Abstract
CD133 + cancer stem cells mediate chemoresistance in multiple aggressive cancers, and anti-CD133 chimeric antigen receptor T (CAR-T) cells are designed to selectively target cisplatin-resistant gastric cancer stem cells in this investigation. The relative CD133 expression was detected in gastric cancer patients before and after cisplatin treatment. Anti-CD133 CAR-T cells were incubated with cisplatin-exposed CD133+ BGC-823 cells to evaluate the killing efficacy. At the same time, the canonical T cell activation markers were assayed by fluorescence-activated cell sorting, and the functional cytokine profile was detected with enzyme-linked immunosorbent assays. In addition to the percentage of CD133 positive stem cell-like cells, the volume and weight of subcutaneous tumors in BGC-823, KATO III and MKN-28 xenograft models were measured to evaluate the anti-tumor activity of cisplatin and anti-CD133 CAR-T combination strategy. After cisplatin treatment, both human samples and BGC-823 cells showed up-regulated CD133 expression. Anti-CD133 CAR-T cells exhibited pronounced killing efficiency against cisplatin-exposed CD133+ BGC-823 cells with up-regulated activation markers and cytotoxicity cytokine production. Moreover, cisplatin and anti-CD133 CAR-T combination treatment inhibited tumor progression in three different xenograft models with diminished CD133 positive stem cell-like cell infiltration. These results indicate that cisplatin and anti-CD133 CAR-T combination strategy can simultaneously target normal and stem cell-like gastric cancer cells to improve the treatment outcome.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00262-021-02891-x.
Keywords: Cisplatin, CAR-T, CD133, Gastric cancer
Introduction
Cisplatin is the first-line chemotherapeutic drug against gastric cancer, especially for patients in the advanced stage. However, cisplatin resistance and reduced response rate often limit the clinical utilization of cisplatin and may lead to the relapse and metastasis of gastric cancer [1, 2]. Although cisplatin resistance has been attributed to heterogeneous molecular pathogenesis, metabolic modification, drug efflux and dysregulated DNA repair, no effective treatment modality can be introduced to the clinical practice [3].
Recent research indicates that most chemotherapy can only diminish the primary tumor load, for cancer stem cells possess intrinsic mechanisms to resist chemotherapy, and the resting cancer stem cells are responsible for tumor initiation, recurrence and metastasis [4–7]. CD133 positive cells are reported with tumor-initiating or stem cell properties in brain, colon, lung, liver, pancreatic and prostate cancers [8–10]. Thus, CD133 is utilized to as a marker for cancer stem cells in these tumors. Although the accuracy of CD133 as a biomarker for gastric cancer stem cells is highly controversial, several groups have reported that CD133 positive gastric cancer stem cell-like cells can mediate the cisplatin resistance in gastric cancer [11, 12]. These studies indicate that the CD133 target strategy may greatly benefit the clinical treatment outcome.
Chimeric antigen receptor T cells (CAR-T) are genetically edited to construct the artificial T-cell receptor for antigen-specific target in immunotherapy [13], which has been shown to be successful in hematologic tumors. Along with the presence of CD133 positive gastric cancer stem cell-like cells, a growing number of studies indicate that CD133 expression is negatively correlated with the overall survival and prognosis of gastric cancer patients [12, 14, 15], suggesting a promising strategy using anti-CD133 CAR-T treatment against gastric cancer.
In clinical practice, both the alleviation of tumor load with surgery and/or chemical therapy, as well as the inhibition of tumor invasion and metastasis, are urgent. Thus, cisplatin and anti-CD133 CAR-T combination strategy is chosen in this study to address the possibility and efficiency of improving gastric cancer prognosis. To our surprise, cisplatin treatment increased the proportion of CD133 positive stem cell-like cancer cells, which could be repressed by cisplatin and anti-CD133 CAR-T combination strategy hence showing improved prognosis. All of these results supported the promising treatment strategy to combine cisplatin with anti-CD133 CAR-T cells.
Methods and materials
Patients
The three patients who received FP treatment (1, 5-FU 500 mg/m2 IV continuous infusion over 24 h on day 1–5, cisplatin 75 mg/m2 IV on day 1–5, cycled every 14 days) but did not respond to this therapeutic regimen were selected in this investigation. The pre-and post-treatment specimens were both endoscopic biopsy samples, which were collected before and 14 days after the first treatment. Antigen retrieval was performed by microwave-heating and labeling with primary anti-CD133 antibody (ab19898, 1:100; Santa Cruz Biotechnology Inc., Santa Cruz, CA). All the experiments were approved by the Ethics Committee of Fudan University, and written consents were acquired.
