Abstract
Esophageal cancer (EC) is a deadly malignancy. Small extracellular vesicles (sEVs) with programmed death ligand 1 (sEV-PDL1) induce immune escape to promote tumor progression. Furthermore, the imbalance between circulating follicular helper T (Tfh) and circulating follicular regulatory T (Tfr) cells is related to the progression of many malignant tumors. However, the role of the EC-derived sEV-PDL1 in circulating Tfh/Tfr is unknown. Circulating Tfh and Tfr cells were detected by flow cytometry. sEVs were isolated through differential centrifugation and cultured for cell expansion assays. Naïve CD4+ T cells were isolated, stimulated, and cultured with sEVs to evaluate the frequencies, phenotypes, and functions of Tfh and Tfr cells. The proportion of circulating Tfh in patients with EC was lower than that in healthy donors (HDs), whereas that of circulating Tfr was higher. The EC group showed significantly lower circulating Tfh/Tfr and a higher level of sEV-PDL1 than HDs. Notably, sEV-PDL1 was negatively correlated with circulating Tfh/Tfr in the EC group. In vitro assays, sEV-PDL1 inhibited Tfh expansion, enhanced the cytotoxic T lymphocyte-associated antigen 4+ (CTLA4+) Tfh cell percentage, decreased the levels of interleukin (IL)-21 and interferon-γ, and increased IL-10. sEV-PDL1 promoted the expansion and immunosuppressive functions of circulating Tfr; the increased percentages of CTLA4+ Tfr and inducible T cell co-stimulator+ Tfr were accompanied with high IL-10. However, applying an anti-PDL1 antibody significantly reversed this. Our results suggest a novel mechanism of sEV-PDL1-mediated immunosuppression in EC. Inhibiting sEV-PDL1 to restore circulating Tfh/Tfr balance provides a novel therapeutic approach for EC.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00262-023-03561-w.
Keywords: Esophageal cancer, Follicular regulatory T cells, Follicular helper T cells, sEV-PDL1, Immunosuppression
Introduction
Esophageal cancer (EC) is a common but deadly malignancy even when treated with immune checkpoint inhibitors (ICIs) [1]. Cancer-derived small extracellular vesicles (sEVs) modulate the immune systems by triggering an immunosuppressive response [2], which in turn promotes tumor progression [3]. These sEVs regulate the functions of immune cells by expressing immunosuppressive or pro-apoptotic molecules on their surface, including programmed death ligand 1 (PDL1) and cytotoxic T lymphocyte-associated antigen-4 (CTLA4) [4]. Furthermore, sEVs derived from tumors reflect the characteristics of their parent cells and promote tumor progression by presenting PDL1. Notably, small extracellular vesicles programmed death ligand 1 (sEV-PDL1) may be a more potent immunosuppressant than other forms of extracellular PDL1 [5], and PDL1 levels on circulating sEVs appear to be a more reliable prognostic marker than PDL1 expression detected in tumor biopsies. Thus, monitoring circulating sEV-PDL1 could serve as a predictor of tumor response to treatment and the clinical outcome [6]. The role of sEV-PDL1 in the formation of systemic immunosuppression may be more important than previously assumed [7].
Circulating follicular helper (Tfh) and regulatory (Tfr) T cells share many characteristics [8]. Specifically, they co-express programmed death 1 (PD1) and chemokine receptor 5 (CXCR5), as well as produce interleukin (IL)-21, IL-10, and interferon (IFN)-γ [8]. Tfr cells play a suppressive role during the generation of CD38+CD27high plasma blasts, leading to immunosuppression [9]. Tfh cells represent a distinct lineage of helper CD4+ T cells; they express CXCR5 and migrate into B cell follicles. Inducible T cell co-stimulator (ICOS), a cell surface co-stimulatory molecule, is highly expressed on Tfh cells and crucial for interactions between Tfh with B cells [10]. Tfh cells play an important role in tumor immune protection [11]. A subset of FoxP3+ regulatory T cells that suppress various immune responses are known to be Tfr cells [12, 13], acting as a regulatory counterpart for Tfh cells [14]. Tfr cells regulate germinal center responses and prevent antibody-mediated autoimmunity, promoting the formation of an immunosuppressive microenvironment [13, 15]. Circulating Tfh and Tfr cells were home to germinal centers and persisted for long periods in vivo; they produced more cytokines than did effector lymph node Tfh and Tfr cells [16]. Given the opposing functions of Tfh and Tfr cells, a balance of their actions is critical for immune homeostasis [17]. Therefore, circulating Tfh/Tfr could be used as a tumor progression marker in several kinds of cancers [12, 18–21]. However, whether EC is characterized by imbalanced circulating Tfh/Tfr remains unknown.
Cancer releases sEVs to stimulate the expansion of regulatory T cells [3], which causes immunosuppression in the tumor microenvironment by impairing the function of antitumorigenic T cells [22]. Regulatory T cells are the precursors of Tfr cells; however, it is unclear whether EC-derived sEVs regulate Tfr cells to promote immunosuppression in EC. Furthermore, the relationship between sEV-PDL1 and circulating Tfh/Tfr in EC has not been clarified. Therefore, this study was designed to assess the effect of EC-derived sEVs on circulating Tfh/Tfr balance. Furthermore, we aimed to evaluate whether these sEVs act via PDL1 to cause immunosuppression and promote the development of EC.
Materials and methods
Patient samples and cell lines
Fresh blood samples were obtained from 45 patients with EC and 33 healthy donors (HDs) visiting the Fourth Hospital of Hebei Medical University from August 2021 to April 2022. None of the EC patients had received anti-cancer therapy before surgical resection. Patients with concurrent human immunodeficiency virus infection, other cancers, or autoimmune diseases were excluded. Among the included patients, the plasma samples of 26 patients with EC and 17 HDs were used to detect sEV-PDL1. The clinical stages were classified according to the guidelines of the International Union Against Cancer, and the patient characteristics are listed in Table S1. The human EC (Eca) 109 cells, used in this study, were obtained from ATCC. All experiments were performed with mycoplasma-free cells.
