Abstract
Background
Standard care for patients with high-risk myelodysplastic syndrome (MDS) is hypomethylating agents such as azacitidine (AZA), which can induce expression of methylated tumor-associated antigens and therefore potentiate immunotherapeutic targeting.
Method
In this phase 1 trial, we combined AZA with a therapeutic peptide vaccine targeting antigens encoded from NY-ESO-1, MAGE-A3, PRAME, and WT-1, which have previously been demonstrated to be upregulated by AZA treatment.
Result
Five patients who had responded to AZA monotherapy were included in the study and treated with the vaccine. The combination therapy showed only few adverse events during the study period, whereof none classified as serious. However, no specific immune responses could be detected using intracellular cytokine staining or ELISpot assays. Minor changes in the phenotypic composition of immune cells and their expression of stimulatory and inhibitory markers were detected. All patients progressed to AML with a mean time to progression from inclusion (TTP) of 5.2 months (range 2.8 to 7.6). Mean survival was 18.1 months (range 10.9 to 30.6) from MDS diagnosis and 11.3 months (range 4.3 to 22.2) from inclusion. Sequencing of bone marrow showed clonal expansion of malignant cells, as well as appearance of novel mutations.
Conclusion
The patients progressed to AML with an average time of only five months after initiating the combination therapy. This may be unrelated to the experimental treatment, but the trial was terminated early as there was no sign of clinical benefit or immunological response.
Why the manuscript is especially interesting
This study is the first to exploit the potential synergistic effects of combining a multi-peptide cancer vaccine with epigenetic therapy in MDS. Although our results are negative, they emphasize challenges to induce immune reactivity in patients with high-risk MDS.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00262-021-02993-6.
Keywords: Myelodysplastic syndrome, Immunotherapy, Vaccine, Cancer testis antigens, Epigenetic therapy, Clinical trial
Introduction
Myelodysplastic syndrome (MDS) is a clonal hematopoietic malignancy of the bone marrow with an incidence of around 4 per 100.000. It mostly affects the elderly, with a median age of 77 [1]. The only curative treatment for MDS is allogeneic bone marrow transplantation, which is a procedure with considerable treatment-related morbidity and few eligible candidates. In high-risk MDS patients, standard treatment is the hypomethylating agent (HMA) azacitidine (AZA), a DNA methyltransferase inhibitor (DNMTi) which covalently binds to DNA methyltransferase, and thereby inhibits copying of the methylation pattern to newly synthesized DNA as the cell divides [2, 3]. There are several hypotheses to AZA’s anti-neoplastic effects on malignant clones in the bone marrow[4]; including demethylation and reactivation of tumor suppressor genes [5], upregulation of HLA class I in tumor cells [6], depletion of myeloid-derived suppressor cells (MDSCs) [7], and as more recently discovered, activation of viral defense pathways through expression of endogenous retroviruses [8].
It is well-documented that HMAs induce expression of cancer-testis antigens (CTAs) in tumor cells in vitro [9–14], which could be an important contributor to the mechanism of AZA’s clinical efficacy. CTAs are downregulated by promoter hypermethylation and are normally only expressed during fetal development or at immune-privileged sites. In cancer cells, epigenetic instability may lead to hypomethylation and expression of CTAs, which leads to the presentation of its peptide fragments on HLA class I molecules and, due to lack of central tolerance, recognition by cytotoxic T-cells [9–13]. Both cellular and humoral spontaneous immunogenicity toward CTAs have been recorded in many cancer types and have in some cases been associated with remission of disease [15–17]. Other studies have successfully used vaccines to induce T-cell responses toward CTAs in cancer patients [18–20], however, its clinical benefit remains to be confirmed in larger clinical trials. There is, therefore, rationale for testing the combination of a HMA with immunotherapy targeting CTAs, since those treatments could have synergistic effects. The approach is being investigated for solid malignancies, and results from initial trials are encouraging [21, 22].
Here, we report the results of a Phase 1 clinical trial combining AZA with a peptide vaccine targeting four tumor-associated antigens (NY-ESO-1, MAGE-A3, PRAME, and WT-1) in patients with high-risk MDS. It is to our knowledge the first study of a HMA in combination with a multi-peptide vaccine in a hematological malignancy.
Method
Clinical trial design
The study was an open-label uncontrolled clinical trial (EudraCT no. 2014-002,432-14; ClinicalTrials.gov identifier: NCT02750995). Patients were recruited from the Department of Hematology, Copenhagen University Hospital, Herlev, Denmark. Inclusion criteria were age above 18 years, with histologically confirmed high-risk MDS[23] or acute myeloid leukemia (AML). Prior to inclusion, the patients should have received six courses of AZA and achieved a clinical response of at least stable disease (SD), as determined by the 2006 International working group (IWG) response criteria [24]. If there was an indication for continued AZA therapy following the six treatment cycles, and the bone marrow was not hypocellular, the patient could be included in the study.
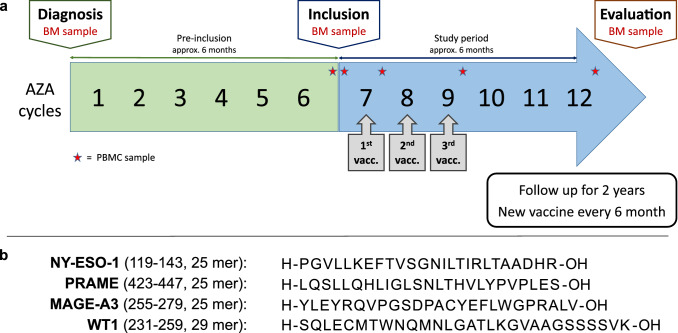
Vaccinations were given on day one of the following three courses of AZA (Fig. 1a). A bone marrow evaluation was performed after six courses or earlier if deemed necessary by attending physicians. Progressive disease (PD) at any time point resulted in discontinuation from the trial. If the patient did not have PD at the evaluation time point then he/she could continue in the study, with a new vaccination every six months for up to two years.
Fig. 1.
Trial and vaccine design. a Trial design. All participants received six courses of AZA prior to inclusion and were evaluated with bone marrow biopsy for treatment response. Vaccination was given together with the next three courses of AZA. b Vaccine composition. Synthetic long peptides from NY-ESO-1, PRAME, MAGE-A3, and WT-1 were emulsified in adjuvant Montanide ISA 51
The primary endpoint was safety, as measured by adverse events (AEs). Secondary endpoint was induced immune response toward vaccine peptides, and third endpoint time-to-progression (TTP) and survival.
