Abstract
Purpose
The most frequent mutation in advanced non-small–cell lung cancer (NSCLC), Kirsten rat-sarcoma viral oncogene (KRAS) is found in 20–25% of these patients’ tumors. While phase III trials on therapies targeting KRAS, especially KRASG12C, are ongoing, the clinical efficacy of anti-programmed death protein-1 (PD-1) or its ligand (PD-L1) against KRAS-mutant NSCLCs remains a topic of debate.
Methods
This meta-analysis examined randomized-trial data comparing first- or second-line anti-PD-(L)1 with or without chemotherapy vs. chemotherapy alone for advanced KRAS-mutant NSCLCs. Outcome measures included overall survival (OS) and progression-free survival (PFS). Analyses were computed using the Cochrane method of collaboration for meta-analyses, with Review Manager software (RevMan version 5.3; Oxford, UK).
Results
We analyzed 3 first-line trials (IMpower-150, Keynote-189 and Keynote-042) and 3 second-line trials (Oak, Poplar and CheckMate-057) that included 1313 NSCLCs (386 KRAS-mutant and 927 KRAS wild-type tumors). For KRAS-mutant NSCLCs, anti-PD-(L)1 with or without chemotherapy was significantly associated (hazard ratio [95% confidence interval]) with prolonged OS (0.59 [0.49–0.72]; p < 0.00001) and PFS (0.58 [0.43–0.78]; p = 0.0003) compared to chemotherapy alone. OS benefited in both first- and second-line trials. OS for patients with KRAS-mutant NSCLCs was significantly longer than that for those with KRAS wild-type tumors (p = 0.001).
Conclusions
Anti-PD-(L)1 with or without chemotherapy seemed to achieve longer OS and PFS than chemotherapy alone for patients with KRAS-mutant and wild-type KRAS advanced NSCLCs, with an even greater OS benefit for the former.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00262-021-03031-1.
Keywords: KRAS, Non-small-cell lung cancer, Meta-analysis, Immunotherapy
Introduction
The last few years have been marked by major progress in better identifying subgroups of advanced non-small–cell lung cancers (NSCLCs) [1]. KRAS (Kirsten rat sarcoma viral antigen) mutations are the most frequent, representing 25–30% of the adenocarcinoma group in Western countries [2] and 10–15% of patients with adenocarcinomas in Asia. Specific targeted therapies are still available only for participants in clinical trials. KRAS mutations, mostly single-point mutations in exon 2 codons 12 and 13, and exon 3 codon 61, lead to constitutive activation of KRAS proteins [3]. The most frequent point mutation—G12C—accounts for more than 30%, followed by G12V and G12D. KRAS-mutation frequencies vary according to patient ethnicity and sex; they are more common in current or former smokers than non-smokers [4]. KRAS mutations are mutually exclusive with epidermal growth factor receptor (EGFR) mutations, other identified oncogene mutations or translocations, suggesting that KRAS mutations define a distinct NSCLC subset.
The prognostic value of KRAS mutation in patients with advanced NSCLCs has not yet been clearly elucidated. A meta-analysis was conducted on 43 studies that had included 8339 (range, 31–986) patients participating in randomized trials and 2826 (range, 97–1036) patients enrolled in observational studies [5]. In this meta-analysis, overall survival (OS) did not differ significantly between patients with a KRAS mutation and those without. In contrast, OS was significantly shorter in observational studies with a hazard ratio (HR) of 1.71 (95% confidence interval (CI): 1.07–2.84), but heterogeneity among the studies was high. The prognosis of patients with tumors harboring a KRAS mutation seemed to vary according to smoker status and associated co-mutations (tumor protein-53 (TP53) or serine/threonine kinase-11 (STK11)). Notably, that meta-analysis did not find significant progression-free survival (PFS) differences between patients with KRAS-mutant tumors and those without, but none of the trials included anti-programmed death protein-1 (PD-1) or its ligand (PD-L1) therapies.
