Abstract
Background
The development of tumor tissue-infiltrating regulatory T cell (Treg) is incompletely understood. This study investigates the role of retinoblastoma cell (Rbc)-derived Twist‑related protein 1 (Twist) in the Treg development.
Methods
The surgically removed Rb tissues were collected. Rbcs were cultured with CD4+ T cells to assess the role of Rbc-derived Twist in the Treg generation.
Results
We found that more than 90% Rbcs expressed Twist. Foxp3+ Tregs were detected in the Rb tissues that were positively correlated with the Twist expression in Rbcs, negatively associated with Rb patient survival and sight survival. Treating Rbcs with hypoxia promoted the Twist expression that could be detected in the cytoplasm, nuclei and on the cell surface. Twist activated CD4+ T cells by binding the TLR4/myeloid differentiation factor 2 complex and promoted the transforming growth factor-β-inducible early gene 1 product and Foxp3 expression. These Rbc-induced Foxp3+ Tregs showed immune-suppressive function on CD8+ T cell proliferation.
Conclusions
Rbcs express Twist, that induces IL-4+ Foxp3+ Tregs; the latter can inhibit CD8+ cytotoxic T cell activities. Therefore, Twist may play an important role in the pathogenesis of Rb.
Keywords: Retinoblastoma, Regulatory T cell, Apoptosis, Twist, Tumor tolerance
Introduction
Retinoblastoma (Rb) is a pediatric retinal cancer that affects one eye or both eyes [1]. Rb is also thought as an intrinsic brain tumor that originates from neuroglial stem or progenitor cells [2]. The prevalence of Rb is about 1/16,000 newborns worldwide [1]. The pathogenesis of Rb is unclear; genetic factors play an important role in Rb [3]. In the most Rb cases, the RB1 gene is damaged. The RB1 gene locates on chromosome 13q14; this gene encodes the RB protein. RB protein is a tumor suppressor. Loss of RB protein results in uncontrolled cell division. Thus, the loss of RB protein may induce retinal cone cell precursors to divide in an uncontrolled fashion and develop into Rb [4]; the underlying mechanism remains to be further investigated.
In general, the immune system can recognize tumor antigens to elicit antigen-based immune response to destroy tumor cells in the body. CD8+ cytotoxic T cells are the most important immune cell fraction in the tumor immune responses [5]. By releasing cytotoxic molecules, such as perforin and granzyme B, CD8+ cytotoxic cells induce targeted tumor cell apoptosis and death [5]. However, the anti-tumor mechanism may be compromised by the phenomenon of tumor tolerance [6]. Regulatory T cells (Treg) are one of the major cell fractions to counteract with the anti-tumor system [7]. Upon activation, Tregs release immune-suppressive mediators, such as transforming growth factor (TGF)-β, to induce anti-tumor cell, such as CD8+ cytotoxic T cell, apoptosis. Whether Tregs play a role in Rb pathogenesis remains further investigated. Although the role of Treg in contributing to tumor tolerance has been extensively studied [8], the Treg development under a tumor environment, such as in Rb tissues, remains incompletely understood.
Twist protein 1 (Twist, in short) is an apoptosis inhibitor protein; it plays an important role in the carcinogenesis, cancer cell metastasis and drug resistance. By interfering with apoptosis machinery, Twist promotes cancer metastasis [9]. The over-expression of Twist has been found in many cancer tissues, such as breast, prostate and gastric carcinomas, melanomas, osteosarcomas, rhabdomyosarcomas [9]; whether Twist is also expressed by Rb tissues is unclear. Based on the information above, we hypothesize that Twist may play an important role in the Rb pathogenesis. In this study, we collected the surgically removed Rb tissues from the clinic. By multiple immunologic and biochemistry approaches, we found that Rb cells (Rbc) expressed high levels of Twist. The results also demonstrated that Twist played a critical role in promoting the Treg development, that suppresses CD8+ T cell activities.
Materials and methods
Reagents
Fluorescence-labeled antibodies of EpCAM (Alexa Fluor® 488), Twist (Alexa Fluor® 594), CD4 (Alexa Fluor® 546), CD25 (Alexa Fluor® 594), CD3 (Alexa Fluor® 488), CD8 (Alexa Fluor® 790), Foxp3 (Alexa Fluor® 680), CD127 (Alexa Fluor® 680), and HIF-1α (Alexa Fluor® 790), RNAi reagent kits of HIF-1α, Twist, TLR4, MD-2 and TIEG1, antibodies of HIF-1α (Clone#: 28b), Twist (Twist2C1a), TLR4 [25], CD103 (BP6), CD3 (PS1), CD28 (3H1179), Foxp3 (F-9) and TIEG1 (95-D) were purchased from Santa Cruz Biotech (Santa Cruz, CA). Anti-TLR4/MD2 complex antibody (7E3) was purchased from abcam (Cambridge, MA). Reagent kits of annexin v, IL-2 protein and ChIP were purchased from Sigma Aldrich (St. Louis., MO). Materials and reagents for RT-qPCR and Western blotting were purchased from Invitrogen (Carlsbad, CA).
Patients
The experimental procedures were approved by the Human Ethical Committee at Shenzhen University. A written informed consent was obtained from each patient’s guardian. From July 2015 to June 2019, patients with Rb were recruited at the Department of Ophthalmology in the Affiliated First, Second, Third and Tumor Hospitals of Shenzhen University. The Rbc diagnosis and management were carried out by our ophthalmologists. In total, 50 Rb patients, including 35 males (70%), 15 females (30%), were recruited into this study. Age: 3–96 months (median: 15.5 months). Rbs were found in the left eye (24 cases; 48%) or the right eye (20 cases; 40%), or both eyes (6 cases; 12%). Tumor stages (based on published criteria [10]) and cases: I, 6 (12%); II, 15 (30%); III, 27 (54%); IV, 2 (4%). Tumor size and cases: ≥ 2 cm, 22 (44%); ≤ 2 cm, 28 (56%). Tumor metastasis: 7 cases (14%). Tumor differentiated: 43 cases (86%); non-differentiated: 7 cases (14%). All Rb cases were treated with surgery by our ophthalmologists. The Rb tissues were collected in the operation facilitates for further experiments.
