Abstract
Previously we reported that administration of IgG could inhibit tumor progression in mouse models. At the same time, we also found that some IgGs have glycosylation modifications on their Fab fragments, which may have different biological functions than non-glycosylated IgG. In this study, we employed mouse tumor models to explore the roles of two different forms of IgG, i.e. Fab-glycosylated and Fab-non-glycosylated IgG, in tumor progression. The two types of IgGs were separated with ConA absorption which could react with glycan on the Fab arm but could not access glycan on the Fc fragment. In addition, we performed cytokine array, ELISA, western blotting, immunocytochemistry and other techniques to investigate the possible mechanisms of the actions of Fab-glycosylated IgG in the models. We found that Fab-glycosylated IgG, unlike Fab-non-glycosylated IgG, did not inhibit tumor growth and metastasis in the model. On the contrary, Fab-glycosylated IgG may bind to antigen-bound IgG molecules and macrophages through the glycosidic chain on the Fab fragment to affect antigen–antibody binding and macrophage polarization, which are likely to help tumor cells to evade the immune surveillance. A new mechanism of immune evasion with Fab-glycosylated IgG playing a significant role was proposed.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00262-020-02809-z.
Keywords: Immunoglobulin G, Glycosylation, Mouse tumor model, Macrophage, Immune evasion
Introduction
Immunoglobulin G (IgG) molecule is constituted by two heavy chains (H) and two light chains (L) linked by disulfide bonds and non-covalent bonds [1]. IgG molecules have two regions based on their chemical and biological properties: antigen-binding region (Fab) and crystallizable region (Fc). The Fab or F(ab′)2 fragment could be obtained by digesting IgG with papain or pepsin. The Fc fragment comprises a ligand interaction site that initiates subsequent immune responses [2]. IgG is a glycoprotein with conserved glycosylation sites in its Fc fragment, and some have glycosidic chains in its Fab region [3, 4]. Margni et al. used Concanavalin A (ConA) to extract a specific IgG from maternal blood of pregnant women, that had additional mannose glycoside chains attached to the Fab fragment and named it asymmetric IgG as the two Fab arms were not symmetrically shaped. The Fab-non-glycosylated IgG was named symmetric IgG [5–8]. Recently, we found that although there was an N-glycoside oligosaccharide chain at the Asn 297 residue of the Fc fragment when the IgG was in a natural folded state, the N-glycoside oligosaccharide chain was located inside the molecule and could not be accessed by the ConA affinity chromatography column. Therefore, one could use ConA to extract Fab-glycosylated IgG without being affected by the glycosylation on the Fc fragment. In addition, we found that Fab-glycosylation might occur in both arms of IgG and Fab-glycogen lection might not be always “asymmetric” [9]. Therefore, we called it ConA+ IgG, while Fab-non-glycosylated IgG was named ConA− IgG. We found that both IgG forms were present in the serum of normal individuals. The concentration of ConA+ IgG is low in normal human serum but is increased in serum of tumor patients and pregnant women [10].
Tumor tissues are infiltrated by leukocytes including macrophages. These macrophages are closely related to the development of tumors and are called tumor-associated macrophages (TAM) [11, 12]. When macrophages are activated in a classical manner by stimulation with lipopolysaccharide (LPS) and interferon-gamma (IFN-γ), they tend to polarize into the M1 subtype, which has antigen presentation ability and tumor cytotoxicity. When the immune system responds to parasites and allergens, cytokines such as IL-4 and IL-13 are produced, allowing macrophages to polarize towards the M2 subtype [13]. Previous studies showed that, in the tumor microenvironment, tumor cells could polarize macrophages and render monocytes into a tumor-friendly M2 subtype [14, 15]. The characteristics of M2 macrophages include high expression of IL-10, scavenger receptor (CD163) and mannose receptor (MR, CD206) [14]. Amin et al. found that M2 macrophages triggered DC-SIGN-dependent B-cell receptor activation by highly mannosylated IgM in follicular lymphoma B cells [16]. And the tumor-associated Fab glycans are usually high-mannose structures [4]. Then we speculate that Fab glycans may interact with macrophages through the mannose receptor, thus affecting the progression of tumor.
We previously found that in an immune potent mouse tumor model, administration of total IgG (IVIg, intravenous immunoglobulin G) has an anti-tumor effect. We further demonstrated that IgG could promote macrophages to switch from tumor-friendly M2 subtype to tumor-inhibiting M1 subtype, which secretes anti-tumor cytokines and inhibits tumor growth and invasion [17]. In this study, we examined the effects of ConA+ IgG and ConA− IgG in mouse tumor models to study their roles in tumor progression. The expressions of cytokines in tumor-bearing mice after treatment were examined. We found that ConA+ IgG does not inhibit the development of tumors, and on the contrary, it may inhibit the anti-tumor effect of IgG by binding to total IgG. In addition, ConA+ IgG may act on macrophages through mannose receptors to promote tumor progression.
Materials and methods
Animals and IgG preparation
Adult female BALB/c mice and C57BL/6 mice were obtained from Beijing Vital River Laboratory Animal Technology Co., Ltd. (Beijing, China), and housed in the Laboratory Animal Center of Shantou University Medical College. Animal experiments were performed in accordance with the Guide for the Care and Use of Laboratory Animals of Shantou University Medical College. The protocol was approved by the Medical Animal Care and Welfare Committee of Shantou University Medical College (SUMC 2018-128). Mice aged between 6 and 8 weeks and weighing 22 ± 2 g were used in all experiments.