In a matter of several years, anti-PD-(L)1 immunotherapy has become the standard second-line treatment of advanced NSCLCs and, more recently, first-line therapy for patients without EGFR mutations or anaplastic lymphoma kinase (ALK) or proto-oncogene tyrosine-protein kinase (ROS) translocation [1). Pembrolizumab monotherapy is indicated for patients whose tumor cells express > 50% PD-L1 in the absence of contradiction; pembrolizumab combined with platin-based chemotherapy is indicated regardless of the PD-L1–expression level. Few data are available on efficacy of first-line or second-line anti-PD-(L)1 immunotherapy for the subgroup of KRAS-mutant NSCLCs [6, 7]. A meta-analysis of 3 trials on second-line immunotherapy vs. chemotherapy, published in 2017, did not find efficacy differences assessed as OS for patients with a KRAS mutation or without [8].
The objective of this meta-analysis was to update efficacy evaluation of first-line or second-line anti-PD-(L)1 in patients with advanced KRAS-mutant NSCLCs.
Materials and methods
This meta-analysis, based on published data, assessed PFS and OS of patients whose NSCLCs harbored a KRAS mutation treated with anti-PD-(L)1 with chemotherapy or without vs. chemotherapy alone.
Research strategy
PubMed and Cochrane databases were screened on 15 January 2021. We also manually searched the abstracts accepted for AACR, ASCO, ESMO, WLCC and ELCC congresses until January 2021. The search terms used were: “immune checkpoint inhibitor or immunotherapy”, “nivolumab, pembrolizumab, cemiplimab, sintilimab, camrelizumab, atezolizumab, avelumab or durvalumab”, “advanced or metastatic”, non-small–cell lung cancer or NSCLC” “KRAS” and “randomized–controlled trial”. Only phase II or III trials on anti-PD-(L)1 with or without chemotherapy compared to chemotherapy alone were retained (supplementary Methods).
Data extraction
All study data were extracted independently by two readers (TL and BD) using a predefined protocol; divergences were resolved by discussion with a third reader (CC). The following information was collected: each patient’s characteristics (age, sex, descriptions and doses of treatments administered, tumor histology), tumor stage and KRAS-mutation status.
Efficacy criteria analyzed were OS and PFS for patients with a KRAS mutation or without. The principal analysis concerned all treatment lines combined; secondary analyses separately addressed first-line and second-line studies.
Statistical analyses
The analyses were conducted according to the Cochrane method for meta-analyses and computed with Review Manager software (RevMan version 5.3; Oxford, U.K.). Statistical heterogeneity was assessed with χ2 tests and I2 statistics, with p < 0.10 in a χ2 test defining the presence of heterogeneity. The I2 statistic assesses heterogeneity between studies; a value of 30–60% indicates heterogeneity is moderate. A fixed-effect model was used to calculate the cumulative risk ratio (HR), when between-study heterogeneity was weak; when heterogeneity was strong, a randomized model was used. Meta-analysis results for OS and PFS are reported as HR [95% confidence interval (CI)]. All tests were bilateral, with p < 0.05 defining significance. A Begg’s funnel plot was used to analyze heterogeneity among the populations studied.
Results
Database screening identified 400 references and the manual search of congress abstracts added 34 more (Fig. 1). Eight studies meeting the inclusion criteria were selected for this analysis but the IMpower-130 and -132 trials were finally excluded [9, 10] because their data for the subgroup of patients with KRAS-mutant NSCLCs were not published.
Fig. 1.
PRISMA flow diagram
Thus, this meta-analysis included 6 studies (7 experimental arms), 5 phase II and 1 phase II randomized trials; 3 each concerned first-line [11–13] or second-line therapy [14–16] (Table 1). Those 6 studies had enrolled 1313 advanced non-squamous NSCLCs: 927 KRAS wild-type and 386 KRAS-mutant. Participants were mostly men (60%), smokers or ex-smokers (80%), whose median age was 64 years. The Begg’s funnel plot (supplemental Fig. S1) indicated the absence of heterogeneity among the populations included.
Table 1.
