Abstract
Background
Sequential tyrosine kinase inhibitors (TKIs) following immune checkpoint inhibitors (ICIs) increases the incidence of serious adverse events (SAEs). However, the factors and the types of TKIs that affect the incidence of SAEs remain unknown.
Methods
We retrospectively reviewed advanced non-small cell lung cancer (NSCLC) patients who received sequential TKIs following ICIs between November 2015 and April 2021. All AEs were evaluated using Common Terminology Criteria for Adverse Events (CTCAE) ver 5.0.
Results
Among 1,638 NSCLC patients who received ICIs, 63 patients received sequential TKIs following ICIs. The types of TKIs included EGFR-TKIs in 48 patients, ALK-TKIs in 10 patients, and others in 5 patients. The median dosing interval was 57 days (range: 7–698). Eighteen (28.6%) patients developed SAEs (Grade 3/4 or hospitalized). The incidence of SAEs and withdrawal of TKIs due to AEs were significantly higher in patients (n = 40) who initiated TKI treatment within 3 months after ICIs than in patients (n = 23) who initiated TKI treatment 3 months after ICIs (SAEs, 40.0% vs. 4.3%, p < 0.01; withdrawal rate: 57.5% vs. 21.7%, p < 0.01). There was no significant difference in the incidence of SAEs and withdrawal rate due to AEs between EGFR-TKIs and other TKIs (SAE, 22.9% vs. 40.0%, p = 0.20; withdrawal rate: 41.7% vs. 53.3%, p = 0.55).
Conclusion
The dosing interval from last ICI to the initiation of TKI treatment can affects the incidence of SAEs and the withdrawal rate due to AEs regardless of the types of TKIs.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00262-023-03429-z.
Keywords: Non-small cell lung cancer, Sequential therapy, Dosing interval, Serious adverse events
Introduction
Immune checkpoint inhibitors (ICIs), such as nivolumab, pembrolizumab, atezolizumab, and durvalumab, have been used as standard treatment in patients with non-small cell lung cancer (NSCLC) without oncogenic driver mutations. In contrast, in patients with oncogenic driver alterations (such as epidermal growth factor receptor (EGFR) mutation, anaplastic lymphoma kinase (ALK) rearrangement, c-ros oncogene 1 (ROS1) rearrangement, and BRAF V600E mutation), tyrosine kinase inhibitors (TKIs) are used as standard treatment in front-line setting [1–4] because ICI monotherapy is less effective in such patients. However, the IMpower 150 trial recently reported that the addition of atezolizumab to carboplatin, paclitaxel, and bevacizumab (ABCP) showed survival benefit in EGFR-mutant NSCLC patients. The addition of vascular endothelial growth factor (VEGF) inhibition using bevacizumab, and chemotherapy could enhance the T-cell–mediated cancer-cell killing action of ICIs including atezolizumab by reversing VEGF-mediated immunosuppression and chemotherapy-induced cell death [5]. Further studies that aim to elucidate the clinical benefits of combination therapy with ICIs such as ABCP regimen after targeted therapy in patients with tumors harboring oncogenic drivers, such as ALK, ROS1, and BRAF may be warranted.
Recently, several studies have reported the therapeutic efficacy of TKI re-administration after standard treatment with ICIs in NSCLC patients with oncogenic driver alterations [6–10]. Additionally, durvalumab after chemoradiotherapy as a maintenance treatment has been the standard of care for locally advanced NSCLC patients, and treatment with TKIs is selected for use after durvalmab failure in the patients having oncogenic driver alterations [11]. Thus, the use of sequential ICIs followed by TKI treatment has become more frequent in treating advanced NSCLC.
The combination of ICIs and TKIs has been associated with an increased risk of toxicity. In the TATTON trial assessing the safety and tolerability of osimertinib in combination with other therapies, a significant increase in incidence of interstitial lung disease (ILD) has been reported when combining osimertinib with durvalumab [12]. Enrollment in the osimertinib plus durvalumab cohort was terminated prematurely. Another study investigating EGFR-TKI combined with ICI treatment similarly reported high rates of toxicities in patients with advanced EGFR-mutant NSCLC [13]. Treatment with osimertinib has been associated with the occurrence of serious adverse events (SAEs) among EGFR-mutant patients who recently received programmed death ligand-1 (PD-(L)1) blockade [14]. In ALK-positive NSCLC patients, sequential ICI and crizotinib treatment is associated with a significantly increased risk of hepatotoxicity [15]. Furthermore, hypersensitivity reactions are increased when using selpercatinib after ICI treatment in patients with RET fusion-positive NSCLC [16]. Thus, the combination of ICIs and TKIs as well as sequential TKIs following ICI treatment can increase the incidence of SAEs. However, the effects of factors including dosing interval between ICI and TKI treatment and the types of TKIs that affect the incidence of SAEs remain unknown. Therefore, we aimed to investigate the incidence and risk factors of SAEs induced by sequential TKI following ICI treatment by evaluating the details of SAEs and withdrawal rate in TKI treatment due to AEs.
