Abstract
Background
We aimed to evaluate the prognostic value of natural killer (NK) cell activity for patients with HER2 + advanced gastric cancer (AGC) treated with first-line fluoropyrimidine–platinum doublet plus trastuzumab.
Methods
Forty-one patients with HER2 + AGC who received fluoropyrimidine–platinum doublet plus trastuzumab as first-line treatment were prospectively enrolled. NK cell activity was evaluated using the NK Vue®.
Results
The median age was 63.5 years, and 31 patients (75.6%) were male. Patients with low baseline NK cell activity (≤ median, n = 21) were associated worse progression-free survival (PFS) and overall survival (OS) compared with patients with high baseline NK cell activity (> median, n = 20) with a median PFS of 4.21 vs. 9.53 months (P < 0.001), and median OS of 8.15 months vs. 17.82 months (P = 0.025), respectively. In the multivariate analysis, low baseline NK cell activity was independently associated with poor PFS (HR 4.35, P = 0.007). NK cell activity recovered to a normal range in nine patients (47.4%) with a low baseline NK cell activity (n = 19) after two cycles of treatment. The median PFS and OS among patients with recovered NK cell activity were significantly better than that among patients with persistently low NK cell activity (PFS, P = 0.038; OS, P = 0.003).
Conclusion
Our results demonstrated the prognostic value of baseline NK cell activity for patients with HER2 + AGC treated with fluoropyrimidine–platinum doublet plus trastuzumab. The association between treatment outcomes and dynamic changes in NK cell activity suggests that NK cell treatment may improve treatment outcomes, especially for patients with low baseline NK cell activity.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00262-021-03035-x.
Keywords: Advanced gastric cancer, Trastuzumab, Natural killer cell activity
Introduction
Globally, gastric cancer is the sixth most common cancer and the third most common cause of cancer-related deaths, with the highest incidence reported in Asia [1]. It is often diagnosed at an advanced stage globally, and only approximately 25% of patients have resectable disease at presentation [2]. Although the survival outcomes of patients with inoperable, recurrent, or metastatic disease have improved over time, overall survival (OS) still remains poor, with a median OS less than 12 months [3]. In particular, according to our previously reported prognostic model, we found that gastric cancer patients in the poor risk group had a median OS of 5.1 months [4]. During the past decade, an increasing understanding of the molecular characteristics of gastric cancer has opened a new horizon for the treatment of gastric cancer through the development and the use of biological targeted agents [5–7].
The addition of trastuzumab, a monoclonal antibody that targets human epidermal growth factor receptor 2 (HER2), to conventional fluoropyrimidine–platinum doublet chemotherapy improved the survival outcomes of patients with HER2 + advanced gastric cancer in the pivotal phase III ToGA trial, and this combination is currently being used as the standard of care for first-line treatment of HER2 + advanced gastric cancer [5]. One of the important mechanisms of action of trastuzumab is antibody-dependent cellular toxicity (ADCC) [8]. A previous preclinical study reported that natural killer (NK) cell dysfunction contributes to the impairment of ADCC mediated by trastuzumab among patients with advanced gastric cancer and that trastuzumab-mediated ADCC was restored after activating NK cells with interleukin-2 [9].
NK cells are a part of the innate immune system, which plays an important role in early immune responses to tumor cells [10]. Several studies have demonstrated that NK cell activity is decreased among patients with cancer and is associated with poor survival outcomes [11, 12]. The gold-standard assay for NK cell activity has been the Chromium 51 (51Cr) release assay, which measures the target cell–killing capacity of NK cells [12]. However, this assay is not used in routine clinical practice due to several drawbacks, such as manipulation of hazardous radioactivity, high cost, and inter-laboratory variability. More recently, a simpler assay for measuring NK cell activity has been developed (NK Vue®; NKMAX, Seongnam-si, Republic of Korea), and decreased NK cell activity evaluated by the NK Vue® has been associated with poor survival outcome among patients with advanced cancer [13].
This study aimed to evaluate the prognostic value of NK cell activity measured by the NK Vue® for patients with HER2 + advanced gastric cancer treated with first-line fluoropyrimidine–platinum doublet plus trastuzumab.
Methods
Patients
Between July 2018 and September 2019, 44 patients with histologically documented HER2 + advanced gastric cancer received fluoropyrimidine–platinum doublet and trastuzumab as first-line treatment at Asan Medical Center, Seoul, Republic of Korea. Among these, 41 patients provided written informed consent and enrolled in this retrospective study of a prospectively collected database. The institutional review board of Asan Medical Center (2014–0301) approved this study.
The following patient data were extracted from the medical records and analyzed: age; sex; Eastern Cooperative Oncology Group Performance Status (ECOG PS); tumor pathology; history of prior gastrectomy; presence of peritoneal, bone, or lung metastasis; alkaline phosphatase (ALP) level; serum albumin level; total bilirubin level; lymphocyte count; and neutrophil count. Patients were stratified into good (1–2 risks), moderate (2–3 risks), or poor (≥ 4 risks) risk group according to the prognostic model described in our previous article based on ECOG PS; history of prior gastrectomy; peritoneal, bone, or lung metastasis; ALP; serum albumin; and total bilirubin level [4].
