 ligands. Moreover, migration indices did not significantly differ, indicating that ibrutinib had no (or only a marginal) effect on CCR7-induced migration. To further confirm this, we performed additional in vitro chemotaxis assays where CLL cells from naïve patients were pre-incubated with increasing concentrations of ibrutinib before exposure to CCR7 ligands (Fig. 2b and Supplementary Fig. 2). In these settings, no impact on CLL cells migration was attributable to an ibrutinib-induced reduction in cell viability (Supplementary Fig. 2-A). Again, no dose–effect relation on migration was observed; migration indices with ibrutinib pre-treatment were comparable to control migration induced by CCR7 ligands (Fig. 2b and Supplementary Fig. 2-A). All together, these results indicated that CLL cells migration induced by CCR7 was not affected by previous in vivo or in vitro treatments with ibrutinib. In other words, effects mediated by the BTKi proved to be insufficient to abrogate CCR7-mediated migration, thus suggesting that specific agents blocking CCR7 are necessary to achieve a complete neutralization of CCR7-mediated migration. Therefore, to test the utility of CAP-100 as a blocking agent in patients receiving ibrutinib and to investigate whether the slight down-modulation of CCR7 surface levels could impair the activity of the anti-CCR7 mAb, we conducted comparative chemotaxis assays between CLL cells obtained from patients on treatment with the BTKi and naïve patients. We also compared migration in cells derived from patients with relapsed/refractory disease to ibrutinib. As seen in Fig. 2c, CLL cells from these groups of patients responded similarly to CCR7 ligands and CAP-100 displayed a clear, equal dose–response inhibitory activity. These results confirmed that CAP-100 blocking abilities were maintained irrespective of previous or current treatment with ibrutinib and that anti-CCR7 therapy seems the most effective way to impair migration induced by CCR7.
Fig. 2.
CAP-100, but not ibrutinib, effectively neutralizes in vitro migration mediated by CCR7 in CLL cells from naïve patients, patients under current treatment with ibrutinib and patients with relapsed/refractory disease to this BTK inhibitor. a Comparative analysis of migration indices (% of input) in CLL cells obtained from naïve untreated patients (N; n = 7, basal, CCL19 and CCL21) versus cells obtained from CLL patients receiving ibrutinib (OT; n = 10, basal-OT, CCL19-OT and CCL21-OT). Migration was achieved with exposure of CLL cells to CCR7 ligands (1 µg/ml). b Percentage of input in CLL cells from naïve untreated patients (n = 7) that were exposed for 3 h to different final concentrations of ibrutinib [0 (vehicle, DMSO), 0.01, 0.1, 1, 10 µM] prior to exposure to CCL19 or CCL21 (1 µg/ml). c The specific blocking of CCR7-mediated migration by CAP-100, expressed as a percentage of inhibition in migration, is shown for CLL cells obtained from naïve untreated patients (N, n = 6), patients receiving ibrutinib (OT, n = 6), or patients who failed to this treatment (RR, n = 6). CLL cells were incubated for 0.5 h with CAP-100 at different final concentrations (100, 10, 1, 0.1, 0.01, and 0 µg/ml) before exposure to CCL19 and CCL21 (1 µg/ml). In A, B, and C, bars represent mean ± standard error of the mean (SEM). ns, not significant; *, p < 0.05. **, p < 0.01)
3.3. Ibrutinib-induced down-modulation of CCR7 surface levels in CLL cells does not impact on killing activity of CAP-100
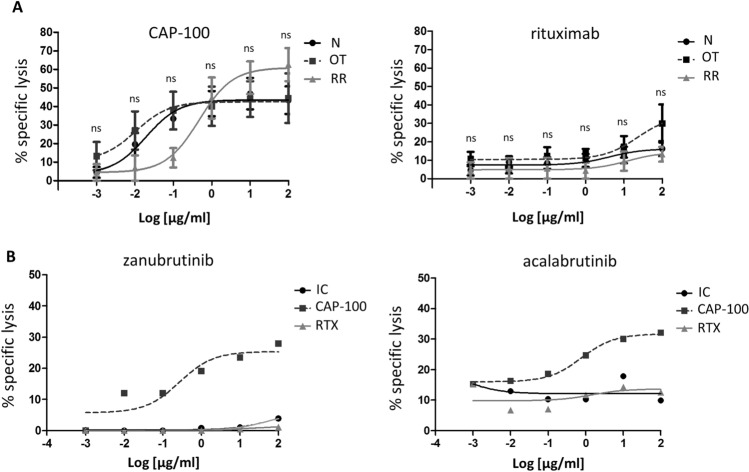