Vaccine formulation
Four long synthetic peptides, from tumor-associated antigens, containing previously described class I and class II epitopes for a variety HLA types (Fig. 1b, see Supplementary Fig. 1 for mapping of known T cell epitopes to the peptide sequences in the vaccine). NY-ESO-1119–143[25, 26], PRAME423-447[27, 28], and MAGE-A3255-279[26, 29] are well-described CTAs known to be upregulated in response to HMAs. WT1231-259[30–32] is another antigen associated with hematological malignancies and is also heavily methylated under normal conditions [33–35]. Clinical grade peptides (sequences PGVLLKEFTVSGNILTIRLTAADHR, LQSLLQHLIGLSNLTHVLYPVPLES, YLEYRQVPGSDPACYEFLWGPRALV, and SQLECMTWNQMNLGATLKGVAAGSSSSVK) were purchased from Pepscan (Lelystad, Netherlands) at a purity of > 97% measured by high-performance liquid chromatography (HPLC). Stability tests were performed continuously to ensure purity > 90%. Lyophilized peptides were dissolved in sterile dimethyl sulfoxide (DMSO, CryoSure) and sterile filtered through a 0.20 µm RC-membrane filter (Corning). The dissolved peptides were then pooled and cryopreserved so that one tube contained one dose of vaccine, corresponding to 50 µg of each of the four peptides. Each batch was evaluated for endotoxins (using Endosafe®, threshold < 500 EU/ml) and sterility tested at the department for clinical microbiology before release. At days of vaccination, tubes of peptide-mixture were thawed in sterile PBS and emulsified with adjuvant Montanide ISA 51VG (SEPPIC, France)[30] using dual syringes and an I-connector (Promepla) shortly before administration to the patient. The vaccine was injected subcutaneously in the deltoid or triceps region.
Safety monitoring
All AEs were registered from the time of the first vaccination and were evaluated for seriousness, causality, and toxicity grade (CTCAE version 4). Disease progression was not recorded as an adverse event (AE) but as an endpoint leading to discontinuation from the study. Due to the nature of the disease studied, and as stated in our protocol, an abnormal laboratory value was only considered to be an AE if the abnormality: (1) resulted in discontinuation from the study; (2) required treatment, or any other therapeutic intervention; or (3) was deemed to be of clinical importance.
Biomaterial
Bone marrow aspirates (10 ml) were taken at the inclusion time point (following the patient’s sixth AZA treatment after MDS diagnosis) and again after receiving an additional six courses of AZA and three vaccinations. If there were signs of disease progression, a bone marrow biopsy was performed ahead of schedule. Blood samples for biobanking were taken at multiple time points (see Fig. 1a). Bone marrow was split into three fractions, where the first two parts of the bone marrow aspirate were treated with red blood cell lysis (Ortho-Lysing buffer), and the third part was used for isolating mononuclear cells by centrifugation through Leucosep® tubes. Peripheral blood mononuclear cells were isolated from whole blood using Leucosep®. All cells were frozen in 90% human AB serum + 10% DMSO to preserve high viability, except for one part of the bone marrow aspirate, intended for sequencing, which was snap-frozen.
Laboratory analysis
Intracellular cytokine staining
Frozen cells from before vaccinations (baseline) and from after the first and third vaccination were thawed in preheated RPMI 1640, Gibco, with 10% fetal calf serum (FCS), Gibco, (R10), then washed and resuspended in X-vivo media (Lonza) with 5% human serum (Sigma-Aldrich) to a concentration of 1 million cells per 100 µl and split to wells on 96-well plate. In order to stimulate the cells, a pool of vaccine peptides (NY-ESO-1, PRAME, MAGE-A3 and WT-1) were added to cells at a concentration of 1 µg/ml per peptide. Two controls were used per time point; one well with an irrelevant peptide (HIV derived) and one without any peptide. Cells incubated for two hours, after which additional media containing GolgiPlug™ (BD Biosciences) at a concentration of 0.1 µl per 100 µl cells was added together with 5 µl of anti-CD107a-BV711 antibody (Biolegend). Thereafter cells were incubated at 37 °C for 10 h. After incubation, cells were washed and stained with surface lineage markers and then fixed and permeabilized using Intracellular Fixation & Permeabilization Buffer Set (eBioscience™). Intracellular cytokine staining (ICS) was performed using anti-IFN-y-PE (BD Biosciences) and anti-TNF-a-BV650 (BD Biosciences). Samples were then acquired and analyzed using LSR Fortessa (BD Biosciences). Peripheral Blood Mononuclear Cells (PBMCs) from healthy donors stimulated with Leucocyte Activation Cocktail (LAC, BD Biosciences) were stained with the fluorescent antibodies and analyzed prior to running experiments with patient samples to validate the assay.
Enzyme-Linked Immune absorbent Spot
Enzyme-Linked Immune absorbent Spot (ELISpot) was performed using cells pre-stimulated with vaccine or control peptides. First, cells were thawed in preheated R10, washed, and resuspended in X-vivo + 5% human serum + IL-15 and IL-21, before split into wells. Vaccine peptides were added to wells on day two at a final concentration of 0.5 µg/ml. A mix of peptides from cytomegalovirus, Epstein-Barr virus, influenza virus, and tetanus toxin (CEFT) was used as a positive control. Cells were cultured in wells for ten days, with new media and cytokines (IL-2, IL-15 and IL-21) added every second or third day as cells expanded. After pre-stimulation, cells were rested overnight in X-vivo media with 5% human serum without cytokines. ELISpot was next performed in triplicates using human IFN-γ ELISPOT, ELISPOT Streptavidin Horseradish Peroxidase, and ELISPOT AEC Substrate Set (BD Biosciences) according to the manufacturer manual. An irrelevant murine peptide and medium without peptide were used as negative controls, while CEFT peptides or phytohaemagglutinin (PHA) were added to positive controls. For one patient (P4), all vaccine peptides were pooled, and experiments were performed in duplicates due to low cell count and poor viability of the PBMCs.
Exploratory analysis of immune subsets
Patient samples were stained with fluorochrome-labeled antibodies to distinguish changes in CD4 and CD8 T cell memory and effector subsets and their expression of markers associated with activation and inhibition. We also investigated regulatory T-cells, NK and NKT-cells, monocytes, dendritic cells (DC), myeloid-derived suppressor cells (MDSCs), and CD34 + hematopoietic stem cells. Samples were acquired on LSR Fortessa (BD bioscience) and gated in FlowJo (LLC, version 10.6). Percentage of parent populations were exported and analyzed in R (version 3.6.1) using the Tidyverse packages (version 1.2.1) [36]. See Supplementary Table 1 for a definition of cell subtypes analyzed and Supplementary Table 2 for the full list of antibodies.