Response assessment
Computed tomography (CT) scans were performed every 6–8 weeks and at any time when tumor progression was suspected. Responses were assessed according to the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1.
NK cell activity analysis
The methods of measuring NK cell activity described below have been reproduced in part from a recent publication [13]. Peripheral blood was sampled before the initiation of treatment (baseline) and after two cycles of treatment. The principle of the NK Vue® is the sampling of peripheral blood into a patented test tube, incubation, and estimation based on enzyme-linked immunosorbent assay (ELISA) findings. Specifically, 1 mL of whole blood was drawn from the patient into NK Vue® tubes (NKMAX, Seongnam-si, Republic of Korea) and placed in an incubator at 37 °C within 15 min of sampling. Following 24 h of stimulation, the plasma interferon-γ level was measured using the NK Vue® Gold (NKMAX ELISA) as a surrogate marker of NK cell activity. The normal range of NK cell activity in healthy individuals measured by the NK Vue® is > 250 pg/mL. As patients with cancer have lower NK cell activity, the cut-off value for baseline high and low NK cell activity in the current study was determined using the median value instead of the cut-off for normal range in healthy individuals (250 pg/mL). The low baseline NK group included patients with baseline NK cell activity levels ≤ median, and the high baseline NK group included patients with baseline NK cell activity levels > median. According to dynamic changes in NK cell activity, patients were further classified as follows: patients with high baseline NK cell activity (> median, high baseline NK group); patients with initially low baseline NK cell activity (≤ median) which recovered to within the normal range (> 250 pg/mL) after two cycles of treatment (recovered NK group); patients with initially low baseline NK cell activity (≤ median) which did not recover to within the normal range (> 250 pg/mL) after two cycles of treatment (persistently low NK group). NKMAX Korea provided NK Vue test kits free of charge for use in the study.
Statistical analysis
Progression-free survival (PFS) was defined as the time from the beginning of first-line fluoropyrimidine–platinum doublet plus trastuzumab administration to disease progression or death, whichever occurred first. Overall survival (OS) was defined as the time interval between the beginning of first-line fluoropyrimidine–platinum doublet plus trastuzumab administration and the date of death from any cause or the last follow-up visit. The duration of response (DOR) was defined as the time from the date of the first documentation of an objective response (complete response [CR] or partial response [PR]) to the date of the first documentation of disease progression. DOR was measured for responding patients (CR or PR) only. Survival rates and corresponding standard errors were estimated using the Kaplan–Meier method, and survival curves were compared using the log-rank test. Baseline characteristics of the groups were compared using Pearson’s chi-square test or Fisher’s exact test for categorical variables and Student’s t-test for continuous variables, as appropriate. Univariate and multivariate analyses were performed using Cox proportional hazards regression modeling. In the multivariate analysis, backward selection was applied and variables exhibiting a potential association with survival (P < 0.25) in the univariate analysis, along with age and sex, were included. The Wilcoxon signed-rank test was used to compare the NK cell activity at baseline with that after the second cycle of treatment. All statistical calculations were conducted using R version 3.6.2. (R Foundation for Statistical Computing, Vienna, Austria, https://www.R-project.org/).
Results
Patient characteristics
The baseline characteristics of the patients are presented in Table 1. The median age was 63.5 (range, 43–79), and 31 patients (75.6%) were male. Most patients had an ECOG PS of 0 or 1 (n = 37; 90.2%) and HER2 immunohistochemistry (IHC) status of 3 + (n = 33; 80.5%). The median baseline NK cell activity was 74.1 pg/mL (range, 40–2238.4). A higher proportion of patients in the low baseline NK group belonged to the moderate and poor risk groups (85.7% vs. 55.0%, P = 0.043), and the low baseline NK group had a higher neutrophil-to-lymphocyte ratio (NLR) value (median 3.0 vs. 2.0, P = 0.048) compared with the high baseline NK group; otherwise, the baseline characteristics were similar between the two groups.
Table 1.