In therapies based on antibodies, high target surface levels are mandatory to achieve an effective killing activity mediated by effector immune mechanisms such as ADCC or CDC [30]. Our results on expression demonstrated that surface CCR7 levels were slightly reduced in patients on treatment with ibrutinib though no reduction on the proportion of tumor cells expressing CCR7 was observed. For this reason, we determined whether the ibrutinib-induced reduction in CCR7 RMFI could have a negative impact on killing activities mediated by CAP-100. To this end, we performed a set of ADCC assays in which target CLL cells from naïve patients, patients on ibrutinib treatment, or from ibrutinib relapsed/refractory patients were incubated with increasing concentrations of CAP-100, rituximab (a reference therapeutic antibody), or an IC (Fig. 3a). In all three groups of patients, CAP-100 demonstrated a potent and comparable ADCC activity and consistently outperformed rituximab. CAP-100 ADCC activity was also observed in patients on treatment with another BTKi such as zanubrutinib or acalabrutinib (Fig. 3b). These results confirmed that the effect of ibrutinib on surface CCR7 had no negative impact on killing activities mediated by CAP-100 which was also shown to be an effective alternative for patients who failed to ibrutinib.
Fig. 3.
CCR7 expression in CLL cells from patients on treatment with BTK inhibitors or with relapsed/refractory disease to these compounds can effectively induce cell death (ADCC) upon binding of CAP100. a ADCC activity was assayed in naïve, untreated patients (N; n = 5), in patients receiving treatment with ibrutinib (OT; n = 5), and in patients with relapsed/refractory disease to ibrutinib (RR; n = 4). CLL cells were incubated in the presence of CAP-100 or the reference anti-CD20 antibody (rituximab). Antibodies were tested at different final concentrations (0, 0.01, 0.1, 1, 10, and 100 µg/ml). b ADCC activity was assayed in one patient receiving treatment with zanubrutinib and in another one receiving treatment with acalabrutinib. CLL cells were incubated in the presence of an irrelevant matched isotype control, the reference anti-CD20 antibody (rituximab), or with CAP-100. To perform ADCC, in A and B, isolated PBMCs from healthy donors were used as effector cells at a fixed E/T ratio of 10:1. The percentage of CLL cells killed by ADCC (specific lysis) was determined by flow cytometry based on the incorporation of 7-aminoactinomycinD (7-AAD). The mean proportion of specific lysis induced by the antibodies ± standard error of the mean (SEM) is shown. ns, not significant
Discussion
Compelling evidence suggests an essential role of CCR7 in the pathobiology of CLL [4–16]. Aiming to disrupt the CCR7-driven homing of CLL into LN tissues, we generated CAP-100, an anti-hCCR7 mAb targeting the ligand binding site of this receptor. Pre-clinical studies demonstrated CAP-100 to effectively inhibit CCR7-induced functions and to trigger a strong host-mediated ADCC against CLL cells [17, 18]. These data contributed to the FDA approval of the first-in-human evaluation of CAP-100 in relapsed/refractory CLL patients (NCT04704323). Since patients who had failed to ibrutinib [19, 21–23] are likely to be enrolled in this first clinical study, the present study focused on the potential effects of current or prior treatments with this BTKi on CCR7 expression and the subsequent impact on the expected efficacy of CAP-100. In this regard, recent reports in a limited number of CLL samples proposed that ibrutinib might impair CCR7 expression and its functionality [9, 26, 31, 32]. Here, we confirm in a larger cohort that ibrutinib treatment leads to some down-modulation in surface levels of CCR7 in CLL cells but does not affect the overall proportion of target-expressing cells which was shown to stay around to 100%. Notably, this effect seems specific of ibrutinib as treatment with other BTKi did not down-regulate CCR7, although further validation of these data is needed since we tested a few patients under alternative BTKi therapies. Interestingly, CCR7 expression levels in ibrutinib relapsed/refractory patients were similar to control patients thus suggesting the reduction in CCR7 is transitory and levels restore upon ibrutinib cessation. In agreement, follow-ups on CCR7 expression in five patients from our relapsed/refractory cohort suggested a time-related recovery of CCR7 after ibrutinib discontinuation; future work is needed to validate these findings.