Characteristics of the studies included in the meta-analysis
| Study | Experimental arm | Patients, n |
Median age, yr |
Males (%) |
nSq (%) |
PS 0/1 (%) |
Smokers (%) |
KRAS-mutant NSCLCs, n (%) |
Line | Primary outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| Keynote-042 | Pembrolizumab | 1,274 | 63 | 71% | 65 | 100 | 78 | 69 (23) | 1st | OS |
| Keynote-189 |
Pembrolizumab + ChT |
616 | 64 | 62% | 100 | 100 | 88 | 89 (31) | 1st | OS, PFS |
| IMpower-150 | Atezolizumab + ChT + bevacizumab | 1,200 | 63 | 60% | 100 | 100 | 79 | 80 (38) | 1st | OS, PFS |
| CheckMate-057 | Nivolumab | 582 | 62 | 55% | 100 | 100 | 79 | 62 (33) | 2nd | OS |
| Poplar | Atezolizumab | 287 | 62 | 60% | 66 | 100 | 80 | 27 (37) | 2nd | OS |
| Oak | Atezolizumab | 850 | 63 | 61% | 74 | 100 | 82 | 59 (23) | 2nd | OS |
nSq non-squamous cell; PS Eastern Cooperative Oncology Group performance status; ChT chemotherapy, OS overall survival, PFS progression-free survival
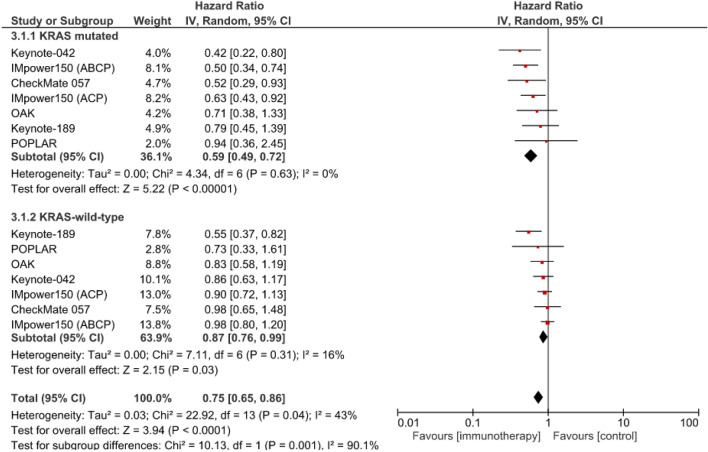
OS was significantly longer for patients with KRAS-mutant NSCLCs who had received anti-PD-(L)1 with chemotherapy or without vs. chemotherapy alone (HR 0.59 [95% CI: 0.49–0.72]; p < 0.00001); the significant benefit was also found for patients with KRAS wild-type tumors (HR 0.87: [95% CI: 0.76–0.99]; p < 0.03) (Fig. 2). The efficacy of anti-PD-(L)1 with chemotherapy or without vs. chemotherapy alone seemed to be significantly higher for patients with KRAS-mutant NSCLCs than those with wild-type KRAS (χ2 = 10.13; p = 0.001).
Fig. 2.
Meta-analysis of overall survival of patients with advanced NSCLCs treated with first- or second-line anti-PD-(L)1 with chemotherapy or without vs. chemotherapy alone (control). ABCP atezolizumab, bevacizumab, carboplatin, paclitaxel), ACP: atezolizumab, bevacizumab, carboplatin, paclitaxel
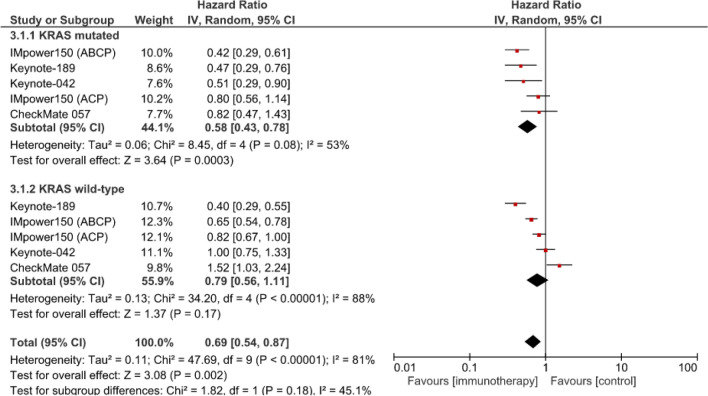
According to the meta-analysis of 4 studies (5 experimental arms), PFS was also significantly longer for the patients with KRAS-mutant NSCLCs treated with anti-PD-(L)1 with chemotherapy or without vs. chemotherapy alone (HR 0.58 [95% CI: 0.43–0.78]; p < 0.0003) but not for those whose tumors carried wild-type KRAS (HR 0.79 [95% CI: 0.56–1.11]; p = 0.17) (Fig. 3). No significant PFS difference was found between patients with KRAS-mutant or wild-type KRAS NSCLCs (χ2 = 1.82; p = 0.18).