Histology
The Rb tissues were fixed with 4% formalin overnight at room temperature. Paraffin sections were cut and stained with hematoxylin and eosin. The histology of the Rb tissues was observed with a light microscope by pathologists.
Immunohistochemistry
A piece of Rb tissue was snap frozen in liquid nitrogen. Cryosections were prepared, dried at room temperature overnight. The sections were fixed with cold acetone for 20 min, incubated with 1% bovine serum albumin for 30 min, incubated with anti-Twist antibody (diluted in 1:100) or isotype IgG (negative control) overnight at 4 °C, washed with phosphate-buffered saline (PBS) 3 times, incubated with peroxidase-conjugated secondary antibody for 2 h at room temperature. The sections were developed by 3,3′-Diaminobenzidine (DAB), mounted with cover slips and observed under a light microscope. All the sections were coded. Observers were not aware of the code to avoid the observer bias.
Rb single-cell preparation
The surgically removed Rb tissues were collected, cut into small pieces (2 × 2×2 mm), incubated with collagenase IV (1 mg/ml) at 37 °C for 30 min with mild agitation. The lysates were filtered through a cell strainer (70 µm first, then 40 µm), centrifuged at 1000g for 5 min. The pellets were resuspended in RPMI1640 culture medium. Cell viability was greater than 99% as checked by Trypan blue exclusion assay.
Collection of peripheral blood samples
Peripheral blood samples were obtained from human subjects (5–10 ml per person) by an ulnar vein puncture. Mononuclear cells were isolated from the samples by the Percoll gradient density centrifugation.
Cell culture
Cells were cultured (106 cells/ml) in DMEM (Dulbecco’s Modified Eagle Medium) or RPMI1640 medium. The medium was supplemented with 10% fetal bovine serum, 100 U/ml penicillin, 0.1 mg/ml streptomycin and 2 mM glutamine. Cell viability was greater than 99% as checked by Trypan blue exclusion assay before handling over further experiments. For hypoxia studies, cells were cultured at 1% oxygen in an O2 Control In Vitro Glove Box (Coy Laboratory Products) for 24 h.
Flow cytometry (FCS)
Single cells were prepared and treated with either surface staining or intracellular staining following established procedures [11]. Cells were analyzed with a flow cytometer (FACSCanto II; BD Bioscience). Data were analyzed with a software package FlowJo (Tree Star Inc., Ashland, OR) with the isotype IgG-staining data as a gating reference. In the analysis, at the SSC and FSC panels, cell populations at the low left quadrant were gated out to eliminate dead cells and cell debris.
Real-time quantitative RT-PCR (RT-qPCR)
Total RNA was extracted from cells collected from experiments. The RNA was converted to cDNA with a reverse transcription reagent kit following the manufacturer’s instruction. The samples were amplified in a qPCR device (CFX96 Touch System; Bio-Rad) with the SYBR Green Master Mix and the presence of primers of Twist (agtcttacgaggagctgcag and aggaagtcgatgtacctggc), or HIF-1α (cagtcgacacagcctggata and ccacctcttttggcaagcat), or TIEG1 (cccgttgtgcagagttcaaa and gggtggctacagatgtgact), or Foxp3 (cccggatgtgagaaggtctt and cttgtcggatgatgccacag). The results were calculated with the 2−∆∆Ct method and presented as relative quantification (RQ).
Protein extraction
Cells were collected from experiments and lysed with a lysis buffer (10 mM HEPES; 1.5 mM MgCl2; 10 mM KCl; 0.5 mM DTT; 1 mM EDTA; 0.05% NP40 and protease inhibitor cocktail). Lysates were centrifuged at 13000 g for 10 min. Supernatant was collected and used as the cytosolic proteins. Pellets were resuspended in a nuclear lysis buffer (5 mM HEPES; 1.5 mM MgCl2SO4; 4.6 M NaCl; 0.2 mM EDTA; 0.5 mM DTT; 26% glycerol) and incubated for 30 min. Lysates were centrifuged at 13,000g for 10 min. Supernatant was collected and used as the nuclear proteins. All procedures were carried out at 4 °C.
Western blotting
Total proteins were extracted from cells collected from experiments, fractioned with sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred onto a polyvinylidene fluoride membrane. After incubating with 5% skim milk to block non-specific binding, the membrane was incubated with the primary antibodies (diluted in 1:500; see figures for individual antibodies) at 4 °C overnight, washed with TBST (Tris-buffered saline containing 0.05% Tween 20) 3 times, incubated with secondary antibodies (conjugated with peroxidase; diluted to 1:5000) at room temperature for 2 h, washed with TBST 3 times. The immunoblots on the membrane were developed with the enhanced chemiluminescence and photographed in an imaging device.
RNA interference (RNAi)
Expression of HIF-1α and Twist in Rbcs, TLR4, MD-2 and TIEG1 in T cells or Rbcs was depleted by RNAi with reagent kits purchased from Santa Cruz Biotech following the manufacturer’s instructions. The RNAi effects were checked in the cells by Western blotting 48 h after RNAi treatment.
Immune cell isolation
EpCAM+ Rbcs, CD3+ CD4+ CD25− T cells, CD3+ CD8+ CD25− T cells and CD3+ CD4+ CD25+ CD127− Tregs were purified for the experiments. Cells were labeled with the indicated antibodies (conjugated with relevant fluorescence) and purified by FCS. Cell purity was checked by FCS. If purity did not reach 95%, FCS was repeated with the cells.
Co-immunoprecipitation (co-IP)
Proteins were extracted from relevant cells and precleared by incubating with protein G Sepharose beads for 2 h. The beads were removed from the samples by centrifugation (3,000 g, 10 min); supernatant was collected and incubated with antibodies of Twist or TLR4/MD-2 (diluted to 1:300) overnight. Immunocomplexes in the samples were precipitated by incubating with protein G Sepharose beads for 2 h. The samples were centrifuged at 13,000 g for 10 min. The beads were collected; proteins on the beads were eluted and analyzed by Western blotting. All the procedures were performed at 4 °C.
Chromatin IP (ChIP)
Cells were fixed with 1% formalin for 15 min to cross-link the DNA with surrounding proteins. The cells were lysed with a lysis buffer and followed by sonication to shear the DNA into small pieces. The samples were then processed with the IP procedures as described above. DNA/protein complexes were eluted from the Sepharose beads, DNA was recovered with a DNA extraction reagent kit following the manufacturer’s instruction, and then analyzed by qPCR in the presence of Twist promoter primers (tgaatggtttgggaggacga and aggctcttatacctccgtgc). The results were presented as fold change against the input.