As for the mechanisms underlying this slight down-modulation of CCR7, a recent work by Patrussi et al. [31] demonstrated that ibrutinib reduced surface CCR7 expression in CLL through re-activating the expression of the proapoptotic protein p66Shc. Deficiency of this cytoplasmic protein is a feature in CLL and directly contributes to CCR7 overexpression in two main ways: First, lack of p66Shc protein favors CCR7 gene transcription [33], and second, defect of p66Shc disrupts the homeostatic recycling of membrane-expressed CCR7, resulting, therefore, in a rapid CCR7 recycling and receptor accumulation in CLL cell membrane [9, 31]. Likewise, direct inhibition of the BCR-BTK signaling may also restore surface CCR7 levels in CLL. Indeed, it has been reported that BCR activation might directly up-regulate CCR7 in CLL [34] as it does in healthy B cells, where CCR7 expression is augmented through BCR-BTK signaling [35]. Finally, it is also likely that ibrutinib partially inactivates transcription factors regulating expression of CCR7 gene such as NFATC1, AP-1, or NF-κB, which are well-known targets of the BTKi [34, 36–38]. Additional genetic or epigenetic studies addressing the effect of ibrutinib on these CCR7 gene regulatory factors would be of great interest.
Our data also indicate that the effect of ibrutinib on CCR7 is markedly different from other therapeutic targets. For example, compared to CD20, a receptor known to be negatively controlled by ibrutinib at both mRNA [28] and protein [29] levels, we show that CCR7 remained highly stable. Interestingly, our results mirror those previously published by Pavlasova et al. [28] or Skarzynski et al. [29] where CLL cells lost about 75% of surface CD20 receptors in patients receiving ibrutinib. Similarly, CCR7 seems to behave differently from other chemokine receptors such as CXCR4 which was shown to be strongly down-modulated in CLL and in acute myeloid leukemia cells upon exposure to ibrutinib [39, 40]. Differences between CCR7 and CXCR4 sensitivity to ibrutinib in CLL might be associated with their distinct recycling pathways [9, 31, 41, 42].
Related to the impact of ibrutinib on CCR7 functionality, our results do not support the hypothesis that ibrutinib-induced down-modulation of CCR7 might affect migration of CLL cells toward CCR7 ligands. Opposite to a previous study where a partial reduction—with a high inter-patient variability—of ~ 30% in CCR7-driven migration of CLL cells was reported [26], our data indicate that prior or current treatment with ibrutinib is not clearly affecting chemotaxis toward CCL19 or CCL21. This fact is not surprising since many molecular cascades (e.g., MEK/ERK, PI3K/AKT, or Rho/ROCK) simultaneously govern migration of CLL cells toward CCR7 ligands [16]; therefore, a partial inhibition of some of these proteins might be compensated by the others. In this sense, our results also confirm the neutralization of the ligand binging site of CCR7 by CAP-100 as the best way to completely abrogate CCR7-mediated migration of CLL cells irrespective of current or previous treatments with ibrutinib. Of course, we cannot obviate that ibrutinib may attenuate chemokine- and BCR-controlled adhesion of CLL cells [26, 43] and down-modulate production of CCL19 by stromal cells [38]. Therefore, we can speculate that both compounds could exert at least complementary mechanisms to impair CCR7-driven chemotaxis in patients.
In this study, we also confirmed that CAP-100 killing properties are maintained against target cells derived from CLL patients receiving ibrutinib or that failed to the BTKi, indicating that CCR7 levels are consistently high enough to allow specific high affinity binding of CAP-100 to its target. These results with ibrutinib are in line with previous findings from our group where fludarabine-based chemoimmunotherapy also down-modulated surface CCR7 in CLL patients but did not affect the killing properties of anti-CCR7 commercial antibodies [8]. Therefore, although CCR7 down-modulation is a common finding in CLL cells from treated patients, the net effect is moderate and allows a highly effective anti-CCR7 therapy in high-risk CLL and/or heavily treated patients, where CCR7 over-expression remains stable.