Fig. 3.
Meta-analysis of progression-free survival of patients with advanced NSCLCs treated with first- or second-line anti-PD-(L)1 with chemotherapy or without vs. chemotherapy alone (control). ABCP atezolizumab, bevacizumab, carboplatin, paclitaxel), ACP: atezolizumab, bevacizumab, carboplatin, paclitaxel
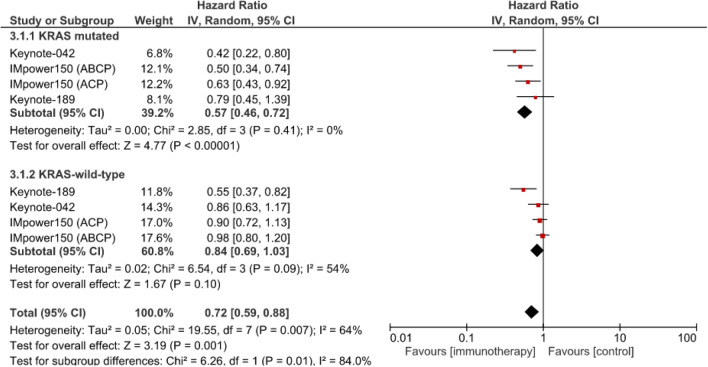
The meta-analysis of 3 studies on first-line anti-PD-(L)1 with chemotherapy or without vs. chemotherapy alone revealed a significant OS benefit for patients with KRAS-mutant NSCLCs (HR 0.57 [95% CI: 0.46–0.72]; p < 0.00001) but not for those with wild-type KRAS (HR 0.84 [95% CI 0.69–1,03]; p = 0.10) (Fig. 4). The efficacy of first-line anti-PD-(L)1 with chemotherapy or without was significantly better for patients with KRAS-mutant NSCLCs than those with wild-type KRAS-expressing tumors (χ2 = 6.26; p = 0.01).
Fig. 4.
Meta-analysis of overall survival of patients with advanced NSCLCs treated with first-line anti-PD-(L)1 with chemotherapy or without vs. chemotherapy alone (control). ABCP atezolizumab, bevacizumab, carboplatin, paclitaxel), ACP: atezolizumab, bevacizumab, carboplatin, paclitaxel
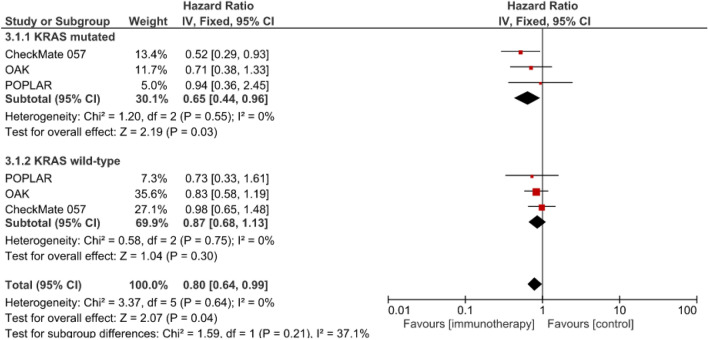
The meta-analysis of 3 studies on second-line anti-PD-(L)1 compared to chemotherapy (HR 0.65, 95% CI: 0.44–0.96; p = 0.03) showed a statistically significant benefit for patients with KRAS-mutant NSCLCs but not for those with wild-type KRAS-harboring tumors (HR 0.87 [95% CI: 0.68–1.13]; p = 0.30) (Fig. 5). For second-line therapy, no significant difference was found between KRAS-mutant vs wild-type subgroups (χ2 = 1.59; p = 0.21).
Fig. 5.
Meta-analysis of overall survival of patients with advanced NSCLCs treated with second-line anti-PD-(L)1 with chemotherapy or without vs. chemotherapy alone (control). ABCP atezolizumab, bevacizumab, carboplatin, paclitaxel), ACP: atezolizumab, bevacizumab, carboplatin, paclitaxel
Discussion
This meta-analysis showed that, compared to chemotherapy alone, combination therapy with anti-PD-(L)1 with chemotherapy or without obtained a significantly greater benefit for patients with KRAS-mutant NSCLCs than those with wild-type KRAS-expressing tumors. That benefit can be explained by higher PD-L1 expression by KRAS-mutant NSCLCs than wild-type KRAS-harboring tumors or a higher mutation load.
