Abstract
Ocular surface squamous neoplasia (OSSN) is the major cause of corneal cancer in man and horses worldwide, and the prevalence of OSSN is increasing due to greater UVB exposure globally. Currently, there are no approved treatments for OSSN in either species, and most patients are managed with surgical excision or off-label treatment with locally injected interferon alpha, or topically applied cytotoxic drugs such as mitomycin C. A more broadly effective and readily applied immunotherapy could exert a significant impact on management of OSSN worldwide. We therefore evaluated the effectiveness of a liposomal TLR complex (LTC) immunotherapy, which previously demonstrated strong antiviral activity in multiple animal models following mucosal application, for ocular antitumor activity in a horse spontaneous OSSN model. In vitro studies demonstrated strong activation of interferon responses in horse leukocytes by LTC and suppression of OSSN cell growth and migration. In a trial of 8 horses (9 eyes), treatment with topical or perilesional LTC resulted in an overall tumor response rate of 67%, including durable regression of large OSSN tumors. Repeated treatment with LTC ocular immunotherapy was also very well tolerated clinically. We conclude therefore that ocular immunotherapy with LTC warrants further investigation as a novel approach to management of OSSN in humans.
Keywords: Ocular surface squamous neoplasia, Immunotherapy, Horse
Introduction
Ocular surface squamous neoplasia (OSSN) represents the most common tumor of the conjunctiva in humans and horses [1–3]. The tumor is often locally invasive and frequently results in loss of vision and necessitates removal of the globe [4]. Although the etiology of OSSN is not fully understood, predisposing factors include absence of pigmentation, high solar radiation exposure, prior viral exposure, hormonal or immunological influences, acquired p53 mutations or repeated physical irritation [4–7]. Currently, there are multiple treatment options for equine adnexal and ocular OSSN, including surgical excision, laser surgery, radiation therapy, immunotherapy, cryotherapy, radiofrequency hyperthermia, intralesional chemotherapy or multimodal therapies, though none of these treatments are approved by the FDA [5, 8–19]. Success rates with treatment in horses with various modalities have been reported to range from 30 to 90%, with success defined as lack of recurrence for up to 5 years [20]. The highest rates of success have occurred following the combination of surgical excision combined with local irradiation or chemotherapy [12, 13, 20–22]. However, disease recurrences are observed with all currently available treatment modalities [10–17].
Horses with OSSN provide an excellent large animal model for human OSSN, as the ocular pathology in horses is very analogous to human OSSN, including similar gross and histologic appearance, shared risk factors and common lesion progression times [1, 23, 24]. Exposure to UV light is a major risk factor for development of OSSN in the horse, since many horses spend the majority of the day outdoors. Ocular OSSN most commonly occurs in older horses, and castrated males are at a higher risk than intact males or females. Lack of periocular pigmentation and certain coat colors are also predisposing factors [1, 25, 26].
Recently, a heritable genetic mutation in several equine breeds has been identified as another predisposing factor to OSSN. Damage-specific DNA binding protein 2 (DDB2) identifies UV-damaged DNA, and horses who are homozygous for mutated DDB2 have been shown to be at higher risk for OSSN [25, 27, 28]. Xeroderma pigmentosum (XP) in humans is caused by mutations in the same risk locus as DDB2 and horses and people with XP have an increased risk of cutaneous squamous cell carcinoma [26, 27]. Another major risk factor for the development of OSSN in humans includes human papillomavirus infection. Equine papillomavirus type 2 is a risk factor for genital SCC in horses, but the role of this papillomavirus in the development of ocular SCC in horses has yet to be fully elucidated [6, 29, 30]. Ocular SCC behaves similarly in the horse as in humans in that it is locally invasive but does not tend to metastasize. The rate of metastasis to local lymph nodes in humans is reported to be < 1%, and this rate is more variable in horses and is dependent on tumor location. Thus, the remarkable similarities in biological behavior and risk factors in OSSN in horses and humans indicate that the horse OSSN model may be particularly valuable for translational studies of new ocular therapies.
Our group has previously described the local innate immune responses to mucosal treatment with liposome-TLR ligand complexes (LTC) in rodent models and in studies in dogs, cats, and cattle [31–33]. These studies demonstrated that LTC potently activated innate immune responses, most notably induction of both type I and II interferon responses [31–34]. Liposome-TLR complexes engineered for mucosal delivery were shown to adhere well to epithelial cells and other mucosal surfaces and to efficiently deliver TLR ligands to surface epithelial and immune target cells for immune activation [32]. Earlier studies of a precursor formulation to the current LTC described immune mechanisms of action and dependency on NK cell activation and IFN-g production for generating antitumor immunity [35, 36].
The mucosal immune activating properties and induction of type I and II interferons (each with potent antitumor activity) suggested that LTC might have utility as a novel immunotherapy for equine and human OSSN. Therefore, the objective of the work reported here was to evaluate the safety and efficacy of topically applied and locally injected LTC for the treatment of OSSN, as evaluated in an equine OSSN model. The ability of LTC to activate equine leukocytes and to directly inhibit growth OSSN tumor cells was assessed using in vitro studies. Studies were also conducted in clinical studies in horses with OSSN. Study animals were treated by topical and/or intralesional administration of LTC, and tumor responses were assessed by serial photography and quantitative image analysis. Key study findings were that LTC activated innate immune responses in horse leukocytes and tumor cells and that in vivo treatment induced durable tumor regression in the majority of treated animals. These findings provide evidence for the potential effectiveness of eye drops and perilesional injection of LTC as a novel immunotherapy for treatment of OSSN in horses and humans.
Materials and methods
Study animals The clinical studies described here were approved by the Colorado State University Clinical Review Board (protocol VCS#2019-204). All horses enrolled in the study were screened to assure no history of ongoing systemic illness, using both history, physical examination, and screening bloodwork. A total of 8 horses (a total of 9 eyes; one horse had bilateral OSSN tumors) including 3 mares and 5 geldings with OSSN were included in this study. Affected horses presented at an average age of 15.2 years (range 10–21 years). Horse breeds represented included 3 American Quarter Horses, 2 Missouri Fox Trotters and one each of American Paint Horse, Appaloosa, Draft cross and Belgian. Horses were enrolled with informed owner consent. Horses were eligible for the study if there were no signs of concurrent systemic disease and histopathology confirmed SCC with tumor localized to the limbus. Horses were not included if lesions of the primary eyelids or the third eyelids were noted.
Horses presenting to the Colorado State University Veterinary Teaching hospital with suspect OSSN received complete physical and ophthalmic examinations by the primary investigator (KW). Complete ophthalmic examination was done using sedation with detomidine hydrochloride (Pfizer Animal Health, Exton, Pennsylvania, USA) and auriculopalpebral nerve block prior to slit-lamp biomicroscopy (Kowa SL-15, Kowa, Tokyo, Japan), direct ophthalmoscopy (Welch Allen direct ophthalmoscope, Welch Allyn Distributors, Skaneateles Falls, NY), fluorescein stain (fluorescein sodium ophthalmic strips, OptiTech Eyecare, Allahabad, India), and rebound tonometry (TonoVet®, Jorgensen Distributors, Fort Collins, CO). Biopsies of an accessible portion of the mass (either corneal or conjunctival) were obtained using topical anesthetics. The biopsy site was prepared with dilute betadine solution (1:50), and a portion of the mass was removed with Westcott tenotomy scissors and sent for histopathologic analysis through the Colorado State University Diagnostic Lab.
Horses were placed in one of two treatment groups, based on the size of the initial OSSN lesions. Group 1 animals consisted of horses with OSSN largely confined to the corneal stroma with minimal conjunctival involvement. This group was treated with topical LTC consisting of two drops applied to the affected eye every other day. Horses in Group 2 had more advanced OSSN lesions and were treated by both subconjunctival perilesional injections of LTC once every 2 weeks for 3 treatments, and as well with every other day eye drop administration of LTC. Treatment duration varied depending on the severity of the lesion and ranged from 42 to 70 days.