Baseline patient and disease characteristics (n = 41)
| Total (n = 41) |
Low baseline NK cell activity (n = 21) |
High baseline NK cell activity (n = 20) |
P value | |
|---|---|---|---|---|
| Age (mean ± SD) | 63.5 ± 9.0 | 63.5 ± 9.4 | 63.5 ± 8.8 | 0.980 |
| Sex | 0.067 | |||
| Female | 10 (24.4%) | 8 (38.1%) | 2 (10.0%) | |
| Male | 31 (75.6%) | 13 (61.9%) | 18 (90.0%) | |
| HER2 status | 0.697 | |||
| IHC 2 + / FISH + | 8 (19.5%) | 5 (23.8%) | 3 (15.0%) | |
| IHC 3 + | 33 (80.5%) | 16 (76.2%) | 17 (85.0%) | |
| Risk group | 0.043 | |||
| Good | 12 (29.3%) | 3 (14.3%) | 9 (45.0%) | |
| Moderate or poor | 29 (70.7%) | 18 (85.7%) | 11 (55.0%) | |
| ECOG PS | 0.606 | |||
| 0–1 | 37 (90.2%) | 18 (85.7%) | 19 (95.0%) | |
| ≥ 2 | 4 (9.8%) | 3 (14.3%) | 1 (5.0%) | |
| Prior gastrectomy | 0.341 | |||
| No | 15 (36.6%) | 6 (28.6%) | 9 (45.0%) | |
| Yes | 26 (63.4%) | 15 (71.4%) | 11 (55.5%) | |
| Peritoneal metastasis | 0.062 | |||
| No | 18 (43.9%) | 6 (28.6%) | 12 (60.0%) | |
| Yes | 23 (56.1%) | 15 (71.4%) | 8 (40.0%) | |
| Bone metastasis | 1.000 | |||
| No | 38 (92.7%) | 19 (90.5%) | 19 (95.0%) | |
| Yes | 3 (7.3%) | 2 (9.5%) | 1 (5.0%) | |
| Lung metastasis | 1.000 | |||
| No | 36 (87.8%) | 18 (85.7%) | 18 (90.0%) | |
| Yes | 5 (12.2%) | 3 (14.3%) | 2 (10.0%) | |
| Elevated ALP | 0.744 | |||
| No | 28 (68.3%) | 15 (71.4%) | 13 (65.0%) | |
| Yes | 13 (31.7%) | 6 (28.6%) | 7 (35.0%) | |
| Elevated total bilirubin | 0.488 | |||
| No | 39 (95.1%) | 19 (90.5%) | 20 (100%) | |
| Yes | 2 (4.9%) | 2 (9.5%) | 0 (0%) | |
| Hypoalbuminemia | 0.326 | |||
| No | 27 (65.9%) | 12 (57.1%) | 15 (75.0%) | |
| Yes | 14 (34.1%) | 9 (42.9%) | 5 (25.0%) | |
| NLR, median (IQR) | 2.4 (1.6–3.3) | 3.0 (2.2–4.9) | 2.0 (1.6–2.4) | 0.048 |
| First-line regimen | 0.697 | |||
| FP + trastuzumab | 7 (17.1%) | 3 (14.3%) | 4 (20.0%) | |
| XP + trastuzumab | 34 (82.9%) | 18 (85.7%) | 16 (80.0%) |
NK, Natural killer; SD, Standard deviation; IHC, Immunohistochemistry; FISH, Fluorescence in situ hybridization; ECOG PS, Eastern Cooperative Oncology Group Performance Status; ALP, Alkaline phosphatase; NL
R, Neutrophil to lymphocyte ratio; IQR, Interquartile range; FP, 5-fluorouracil + cisplatin; XP, capecitabine + cisplatin
Treatment outcomes according to baseline NK cell activity
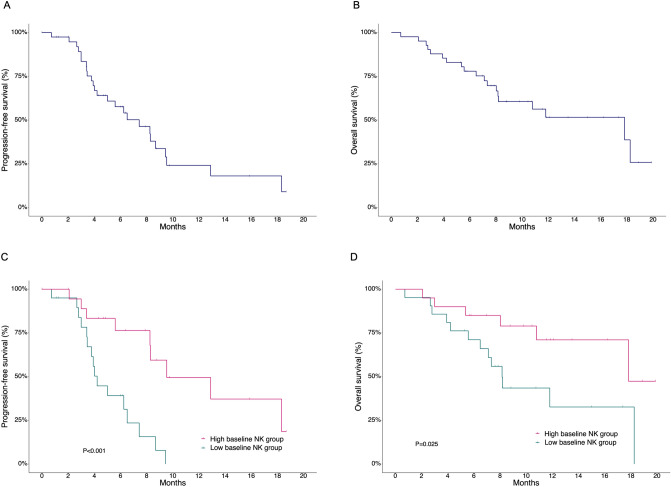
The overall response rate among all patients was 68.2%, with a median follow-up duration of 10.6 months (range, 5.3–19.9) for surviving patients. The median PFS and OS were 7.43 months (95% confidence interval [CI], 5.0–9.5) and 17.82 months (95% CI, 8.2–not applicable), respectively (Fig. 1A, B). There was no significant difference in overall response rate (86.7% vs. 85.7%, P > 0.999) between the patients in the low and high baseline NK groups with measurable disease (Supplementary Table S1). The DOR among responding patients (n = 25) in the high baseline NK group was significantly longer than that in the low baseline NK group (median DOR: 8.28 vs. 3.29 months, P < 0.001). Patients in the low baseline NK group had a significantly worse PFS than those in the high baseline NK group (median PFS: 4.21 vs. 9.53 months, P < 0.001; Fig. 1C). As with PFS, OS was significantly worse in the low baseline NK group compared with the high baseline NK group (median OS: 8.15 vs. 17.82 months, P = 0.025; Fig. 1D).
Fig. 1.