A recent international, retrospective, observational study assessing the contribution of anti-PD-(L)1 as treatment of advanced NSCLCs enrolled 551 patients from 24 centers and 10 countries [17]; 214 (38.9%) of them had known PD-L1 status. Among the 80 patients with KRAS-mutant NSCLCs, 32.5% had > 50% PDL1 expression and the overall response rate (ORR) was 26%, much better than for the other oncogene drivers. The included patients with KRAS-mutant NSCLCs were more frequently current or ex-smokers, which also seemed to be a factor predictive of response to anti-PD-(L)1.
A multicenter, retrospective study [18] examined the impact of oncogene-driver subtype, PD-L1 status and smoker status on 189 oncogene-positive patients. PD-L1 positivity (≥ 1%) was higher for patients with KRAS-mutant NSCLCs (p = 0.031), smokers (p = 0.006) and non-Asian ethnicity (p = 0.002). Their multivariable analysis retained only smokier status (p = 0.008) as a significant predictor when PD-L1 expression ≥ 1% was included in the model. But, when ≥ 50% PD-L1 expression was entered into the model, smoker status (p = 0.001) and PD-L1 status (p = 0.028) were independent predictors of OS. Those findings agree with the emerging notion that immunotherapy is more beneficial against tumors carrying a high mutational burden, i.e., because TP53 and KRAS mutations are linked to smoking, more somatic mutations and neoantigen expression [19]. In contrast, a retrospective study [20] assessing the routine efficacy of first-line-or-more anti-PD-(L)1 immunotherapy in 282 advanced-NSCLC patients, including 162 (57.4%) KRAS-mutant tumors, 27 (9.6%) with other mutations and 93 (33%) harboring wild-type KRAS, no significant ORR, PFS or OS difference was found between KRAS-mutant and other NSCLC subgroups.
Our meta-analysis did not take into consideration the KRAS-mutation subtype. The impact of KRAS-mutation subtype and concurrent pathogenic mutations on NSCLCs are starting to be elucidated, even though the findings are contradictory. According to the retrospective analysis of 186 patients with KRAS-mutant NSCLCs—35% G12C and 17% G12D–concomitant pathogenic TP53, STK11, Kelch-like ECH-associated protein 1 (KEAP1) and/or phosphatidylinositol-4, 5-bisphosphate 3-kinase catalytic subunit-alpha (PIK3CA) mutations were identified, respectively, in 39%, 12%, 8% and 4% of the subjects. Multivariable analysis retained KRASG12D mutations as being significantly associated with poor OS [HR 2.43 [95% CI 1.15–5.16]; p = 0.021), as were STK11 co-mutations (HR 2.95 [95% CI 1.27–6.88]; p = 0.012). KRASG12C mutations were also significantly associated with positive but low (1–49%) PD-L1 expression [odds ratio 4.94 [95% CI 1.07–22.85]; p = 0.041), and TP53 co-mutations with ≥ 50% PD-L1 expression (odds ratio 6.36 [95% CI 1.84–22.02]; p = 0.004) [21]. More recently, among 770 patients with recurrent or metastatic KRAS-mutant NSCLCs identified through routine next-generation sequencing, 46% had KRASG12C mutations [22]. The tumor mutation burden (median 8.8 vs. 7 mutations/Mb; p = 0.006) and median PD-L1 expression (5% vs. 1%) were higher for patients harboring the KRASG12C mutation. STK11 and KEAP1 co-mutation patterns were similar for patients whose NSCLCs carried the KRASG12C or other KRAS non-G12C mutations. Median OS durations post-diagnosis according to KRASG12C or other-KRAS mutation status did not differ significantly: 13.4 and 13.1 months, respectively. For patients with NSCLCs expressing ≥ 50% PD-L1, ORRs to t immune-checkpoint inhibitor monotherapy also did not differ significantly for those with KRASG12C mutations vs. other non-KRASG12C mutations (40% vs. 58%, respectively; p = 0.07). Among the studies included in this meta-analysis, only 2 specified the type of KRAS mutation [11, 12], which precluded drawing any definitive conclusion.