Materials and methods
Patient characteristics
We retrospectively reviewed the records of patients with advanced or recurrent NSCLC and oncogenic driver alterations such as EGFR, ALK, RET, neuregulin 1 (NRG1), c-Met (MET), ROS1, BRAF V600E, and human epidermal growth factor receptor 2 (HER2) who received sequential TKI following ICI treatment at the National Cancer Center Hospital (Tokyo, Japan) between November 2015 and April 2021. The following clinical data were evaluated: age, sex, Eastern Cooperative Oncology Group (ECOG) performance status (PS), smoking history, histological type, stage, driver mutation status, PD-L1 expression data if available, treatment line, types of ICIs and TKIs, duration of ICI administration, dosing interval between ICIs and TKIs, frequency of SAEs, and withdrawal of TKI treatment. The time interval between ICI and TKI treatment was measured from the last day of ICI administration to TKI initiation.
Assessment of AEs
All AEs were evaluated using Common Terminology Criteria for Adverse Events (CTCAE) version 5.0 [17]. SAEs were defined as grade ≥ 3 AEs or AEs requiring hospitalization or withdrawal of TKI treatment due to AEs as leading to discontinuation of TKI treatment regardless of the grade. Data were analyzed until patient death or data cutoff date of 30 December, 2021. The proposed study was accepted by the Ethics Committee of the National Cancer Center Hospital (Tokyo, Japan).
Statistical analysis
Fisher’s exact test was performed to evaluate differences in categorical data. Statistical analysis was performed using EZR version 1.53 [17]. p value < 0.05 was considered significant.
Results
Patient characteristics
Among the 1,638 NSCLC patients who received ICIs, 63 patients received sequential TKIs following ICI treatment (Fig. 1). Patient baseline characteristics are described in Table 1. The median age was 64 years (range: 32–84). The majority of the patients were female (41 [65.1%]) and light or non-smokers (49 [77.8%]). Most of the patients were classified as ECOG PS 0–1 (49 [77.8%]). The major driver mutation was EGFR (n = 44, 69.8%) followed by ALK (7, 11.1%), RET (4, 6.3%), NRG (3, 4.8%), MET (2, 3.2%), ROS1 (1, 1.6%), BRAF V600E (1, 1.6%), and HER2 (1, 1.6%). Among the 63 patients, 20 (31.7%) received cytotoxic chemotherapy during the interval between ICI and TKI treatment.
Fig. 1.

CONSORT diagram of the study. The CONSORT diagram represents the process used to select the patients included in the study. NSCLC, non-small cell lung cancer; ICI, immune checkpoint inhibitor; TKI, tyrosine kinase inhibitor
Table 1.
Patient baseline characteristics (N = 63)
| Total N = 63 | |
|---|---|
| Age, median (range) (years) | 64 (32–84) |
| Sex (male/ female), n (%) | 22 (35)/41 (65) |
| PS (0–1/2), n (%) | 49 (78)/14 (22) |
| Smoking history (> 10 pack years), (yes/no), n (%) | 14 (22)/49 (78) |
| Histological type, (Ad/ non-Ad), n (%) | 61 (97)/2 (3) |
| Stage (III-IV/ recurrence), n (%) | 11 (17)/52 (83) |
| Driver mutation | |
| EGFR/ ALK/ RET/ NRG/ MET/ ROS1/ BRAF V600E/ HER2, n (%) | 44 (70)/7 (11)/4 (6)/3 (4)/2 (3)/1 (2)/1 (2)/1 (2) |
| PD-L1 expression TPS (unknown/0/1–49/ ≥ 3), n (%) | 9(14)/21 (33)/13 (21)/20 (32) |
| Prior treatment line (< 3/ ≥ 3), n (%) | 8 (13)/55 (87) |
| Duration of ICI administration, median (range) (days) | 50 (14–917) |
| Dosing interval between ICIs and TKIs, median (range) (days) | 57 (7–698) |
| Duration of TKI treatment, median (range) (days) | 268 (13–1734) |
PS performance status, Ad adenocarcinoma, TPS tumor proportion score, ICI immune checkpoint inhibitor, TKI tyrosine kinase inhibitor, PD-L1 programmed death ligand-1
The types of ICIs and TKIs are shown in Fig. 2. Among the 63 patients, 54 (85.7%) received ICI monotherapy, and 9 (14.3%) received chemotherapy in combination with ICIs. The types of ICIs administered included nivolumab (n = 29, 46.0%), pembrolizumab (n = 15, 23.8%), atezolizumab (n = 14, 22.2%), and durvalumab (n = 5, 8.0%). The types of TKIs administered included EGFR-TKIs in 48 patients (n = 21, osimertinib [33.3%]; 14, afatinib [22.2%]; 9, erlotinib [14.3%]; and 4, gefitinib [6.3%]), ALK-TKIs in 10 patients (4, alectinib [6.3%]; 3, crizotinib [4.8%]; 2, brigatinib [3.2%]; and 1, lorlatinib [1.6%]), and others in 5 patients (3, selpercatinib [4.8%]; 1, lenvatinib [1.6%]; and 1, dabrafenib/trametinib [1.6%]). Most patients received ICIs after 3rd line treatment (55 [87.3%]). The median follow-up time from the initiation of ICI is 508 days (range: 71–2038). The median dosing interval between ICIs and TKIs was 57 days (range: 7–698). Median duration of TKI treatment was 268 days (range: 13–1734) (Table 1).