Human IgG preparation used in the study was purchased from Shanghai RAAS blood products Co., Ltd. Mouse IgG preparation used in the study were purchased from Beijing Solarbio Science & Technology Co., Ltd. Goat IgG and rabbit IgG used in the study was purchased from Beijing ZSGB-BIO Co., Ltd. Insulin Ab (ZM-0155) and Glucagon Ab (ZA-0119) used in the study were purchased from Beijing ZSGB-BIO Co., Ltd. Human IgG, Insulin Ab and Glucagon Ab were fractionated with lectin affinity chromatography using ConA Sepharose 4B following the manufacturer’s instruction (GE Healthcare, Connecticut, USA). In brief, IgG was diluted with Binding Buffer and loaded onto 2 ml of the Sepharose 4B-linked ConA column. The column was further washed with Binding Buffer to remove the unbound IgG, and all these unbound fractions were mixed to obtain the unbound IgG named ConA− IgG. The fraction bound to the ConA column was orderly eluted with Elution Buffer. The fraction isolated by elution with Elution Buffer was named ConA+ IgG. The concentration of all fractions was prepared with Amicon Ultra-4 Centrifugal Filter Units, 10 kDa (Millipore, Massachusetts, USA), quantified with BCA protein assay kit (Pierce™, Thermo Fisher Scientific, Waltham, MA, USA), filtered with 0.22 μm filter and stored at − 20 °C.
Cell culture
Mouse breast cancer cell line 4T1 and mouse colon cancer cell line CT26 were obtained from Stem Cell Bank, Chinese Academy of Sciences (Shanghai, China). Mouse macrophage line RAW246.7 was purchased from Shanghai Gefan Biotechnology Co., Ltd. All cell lines used in this study were grown in Roswell Park Memorial Institute (RPMI)-1640 medium (SH30809.01, Hyclone, USA) supplemented with 10% fetal bovine serum (FBS) (Gibco, USA) and 100 IU/ml penicillin and 100 μg/ml streptomycin (Life Technologies, USA) at 37 °C in a 5% CO2 atmosphere.
Animal experimental design
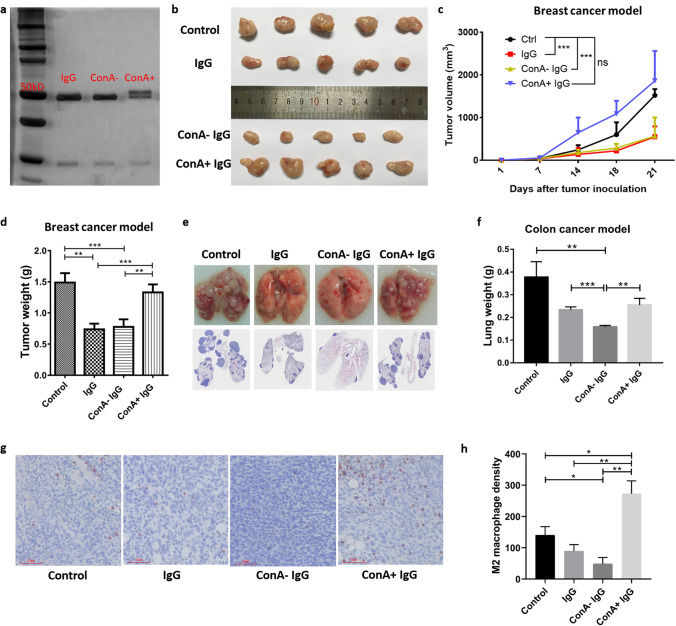
For 4T1 breast cancer model, mice were injected subcutaneously (into mammary fat pad) with 1 × 105 4T1 breast cancer cells on day 0 and were injected intravenously (tail vein) with IgG, ConA− IgG, ConA+ IgG (50 mg/kg) or PBS (control) on day 5, 10, 15, and 20. Mice were sacrificed on day 21 and tumors were weighed (n = 15/group, Fig. 2b–d).
Fig. 2.
ConA+ IgG affects tumor progression by regulating TAMs polarization. The difference in molecular weight of ConA+ IgG and ConA− IgG was examined with electrophoresis and silver stain. ConA+ IgG had higher molecular weight bands above the 50 kDa heavy chain as extra N-glycans attached (a). IgG and ConA− IgG injection inhibited tumor growth in the breast cancer model (b–d) and colon cancer model (e, f), ConA+ IgG did not inhibit the growth of tumor but promoted tumor growth. The changes in tumor volume in four groups as time went on (c). CD206 immunostaining (g, h) revealed more M2 macrophages in ConA+ IgG-treated 4T1 tumor tissues than in other groups (n = 5, bar: 60 µm). All data were shown as the mean ± SEM (*p < 0.05, **p < 0.01, ***p < 0.001, ns p > 0.05)
For CT26 colon cancer model, mice were injected intravenously (tail vein) with 3 × 105 tumor cells on day 0 and given IgG, ConA− IgG, ConA+ IgG (100 mg/kg) or PBS (control) on day 0, 4, 8, 12, 16. Mice were sacrificed on day 21 and lungs were weighed (n = 7/group, Fig. 2e, f).
Mouse cytokine array
Mice serum was measured for the presence of cytokines using Proteome Profiler™ Mouse XL Cytokine Array (ARY028, R&D, USA). It was performed following the protocol supplied by the manufacturer.
ELISA
Expressions of cytokines including sICAM-1, IL-23, IL-10, MMP9 in mice serum or culture supernatants of macrophages were measured with ELISA Kits (4A Biotech Co. Ltd, Beijing, China) according to the manufacturer’s instructions.
Deglycosylation enzymatic treatment
The deglycosylation enzymatic treatment of ConA+ IgG was performed according to the manufacturer’s instructions of the Native Protein Deglycosylation Kit (Sigma-Aldrich, Merck KGaA, Darmstadt, Germany) and PNGase F (NEB, Massachusetts, USA).
Silver staining
IgG, ConA− IgG, ConA+ IgG and ConA+ IgG with or without deglycosylation enzymatic treatment were separated with SDS PAGE using 8% polyacrylamide BisTris gels. After SDS PAGE, the gels were stained as described previously [9].