A, B Progression-free survival (PFS) and overall survival (OS) among all patients. C, D PFS and OS according to baseline natural killer (NK) cell activity
Table 2 summarizes the results of the univariate and multivariate analyses of the potential prognostic factors in terms of PFS and OS. In the univariate analysis, HER2 IHC status showed a potential association (P < 0.25) with PFS; age, risk group, NLR, and baseline NK cell activity showed potential associations with both PFS and OS. In the multivariate analysis, low baseline NK cell activity was independently associated with poor PFS (vs. high baseline NK group; HR 4.35, P = 0.007) along with HER2 IHC2 + (vs. IHC3 + ; HR 5.79, P = 0.003) and poor risk group (vs. good or moderate risk group; HR 14.55, P < 0.001). For OS, age > 65 years (vs. ≤ 65; HR 3.68, P = 0.014), poor risk group (vs. good or moderate risk group; HR 3.68, P = 0.014), and high NLR (vs. ≤ median; HR 4.86, P = 0.014) were independently associated with poor OS.
Table 2.
Univariate and multivariate analysis for PFS and OS (n = 41)
| PFS | OS | |||||||
|---|---|---|---|---|---|---|---|---|
| Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | |||||
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | |
| Age | ||||||||
| ≤ 65 | Reference | – | – | Reference | Reference | |||
| > 65 | 1.70 (0.76–3.81) | 0.197 | – | – | 2.12 (0.85–5.31) | 0.108 | 3.68 (1.30–10.43) | 0.014 |
| Sex | ||||||||
| Female | Reference | – | – | Reference | – | – | ||
| Male | 1.19 (0.47–3.04) | 0.710 | – | – | 1.64 (0.47–5.75) | 0.438 | – | – |
| HER2 status | ||||||||
| IHC3 + | Reference | Reference | Reference | – | – | |||
| IHC2 + /FISH + | 2.33 (0.93–5.87) | 0.072 | 5.79 (1.83–18.26) | 0.003 | 1.42 (0.50–3.98) | 0.507 | – | – |
| Baseline NK cell activity | ||||||||
| High | Reference | Reference | Reference | – | – | |||
| Low | 4.87 (1.85–12.85) | 0.001 | 4.35 (1.48–12.78) | 0.007 | 2.92 (1.01–7.78) | 0.032 | – | – |
| Risk group | ||||||||
| Good or moderate | Reference | Reference | Reference | Reference | ||||
| Poor | 11.02 (3.40–35.67) | < 0.001 | 14.55 (3.67–57.71) | < 0.001 | 6.88 (2.60–18.20) | < 0.001 | 3.35 (1.07–10.52) | 0.039 |
| NLR | ||||||||
| ≤ median | Reference | – | – | Reference | Reference | |||
| > median | 6.64 (2.54–17.34) | < 0.001 | – | – | 5.65 (2.00–15.94) | 0.001 | 4.86 (1.39–17.05) | 0.014 |
PFS, Progression free survival; OS, Overall survival; CI, Confidence interval; IHC, Immunohistochemistry; FISH, Fluorescence in situ hybridization; NK, Natural killer; NLR, Neutrophil to lymphocyte ratio
Dynamic changes in NK cell activity following two cycles of fluoropyrimidine–platinum doublet plus trastuzumab
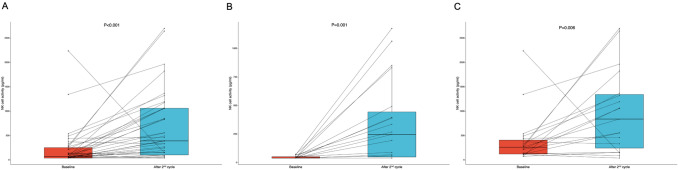
We additionally evaluated dynamic changes in NK cell activity following two cycles of fluoropyrimidine–platinum doublet plus trastuzumab among 38 patients whose data were available for NK cell activity evaluation after two cycles of treatment. Significant increases in NK cell activity were observed not only among all patients (median NK cell activity at baseline vs. after the second cycle; 74.1 vs. 393.7, P < 0.001), but also individually within the low and high baseline NK groups (median NK cell activity at baseline vs. after the second cycle; low baseline NK group, 40.0 vs. 247.1, P = 0.001; high baseline NK group, 246.1 vs. 840.6, P = 0.006), respectively (Fig. 2A–C). Among patients in the low baseline NK group (n = 19), the NK cell activity increased to within the normal range (> 250 pg/mL) in nine patients (47.4%). According to dynamic changes in NK cell activity, patients were further classified as high baseline NK group (n = 19), recovered NK group (n = 9), and persistently low NK group (n = 10). The baseline characteristics of these patients are summarized in Supplementary Table S2. A significantly higher proportion of patients in the recovered NK group were female (55.6%) compared with the high baseline NK group (10.5%) and persistently low NK group (10.0%) (P = 0.015). Additionally, all of the patients in the persistently low NK group were classified as moderate or poor risk group, whereas 52.6% and 66.7%, respectively, of the patients in the high baseline and recovered NK groups were classified as such (P = 0.033). Otherwise, the baseline characteristics were similar among all three groups.
Fig. 2.