Preparation of LTC Liposome TLR complexes (LTC), which consist of cationic liposomes complexed to TLR3 and TLR9 nucleic acid agonists, were prepared as described previously [33]. Briefly, cationic liposomes were complexed to poly-inosinic, poly-cytidylic acid (InVivoGen, San Diego, CA) and non-coding plasmid DNA (PCR2.1, Thermo Fisher Scientific, Waltham, MA). Carboxymethylcellulose (CMC; Sigma-Aldrich, St. Louis, MO) was added to the pre-formed complexes to produce LTC for study. For topical application, the LTC was mixed 1:1 (v/v) with high viscosity CMC to produce a gel capable of more prolonged adherence to the cornea and adnexal tissues. For in vitro studies of LTC uptake by cell lines, cholesterol was replaced with BODIPY® 542/563 cholesteryl ester (Thermo Fisher Scientific, Waltham, MA) to generate fluorescent liposomes suitable for confocal microscopy and flow cytometric measurement.
LTC administration by topical or injectable routes Topical treatment with LTC was administered as 2 drops to the affected eye, once every other day, for up to 70 days of continuous treatment (Group 1, 3 horses). For intralesional injections of LTC, animals were injected at two-week intervals for 4 to 6 total injections (Group 2, 6 horses). The total number of LTC injections was determined at the discretion of the primary investigator (KW) based on responses to treatment. Prior to each perilesional injection, horses were sedated with detomidine hydrochloride (Pfizer Animal Health, Exton, Pennsylvania, USA) for complete ophthalmic examination, photographs of the affected eye(s) (Nikon D160 DSLR, micro Nikkor 105 mm lens, Nikon Inc., Melville, NY). In preparation for the injection, the area of affected conjunctiva was cleaned with dilute betadine solution (1:50) and prepared with topical 2% ophthalmic lidocaine gel and 0.5% proparacaine. All perilesional injections were performed sterilely by a single investigator (KW) using a tuberculin syringe with 27-gauge needle. A total of 1 mL of LTC was injected at each time point regardless of tumor size. The injection was directed dorsal portion of the lesion when possible and a bleb of LTC formed in and around the lesion. Horses were discharged with instructions for the owner to apply two LTC drops to the affected eye every other day.
Tumor monitoring At each visit, prior to injection, horses were photographed with a ruler in the frame. ImageJ software (https://imagej.nih.gov/ij/) was used to analyze tumor size (cm2). Approximately, one and three months after the final LTC injection, horses returned for follow-up examinations during which a full ophthalmic examination was performed. Follow-up at one year after the final injection was performed via a combination of phone, photographs, and ophthalmic examinations. Horses were monitored for local or systemic reactions for three hours following the injection. Heart rate, respiratory rate, and rectal temperature were recorded every hour for three hours following sedation and injection. Conjunctival hyperemia scores were assigned to each eye at initial exam and prior to each treatment. Eyes were scored (0–4 +) independently by the investigators (KW, BM) according to the semiquantitative preclinical ocular toxicology score (SPOTS) system [37]. Images of patient eyes were obtained with a digital camera (Nikon D160 DSLR, micro Nikkor 105 mm lens, Nikon Inc., Melville, NY). ImageJ software was used to first calibrate imaged area using ruler monuments and then to determine region of interest areas. Data were plotted using Prism 9 software (GraphPad, San Diego, CA).
Tear sampling and cytokine analysis Tears were collected from the normal eye and the affected eye from each horse prior to starting treatment and then collected again 7 and 14 days after the initial treatment. Tears were collected from the palpebral conjunctiva and placed into Eppendorf tubes and frozen at – 80 C until analysis. Cytokine concentrations in tears were measured using an Equine Inflammation/Immunology Bead-Based Multiplex cytokine Assay kit (MilliporeSigma, Burlington MA). Tears were added without dilution to multiplex plates and run according to manufacturers’ instructions. Plates were run on an MAGPIX® System and analyzed using xPONENT® software (Luminex Corporation, Austin TX). Cytokine concentrations in cell culture supernatants were run on the same assay, using the same standards, also using undiluted samples.
Generation of equine OSSN cell lines Tumor biopsies from three study animals were minced and then digested with collagenase (Sigma Aldrich; 1 mg/ml) for 30 min at 37 °C. After washing, digested cells were cultured in serum free media DMEM/F12, non-essential amino acids, essential amino acids, Glutamax and penicillin/streptomycin solution (Gibco, Carlsbad, CA), along with human FGF basic (10 ng/mL) and human EGF (10 ng/mL) (PeproTech, Cranbury, NJ). Cultures were maintained in serum-free conditions for up to 30 days to assure fibroblast elimination. Cells were then converted to culture in serum-containing medium (DMEM) with10% fetal bovine serum (VWR, Radnor, PA) for further expansion and in vitro assays. Tumor cells were used at passage 10 or later, and cell appearance was assessed by visual examination and immunostaining for EpCAM expression to assure epithelial features (data not shown).
Leukocyte activation by LTC Equine leukocyte (peripheral blood mononuclear cell; PBMC) cultures were generated from blood of healthy horses, using previously published Ficoll gradient leukocyte isolation protocols [32]. The PBMC was plated at a concentration of 2 × 106 cells per mL in complete media in 24-well cell culture plates (Corning Inc. Corning, NY) and then incubated with LTC at concentrations indicated for up to 48 h (37 °C, 5%CO2). For analysis of cytokine gene expression, cells were collected after 8 h of incubation with LTC in vitro, in triplicate cultures, and subjected to RT-PCR amplification as noted below. In addition, conditioned medium (CM) was collected and centrifuged for 5 min at 300 g at room temperature to remove cellular debris and then frozen at -80C prior to analysis and use in cell culture or cytokine studies.
For analysis of cytokine gene expression, primers, and probes for equine IFN-α, IFN-ß, and the housekeeping gene GAPDH were generated using IDT PrimerQuest™ Tool (Integrated DNA Technologies, Inc, Coralville, IA). Primer-probe sets were verified at > 95% efficiency by standard curve. RNA was extracted from 2 × 106 cells/ well after 8-h incubation with LTC using RNeasy mini kit (Qiagen, Valencia, CA) according to manufacturers’ instructions. Quality and quantity of RNA were verified on NanoDrop™ One/OneC Microvolume UV–Vis Spectrophotometer (ThermoFisher Scientific, Waltham, MA). The RNA (50 ng) was then combined with master mix components from GoTaq® Probe 1-Step RT-qPCR kit (Promega, Madison, WI). A concentration of 900 nM forward and reverse primer and 250 nM probe was used in each reaction, under regular cycling conditions following manufactures recommendation. The RT-PCR was run on QuantStudio 3 Real-Time PCR System, and fold change was analyzed using QuantStudio Design and Analysis Software v1.5.1 (ThermoFisher Scientific, Waltham, MA). Primer and probe sequences were as follows (Table 1).
Table 1.