CAR-T cell construction
The humanized anti-CD133 single-chain variable fragment (scFv) was selected against recombinant CD133 via phage display from the Tomlinson I and J libraries, as previous report indicated [16]. The chosen anti-CD133 scFv sequence was synthesized and purified by Chinese Peptide Company (Hangzhou, China), and then cloned into a lentiviral vector containing the CD28 transmembrane region, CD28 cytoplasmic region, and D10 intracellular domains. Packaged with psPAX2 and pMD2g, an anti-CD133 plasmid was co-transfected into 293 T cells with polyethyleneimine (Sigma-Aldrich, St Louis, MO, USA). The cellular supernatant was collected 72-h post-transfection and filtered with 0.45 μm filters.
PBMCs were extracted from the whole blood obtained from healthy volunteers with Ficoll-Paque PLUS (GE Healthcare Life Sciences). The Pan-T Isolation Kit (Miltenyi Biotec, Germany) was used to isolate T cells from PBMCs. The enriched T cells were stimulated with CD3/CD28/CD2-coated microbeads (Miltenyi Biotec, Germany) at a 1:1:1 ratio for 48 h, which were transfected with lentivirus particles collected in the supernatant for 6–8 h and maintained in RPMI-1640 medium (10% fetal bovine serum, 300 IU/ml interleukin-2).
Flow cytometry
The cells cultured in six-well plates or the tumor cells dissociated with gentle MACS (1 × 106) were harvested and suspended with 100 μl phosphate-buffered saline (PBS), and then incubated with labeled primary antibodies at 4 °C for 30 min and analyzed with NovoCyteTM (ACEA Biosciences). The data were analyzed with FlowJo software (FlowJo, LLC, Ashland, OR, USA). The antibodies utilized in this study included anti-human CD133-APC antibody (ab253259, Santa Cruz Biotechnology, clone 293C3), anti-human CCR7-FITC antibody (Biolegend, clone 3D12), anti-human CD62L-PE-Cy7 antibody (Biolegend, clone DREG-56), anti-human CD45RA-BV510 antibody (Biolegend, clone HI100), anti-human CD45RO-PE-Cy7 antibody (Biolegend, clone UCHL1), anti-human CD27-BV650 antibody (Biolegend, clone M-T271), anti-human CD69-APC antibody (Biolegend, clone FN50), and anti-human CD3-BV510 antibody (Biolegend, clone UCHT1).
Cytotoxicity assays
BGC-823, MKN-28 and KATO III cell lines were obtained from the Institute of Biochemistry and Cell Biology (Shanghai, China). A lentivirus vector expressing green fluorescent protein-luciferase (GL) was transduced into the BGC-823 cell line to generate GL-labeled cells. The cytotoxicity activity of anti-CD133 CAR-T cells against GL-labeled BGC-823 cells and cisplatin-exposed CD133+ GL-labeled BGC-823 cells at the indicated effector-to-target (E-T) ratios was evaluated after 24-h incubation. Then, 100 μl D-luciferin (100 μg/ml) was added to the triplicate wells of 96-well plates. The fluorescence was measured with SpectraMax® M5 Multi-Mode Microplate Reader. The percent viability (%) was calculated as experimental signal/maximal signal × 100, and the percent lysis was equal to 100% viability.
Cytokine release assays
The concentration of interferon (IFN)-γ, IL-2, tumor necrosis factor (TNF)-α, and granulocyte–macrophage colony-stimulating factor (GM-CSF) in the supernatants of anti-CD133 CAR-T cells after coculture with normal and CD133 + BGC-823 cells overnight (at an E-T ratio of 1:1) were assayed with enzyme-linked immunosorbent assay (ELISA) kits ordered from eBioscience (R&D Systems, Minneapolis, MN), and all the operation followed the protocol suggested by the manufacturer. A SpectraMax M5 microplate reader (Molecular Devices) was utilized to read the signal at the wavelength of 450 nm.
Western blot
Cellular lysate (30 µg) was separated with 12% sodium dodecyl sulfate–polyacrylamide gel and transferred to polyvinylidene fluoride membranes, which was further incubated with anti-CD133 antibody (ab19898, 1:1000; Santa Cruz Biotechnology Inc., Santa Cruz, CA) at 4 °C overnight. Peroxidase-conjugated secondary antibody (Sigma-Aldrich, 1:1000 dilution) and ECL system (GE Healthcare Life Sciences) were utilized to develop the signal. The relative intensity of CD133 was normalized to β-actin.