Cytogenetics and sequencing
Cytogenetic analyses were performed on bone marrow samples, in accordance with routine clinical practice, at diagnosis, inclusion, and evaluation time points. Samples were analyzed at the Cytogenetic Laboratory, Department of Clinical Genetics, Rigshospitalet, Copenhagen, Denmark. For next-generation sequencing (NGS), snap-frozen pellets from the bone marrow samples were dissolved in 200 µL PBS and DNA isolated with QiaAmp® DNA Blood Mini kit (Qiagen). NGS library preparation on DNA was done with the Ion Torrent™ Oncomine™ Myeloid Research Assay (Thermo Fisher) with DNA input of 10–30 ng / reaction. The library concentrations were normalized using Ion Library Equalizer™ kit before template preparation on the Ion Chef™ System (Thermo Fisher). Sequencing was performed on Ion S5™ XL System, and data analyzed with Ion Reporter software (Thermo Fisher, version 5.10) using the default workflow settings. In addition to the default filter, the data were filtered with a local filter setting to include splice site variants and more clinically relevant variants. Raw data on all detected variants were reviewed manually using the Integrative Genomics Viewer (http://software.broadinstitute.org, version 5.01) and classified using Varsome [37].
Result
Patient characteristics
The trial was planned to include 15 patients treated with a minimum of three vaccinations each. Due to a pause in inclusion to investigate whether bone marrow hypoplasia was related to the vaccine and slower recruitment than anticipated, only five patients entered the trial and received the experimental treatment. All patients were classified as high-risk MDS, with an IPSS score of at least 1.5 (Table 1).
Table 1.
Patients Characteristics
| P1 | P2 | P3 | P4 | P5 | |
|---|---|---|---|---|---|
| Diagnosis | |||||
| Age at diagnosis | Mean: 79 years (all > 75) | ||||
| Blasts in BM (%) | 2 | 12 | 15 | 18 | 12 |
| IPSS | 1.5 | 3 | ≥ 2 * | 2 | 2 |
| IPSS-R | 4.5 | 9 | ≥ 4 * | 5 | 6 |
| Cytogenetics | Complex | Complex | NA | Normal | Normal |
| MDS subtype | MLD | EB-2 | EB-2 | EB-2 | EB-2 |
| Blasts peripheral (%) | 0 | 10 | 0 | 1 | 2 |
| ECOG performance status | 1 | 2 | 0 | 1 | 1 |
| Inclusion | |||||
| Age at inclusion | Mean: 80 years (all > 75) | ||||
| AZA cycle | 6 | 6 | 6 | 6 | 6 |
| Blasts in BM (%) | 1 | 2 | 2 | 16 | 0 |
| Cytogenetics | Normal | Complex | NA | NA | NA |
| IWG response criteria | CR with dysplasia | CR with dysplasia | CR with dysplasia | HI-E | CR with dysplasia |
| Blasts peripheral (%) | 0 | 0 | 0 | 0 | 0 |
| ECOG performance status | 1 | 1 | 0 | 1 | 1 |
* = Cytogenetic analysis at diagnosis failed for P3. IPSS and IPSS-R risk score is therefore based on percentage of blast and degrees of peripheral cytopenia for this patient
NA = Cytogenetic analysis not available at time point
Complex = Three or more cytogenetic abnormalities
The average age at inclusion was 80 years (all patients were over 75). Both men and women were included. All patients had been diagnosed within ten months prior to inclusion and had received exactly six courses of AZA treatment at full or reduced AZA dose. At the inclusion time point, all but one patient (P4) had achieved complete remission (CR) with persisting bone marrow dysplasia (due to the underlying MDS) and normalization of peripheral cytopenias. The response of P4 was characterized as stable disease (SD) with hematological improvement in the erythrocyte compartment (HI-E). Three patients had a bone marrow cellularity of 20% at the inclusion time point, which were not assessed to be pathological due to the high age of the participants and normal peripheral hematology.
Safety
AEs with probable causality to the vaccine were injection site reactions, localized edema, and pruritus, while AEs with possible causality were vomiting, dizziness, chills, malaise, and all registered blood and lymphatic system disorders, such as bone marrow hypoplasia and decreased neutrophil and platelet counts. One case of grade 4 toxicity (neutropenia, resulting in administration of prophylactic antibiotics), two cases of grade 3 toxicity (thrombocytopenia and neutropenia), and two cases of bone marrow hypoplasia were recorded (assessed by histological examination of the trephine biopsies). Three out of five patients experienced bone marrow hypoplasia or decreased platelet or neutrophil count. No AEs in the clinical trial were classified as serious, and all AEs registered (apart from one grade 1 case of eye floaters) have previously been described in single-agent AZA studies. A detailed summary of safety data can be seen in Supplementary Table 3.
Clinical and molecular outcome
Two patients were evaluated at cycle 12. P4, who were in SD at inclusion, were still in SD at the evaluation following 12 cycles of AZA and three vaccinations and could therefore continue to receive a fourth vaccination. The patient continued in the trial for another three months before showing signs of PD. P1 showed signs of disease progression at cycle 12 and shortly thereafter developed secondary AML. After discontinuing the study, P1 continued with AZA for a few cycles and was then shifted to low dose cytarabine, and managed to survive 30.6 months from MDS diagnosis, which was twice as long as the average survival of the four other participants. The remaining three patients (P2, P3, and P5) showed signs of PD after AZA cycle 9, which was confirmed by a bone marrow biopsy. P3 had a hypoplastic bone marrow when progressing to AML (see Supplementary Table 5) and the AML diagnosis was therefore based on a high prevalence of blasts in the peripheral blood.
The average time to progression (TTP) from inclusion was 5.2 months (range 2.8 to 7.6), while average survival was 11.3 months (range 4.3 to 22.2). The average survival from time of diagnosis was 18.1 months (range 10.9 to 30.6) in our patient cohort (Table 2).
Table 2.