To summarize, in this study we demonstrate that ibrutinib, a current non-chemotherapeutic standard-of-care in CLL, down-modulates CCR7 surface levels in malignant cells but is not affecting CCR7-mediated migration. In addition, we confirm CCR7 as an ideal potential target for a next line therapy in CLL patients that have failed to BTK inhibitors such as ibrutinib, and we validate the utility of CAP-100 as a single agent for these patients. Finally, our results highlight the potential utility of a combination regimen consisting of a BTKi with CAP-100. This type of combination would be expected not only to reduce the appearance of resistances but also to improve the therapeutic outcome in patients in two ways: First, CAP-100 potently adds on the inhibition of migration of malignant cells to the LNs or other SLO, preventing therefore their escape to survival niches and becoming more accessible and vulnerable to by-stander therapies; second, CAP-100 provides additional cell killing through ADCC against accumulated CLL cells in bloodstream. In early 2021, a phase I clinical trial with CAP-100 in relapsed/refractory CLL will start, and the first-in-human data about anti-tumor activity of this novel therapy will be available soon.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to thank Dr. Marty Wulferink and Dr. Wiebe Olive for advice. The authors are also grateful to Dr. Francisco Sánchez-Madrid for reagents and advices and Lawrence Baron for proofreading and editing of the manuscript.
Authors’ contribution
TMA, RJS, JL, WM, and CCM carried out in vitro and ex vivo assays and analyzed data. FT, WM, CMC, and CCM designed the study, developed experimental procedures, analyzed data, carried out the statistical design and analysis, discussed results, and wrote the manuscript. All the authors reviewed the manuscript. All the authors approved the submission of the manuscript.
Funding
None of the authors received grants for this work.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Conflict of interest
CCM is an employee of Catapult Therapeutics and of Immunological and Medical Products (IMMED S.L.) and a shareholder in IMMED. JL has received honoraria from Abbvie, Janssen, BeiGene, and Astra-Zeneca. FT and WM are managing directors of Catapult Therapeutics, and FT is CEO of IMMED.S.L. and a shareholder in the same company and Catapult Therapeutics. CMC is a consultant for IMMED S.L., held a patent for the use of therapeutic antibodies targeting CCR7 in cancer and has received research funds from IMMED.S.L. and Catapult Therapeutics. She also holds shares in IMMED S.L. RJS is an employee of IMMED S.L. The other authors declare that they have no competing interests.
Ethics approval
The clinical study was performed in accordance with the principles of the Declaration of Helsinki and was approved and supervised by the Ethics Committee of Hospital Universitario de la Princesa (PI-352). Written informed consent was obtained from each patient before they entered the study.
Consent to participate
Written informed consent was obtained from each patient before they entered the study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.HauserLegler MADF. Common and biased signaling pathways of the chemokine receptor CCR7 elicited by its ligands CCL19 and CCL21 in leukocytes. J Leukoc Biol. 2016;99:869–882. doi: 10.1189/jlb.2MR0815-380R. [DOI] [PubMed] [Google Scholar]