Cell-derived xenograft models for CAR-T cells treatment
NOD-SCID-IL2Rg−/− (NSI) mice (6 weeks old) were ordered from Beijing Vitalstar Biotechnology Co., Ltd. BGC-823, KATO III, and MKN-28 tumor cells (1 × 106,150 μl) were subcutaneously injected into the left flanks to establish xenograft models. When the tumor nodules were larger than 50 mm3, the mice were treated with cisplatin (every three days, 2.5 mg/kg, intravenous injection), while 2.5 × 106 anti-CD133 CAR-T cells were further intravenously injected on day 3 and day 12. Tumor volume and weight were measured every 3 days for a total of 21 days. All the operation performed on the animal was approved by the Institutional Animal Care and Use Committee.
Statistics
Student’s t test, one- or two-way ANOVA analysis with a Tukey’s post hoc test was performed to evaluate the statistical significance. The level of significance was set to p < 0.05.
Results
Cisplatin treatment induces CD133 expression both on gastric cancer patients and gastric cancer cell lines
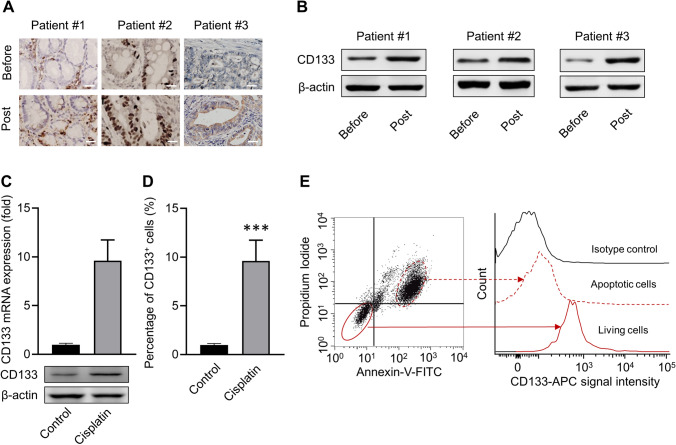
Although the three gastric cancer samples showed heterogeneity in the proportion of CD133 positive stem cell-like cells, cisplatin treatment could up-regulate the relative fraction and expression as detected with immunohistochemistry (Fig. 1a) and Western blot (Fig. 1b). Cisplatin-exposed BGC-823 cells showed up-regulated CD133 expression both in mRNA and protein levels, as detected with PCR and Western blot (Fig. 1c), respectively, and increased CD133 positive stem cell-like cancer cell proportion was assayed by flow cytometry (Fig. 1d). Furthermore, the expression of CD133 in the cisplatin-exposed non-apoptotic BGC-823 cells was significantly higher than that in apoptotic cells (Fig. 1e). These results indicated that cisplatin treatment could increase the proportion of CD133 positive tumor stem cell-like cancer cells, which might escape from cisplatin treatment.
Fig. 1.
Cisplatin treatment induces enrichment of stem cell-like gastric cancer cells. a Immunohistochemical staining for CD133 in three primary gastric cancer (GC) samples before and post-cisplatin treatment. Scale bar = 100 μm. b Detection of CD133 expression in three human GC samples via western blot. c Detection of CD133 expression in cisplatin-exposed human BGC-823 cell line. BGC-823 was treated with 7.5 μM cisplatin (the value of IC50) for 48 h, and then the mRNA and protein levels of CD133 were assessed by western blot. d Percentages of CD133 positive cells post-cisplatin treatment. BGC-823 cells were treated as indicated above and collected for CD133 antibody staining. The percentage of CD133 positive cells were examined by flow cytometry. f CD133 level in apoptotic and living BGC-823 cells post-cisplatin treatment. BGC-823 was treated with 7.5 μM cisplatin (the value of IC50) for 48 h, then stained with Annexin-V-FITC, propidium iodide, and anti-CD133 antibody. Data represent means ± SD. n = 3 samples. ***p < 0.001, versus PBS control group. A two-way ANOVA analysis with Tukey’s post hoc test was performed to evaluate the statistical significance
Generation and characterization of anti-CD133 CAR-T cells
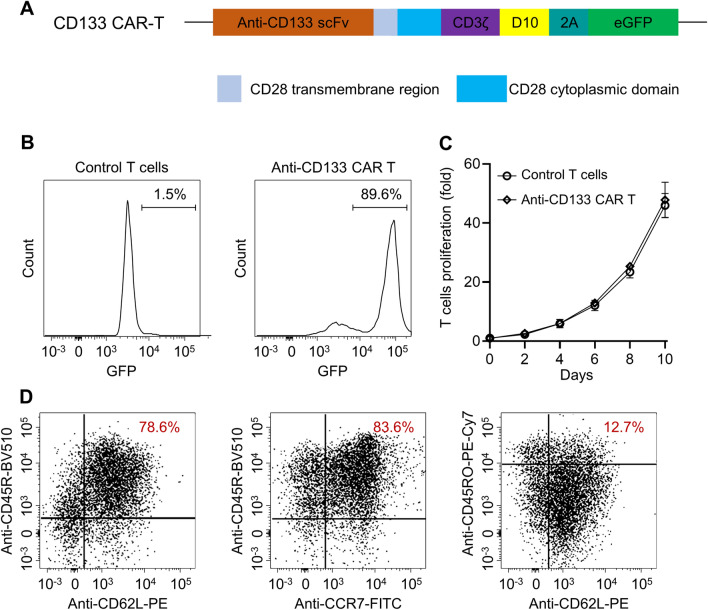
The third-generation CAR-T cells were constructed with the humanized scFv fragment and lentivirus vector composing a CD3ζ intracellular domain and CD28 and D10 costimulatory domains (Fig. 2a). Flow cytometry confirmed the successful transduction (with 90% efficiency) (Fig. 2b), which did not affect the proliferation of the transduced cells, as shown in Fig. 2c. A high ratio of CD45RO+CCR7+CD62Lhigh anti-CD133 CAR-T cells was generated (12.7%, Fig. 2d), and such phenotype was reported to have sustainable anti-tumor potential in vivo.