Western blot
The ConA− IgG, ConA+ IgG and ConA+ IgG with endoglycosidase treatment were labeled with biotin following the manufacturer’s instruction of AnaTag™ Biotin Protein Labeling Kit (AnaSpec Corporate, San Jose, CA, USA). For neutralization experiment, biotin-labeled ConA+ IgG (50 µg/ml) was pre-mixed with 0 µg/ml, 500 µg/ml or 2.5 mg/ml human IgG for 2 h at room temperature before use. The human IgG, mouse IgG, goat IgG, and rabbit IgG were separated with SDS-PAGE using 10% polyacrylamide BisTris gels. The proteins were transferred to nitrocellulose membranes (Whatman, Dassel, Germany). The blots were blocked with 5% skim milk in TBST for 1 h at room temperature and incubated overnight with biotin-labeled IgGs (50 µg/ml), and then incubated with (HRP)-labeled streptavidin (Beyotime Biotechnology, Shanghai, China) for 1 h at room temperature. The blots were examined with the Tanon imaging system (Tanon Science & Technology, Shanghai, China).
Immunocytochemistry and immunohistochemistry
Mouse macrophages were fixed onto slides and preincubated with 0 µg/µl or 500 µg/µl d-mannose for 2 h at room temperature. ICC was performed with biotin-labeled ConA− IgG and ConA+ IgG as described above, and the slides then were incubated with HRP-labeled streptavidin.
Mouse breast tumor tissues were fixed in 4% paraformaldehyde and embedded in paraffin. Tissue sections at 4 µm were immunostained with CD206 antibody (ab64693, Abcam, Cambridge, UK). Goat anti-rabbit IgG conjugated with HRP (Boster, Wuhan, China) was applied as the secondary antibody.
Pancreatic tissue was obtained at autopsy and fixed in 4% paraformaldehyde and embedded in paraffin. Tissue sections at 4 µm were immunostained following standard procedures. We used insulin antibodies (including ConA+ insulin antibody, ConA− insulin antibody or un-fractionated insulin antibody) and glucagon antibodies (including ConA+ glucagon antibody, ConA− glucagon antibody or un-fractionated glucagon antibody) as primary antibodies. They were used at the same working concentration. The poly-horseradish peroxidase anti-mouse/rabbit IgG detection system (PV-9000; ZSGB-Bio Co., Beijing, China) was applied as the secondary antibody.
Positive reactions were colorized with AEC kit (Zymed Laboratories, South San Francisco, CA, USA) and counterstained with hematoxylin. The slides were photographed with light microscopes (Motic, Xiamen, China; Leica, Germany).
Macrophage quantification: The density of M2 macrophages was evaluated using an image analyzing software Image-Pro Plus 6.0 (Media Cybernetics Inc.). For each section, the CD206-positive macrophages (M2 macrophages) were counted in five randomized high-power fields (HPFs, 200 ×). The density of M2 macrophages was calculated as the mean cell number per mm2.
Fluorescence-activated cell sorting
The phycoerythrin-labeled ConA+ IgG was produced according to the manufacturer’s instruction of the P-PE-Antibody conjugation Kit (APL001-200, 4A Biotech Co. Ltd, Beijing, China). RAW246.7 cells were incubated with 0 µg/ml, 100 µg/ml and 1000 µg/ml d-mannose for 2 h at 37 °C in a 5% CO2 atmosphere. Prepared single-cell suspension of RAW246.7 and incubated with PE-labeled ConA+ IgG at 4 °C for 20 min, washed away excess fluorescent protein and used BD Accuri™ C6 Flow Cytometer (BD Biosciences, San Jose, USA) to measure cell fluorescence intensity.
Lectin staining
For SNA, GNA, PNA, MAA, DSA staining, the experiment was performed using Dig Glycan Differentiation Kit following the manufacture’s instructions (Roche, Basel, Switzerland).
For ConA staining, the experiment was performed using the biotin-labeled ConA (Vector labs, California, USA), VECTASTAIN ABC-AP kit (Vector Labs, California, USA) and BCIP/NBT substrate kit (Vector Labs, California, USA) as described previously [9].
Statistical analyses
Statistical analyses and graphics generation were performed using GraphPad Prism 7.0. Statistical significance was calculated with Student’s t test, setting p < 0.05 as statistically significant.
Results
ConA+ IgG affects tumor progression by regulating TAMs polarization
As shown in Supplementary Fig. 1, the glycan of IgG can react with SNA and ConA. And in our previous study, we found a fraction of IgG in human serum was glycosylated at its Fab region [9, 18]. This fraction of IgG can be separated from non-glycosylated IgG by binding to a ConA column. Moreover, a higher concentration of ConA+ IgG was found in cancer patients and pregnant women, suggesting that ConA+ IgG might be associated with the immune response of the host. As the amount of ConA+ IgG in mouse is very low, and it has been found that human IgG can be used in mouse models [19], in this study, we used human IgG instead. We first fractionated human intravenous immune globulin (IVIg), which was obtained by pooling IgG from thousands of healthy donors, with ConA Sepharose 4B. We further purified the protein via Protein G. The difference in molecular weight of ConA+ and ConA− IgG was examined with Westen blot electrophoresis and silver staining (Fig. 2a). In a 4T1 breast cancer mouse model and a CT26 colon cancer pulmonary metastasis mouse model, IgG, ConA+ IgG, and ConA− IgG were administrated for a period of 3 weeks, and PBS was used as a control. The experimental design is shown in Fig. 1.
Fig. 1.