This meta-analysis has several limitations: it was conducted on published data and not individual patient’s information, thereby restricting its internal validity; no distinction was made between anti-PD1 and anti-PD-L1, which might have had minor efficacy differences; and, finally, it included combination therapies with chemotherapy. Nevertheless, the results of this meta-analysis confirmed that anti-PD-(L)1 with chemotherapy or without was better than chemotherapy alone, with greater OS benefit for the subgroup with KRAS-mutant NSCLCs than wild-type KRAS tumors. Thus, anti-PD-(L)1 with chemotherapy or without constitutes a reference therapy for the evaluation of targeted therapies being developed for patients with KRAS-mutant NSCLCs, especially the subgroup with the KRASG12C mutation [23].
Supplementary Information
Below is the link to the electronic supplementary material.
Funding
None.
Declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Planchard D, Popat S, Kerr K, Novello S, Smit EF, Faivre-Finn C, et al. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol Off J Eur Soc Med Oncol. 2018;29(Suppl 4):192–237. doi: 10.1093/annonc/mdy275. [DOI] [PubMed] [Google Scholar]
- 2.El Osta B, Behera M, Kim S, Berry LD, Sica G, Pillai RN, et al. Characteristics and outcomes of patients with metastatic kras-mutant lung adenocarcinomas: the lung cancer mutation consortium experience. J Thorac Oncol Off Publ Int Assoc Study Lung Cancer. 2019;14(5):876–889. doi: 10.1016/j.jtho.2019.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jordan EJ, Kim HR, Arcila ME, Barron D, Chakravarty D, Gao J, et al. Prospective comprehensive molecular characterization of lung adenocarcinomas for efficient patient matching to approved and emerging therapies. Cancer Discov juin. 2017;7(6):596–609. doi: 10.1158/2159-8290.CD-16-1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dogan S, Shen R, Ang DC, Johnson ML, D’Angelo SP, Paik PK, et al. Molecular epidemiology of EGFR and KRAS mutations in 3,026 lung adenocarcinomas: higher susceptibility of women to smoking-related KRAS-mutant cancers. Clin Cancer Res Off J Am Assoc Cancer Res. 2012;18(22):6169–6177. doi: 10.1158/1078-0432.CCR-11-3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goulding RE, Chenoweth M, Carter GC, Boye ME, Sheffield KM, John WJ, et al. KRAS mutation as a prognostic factor and predictive factor in advanced/metastatic non-small cell lung cancer: a systematic literature review and meta-analysis. Cancer Treat Res Commun. 2020;24:100200. doi: 10.1016/j.ctarc.2020.100200. [DOI] [PubMed] [Google Scholar]
- 6.Agyeman A, Vallejo JJ, Myers A, Blumenthal GM. (2018). Meta-analysis exploring the effect of oncogenic driver mutations on outcome of metastatic non-small cell lung cancer (mNSCLC) patients (pts) treated with immune checkpoint inhibitors (ICI) or docetaxel (doc). J Clin Oncol. 36(15_suppl): 9029‑9029.
- 7.Torralvo J, Friedlaender A, Achard V, Addeo A. The activity of immune checkpoint inhibition in kras mutated non-small cell lung cancer: a single centre experience. Cancer Genomics Proteomics. 2019;16(6):577–582. doi: 10.21873/cgp.20160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee CK, Man J, Lord S, Cooper W, Links M, Gebski V, et al. Clinical and molecular characteristics associated with survival among patients treated with checkpoint inhibitors for advanced non-small cell lung carcinoma: a systematic review and meta-analysis. JAMA Oncol. 2018;4(2):210–216. doi: 10.1001/jamaoncol.2017.4427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nishio M, Barlesi F, West H, Ball S, Bordoni R, Cobo M, et al. (2020). Atezolizumab Plus Chemotherapy for First-Line Treatment of Non-Squamous Non-Small Cell Lung Cancer: Results From the Randomized Phase III IMpower132 Trial. J Thorac Oncol Off Publ Int Assoc Study Lung Cancer. [DOI] [PubMed]