Clinical outcome
| P1 | P2 | P3 | P4 | P5 | ||
|---|---|---|---|---|---|---|
| Evaluation time point | AZA cycles received (total) | 12 | 9 | 9 | 12 | 9 |
| Vaccinations received | 3 | 3 | 3 | 3 | 3 | |
| Blasts in BM | 5 | 67 | 30 | 19 | 29 | |
| Blasts peripheral | 0 | 8 | 8 | 1 | 1 | |
| Cytogenetics | Complex | NA | Trisomy 8, -Y | NA | Trisomy 8 | |
| Progression time point | AZA cycles received (total) | 12 | 9 | 9 | 15 | 9 |
| Vaccinations received | 3 | 3 | 3 | 4 | 3 | |
| TTP and survival | Time to progression (months from inclusion) | 7.5 | 2.8 | 4.8 | 7.6 | 3.5 |
| Survival (months from inclusion) | 22.2 | 4.3 | 12.8 | 8.7 | 8.3 | |
| Survival (months from diagnosis) | 30.6 | 10.9 | 19 | 14.2 | 15.7 | |
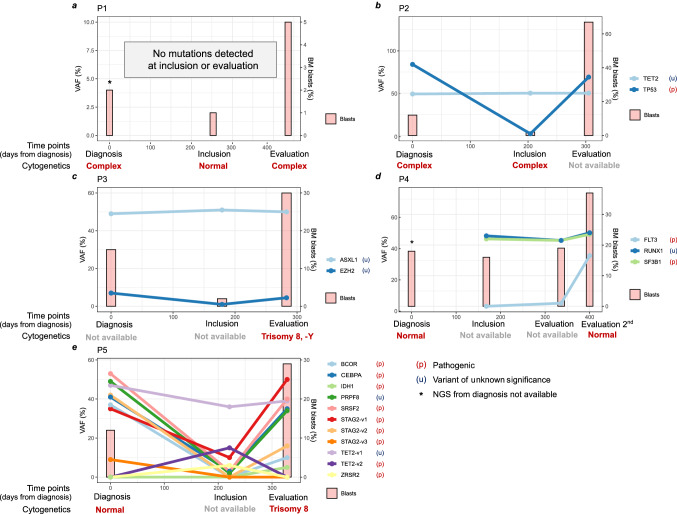
Targeted NGS for common mutations in MDS showed a decrease in variant allele frequency (VAF) for most mutations during the period from diagnosis to inclusion; however, the frequency of a few variants remained around 50% in P2, P3, and P4 (Fig. 2). At the evaluation time points, when patients progressed to AML, many of the mutations present at diagnosis had a secondary increase to high VAF levels. P5 had the highest mutational load and showed both reoccurrence of mutations present at diagnosis and new mutations in classical tumor suppressors and oncogenes at the evaluation time point. P5 had also developed de-novo trisomy 8 during progression to AML, which, interestingly, is associated with inflammatory disease, high WT-1 levels and T-cell induced suppression of hematopoiesis in some patients [38]. P3 also showed trisomy 8 at the evaluation time point, but due to missing data at diagnosis and inclusion, we do not know if the aberration also was present earlier. See Supplementary Table 4 for a detailed list of mutations and frequencies.
Fig. 2.
Molecular outcome. Evolution of genetic clones, as measured by targeted NGS, together with the percentage of bone marrow blasts and cytogenetic profile for patients 1–5(a–e). Missing data for cytogenetics is due to poor sample quality or because the cytogenetic analysis was not performed. P4 did not have clinical progression at the first evaluation time point and was consequently re-evaluated later, showing progression to AML
Specific immune responses
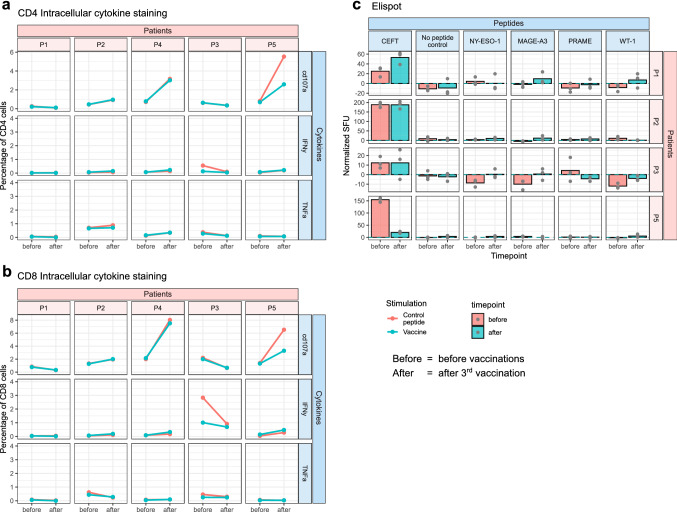
No vaccine-specific immune response could be detected for patients included in this clinical trial. For ICS, cells were stimulated with vaccine peptides for 12 h before analysis. There was no detectable secretion of IFNy and TNFa nor increased expression of CD107a (a sign of degranulation) compared to the control peptides, and there was no trend for increased signal intensity at later time points compared to baseline (Fig. 3a, b). ICS was also performed on bone marrow samples from the various time points, and no differences from control peptides were found in this material (Supplementary Fig. 2).
Fig. 3.
Immune response monitoring. Detection of specific immune responses. (a + b) Intracellular cytokine staining (ICS) performed on mononuclear cells from time points before and after vaccination, stimulated with either an irrelevant control peptide (red) or a pool of peptides from the vaccine (blue). Gated on (a) CD4 or (b) CD8 cells positive for CD107a, Interferon-gamma (IFNy) or Tumor Necrosis Factor-alpha (TNFa). (c) ELISpot comparing T cell reactivity before (red) and after (blue) vaccination. Experiment performed on PBMCs pre-stimulated with the individual vaccine peptides, an irrelevant peptide control, no peptide, or a cocktail of viral peptides (CEFT). Spot forming units (SFU) per 100.000 cells were normalized by subtracting the background signal from wells containing the irrelevant control peptide. ELISpot experiments were performed in triplicates. ELISpot for P4 is showed separately in Supplementary Fig. 3
For ELISpot, where cells were pre-stimulated for ten days, there was no significant difference from control peptides (Fig. 3c). For P4, stimulation was done with a pool of vaccine peptides instead of individual peptides due to the limited availability of frozen viable cells. Here, there was increased unspecific activation at the time point before vaccination compared to after vaccination; however, no significant difference was observed when comparing the pool of vaccine peptides to the irrelevant peptide control (Supplementary Fig. 3).
Changes in immune subsets
Exploratory multicolor flow cytometry revealed changes in the prevalence of several immune cell populations and expression of inflammatory markers at the evaluation time point compared to baseline (Supplemetary Fig. 4). However, the results were not significant, which partly could be due to the low sample size. There was a tendency for a decrease in central memory (CM: CD45RA− CCR7+) cells and an increase in naïve (CD45RA+ CCR7+) cells in the CD4 T cell population. In the CD8 population, a tendency for increased terminal effector (TE: CD45RA+ CCR7−) cells, and decreased CM, naïve, and effector memory (EM: CD45RA− CCR7−) cells were seen. Tregs remained unchanged, as did both checkpoint markers and markers of activation on CD8 cells. Not surprisingly, there was also an increase in CD34 positive cells at the later time point, reflecting the patients’ disease progression to AML. The fraction of circulating myeloblasts increased, as indicated by standard evaluation (Table 2), and more pronounced so on flowcytometric evaluation of CD34 + cell counts in the fraction of myeloid (CD3- and CD19-) PBMCs (Supplemetary Fig. 4). Comparing the flow cytometry data at the inclusion time point to PBMC from one healthy donor (HD), which was included in the experiment as a technical quality control, indicated similar exhaustion profiles in the MDS patient and the HD, but with more terminally differentiated T cell subsets in the MDS patients (see Supplementary Fig. 5).