A diagram shows the experimental design of various groups of mice treated with different protocols
We found that IgG and ConA− IgG inhibited tumor progression, but ConA+ IgG did not. In the breast cancer mouse model, application of IgG and ConA− IgG led to a significant reduction in the growth of subcutaneous breast tumors as time went on (Fig. 2b, c). On day 21, the weights of tumors in the IgG group and the ConA− IgG group were less than those of the ConA+ IgG and the control groups (Fig. 2d). In addition, IgG and ConA− IgG injection also significantly inhibited pulmonary metastasis in the CT26 model, but ConA+ IgG did not have this inhibitory effect (Fig. 2e, f). To investigate the role of ConA+ IgG in tumor progression, we detected TAM infiltration in breast cancer tissues with immunohistochemistry (Fig. 2g, h). The results showed that more M2 macrophages were infiltrated in ConA+ IgG-treated breast cancer tissue than in tissues of the other three groups, which suggested that Fab-glycosylated IgG tends to protect tumor instead of inhibiting tumor proliferation by driving M2 polarization of TAMs.
ConA+ IgG changed the expression of multiple tumor-associated cytokines
To explore the role of ConA+ IgG in the mouse tumor model, we collected serum from four groups of mice and tested the expression of tumor-associated cytokines with Mouse XL Cytokine Array (Fig. 3a). Through gray value analysis, we selected 12 cytokines with obvious differences in expression (Fig. 3b) for further analysis.
Fig. 3.
Array analysis of the sera of tumor-bearing mice. The sera of mice treated with PBS (control), IgG, ConA− IgG and ConA+ IgG were measured with Mouse XL Cytokine Array analysis (a). 12 cytokines with obvious differences in expression were selected for further analysis (b). The expressions of cytokines associated with angiogenesis were increased in the ConA+ IgG group (c), which suggested that ConA+ IgG could promote tumor angiogenesis. In addition, the expressions of tumor-associated cytokines except IL-23 were increased in the ConA+ IgG groups (d), also suggested that ConA+ IgG played a role in tumor progression. The graphs (c, d) show the relative fold changes of protein concentration with significant difference upon IgG treatment. Pretreatment control was normalized to 1
First, we found that angiogenesis-related cytokines, such as Angiopoietin-1, Angiopoietin-2, and Angiopoietin-like 3 were increased in the serum of mice that were treated with ConA+ IgG. The expression levels were higher than those of the PBS (control) group, IgG group, and ConA− IgG groups. Another angiogenesis-related cytokine, thrombopoietin, was expressed similarly in the PBS group and the ConA+ IgG group, and both of them were higher than those in the total IgG group and the ConA− IgG group (Fig. 3c).
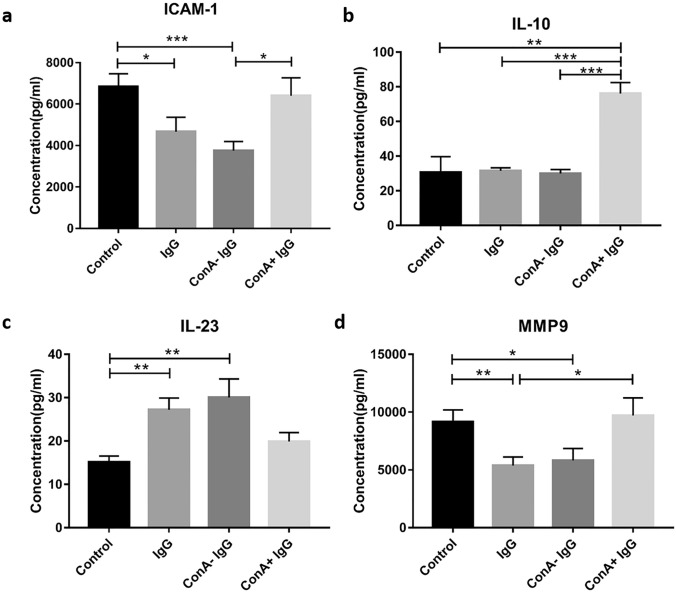
Moreover, tumor-associated cytokines such as Dickkopf-1, ICAM-1, LDL R, and interleukin-10 in the serum of the ConA+ IgG group were higher than those of the other three groups. And the expression of Resistin, MMP2, and MMP9 in the control group and the ConA+ IgG group were higher than the other two groups. But the expression of interleukin 23 was higher in the sera of the IgG group and the ConA− IgG group than the other two groups, (Fig. 3d). Among these cytokines, ICAM-1, IL-10, IL-23, and MMP9 are closely related to tumor development and metastasis. We further verified them with ELISA assay. The results showed that the expressions of ICAM-1 were higher in the control group and the ConA+ IgG group than those in the IgG group and the ConA− IgG group (Fig. 4a). The expression of IL-10 increased significantly in only the ConA+ IgG group (Fig. 4b). IgG and ConA− IgG injection led to an increase of IL-23 and the reduction of MMP9 (Fig. 4c, d).
Fig. 4.
ELISA analysis of the sera of tumor-bearing mouse. The sera of mice treated with PBS (control), IgG, ConA− IgG and ConA+ IgG were measured by ICAM-1 (a), IL-10 (b), IL-23 (c), and MMP9 (d) ELISA kit (*p < 0.05, **p < 0.01, ***p < 0.001). The results showed that the expressions of ICAM-1 and MMP9 were reduced in the IgG group and the ConA− IgG group, and the expression of IL-23 was increased in the IgG group and the ConA− IgG group. The expression of IL-10 was increased significantly in the ConA+ IgG group
The glycan on the Fab fragment of ConA+ IgG affects the function of IgG
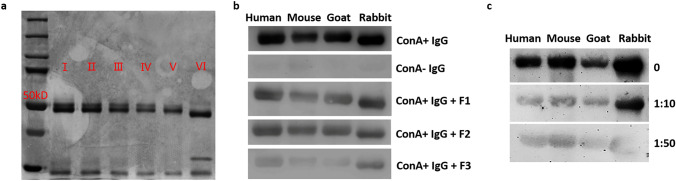
ConA+ IgG has an additional N-glycoside chain attached to the F(ab′)2 while ConA− IgG has not, so we speculated that ConA+ IgG might exert its biological function through its glycoside chain. To verify this hypothesis, we treated purified ConA+ IgG with endoglycosidase F1, F2, and F3 which have different cleavage sites on the glycosidic chains without affecting the activity of the protein. Based on the results of silver staining, endoglycosidase F1 and F2 only removed parts of glycosidic chains attached to ConA+ IgG, and endoglycosidase F3 removed most of the glycosidic chains attached to ConA+ IgG (Fig. 5a). We labeled ConA+ IgG, ConA− IgG, and ConA+ IgG after endoglycosidase F1, F2, F3 treatments with biotin. IgGs of four species, i.e. human, mouse, goat and rabbit were transferred to the NC membrane in Western blot, and reacted with the above biotin-labeled IgGs. These were then reacted to horseradish peroxidase (HRP) and detected with chemiluminescence detection (Fig. 5b). We found that ConA+ IgG bound to IgG of different species, while ConA− IgG did not. Removing the glycosidic chain attached to ConA+ IgG effectively reduced the reaction between ConA+ IgG and IgG of other species. We also mixed the biotin-labeled ConA+ IgG with human IgG in different proportions, and then reacted with IgG from different species (Fig. 5c). We found that as the proportion of human IgG increased, the ability of ConA+ IgG to bind to IgG molecules decreased. The results indicate that ConA+ IgG reacts to IgG of different species via the glycosidic chains, and the reaction is specific.