- 10.West H, McCleod M, Hussein M, Morabito A, Rittmeyer A, Conter HJ, et al. Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol juill. 2019;20(7):924–937. doi: 10.1016/S1470-2045(19)30167-6. [DOI] [PubMed] [Google Scholar]
- 11.Herbst RS, Lopes G, Kowalski DM, Kasahara K, Wu Y-L, Castro GD, et al. LBA4 Association of KRAS mutational status with response to pembrolizumab monotherapy given as first-line therapy for PD-L1-positive advanced non-squamous NSCLC in Keynote-042. Ann Oncol. 2019;30:xi63–xi64. doi: 10.1093/annonc/mdz453.001. [DOI] [Google Scholar]
- 12.Gadgeel S, Rodriguez-Abreu D, Felip E, Esteban E, Speranza G, Reck M, et al. LBA5–KRAS mutational status and efficacy in KEYNOTE-189: Pembrolizumab (pembro) plus chemotherapy (chemo) vs placebo plus chemo as first-line therapy for metastatic non-squamous NSCLC. Ann Oncol. 2019;30:xi64–xi65. doi: 10.1093/annonc/mdz453.002. [DOI] [Google Scholar]
- 13.Socinski MA, Jotte RM, Cappuzzo F, Orlandi F, Stroyakovskiy D, Nogami N, et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med. 2018;378(24):2288–2301. doi: 10.1056/NEJMoa1716948. [DOI] [PubMed] [Google Scholar]
- 14.Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373(17):1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fehrenbacher L, Spira A, Ballinger M, Kowanetz M, Vansteenkiste J, Mazieres J, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet Lond Engl. 2016;387(10030):1837–1846. doi: 10.1016/S0140-6736(16)00587-0. [DOI] [PubMed] [Google Scholar]
- 16.Rittmeyer A, Barlesi F, Waterkamp D, Park K, Ciardiello F, von Pawel J, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet Lond Engl. 2017;389(10066):255–265. doi: 10.1016/S0140-6736(16)32517-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mazieres J, Drilon A, Lusque A, Mhanna L, Cortot AB, Mezquita L, et al. Immune checkpoint inhibitors for patients with advanced lung cancer and oncogenic driver alterations: results from the IMMUNOTARGET registry. Ann Oncol Off J Eur Soc Med Oncol. 2019;30(8):1321–1328. doi: 10.1093/annonc/mdz167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ng TL, Liu Y, Dimou A, Patil T, Aisner DL, Dong Z, et al. Predictive value of oncogenic driver subtype, programmed death-1 ligand (PD-L1) score, and smoking status on the efficacy of PD-1/PD-L1 inhibitors in patients with oncogene-driven non-small cell lung cancer. Cancer. 2019;125(7):1038–1049. doi: 10.1002/cncr.31871. [DOI] [PubMed] [Google Scholar]
- 19.Scheel AH, Ansén S, Schultheis AM, Scheffler M, Fischer RN, Michels S, et al. PD-L1 expression in non-small cell lung cancer: correlations with genetic alterations. Oncoimmunology. 2016;5(5):e1131379. doi: 10.1080/2162402X.2015.1131379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jeanson A, Tomasini P, Souquet-Bressand M, Brandone N, Boucekine M, Grangeon M, et al. Efficacy of Immune checkpoint inhibitors in kras-mutant non-small cell lung cancer (NSCLC) J Thorac Oncol Off Publ Int Assoc Study Lung Cancer. 2019;14(6):1095–1101. doi: 10.1016/j.jtho.2019.01.011. [DOI] [PubMed] [Google Scholar]
- 21.Aredo JV, Padda SK, Kunder CA, Han SS, Neal JW, Shrager JB, et al. Impact of KRAS mutation subtype and concurrent pathogenic mutations on non-small cell lung cancer outcomes. Lung Cancer Amst Neth juill. 2019;133:144–150. doi: 10.1016/j.lungcan.2019.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arbour KC, Rizvi H, Plodkowski AJ, Hellmann MD, Knezevic A, Heller G, et al. Treatment Outcomes and Clinical Characteristics of Patients with KRAS-G12C-Mutant Non-Small Cell Lung Cancer. Clin Cancer Res Off J Am Assoc Cancer Res. 8 févr; [DOI] [PMC free article] [PubMed]
- 23.Hong DS, Fakih MG, Strickler JH, Desai J, Durm GA, Shapiro GI, et al. KRASG12C inhibition with sotorasib in advanced solid tumors. N Engl J Med. 2020;383(13):1207–1217. doi: 10.1056/NEJMoa1917239. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.