Primer and probe sequences for RT-PCR equine IFN-α, IFN-β, and GAPDH
| IFN-α NM_001099441 | Fwd | TGC CTG AAG GAC AGA AAT G |
| Rev | TGC TGA AGA GGT GGA AGA | |
| Probe | /56-FAM/TC AAG CCA T/ZEN/C TCT GCG GTC CAT /3IABkFQ/ | |
| IFN-ß NM_001099440.1 | Fwd | GAT GCT GCA TTG GTC ATC TA |
| Rev | CCA CAA GGA GGT TCT TAA CG | |
| Probe | /56-FAM/AG CAC TGG C/ZEN/T GGA ATG AGA CCA T/3IABkFQ/ | |
| GAPDH NM_001163856 | Fwd | CTG GAG AAA GCT GCC AAA TAC |
| Rev | CAA CCT GGT CCT CAG TGT AG | |
| Probe | /56-FAM/TCA AGA AGG/ZEN/TGG TGA AGC AGG CAT/3IABkFQ/ |
Tumor growth suppression by conditioned medium (CM) from LTC-activated PBMC Conditioned medium from LTC-activated leukocytes, generated as described above, was added to triplicate cultures (24-well plates) of 3 different equine OSSN cell lines (EQOSSN-LC177, EQOSSN-LC653, EQOSSN-LC914) at the indicated CM dilutions. Controls included tumor cells not treated with CM, and tumor cells treated with CM from non-activated leukocytes. Cells were incubated for 48 additional hours, and then cell viability was assessed using MTT assay.
LTC uptake by OSSN cells and by normal corneal epithelium To assess LTC uptake by OSSN cell lines, OSSN cells were plated in 24-well cell culture plates (Thermo Fisher Scientific, Waltham MA), containing round coverslips (Chemglass Life Sciences LLC, Vineland, NJ). Then, fluorescently-labeled LTC (10 µL dissolved in 1 mL volume) were added directly to OSSN cells, which were then gently agitated to ensure even LTC distribution at room temp for 10 min. After 10 min of incubation, wells were washed with sterile PBS and then fixed with 4% paraformaldehyde for 15 min at room temperature. Coverslips were then removed and mounted to a microscope slide (Fisher Scientific, Hampton, NH) using ProLong™ Diamond Antifade Mountant (ThermoFisher) and imaged on Olympus IX83 spinning disk confocal microscope, as noted above.
To determine the uptake and retention of LTC by normal corneal epithelial cells, corneal explants were obtained from euthanized horses and placed in tissue culture medium in 24-well plates. Within 1 h of tissue collection, fluorescently labeled LTC was added to the corneal surface, then incubated at 37C 5%CO2 for one hour. Corneal tissues were then washed with sterile PBS to remove residual labeled LTC and then fixed with 4% paraformaldehyde (Sigma-Aldrich, St. Louis, MO) for 15 min and then imaged on an Olympus IX83 spinning disk confocal microscope.
Tumor cell responses to secreted factors from LTC-activated leukocytes Two assays were used to assess the effects of cytokines released from LTC-stimulated PBMC on tumor viability and migration. An MTT assay [38] was used to assess the impact of PBMC CM on tumor cell metabolic activity, using two equine OSSN cell lines (EQOSSN-LC653 and EQOSSN-LC914). Briefly, OSSN cells were seeded in triplicate wells of 24-well plates at a density of 2.5 X 105 cells per well and then incubated with PBMC CM generated as noted above. Tumor cell responses to LTC-activated PBMC CM were compared to tumor cell responses to CM from non-activated PBMC and to responses of untreated OSSN cells.
To assess the impact of LTC activated PBMC CM on OSSN cell migration, an IncuCyte® Zoom system (Essen BioScience Inc. Ann Arbor, MI) was used. First, 2 × 104 OSSN cells were plated on an Imageock plate and allowed to adhere and proliferate in complete media for 48 h. After 48 h, an area of wounding (scratch) was created on a confluent monolayer of OSSN cells following manufacturers’ instructions for Incucyte® Scratch Wound Assay (Sartorius AG, Göttingen Germany). Then, PBMC CM was diluted 1:1 in complete tissue culture medium and added to the cell cultures and the impact of activated and non-activated CM on cell migration was assessed over 72 h using IncuCyte® Scratch Wound Cell Migration Software Module (Sartorius AG, Göttingen Germany).
Statistical analysis Statistical comparisons between two treatment groups were made using nonparametric t tests (Mann–Whitney test). Comparisons between more than 3 treatment groups were completed using one‐way ANOVA, followed by Tukey multiple means post‐test. Values were considered statistically significant for p < 0.05 and were corrected for multiple comparisons. Statistical analysis was performed using Prism 9 software (GraphPad Prism, San Diego, CA).
Results
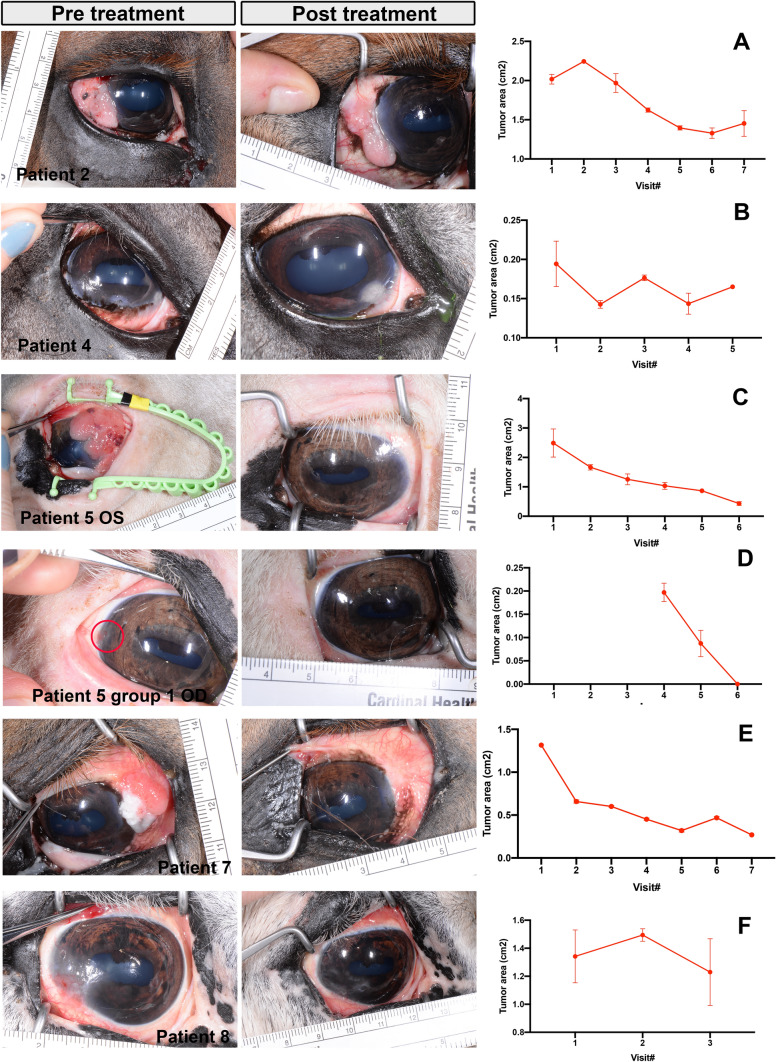
In vivo responses of OSSN tumors to treatment with LTC Horses with spontaneous OSSN were enrolled in a clinical pilot study designed to assess both safety and tumor responses to repeated LTC treatment. A total of 8 animals were enrolled in the study, each with a variety of different starting tumor sizes. Of the 3animals treated in Group 1 with smaller tumors (topical treatment only), the OSSN lesions in one animal responded completely to topical therapy and did not require further intervention (Fig. 1D). In the other two treated animals in Group 1, there was no response to treatment, and the OSSN lesions were later removed by keratectomy. For the 6 animals in Group 2 with larger tumors (treated with topical and perilesional LTC injection), there were 5 animals with measurable tumor responses. One animal had a 35% decrease in total tumor surface area (Fig. 1A). A second animal had a tumor area that decreased by 26% (Fig. 1B), while two additional animals (horse 5 and 7) had the largest decreases in total tumor area, with 83% reduction in tumor surface area compared to pretreatment values (Fig. 1C) for horse 5 and 80% tumor surface area decrease for horse 7 (Fig. 1E). Horse 8 had a decrease of 8% tumor area after one treatment and did not return for further treatment or evaluation (Fig. 1F). The remaining two animals in Group 2 did not respond to treatment.