Lentivirus infection
Short hairpin RNA (shRNA) was introduced using a lentivirus vector under a CMV promoter/enhancer (Gene Pharma, Shanghai, China). The vector carried a GFP encoding gene for visualization and a puromycin resistance gene for selection. Cells were grown in six-well plates for 24 h until reaching approximately 50% confluence. Cells were washed with PBS, and viral transfection solution was added along with 3 ml polybrene. Cells were incubated at 37 °C in 5% CO2 for 24 h before normal medium was replenished. Transfection efficiency was estimated by observing green fluorescence under a fluorescence microscope at 48 h post-transfection. Puromycin was added at 48 h post-transfection to maintain stably transfected cells. The shRNA sequences were as follows: shRNA1: CTGACATTCATCTTCCGTTTA, shRNA2: CGAATTACTGTGAAAGTCAAT.
sEVs isolation
sEVs were isolated through differential centrifugation of conditioned media collected from human plasma or the supernatant of Eca109 cells in which PDL1 had been knocked down (Eca109-PDL1kd), while normal Eca109 cells were used as the negative control (Eca109-PDL1nc). Cell cultures were treated with sEVs-depleted media prepared via ultracentrifugation of fetal bovine serum for 3 h at 200,000g. All culture medium contained polymyxin B (20 μg/ml, Sigma-Aldrich) to eliminate endotoxin contamination. The cells were grown in their respective conditioned media until reaching 70–80% confluence. Briefly, cells were cultured for 72 h, the culture medium was centrifuged at 300g for 10 min to remove dead cells and debris, followed by centrifugation at 2000g for 10 min. Subsequently, the supernatant was centrifuged at 100,000g for 60 min to pellet the sEVs. The sEVs pellet was washed in a large volume of PBS to eliminate contaminating proteins and centrifuged for a final time at 100,000g for another 60 min. Finally, the sEVs pellet was then re-suspended in 100ul PBS, and the total sEVs protein concentration was determined using the BCA Protein Assay kit (Thermo Fisher Scientific, Santa Clara, CA).
sEVs analysis
Transmission electron microscopy (HITACHI H-7650, Tokyo, Japan) was used to analyze the samples of sEVs processed using a standard protocol: About 30 μl of sEVs samples was dropped onto a carbon-plated copper mesh support film which was then placed on the sealing film for 3 min. Subsequently, the excess solution was absorbed from the edge, and the film was placed on filter paper filter paper for 8–10 min. After support film dried, a drop of uranyl acetate dye solution was dripped and left to stain for 90 s. Then, the excess dye solution was absorbed and support film was clamped on the filter paper and dried for 3 h to observe. Protein estimation and particle number of sEVs were determined using the protein micro-BCA assay kit (Invitrogen, Santa Clara, CA) and nanoparticle tracking analysis (NTA, Particle Metrix Zetaview, Meerbusch, Germany) or Flow NanoAnalyzer (NanoFCM, Xiamen, China).The expression levels of CD63, CD9, Tsg101,Alix Calnexin, and PDL1 on sEVs were evaluated by Western blot analysis.
Peripheral blood mononuclear cell (PBMC) isolation
PBMCs were isolated from fresh blood samples within 1 h using a lymphocyte separation medium (Cedarlane Corporation, Ontario, Canada) to ensure that the living cells comprised more than 90% of the total cells. Briefly, 10 ml fresh blood was mixed with 10 ml PBS, and the mixture was carefully added to 10 ml lymphocyte separation medium. The mixed solution was centrifuged at 400g for 25 min at 20–25 °C, and the buffy coat was removed and washed three times with PBS to obtain PBMCs. Naïve CD4+ T cells were purified using a Naïve CD4+ T Cell Isolation Kit (BioLegend, New York, America), and cell purity was > 90% (Figure S1).
Cell stimulation and culture
All cells were cultured in a humidified incubator at a temperature of 37ºC in an atmosphere of 5% CO2. Briefly, 96-well plates were coated with anti-CD3 (1 µg/ml, 100 µl) and anti-CD28 antibodies (0.5 µg/ml, 100 µl) overnight at 4 °C. Then, the antibodies solution was removed, and the plate was washed twice with PBS to remove unbound antibodies. Next, naïve CD4+T cells were plated (2 × 105 cells/well) in above-mentioned TCR (anti-CD3 and anti-CD28) antibodies-coated wells. After 48 h, isolated sEVs (10 μg/ml) or sEVs that were preincubated with 20 μg/ml anti-PDL1 or anti-IgG for 1 h at 37 °C were added to cell cultures. Then, the CD4+T cells were cultured for an additional 72 h before being harvested and analyzed using flow cytometry. Phenotypic and intracellular cytokine analyses of CD4+T cells, with or without stimulation, were carried out. The reagents used in this experiment are listed in Tables S2 and S3.
Cell viability assay
The Cell Counting Kit-8 (CCK-8) assay was used to examine naïve CD4+ T cell viability. Briefly, 96-well plates were coated with anti-CD3 (1 µg/ml, 100 µl) and anti-CD28 antibodies (0.5 µg/ml, 100 µl) overnight at 4 °C as before. Then, the antibodies solution was removed, and the plate was washed twice with PBS to remove unbound antibodies. Next, naïve CD4+T cells were plated (2 × 105 cells/well) in above-mentioned TCR (anti-CD3 and anti-CD28) antibodies-coated wells. After 48 h, isolated PDL1kd-sEVs and PDL1nc-sEVs (10 μg/ml) were added to cell cultures. Then, the naïve CD4+T cells were cultured for an additional 72 h. After that, we added 10 μl of the CCK8 reagent to each well and incubated it for 3 h. Absorbance was read at 450 nm on a microplate reader (Dynex Technologies, Chantilly, USA) in order determine the optical density. Cell viability was standardized to that of the untreated controls.