- 2.ComerfordHarata-LeeBuntingGregorKaraMcColl IYMDCEESR. A myriad of functions and complex regulation of the CCR7/CCL19/CCL21 chemokine axis in the adaptive immune system. Cytokine Growth Factor Rev. 2013;24:269–283. doi: 10.1016/j.cytogfr.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 3.ForsterDavalos-MisslitzRot RACA. CCR7 and its ligands: balancing immunity and tolerance. Nat Rev Immunol. 2008;8:362–371. doi: 10.1038/nri2297. [DOI] [PubMed] [Google Scholar]
- 4.TillLinZuzelCawley KJKMJC. The chemokine receptor CCR7 and alpha4 integrin are important for migration of chronic lymphocytic leukemia cells into lymph nodes. Blood. 2002;99:2977–2984. doi: 10.1182/blood.V99.8.2977. [DOI] [PubMed] [Google Scholar]
- 5.De Lopez-GiralQuintanaCabrerizoAlfonso-PerezSala-ValdesSoriaFernandez-RanadaFernandez-RuizMunoz SNEMMMVGJMEC. Chemokine receptors that mediate B cell homing to secondary lymphoid tissues are highly expressed in B cell chronic lymphocytic leukemia and non-Hodgkin lymphomas with widespread nodular dissemination. J Leukoc Biol. 2004;76:462–471. doi: 10.1189/jlb.1203652. [DOI] [PubMed] [Google Scholar]
- 6.RichardsonMatthewsCatherwood SJCMA, et al. ZAP-70 expression is associated with enhanced ability to respond to migratory and survival signals in B-cell chronic lymphocytic leukemia (B-CLL) Blood. 2006;107:3584–3592. doi: 10.1182/blood-2005-04-1718. [DOI] [PubMed] [Google Scholar]
- 7.YanDozmorovLi XJIW, et al. Identification of outcome-correlated cytokine clusters in chronic lymphocytic leukemia. Blood. 2011;118:5201–5210. doi: 10.1182/blood-2011-03-342436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cuesta-MateosLoscertalesKreutzmanColom-FernandezPortero-SainzPerez-VillarTerronMunoz-Calleja CJABIJJFC. Preclinical activity of anti-CCR7 immunotherapy in patients with high-risk chronic lymphocytic leukemia. Cancer Immunol Immunother. 2015;64:665–676. doi: 10.1007/s00262-015-1670-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.PatrussiCapitaniMartini LNV, et al. Enhanced chemokine receptor recycling and impaired S1P1 expression promote leukemic cell infiltration of lymph nodes in chronic lymphocytic leukemia. Cancer Res. 2015;75:4153–4163. doi: 10.1158/0008-5472.CAN-15-0986. [DOI] [PubMed] [Google Scholar]
- 10.Redondo-MunozJose TerolGarcia-MarcoGarcia-Pardo JMJAA. Matrix metalloproteinase-9 is up-regulated by CCL21/CCR7 interaction via extracellular signal-regulated kinase-1/2 signaling and is involved in CCL21-driven B-cell chronic lymphocytic leukemia cell invasion and migration. Blood. 2008;111:383–386. doi: 10.1182/blood-2007-08-107300. [DOI] [PubMed] [Google Scholar]
- 11.RehmMensenSchradi AAK, et al. Cooperative function of CCR7 and lymphotoxin in the formation of a lymphoma-permissive niche within murine secondary lymphoid organs. Blood. 2011;118:1020–1033. doi: 10.1182/blood-2010-11-321265. [DOI] [PubMed] [Google Scholar]
- 12.GirblHinterseerGrossinger TEEM, et al. CD40-mediated activation of chronic lymphocytic leukemia cells promotes their CD44-dependent adhesion to hyaluronan and restricts CCL21-induced motility. Cancer Res. 2013;73:561–570. doi: 10.1158/0008-5472.CAN-12-2749. [DOI] [PubMed] [Google Scholar]
- 13.GanghammerHuttererHinterseer SEE, et al. CXCL12-induced VLA-4 activation is impaired in trisomy 12 chronic lymphocytic leukemia cells: a role for CCL21. Oncotarget. 2015;6:12048–12060. doi: 10.18632/oncotarget.3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.LauferLyckLegler JMRDF. ZAP70 expression enhances chemokine-driven chronic lymphocytic leukemia cell migration and arrest by valency regulation of integrins. FASEB J. 2018;32:4824–4835. doi: 10.1096/fj.201701452RR. [DOI] [PubMed] [Google Scholar]