Assessment of CD8+ T cell proliferation
Naive CD8+ T cells were isolated from healthy person blood samples by FCS, labeled with carboxyfluorescein succinimidyl ester (CFSE; 1 µmol/ml for 8 min at 37 °C). CD8+ T cells were cultured with Tregs at a ratio of 1:1 for 3 days in the presence of anti-CD3/CD8 Abs (T cell activators; 5 µg/ml each). Proliferation of CFSE-labeled cells was analyzed with a flow cytometer.
Assessment of cell apoptosis
Cells were stained with propidium iodide (2 µg/ml) and Annexin V-PE reagents following the manufacturer’s instruction. The cells were analyzed with a flow cytometer. Annexin V+ or PI+ Annexin V+ cells were regarded as apoptotic cells.
Rb patient follow-up
After surgery, all the Rb patients were followed up for every 3 months by email or telephone. If necessary, patients were asked to come to hospital to do relevant examinations.
Statistics
Each experiment was repeated at least 3 times. Each sample was analyzed in triplicate; average of the 3 readouts was used as one datum. Data are presented as mean ± SEM or median (IQR). The difference between two groups was determined by the Student t test or the Mann–Whitney test. Multiple comparisons were carried out with ANOVA followed by the Dunnett’s test or the Bonferroni test. Pearson correlation coefficient test was performed between two group data if necessary. P < 0.05 was set as a significant criterion.
Results
Rbcs express Twist
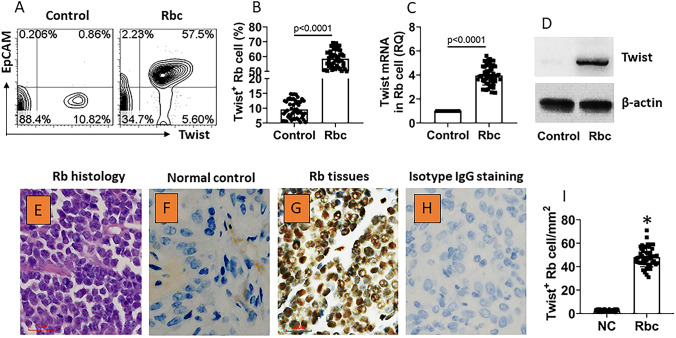
The Rbc samples were collected from surgically removed Rb tissues. The marginal non-tumor tissues (determined by pathologists) were used as normal controls (NC). Single cells were prepared with the tissues and analyzed by flow cytometry (FCS). The results showed that almost all Rbcs were Twist positive, while less than 10% Twist+ cells were detected in control cells (Fig. 1a, b). Using epithelial cell adhesion molecule (EpCAM) as the cell marker of Rbcs [12], Rbcs were isolated by FCS; the cells were analyzed by RT-qPCR and Western blotting. The results showed that the Twist expression was markedly higher in Rbcs than that in control cells at both mRNA and protein levels (Fig. 1c, d). Each sample was assessed by histology that showed the typical Rb histology structure (Fig. 1e). We also found Twist expression in Rb cells by immunohistochemistry (Fig. 1f–i). The results indicate that Rbcs express high Twist levels.
Fig. 1.
Rb cells express Twist. Surgically removed Rb tissues (n = 50) were collected; single cells were prepared from the samples and analyzed by flow cytometry (FCS). a The plots in upper right quadrant show Twist+ Rb cells (EpCAM is regarded as a specific Rb cell marker). b The bars show summarized Twist+ cell counts. c, d Rb cells were isolated from the samples and analyzed by RT-qPCR and Western blotting. c The bars show Twist mRNA levels in the samples. d The immunoblots show Twist protein levels in the samples. e A representative Rb histology image. f, g Representative immunohistochemistry staining images show Twist positive staining in normal control (NC) tissues (F), Rb tissues (G). h A representative image shows negative staining (with isotype IgG). Original magnification: × 400 (the bar 20 µm). I, bars show mean ± SEM (*, p < 0.01, compared with the NC group) of positive stained cell counts of panels e, f. Data of b, c are presented as median with interquartile range (IQR). Statistics: The Mann–Whitney test. Data of a, e–h are from one experiment that represent 50 independent experiments
Foxp3+ Tregs are detected in the Rb tissues
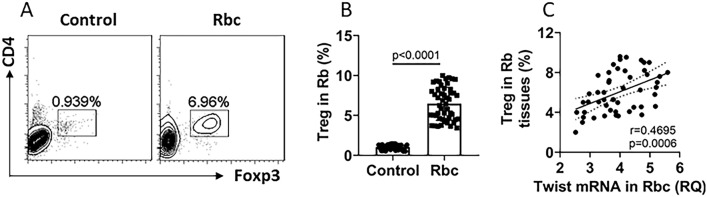
Tregs are the important cellular component in the tumor tolerance [13]. We next assessed Tregs in the Rb tissues. By FCS, about 8% Tregs were detected in the single cells isolated from the Rb tissues (Fig. 2a, b). A positive correlation was detected between Twist mRNA levels in Rbcs and the Treg counts in the Rb tissues (Fig. 2c). The results suggest that Rbc-derived Twist may be involved in the Treg development in Rb tissues.
Fig. 2.