Fig. 1.
Tumor responses to LTC treatment. Pre- and post-treatment photographs of OSSN effected eye in responder patients—patient 2, patient 4 and patients 5 (OS), patients 5 (OD), patients 7 and 8 from top to bottom. Right column shows tumor area calculations, hand drawn on 3 photographs per visit and calculated using ImageJ as described in methods. A Tumor area in patient 2 from visit 1 to 7, each visit approximately 2 weeks apart. B tumor area calculations from patient 4 from visit 1 to 5. C Tumor area calculations from patient 5 from visit 1 to 6. D Tumor area in patient 5 (OD) from group 1 from visit 4 to 6. Horse 5 developed tumor in right eye after 4 visits. E tumor area calculations from patient 7 from visit 1 to 7. F Tumor area calculations from patient 8 from visit 1 to 3
In the two animals with partial tumor responses, tumor growth ceased, but the tumors did not fully regress, and were ultimately removed by keratectomy and did not recur following surgical excision. Thus, the overall response rate (partial or complete tumor regression) for the 9 treated animals was 67% (6 of 9 responders). The response rate was higher in the Group 2 animals (83%) than in the Group 1 animals (33%).
Importantly, adverse effects from treatment with LTC were not observed in any of the treated animals at any time point. For example, conjunctival hyperemia scores in treated animals did not differ significantly from baseline values in except in one horse which showed a significant decrease in the conjunctival score from baseline compared to the final observation, which coincided with OSSN lesion regression.
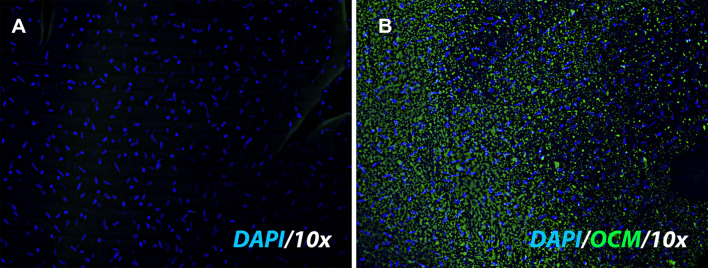
Uptake of LTC by corneal explants To assess the ability to LTC to be taken up by corneal epithelial cells, corneal explants were obtained from cadaveric horses and placed in short term in vitro culture. The explants were incubated with fluorescently labeled LTC for 30 min, as described in Methods. The explant cultures were then sectioned and examined by confocal microscopy to evaluate TLC uptake and localization (Fig. 2A, B). We observed uptake of the labeled LTC directly by corneal epithelial cells, with liposomes visualized in the cytoplasm of corneal epithelial cells.
Fig. 2.
LTC uptake by corneal epithelial cells. A corneal explant tissue obtained from a normal horse eye was incubated with fluorescently labeled LTC, as described in Methods. A Explant without the addition of labeled LTC, stained with DAPI (blue) to visualize nuclei. B Explant incubated with labeled (green) LTC for 30 min
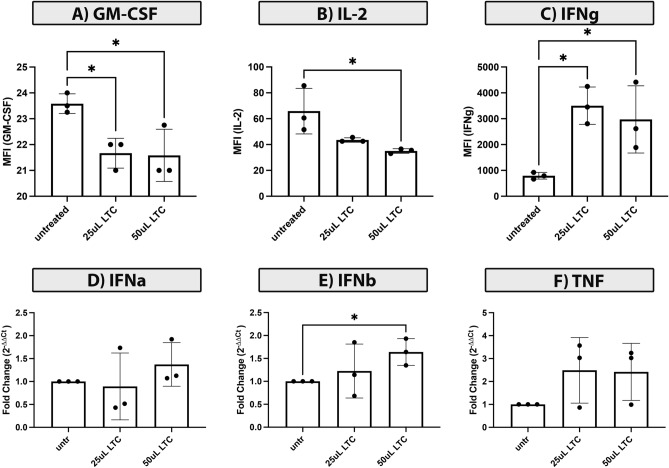
Immune responses to LTC treatment Previous studies have established that multiple different species, including humans, mice, dogs, cats, cattle all respond to innate immune activation by LTC [31–33, 39]. To determine whether LTC elicited similar innate immune responses in horses, equine PBMCs from several different horses were treated in vitro for 24 h with increasing concentrations of LTC, ranging from 10 to 50 ul/ml. Cytokine analysis of supernatants from LTC treated PBMC, using equine cytokine multiplex assays, revealed a significant decrease in GM-CSF (granulocyte–macrophage colony-stimulating factor) (Fig. 3A), significant decrease in IL-12 release (Fig. 3B); and a significant increase in secreted IFN-γ (Fig. 3C). Several other cytokines were also numerically increased in LTC-treated cultures though the differences did not reach statistical significance. By qRT-PCR analysis, there was significant upregulation of expression of IFN-β by LTC activated PBMC (Fig. 3E). Expression of IFN-α and TNF-α was increased numerically, but the increase did not reach the level of statistical significance (Fig. 3D, F).
Fig. 3.
Activation of innate immune responses in blood leukocytes by LTC. PBMC cultures prepared from blood of normal horses were stimulated with LTC in vitro, as described in Methods, and cytokine production assessed by qRT-PCR or by ELISA. In 24 h CM from LTC stimulated cells, there was a significant decrease in concentrations of GM-CSF (A), IL-12 (B) and significant increase of IFN-γ (C). The impact of LTC activation of PBMC on expression of key antitumor cytokines was also assessed by qRT-PCR IFN-a (D) and IFN-b (E), and TNFa (F). Statistical comparisons between two treatment groups were made using nonparametric t tests (Mann–Whitney test). Statistical significance was noted for p < 0.05
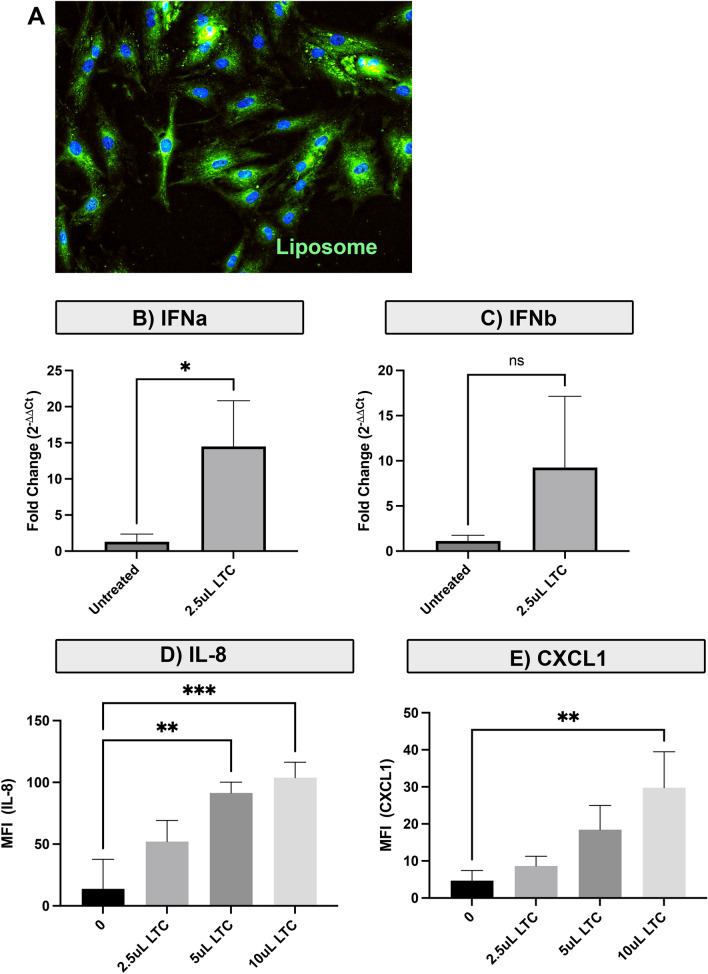
LTC treatment stimulates interferon production by OSSN cell lines The other potential target for LTC treatment, in addition to corneal cells and periocular immune cells, is the OSSN tumor cells themselves. Therefore, we assessed the direct impact of LTC treatment on OSSN innate immune responses. Uptake of LTC by equine OSSN cells was confirmed in vitro (Fig. 4A). Next, OSSN cells were incubated for 24 h with varying concentrations of LTC, and then, cytokine transcript responses were assessed. These studies revealed that OSSN cells significantly upregulated IFN-α and IFN-β expression following treatment with LTC (Fig. 4B, C). These type I interferons are important cytokines for suppressing tumor growth directly, and also for inhibiting tumor angiogenesis locally, and for stimulating the antigen presenting capabilities of tumor cells [40]. We also observed that LTC-stimulated OSSN cells produced significantly more IL-8 and CXCL1 following 24 h of in vitro stimulation. (Fig. 4D, E).