Discussion
In this clinical trial, we combined epigenetic therapy in the form of AZA with a peptide vaccine composed of long synthetic peptides from NY-ESO-1119–143, PRAME423-447, MAGE-A3255-279, and WT1231-259, in patients with high-risk MDS. The peptides chosen represent antigens upregulated in malignant hematopoietic cells when exposed to HMAs [9–13]. As an adjuvant, Montanide ISA56 VG was used because of its well-established safety profile and capability of eliciting cellular immune responses in cancer patients [30, 39, 40].
Blood samples and bone marrow aspirates from before and after vaccination were analyzed using two independent methods for detecting immune responses toward the vaccine peptides. Neither ICS nor ELISpot showed a difference in response compared to control peptides that would support the induction of a vaccine-specific immune response in the trial participants. There were some changes in immune subsets during the trial, e.g., a reduction in CM cells in the CD4 population, an increase in TE cells in the CD8 population, and reduced expression of common immune checkpoint markers on both CD4 and CD8 cells. The changes were, however, not significant across the cohort, and since no specific immune response was found, it likely reflects the participants’ disease progression or could be secondary to the AZA treatment rather than a response induced by the vaccine.
Our primary endpoint was safety. In accordance with previously reported clinical trials investigating therapeutic cancer vaccination in patients with myeloid malignancies, [22, 30, 32, 35, 41, 42] the vaccine was well tolerated. AEs in this clinical trial were few, and there were none that classified as serious, indicating the vaccine was well tolerated. Two cases of bone marrow hypoplasia were reported by the pathology department in accordance with standard protocols. Since bone marrow hypoplasia had not been anticipated, further inclusion of patients to the clinical trial was put on hold. However, a second evaluation of all collected bone marrow samples with a semi-quantitative assessment of the cellularity by a hematopathologist later showed that only one of the reported cases was hypoplastic (Supplementary Table 5). Indeed, the observed myelosupression post-vaccination could be an early indication of vaccine efficacy, however, due to the lack of specific immune responses in laboratory assays and confounding factors, such as the myelosupressive potential of HMAs, the causality is graded as possible, yet unlikely.
All five patients progressed to AML after the third or fourth vaccination, with a mean TTP of 5.2 months (range 2.8 to 7.6) from inclusion. Survival was 18.1 months (range 10.9 to 30.6) from MDS diagnosis. Sequencing data from the different time points indicated that some clones that were suppressed by the AZA treatment reappeared at the evaluation time point when patients progress to AML, while other clones were unchanged throughout the treatment period.
There are conflicting results reported in the literature regarding the efficacy of vaccinations against CTAs and WT1 and their ability to generate a sustainable immune response.
Anguille et al. treated 30 patients with AML in post-remission phase with three DC vaccines loaded with different WT1 mRNA constructs. WT1 specific T cell responses increased > 1.5 fold in 6 out of 12 of their HLA-A0201 positive patients, and 13 of the 30 patients (43%) registered normalization of their WT1 transcript levels. They also found that patients receiving vaccination in their first complete remission had a relapse reduction rate of 25% [42]. In a study by Ueda et al., 26 MDS patients were treated with an affinity matured WT1 peptide in a water/oil emulsion. Here there was no clear relationship between outcome and induced T cell response, but they noted that 11 patients who were azacitidine non-responders survived for ≥ six months after termination of the vaccination, which was longer than predicted based on their risk profile [32]. Liu et al. randomized seven patients with AML, MDS, and CML to peptide vaccination against WT1 with either Montanide or poly-ICLC as an adjuvant. In their cohort, only one patient in the Montanide arm developed a specific immune response and none in the poly-ICLC group [30]. Van de Loosdrecht et al. matured DCs from an allogeneic AML cell line to create an off-the-shelf cancer vaccine. When treating 12 elderly AML patients in a phase I study, they found that four out of eight evaluable patients developed specific T cell responses against WT1, PRAME, NY-ESO-1, or MAGE-A3 [41]. They also found that patients with low blast count were more likely to develop an immune response, implying that anti-cancer vaccination should be given to patients with less advanced disease stage.
Few clinical trials have investigated the combination of HMAs with anti-cancer vaccinations.
In a phase I trial investigating the combination therapy of the HMA decitabine and a DC vaccine targeting MAGE‑A1, MAGE‑A3, and NY‑ESO‑1 in children with relapsed or therapy‑refractory neuroblastoma and sarcoma, Krishnadas et al. reported treatment success in two out of ten patients. One patient had a complete response following the combination therapy and remained disease-free 3.5 years after therapy, and the other, who was in remission at trial initiation, remained disease-free two years post-therapy [21]. One trial applied a similar approach in patients with MDS. Griffiths et al. treated 9 MDS patients with a combination of decitabine and a vaccine containing a full-length NY-ESO-1 protein fused to an antibody that selectively binds DCs. Out of the seven patients that reached the end of the study (two patients were lost to unrelated AEs), 86% developed a CD4 T cell response, as measured by ELISpot, compared to 33% at pre-treatment. 57% of patients developed a CD8 response, which was absent in all patients pre-treatment [22].