Rb tissue Treg counts are positively correlated with Twist expression in Rbs. Rb samples (n = 50) were collected, single cells were prepared and analyzed by FCS. a Gated FCS plots show Treg counts (these cells were also CD25+; not shown). b Bars show summarized Treg counts. c Scatter dot plots show positive correlation between Treg counts and Twist mRNA levels (data are presented in Fig. 1) in Rb cells. Data of panel a are from one experiment that represent 50 independent experiments. Data of bars are presented as median with interquartile range (IQR). Statistics: the Mann–Whitney test
Hypoxia promotes Twist expression in Rbcs
Hypoxia is a common phenomenon in cancer tissues, in which the hypoxia-inducible factor-1α (HIF-1α) is abnormally active [14]. Thus, we wondered whether hypoxia and HIF-1α were associated with the high Twist expression in Rbcs. To this end, Rbcs and normal cells (NC, isolated from the adjacent normal tissues of Rb) were prepared and analyzed by RT-qPCR. The results showed that a positive correlation was detected between the Twist mRNA levels and the HIF-1α mRNA levels in Rbcs (Fig. 3a), indicating that the HIF-1α may be involved in the Twist expression in Rbcs. To test this, Rbcs and NCs were treated with hypoxia or normoxia in the culture. As compared with NCs, hypoxia significantly increased the Twist and HIF-1α expression in Rbcs (Fig. 3b–d); the HIF-1α levels were markedly increased in the TWIST1 gene promoter (Fig. 3e). The increase in the Twist expression in Rbcs was abolished by knocking down of HIF-1α (Fig. 3b–f). As shown by FCS, the Twist expression was detectable on the surface of Rbc cells (stained by the surface-staining approach), that was markedly up-regulated by hypoxia and abolished by the HIF-1α knockdown (Fig. 3g, h). The results indicate that hypoxia can increase the Twist expression in Rbcs through up-regulating the HIF-1α expression. Twist can be detected on the surface of Rbcs, implicating that Twist may interact with other immune cells in a cell–cell-contact manner.
Fig. 3.
Hypoxia induces the Twist expression in Rbcs. a Scatter plots show a positive correlation between the expression of Twist and HIF-1α in Rbcs. b–d Rbcs and NCs (normal cells isolated from the adjacent tissue of Rb) were treated with hypoxia or normoxia for 24 h. Bars show HIF-1α (b) and Twist (c) mRNA levels. Immunoblots show Twist protein levels in Rbc cytoplasm (d) and nuclei (e). f Bars show HIF-1α levels at the TWIST1 promoter locus. g HIF-1α RNAi results. h Bars show Twist+ Rbc counts in panel I (the group labels are the same as panel h). i Gated FCS plots show Twist-staining on the surface of Rbcs. RNAi: HIF-α RNAi. cRNAi: Control RNAi. Data of bars are presented as mean ± SEM. *, p < 0.01, compared with the NC/normoxia group. #, p < 0.01, compared with the Rbc/hypoxia group. $, p < 0.01, compared with the Rbc/hypoxia/RNAi group. Statistical methods: The Pearson correlation coefficient test (a) or ANOVA followed by the Bonferroni test (b, c, f, h)
Hypoxia-primed Rbcs induce Treg development
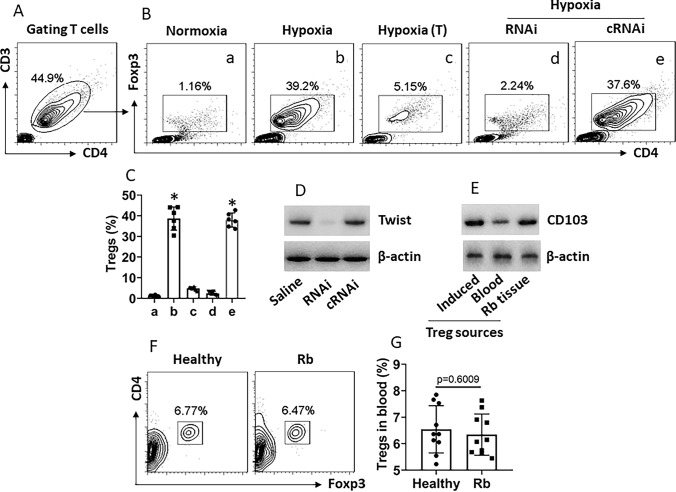
Since the Treg counts in the Rb tissues are positively correlated with the Twist expression in Rbcs as shown by Fig. 1 and Twist can be detected on the surface of Rbcs (Fig. 3), we hypothesize that Rbc-derived Twist may promote the Treg development. To test this, Rbcs were treated with or without hypoxia to up-regulating the Twist expression and cultured with CD4+ CD25− T cells (isolated from the peripheral blood of healthy persons) in the presence of IL-2 for 5 days. As analyzed by FCS, the incubation with hypoxia-primed Rbcs induced close to 40% CD4+ T cells into Tregs (Fig. 4a–c), which was abolished by depleting the Twist expression (Fig. 4d) or CD4+ CD25− T cells were separated from hypoxia-primed Rbcs in Transwells (Fig. 4a–c). The induced Tregs and Tregs isolated from the Rb tissues showed higher CD103 protein levels, a molecular marker expressed by residential T cells [15] (Fig. 4e). Culture with the normoxia-primed Rbcs did not induce Treg development (Fig. 4a, b). Additionally, Treg counts in peripheral blood were not significantly different between Rb patients and healthy controls (Fig. 4e, f). The results demonstrate that hypoxia-primed Rbcs can induce Treg development, in which Rbc-derived Twist plays a critical role.
Fig. 4.