Fig. 2.
Generation of anti-CD133 CAR-T cells. a The discrete CAR units of anti-CD133 CAR-T cells. b Representative plot of flow cytometry detection of transfected T cells. c The rate of anti-CD133 CAR-T cells proliferation within 10 days. d T cell activation markers, such as CCR7, CD62L, CD45RA, and CD45RO, were detected with flow cytometry
Anti-CD133 CAR-T cells exhibit increased cytotoxicity against GC cell lines
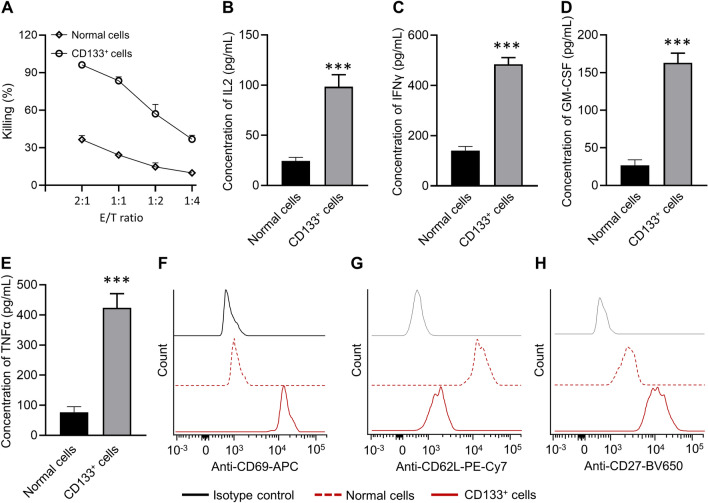
GL-labeled BGC-823 cells were treated with cisplatin for 48 h, and then cisplatin-exposed CD133+ BGC-823 cells were sorted by flow cytometry, which was further incubated with anti-CD133 CAR-T cells at different E-T ratios indicated. The results showed that anti-CD133 CAR-T cells exhibited dramatically increased cytotoxicity (up to 90%) when incubated with cisplatin-exposed CD133+ BGC-823 cells, as compared with the normal BGC-823 cells (Fig. 3a) with up-regulated secretion of IL-2 (Fig. 3b), IFN-γ (Fig. 3c), GM-CSF (Fig. 3d) and TNF-α (Fig. 3e). The above results indicated that anti-CD133 CAR-T cells exhibited the antigen-specific killing effect as expected. Flow cytometry analysis also indicated that anti-CD133 CAR-T cells showed memory phenotype characterized by up-regulated CD69 (Fig. 3f), down-regulated CD62L (Fig. 3g), and up-regulated CD27 expression (Fig. 3h) when incubated with cisplatin-exposed CD133+ BGC-823 cells, as compared with the normal BGC-823 cells at a 1:1 ratio (Fig. 3f–h). It was worth noting that as a homing receptor, CD62L was highly expressed in central memory T lymphocytes but was not found in effector memory T lymphocytes. Collectively, these experiments indicated that anti-CD133 CAR-T cells showed effective memory phenotype and robust cytotoxicity by producing traditional cytotoxic cytokines.
Fig. 3.