Cell apoptosis analysis
According to the manufacturer’s protocol, we used the Annexin V-FITC cell apoptosis detection kit to determine the number of apoptotic cells. Briefly, we collected the naïve CD4+T cells as described in section “Cell viability assay”, 2 × 105 cells were collected to prepare a single-cell suspension. Then, the supernatant was discarded in the dark, and 5 μl/sample of FITC labeled Annexin-V was added and incubated for 30 min. Following this, 5 μl/sample of PI was added and allowed to react for 5 min. The number of apoptotic cells was counted using flow cytometry.
Flow cytometry
We identified CD3+CD4+CD45RA−CXCR5+PD1+FoxP3−/+T cells as “Tfh” “Tfr.” Cells were stained with specific antibodies for 30 min; antibodies used in this experiment are listed in Tables S2 and S3. For intracellular staining, cells were fixed and permeabilized for 30 min at 4 °C using a Transcription Factor Buffer Set (BD Biosciences, New York, USA) and then, washed with Perm/Wash Buffer. For intracytoplasmic staining, cells were stimulated for 5 h with phorbol 12-myristate 13-acetate (50 ng/ml), ionomycin (1 μg/ml), and brefeldin A (2.5 μg/ml). Isotype-matched control antibodies were used as the background control. Flow cytometric analysis was finally performed using BD FACSAria and analyzed using FlowJo software version 10.
Enzyme-linked immunosorbent assay (ELISA)
The level of PDL1 expression on sEVs isolated from plasma of patients with EC and HDs was determined using human ELISA kits (Abcam, Cambridge, UK) according to the manufacturer’s instructions.
Statistical analysis
The results are expressed as mean and standard error (mean ± SE). The statistical significance of differences between the EC and HD groups was analyzed using the log-rank test or Student’s t-test. Correlations between Tfh/Tfr, Tfh, Tfr and sEV-PDL1 were assessed using Spearman’s correlation analysis, respectively. All data were analyzed using two-tailed tests, and results with P < 0.05 were considered statistically significant. Data analyses and plotting were performed in R v. 4.1.0, using vegan and ggplot2.
Results
Patients with EC with progressive clinical characteristics have imbalanced circulating Tfh/Tfr
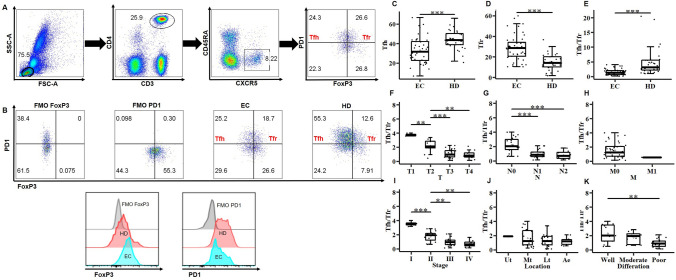
First, we obtained PBMCs from fresh peripheral blood samples of 45 patients with EC and 33 HDs. CD3+CD4+CD45RA−CXCR5+PD1+FoxP3− Tfh cells and CD3+CD4+CD45RA−CXCR5+PD1+FoxP3− Tfr cells were identified using flow cytometry (Fig. 1A, B). The proportion of circulating Tfh cells was significantly low in the EC group (Fig. 1C), whereas that of circulating Tfr cells was significantly enhanced (Fig. 1D). Furthermore, circulating Tfh/Tfr was significantly lower in the EC group than in the HD group (Fig. 1E).
Fig. 1.
Circulating follicular helper T/regulatory T (Tfh/Tfr) cells in patients with esophageal cancer (EC) with progressive clinical characteristics. A Representative flow cytometric staining of peripheral blood Tfh (CD3+CD4+CD45RA−CXCR5+PD1+FoxP3−) and Tfr (CD3+CD4+CD45RA−CXCR5+PD1+FoxP3+) cells. B Gating strategy of Tfh and Tfr and histograms of programmed death 1 (PD1) and FoxP3 expressed by the fluorescent minus one method (FMO) in the EC and healthy donor (HD) subsets. C-E, Proportions of circulating Tfh and Tfr (C, D) and circulating Tfh/Tfr (E) between the EC and HD groups. F–K, The correlation of circulating Tfh/Tfr with tumor TNM stage, location, and differentiation status. Data are presented as mean ± SD; Statistical significance: *p < 0.05, **p < 0.01, ***p < 0.001
To determine the clinical relevance of our findings, we checked the correlation between the circulating Tfh/Tfr and the tumor TNM stage, location, and differentiation status. Notably, a significant negative correlation was observed between circulating Tfh/Tfr and the tumor size or lymph node infiltration. Patients with EC with a high TNM stage had low circulating Tfh/Tfr (Fig. 1F–I). No correlation was observed between circulating Tfh/Tfr and the tumor location (Fig. 1J). Furthermore, patients with EC with poor tumor differentiation had lower circulating Tfh/Tfr than those with well-or-moderately differentiated tumors (Fig. 1K). Thus, it can be inferred that the low circulating Tfh/Tfr value predicts a worse clinical outcome and may negatively impact antitumor immunity in patients with EC.