Hypoxia-primed Rb cells induce Treg development. CD4+ CD25− T cells were isolated from healthy human blood samples and cultured with Rbcs (with or without being primed by hypoxia) in the presence of IL-2 (10 ng/ml) for 5 days. a T cells were gated first. b Gated FCS plots show generated Tregs. c Bars show mean ± SEM of summarized Tregs of 3 experiments. d Immunoblots show Twist protein in Rbcs. RNAi or cRNAi: Rbcs were treated with Twist RNAi or control RNAi reagents. e Immunoblots show CD103 protein levels in Tregs. f, g Peripheral blood samples were collected from healthy control subjects and Rb patients. Mononuclear cells were isolated from the samples and analyzed by FCS. Gated plots show Treg counts. Bars show summarized Treg counts. These Tregs are also CD25+ (not shown). The data represent 3 (a–e) or 10 (f, g) independent experiments. Statistics: ANOVA followed by the Bonferroni test. (T): Rbcs were cultured in upper chambers of Transwells and T cells in basal chambers. *, p < 0.01 (ANOVA followed by the Dunnett’s test), compared with group a
Rbc-derived Twist interacts with TLR4/MD-2 complex to promote Foxp3 expression in CD4+ T cells
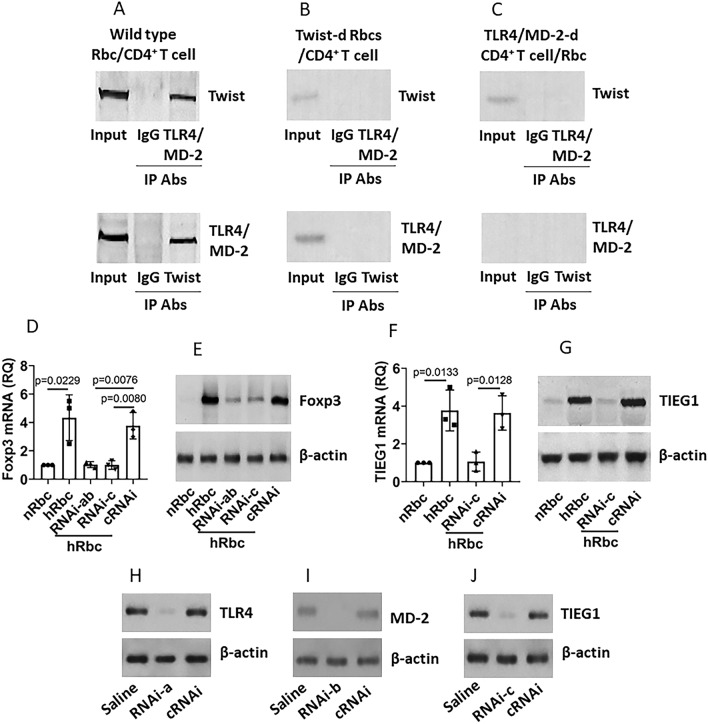
CD4+ T cells have the TLR4/MD-2 complexes that can communicate with extrinsic events, e.g., Gram− bacterial products, to regulate CD4+ T cell activities [16]. We wonder if Twist also communicates with the TLR4/MD-2 complexes to interact with the CD4 T cells to favor their conversion into Treg. To this end, CD4+ CD25− T cells were isolated from healthy person’s blood samples and cultured with hypoxia-primed Rbcs for 48 h. We found that a complex of Twist/TLR4/MD-2 was formed in the CD4+ T cells, but not in CD4+ T cells cultured with Twist-deficient Rbcs, or TLR4/MD-2-deficient CD4+ T cells cultured with Rbcs (Fig. 5a–c). Since the TGF-β inducible early gene 1 product (TIEG1) and Foxp3 are the canonical components in the Treg differentiation [17], we assessed the expression of TIEG1 and Foxp3 in CD4+ T cells after culturing with hypoxia-primed Rbcs. The results showed that the TIEG1 and Foxp3 expression in CD4+ T cells was markedly up-regulated by the hypoxia-primed Rbcs, which was abolished (Fig. 5d–g) by depleting the TLR4/MD-2 expression (Fig. 5h, i) or the TIEG1 expression (Fig. 5j). Since Foxp3 is a landmark of Treg development, the results indicate that the TLR4/MD-2 complex mediates the effects of Twist on activating the CD4+ T cells transition into Treg in RB.
Fig. 5.
Rb cell-derived Twist regulates Foxp3 expression in CD4+ T cells. CD4+ CD25− T cells [or TLR4/MD-2-d (deficient) CD4+ CD25− T cells] were isolated from healthy human blood samples and cultured with hypoxia-primed Rbcs (hRbcs, or Twist-d hRbcs) or normoxia-primed Rbcs (nRbcs) for 48 h in the presence of IL-2 (10 ng/ml). a–c Co-immunoprecipitation results show a complex of Twist and TLR4/MD-2 in CD4+ T cells. d, e TIEG1 expression in CD4+ T cells. f, g Foxp3 expression in CD4+ T cells. h–j CD4+ T cells were treated with the RNAi to deplete the TLR4 expression (h; RNAi-1), or the TIEG1 expression (i, j; RNAi-2). The RNAi effects were assessed by Western blotting and FCS 48 h after the treatment. Data of bars are presented as mean ± SEM. Statistics: ANOVA followed by the Bonferroni test. RNAi-ab: CD4+ T cells were treated with both RNAi-a and RNAi-b
Rbc-derived Twist-induced Tregs suppress CD8+ T cell proliferation and induce CD8+ T cell apoptosis
Next, we assessed the capacity of Rbc-derived Twist in suppressing CD8+ T cell activities. Tregs were induced by coculturing naive CD4+ T cells with hypoxia-primed Rbcs, or Tregs were isolated from the peripheral blood. CD8+ T cells were isolated from healthy person’s blood samples. CD8+ T cells were labeled with carboxyfluorescein succinimidyl ester (CFSE) and cultured with Tregs in the presence of activators (anti-CD3/CD28 Abs) for 3 days. The cells were analyzed by FCS, the CFSE-dilution assay. T cell proliferation was recorded and regarded as an indicator of CD8+ T cell activities. The results showed that the presence of Rbc-generated Tregs or blood-isolated Tregs significantly suppressed the CD8+ T cell proliferation (Fig. 6a, b), indicating that Rbc-induced Tregs have the immune-suppressive effects on CD8+ T cells. Additionally, we also found that after culturing with Tregs, abundant apoptotic CD8+ T cells were detected (Fig. 6c, d). The results indicate that Rbc-induced Tregs are capable of suppressing CD8+ T cell proliferation and inducing CD8+ T cell apoptosis.
Fig. 6.
Rb cell-induced Tregs suppress CD8+ T cells. Treg-1: isolated from the peripheral blood. Treg-2: Generated by culturing with hypoxia-primed Rbcs. CD8+ CD25− T cells were isolated from healthy person’s blood samples and cultured with the additional treatment denoted above each subpanel of a in the presence of anti-CD3/CD28 Ab or saline. Treg (or naive CD4+ T cells.): CD8+ T cells (labeled with CFSE) were cultured with Tregs (or naive CD4+ T cells) at a ratio of 1:1 for 3 days. a Gated histograms show proliferating CD8+ T cells. b Bars show summarized proliferating CD8+ T cells. c, d The cells were stained with Annexin V reagents and propidium iodide (PI). Gated FCS plots show apoptotic cell counts. Bars show summarized apoptotic cell counts. Data of bars are presented as mean ± SEM. Statistics: ANOVA followed by the Bonferroni test. The data represent 3 independent experiments
Rbc Twist expression shows prognosis value in Rb patient survival and sight
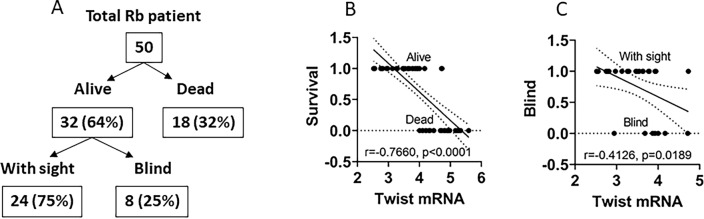
All Rb patients were followed up regularly. In the data of 24-month follow-up, 32 (64%) patients were alive and 18 (32%) patients were dead. A negative correlation was detected between the Rbc Twist expression and the survival rate. Within the alive patients, only 24 patients with sight and 8 patients were blind. A negative correlation was detected between the Rbc Twist expression and sight survival. The results demonstrate that the Rbc Twist expression is associated with Rb patient and sight survival (Fig. 7).