Anti-CD133 CAR-T cells exhibit dramatic antitumor efficacy ex vivo. a BGC-823 cells were treated with cisplatin for 48 h, and the CD133+ cells were isolated via flow cytometry and then incubated with anti-CD133 CAR-T cells at the indicated effector-to-target ratios. Cytotoxicity assays evaluated the cytotoxicity of T cells. The concentrations of IL-2 b, IFN-γ c, GM-CSF d, and TNFα e released by anti-CD133 CAR-T cells after coculture with normal and CD133+ BGC-823 cells overnight at an E/T ratio of 1:1 were shown. Data represent means ± SD. n = 3 samples. ***p < 0.001. f, g Canonical T cell markers were detected by flow cytometry at a recommended E: T ratio of 1:1 after coculturing anti-CD133 CAR-T cells with target cells. A two-way ANOVA analysis with Tukey’s post hoc test was performed to evaluate the statistical significance
Cisplatin and anti-CD133 CAR-T cells combination shows pronounced anti-tumor activity
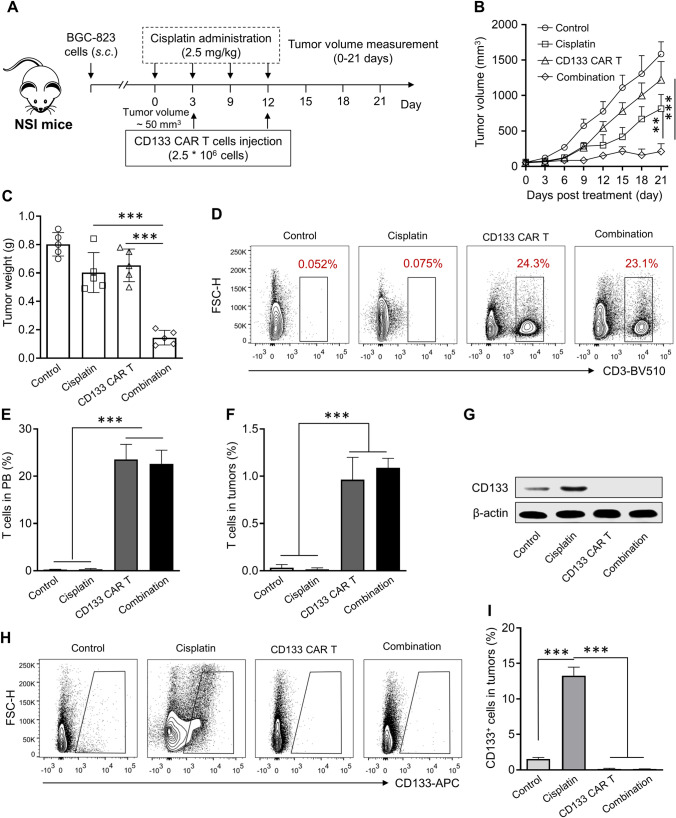
The combined therapeutic effect of cisplatin and anti-CD133 CAR-T cells in vivo was investigated by establishing subcutaneous gastric cancer xenograft models, and the treatment strategy is shown in Fig. 4a. Compared with the control group, the anti-CD133 CAR-T group or cisplatin treatment group showed significant anti-tumor efficacy as indicated by decreased tumor volume (Fig. 4b) and weight (Fig. 4c). It was worth noting that the cisplatin and anti-CD133 CAR-T combination group showed more significant anti-tumor efficacy than the single treatment strategy. There was no significant change in the proportion of anti-CD131 CAR-T cells in the peripheral blood (Fig. 4d and e) or tumor (Fig. 4f) when co-administrated with cisplatin, which indicated that anti-CD133 CAR-T cells could tolerate the dose of cisplatin utilized, and observed beneficial treatment outcome could be attributed to the combination of cisplatin and anti-CD133 CAR-T cells. It was further revealed that cisplatin treatment could up-regulate the expression of CD133 (Fig. 4g) and the percentage of CD133 positive stem cell-like cancer cells in the tumor (Fig. 4h and i), which indicated that CD133 positive cells could tolerant the traditional chemotherapy and became enriched. In terms of CD133 positive cells, both anti-CD131 CAR-T cell treatment and the combination treatment could reduce the relative expression of CD133 (Fig. 4g) and the percentage of CD133 positive cells in the tumor (Fig. 4h and i).
Fig. 4.