Correlation of sEV-PDL1 with circulating Tfh/Tfr
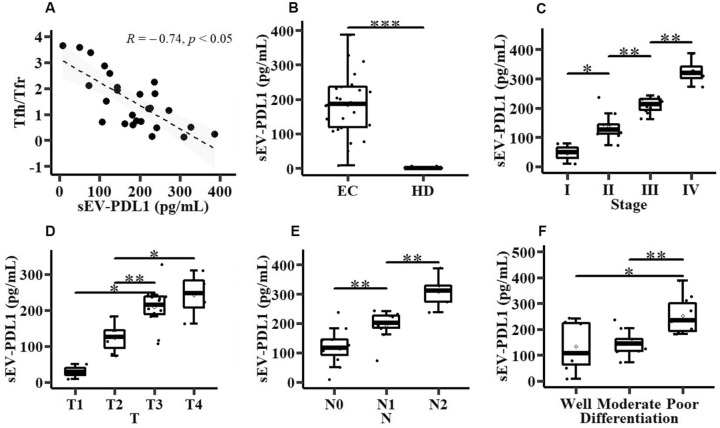
Tumor cell-derived sEVs may induce Tfr cell expansion and the expression of the immunosuppressive marker PDL1 to trigger an inhibitory signal [23]. Therefore, exploring the role of sEV-PDL1 is crucial to understand its role as the inhibitor of immune responses and its potential as a biomarker. In this study, we isolated and characterized sEVs from the plasma of 26 patients with EC and 17 HDs (Figure S2). Then, we detected sEV-PDL1 of these sEVs. sEV-PDL1 was negatively correlated with circulating Tfh and positively correlated with circulating Tfr (Figure S3). Patients with EC showed a clear negative correlation between sEV-PDL1 and circulating Tfh/Tfr and exhibited higher sEV-PDL1 levels than those of the HD group (Fig. 2A, B). Additionally, patients with EC with a high TNM stage had high sEV-PDL1 levels (Fig. 2C–E). EC patients with poor tumor differentiation had a higher sEV-PDL1 level than those with well-or-moderately differentiated tumors (Fig. 2F). Based on these findings, we hypothesized that the observed decrease in circulating Tfh/Tfr might be attributed to EC-derived sEV-PDL1.
Fig. 2.
Correlation between programmed death ligand 1 of the small extracellular vesicles (sEV-PDL1) and circulating Tfh/Tfr in patients with EC. A Correlation of plasma sEV-PDL1 level with circulating Tfh/Tfr in patients with EC (n = 26). B Relative plasma sEV-PDL1 level in 17 HDs and 26 patients with EC. C–F Correlation of sEV-PDL1 with the TNM stage (C), tumor T (D), tumor N (E), and tumor differentiation (F). Data are presented as mean ± SD; Statistical significance: *p < 0.05, **p < 0.01, ***p < 0.001
Isolation and characterization of EC cell-derived sEVs
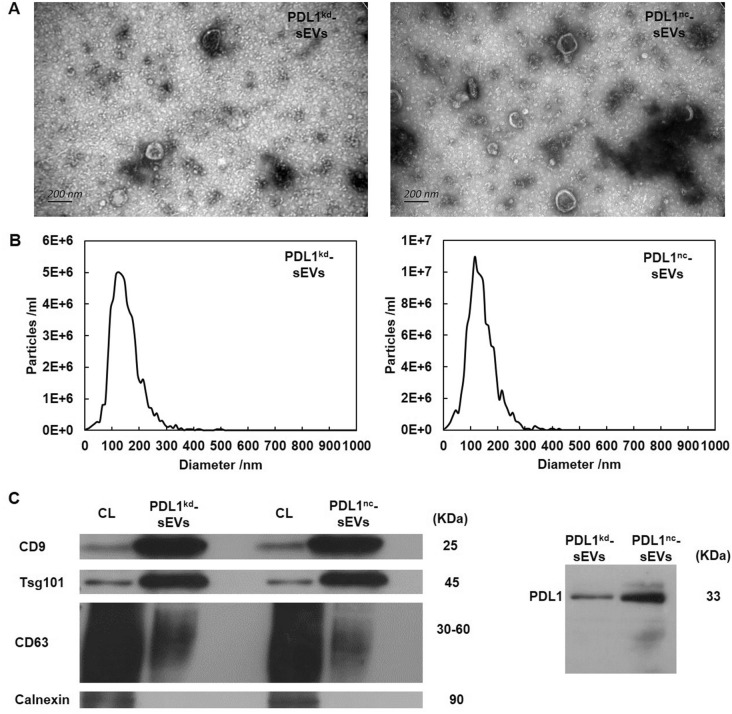
To determine whether sEV-PDL1 can decrease circulating Tfh/Tfr, we knocked down PDL1 in Eca109 cells and acquired PDL1-deficient cell clones that secreted sEVs without PDL1. sEVs obtained from the culture supernatant of Eca109-PDL1kd and Eca109-PDL1nc cells showed vesicle-like morphology with a lipid bilayer (Fig. 3A). NTA showed that the extracellular vesicles were approximately 100 nm in diameter (Fig. 3B). PDL1nc-sEVs and PDL1kd-sEVs were positive for the multivesicular body-related proteins CD63, CD9, and Tsg101, but not for Calnexin. As expected, PDL1nc-sEVs were more positive for PDL1 than PDL1kd-sEVs (Fig. 3C). Raw Western blots and NTA files were provided as supplementary files.
Fig. 3.