Fig. 2.
Proportion of ICIs and TKIs administered. A Proportion of ICIs administered. Nivolumab was the most commonly administered ICI followed by pembrolizumab and atezolizumab. B Proportion of TKIs administered. Osimertinib was the most commonly administered TKI followed by afatinib and erlotinib. ICI, immune checkpoint inhibitor; TKI, tyrosine kinase inhibitor
Incidence and timing of SAEs
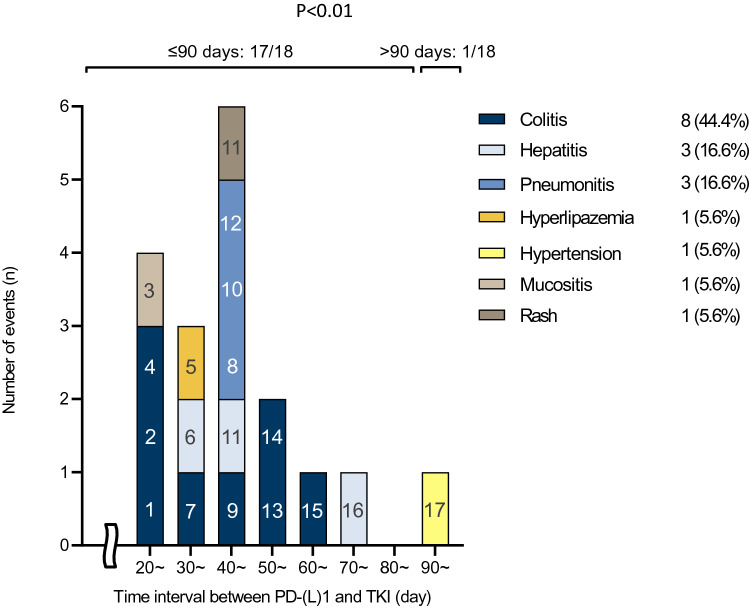
Among the 63 patients, 17 patients (27.0%) developed a total of 18 SAEs including colitis (8 cases, 44.4%), hepatitis (3, 16.6%), pneumonitis (3, 16.6%), lipase increased (1, 5.6%), hypertension (1, 5.6%), mucositis (1, 5.6%), and rash (1, 5.6%). The incidence of SAEs based on the time interval is shown in Fig. 3. The SAEs occurred within 90 days, except in one case. The incidence of SAEs and withdrawal rate due to AEs in patients who initiated TKI treatment within 90 days after ICIs (n = 40) was significantly higher than those in patients who initiated TKI treatment 90 days after ICIs (n = 23) (SAEs: 40.0% ([16/40] vs. 4.3% [1/23], p < 0.01; withdrawal rate: 57.5% ([23/ 40] vs. 21.7% [5/23], p < 0.01) (Table 2).
Fig. 3.
Incidence and timing of SAEs. The most frequently reported SAE was diarrhea. Almost all SAEs occurred in a time interval of 90 days except for one case. SAE, severe adverse advent. Hyperlipazemia; Lipase increased
Table 2.