Fig. 4.
LTC activation of OSSN tumor innate immune responses. Equine OSSN cells were incubated with labeled LTC to assess cell uptake of the complexes, and the impact of LTC stimulation on direct tumor innate immune activation was assessed as well, as described in Methods. A Uptake of labeled LTC visualized in OSSN cells. B, C Assessment of immune activation of OSSN cells by RT PCR. B Increase in expression of IFNa transcript following treatment with 25 uL/mL LTC. (3C) Increase in expression of IFNb transcript. D LTC concentration dependent Increase in secretion of IL-8 and CXCL1 by LTC activated OSSN cells evaluated by cytokine multiplex ELISA. Statistical comparison was done using one‐way ANOVA, followed by Tukey multiple means post‐test. Statistical significance was assigned for p < 0.05
LTC activation of PBMC stimulates production of cytokines with tumor suppressive activity The interaction between ocular and periocular immune cells and OSSN tumor cells is considered an important component of the overall LTC treatment effect [41]. Therefore, experiments were conducted wherein secreted cytokines from equine PBMC were incubated with OSSN cells to assess the indirect effects of secreted cytokines.
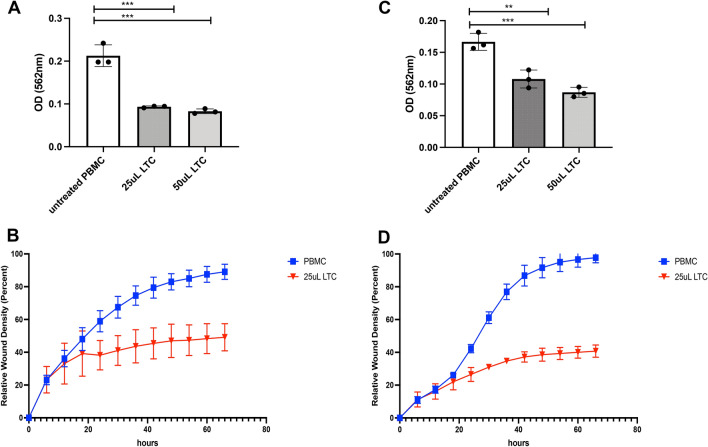
The two OSSN lines EQOSSNLC653, EQOSSNLC914 were co-cultured with supernatants collected from LTC-activated equine PBMCs. Both EQOSSNLC653 (Fig. 5A, B) and EQOSSNLC914 (Fig. 5B, C) cells displayed significantly inhibited metabolic activity (assessed by MTT assay) from cytokines secreted from LTC-activated PBMCs (Fig. 5A, C), compared to cytokines produced by non-activated PBMC. In as early as 24 h, there was a significant decrease in proliferation and migratory ability in the cells treated with LTC-activated PBMC supernatants (Fig. 5B, D) as shown by the IncuCyte scratch assay described in methods. This reduction in proliferation and migration persisted for > 48 h, during which time OSSN tumor cells were still unable to recover from the effects of tumoricidal cytokines.
Fig. 5.
Suppression of OSSN tumor cell growth and migration by cytokines released from LTC activated PBMC. Indirect effects of leukocyte cytokines on OSSN tumor growth were assessed by incubating tumor cells with cytokines released from LTC activated PBMC 24-h metabolic activity assay, using EQOSSNLC653 (A), and EQOSSNLC914 (C) cell line treated with n = 3 PBMC conditioned medium (CM), from either LTC activated or resting PBMC cultures. B, D Assessment of migration and proliferation using Incucyte scratch assay as described in Methods. Blue line depicts migration of EQOSSNLC653 cell line + untreated PBMC supernatants across the scratch area, red depicts the migration of EQOSSNLC653 cell line + PBMC supernatants treated with 25uL/mL of LTC. 4D Migration of EQOSSNLC914 incubated with PBMC-CM. Comparisons between more than 3 treatment groups were completed using one‐way ANOVA, followed by Tukey multiple means post‐test. Statistical significance was assigned at p < 0.05
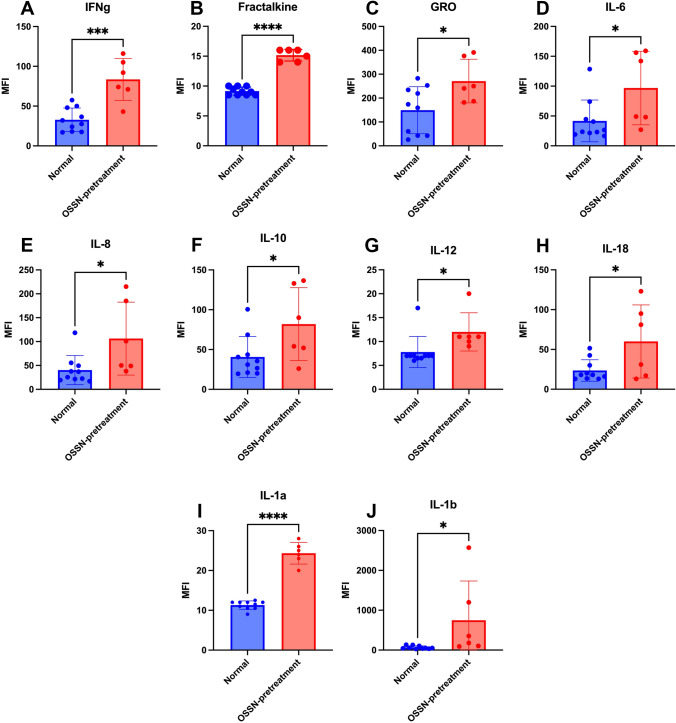
Cytokines in tears of horses with OSSN and response to treatment with LTC To evaluate local immune responses in the cornea and periocular tissues to LTC treatment, we next measured cytokine concentrations in tears of study animals, using tear samples obtained pretreatment and at 2-week intervals, as noted in Methods. We observed that compared to healthy horse tears, tears from the eyes of horses with OSSN had significant increases in the concentrations of IFN-γ, IL-1β, IL-1α, IL-6, IL-12, and IL-18, as well as the anti-inflammatory cytokines IL-10 and the chemotactic cytokines GRO and IL-8 (Fig. 6). To compare pre-treatment and post-treatment tear cytokine concentration from study animal with OSSN treated with LTC, data were normalized for each animal. Tear concentrations of IL1-ß, CXCL2, IFN-γ, IL-10, and TNF-α were numerically higher in tears from animals that responded to LTC treatment compared to those that did not (data not shown), though the differences did not reach the level of statistical significance.
Fig. 6.