Fig. 7.
Prognosis value of the Rb Twist expression. a A schematic shows follow-up results of survival and sight of Rb patients 24 months after the surgery. b A negative correlation between Rbc Twist mRNA levels and survival. c A negative correlation between sight condition and Rbc Twist mRNA levels
Discussion
The present data show that Rbcs express Twist that can be up-regulated by hypoxia and presented on the cell surface. Twist interacts with the TLR4/MD-2 complexes on CD4+ T cells to regulate the Foxp3 expression and promotes the Treg development. The induced Tregs have immune-suppressive effects on CD8+ T cell activities. The Twist expression is negatively associated with Rb patient survival and the sight survival.
Twist is expressed in different types of tumor cells, including carcinomas, sarcomas, and lymphomas. Published data showed that Twist could be detected in breast, prostate and gastric carcinomas, melanomas, osteosarcomas, rhabdomyosarcomas and played a critical role in cancer cell metastasis [18]. In line with those pioneer studies, our novel findings indicate that Rbcs expressed Twist. We also found that exposure of Rbcs to hypoxia promoted the Twist expression, in which HIF-1α mediated the hypoxia effects on up-regulating the Twist expression. It is well-known that HIF-1α contributes to the cancer pathogenesis, such as to promote cancer cell invasiveness that can be counteracted by GATA3 [19]. HIF-1α can be up-regulated by other factors, such as Aldolase A can increase the HIF-1α expression; through activating the downstream factor matrix metallopeptidase 9, HIF-1α promotes the cancer cell metastasis [20]. Our data expand the HIF-1α study by revealing that HIF-1α can promote the Twist expression in Rbcs. Previous studies recognize that twist is a transcription factor [21]; while we found that this protein also localized on the Rbc cell surface, although could not be released into the micro-environment.
We found abundant Tregs in the Rb tissues. Tregs are one of the important cell fractions in the tumor tolerant system. By releasing immune-suppressive mediators, such as TGF-β, Tregs suppress the anti-tumor immunity, thereby compromising immunosurveillance against cancer and attenuating the anti-tumor capacity in tumor-bearing subjects [8]. There are fractions of tumor antigen-specific CD4+ T cells and CD8+ T cells in the body of healthy subjects [22]. These cells are important to eliminate tumor cells [23]. Therefore, it is conceivable that Tregs in the Rb tissues may dampen the anti-tumor CD4+ or CD8+ T cells. Indeed, the Rbc-derived Twist-induced Tregs show the capacity to suppress CD8+ cytotoxic T cells as shown by the present study. Therefore, removing Tregs from the body may prevent such a phenomenon. However, apart from dampening the anti-tumor effects, Tregs are also important in preventing autoimmune disorders in the body [24]. Inhibition of Treg has dual functions in cancer-bearing hosts.
To our knowledge, this is the first report which shows that Rbc-derived Twist can generate Tregs. Specifically, we found that, in response to hypoxia, the Twist expression was up-regulated in Rbcs, and more importantly, the Twist protein could be found in the cytoplasm, nuclei and was detectable on the surface of Rbcs. The data provide the mechanistic evidence to explain that the peripheral Treg frequency in Rb patients was similar to that in healthy control subjects, while abundant Tregs were found in the Rb tissues. The data demonstrate that Rbc-derived Twist induces Tregs in situ in a cell–cell-contact manner. The induced Tregs show the residential feature that express high levels of CD103 [15].
After coculture with hypoxia-primed Rbcs, a complex of Twist and TLR4/MD-2 was detected in CD4+ T cells, while exposure to normoxia-primed Rbcs did not. Cancer cells are featured as high metabolic levels, a condition which needs more oxygen supply, that forms a hypoxic environment in the tumor tissues [25]. The HIF-1α expression can be up-regulated by hypoxia [26]. HIF-1α can promote tumor growth, expand cancer stem cell activities and can be an indicator of tumor prognosis [26]. Our data are in line with published data by showing that Rbcs produce more HIF-1α in a hypoxic environment; the data also provide further and novel information to the HIF-1α studies by showing that the hypoxia-primed Rbcs are capable of inducing Tregs through a novel signal transduction pathway of hypoxia/HIF-1α/Twist/TLR4/MD-2/TIEG1/Foxp3/Treg.
The TLR4/MD-2 complex was found in monocytes that recognizes microbial signal such as LPS. This complex works as a link between microbial stimuli and cellular defensive system [16, 27]. Latterly, the TLR4/MD-2 complex was also found in other cell types, such as marginal B cells, plasma cells [28], cancer cells [29]. Our data show that the TLR4/MD-2 complex also can be detected in CD4+ T cells. Besides being bounded by LPS, the TLR4/MD-2 complex also can be bound by other substances, such as procyanidin B3, a substance in the diet that has anti-inflammation and anti-cancer function and also can bind to TLR4/MD-2 complex [30]. CD83 binds to TLR4/MD-2 on monocytes can inhibit T cell activation [31], implicating that activating TLR4/MD-2 complex links to the immune regulation, that has been expanded by our data. We found that the Rb-derived Twist also could bind to TLR4/MD-2 to form a complex.
The data show that by binding TLR4/MD-2 in CD4+ T cells, Twist induced the Treg differentiation, that was mediated by TIEG1. By promoting the Foxp3 expression, TIEG1 plays a critical role in the Treg generation that is beneficial for tumor growth [17]. Our data are in line with this report by showing that activating TIEG1 induced Foxp3 expression. Besides, TIEG1 also has other functions, such as maintaining mitochondrial homeostasis [32], playing a role in Langerhans cell maturation [33] and regulating osteoblast activities [34]. However, the regulatory factors for TIEG1 remain to be further investigated. Our data show that Rb-derived Twist can up-regulate the TIEG1 expression in CD4+ T cells, by which Twist induces Foxp3 expression and induces Treg development.