Characterization of sEVs derived from human Eca109-PDL1kd and Eca109-PDL1nc cells. A sEVs morphology determined using transmission electron microscopy. Scale, 200 nm. B Size distribution of the sEVs was determined using nanoparticle tracking analysis (NTA). C Surface proteins on Eca109-PDL1kd and Eca109-PDL1nc cell-derived sEVs were detected using Western blotting
EC-derived sEVs decrease circulating Tfh/Tfr via sEV-PDL1
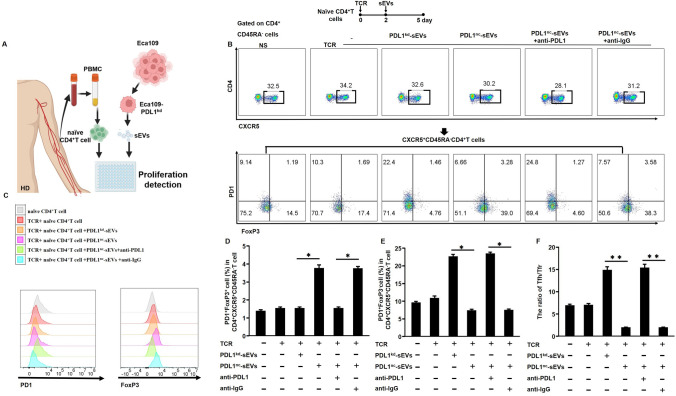
sEVs were isolated from Eca109-PDL1kd and Eca109-PDL1nc cell supernatants; naïve CD4+T cells were separated from PBMCs of HDs and cultured for use in subsequent assays (Fig. 4A, B). It indicated that PDL1nc-sEVs could significantly reduce cell viability (Figure S4) and induce apoptosis of naïve CD4+T cells (Figure S5). We analyzed the expression level of PD1 and FoxP3 in these groups. PDL1nc-sEVs group and PDL1nc-sEVs + anti-IgG group expressed the highest amount of FoxP3 but weakly produced PD1. The PDL1kd-sEVs and PDL1nc-sEVs + anti-PDL1 groups weakly expressed FoxP3 (Fig. 4C). For the PDL1nc-sEVs group, a high proportion of Tfr cells and low proportion of Tfh cells was observed (Fig. 4D, E), resulting in a low circulating Tfh/Tfr (Fig. 4F), while the PDL1kd-sEVs group showed the opposite trend (Fig. 4D–F). Moreover, the neutralization of sEV-PDL1 by anti-PDL1 reversed the circulating Tfh/Tfr induced by PDL1nc-sEVs (Fig. 4F). Additionally, the PDL1nc-sEVs + anti-PDL1 group had similar results as that observed in the PDL1kd-sEVs group (Fig. 4F). EC cell-derived sEVs showed a strong potential to decrease Tfh cell expansion while also inducing Tfr cell expansion via sEV-PDL1, leading to a low circulating Tfh/Tfr. Notably, these results suggest that reversing circulating Tfh/Tfr imbalance in EC may be one of mechanisms for anti-PDL1 drugs to achieve ICI efficiency in clinical settings [24].
Fig. 4.
sEV-PDL1 and disturbed circulating Tfh/Tfr. A Schematic illustration of human naïve CD4+T cell proliferation in response to EC-derived sEVs. B Representative flow cytometry plots showing the distribution of circulating Tfh and Tfr. C Histograms showing the expression levels of PD1 and FoxP3 after incubation with PDL1kd-sEVs or PDL1nc-sEVs. D-F, Representative histograms showing the proportions of Tfr (D) and Tfh (E) and circulating Tfh/Tfr (F). Data are presented as mean ± SD; Statistical significance: *p < 0.05, **p < 0.01, ***p < 0.001
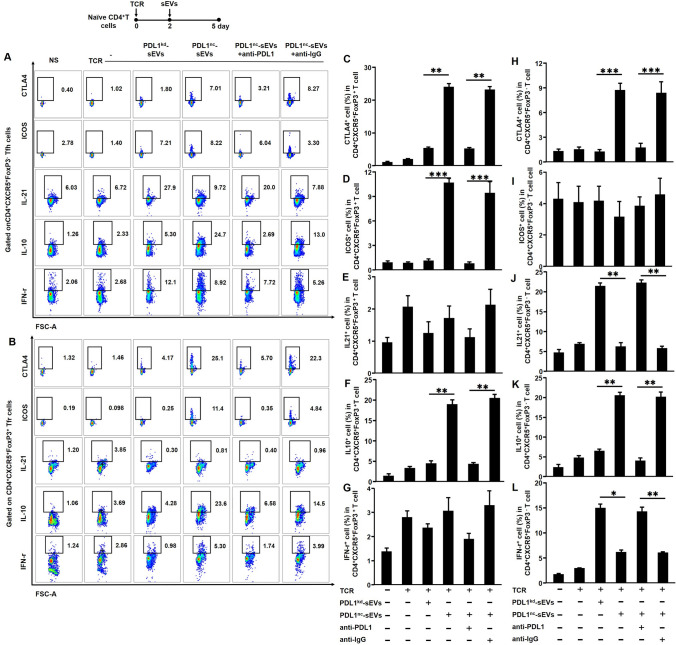
sEV-PDL1 induces the immunosuppressive phenotypes and functions of circulating Tfh and Tfr cells
Thus far, the results suggest that EC-derived sEV-PDL1 may have the potential to decrease the levels of circulating Tfh/Tfr via PD1-PDL1 interaction. Additionally, we found that blocking PD1-PDL1 interaction reversed this effect. After sEVs was incubated with naïve CD4+T cells for 72 h, we analyzed the expression levels of CTLA4 and ICOS on circulating CD4+CXCR5+FoxP3−Tfh cells and circulating CD4+CXCR5+FoxP3+Tfr cells. Levels of IL-10, IL-21, and IFN-γ secreted from circulating Tfh and Tfr cells were detected (Fig. 5A, B). After incubation with PDL1nc-sEVs, the proportion of CD4+CXCR5+FoxP3+CTLA4+Tfr cells and CD4+CXCR5+FoxP3+ICOS+Tfr cells increased (Fig. 5C, D), whereas for Tfh, only CD4+CXCR5+FoxP3+CTLA4+Tfh cells increased; CD4+CXCR5+FoxP3+ICOS+Tfh cells were not affected (Fig. 5H, I). Concurrently, after incubation with PDL1nc-sEVs, Tfr cells expressed a higher level of IL-10; the levels of IL-21 and IFN-γ were not affected (Fig. 5E–G), whereas circulating Tfh cells secreted lower levels of IL-21 and IFN-γ and a higher level of IL-10 (Fig. 5J–L) than those incubated with PDL1kd-sEVs. Overall, Tfh and Tfr cells acquired immunosuppressive phenotypes and functions after coculture with PDL1nc-sEVs. Next, to elucidate whether these trends were induced by PD1-PDL1 interaction, we incubated sEVs with an anti-PDL1 antibody, which significantly reversed the results.