Difference in SAE and treatment discontinuation between the two groups which started TKI treatment within 90 days after last ICI (≤ 90 days) vs. 90 days or more after last ICI (> 90 days)
| SAE, n (%) | No SAE, n (%) | p value | |
|---|---|---|---|
| Difference in SAE occurrence between the two groups which started TKI treatment within 90 s days after last ICI (≤ 90 days) versus 90 days or more after last ICI (> 90 days) | |||
| Time interval ≤ 90 days | 16 (40.0) | 24 (60.0) | |
| Time interval > 90 days | 1 (4.3) | 22 (95.7) | < 0.01 |
| Total | 17 (27.0) | 46 (73.0) | |
| Discontinuation, n (%) | No Discontinuation, n (%) | p value | |
|---|---|---|---|
| Difference in treatment discontinuation due to AEs between the two groups which started TKI treatment within 90 s days after last ICI (≤ 90 days) versus 90 days or more after last ICI (> 90 days) | |||
| Time interval ≤ 90 days | 23 (57.5) | 17 (42.5) | |
| Time interval > 90 days | 5 (21.7) | 18 (78.3) | < 0.01 |
| Total | 28 (44.4) | 35 (55.6) | |
Time interval; the duration between the last day of ICI administration to the initiation of TKI
SAE severe adverse event
Incidence of SAEs and withdrawal rate between EGFR-TKIs and other TKIs
We evaluated whether the different types of TKIs affected the incidence of SAEs. The SAEs observed in 17 patients are described in Table 3 and Fig. 3. Among 48 patients receiving EGFR-TKIs, 11 (22.9%) patients reported SAEs. SAEs were observed in patients receiving EGFR-TKIs, including afatinib (7), osimertinib (2), erlotinib (1), and gefitinib (1). Among the patients showing SAEs, 3 (27.3%) patients could continue TKI treatment with management such as steroid combination, dose reduction, or observation. Among 15 patients using other TKIs, 6 (40.0%) reported SAEs. SAEs were observed in patients receiving other TKIs including brigatinib (2), dabrafenib/trametinib (1), selpercatinib (1), crizotinib (1), and lenvatinib (1). Among the patients showing SAEs, 4 (66.7%) patients could continue TKI treatment. There was no significant difference in the incidence of SAEs and withdrawal rate due to AEs between patients receiving EGFR-TKIs and other TKIs (ALK-TKIs and others) (SAEs: 22.9% ([11/48] vs. 40.0% [6/15], p = 0.20; withdrawal rate: 41.7% ([20/ 48] vs. 53.3% [8/ 15], p = 0.55) (TSupplemental Table II). On the other hand, the incidence of SAE in patients treated with afatinib following ICIs was significantly higher than that of other TKIs (50% vs. 20.4%, p = 0.04). Almost all of the SAEs due to afatinib were Grade 3 diarrhea (Fig. 4).
Table 3.
Details of 17 patients who experienced SAEs
| Pt No | Mutation type | ICI regimen | TKI | Interval (days) | SAE (Grade) | Response to steroid | Continuation of TKI (reduction) |
|---|---|---|---|---|---|---|---|
| 1 | BRAF V600E | ABCP | Dab/Tra | 21 | Colitis (3) | Yes | Yes (No) |
| 2 | EGFR ex19del | Atezolizumab | Afatinib | 21 | Colitis (3) | Yes | No (Yes) |
| 3 | NRG1-fusion | PC, Pembrolizumab | Afatinib | 25 | Mucositis (3) | - | No (Yes) |
| 4 | EGFR ex19del | Pembrolizumab | Afatinib | 29 | Colitis (3) | - | No (Yes) |
| 5 | ALK-fusion | Nivolumab | Brigatinib | 32 | Lipase increased (3) | - | Yes (No) |
| 6 | EGFR L858R/T790M | Nivolumab | Osimertinib | 35 | Hepatitis (3) | - | Yes (Yes) |
| 7 | EGFR ex19del | Nivolumab | Afatinib | 37 | Colitis (3) | Yes | Yes (Yes) |
| 8 | EGFR ex19del | Nivolumab | Osimertinib | 40 | Pneumonitis (3) | Yes | No |
| 9 | NRG1-fusion | Nivolumab | Afatinib | 43 | Colitis (3) | Yes | Yes (Yes) |
| 10 | EGFR L858R | Pembrolizumab | Erlotinib | 43 | Pneumonitis (4) | - | No |
| 11 | RET-fusion | PC, Pembrolizumab | Selpercatinib | 45 | Hepatitis (3), Rash (3) | Yes | No (Yes) |
| 12 | MET-fusion | PC, Pembrolizumab | Crizotinib | 47 | Pneumonitis (4) | No | No |
| 13 | EGFR T790M | Nivolumab | Afatinib | 50 | Colitis (3) | - | No |
| 14 | EGFR L858R/cMET | Atezolizumab | Gefitinib | 57 | Colitis (3) | - | No |
| 15 | EGFR ex20ins | Nivolumab | Afatinib | 61 | Colitis (3) | - | No (Yes) |
| 16 | ALK-fusion | ABCP | Brigatinib | 77 | Hepatitis (3) | - | Yes (Yes) |
| 17 | RET-fusion | Pembrolizumab | Lenvatinib | 91 | HT (3) | - | Yes (Yes) |
Interval; the duration between the last day of ICI administration to the initiation of TKI
ICI immune checkpoint inhibitor, TKI tyrosine kinase inhibitors; SAE: severe adverse event, PC Pemetrexed and Carboplatin, ABCP Atezolizumab, Bevacizumab, Carboplatinand Paclitaxel, Dab/Tra: Dabrafenib/ Trametinib, HT Hypertension, – no use
Fig. 4.