Fig. 5.
ConA+ IgG reacts to IgG molecules from different species. ConA+ IgG treated with endoglycosidase was examined with electrophoresis and silver stain (a): ConA+ IgG (I), ConA+ IgG treated with reaction buffer (control, II), ConA+ IgG treated with endoglycosidase F1 (III), ConA+ IgG treated with endoglycosidase F2 (IV), ConA+ IgG treated with endoglycosidase F3 (V), ConA+ IgG treated with PNGase F (VI). It showed that endoglycosidase F3 removed most of the glycosidic chains attached to ConA+ IgG. ConA+ IgG, ConA− IgG, and ConA+ IgG treated with endoglycosidase F1, F2, F3 were used as the primary antibodies. The results showed that ConA+ IgG, but not ConA− IgG, reacted to IgG molecules from different species. As endoglycosidase F3 can remove the glycosidic chain of ConA+ IgG, the reaction between ConA+ IgG and IgG molecules from different species was the weakest (b). The reaction between ConA+ IgG and IgG molecules from different species was weakened after ConA+ IgG was neutralized with a large number of IgG molecules (c), indicating that ConA+ IgG reacted with IgG molecules specifically
To verify the effect of ConA+ IgG on antigen binding, we fractionated immunohistochemical primary antibodies, including insulin antibody and glucagon antibody, to separate ConA+ antibody and ConA− antibody with ConA. Then we performed immunostaining with these antibodies. As shown in Supplementary Fig. 2, the positive intensities of the staining with ConA+ insulin antibody and ConA+ glucagon antibody were weaker than those obtained with ConA− antibodies and un-fractionated antibodies. The above results suggest that ConA+ IgG in the human body may affect the antigen-binding property of IgG.
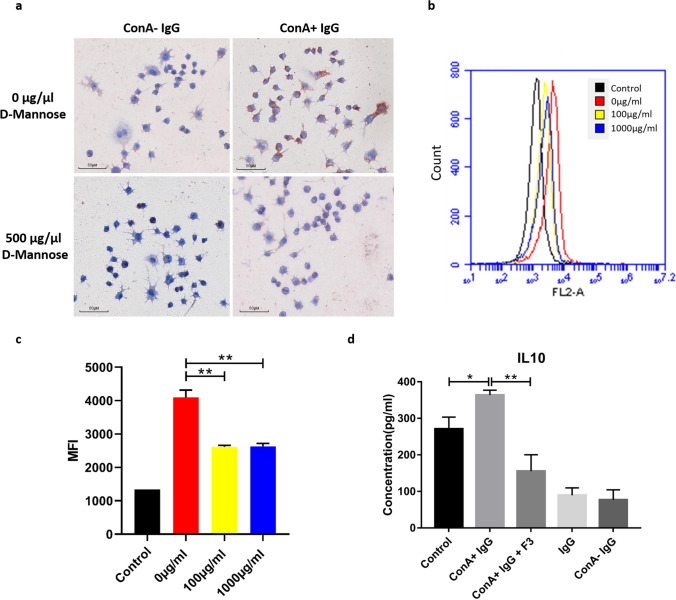
ConA+ IgG acts on macrophages via mannose receptors
Our previous study found that IgG could act on macrophages, and polarize macrophages from tumor-friendly M2 subtype to anti-tumor M1 subtype. The results of the current study showed that ConA+ IgG increased the expression of IL-10 in the serum of tumor-bearing mice significantly, which is the marker of M2 macrophage. This suggested that ConA+ IgG might also play a role in macrophage polarization. We used biotin-labeled ConA+ IgG and ConA− IgG to perform immunocytochemical staining of a mouse macrophage cell line RAW246.7 (Fig. 6a). Both ConA+ IgG and ConA− IgG reacted with macrophages, but the positive signal of ConA+ IgG was stronger. After blocking the macrophages with mannose, the positive signal of ConA+ IgG was weakened. We obtained similar results with fluorescence-activated cell sorting (FACS). RAW246.7 cells were incubated with 0 µg/ml, 100 µg/ml and 1000 µg/ml d-mannose for 2 h first to block the MR of macrophages. Then we used PE-labeled ConA+ IgG to measure cell fluorescence intensity (Fig. 6b, c). We found that the mean cell fluorescence intensity became weaker after d-mannose blocking. The above results indicated that ConA+ IgG could bind to macrophages via mannose receptors. In addition, RAW246.7 cells stimulated with ConA+ IgG were found to express more IL-10, while IL-10 expression was reduced after the glycosidic chains were removed (Fig. 6d). This observation showed that ConA+ IgG promoted the expression of IL-10 via mannose receptor to polarize macrophage to M2 subtype, which plays a potential role in promoting the development of tumor.