Dynamic changes in natural killer (NK) cell activity following two cycles of fluoropyrimidine–platinum doublet plus trastuzumab in A all patients, B the low baseline NK group, C the high baseline NK group. Boxplots represent the medians, as well as 25th and 75th percentiles; the whiskers extend from the hinge to the largest value no further than 1.5 × interquartile range from the hinge
Treatment outcomes according to changes in NK cell activity
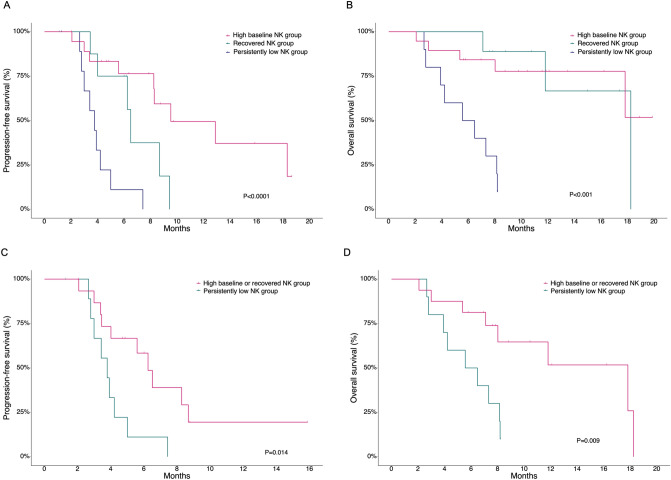
There was no significant difference in ORR among patients in the high baseline (85.7%), recovered (87.5%), and persistently low (85.7%) NK groups with measurable disease (n = 29) (P > 0.999) (Supplementary Table S3). The DOR among responding patients (n = 25) were significantly different among these groups with a median DOR of 8.28, 5.72, and 2.68 months in the high baseline, recovered, and persistently low NK groups, respectively (P < 0.001). The median PFS according to high baseline, recovered, and persistently low NK groups were 9.53, 6.51, and, 3.78 months, respectively (P < 0.001, Fig. 3A). The PFS of the recovered NK group was comparable to that of the high baseline NK group and significantly better than that of the persistently low NK group (high baseline NK group vs. recovered NK group, P = 0.195; high baseline NK group vs. persistently low NK group, P < 0.001; recovered NK group vs. persistently low NK group, P = 0.038). The median OS was not reached in the high baseline NK group, 18.25 months in the recovered NK group, and 6.02 months in the persistently low NK group (P < 0.001, Fig. 3B). The recovered NK group demonstrated OS that was comparable to that of the high baseline NK group and significantly better than that of the persistently low NK group (high baseline NK group vs. recovered NK group, P > 0.999; high baseline NK group vs. persistently low NK group, P < 0.002; recovered NK group vs. persistently low NK group, P = 0.003). Given the favorable survival outcomes in the high baseline NK group and recovered NK group compared with the persistently low NK group, we further evaluated the prognostic value of dynamic changes in NK cell activity within the moderate to poor risk group (n = 26). All patients in the good risk group were in the high baseline or recovered NK group. Patients in the high baseline NK group or recovered NK group had significantly better PFS and OS outcomes compared with patients in the persistently low NK cell group, with a median PFS of 6.25 vs. 3.78 months (P = 0.014) and a median OS of 17.82 vs. 6.02 months (P = 0.009), respectively (Fig. 3C, D).
Fig. 3.
Survival outcomes according to changes in NK cell activity among patients with NK cell activity data after two cycles of treatment (n = 38). A, B Progression-free survival (PFS) and overall survival (OS) in the high baseline, recovered, and persistently low NK groups. C, D PFS and OS in the high baseline or recovered NK group and the persistently low NK group among patients with moderate to poor risk (n = 26)
Discussion
To our knowledge, this was the first study to evaluate the prognostic value of NK cell activity, which was measured by a novel and simple blood test (NK Vue®), for patients with HER2 + advanced gastric cancer treated with first-line fluoropyrimidine–platinum doublet plus trastuzumab. Low baseline NK cell activity was significantly associated with both poor PFS and OS. In addition, NK cell activity significantly increased after two cycles of treatment in most patients, and dynamic changes in NK cell activity were associated with survival outcomes.
In this study, although there was no significant association between baseline NK cell activity and treatment response rates, low baseline NK cell activity was independently associated with poor PFS in both univariate and multivariate analyses, most likely due to shorter duration of response. Similar findings have been observed in previous studies investigating various cancer types, including gastric cancer, demonstrating the association between NK cell activity and treatment outcomes [11, 14, 15]. However, these studies used the 51Cr release assay to evaluate the NK cell activity, which limits the clinical applicability of the study findings. Considering the prognostic value of the NK Vue® in this study and the simplicity of the test, it may be more practical to use the NK Vue® than the 51Cr release assay for the evaluation of NK cell activity among patients with advanced gastric cancer. Although there was a significant association between NK cell activity and OS in the univariate analysis, the prognostic value of NK cell activity did not remain significant for OS in the multivariate analysis. This may be due in part to a relatively short follow-up duration, small sample size, recovery of NK cell activity after chemotherapy in a fraction of patients, and the effect of subsequent lines of treatment without anti-HER2 antibody.