Time series of SAEs. All SAEs occurred early after initiation of TKI treatment. SAE, severe adverse advent; TKI, tyrosine kinase inhibitor
Discussion
We demonstrated that SAEs occurred in 27.0% (17/63) of the patients who received sequential ICIs and TKIs. The dosing interval between the last ICI to the initiation of TKI treatment affects the incidence of SAEs and the withdrawal rate due to AEs regardless of the type of TKI.
Several studies have focused on the safety of the combination of TKIs and ICIs and on sequential TKI following ICI therapy in EGFR-mutant NSCLC patients and those with other driver oncogenic alterations (Supplemental Table I). Several early-phase clinical trials have reported an increase in occurrence of grade 3 or higher toxicities in EGFR-mutant NSCLC patients treated with EGFR-TKI, especially osimertinib, in combination with ICIs. These results have led to discontinuation of this combination therapeutic approach in EGFR-mutant NSCLC. Additionally, the sequential use of EGFR-TKIs including osimertinib after ICI treatment was reported to relate to an increased risk of SAEs (1). In EGFR-mutant NSCLC, concerns regarding the increased risks with sequential administration of ICIs and EGFR-TKIs have been on the rise. In ALK-positive NSCLC, a relatively higher risk of hepatotoxicity has been reported with sequential use of ALK-TKIs after ICI treatment. Lin et al. reported that 5 of 11 (45.5%) patients treated with crizotinib after receiving previous treatment with an ICI developed grade 3 or 4 liver toxicity, leading to permanent discontinuation of crizotinib in four of five patients [15]. Furthermore, McCoach et al. recently reported an increase in hypersensitivity reactions when using selpercatinib after ICIs in patients with RET fusion-positive NSCLC [16]. In melanoma, BRAF V600E-TKIs, such as vemurafenib and dabrafenib/ trametinib (dab/tra), have been reported to increase SAE occurrence when used after ICIs [18–20]. Indeed, our study showed that there was no significant difference in incidence of SAEs between EGFR-TKIs and other TKIs in patients who received TKIs after ICIs. Our findings have important clinical implications in the potential risk of TKI treatment after ICI use regardless of the types of ICIs.
Regarding the association between the treatment-free interval and development of SAEs, Schoenfeld et al. [14] reported that development of SAEs was most common among patients who began osimertinib treatment within 3 months of prior PD-(L)1 blockade compared with > 3–12 months and > 12 months. Gemma et al. [21] reported that in patients who received a previous nivolumab treatment, ILD development was the highest in patients who discontinued nivolumab treatment within the first month before initiating osimertinib treatment. The trends for decreasing incidence and proportion are observed with an increasing duration between the end of nivolumab treatment and the initiation of osimertinib treatment. Additionally, several case series showed that almost all of the SAEs occurred within 90 days of time interval in patients treated with TKIs following ICIs (Supplemental Table I). Our study showed that the incidence of SAEs in patients who initiated TKI treatment within 90 days after ICIs was significantly higher than that in those who initiated TKI 90 days or later after ICI treatment. Thus, three months of TKI-free interval after ICI treatment may be appropriate when TKI treatment after ICI is initiated. TKI treatment within 90 days after the last ICI treatment, especially within 30 days, needs to be avoided, and alternative treatments such as interval chemotherapy may be administered. On the other hand, it remains unclear whether 90 days after last ICI treatment is an appropriate cut-off interval in terms of reducing the safety risk of subsequent TKIs treatment after that of ICIs. TKI treatment in TKI-naïve NSCLC patients with oncogenic driver mutations is more effective than cytotoxic chemotherapy. Additionally, re-administration of TKIs can be a treatment option for patients with brain metastases, even if previously treated with TKIs. There are some patients for whom TKIs must be administered shortly after their last ICI treatment. In such patients, initiation of TKI therapy should consider the interval from the last ICI treatment and the risk of SAE caused by TKIs following ICI treatment. Further investigation on the appropriate treatment sequence after ICI failure in advanced NSCLC patients with oncogenic driver mutations will be needed.