In our study, AZA was used instead of decitabine since AZA is the standard of care for high-risk MDS patients in the EU. It is worth considering if the efficacy of our vaccine would have been better in combination with decitabine instead of AZA, but pre-clinical studies suggest that the two HMAs are comparable in their capacity to upregulate methylated tumor antigens [14]. Trial participants entered the trial following six cycles of AZA in order to exclude those who tolerated AZA poorly and enrich the study with clinical responders. We hypothesized that AZA more likely would induce expression of the selected vaccine antigens in the AZA responding patients compared to the non-responders and that the responding patients, therefore, more likely would benefit from the vaccine. Recent data indicate that waiting six months after AZA initiation before treating patients with a therapeutic cancer vaccine, can negatively influence a vaccine’s ability to mobilize T cell responses toward the tumor cells [43, 44]. López et al. performed longitudinal studies on the expression of CTAs following HMA therapy and found that in AZA responding patients, the peak derepression of CTAs on both gene and protein level occurred already after completion of the first AZA series [43]. The authors concluded that the ideal timing for a CTA-targeted vaccine would be early during the AZA treatment. Another argument for reducing the time from MDS diagnosis to immunotherapy initiation is that the MDS microenvironment may become more suppressive with time, with an expansion of inhibitory mesenchymal stem cells, MDSCs and regulatory effector cells, as well as immune tolerance due to continuous exposure of tumor antigens and impaired DC function. [44, 45]
The reasons why our combinatorial approach failed to induce a detectable immune response or clinical benefit are presumably multifactorial. Firstly, the vaccine epitopes chosen from the full-length proteins, based on earlier described immune responses in cancer patients, might not have been immunogenic enough. Since the planning of this clinical trial commenced, studies have emerged with epitopes that could be better for therapeutic vaccination. However, the peptide sequences utilized for vaccination in this clinical trial do contain several strong and weak binders for all HLA-types of the trial participants when evaluated using NetMHCpan 4.0 [46]. Secondly, although the targeted peptides in our vaccine were chosen based on their upregulation following AZA treatment, the patients in our trial were not included based on verification of their expression in the individual patient. Thirdly, the immune system of high-risk MDS patients is more dysfunctional than that of patients with other cancer types where therapeutic cancer vaccination reportedly has been successful [44, 45, 47]. Although AZA also can induce demethylation of genes coding for inhibitory checkpoint molecules by itself which could aggravate T cell exhaustion [48], no difference in expression of checkpoint markers was evident when patients in our study were compared to a healthy donor control. Finally, even though some studies have shown that it is possible to induce immunological responses in patients with MDS [22, 32, 35, 49], there is reason to speculate if a therapeutic cancer vaccine strategy would be better suited for patients with lower-risk MDS. Another solution could be to co-treat patients with a potent immune stimulant, such as a checkpoint inhibitor, or by including epitopes in the vaccine that target immune regulatory pathways [50].
Supplementary Information
Below is the link to the electronic supplementary material.
Funding
Financial support to this study was provided by the Danish Cancer Society, grant no. R72-A4531 and R146-A9531-16-S2; Herlev-Gentofte hospital research grant and from an unrestricted research grant from Celgene inc.
Availability of data and material
Case report forms, trial master files, and experimental data are stored in accordance with EU regulation.
Code availability
R code for experimental statistical analysis is available upon request.
Declaration
Conflicts of interest
Celgene provided an unrestricted research grant for this clinical trial. They were allowed to read the final manuscript prior to publication but did not have any influence on the planning, conduction, or publishing of this clinical trial. There were no other conflicts of interest to report.
Ethics approval
The study was conducted in accordance with the Helsinki Declaration and ICH-GCP, and approved by the Danish Medicines Agency and the regional research ethics committee in Denmark. The trial is registered in the EU Clinical Trials Register (2014–002432-14) and clinicaltrials.gov (NCT02750995).
Consent to participate
Written informed consent was obtained from all patients in the clinical trial following oral and written information.
Consent for publication
All patients consented to the publication of anonymized data in scientific journals prior to receiving written and oral information hereof.
Footnotes
Sine Reker Hadrup and Daniel El Fassi are shared senior authors.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Sine Reker Hadrup, Email: sirha@dtu.dk.
Daniel El Fassi, Email: daniel.el.fassi.01@regionh.dk.
References
- 1.Zeidan AM, Shallis RM, Wang R, Davidoff A, Ma X. Epidemiology of myelodysplastic syndromes: why characterizing the beast is a prerequisite to taming it. Blood Rev. 2019;34:1–15. doi: 10.1016/j.blre.2018.09.001. [DOI] [PubMed] [Google Scholar]
- 2.Fenaux P, Mufti GJ, Hellstrom-Lindberg E, Santini V, Finelli C, Giagounidis A, et al. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: a randomised, open-label, phase III study. Lancet Oncol. 2009;10:223–232. doi: 10.1016/S1470-2045(09)70003-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bender CM, Pao MM, Jones PA. Inhibition of DNA methylation by 5-Aza-2’-deoxycytidine suppresses the growth of human tumor cell lines. Cancer Res. 1998;58:95–101. [PubMed] [Google Scholar]
- 4.Ørskov AD, Grønbæk K. DNA methyltransferase Inhibitors in myeloid cancer: clonal eradication or clonal differentiation? Cancer J. 2017;23:277–285. doi: 10.1097/PPO.0000000000000282. [DOI] [PubMed] [Google Scholar]
- 5.Mund C, Hackanson B, Stresemann C, Lübbert M, Lyko F. Characterization of DNA demethylation effects induced by 5-Aza-2′-deoxycytidine in patients with myelodysplastic syndrome. Cancer Res. 2005;65:7086–7090. doi: 10.1158/0008-5472.CAN-05-0695. [DOI] [PubMed] [Google Scholar]
- 6.Fonsatti E, Nicolay HJM, Sigalotti L, Calabrò L, Pezzani L, Colizzi F, et al. Functional up-regulation of human leukocyte antigen class I antigens expression by 5-aza-2′-deoxycytidine in cutaneous melanoma: immunotherapeutic implications. Clin Cancer Res. 2007;13:3333–3338. doi: 10.1158/1078-0432.CCR-06-3091. [DOI] [PubMed] [Google Scholar]