The level of NK cell activity significantly increased after two cycles of treatment in most patients. Several preclinical studies have demonstrated that conventional chemotherapeutic agents, including 5-FU and cisplatin, may increase the activity of NK cells [16, 17]. Moreover, trastuzumab enhances the anti-tumor activity of NK cells by promoting NK cell proliferation, increasing cytotoxicity, and expanding the cytotoxic population of NK cells [18, 19]. NK cell activity normalized (> 250 pg/mL) after two cycles of treatment in the subgroup of patients with low baseline NK cell activity (≤ median [74.1 pg/mL]), and this normalization was associated with good survival outcomes, comparable to those achieved for patients with high baseline NK cell activity levels and significantly better than those achieved for patients with persistently low NK cell activity levels. This is in line with previous studies, which demonstrated that increases in NK cell activity were associated with better treatment outcomes among patients with advanced cancer [13, 20]. Of note, the median baseline NK cell activity was significantly lower among patients in the moderate to poor risk group, and a subgroup of these patients had persistently low NK cell activity, while none of the patients in the good risk group had persistently low NK cell activity. The improved survival outcomes associated with NK cell activity recovery remained significant within the moderate to poor risk group of patients. These results suggest that treatment strategies to augment NK cell activity might be beneficial among patients with HER2 + advanced gastric cancer, especially among those with low baseline NK cell activity levels in the moderate to poor risk group whose NK cell activity might not recover after chemotherapy.
Based on the data from preclinical studies demonstrating the anti-tumor activity of NK cells, various strategies are being studied to introduce NK cell therapy into the field of cancer treatment [21]. Novel checkpoint inhibitors, such as anti-NKG2A monoclonal antibody and anti-TIGIT antibody, have been demonstrated to promote anti-tumor immunity through the reactivation of NK cells in preclinical models [22, 23]. In addition, a recent phase I study reported that the addition of adoptive transfer of expanded NK cell populations to doublet chemotherapy (S-1 + cisplatin) plus trastuzumab for patients with advanced gastric cancer was safe, feasible, and associated with preliminary anti-tumor activity [24]. Moreover, NK cell adoptive therapy in combination with an anti-PD-1 inhibitor was associated with improved survival outcomes among heavily pretreated patients with PD-L1+ non-small cell lung cancer in a recent phase I/II trial [25]. Recently, a preliminary report of a phase I/II trial demonstrated that the addition of anti-PD-1 inhibitor to fluoropyrimidine–platinum doublet in combination with trastuzumab as first-line therapy in HER2 + advanced gastric cancer was associated with encouraging anti-tumor efficacy, and a phase III trial based on this regimen is currently ongoing [26]. Considering the results of these recent reports, there may be opportunities for the use of NK cell adoptive treatment alone or in combination with anti-PD-1 inhibitor, trastuzumab, and fluoropyrimidine–platinum doublet to enhance anti-tumor efficacy.
The anti-tumor activity of NK cells is mostly determined by the interaction between its inhibitory or activating receptors and the tumor microenvironment [27]. Recent studies have focused on the association between specific phenotypes of NK cells and anti-tumor activity. Overall, there is a lot of evidence suggesting that activated NK cells in tissues are associated with a good prognosis among patients with cancer. However, several studies have reported contradictory results with no or even negative prognostic effects of tumor-infiltrating NK cells [28]. Additionally, the distribution of NK cell infiltration is also important; a recent study demonstrated that while intra-tumoral NK cell density was associated with survival outcomes, no such association was observed with stromal NK cell density among patients with gastric cancer [29]. These results suggest that evaluating NK cell within the tumor is complex and might not be readily translated into routine clinical practice. In this regard, the NK cell activity test used in the present study has strengths in that it is non-invasive, simple, feasible, and may overcome spatial and temporal tumor heterogeneity.
This study had several limitations. As expected for any retrospective study, there may have been selection bias. In addition, the median follow-up duration of the study was relatively short, and the sample size was small. Moreover, the correlations between NK cell activity level, tumor microenvironment, and NK cell phenotypes were not evaluated. Thus, the prognostic value of NK cell activity needs to be further evaluated in studies with larger sample sizes with longer follow-up. Despite these limitations, this study had several strengths. The results of this study were exclusively based on patients with HER2 + advanced gastric cancer treated with fluoropyrimidine–platinum doublet plus trastuzumab. The relatively homogeneous patient population in the present study might have mitigated the potential confounding factors. Furthermore, by evaluating NK cell activity after the treatment as well as at baseline, this study provided insight into the potential role of NK cell treatment among patients with low baseline NK cell activity.
The results of our study demonstrate the prognostic value of NK cell activity for patients with HER2 + advanced gastric cancer treated with fluoropyrimidine–platinum doublet plus trastuzumab, which may inform clinical decisions. Patients with low baseline NK cell activity, especially those in the moderate to poor risk group, maybe a potential candidate for NK cell treatment.
Supplementary Information
Below is the link to the electronic supplementary material.
Availability of data and material
Data are available on reasonable request.
Declarations
Conflict of interest
The authors declare that they do not have any conflict of interest.
Ethics approval
This study was approved by the ethics committee, and all patients provided written informed consent.