Cytokine concentrations in tears from healthy horses and horses with OSSN. The immunological effects of the presence of OSSN lesions on local cytokine concentrations in tears were assessed using tear samples and a cytokine multiplexing assay, as described in Methods. Tears were collected from n = 10 healthy horses and n = 6 horses with OSSN (collected from the affected eye) and analyzed by multiplex kit. Significant increases in several cytokines were noted in tears from horses with OSSN, whereas no decreases in cytokine concentrations were observed, when healthy horses were compared to horses with OSSN. Cytokine concentrations are depicted in mean fluorescent intensity (MFI) units. A IFN-γ, B fractalkine, C GRO D IL-6 E IL-8, F IL-10, G IL-12, H IL-18, I IL-1a, J IL-1b
Discussion
Here, we report that topical and perilesional administration of a potent innate immune agonist (LTC) could induce significant and sustained lesion regression in a large animal spontaneous model of OSSN. The majority of treated animals, especially those treated with perilesional injections of LTC, responded to treatment with marked and durable tumor regression. This is the first report to our knowledge of a biologic response modifier such as LTC being administered topically or perilesionally to the eye for treatment of OSSN, and as such represents a potentially important advance in the development of new ocular immunotherapies.
Non-specific immunotherapies with biologic response modifiers such as TLR agonists have been used for years to induce tumor immunity [42–44]. The most effective biologic response modifiers are typically those capable of generating strong interferon responses, along with activation of innate immune effector cells, especially macrophages and NK cells. In this regard, several liposome-nucleic acid immunotherapeutics are known to be uniquely potent inducers of both type I and II interferons, in multiple different species, including humans [34, 45, 46]. For example, in prior cancer immunotherapy studies, LTC immunotherapy administered by systemic routes (i.v. or i.p.) induced potent suppression of tumor growth and metastasis [34].
More recently, we reported that a new formulation of LTC optimized for mucosal delivery was active in stimulating innate immune responses at mucosal surfaces, including the nasal cavity and the pharyngeal region [32]. Thus, we hypothesized that LTC immunotherapy would also be active in stimulating innate immune responses at ocular surfaces. We reasoned that the likely responding cell populations at the corneal surface of the eye would include corneal epithelium, squamous epithelium in the conjunctiva, and leukocytes in superficial vessels in adnexal tissues. Our in vitro studies demonstrated that LTC activated equine leukocytes and triggered production of key innate immune cytokines, including especially type I interferons, which are known to play critical roles in stimulating antitumor immunity [47–49]. However, we were unable to determine which cells produced the measured cytokines using the assays performed. However, it is likely that multiple cell types, including monocytes, NK cells, and T cells would have contributed [50].
In the current study, it is not clear which of these cell sources (e.g., corneal epithelial cells, limbal or perilimbal tissues, or conjunctival tissues) of antitumor cytokines may be the most important, and the overall tumor response likely represents the combined effects of cytokines released from each source. Injection of LTC into perilesional tissues appeared to stimulate greater antitumor immunity, as evidenced by the greater response rate (67%) in animals with larger tumors treated by perilesional LTC injection, compared to a 33% response rate in animals treated topically only, though study numbers were small, and the topical treatment groups were limited to only a single, every other day treatment. In humans with OSSN, topical IFN-α is given much more frequently. This difference in topical versus injection responses may also reflect prolonged engagement of more immune cells following LTC injection in highly vascularized subconjunctival tissues, whereas widely spaced (48 h) topical treatment would primarily activate the corneal epithelium and superficial conjunctival cells. Furthermore, the inability to detect consistent tear cytokine responses to LTC treatment may be a reflection of the post-treatment tear sampling time point (14 days) occurring too long after treatment, where we have shown previously these responses typically peak at between 48 and 72 h after administration [45].
Interferons such as those induced by LTC treatment can exert a number of antitumor effects. These include suppression of tumor cell proliferation, induction of direct tumoricidal activity by NK cells, upregulation of antigen presentation for improved recognition by T cells, and inhibition of tumor angiogenesis and cell migration [41]. Corneal epithelial cells are usually short lived and shed from the corneal surface; therefore, full tumor regression may ultimately depend on limiting the proliferation of the malignant limbal epithelial stem population which sustains OSSN tumor growth [23]. In support of this idea, we observed that conditioned medium from LTC- treated PBMCs contained a mixture of cytokines, including in particular type I and type II interferons, that were able to kill or suppress OSSN cell proliferation, as well as inhibiting OSSN cell migration.
Horses with spontaneous OSSN are an excellent animal model for OSSN in humans, due to similarities in gross and histologic appearance, shared risk factors, and comparable lesion progression times [1, 23, 24]. Horses also appear to share many of the same immunological properties of tumors and leukocytes upon stimulation with liposomes or TLR agonists [51]. In this study, we found that LTC stimulated both type I and II interferon responses in horses, both of which are important antitumor cytokines. The response of OSSN tumors to LTC immunotherapy may be mediated by antitumor cytokines generated either locally from tumor tissues themselves or produced by leukocytes in adjacent adnexal tissues. For example, both human and equine OSSN have been shown to respond to intralesional injections of IFN-α [52, 53], with up to an 81% response rate in human studies [54]; and the responses to LTC immunotherapy in OSSN in horses are likely involve similar mechanisms.
These studies provide initial evidence that LTC immunotherapy is active in inducing lesion regression in horses with OSSN, including in some animals with advanced tumors. Caveats to this study should be noted, including the small sample size examined, the lack of an untreated control group, or a cohort group for comparison that was treated with a current approach for equine OSSN, such as keratectomy.
Despite the study limitations, the horse OSSN model is considered an excellent spontaneous animal model for OSSN in humans, including shared risk factors such as UV light exposure, similar lesion progression times, and analogous pathology, with similar gross and histologic appearance [1, 23, 24]. For these reasons, the horse OSSN model is valuable animal model with which to further improve the LTC treatment approach, prior to initiating trials in human OSSN patients.
For example, the optimal dosing protocol for topical treatment of OSSN will likely require more frequent eye drop administrations, with at least twice daily administration suggested, based on similar studies in cats (Lappin et al., manuscript accepted)). In addition, more frequent topical LTC administration may improve responses to perilesional LTC injections. Stimulation of broad innate immune responses, including sustained induction of type I and II interferon responses, may ultimately prove more active in treatment of OSSN than repeated injections of a single cytokine such as IFN-α. Studies are currently underway in the horse OSSN model to directly compare the effectiveness of the two approaches.
In summary, the findings of this study demonstrated that LTC immunotherapy was active in inducing lesion regression in the majority of treated horses with OSSN and was clinically well tolerated. Therefore, the novel LTC biological response modifier merits further investigation in the equine OSSN model as a model to optimize and predict treatment responses in human OSSN.
Acknowledgements
The authors acknowledge and thank the clinical staff of the Colorado State University Veterinary Teaching Hospital who provided care and assisted with procedures described.
Author contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by LC, KW, BM. All clinical trials and related sample collection were performed by KW, BM. LP performed manuscript writing and data verification. The final version of manuscript was approved by all authors.
Funding
This study was funded by the Charles Shipley Family Foundation and by a grant from the Translational Medicine Institute and CSU Ventures at Colorado State University. Dr. Pezzanite was also supported by NIH grants 5TL1TR002533-02 and 5T32OD010437-19.
Data availability
All relevant data are within the manuscript.
Declarations
Competing interests
Authors Steven Dow and Lyndah Chow declare that they are among several holders of an issued US patent related to the LTC technology. All other authors declared no potential conflicts of interest.