Anti-CD133 CAR-T cells and cisplatin efficiently reduce tumor progression in BGC-823 xenograft models. a Schematic representation of the treatment strategy. The subcutaneous BGC-823 tumor model was established by injecting 1*106 BGC-823 cells into the right flank, and the antitumor study started when tumor volume reached about 50 mm3, five mice per group. b Tumor growth curve. Data represent means ± SD. n = 5 mice. **p < 0.01, ***p < 0.001. c Tumor weight was assayed. Mice were sacrificed at the 22-day post the first treatment, and tumor tissues were collected and weighted. Data represent means ± SD. n = 5 samples. ***p < 0.001. Representative FACS plots d and the percentage of T cells in the peripheral blood e in different groups at the end of the antitumor study. Data represent means ± SD. n = 5 samples. ***p < 0.001. f Percentage of T cells in the tumor tissues in different groups at the end of the antitumor study. Data represent means ± SD. n = 5 samples. ***p < 0.001. g The protein level of CD133 in the tumor tissues receiving different treatments. Representative FACS plots h and percentages of CD133 positive cells in tumor tissues i in different groups at the end of the antitumor study. Data represent means ± SD. n = 5 samples. ***p < 0.001
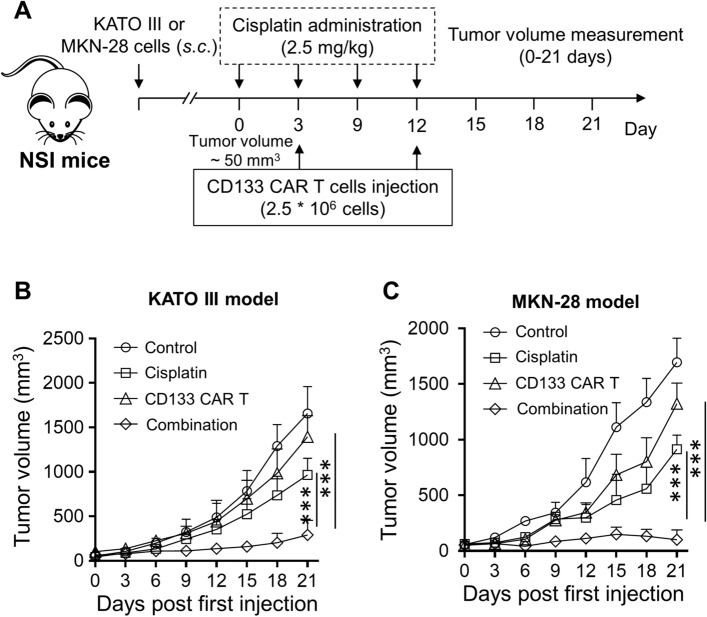
Then, to further validate the generality of results, the KATO III and MKN-28 xenograft models were established, and the same treatment strategy was applied (Fig. 5a). Robust anti-neoplastic capacity was observed when anti-CD133 CAR-T cells intravenously deliver into KATO III (Fig. 5b) and MKN-28 xenograft mice (Fig. 5c), when compared with the control group and cisplatin treatment group. As expected, the beneficial treatment outcome could be achieved by the combination treatment of cisplatin and anti-CD133 CAR-T cells. In summary, our data demonstrated the remarkable anti-tumor efficacy of the cisplatin and anti-CD133 CAR-T cell combination treatment in vivo.
Fig. 5.
Anti-CD133 CAR-T cells and cisplatin efficiently reduce tumor progression in KATO III and MKN-28 xenograft models. a Schematic representation of the time course of the experiment. Tumor volume of KATO III b and MKN-28 c subcutaneously injected mice. Data represent means ± SD. n = 5 mice. ***p < 0.001. A two-way ANOVA analysis with Tukey’s post hoc test was performed to evaluate the statistical significance
Discussion
As the major chemotherapeutic drug utilized in gastric cancer, cisplatin could cross-link the DNA fragments, induce DNA damage, and trigger apoptosis to reduce cancer load and eliminate the rapidly proliferating cells [17, 18]. However, tumor stem cells can escape such elimination, propagate after chemotherapy and subsequently give rise to the neogenetic tumor [19, 20]. Furthermore, in lung adenocarcinoma, cisplatin treatment could significantly promote the enrichment of CD133 positive cells through the Notch signaling pathway, which can further acquire resistance to doxorubicin and paclitaxel treatment by expressing ABCB1 and ABCG2 [21]. These reports indicate that cancer stem cells may mediate the chemoresistance to cisplatin and contribute to the recurrence and metastasis of tumors.
The universality of CD133 as a gastric cancer stem cell biomarker still needs further investigation [14, 22–24]. Recent research has indicated that CD133 positive cancer stem cells contribute to cisplatin resistance in gastric cancer [11]. The cellular source (de novo or re-distribution) of increased CD133 positive stem cell-like cells is not directly investigated in this study. Our results confirm that cisplatin treatment could significantly increase the relative CD133 mRNA expression in BGC-823. Therefore, we speculate that cisplatin treatment could induce de novo CD133 expression in cancer cells or selective enrichment of pre-existing CD133 + cells as previously reported [21, 25]. We have further verified that cisplatin and CAR-T combination treatment could decrease the volume and weight of BGC-823, KATO III and MKN-28 xenograft tumors with reduced infiltration of CD133 positive cancer stem cells. In terms of anti-CD133 CAR-T cells, up-regulated activation markers, such as CD69 and CD27, and abundantly secreted cytokines critical for T cell immunity upon coculture with BGC-823 cells were observed. These data suggest the feasibility of cisplatin and CAR-T cell combination treatment to treat advanced gastric cancer.