Fig. 5.
sEV-PDL1 and immunosuppressive phenotypes and functions of circulating Tfh and Tfr. A-B, Representative flow cytometry plots showing expression levels of cytotoxic T lymphocyte-associated antigen 4+ (CTLA4+), inducible T cell co-stimulator (ICOS), interleukin (IL)-21, IL-10, and interferon (IFN)-γ in CD4+ chemokine receptor 5 (CXCR5+) FoxP3−Tfh (A) and CD4+CXCR5+FoxP3+Tfr (B). C-L, Bar graphs showing expression levels of CTLA4, ICOS, IL-21, IL-10, and IFN-γ (C–G) in Tfr and Tfh (H–L). Data are presented as mean ± SD; Statistical significance: *p < 0.05, **p < 0.01, ***p < 0.001
Collectively, these findings indicate that sEV-PDL1 induces not only the immunosuppressive phenotypes of circulating Tfh and Tfr cells but also suppressive functions. sEV-PDL1 can, therefore, play a key role in immunosuppression by involving PD1-PDL1 and decreasing circulating Tfh/Tfr in patients with EC.
Discussion
As circulating Tfh and Tfr cells play opposing roles in maintaining germinal center responses, their balanced activities are critical for immune homeostasis [16]. In this study, we investigated the role of EC-derived sEVs in regulating the balance of circulating Tfh/Tfr. Patients with EC had a significantly lower circulating Tfh/Tfr than did HDs. Furthermore, circulating Tfh/Tfr significantly correlated with tumor TNM stage and differentiation status, suggesting that circulating Tfh/Tfr may be a mechanism to induce EC carcinogenesis and could therefore be used as a tumor prediction marker for EC. Our results are consistent with findings from previous studies, which reported that Tfh/Tfr imbalance is related to the progression of malignant tumors, such as non-small cell lung cancer [18], breast cancer [25], hepatocellular carcinoma [26], colorectal cancer [19] and pancreatic cancer [27].
sEV-PDL1 mediates PD1 crosslinking and immunosuppression as it has a similar extracellular membrane topology as its cell surface counterpart [5]. sEVs suppress T cells in a PDL1-dependent fashion [28], suggesting that sEVs are a significant source of extra-tumoral PDL1 and may contribute to PD1 antibody treatment resistance [29, 30]. Immunosuppressive signaling mediated by sEV-PDL1 reportedly plays a crucial role in inhibiting antitumor immunity [31]. Accordingly, sEV-PDL1 predicts poor survival, reflects immune status in gastric cancer, and correlates with the clinical stage of breast cancer, head and neck cancer, and melanoma [32–35]. Similar results were reported for MC38 colon cancer, melanoma, and TRAMP-C2 prostate cancer mouse models [5, 7, 29]. We noted a significant correlation between sEV-PDL1 and the tumor TNM stage and differentiation status for EC patients; in addition, an increased sEV-PDL1 expression level reportedly negatively correlates with circulating Tfh/Tfr. Based on these findings, we infer that EC-derived sEV-PDL1 may be one of the mechanisms underlying circulating Tfh/Tfr imbalance.
There were almost no methods for selectively modulating Tfr cells in clinical settings [32]. Our findings suggest the possibility that tumor-derived sEVs may serve as a therapeutic target, while sEV-PDL1 could modulate circulating Tfh/Tfr in clinical settings. In vitro coculture of PDL1+sEVs with T cells was found to suppress T cell activation[7]. Here, we confirmed that sEV-PDL1 induced the decreased circulating Tfh/Tfr. It is possible that the greater suppressive capacity of circulating Tfr cells, together with the decreased circulating Tfh/Tfr, inhibits circulating Tfh cell function[36].
CTLA4 inhibits T cell proliferation, as well as initiates inhibitory signal transduction. CTLA4 loss on Tfh cells was found to result in strong B cell responses, whereas CTLA4 loss on Tfr cells resulted in defective suppression of antigen-specific antibody responses [37, 38]. Furthermore, ICOS is required for Tfr cell differentiation [36]. ICOS ligand on the B cell surface also binds to ICOS on the Tfr cell surface, which may lead to dysfunctional B cells [39]. Our results showed that PDL1nc-sEVs changed CTLA4 and ICOS expression, implying that antigen-specific antibody responses and B cell responses were significantly suppressed, which may be one of the reasons accounting for the EC immunosuppressive microenvironment. IL-10 derived from Tfr cells could inhibit the ability of Tfh cells to proliferate and secrete cytokines [8]. IL-21 derived from Tfh cells can also inhibit the expansion of Tfr cells in the germinal center [12]. In our study, circulating Tfh and circulating Tfr cells acquired immunosuppressive phenotypes and functions after coculture with PDL1nc-sEVs. These changes in cytokine levels accelerated the imbalance of circulating Tfh/Tfr and defected humoral immunity.
PD1 controls the generation and function of suppressive Tfr cells [36]. Our data showed that when sEV-PDL1 was neutralized by the anti-PDL1 antibody, the circulating Tfh/Tfr imbalance could be reversed. Thus, anti-PDL1 treatment is known to abolish sEVs immunosuppression mediated by PDL1, thereby activating antitumor immunity and attenuating the immune suppression [40]. Our results are consistent with those findings, showing that sEV-PDL1 can function as a systemic immunosuppressant [41, 42]. For immune inhibition, PDL1 engages PD1 in close proximity to the TCR-CD28 signal so as to localize the SHP2 phosphatase activity. If peptide-MHC molecules and PDL1 are co-expressed on sEVs, this brings them into the close proximity needed for effective immune inhibition [5, 43, 44]. In addition, the SHP2 phosphatase activity may affect the expression of FoxP3 [13, 36], leading to the expansion of Tfr cells and, finally, the disturbed circulation of Tfh/Tfr.