- 15.TicchioniEssafiJeandelDaviCassutoDeckertBernard MMPYFJPMA. Homeostatic chemokines increase survival of B-chronic lymphocytic leukemia cells through inactivation of transcription factor FOXO3a. Oncogene. 2007;26:7081–7091. doi: 10.1038/sj.onc.1210519. [DOI] [PubMed] [Google Scholar]
- 16.de Cuesta-MateosLopez-GiralAlfonso-PerezSoriaLoscertalesGuasch-VidalBeltranZapataMunoz-Calleja CSMVGJSAEJMC. Analysis of migratory and prosurvival pathways induced by the homeostatic chemokines CCL19 and CCL21 in B-cell chronic lymphocytic leukemia. Exp Hematol. 2010;38:756–764, 64 e1-4. doi: 10.1016/j.exphem.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 17.CuestaMunoz-CallegaLoscertalesTerronMol CCJFW. CAP-100: First-in-class antibody for CCR7+ hematological malignancies. J Clinic Oncol. 2019;37:e19008-e. doi: 10.1200/JCO.2019.37.15_suppl.e19008. [DOI] [Google Scholar]
- 18.Cuesta-MateosMuñoz-CallejaLoscertalesTerronMol CCJFW. Abstract 4849: CAP-100: first-in-class anti-CCR7 antibody for CLL. Cancer Res. 2019;79:4849. doi: 10.1158/1538-7445.AM2019-4849. [DOI] [Google Scholar]
- 19.KaurSwami VA. Ibrutinib in CLL: a focus on adverse events, resistance, and novel approaches beyond ibrutinib. Ann Hematol. 2017;96:1175–1184. doi: 10.1007/s00277-017-2973-2. [DOI] [PubMed] [Google Scholar]
- 20.CameronSanford FM. Ibrutinib: first global approval. Drugs. 2014;74:263–271. doi: 10.1007/s40265-014-0178-8. [DOI] [PubMed] [Google Scholar]
- 21.Brown JR. Relapsed CLL: sequencing, combinations, and novel agents. Hematology Am Soc Hematol Educ Program. 2018;2018:248–255. doi: 10.1182/asheducation-2018.1.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jain N. Selecting Frontline Therapy for CLL in 2018. Hematology Am Soc Hematol Educ Program. 2018;2018:242–247. doi: 10.1182/asheducation-2018.1.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.HallekChesonCatovsky MBDD, et al. iwCLL guidelines for diagnosis, indications for treatment, response assessment, and supportive management of CLL. Blood. 2018;131:2745–2760. doi: 10.1182/blood-2017-09-806398. [DOI] [PubMed] [Google Scholar]
- 24.MaddocksRuppertLozanski KJASG, et al. Etiology of Ibrutinib Therapy Discontinuation and Outcomes in Patients With Chronic Lymphocytic Leukemia. JAMA Oncol. 2015;1:80–87. doi: 10.1001/jamaoncol.2014.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.MatoRoekerAllan ARLEJN, et al. Outcomes of front-line ibrutinib treated CLL patients excluded from landmark clinical trial. Am J Hematol. 2018;93:1394–1401. doi: 10.1002/ajh.25261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de RooijKuilGeestElderingChangBuggyPalsSpaargaren MFACREBYJJSTM. The clinically active BTK inhibitor PCI-32765 targets B-cell receptor- and chemokine-controlled adhesion and migration in chronic lymphocytic leukemia. Blood. 2012;119:2590–2594. doi: 10.1182/blood-2011-11-390989. [DOI] [PubMed] [Google Scholar]
- 27.Alfonso-PerezLopez-GiralQuintanaLoscertalesMartin-JimenezMunoz MSNEJPC. Anti-CCR7 monoclonal antibodies as a novel tool for the treatment of chronic lymphocyte leukemia. J Leukoc Biol. 2006;79:1157–1165. doi: 10.1189/jlb.1105623. [DOI] [PubMed] [Google Scholar]
- 28.PavlasovaBorskySeda GMV, et al. Ibrutinib inhibits CD20 upregulation on CLL B cells mediated by the CXCR4/SDF-1 axis. Blood. 2016;128:1609–1613. doi: 10.1182/blood-2016-04-709519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.SkarzynskiNiemannLee MCUYS, et al. Interactions between Ibrutinib and Anti-CD20 Antibodies: Competing Effects on the Outcome of Combination Therapy. Clinical Cancer Res Official J Am Assoc Cancer Res. 2016;22:86–95. doi: 10.1158/1078-0432.CCR-15-1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.ScottWolchokOld AMJDLJ. Antibody therapy of cancer. Nat Rev Cancer. 2012;12:278–287. doi: 10.1038/nrc3236. [DOI] [PubMed] [Google Scholar]