The follow-up data show that, 2 years after surgery, about 64% Rb patients were still alive, in which about 75% patients were with sight survival. This is in line with other reports. Waddell et al. reported two-year Rb patient survival rate was 45% in Rb patients who received pre-chemotherapy and 65% in those who received post-chemotherapy [35]. Our data also show a negative correlation between the Rbc Twist expression and the Rb patient survival and the sight survival. It seems that the Rbc Twist expression may prognose the outcomes of Rb treatment; this needs to be further investigated.
In summary, the present data show that Rbcs express Twist. The Rbc-derived Twist can induce the Treg development through the Twist/TLR4/MD-2/TIEG1/Foxp3 signal transduction pathway. Therefore, Twist may be a therapeutic target for the Rb treatment.
Acknowledgements
This study was supported by grants of the National Nature and Science Foundation of China (81870706, 31570932, U1801286, 31000713, 31700805), Guangdong Provincial Key Laboratory of Regional Immunity and Diseases (2019B030301009), Cross Innovation Research Initiative Funds of Shenzhen University and Shenzhen science, technology and innovation committee (201002052, KQTD20170331145453160 and KQJSCX20180328095619081).
Author contributions
RZ, YNS, XD, ZG, YS, ZZ and YL performed experiments, analyzed data and reviewed the manuscript. GN, PCY and BPM organized the study and supervised experiments. PCY designed the project and prepared manuscript.
Compliance with ethical standards
Conflict of interest
The author declares that there is no competing interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Ruishi Zhang and Yan-Nan Song equally contributed to this work.
Contributor Information
Beiping Miao, Email: miaobeiping@163.com.
Ping-Chang Yang, Email: pcy2356@szu.edu.cn.
Guohui Nie, Email: nghui@21cn.com.
References
- 1.Dimaras H, Corson TW, Cobrinik D, et al. Retinoblastoma. Nat Rev Dis Prim. 2015;1:15021. doi: 10.1038/nrdp.2015.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Le Rhun E, Preusser M, Roth P, Reardon DA, van den Bent M, Wen P, Reifenberger G, Weller M. Molecular targeted therapy of glioblastoma. Cancer Treat Rev. 2019;80:101896. doi: 10.1016/j.ctrv.2019.101896. [DOI] [PubMed] [Google Scholar]
- 3.Mendoza PR, Grossniklaus HE. The biology of retinoblastoma. Prog Mol Biol Transl Sci. 2015;134:503–516. doi: 10.1016/bs.pmbts.2015.06.012. [DOI] [PubMed] [Google Scholar]
- 4.Xu XL, Singh HP, Wang L, Qi DL, Poulos BK, Abramson DH, Jhanwar SC, Cobrinik D. Rb suppresses human cone-precursor-derived retinoblastoma tumours. Nature. 2014;514:385–388. doi: 10.1038/nature13813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andre P, Denis C, Soulas C, et al. Anti-NKG2A mAb is a checkpoint inhibitor that promotes anti-tumor immunity by unleashing both T and NK cells. Cell. 2018;175:1731–43.e13. doi: 10.1016/j.cell.2018.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schietinger A, Greenberg PD. Tolerance and exhaustion: defining mechanisms of T cell dysfunction. Trends Immunol. 2014;35:51–60. doi: 10.1016/j.it.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ali K, Soond DR, Pineiro R, et al. Inactivation of PI(3)K p110delta breaks regulatory T-cell-mediated immune tolerance to cancer. Nature. 2014;510:407–411. doi: 10.1038/nature13444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Togashi Y, Shitara K, Nishikawa H. Regulatory T cells in cancer immunosuppression—implications for anticancer therapy. Nat Rev Clin Oncol. 2019;16:356–371. doi: 10.1038/s41571-019-0175-7. [DOI] [PubMed] [Google Scholar]
- 9.Puisieux A, Valsesia-Wittmann S, Ansieau S. A twist for survival and cancer progression. Br J Cancer. 2006;94:13–17. doi: 10.1038/sj.bjc.6602876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaliki S, Patel A, Iram S, Palkonda VAR. Clinical presentation and outcomes of stage III or stage IV retinoblastoma in 80 Asian Indian patients. J Pediatr Ophthalmol Strabismus. 2017;54:177–184. doi: 10.3928/01913913-20161019-01. [DOI] [PubMed] [Google Scholar]
- 11.Xu LZ, Xie RD, Xie H, et al. Chimeric specific antigen epitope-carrying dendritic cells induce interleukin-17(+) regulatory T cells to suppress food allergy. Clin Exp Allergy. 2020;50:231–243. doi: 10.1111/cea.13528. [DOI] [PubMed] [Google Scholar]
- 12.Krishnakumar S, Mohan A, Mallikarjuna K, Venkatesan N, Biswas J, Shanmugam MP, Ren-Heidenreich L. EpCAM expression in retinoblastoma: a novel molecular target for therapy. Invest Ophthalmol Vis Sci. 2004;45:4247–4250. doi: 10.1167/iovs.04-0591. [DOI] [PubMed] [Google Scholar]
- 13.Takeuchi Y, Nishikawa H. Roles of regulatory T cells in cancer immunity. Int Immunol. 2016;28:401–409. doi: 10.1093/intimm/dxw025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pezzuto A, Carico E. Role of HIF-1 in cancer progression: novel insights. A review. Curr Mol Med. 2018;18:343–351. doi: 10.2174/1566524018666181109121849. [DOI] [PubMed] [Google Scholar]
- 15.Mami-Chouaib F, Tartour E (2019) Editorial: tissue resident memory T cells. 10. 10.3389/fimmu.2019.01018 [DOI] [PMC free article] [PubMed]