- 7.Zhou J, Yao Y, Shen Q, Li G, Hu L, Zhang X. Demethylating agent decitabine disrupts tumor-induced immune tolerance by depleting myeloid-derived suppressor cells. J Cancer Res Clin Oncol. 2017;143:1371–1380. doi: 10.1007/s00432-017-2394-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chiappinelli KB, Strissel PL, Desrichard A, Chan T, Baylin SB, Correspondence S. Inhibiting DNA methylation causes an interferon response in cancer via dsrna including endogenous retroviruses. Cell. 2015;162:974–986. doi: 10.1016/j.cell.2015.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Almstedt M, Blagitko-Dorfs N, Duque-Afonso J, Karbach J, Pfeifer D, Jäger E, et al. The DNA demethylating agent 5-aza-2’-deoxycytidine induces expression of NY-ESO-1 and other cancer/testis antigens in myeloid leukemia cells. Leuk Res. 2010;34:899–905. doi: 10.1016/j.leukres.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 10.Goodyear O, Agathanggelou A, Novitzky-Basso I, Siddique S, McSkeane T, Ryan G, et al. Induction of a CD8+ T-cell response to the MAGE cancer testis antigen by combined treatment with azacitidine and sodium valproate in patients with acute myeloid leukemia and myelodysplasia. Blood. 2010;116:1908–1918. doi: 10.1182/blood-2009-11-249474. [DOI] [PubMed] [Google Scholar]
- 11.Siebenkäs C, Chiappinelli KB, Guzzetta AA, Sharma A, Jeschke J, Vatapalli R, et al. Inhibiting DNA methylation activates cancer testis antigens and expression of the antigen processing and presentation machinery in colon and ovarian cancer cells. PLoS ONE. 2017;12:e0179501. doi: 10.1371/journal.pone.0179501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Srivastava P, Paluch BE, Matsuzaki J, James SR, Collamat-Lai G, Blagitko-Dorfs N, et al. Induction of cancer testis antigen expression in circulating acute myeloid leukemia blasts following hypomethylating agent monotherapy. Oncotarget. 2016;7:12840–12856. doi: 10.18632/oncotarget.7326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gang AO, Frøsig TM, Brimnes MK, Lyngaa R, Treppendahl MB, Grønbæk K, et al. 5-Azacytidine treatment sensitizes tumor cells to T-cell mediated cytotoxicity and modulates NK cells in patients with myeloid malignancies. Blood Cancer J. 2014;4:e197. doi: 10.1038/bcj.2014.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saleh MH, Wang L, Goldberg MS. Improving cancer immunotherapy with DNA methyltransferase inhibitors. Cancer Immunol Immunother. 2016;65:787–796. doi: 10.1007/s00262-015-1776-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Atanackovic D, Arfsten J, Cao Y, Gnjatic S, Schnieders F, Bartels K, et al. Cancer-testis antigens are commonly expressed in multiple myeloma and induce systemic immunity following allogeneic stem cell transplantation. Blood. 2007;109:1103–1112. doi: 10.1182/blood-2006-04-014480. [DOI] [PubMed] [Google Scholar]
- 16.Mischo A, Kubuschok B, Ertan K, Preuss K-D, Romeike B, Regitz E, et al. Prospective study on the expression of cancer testis genes and antibody responses in 100 consecutive patients with primary breast cancer. Int J Cancer. 2006;118:696–703. doi: 10.1002/ijc.21352. [DOI] [PubMed] [Google Scholar]
- 17.Goodyear O, Piper K, Khan N, Starczynski J, Mahendra P, Pratt G, et al. CD8+ T cells specific for cancer germline gene antigens are found in many patients with multiple myeloma, and their frequency correlates with disease burden. Blood. 2005;106:4217–4224. doi: 10.1182/blood-2005-02-0563. [DOI] [PubMed] [Google Scholar]
- 18.Baumgaertner P, Costa Nunes C, Cachot A, Maby-El Hajjami H, Cagnon L, Braun M, et al. Vaccination of stage III/IV melanoma patients with long NY-ESO-1 peptide and CpG-B elicits robust CD8 + and CD4 + T-cell responses with multiple specificities including a novel DR7-restricted epitope. Oncoimmunology. 2016;5:e1216290. doi: 10.1080/2162402X.2016.1216290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Connerotte T, Pel AV, Godelaine D, Tartour E, Schuler-Thurner B, Lucas S, et al. Functions of anti-MAGE T-cells induced in melanoma patients under different vaccination modalities. Cancer Res. 2008;68:3931–3940. doi: 10.1158/0008-5472.CAN-07-5898. [DOI] [PubMed] [Google Scholar]
- 20.Jäger E, Karbach J, Gnjatic S, Neumann A, Bender A, Valmori D, et al. Recombinant vaccinia/fowlpox NY-ESO-1 vaccines induce both humoral and cellular NY-ESO-1-specific immune responses in cancer patients. Proc Natl Acad Sci. 2006;103:14453–14458. doi: 10.1073/pnas.0606512103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krishnadas DK, Shusterman S, Bai F, Diller L, Sullivan JE, Cheerva AC, et al. A phase I trial combining decitabine/dendritic cell vaccine targeting MAGE-A1, MAGE-A3 and NY-ESO-1 for children with relapsed or therapy-refractory neuroblastoma and sarcoma. Cancer Immunol Immunother. 2015;64:1251–1260. doi: 10.1007/s00262-015-1731-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Griffiths EA, Srivastava P, Matsuzaki J, Brumberger Z, Wang ES, Kocent J, et al. NY-ESO-1 vaccination in combination with decitabine induces antigen-specific T-lymphocyte responses in patients with myelodysplastic syndrome. Clin Cancer Res. 2017;24:1019–1029. doi: 10.1158/1078-0432.CCR-17-1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Greenberg PL, Tuechler H, Schanz J, Sanz G, Garcia-Manero G, Solé F, et al. Revised international prognostic scoring system for myelodysplastic syndromes. Blood. 2012;120:2454–2465. doi: 10.1182/blood-2012-03-420489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheson BD, Greenberg PL, Bennett JM, Lowenberg B, Wijermans PW, Nimer SD, et al. Clinical application and proposal for modification of the international working group (IWG) response criteria in myelodysplasia. Blood. 2006;108:419–425. doi: 10.1182/blood-2005-10-4149. [DOI] [PubMed] [Google Scholar]
- 25.Zarour HM, Maillere B, Brusic V, Coval K, Williams E, Pouvelle-Moratille S, et al. NY-ESO-1 119–143 Is a promiscuous major histocompatibility complex class II T-helper epitope recognized by Th1- and Th2-type tumor-reactive CD4+ T Cells. Cancer Res. 2002;62:213–218. [PubMed] [Google Scholar]