Footnotes
Hyungwoo Cho and Min-Hee Ryu are Co-first authors.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Hyungwoo Cho and Min-Hee Ryu equally contributed to this work.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Wagner AD, Syn NL, Moehler M, Grothe W, Yong WP, Tai BC, Ho J, Unverzagt S. Chemotherapy for advanced gastric cancer. Cochrane Database Syst Rev. 2017 doi: 10.1002/14651858.CD004064.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koo DH, Ryu MH, Ryoo BY, Seo J, Lee MY, Chang HM, Lee JL, Lee SS, Kim TW, Kang YK. Improving trends in survival of patients who receive chemotherapy for metastatic or recurrent gastric cancer: 12 years of experience at a single institution. Gastric Cancer. 2015;18:346–353. doi: 10.1007/s10120-014-0385-8. [DOI] [PubMed] [Google Scholar]
- 4.Koo DH, Ryoo BY, Kim HJ, Ryu MH, Lee SS, Moon JH, Chang HM, Lee JL, Kim TW, Kang YK. A prognostic model in patients who receive chemotherapy for metastatic or recurrent gastric cancer: validation and comparison with previous models. Cancer Chemother Pharmacol. 2011;68:913–921. doi: 10.1007/s00280-011-1561-8. [DOI] [PubMed] [Google Scholar]
- 5.Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T, Aprile G, Kulikov E, Hill J, Lehle M, Rüschoff J, Kang YK, Investigators TT. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–697. doi: 10.1016/S0140-6736(10)61121-X. [DOI] [PubMed] [Google Scholar]
- 6.Fuchs CS, Tomasek J, Yong CJ, Dumitru F, Passalacqua R, Goswami C, Safran H, Dos Santos LV, Aprile G, Ferry DR, Melichar B, Tehfe M, Topuzov E, Zalcberg JR, Chau I, Campbell W, Sivanandan C, Pikiel J, Koshiji M, Hsu Y, Liepa AM, Gao L, Schwartz JD, Tabernero J, Trial Investigators REGARD. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet. 2014;383:31–39. doi: 10.1016/S0140-6736(13)61719-5. [DOI] [PubMed] [Google Scholar]
- 7.Wilke H, Muro K, Van Cutsem E, Oh SC, Bodoky G, Shimada Y, Hironaka S, Sugimoto N, Lipatov O, Kim TY, Cunningham D, Rougier P, Komatsu Y, Ajani J, Emig M, Carlesi R, Ferry D, Chandrawansa K, Schwartz JD, Ohtsu A, RAINBOW Study Group Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol. 2014;15:1224–1235. doi: 10.1016/S1470-2045(14)70420-6. [DOI] [PubMed] [Google Scholar]
- 8.Hudis CA. Trastuzumab — mechanism of action and use in clinical practice. N Engl J Med. 2007;357:39–51. doi: 10.1056/NEJMra043186. [DOI] [PubMed] [Google Scholar]
- 9.Kono K, Takahashi A, Ichihara F, Sugai H, Fujii H, Matsumoto Y. Impaired antibody-dependent cellular cytotoxicity mediated by herceptin in patients with gastric cancer. Cancer Res. 2002;62:5813–5817. [PubMed] [Google Scholar]
- 10.Du Y, Wei Y. Therapeutic potential of natural killer cells in gastric cancer. Front Immunol. 2019;9:3095. doi: 10.3389/fimmu.2018.03095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takeuchi H, Maehara Y, Tokunaga E, Koga T, Kakeji Y, Sugimachi K. Prognostic significance of natural killer cell activity in patients with gastric carcinoma: a multivariate analysis. Am J Gastroenterol. 2001;96:574–578. doi: 10.1111/j.1572-0241.2001.03535.x. [DOI] [PubMed] [Google Scholar]
- 12.Jobin G, Rodriguez-Suarez R, Betito K. Association between natural killer cell activity and colorectal cancer in high-risk subjects undergoing colonoscopy. Gastroenterology. 2017;153:980–987. doi: 10.1053/j.gastro.2017.06.009. [DOI] [PubMed] [Google Scholar]
- 13.Hansen TF, Nederby L, Zedan AH, Mejlholm I, Henriksen JR, Steffensen KD, Thomsen CB, Raunkilde L, Jensen LH, Jakobsen A. Correlation between natural killer cell activity and treatment effect in patients with disseminated cancer. Transl Oncol. 2019;12:968–972. doi: 10.1016/j.tranon.2019.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kondo E, Koda K, Takiguchi N, Oda K, Seike K, Ishizuka M, Miyazaki M. Preoperative natural killer cell activity as a prognostic factor for distant metastasis following surgery for colon cancer. Dig Surg. 2003;20:445–451. doi: 10.1159/000072714. [DOI] [PubMed] [Google Scholar]
- 15.Liljefors M, Nilsson B, Hjelm Skog AL, Ragnhammar P, Mellstedt H, Frödin JE. Natural killer (NK) cell function is a strong prognostic factor in colorectal carcinoma patients treated with the monoclonal antibody 17–1A. Int J Cancer. 2003;105:717–723. doi: 10.1002/ijc.11139. [DOI] [PubMed] [Google Scholar]
- 16.Cifaldi L, Locatelli F, Marasco E, Moretta L, Pistoia V. Boosting natural killer cell-based immunotherapy with anticancer drugs: a perspective. Trends Mol Med. 2017;23:1156–1175. doi: 10.1016/j.molmed.2017.10.002. [DOI] [PubMed] [Google Scholar]