In this investigation, anti-CD133 CAR-T cells remain cytotoxic and active when combined with cisplatin. The sequential treatment strategy using cisplatin to shrink tumor mass and CAR-T cells to eliminate the CD133 positive cancer stem cell-like cells has led to the most potent inhibiting or killing effect in this study. Whether concomitantly administrating anti-CD133 CAR-T cells and cisplatin can yield better treatment outcome is still worthy of clinical investigation. A previous study has indicated that cisplatin could up-regulate the expression of NKG2D ligand in gastric cancer cells, which may make the gastric cancer cells more susceptible to NKG2D-CAR-T mediated cytotoxicity [26]. The precise mechanism for cisplatin to induce the up-regulated expression of CD133 needs further analysis.
There are also some limitations that should be indicated. The underlying mechanism leading to the increased proportion of CD133 positive cancer stem cell-like cells needs further detailed analysis. Only the cellular killing effect of cisplatin and CAR-T cell sequential treatment strategy was investigated in this study, and long-term observation is needed to investigate whether the cisplatin-resistant and or CD133-negative tumor cells could lead to invasion and metastasis of gastric cancer.
Our data collectively indicate that cisplatin treatment can enrich CD133 positive tumor stem cell-like cells, which could be attenuated by cisplatin and CAR-T cell sequential treatment strategy to improve the prognosis of gastric cancers.
Conclusion
In summary, our findings indicate that anti-CD133 CAR-T cells can selectively target cisplatin-induced CD133 positive tumor stem cell-like cells both in vitro and in vivo, suggesting a promising cisplatin and CART sequential treatment strategy against gastric cancer.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgment
This work was supported by Shanghai Anticancer Association “Aoxiang” Project (SACA-AX201901).
Data availability
Data will be made available upon reasonable request.
Declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Human or animals rights
All the performance was approved by the Ethics Committees of Fudan University, and the written consents were acquired.
Informed consent
All participants in this study were informed and gave written consent.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yang Han and Bo Sun these authors contributed equally to this work.
Change history
12/9/2021
Electronic Supplementary Material is missing
Contributor Information
Hong Cai, Email: caihong450@hotmail.com.
Yi Xuan, Email: xuanyi0118@126.com.
References
- 1.Avendaño FM, Aguilar MM, Bermudez M, Lizárraga VE, López CC, Ramos PR. Refocusing the use of psychiatric drugs for treatment of gastrointestinal cancers front. Oncol. 2020;10:1452. doi: 10.3389/fonc.2020.01452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reutovich M, Krasko O, Sukonko O. Efficacy of adjuvant systemic chemotherapy combined with radical surgery and hyperthermic intraperitoneal chemotherapy in gastric cancer treatment. Indian J Surg Oncol. 2020;11:337–343. doi: 10.1007/s13193-020-01102-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang D, Duan H, Huang H, Tong X, Han Y, Ru G, Qu L, Shou C, Zhao Z (2016) Cisplatin resistance in gastric cancer cells is associated with HER2 upregulation-induced epithelial-mesenchymal transition. Sci. Rep10.1038/srep20502 [DOI] [PMC free article] [PubMed]
- 4.Courtois S, Haykal M, Bodineau C, et al. Autophagy induced by Helicobacter pylori infection is necessary for gastric cancer stem cell emergence. Gastric Cancer. 2020 doi: 10.1007/s10120-020-01118-9. [DOI] [PubMed] [Google Scholar]
- 5.Sun LF, Yang K, Wang YG, et al. The role of HER2 in self-renewal, invasion, and tumorigenicity of gastric cancer stem cells. Front Oncol. 2020;10:1608. doi: 10.3389/fonc.2020.01608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xiao S, Zhou L. Gastric stem cells: physiological and pathological perspectives. Front Cell Dev Biol. 2020;8:571536. doi: 10.3389/fcell.2020.571536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu L, Yin S, Brobbey C, Gan W. Ubiquitination in cancer stem cell: roles and targeted cancer therapy. STEMedicine. 2020;1:e37. doi: 10.37175/stemedicine.v1i3.37. [DOI] [Google Scholar]
- 8.Dzobo K, Ganz C, Thomford NE, Senthebane DA. Cancer Stem cell markers in relation to patient survival outcomes: lessons for integrative diagnostics and next-generation anticancer drug development. OMICS. 2020 doi: 10.1089/omi.2020.0185. [DOI] [PubMed] [Google Scholar]