Fig. 6.
ConA+ IgG binds to macrophages via mannose receptors to promote tumor progression. ConA+ IgG as a primary antibody reacted strongly with mouse macrophages, but the reaction was weakened after the macrophages were blocked by mannose (a). RAW246.7 cells were incubated with 0 µg/ml, 100 µg/ml and 1000 µg/ml d-mannose for 2 h first. Then we used PE-labeled ConA+ IgG to measure cell fluorescence intensity with Flowcytometry. Mean fluorescence intensity (MFI) represents the average binding affinity of ConA+ IgG on RAW246.7 (1 × 104 cells), and the binding affinity of ConA+ IgG became lower after d-mannose blocking (b, c, **p < 0.01). ConA+ IgG promoted the secretion of IL-10 by mouse macrophages, but this promotion was weakened after the ConA+ IgG was treated with endoglycosidase F3 to remove the glycosidic chain (d, *p < 0.05, **p < 0.01)
Discussion
Shoenfeld et al. reported that human IgG inhibited the growth of tumor in tumor-bearing mice with a normal immune system [20, 21]. In our previous studies, we found that in mouse tumor models, mouse IgG promoted the expression of various anti-tumor cytokines by polarizing macrophage from M2 to M1 subtype [17]. We also found that IgG played a role in inhibiting the growth and migration of tumor cells. In this study, we used the ConA column to separate IgG of normal human serum into ConA+ IgG and ConA− IgG, and administered them into an established mouse subcutaneous breast cancer model and a mouse colon cancer lung metastasis model. The effects of IgG, ConA− IgG and ConA+ IgG in treating cancer were compared in these animal models. We demonstrated that both IgG and non-glycosylated IgG inhibited tumor growth and metastasis, but glycosylated IgG, which drove M2 polarization of TAMs, did not produce such effects.
The cell adhesion factor ICAM-1, including sICAM-1 and mICAM-1, mediates the binding and adhesion between cells as well as between extracellular matrix and cells. Recent studies reported that ICAM-1 played an important role in invasion and metastasis of malignant tumor, and mediated hematogenous and lymphatic metastases of tumor cells. At the same time, sICAM-1 inhibited the immune recognition of tumor cells, inhibited the activities of immune effector cells such as NK cells and killer T cells to kill tumor cells, and promoted immune escape of tumor cells from immune surveillance [22]. MMP9 is an important member of the matrix metalloproteinase family. MMPs are expressed in almost all cancer types, and they promote angiogenesis, tumor growth and metastasis and affect tumor immune regulation. MMPs are closely related to tumor invasion, staging and patient prognosis [23]. IL-23 is mainly produced by dendritic cells and macrophages. It is a member of the IL-12 family and has strong anti-tumor effects. Verreck et al. found that IL-23 stimulated mouse T cells to secrete IFN-γ and GM-CSF, recruit monocytes and polarize them to M1 macrophages [24]. In this study, we used Cytokine Array and ELISA kits to examine the expression of cytokines in serum of tumor-bearing mice. Both IgG and ConA− IgG were found to down-regulate the expression of ICAM-1 and MMP9, and up-regulate the expression of IL-23, suggesting a strong anti-tumor effect. However, ConA+ IgG significantly promoted the expression of IL-10. IL-10 is secreted by macrophages and lymphocytes and is a marker of M2 macrophages. Most previous studies suggested that IL-10 was an immunosuppressive molecule secreted by tumor cells or tumor-associated macrophages. It helps tumors to evade immune surveillance [25]. Our results indicate that ConA+ IgG does not take part in the process of suppressing tumor growth. Instead, it appears to help tumor cells to escape the attack of the immune system.
To date, most studies have focused on IgG Fc glycans, but few have paid close attention to the role of glycans on IgG Fab in cancer progression and immune modulation. Gercel-Taylor et al. and Kodar et al. found that the ratio of glycosylated IgG, which can bind to ConA in the serum of patients with ovarian cancer and gastric cancer, was elevated, and they believed that the glycosylation site was located on the Fc fragment [26, 27]. However, we found that ConA+ and ConA− IgG under denaturation and reduction conditions could react with SNA and ConA (Supplementary Fig. 1). Although both Fab and Fc fragments could react with ConA, the N-glycoside oligosaccharide chain on the Fc fragment was located inside the molecule and could not be accessed by the ConA affinity chromatography column in a natural folded state [9].
Margni et al. found asymmetric IgG increased in the serum of pregnant women. They reported that the immune complex formed by asymmetric IgG could not effectively clear the antigen, trigger antibody-dependent cell-mediated cytotoxicity, and activate downstream immune responses such as complement activation [28]. There are studies reporting that the presence of glycosylation sites in the Fab fragments can influence antigen binding [29–31]. Chavele et al. found that in a mouse glomerulonephritis model, sheep nephrotoxic globulin which has enriched mannose on the Fab fragment could bind to the mesangial cells via mannose receptor, triggering the downstream immune response [32]. These suggest that the glycans on IgG Fab may affect the function of IgG.
In this study, we find that ConA+ IgG reacts to non-specific IgG via the glycosidic chains, and Fab glycosylation may weaken or nullify the antigen–antibody binding (Supplementary Fig. 2). These observations imply that Fab glycosylation affects the function of IgG including antigen binding and immune complex formation, resulting in tumor survival without being effectively and timely detected and destroyed by IgG-mediated immunity as Fab-glycosylated antibodies may not be able to recognize and bind to tumor antigens. We also found that ConA+ IgG could bind to the mannose receptor on macrophages via the glycoside chain of the Fab fragment. The M2 macrophages were recognized by their ability to express more IL-10, and they are involved in immunosuppression. The above results suggest that ConA+ IgG may affect macrophage polarization to suppress the elimination of tumor antigens and immune reactions against cancer in the cancer microenvironment.