Our study had several limitations. First, this was a retrospective, single-center study, and the number of patients was limited. Additionally, the mechanism via which TKI treatment after ICI treatment induces SAEs is not fully understood. One of the reasons may be the prolonged receptor occupancy of ICIs compared with the relatively short half-life of TKIs (for example, half-life of 55 hours) [22, 23]. The similar synergistic effect of ICIs and TKIs may occur when TKIs are administered in combination with ICIs or sequentially after ICIs, especially within 3 months after last ICI treatment, due to the long half-life of ICI antibodies. Another reason may be the enhancement of T cell reactions by TKIs. It is well known that ICIs enhance immunological reactions correlated to CD8+ cytotoxic T-cell lymphocytes against cross antigens in tumors and normal tissues and increase levels of both preexisting autoantibodies and inflammatory cytokines [24–26]. TKIs also exert an immunomodulatory effect. Few studies showed that EGFR inhibitors increase both basal and interferon gamma-induced major histocompatibility complex class-I levels, which enhances recognition and lysis of tumor cells targeted by CD8+ T cells in preclinical models [27, 28]. Additionally, in patients treated with BRAF and MEK inhibitors, mitogen-activated protein kinase (MAPK) pathway inhibition has been reported to have an immunomodulatory effect on the tumor microenvironment. Frederick reported an increase in CD8+ T-cell infiltration and levels of exhaustion markers TIM-3 and PD-1 along with levels of immunosuppressive ligand PD-L1 after BRAF and MEK inhibitor treatment using thirty-five biopsies which were collected from 16 patients with metastatic melanoma [29]. Thus, treatment with not only EGFR-TKIs, but also other TKIs, can enhance the response of T-cell reaction induced by ICIs.
Conclusion
Our study showed that the risk of SAEs and withdrawal rate due to AEs arise regardless of the types of TKIs administered to patients who received ICIs followed by TKIs. Additionally, dosing interval between the last ICI to the initiation of TKI treatment affects the risk of SAEs and withdrawal rate due to AEs. Further investigation on whether 90 days is appropriate as a treatment-free interval and the mechanism via which combination of ICIs and TKIs as well as sequential TKIs following ICI treatment increase SAEs will be needed.
Supplementary Information
Below is the link to the electronic supplementary material.
Abbreviations
- ABCP
Atezolizumab, bevacizumab, carboplatin, and paclitaxel
- ALK
Anaplastic lymphoma kinase
- CTCAE
Common Terminology Criteria for Adverse Events
- Dab/Tra
Dabrafenib/trametinib
- ECOG
Eastern Cooperative Oncology Group
- EGFR
Epidermal growth factor receptor
- HER2
Human epidermal growth factor receptor 2
- ICI
Immune checkpoint inhibitor
- ILD
Interstitial lung disease
- MAPK
Mitogen-activated protein kinase
- MET
C-Met
- NE
Not estimable
- NRG1
Neuregulin 1
- NSCLC
Non-small cell lung cancer
- PD-1
Programmed cell death 1
- PD-L1
Programmed death-ligand 1
- PS
Performance status
- ROS1
C-ros oncogene 1
- SAE
Serious adverse event
- TKI
Tyrosine kinase inhibitor
Authors contribution
YS and TY designed the study. YS collected the clinical data. YS performed statistical data analyses, interpretation of the results, and writing of the manuscript. YS and TY drafted the manuscript. All authors read the manuscript and approved the final version.
Funding
The study did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability
Data are available on reasonable request.
Declarations
Conflict of interest
Dr. Yoshida received grants and personal fees from AstraZeneca and Bristol-Myers Squibb; grants from AbbVie, MSD, Ono Pharmaceutical, and Takeda Pharmaceutical; and personal fees from Chugai and Novartis. Dr. Matsumoto received grants from Grant-in-Aid for Scientific Research on Innovative Areas, Hitachi High-Technologies, Hitachi, Ltd., and National Cancer Center Research and Development Fund and received personal fees from AMCO INC., AstraZeneca, COOK, and Olympus. Dr. Masuda received personal fees from Chugai and AstraZeneca. Dr. Shinno received personal fees from Pfizer, AstraZeneca, and Chugai Pharmaceutical and received grants from Japan Clinical Research Operations K.K., Janssen Pharmaceutical K.K., and Ono Pharmaceutical. Dr. Okuma received grants from AbbVie. Dr. Goto received grants and personal fees from Bristol-Myers Squibb, Daiichi- Sankyo, Eli Lilly, Guardant Health, MSD, Novartis, Ono Pharmaceutical, Pfizer, and Taiho Pharmaceutical; grants from Kyorin; and personal fees from AstraZeneca, Boehringer Ingelheim, Chugai, and Illumina. Dr. Horinouchi received grants and personal fees from AstraZeneca, BMS, Chugai, Eli Lilly, MSD, Taiho Pharmaceutical, and Ono Pharmaceutical and received grants from Astellas, Genomic Health, and Merck Serono. Dr. Yamamoto received grants and personal fees from BMS, Boehringer Ingelheim, Chugai, Eisai, Eli Lilly, Ono Pharmaceutical, Pfizer, and Takeda Pharmaceutical; grants from Astellas, Bayer, Chiome Bioscience Inc., Daiichi-Sankyo, GSK, Janssen Pharma, Kyowa-Hakko kirin, MSD, Merck, Novartis, Otsuka, Taiho Pharmaceutical, Quintiles, and Sumitomo Dainippon; and received personal fees from AstraZeneca, Otsuka, Cimic, and Sysmex. Dr. Ohe received grants and personal fees from AstraZeneca, Bristol-Myers Squibb, Chugai, Eli Lilly, Janssen Pharma, Kyorin, MSD, Nippon Kayaku, Novartis, Ono Pharmaceutical, Pfizer, Taiho Pharmaceutical, and Takeda Pharmaceutical; grants from Kissei; and personal fees from Boehringer Ingelheim, and Celtrion. The remaining authors declare no competing interests.