- 31.PatrussiCapitaniCattaneo LNF, et al. p66Shc deficiency enhances CXCR4 and CCR7 recycling in CLL B cells by facilitating their dephosphorylation-dependent release from beta-arrestin at early endosomes. Oncogene. 2018;37:1534–1550. doi: 10.1038/s41388-017-0066-2. [DOI] [PubMed] [Google Scholar]
- 32.HermanMustafaJonesWongFarooquiWiestner SEMRZJDHMA. Treatment with Ibrutinib Inhibits BTK- and VLA-4-Dependent Adhesion of Chronic Lymphocytic Leukemia Cells In Vivo. Clinical Cancer Res Official J Am Assoc Cancer Res. 2015;21:4642–4651. doi: 10.1158/1078-0432.CCR-15-0781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.CapitaniPatrussiTrentin NLL, et al. S1P1 expression is controlled by the pro-oxidant activity of p66Shc and is impaired in B-CLL patients with unfavorable prognosis. Blood. 2012;120:4391–4399. doi: 10.1182/blood-2012-04-425959. [DOI] [PubMed] [Google Scholar]
- 34.CalpeCodonyBaptistaAbrisquetaCarpioPurroyBoschCrespo ECMJPCNFM. ZAP-70 enhances migration of malignant B lymphocytes toward CCL21 by inducing CCR7 expression via IgM-ERK1/2 activation. Blood. 2011;118:4401–4410. doi: 10.1182/blood-2011-01-333682. [DOI] [PubMed] [Google Scholar]
- 35.HinmanBushanamNicholsSatterthwaite RMJNWAAB. B cell receptor signaling down-regulates forkhead box transcription factor class O 1 mRNA expression via phosphatidylinositol 3-kinase and Bruton's tyrosine kinase. J Immunol. 2007;178:740–747. doi: 10.4049/jimmunol.178.2.740. [DOI] [PubMed] [Google Scholar]
- 36.WolfGardingFilarsky CAK, et al. NFATC1 activation by DNA hypomethylation in chronic lymphocytic leukemia correlates with clinical staging and can be inhibited by ibrutinib. Int J Cancer. 2017;2017:31057. doi: 10.1002/ijc.31057. [DOI] [PubMed] [Google Scholar]
- 37.RodriguezMartinezCamacho ANFI, et al. Variability in the degree of expression of phosphorylated IkappaBalpha in chronic lymphocytic leukemia cases with nodal involvement. Clin Cancer Res. 2004;10:6796–6806. doi: 10.1158/1078-0432.CCR-04-0753. [DOI] [PubMed] [Google Scholar]
- 38.PingDingShi LNY, et al. The Bruton's tyrosine kinase inhibitor ibrutinib exerts immunomodulatory effects through regulation of tumor-infiltrating macrophages. Oncotarget. 2017;8:39218–39229. doi: 10.18632/oncotarget.16836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.ChenChangChangTongHamSherryBurgerRaiChiorazzi SSBYSTSBJAKRN. BTK inhibition results in impaired CXCR4 chemokine receptor surface expression, signaling and function in chronic lymphocytic leukemia. Leukemia. 2016;30:833–843. doi: 10.1038/leu.2015.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.ZaitsevaMurrayShafatLawesMacEwanBowlesRushworth LMYMSMJDJKMSA. Ibrutinib inhibits SDF1/CXCR4 mediated migration in AML. Oncotarget. 2014;5:9930–9938. doi: 10.18632/oncotarget.2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.OteroGroettrupLegler CMDF. Opposite fate of endocytosed CCR7 and its ligands: recycling versus degradation. J Immunol. 2006;177:2314–2323. doi: 10.4049/jimmunol.177.4.2314. [DOI] [PubMed] [Google Scholar]
- 42.MarcheseBenovic AJL. Agonist-promoted ubiquitination of the G protein-coupled receptor CXCR4 mediates lysosomal sorting. J Biol Chem. 2001;276:45509–45512. doi: 10.1074/jbc.C100527200. [DOI] [PubMed] [Google Scholar]
- 43.devan GorterBeulingKersseboomMiddendorpGilsHendriksPalsSpaargaren DJEARSJMRWSTM. Bruton's tyrosine kinase and phospholipase Cgamma2 mediate chemokine-controlled B cell migration and homing. Immunity. 2007;26:93–104. doi: 10.1016/j.immuni.2006.11.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.