In summary, we found that Fab glycosylated IgG might affect macrophage polarization and the antigen-binding ability of IgG, leading to tumor growth and metastasis in a mouse tumor model. These original observations provide new insight into the mechanisms of actions of immunoglobulin in cancer microenvironment and offer new possibilities for intervention.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
This research was funded by the National Natural Science Foundation of China (81872334) and Li Ka Shing Foundation. The authors would like to thank the Laboratory Animal Center and the Center for Core Facilities of Shantou University Medical College for supporting and providing help to this research.
Abbreviations
- ConA
Concanavalin A
- DSA
Datura stramonium agglutinin
- ELISA
Enzyme-linked immunosorbent assay
- GM-CSF
Granulocyte–macrophage colony-stimulating factor
- GNA
Galanthus nivalis agglutinin
- HRP
Horseradish peroxidase
- ICAM-1
Intercellular adhesion molecule 1
- IFN-γ
Interferon gamma
- IL
Interleukin
- IVIg
Intravenous immunoglobulin
- LDL R
Low-density lipoprotein receptor
- LPS
Lipopolysaccharide
- MAA
Maackia amurensis agglutinin
- MFI
Mean fluorescence intensity
- MMP 9
Matrix metallopeptidase 9
- MR
Mannose receptor
- SNA
Sambucus nigra agglutinin
- TAM
Tumor-associated macrophages
- P-PE
P-phycoerythrin
- PNA
Peanut agglutinin
Author contributions
JG coordinated the entire project; JG and QX wrote the manuscript; QX, XD, BZ, CZ, TH, YZ, and ZC performed the experiments; QX, BZ, CZ analyzed the data.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving animals were in accordance with the ethical standards of the Medical Animal Care and Welfare Committee of Shantou University Medical College (SUMC 2018-128).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Poljak RJ. Three-dimensional structure, function and genetic control of immunoglobulins. Nature. 1975;256(5516):373–376. doi: 10.1038/256373a0. [DOI] [PubMed] [Google Scholar]
- 2.Youings A, Chang SC, Dwek RA, Scragg IG. Site-specific glycosylation of human immunoglobulin G is altered in four rheumatoid arthritis patients. Biochem J. 1996;314(Pt 2):621–630. doi: 10.1042/bj3140621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jefferis R. Glycosylation of recombinant antibody therapeutics. Biotechnol Prog. 2005;21(1):11–16. doi: 10.1021/bp040016j. [DOI] [PubMed] [Google Scholar]
- 4.van de Bovenkamp FS, Hafkenscheid L, Rispens T, Rombouts Y. The emerging importance of IgG Fab glycosylation in immunity. J Immunol. 2016;196(4):1435–1441. doi: 10.4049/jimmunol.1502136. [DOI] [PubMed] [Google Scholar]
- 5.Borel IM, Gentile T, Angelucci J, Margni RA, Binaghi RA. Asymmetrically glycosylated IgG isolated from non-immune human sera. Biochem Biophys Acta. 1989;990(2):162–164. doi: 10.1016/S0304-4165(89)80029-7. [DOI] [PubMed] [Google Scholar]
- 6.Barrientos G, Fuchs D, Schrocksnadel K, Ruecke M, Garcia MG, Klapp BF, Raghupathy R, Miranda S, Arck PC, Blois SM. Low levels of serum asymmetric antibodies as a marker of threatened pregnancy. J Reprod Immunol. 2009;79(2):201–210. doi: 10.1016/j.jri.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 7.Gentile T, Borel IM, Angelucci J, Miranda S, Margni RA. Preferential synthesis of asymmetric antibodies in rats immunized with paternal particulate antigens. Effect on pregnancy. J Reprod Immunol. 1992;22(2):173–183. doi: 10.1016/0165-0378(92)90014-U. [DOI] [PubMed] [Google Scholar]
- 8.Margni RA, Perdigon G, Abatangelo C, Gentile T, Binaghi RA. Immunobiological behaviour of rabbit precipitating and non-precipitating (co-precipitating) antibodies. Immunology. 1980;41(3):681–686. [PMC free article] [PubMed] [Google Scholar]
- 9.Huang T, Chen X, Gu H, Zhao C, Liu X, Yan M, Deng X, Zhang Z, Gu J. Fractionation of Fab glycosylated immunoglobulin G with concanavalin A chromatography unveils new structural properties of the molecule. Oncotarget. 2016;7(21):31166–31176. doi: 10.18632/oncotarget.9085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zenclussen AC, Gentile T, Kortebani G, Mazzolli A, Margni R. Asymmetric antibodies and pregnancy. Am J Reprod Immunol. 2001;45(5):289–294. doi: 10.1111/j.8755-8920.2001.450504.x. [DOI] [PubMed] [Google Scholar]
- 11.Chen Y, Tan W, Wang C. Tumor-associated macrophage-derived cytokines enhance cancer stem-like characteristics through epithelial-mesenchymal transition. OncoTargets Ther. 2018;11:3817–3826. doi: 10.2147/OTT.S168317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mantovani A, Locati M. Tumor-associated macrophages as a paradigm of macrophage plasticity, diversity, and polarization: lessons and open questions. Arterioscler Thromb Vasc Biol. 2013;33(7):1478–1483. doi: 10.1161/ATVBAHA.113.300168. [DOI] [PubMed] [Google Scholar]
- 13.Murray PJ. Macrophage polarization. Annu Rev Physiol. 2017;79:541–566. doi: 10.1146/annurev-physiol-022516-034339. [DOI] [PubMed] [Google Scholar]