Ethical approval
The present study with human samples has been approved by the Ethics Committee of the National Cancer Center Hospital, Tokyo, Japan (2019-123).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362:2380–2388. doi: 10.1056/NEJMoa0909530. [DOI] [PubMed] [Google Scholar]
- 2.Zhou C, Wu Y-L, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12:735–742. doi: 10.1016/s1470-2045(11)70184-x. [DOI] [PubMed] [Google Scholar]
- 3.Sequist LV, Yang JC-H, Yamamoto N, et al. Phase III study of Afatinib or Cisplatin Plus pemetrexed in patients with metastatic lung adenocarcinoma With EGFR mutations. J Clin Oncol. 2013;31:3327–3334. doi: 10.1200/jco.2012.44.2806. [DOI] [PubMed] [Google Scholar]
- 4.Solomon BJ, Mok T, Kim DW, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med. 2014;371:2167–2177. doi: 10.1056/NEJMoa1408440. [DOI] [PubMed] [Google Scholar]
- 5.Socinski MA, Jotte RM, Cappuzzo F, et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med. 2018;378:2288–2301. doi: 10.1056/NEJMoa1716948. [DOI] [PubMed] [Google Scholar]
- 6.Kaira K, Naito T, Takahashi T, et al. Pooled analysis of the reports of erlotinib after failure of gefitinib for non-small cell lung cancer. Lung Cancer. 2010;68:99–104. doi: 10.1016/j.lungcan.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 7.Asahina H, Oizumi S, Inoue A, et al. Phase II study of gefitinib readministration in patients with advanced non-small cell lung cancer and previous response to gefitinib. Oncology. 2010;79:423–429. doi: 10.1159/000326488. [DOI] [PubMed] [Google Scholar]
- 8.Cappuzzo F, Morabito A, Normanno N, et al. Efficacy and safety of rechallenge treatment with gefitinib in patients with advanced non-small cell lung cancer. Lung Cancer. 2016;99:31–37. doi: 10.1016/j.lungcan.2016.06.008. [DOI] [PubMed] [Google Scholar]
- 9.Oda N, Ichihara E, Hotta K, et al. Phase II study of the EGFR-TKI rechallenge With Afatinib in patients with advanced NSCLC harboring sensitive EGFR mutation without T790M: Okayama lung cancer study group trial OLCSG 1403. Clin Lung Cancer. 2017;18:241–244. doi: 10.1016/j.cllc.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 10.Yamaguchi O, Kaira K, Mouri A, et al. Re-challenge of afatinib after 1st generation EGFR-TKI failure in patients with previously treated non-small cell lung cancer harboring EGFR mutation. Cancer Chemother Pharmacol. 2019;83:817–825. doi: 10.1007/s00280-019-03790-w. [DOI] [PubMed] [Google Scholar]
- 11.Antonia SJ, Villegas A, Daniel D, et al. Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med. 2018;379:2342–2350. doi: 10.1056/NEJMoa1809697. [DOI] [PubMed] [Google Scholar]
- 12.Ahn MJ, Yang J, Yu H, et al. 136O: Osimertinib combined with durvalumab in EGFR-mutant non-small cell lung cancer: results from the TATTON phase Ib trial. J Thorac Oncol. 2016 doi: 10.1016/s1556-0864(16)30246-5. [DOI] [Google Scholar]
- 13.Gettinger S, Hellmann MD, Chow LQM, et al. Nivolumab plus Erlotinib in patients with EGFR-mutant advanced NSCLC. J Thorac Oncol. 2018;13:1363–1372. doi: 10.1016/j.jtho.2018.05.015. [DOI] [PubMed] [Google Scholar]