- 26.Neek M, Tucker JA, Kim TI, Molino NM, Nelson EL, Wang SW. Co-delivery of human cancer-testis antigens with adjuvant in protein nanoparticles induces higher cell-mediated immune responses. Biomaterials. 2018;156:194–203. doi: 10.1016/j.biomaterials.2017.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kessler JH, Beekman NJ, Bres-Vloemans SA, Verdijk P, van Veelen PA, Kloosterman-Joosten AM, et al. Efficient identification of novel hla-A*0201–presented cytotoxic t lymphocyte epitopes in the widely expressed tumor antigen prame by proteasome-mediated digestion analysis. J Exp Med. 2001;193:73–88. doi: 10.1084/jem.193.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Quintarelli C, Dotti G, Hasan ST, Angelis BD, Hoyos V, Errichiello S, et al. High-avidity cytotoxic T lymphocytes specific for a new PRAME-derived peptide can target leukemic and leukemic-precursor cells. Blood. 2011;117:3353–3362. doi: 10.1182/blood-2010-08-300376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang H-G, Chen H-S, Peng J-R, Shang X-Y, Zhang J, Xing Q, et al. Specific CD8+ T cell responses to HLA-A2 restricted MAGE-A3 p271–279 peptide in hepatocellular carcinoma patients without vaccination. Cancer Immunol Immunother. 2007;56:1945–1954. doi: 10.1007/s00262-007-0338-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu H, Zha Y, Choudhury N, Malnassy G, Fulton N, Green M, et al. WT1 peptide vaccine in montanide in contrast to poly ICLC, is able to induce WT1-specific immune response with TCR clonal enrichment in myeloid leukemia. Exp Hematol Oncol. 2018;7:1. doi: 10.1186/s40164-018-0093-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Doubrovina E, Carpenter T, Pankov D, Selvakumar A, Hasan A, O’Reilly RJ. Mapping of novel peptides of WT-1 and presenting HLA alleles that induce epitope-specific HLA-restricted T cells with cytotoxic activity against WT-1+ leukemias. Blood. 2012;120:1633–1646. doi: 10.1182/blood-2011-11-394619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ueda Y, Ogura M, Miyakoshi S, Suzuki T, Heike Y, Tagashira S, et al. Phase 1/2 study of the WT1 peptide cancer vaccine WT4869 in patients with myelodysplastic syndrome. Cancer Sci. 2017;108:2445–2453. doi: 10.1111/cas.13409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oka Y, Tsuboi A, Nakata J, Nishida S, Hosen N, Kumanogoh A, et al. Wilms’ tumor gene 1 (WT1) peptide vaccine therapy for hematological malignancies: from CTL epitope identification to recent progress in clinical studies including a cure-oriented strategy. Oncol Res Treat. 2017;40:682–690. doi: 10.1159/000481353. [DOI] [PubMed] [Google Scholar]
- 34.Uttenthal B, Martinez-Davila I, Ivey A, Craddock C, Chen F, Virchis A, et al. Wilms’ tumour 1 (WT1) peptide vaccination in patients with acute myeloid leukaemia induces short-lived WT1-specific immune responses. Br J Haematol. 2014;164:366–375. doi: 10.1111/bjh.12637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brayer J, Lancet JE, Powers J, List A, Balducci L, Komrokji R, et al. WT1 vaccination in AML and MDS: a pilot trial with synthetic analog peptides. Am J Hematol. 2015;90:602–607. doi: 10.1002/ajh.24014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wickham H, Averick M, Bryan J, Chang W, McGowan LD, François R, et al. Welcome to the Tidyverse. J Open Source Softw. 2019;4:1686. doi: 10.21105/joss.01686. [DOI] [Google Scholar]
- 37.Kopanos C, Tsiolkas V, Kouris A, Chapple CE, Albarca Aguilera M, Meyer R, et al. Varsome: the human genomic variant search engine. Bioinformatics. 2019;35:1978–1980. doi: 10.1093/bioinformatics/bty897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oka S, Ono K, Nohgawa M. The acquisition of trisomy 8 associated with behçet’s-like disease in myelodysplastic syndrome. Leuk Res Rep. 2020;13:100196. doi: 10.1016/j.lrr.2020.100196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fox CB, Haensler J. An update on safety and immunogenicity of vaccines containing emulsion-based adjuvants. Expert Rev Vaccines. 2013;12:747–758. doi: 10.1586/14760584.2013.811188. [DOI] [PubMed] [Google Scholar]
- 40.Aucouturier J, Ascarateil S, Dupuis L. The use of oil adjuvants in therapeutic vaccines. Vaccine. 2006;24:S44–S45. doi: 10.1016/j.vaccine.2005.01.116. [DOI] [PubMed] [Google Scholar]
- 41.van de Loosdrecht AA, van Wetering S, Santegoets SJAM, Singh SK, Eeltink CM, den Hartog Y, et al. A novel allogeneic off-the-shelf dendritic cell vaccine for post-remission treatment of elderly patients with acute myeloid leukemia. Cancer Immunol Immunother. 2018;67:1505–1518. doi: 10.1007/s00262-018-2198-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Anguille S, Van de Velde AL, Smits EL, Van Tendeloo VF, Juliusson G, Cools N, et al. Dendritic cell vaccination as post-remission treatment to prevent or delay relapse in acute myeloid leukemia. Blood. 2017;130:1713–1721. doi: 10.1182/blood-2017-04-780155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.López AMH, Chen-Liang TH, Zurdo M, Carrillo-Tornel S, Panadero J, Salido EJ, et al. Cancer testis antigens in myelodysplastic syndromes revisited: a targeted RNA-seq approach. OncoImmunology. 2020;9:1824642. doi: 10.1080/2162402X.2020.1824642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Leeuwen-Kerkhoff N van, Westers TM, Poddighe PJ, Povoleri GAM, Timms JA, Kordasti S et al (2021) Reduced frequencies and functional impairment of dendritic cell subsets and non-classical monocytes in myelodysplastic syndromes. Haematologica. 10.3324/haematol.2020.268136 [DOI] [PMC free article] [PubMed]
- 45.Pleyer L, Valent P, Greil R. Mesenchymal stem and progenitor cells in normal and dysplastic hematopoiesis—masters of survival and clonality? Int J Mol Sci. 2016;17:1009. doi: 10.3390/ijms17071009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jurtz V, Paul S, Andreatta M, Marcatili P, Peters B, Nielsen M. NetMHCpan-4.0: improved peptide–MHC class I interaction predictions integrating eluted ligand and peptide binding affinity data. J Immunol. 2017;199:3360–3368. doi: 10.4049/jimmunol.1700893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rezvani K, Yong ASM, Mielke S, Jafarpour B, Savani BN, Le RQ, et al. Repeated PR1 and WT1 peptide vaccination in montanide-adjuvant fails to induce sustained high-avidity, epitope-specific CD8+ T cells in myeloid malignancies. Haematologica. 2011;96:432–440. doi: 10.3324/haematol.2010.031674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ørskov AD, Treppendahl MB, Skovbo A, Holm MS, Friis LS, Hokland M, et al. Hypomethylation and up-regulation of PD-1 in T cells by azacytidine in MDS / AML patients: a rationale for combined targeting of PD-1 and DNA methylation. Oncotarget. 2015;6:9612–9626. doi: 10.18632/oncotarget.3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Di Stasi A, Jimenez AM, Minagawa K, Al-Obaidi M, Rezvani K. Review of the results of WT1 peptide vaccination strategies for myelodysplastic syndromes and acute myeloid leukemia from nine different studies. Front Immunol. 2015;6:36. doi: 10.3389/fimmu.2015.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kjeldsen JW, Iversen TZ, Engell-Noerregaard L, Mellemgaard A, Andersen MH, Svane IM. Durable clinical responses and long-term follow-up of stage III–IV non-small-cell lung cancer (NSCLC) patients treated with ido peptide vaccine in a phase i study—a brief research report. Front Immunol. 2018;9:2145. doi: 10.3389/fimmu.2018.02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Case report forms, trial master files, and experimental data are stored in accordance with EU regulation.
R code for experimental statistical analysis is available upon request.