Conflict of interest
The authors have read the journal’s policy and confirm that the ethical policies of the journal, as noted on the journal’s author guidelines page, have been adhered to. Owners provided consent for enrollment of client-owned patients in this clinical study. SD and LC declare that they are among several holders of an issued US patent related to the LTC technology. All other authors declared no potential conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kafarnik C, Rawlings M, Dubielzig RR. Corneal stromal invasive squamous cell carcinoma: a retrospective morphological description in 10 horses. Vet Ophthalmol. 2009;12(1):6–12. doi: 10.1111/j.1463-5224.2009.00666.x. [DOI] [PubMed] [Google Scholar]
- 2.Wilcock BP (1993) The eye and ear.In: Pathology of domestic animals, pp 441–529
- 3.Blodi FC, Ramsey FK. Ocular tumors in domestic animals. Am J Ophthalmol. 1967;64(3):627–633. doi: 10.1016/0002-9394(67)90568-5. [DOI] [PubMed] [Google Scholar]
- 4.McInnis CL, Giuliano EA, Johnson PJ, Turk JR. Immunohistochemical evaluation of cyclooxygenase expression in corneal squamous cell carcinoma in horses. Am J Vet Res. 2007;68(2):165–170. doi: 10.2460/ajvr.68.2.165. [DOI] [PubMed] [Google Scholar]
- 5.Dugan SJ, Curtis CR, Roberts SM, Severin GA. Epidemiologic study of ocular/adnexal squamous cell carcinoma in horses. J Am Vet Med Assoc. 1991;198(2):251–256. [PubMed] [Google Scholar]
- 6.Sykora S, Brandt S. Papillomavirus infection and squamous cell carcinoma in horses. Vet J. 2017;223:48–54. doi: 10.1016/j.tvjl.2017.05.007. [DOI] [PubMed] [Google Scholar]
- 7.Rassnick KM, Njaa BL. Cyclooxygenase-2 immunoreactivity in equine ocular squamous-cell carcinoma. J Vet Diagn Invest. 2007;19(4):436–439. doi: 10.1177/104063870701900419. [DOI] [PubMed] [Google Scholar]
- 8.Dugan SJ, Roberts SM, Curtis CR, Severin GA. Prognostic factors and survival of horses with ocular/adnexal squamous cell carcinoma: 147 cases (1978–1988) J Am Vet Med Assoc. 1991;198(2):298–303. [PubMed] [Google Scholar]
- 9.King TC, Priehs DR, Gum GG, Miller TR. Therapeutic management of ocular squamous cell carcinoma in the horse: 43 cases (1979–1989) Equine Vet J. 1991;23(6):449–452. doi: 10.1111/j.2042-3306.1991.tb03759.x. [DOI] [PubMed] [Google Scholar]
- 10.Mosunic CB, Moore PA, Carmicheal KP, Chandler MJ, Vidyashankar A, Zhao Y, Roberts RE, Dietrich UM. Effects of treatment with and without adjuvant radiation therapy on recurrence of ocular and adnexal squamous cell carcinoma in horses: 157 cases (1985–2002) J Am Vet Med Assoc. 2004;225(11):1733–1738. doi: 10.2460/javma.2004.225.1733. [DOI] [PubMed] [Google Scholar]
- 11.McCalla TL, Moore CP, Collier LL. Immunotherapy of periocular squamous cell carcinoma with metastasis in a pony. J Am Vet Med Assoc. 1992;200(11):1678–1681. [PubMed] [Google Scholar]
- 12.Théon AP, Pascoe JR, Meagher DM. Perioperative intratumoral administration of cisplatin for treatment of cutaneous tumors in equidae. J Am Vet Med Assoc. 1994;205(8):1170–1176. [PubMed] [Google Scholar]
- 13.Théon AP, Pascoe JR. Iridium-192 interstitial brachytherapy for equine periocular tumours: treatment results and prognostic factors in 115 horses. Equine Vet J. 1995;27(2):117–121. doi: 10.1111/j.2042-3306.1995.tb03046.x. [DOI] [PubMed] [Google Scholar]
- 14.Théon AP, Pascoe JR, Madigan JE, Carlson G, Metzger L. Comparison of intratumoral administration of cisplatin versus bleomycin for treatment of periocular squamous cell carcinomas in horses. Am J Vet Res. 1997;58(4):431–436. [PubMed] [Google Scholar]
- 15.Wilkie DA, Burt JK. Combined treatment of ocular squamous cell carcinoma in a horse, using radiofrequency hyperthermia and interstitial 198Au implants. J Am Vet Med Assoc. 1990;196(11):1831–1833. [PubMed] [Google Scholar]
- 16.English RV, Nasisse MP, Davidson MG. Carbon dioxide laser ablation for treatment of limbal squamous cell carcinoma in horses. J Am Vet Med Assoc. 1990;196(3):439–442. [PubMed] [Google Scholar]
- 17.Walker MA, Schumacher J, Schmitz DG, McMullen WC, Ruoff WW, Crabill MR, Hawkins JF, Hogan PM, McClure SR, Vacek JR, et al. Cobalt 60 radiotherapy for treatment of squamous cell carcinoma of the nasal cavity and paranasal sinuses in three horses. J Am Vet Med Assoc. 1998;212(6):848–851. [PubMed] [Google Scholar]
- 18.De Ridder T, Ruppin M, Wheeless M, Williams S, Reddell P. Use of the intratumoural anticancer drug tigilanol tiglate in two horses. Front Vet Sci. 2020;7:639. doi: 10.3389/fvets.2020.00639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Estell K. Periocular neoplasia in the horse. Vet Clin North Am Equine Pract. 2017;33(3):551–562. doi: 10.1016/j.cveq.2017.08.004. [DOI] [PubMed] [Google Scholar]
- 20.Elce YA, Orsini JA, Blikslager AT. Expression of cyclooxygenase-1 and -2 in naturally occurring squamous cell carcinomas in horses. Am J Vet Res. 2007;68(1):76–80. doi: 10.2460/ajvr.68.1.76. [DOI] [PubMed] [Google Scholar]
- 21.Howarth S, Lucke VM, Pearson H. Squamous cell carcinoma of the equine external genitalia: a review and assessment of penile amputation and urethrostomy as a surgical treatment. Equine Vet J. 1991;23(1):53–58. doi: 10.1111/j.2042-3306.1991.tb02715.x. [DOI] [PubMed] [Google Scholar]
- 22.Hendrix DVH. Equine ocular squamous cell carcinoma. Clin Tech Equine Pract. 2005;4(1):87–94. doi: 10.1053/j.ctep.2005.03.011. [DOI] [Google Scholar]
- 23.Gichuhi S, Ohnuma S-i, Sagoo MS, Burton MJ. Pathophysiology of ocular surface squamous neoplasia. Exp Eye Res. 2014;129:172–182. doi: 10.1016/j.exer.2014.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shank A, Teixeria L, Dubielzig R. Canine, feline, and equine corneal vascular neoplasia: a retrospective study (2007–2015) Vet Ophthalmol. 2018 doi: 10.1111/vop.12571. [DOI] [PubMed] [Google Scholar]
- 25.Crausaz M, Launois T, Smith-Fleming K, McCoy AM, Knickelbein KE, Bellone RR. DDB2 genetic risk factor for ocular squamous cell carcinoma identified in three additional horse breeds. Genes (Basel) 2020 doi: 10.3390/genes11121460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bellone RR. Genetics of equine ocular disease. Vet Clin N Am Equine Pract. 2020;36(2):303–322. doi: 10.1016/j.cveq.2020.03.009. [DOI] [PubMed] [Google Scholar]
- 27.Bellone RR, Liu J, Petersen JL, Mack M, Singer-Berk M, Drögemüller C, Malvick J, Wallner B, Brem G, Penedo MC, et al. A missense mutation in damage-specific DNA binding protein 2 is a genetic risk factor for limbal squamous cell carcinoma in horses. Int J Cancer. 2017;141(2):342–353. doi: 10.1002/ijc.30744. [DOI] [PubMed] [Google Scholar]
- 28.Knickelbein KE, Lassaline ME, Singer-Berk M, Reilly CM, Clode AB, Famula TR, Michau TM, Bellone RR. A missense mutation in damage-specific DNA binding protein 2 is a genetic risk factor for ocular squamous cell carcinoma in Belgian horses. Equine Vet J. 2020;52(1):34–40. doi: 10.1111/evj.13116. [DOI] [PubMed] [Google Scholar]