- 9.Kesh K, Garrido VT, Dosch A, et al. Stroma secreted IL6 selects for "stem-like" population and alters pancreatic tumor microenvironment by reprogramming metabolic pathways. Cell Death Dis. 2020;11:967. doi: 10.1038/s41419-020-03168-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee HY, Hong IS (2020) Targeting Liver Cancer Stem Cells: An Alternative Therapeutic Approach for Liver Cancer. Cancers (Basel). 1210.3390/cancers12102746 [DOI] [PMC free article] [PubMed]
- 11.Lu R, Zhao G, Yang Y, Jiang Z, Cai J, Hu H. Inhibition of CD133 overcomes cisplatin resistance through inhibiting PI3K/AKT/mTOR signaling pathway and autophagy in CD133-positive gastric cancer cells. Technol Cancer Res Treat. 2019;18:1533033819864311. doi: 10.1177/1533033819864311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yiming L, Yunshan G, Bo M, et al. CD133 overexpression correlates with clinicopathological features of gastric cancer patients and its impact on survival: a systematic review and meta-analysis. Oncotarget. 2015;6:42019–42027. doi: 10.18632/oncotarget.5714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang Y, Li L, Liu W, Tang T, Chen L. The progress of CAR-T therapy in cancer and beyond. STEMedicine. 2020;1:e47. doi: 10.37175/stemedicine.v1i3.47. [DOI] [Google Scholar]
- 14.Liu WT, Liu WB, Gao M, Zhang YY, Gu KS. Expression of ALDH1A1 and CD133 is associated with the prognosis and effect of different chemotherapeutic regimens in gastric cancer. Oncol Lett. 2019;18:4573–4582. doi: 10.3892/ol.2019.10798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wen L, Chen XZ, Yang K, Chen ZX, Zhang B, Chen JP, Zhou ZG, Mo XM, Hu JK. Prognostic value of cancer stem cell marker CD133 expression in gastric cancer: a systematic review. PLoS ONE. 2013;8:e59154. doi: 10.1371/journal.pone.0059154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xia J, Zhang Y, Qian J, Zhu X, Zhang Y, Zhang J, Zhao G. Isolation, identification and expression of specific human CD133 antibodies. Sci Rep. 2013;3:3320. doi: 10.1038/srep03320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dasari S, Tchounwou PB. Cisplatin in cancer therapy: molecular mechanisms of action. Eur J Pharmacol. 2014;740:364–378. doi: 10.1016/j.ejphar.2014.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rancoule C, Guy JB, Vallard A, Ben Mrad M, Rehailia A, Magne N. 50th anniversary of cisplatin. Bull Cancer. 2017;104:167–176. doi: 10.1016/j.bulcan.2016.11.011. [DOI] [PubMed] [Google Scholar]
- 19.Galluzzi L, Senovilla L, Vitale I, Michels J, Martins I, Kepp O, Castedo M, Kroemer G. Molecular mechanisms of cisplatin resistance. Oncogene. 2012;31:1869–1883. doi: 10.1038/onc.2011.384. [DOI] [PubMed] [Google Scholar]
- 20.Moitra K. Overcoming multidrug resistance in cancer stem cells. Biomed Res Int. 2015;2015:635745. doi: 10.1155/2015/635745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu YP, Yang CJ, Huang MS, et al. Cisplatin selects for multidrug-resistant CD133+ cells in lung adenocarcinoma by activating Notch signaling. Cancer Res. 2013;73:406–416. doi: 10.1158/0008-5472.CAN-12-1733. [DOI] [PubMed] [Google Scholar]
- 22.Glumac PM, LeBeau AM. The role of CD133 in cancer: a concise review. Clin Transl Med. 2018;7:18. doi: 10.1186/s40169-018-0198-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hashimoto K, Aoyagi K, Isobe T, Kouhuji K, Shirouzu K. Expression of CD133 in the cytoplasm is associated with cancer progression and poor prognosis in gastric cancer. Gastric Cancer. 2014;17:97–106. doi: 10.1007/s10120-013-0255-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rocco A, Liguori E, Pirozzi G, et al. CD133 and CD44 cell surface markers do not identify cancer stem cells in primary human gastric tumors. J Cell Physiol. 2012;227:2686–2693. doi: 10.1002/jcp.23013. [DOI] [PubMed] [Google Scholar]
- 25.Thakur B, Ray P. Cisplatin triggers cancer stem cell enrichment in platinum-resistant cells through NF-kappaB-TNFalpha-PIK3CA loop. J Exp Clin Cancer Res. 2017;36:164. doi: 10.1186/s13046-017-0636-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tao K, He M, Tao F, Xu G, Ye M, Zheng Y, Li Y. Development of NKG2D-based chimeric antigen receptor-T cells for gastric cancer treatment. Cancer Chemother Pharmacol. 2018;82:815–827. doi: 10.1007/s00280-018-3670-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available upon reasonable request.