- 17.Shi L, Lin H, Li G, Sun Y, Shen J, Xu J, Lin C, Yeh S, Cai X, Chang C. Cisplatin enhances NK cells immunotherapy efficacy to suppress HCC progression via altering the androgen receptor (AR)-ULBP2 signals. Cancer Lett. 2016;373:45–56. doi: 10.1016/j.canlet.2016.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tian X, Wei F, Wang L, Yu W, Zhang N, Zhang X, Han Y, Yu J, Ren X. Herceptin enhances the antitumor effect of natural killer cells on breast cancer cells expressing human epidermal growth factor receptor-2. Front Immunol. 2017;8:1426. doi: 10.3389/fimmu.2017.01426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arnould L, Gelly M, Penault-Llorca F, Benoit L, Bonnetain F, Migeon C, Cabaret V, Fermeaux V, Bertheau P, Garnier J, Jeannin JF, Coudert B. Trastuzumab-based treatment of HER2-positive breast cancer: an antibody-dependent cellular cytotoxicity mechanism? Br J Cancer. 2006;94:259–267. doi: 10.1038/sj.bjc.6602930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ménard C, Blay JY, Borg C, Michiels S, Ghiringhelli F, Robert C, Nonn C, Chaput N, Taïeb J, Delahaye NF, Flament C, Emile JF, Le Cesne A, Zitvogel L. Natural killer cell IFN-gamma levels predict long-term survival with imatinib mesylate therapy in gastrointestinal stromal tumor-bearing patients. Cancer Res. 2009;69:3563–3569. doi: 10.1158/0008-5472.CAN-08-3807. [DOI] [PubMed] [Google Scholar]
- 21.Shimasaki N, Jain A, Campana D. NK cells for cancer immunotherapy. Nat Rev Drug Discov. 2020;19:200–218. doi: 10.1038/s41573-019-0052-1. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Q, Bi J, Zheng X, Chen Y, Wang H, Wu W, Wang Z, Wu Q, Peng H, Wei H, Sun R, Tian Z. Blockade of the checkpoint receptor TIGIT prevents NK cell exhaustion and elicits potent anti-tumor immunity. Nat Immunol. 2018;19:723–732. doi: 10.1038/s41590-018-0132-0. [DOI] [PubMed] [Google Scholar]
- 23.André P, Denis C, Soulas C, Bourbon-Caillet C, Lopez J, Arnoux T, Bléry M, Bonnafous C, Gauthier L, Morel A, Rossi B, Remark R, Breso V, Bonnet E, Habif G, Guia S, Lalanne AI, Hoffmann C, Lantz O, Fayette J, Boyer-Chammard A, Zerbib R, Dodion P, Ghadially H, Jure-Kunkel M, Morel Y, Herbst R, Narni-Mancinelli E, Cohen RB, Vivier E. Anti-NKG2A mAb is a checkpoint inhibitor that promotes anti-tumor immunity by unleashing both T and NK cells. Cell. 2018;175(1731–43):e13. doi: 10.1016/j.cell.2018.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ishikawa T, Okayama T, Sakamoto N, Ideno M, Oka K, Enoki T, Mineno J, Yoshida N, Katada K, Kamada K, Uchiyama K, Handa O, Takagi T, Konishi H, Kokura S, Uno K, Naito Y, Itoh Y. Phase I clinical trial of adoptive transfer of expanded natural killer cells in combination with IgG1 antibody in patients with gastric or colorectal cancer. Int J Cancer. 2018;142:2599–2609. doi: 10.1002/ijc.31285. [DOI] [PubMed] [Google Scholar]
- 25.Lin M, Luo H, Liang S, Chen J, Liu A, Niu L, Jiang Y. Pembrolizumab plus allogeneic NK cells in advanced non-small cell lung cancer patients. J Clin Invest. 2020;130:2560–2569. doi: 10.1172/JCI132712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rha SY, Lee CK, Kim HS, Kang B, Jung M, Kyun W, Koo DH, Shin SJ, Jeung HC, Zang DY, Chung HC. Targeting HER2 in combination with anti-PD-1 and chemotherapy confers a significant tumor shrinkage of gastric cancer: a multi-institutional phase Ib/II trial of first-line triplet regimen (pembrolizumab, trastuzumab, chemotherapy) for HER2-positive advanced gastric cancer (AGC) J Clin Oncol. 2020;38:3081. doi: 10.1200/JCO.2020.38.15_suppl.3081. [DOI] [Google Scholar]
- 27.Stabile H, Fionda C, Gismondi A, Santoni A. Role of distinct natural killer cell subsets in anticancer response. Front Immunol. 2017;8:293. doi: 10.3389/fimmu.2017.00293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Melaiu O, Lucarini V, Cifaldi L, Fruci D. Influence of the tumor microenvironment on NK cell function in solid tumors. Front Immunol. 2019;10:3038. doi: 10.3389/fimmu.2019.03038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li T, Zhang Q, Jiang Y, Yu J, Hu Y, Mou T, Chen G, Li G. Gastric cancer cells inhibit natural killer cell proliferation and induce apoptosis via prostaglandin E2. Oncoimmunology. 2016;5:e1069936. doi: 10.1080/2162402X.2015.1069936. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available on reasonable request.