- 29.Neale RE, Weissenborn S, Abeni D, Bavinck JN, Euvrard S, Feltkamp MC, Green AC, Harwood C, de Koning M, Naldi L, et al. Human papillomavirus load in eyebrow hair follicles and risk of cutaneous squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev. 2013;22(4):719–727. doi: 10.1158/1055-9965.EPI-12-0917-T. [DOI] [PubMed] [Google Scholar]
- 30.Zhu KW, Affolter VK, Gaynor AM, Dela Cruz FN, Pesavento PA. Equine genital squamous cell carcinoma: in situ hybridization identifies a distinct subset containing equus caballus papillomavirus 2. Vet Pathol. 2015;52(6):1067–1072. doi: 10.1177/0300985815583095. [DOI] [PubMed] [Google Scholar]
- 31.Wheat W, Chow L, Coy J, Contreras E, Lappin M, Dow S. Activation of upper respiratory tract mucosal innate immune responses in cats by liposomal toll-like receptor ligand complexes delivered topically. J Vet Intern Med. 2019;33(2):838–845. doi: 10.1111/jvim.15426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wheat W, Chow L, Kuzmik A, Soontararak S, Kurihara J, Lappin M, Dow S. Local immune and microbiological responses to mucosal administration of a Liposome-TLR agonist immunotherapeutic in dogs. BMC Vet Res. 2019;15(1):330. doi: 10.1186/s12917-019-2073-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wheat W, Chow L, Rozo V, Herman J, Still Brooks K, Colbath A, Hunter R, Dow S. Non-specific protection from respiratory tract infections in cattle generated by intranasal administration of an innate immune stimulant. PLoS ONE. 2020;15(6):e0235422. doi: 10.1371/journal.pone.0235422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zaks K, Jordan M, Guth A, Sellins K, Kedl R, Izzo A, Bosio C, Dow S. Efficient immunization and cross-priming by vaccine adjuvants containing TLR3 or TLR9 agonists complexed to cationic liposomes. J Immunol. 2006;176(12):7335–7345. doi: 10.4049/jimmunol.176.12.7335. [DOI] [PubMed] [Google Scholar]
- 35.Kamstock D, Guth A, Elmslie R, Kurzman I, Liggitt D, Coro L, Fairman J, Dow S. Liposome–DNA complexes infused intravenously inhibit tumor angiogenesis and elicit antitumor activity in dogs with soft tissue sarcoma. Cancer Gene Ther. 2006;13(3):306–317. doi: 10.1038/sj.cgt.7700895. [DOI] [PubMed] [Google Scholar]
- 36.Regan D, Dow S. Manipulation of innate immunity for cancer therapy in dogs. Vet Sci. 2015 doi: 10.3390/vetsci2040423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eaton JS, Miller PE, Bentley E, Thomasy SM, Murphy CJ. The SPOTS system: an ocular scoring system optimized for use in modern preclinical drug development and toxicology. J Ocul Pharmacol Ther. 2017;33(10):718–734. doi: 10.1089/jop.2017.0108. [DOI] [PubMed] [Google Scholar]
- 38.van Meerloo J, Kaspers GJ, Cloos J. Cell sensitivity assays: the MTT assay. Methods Mol Biol. 2011;731:237–245. doi: 10.1007/978-1-61779-080-5_20. [DOI] [PubMed] [Google Scholar]
- 39.Henderson A, Propst K, Kedl R, Dow S. Mucosal immunization with liposome-nucleic acid adjuvants generates effective humoral and cellular immunity. Vaccine. 2011;29(32):5304–5312. doi: 10.1016/j.vaccine.2011.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aricò E, Castiello L, Capone I, Gabriele L, Belardelli F. Type I interferons and cancer: an evolving story demanding novel clinical applications. Cancers (Basel) 2019 doi: 10.3390/cancers11121943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chulpanova DS, Kitaeva KV, Green AR, Rizvanov AA, Solovyeva VV. Molecular aspects and future perspectives of cytokine-based anti-cancer immunotherapy. Front Cell Dev Biol. 2020;8:402–402. doi: 10.3389/fcell.2020.00402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alexandroff AB, Jackson AM, O'Donnell MA, James K. BCG immunotherapy of bladder cancer: 20 years. Lancet. 1999 doi: 10.1016/S0140-6736(98)07422-4. [DOI] [PubMed] [Google Scholar]
- 43.Studer U, Marti E Fau - Stornetta D, Stornetta D Fau - Lazary S, Lazary S Fau - Gerber H, Gerber H (1997) The therapy of equine sarcoid with a non-specific immunostimulator—the epidemiology and spontaneous regression of sarcoids. (0036–7281 (Print)) [PubMed]
- 44.Papaioannou NE, Beniata OV, Vitsos P, Tsitsilonis O, Samara P. Harnessing the immune system to improve cancer therapy. Ann Transl Med. 2016;4(14):261–261. doi: 10.21037/atm.2016.04.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dow SW, Fradkin LG, Liggitt DH, Willson AP, Heath TD, Potter TA. Lipid-DNA complexes induce potent activation of innate immune responses and antitumor activity when administered intravenously. J Immunol. 1999;163(3):1552. doi: 10.4049/jimmunol.163.3.1552. [DOI] [PubMed] [Google Scholar]
- 46.Dow S. Liposome-nucleic acid immunotherapeutics. Expert Opin Drug Deliv. 2008;5(1):11–24. doi: 10.1517/17425247.5.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Boukhaled GM, Harding S, Brooks DG. Opposing roles of type I interferons in cancer immunity. Annu Rev Pathol. 2021;16(1):167–198. doi: 10.1146/annurev-pathol-031920-093932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cao X, Liang Y, Hu Z, Li H, Yang J, Hsu EJ, Zhu J, Zhou J, Fu Y-X. Next generation of tumor-activating type I IFN enhances anti-tumor immune responses to overcome therapy resistance. Nat Commun. 2021;12(1):5866. doi: 10.1038/s41467-021-26112-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Budhwani M, Mazzieri R, Dolcetti R. Plasticity of type I interferon-mediated responses in cancer therapy: from anti-tumor immunity to resistance. Front Oncol. 2018;8:322–322. doi: 10.3389/fonc.2018.00322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu J, Li Z. Resident innate immune cells in the Cornea. Front Immunol. 2021 doi: 10.3389/fimmu.2021.620284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gao A, Hu X-L, Saeed M, Chen B-F, Li Y-P, Yu H-J. Overview of recent advances in liposomal nanoparticle-based cancer immunotherapy. Acta Pharmacol Sin. 2019;40(9):1129–1137. doi: 10.1038/s41401-019-0281-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Galor A, Karp CL, Chhabra S, Barnes S, Alfonso EC. Topical interferon alpha 2b eye-drops for treatment of ocular surface squamous neoplasia: a dose comparison study. Br J Ophthalmol. 2010;94(5):551–554. doi: 10.1136/bjo.2008.153197. [DOI] [PubMed] [Google Scholar]
- 53.Al Bayyat G, Arreaza-Kaufman D, Venkateswaran N, Galor A, Karp CL. Update on pharmacotherapy for ocular surface squamous neoplasia. Eye Vis. 2019;6(1):24. doi: 10.1186/s40662-019-0150-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kozma K, Dömötör ZR, Csutak A, Szabó L, Hegyi P, Erőss B, Helyes Z, Molnár Z, Dembrovszky F, Szalai E. Topical pharmacotherapy for ocular surface squamous neoplasia: systematic review and meta-analysis. Sci Rep. 2022;12(1):14221. doi: 10.1038/s41598-022-18545-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the manuscript.