- 16.Park BS, Song DH, Kim HM, Choi BS, Lee H, Lee JO. The structural basis of lipopolysaccharide recognition by the TLR4-MD-2 complex. Nature. 2009;458:1191–1195. doi: 10.1038/nature07830. [DOI] [PubMed] [Google Scholar]
- 17.Peng DJ, Zeng M, Muromoto R, Matsuda T, Shimoda K, Subramaniam M, Spelsberg TC, Wei WZ, Venuprasad K. Noncanonical K27-linked polyubiquitination of TIEG1 regulates Foxp3 expression and tumor growth. J Immunol. 2011;186:5638–5647. doi: 10.4049/jimmunol.1003801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khanbabaei H, Teimoori A, Mohammadi M. The interplay between microRNAs and Twist1 transcription factor: a systematic review. Tumour Biol. 2016;37:7007–7019. doi: 10.1007/s13277-016-4960-y. [DOI] [PubMed] [Google Scholar]
- 19.Lin MC, Lin JJ, Hsu CL, Juan HF, Lou PJ, Huang MC. GATA3 interacts with and stabilizes HIF-1alpha to enhance cancer cell invasiveness. Oncogene. 2017;36:4243–4252. doi: 10.1038/onc.2017.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang YC, Chan YC, Chang WM, Lin YF, Yang CJ, Su CY, Huang MS, Wu ATH, Hsiao M. Feedback regulation of ALDOA activates the HIF-1alpha/MMP9 axis to promote lung cancer progression. Cancer Lett. 2017;403:28–36. doi: 10.1016/j.canlet.2017.06.001. [DOI] [PubMed] [Google Scholar]
- 21.Vichalkovski A, Gresko E, Hess D, Restuccia DF, Hemmings BA. PKB/AKT phosphorylation of the transcription factor Twist-1 at Ser42 inhibits p53 activity in response to DNA damage. Oncogene. 2010;29:3554–3565. doi: 10.1038/onc.2010.115. [DOI] [PubMed] [Google Scholar]
- 22.Kishton RJ, Sukumar M, Restifo NP. Metabolic regulation of T Cell longevity and function in tumor immunotherapy. Cell Metab. 2017;26:94–109. doi: 10.1016/j.cmet.2017.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mohanty R, Chowdhury CR, Arega S, Sen P, Ganguly P, Ganguly N. CAR T cell therapy: a new era for cancer treatment (Review) Oncol Rep. 2019;42:2183–2195. doi: 10.3892/or.2019.7335. [DOI] [PubMed] [Google Scholar]
- 24.Romano M, Fanelli G, Albany CJ, Giganti G, Lombardi G. Past, present, and future of regulatory T cell therapy in transplantation and autoimmunity. Front Immunol. 2019;10:43. doi: 10.3389/fimmu.2019.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Joseph JP, Harishankar MK, Pillai AA, Devi A. Hypoxia induced EMT: a review on the mechanism of tumor progression and metastasis in OSCC. Oral Oncol. 2018;80:23–32. doi: 10.1016/j.oraloncology.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 26.Hajizadeh F, Okoye I, Esmaily M, Ghasemi Chaleshtari M, Masjedi A, Azizi G, Irandoust M, Ghalamfarsa G, Jadidi-Niaragh F. Hypoxia inducible factors in the tumor microenvironment as therapeutic targets of cancer stem cells. Life Sci. 2019;237:116952. doi: 10.1016/j.lfs.2019.116952. [DOI] [PubMed] [Google Scholar]
- 27.Ciesielska A, Hromada-Judycka A, Ziemlinska E, Kwiatkowska K. Lysophosphatidic acid up-regulates IL-10 production to inhibit TNF-alpha synthesis in Mvarphis stimulated with LPS. J Leukoc Biol. 2019;106:1285–1301. doi: 10.1002/jlb.2a0918-368rr. [DOI] [PubMed] [Google Scholar]
- 28.Nagai Y, Yanagibashi T, Watanabe Y, et al. The RP105/MD-1 complex is indispensable for TLR4/MD-2-dependent proliferation and IgM-secreting plasma cell differentiation of marginal zone B cells. Int Immunol. 2012;24:389–400. doi: 10.1093/intimm/dxs040. [DOI] [PubMed] [Google Scholar]
- 29.Liu H, Zhang G, Huang J, Ma S, Mi K, Cheng J, Zhu Y, Zha X, Huang W. Atractylenolide I modulates ovarian cancer cell-mediated immunosuppression by blocking MD-2/TLR4 complex-mediated MyD88/NF-kappaB signaling in vitro. J Transl Med. 2016;14:104. doi: 10.1186/s12967-016-0845-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shang P, Tang Q, Hu Z, Huang S, Hu Y, Zhu J, Liu H. Procyanidin B3 alleviates intervertebral disc degeneration via interaction with the TLR4/MD-2 complex. J Cell Mol Med. 2020;24:3701–3711. doi: 10.1111/jcmm.15074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Horvatinovich JM, Grogan EW, Norris M, Steinkasserer A, Lemos H, Mellor AL, Tcherepanova IY, Nicolette CA, DeBenedette MA. Soluble CD83 inhibits T cell activation by binding to the TLR4/MD-2 complex on CD14(+) monocytes. J Immunol. 2017;198:2286–2301. doi: 10.4049/jimmunol.1600802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kammoun M, Piquereau J, Nadal-Desbarats L, et al. Novel role of Tieg1 in muscle metabolism and mitochondrial oxidative capacities. Acta Physiol (Oxf) 2020;228:e13394. doi: 10.1111/apha.13394. [DOI] [PubMed] [Google Scholar]
- 33.Zhang X, Yao Y, Wei WZ, Yang ZQ, Gu J, Zhou L. Impaired epidermal Langerhans cell maturation in TGFbeta-inducible early gene 1 (TIEG1) knockout mice. Oncotarget. 2017;8:112875–112882. doi: 10.18632/oncotarget.22843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Subramaniam M, Pitel KS, Withers SG, Drissi H, Hawse JR. TIEG1 enhances Osterix expression and mediates its induction by TGFbeta and BMP2 in osteoblasts. Biochem Biophys Res Commun. 2016;470:528–533. doi: 10.1016/j.bbrc.2016.01.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Waddell KM, Kagame K, Ndamira A, Twinamasiko A, Picton SV, Simmons IG, Revill P, Johnston WT, Newton R. Improving survival of retinoblastoma in Uganda. Br J Ophthalmol. 2015;99:937–942. doi: 10.1136/bjophthalmol-2014-306206. [DOI] [PubMed] [Google Scholar]