- 14.Schoenfeld AJ, Arbour KC, Rizvi H, et al. Severe immune-related adverse events are common with sequential PD-(L)1 blockade and osimertinib. Ann Oncol. 2019;30:839–844. doi: 10.1093/annonc/mdz077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin JJ, Chin E, Yeap BY, et al. Increased hepatotoxicity associated with sequential immune checkpoint inhibitor and crizotinib therapy in patients with non-small cell lung cancer. J Thorac Oncol. 2019;14:135–140. doi: 10.1016/j.jtho.2018.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McCoach CE, Rolfo C, Drilon A, et al. hypersensitivity reactions to selpercatinib treatment with or without prior immune checkpoint inhibitor therapy in patients with non-small-cell lung cancer in LIBRETTO-001. J Thorac Oncol. 2022 doi: 10.1016/j.jtho.2022.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kanda Y. Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transplant. 2013;48:452–458. doi: 10.1038/bmt.2012.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harding JJ, Pulitzer M, Chapman PB. Vemurafenib sensitivity skin reaction after ipilimumab. N Engl J Med. 2012;366:866–868. doi: 10.1056/NEJMc1114329. [DOI] [PubMed] [Google Scholar]
- 19.Imafuku K, Yoshino K, Ymaguchi K, Tsuboi S, Ohara K, Hata H. Nivolumab therapy before vemurafenib administration induces a severe skin rash. J Eur Acad Dermatol Venereol. 2017;31:e169–e171. doi: 10.1111/jdv.13892. [DOI] [PubMed] [Google Scholar]
- 20.Dimitriou F, Matter AV, Mangana J, Urosevic-Maiwald M, Micaletto S, Braun RP, French LE, Dummer R. Cytokine release syndrome during sequential treatment with immune checkpoint inhibitors and kinase inhibitors for metastatic melanoma. J Immunother. 2019;42:29–32. doi: 10.1097/CJI.0000000000000236. [DOI] [PubMed] [Google Scholar]
- 21.Gemma A, Kusumoto M, Sakai F, et al. Real-world evaluation of factors for interstitial lung disease incidence and radiologic characteristics in patients with EGFR T790M-positive NSCLC treated with osimertinib in Japan. J Thorac Oncol. 2020;15:1893–1906. doi: 10.1016/j.jtho.2020.08.025. [DOI] [PubMed] [Google Scholar]
- 22.Brahmer JR, Drake CG, Wollner I, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010;28:3167–3175. doi: 10.1200/JCO.2009.26.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Janne PA, Yang JC, Kim DW, et al. AZD9291 in EGFR inhibitor-resistant non-small-cell lung cancer. N Engl J Med. 2015;372:1689–1699. doi: 10.1056/NEJMoa1411817. [DOI] [PubMed] [Google Scholar]
- 24.Mamesaya N, Kenmotsu H, Katsumata M, Nakajima T, Endo M, Takahashi T. Osimertinib-induced interstitial lung disease after treatment with anti-PD1 antibody. Invest New Drugs. 2017;35:105–107. doi: 10.1007/s10637-016-0389-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhai X, Zhang J, Tian Y, Li J, Jing W, Guo H, Zhu H. The mechanism and risk factors for immune checkpoint inhibitor pneumonitis in non-small cell lung cancer patients. Cancer Biol Med. 2020;17:599–611. doi: 10.20892/j.issn.2095-3941.2020.0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sullivan RJ, Weber JS. Immune-related toxicities of checkpoint inhibitors: mechanisms and mitigation strategies. Nat Rev Drug Discov. 2021 doi: 10.1038/s41573-021-00259-5. [DOI] [PubMed] [Google Scholar]
- 27.Lizotte PH, Hong RL, Luster TA, et al. A high-throughput immune-oncology screen identifies EGFR inhibitors as potent enhancers of antigen-specific cytotoxic T-lymphocyte tumor cell killing. Cancer Immunol Res. 2018;6:1511–1523. doi: 10.1158/2326-6066.CIR-18-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pollack BP, Sapkota B, Cartee TV. Epidermal growth factor receptor inhibition augments the expression of MHC class I and II genes. Clin Cancer Res. 2011;17:4400–4413. doi: 10.1158/1078-0432.CCR-10-3283. [DOI] [PubMed] [Google Scholar]
- 29.Frederick DT, Piris A, Cogdill AP, et al. BRAF inhibition is associated with enhanced melanoma antigen expression and a more favorable tumor microenvironment in patients with metastatic melanoma. Clin Cancer Res. 2013;19:1225–1231. doi: 10.1158/1078-0432.CCR-12-1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available on reasonable request.