Conclusion
This is the first study to investigate the relationship between circulating Tfh/Tfr, sEV-PDL1, and the clinical characteristics of patients with EC. We found that sEV-PDL1 and circulating Tfh/Tfr serve as independent prognostic biomarkers for EC. Furthermore, a new role for sEV-PDL1 in regulating EC immune responses was noted via modulating circulating Tfh and Tfr cells in the blood. sEV-PDL1 may be one of the effectors by which anti-PDL1 drugs achieve their clinical benefits. Furthermore, the SHP2 phosphatase activity could be the underlying mechanism. Deepening our understanding of how sEV-PDL1 influences the overall immune profile of patients with EC is critical to achieve successful patient outcomes.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We sincerely thank all the people who have provided helpful support.
Abbreviations
- Ae
Abdominal esophagus
- ATCC
American Type Culture Collection
- CCK-8
Cell counting Kit-8
- CTLA4
Cytotoxic T lymphocyte-associated antigen 4
- CXCR5
The chemokine receptor 5
- EC
Esophageal cancer
- Eca109-PDL1kd
PDL1 knocked down Eca109 cells
- Eca109-PDL1nc
PDL1 negative controlled Eca109 cells
- FMO
The fluorescent minus one method
- HD
Healthy donor
- ICIs
Immune checkpoint inhibitors
- ICOS
Inducible T cell co-stimulator
- Lt
Lower thoracic esophagus
- Mt
Middle thoracic esophagus
- NTA
Nanoparticle tracking analysis
- PBMC
Peripheral blood mononuclear cell
- PD1
Programmed death receptor 1
- PDL1
Programmed death ligand 1
- qPCR
Quantitative PCR
- sEVs
Small extracellular vesicles
- sEV-PDL1
PDL1 of the small extracellular vesicle
- Tfr
Follicular regulatory T cell
- Tfh
Follicular helper T cell
- TNM
Tumor size (T), lymph node infiltration (N), metastasis (M)
- Ut
Upper thoracic esophagus
Author contributions
ZW and ZL conducted conceptualization of the study and designed the experiment. ZL performed major Imageflow experiments and mass cytometry experiments and analysis. HH and YZ interpreted and analyzed the data. LC, XZ, and TL assisted with the experiments. YQ provided advice with the experiments. ZL wrote, reviewed, and edited the manuscript. ZW is responsible for the overall content as guarantor.
Funding
This work was supported by the Basic Research Cooperation Project of Beijing, Tianjin, Hebei from the Natural Science Foundation of Hebei (H2020206649), Tianjin (20JCZXJC00070), and Beijing (J200018) and the key Research and Development Program of Hebei Province (21377704D).
Data availability
Data are available upon reasonable request.
Declarations
Conflict of interest
No potential conflict of interest was reported by the authors.
Ethics approval and consent to participate
This study involves human participants. Participants gave informed consent to participate in the study before taking part. This study was approved by the Fourth Hospital of Hebei Medical University Ethics Committee (2020ky241).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Mao YS et al (2020) Analysis of a registry database for esophageal cancer from high-volume centers in China. Dis Esophagus 33(8):doz091 [DOI] [PubMed] [Google Scholar]
- 2.Morrissey SM et al (2021) Tumor-derived exosomes drive immunosuppressive macrophages in a pre-metastatic niche through glycolytic dominant metabolic reprogramming. Cell Metab 33(10):2040-2058 e10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lindau D et al (2013) The immunosuppressive tumour network: myeloid-derived suppressor cells, regulatory T cells and natural killer T cells. Immunology 138(2):105–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raimondo S et al (2020) Extracellular vesicles and tumor-immune escape: biological functions and clinical perspectives. Int J Mol Sci 21(7):2286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Daassi D, Mahoney KM, Freeman GJ (2020) The importance of exosomal PDL1 in tumour immune evasion. Nat Rev Immunol 20(4):209–215 [DOI] [PubMed] [Google Scholar]
- 6.Cordonnier M et al (2020) Tracking the evolution of circulating exosomal-PD-L1 to monitor melanoma patients. J Extracell Vesicles 9(1):1710899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morrissey SM, Yan J (2020) Exosomal PD-L1: roles in tumor progression and immunotherapy. Trends Cancer 6(7):550–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crotty S (2019) T follicular helper cell biology: a decade of discovery and diseases. Immunity 50(5):1132–1148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hao H et al (2021) Conversion of T follicular helper cells to T follicular regulatory cells by interleukin-2 through transcriptional regulation in systemic lupus erythematosus. Arthritis Rheumatol 73(1):132–142 [DOI] [PubMed] [Google Scholar]
- 10.Ma X et al (2018) Expansion of T follicular helper-T helper 1 like cells through epigenetic regulation by signal transducer and activator of transcription factors. Ann Rheum Dis 77(9):1354–1361 [DOI] [PubMed] [Google Scholar]
- 11.Ye Y, Wang M, Huang H (2021) Follicular regulatory T cell biology and its role in immune-mediated diseases. J Leukoc Biol 110(2):239–255 [DOI] [PubMed] [Google Scholar]
- 12.Wing JB, Tanaka A, Sakaguchi S (2019) Human FOXP3(+) regulatory T cell heterogeneity and function in autoimmunity and cancer. Immunity 50(2):302–316 [DOI] [PubMed] [Google Scholar]
- 13.Chung Y et al (2011) Follicular regulatory T cells expressing Foxp3 and Bcl-6 suppress germinal center reactions. Nat Med 17(8):983–988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gonzalez-Figueroa P et al (2021) Follicular regulatory T cells produce neuritin to regulate B cells. Cell 184(7):1775–1789 [DOI] [PubMed] [Google Scholar]