- 14.Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23(11):549–555. doi: 10.1016/S1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 15.Rhee I. Diverse macrophages polarization in tumor microenvironment. Arch Pharm Res. 2016;39(11):1588–1596. doi: 10.1007/s12272-016-0820-y. [DOI] [PubMed] [Google Scholar]
- 16.Amin R, Mourcin F, Uhel F, Pangault C, Ruminy P, Dupre L, Guirriec M, Marchand T, Fest T, Lamy T, Tarte K. DC-SIGN-expressing macrophages trigger activation of mannosylated IgM B-cell receptor in follicular lymphoma. Blood. 2015;126(16):1911–1920. doi: 10.1182/blood-2015-04-640912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu Q, Zhang Z, Chen Z, Zhang B, Zhao C, Zhang Y, Zhao C, Deng X, Zhou Y, Wu Y, Gu J. Nonspecific immunoglobulin G is effective in preventing and treating cancer in mice. Cancer Manag Res. 2019;11:2073–2085. doi: 10.2147/CMAR.S188172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gu J, Lei Y, Huang Y, Zhao Y, Li J, Huang T, Zhang J, Wang J, Deng X, Chen Z, Korteweg C, Deng R, Yan M, Xu Q, Dong S, Cai M, Luo L, Huang G, Wang Y, Li Q, Lin C, Su M, Yang C, Zhuang Z. Fab fragment glycosylated IgG may play a central role in placental immune evasion. Hum Reprod. 2015;30(2):380–391. doi: 10.1093/humrep/deu323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Overdijk MB, Verploegen S, Ortiz Buijsse A, Vink T, Leusen JH, Bleeker WK, Parren PW. Crosstalk between human IgG isotypes and murine effector cells. J Immunol. 2012;189(7):3430–3438. doi: 10.4049/jimmunol.1200356. [DOI] [PubMed] [Google Scholar]
- 20.Fishman P, Bar-Yehuda S, Shoenfeld Y. IVIg to prevent tumor metastases (review) Int J Oncol. 2002;21(4):875–880. [PubMed] [Google Scholar]
- 21.Shoenfeld Y, Fishman P. Gamma-globulin inhibits tumor spread in mice. Int Immunol. 1999;11(8):1247–1252. doi: 10.1093/intimm/11.8.1247. [DOI] [PubMed] [Google Scholar]
- 22.Li M, Gao CJ. An update on beta2 integrin LFA-1 and ligand ICAM-1 signaling. Zhongguo shi yan xue ye xue za zhi. 2008;16(1):213–216. [PubMed] [Google Scholar]
- 23.Reimers N, Zafrakas K, Assmann V, Egen C, Riethdorf L, Riethdorf S, Berger J, Ebel S, Janicke F, Sauter G, Pantel K. Expression of extracellular matrix metalloproteases inducer on micrometastatic and primary mammary carcinoma cells. Clin Cancer Res. 2004;10(10):3422–3428. doi: 10.1158/1078-0432.CCR-03-0610. [DOI] [PubMed] [Google Scholar]
- 24.Verreck FA, de Boer T, Langenberg DM, Hoeve MA, Kramer M, Vaisberg E, Kastelein R, Kolk A, de Waal-Malefyt R, Ottenhoff TH. Human IL-23-producing type 1 macrophages promote but IL-10-producing type 2 macrophages subvert immunity to (myco)bacteria. Proc Natl Acad Sci USA. 2004;101(13):4560–4565. doi: 10.1073/pnas.0400983101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mannino MH, Zhu Z, Xiao H, Bai Q, Wakefield MR, Fang Y. The paradoxical role of IL-10 in immunity and cancer. Cancer Lett. 2015;367(2):103–107. doi: 10.1016/j.canlet.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 26.Gercel-Taylor C, Bazzett LB, Taylor DD. Presence of aberrant tumor-reactive immunoglobulins in the circulation of patients with ovarian cancer. Gynecol Oncol. 2001;81(1):71–76. doi: 10.1006/gyno.2000.6102. [DOI] [PubMed] [Google Scholar]
- 27.Kodar K, Stadlmann J, Klaamas K, Sergeyev B, Kurtenkov O. Immunoglobulin G Fc N-glycan profiling in patients with gastric cancer by LC-ESI-MS: relation to tumor progression and survival. Glycoconj J. 2012;29(1):57–66. doi: 10.1007/s10719-011-9364-z. [DOI] [PubMed] [Google Scholar]
- 28.Canellada A, Gentile T, Dokmetjian J, Margni RA. Occurrence, properties, and function of asymmetric IgG molecules isolated from non-immune sera. Immunol Investig. 2002;31(2):107–120. doi: 10.1081/IMM-120004802. [DOI] [PubMed] [Google Scholar]
- 29.Wright A, Tao MH, Kabat EA, Morrison SL. Antibody variable region glycosylation: position effects on antigen binding and carbohydrate structure. EMBO J. 1991;10(10):2717–2723. doi: 10.1002/j.1460-2075.1991.tb07819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coloma MJ, Trinh RK, Martinez AR, Morrison SL. Position effects of variable region carbohydrate on the affinity and in vivo behavior of an anti-(1–>6) dextran antibody. J Immunol. 1999;162(4):2162–2170. [PubMed] [Google Scholar]
- 31.Schneider D, Duhren-von Minden M, Alkhatib A, Setz C, van Bergen CA, Benkisser-Petersen M, Wilhelm I, Villringer S, Krysov S, Packham G, Zirlik K, Romer W, Buske C, Stevenson FK, Veelken H, Jumaa H. Lectins from opportunistic bacteria interact with acquired variable-region glycans of surface immunoglobulin in follicular lymphoma. Blood. 2015;125(21):3287–3296. doi: 10.1182/blood-2014-11-609404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chavele KM, Martinez-Pomares L, Domin J, Pemberton S, Haslam SM, Dell A, Cook HT, Pusey CD, Gordon S, Salama AD. Mannose receptor interacts with Fc receptors and is critical for the development of crescentic glomerulonephritis in mice. J Clin Investig. 2010;120(5):1469–1478. doi: 10.1172/JCI41560. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.