- 15.Sage PT, Sharpe AH (2015) T follicular regulatory cells in the regulation of B cell responses. Trends Immunol 36(7):410–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sage PT et al (2014) Circulating T follicular regulatory and helper cells have memory-like properties. J Clin Invest 124(12):5191–5204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu C et al (2018) Increased circulating follicular treg cells are associated with lower levels of autoantibodies in patients with rheumatoid arthritis in stable remission. Arthritis Rheumatol 70(5):711–721 [DOI] [PubMed] [Google Scholar]
- 18.Shi W et al (2020) PD-1 regulates CXCR5(+) CD4 T cell-mediated proinflammatory functions in non-small cell lung cancer patients. Int Immunopharmacol 82:106295 [DOI] [PubMed] [Google Scholar]
- 19.Shi W et al (2018) Follicular helper T cells promote the effector functions of CD8(+) T cells via the provision of IL-21, which is downregulated due to PD-1/PD-L1-mediated suppression in colorectal cancer. Exp Cell Res 372(1):35–42 [DOI] [PubMed] [Google Scholar]
- 20.Li L et al (2018) TIM-3 expression identifies a distinctive PD-1(+) follicular helper T cell subset, with reduced interleukin 21 production and B cell help function in ovarian cancer patients. Int Immunopharmacol 57:139–146 [DOI] [PubMed] [Google Scholar]
- 21.Ma Q-Y et al (2016) Function of follicular helper T cell is impaired and correlates with survival time in non-small cell lung cancer. Int Immunopharmacol 41:1–7 [DOI] [PubMed] [Google Scholar]
- 22.Wieckowski EU et al (2009) Tumor-derived microvesicles promote regulatory T cell expansion and induce apoptosis in tumor-reactive activated CD8+ T lymphocytes. J Immunol 183(6):3720–3730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xie F et al (2019) The role of exosomal PD-L1 in tumor progression and immunotherapy. Mol Cancer 18(1):146–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kato SYK (2020) Immuno-oncology for esophageal cancer. Future Oncol 16(32):1749–6694 [DOI] [PubMed] [Google Scholar]
- 25.Faghih Z et al (2014) Immune profiles of CD4+ lymphocyte subsets in breast cancer tumor draining lymph nodes. Immunol Lett 158(1–2):57–65 [DOI] [PubMed] [Google Scholar]
- 26.Wang B et al (2020) Tfr-Tfh index: a new predicator for recurrence of hepatocellular carcinoma patients with HBV infection after curative resection. Clin Chim Acta 511:282–290 [DOI] [PubMed] [Google Scholar]
- 27.Lux A et al (2019) c-Met and PD-L1 on circulating exosomes as diagnostic and prognostic markers for pancreatic cancer. Int J Mol Sci 20(13):3305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Poggio M et al (2019) Suppression of exosomal PD-L1 induces systemic anti-tumor immunity and memory. Cell 177(2):414–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kahlert C, Kalluri R (2013) Exosomes in tumor microenvironment influence cancer progression and metastasis. J Mol Med 91(4):431–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu J et al (2021) The biology, function, and applications of exosomes in cancer. Acta Pharm Sin B 11(9):2783–2797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang L et al (2018) HCV-associated exosomes promote myeloid-derived suppressor cell expansion via inhibiting miR-124 to regulate T follicular cell differentiation and function. Cell Discov 4:51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Del Re M et al (2018) PD-L1 mRNA expression in plasma-derived exosomes is associated with response to anti-PD-1 antibodies in melanoma and NSCLC. Br J Cancer 118(6):820–824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cao J et al (2020) Exosomes in head and neck cancer: roles, mechanisms and applications. Cancer Lett 494:7–16 [DOI] [PubMed] [Google Scholar]
- 34.Fan Y et al (2019) Exosomal PD-L1 retains immunosuppressive activity and is associated with gastric cancer prognosis. Ann Surg Oncol 26(11):3745–3755 [DOI] [PubMed] [Google Scholar]
- 35.Yang Y et al (2018) Exosomal PD-L1 harbors active defense function to suppress T cell killing of breast cancer cells and promote tumor growth. Cell Res 28(8):862–864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sage PT et al (2013) The receptor PD-1 controls follicular regulatory T cells in the lymph nodes and blood. Nat Immunol 14(2):152–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sage PT, Sharpe AHJIR (2016) T follicular regulatory cells. Immunol Rev 271(1):246–259 [DOI] [PubMed] [Google Scholar]
- 38.Sage PT et al (2014) The coinhibitory receptor CTLA-4 controls B cell responses by modulating T follicular helper, T follicular regulatory, and T regulatory cells. Immunity 41(6):1026–1039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stone EL et al (2015) ICOS coreceptor signaling inactivates the transcription factor FOXO1 to promote Tfh cell differentiation. Immunity 42(2):239–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Theodoraki MN et al. (2017) Clinical significance of PD-L1+ exosomes in plasma of head and neck cancer patients. 24(4): 896–905 [DOI] [PMC free article] [PubMed]
- 41.Xing C et al (2021) The roles of exosomal immune checkpoint proteins in tumors. Mil Med Res 8(1):56–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kugeratski FG, Kalluri R (2021) Exosomes as mediators of immune regulation and immunotherapy in cancer. FEBS J 288(1):10–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bennett F et al (2003) Program death-1 engagement upon TCR activation has distinct effects on costimulation and cytokine-driven proliferation: attenuation of ICOS, IL-4, and IL-21, but not CD28, IL-7, and IL-15 responses. J Immunol 170(2):711–718 [DOI] [PubMed] [Google Scholar]
- 44.Hui E et al (2017) T cell costimulatory receptor CD28 is a primary target for PD-1-mediated inhibition. Science 355(6332):1428–1433 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon reasonable request.