Abstract
Recently, the use of nanotechnology in food has gained great interest. Iron nanoparticles with unique chemical, physical and structural properties allow their potential use mainly as iron fortifiers, colorants and antimicrobial agents. However, in the market we can find only supplements and food colorants based on iron nanoparticles. Their use in food fortification has so far been focused only on in vitro and in vivo experimental studies, since the toxicological evaluation of these studies has so far been the basis for the proposals of laws and regulations, which are still in an early stage of development. Therefore, the aim of this work was to summarize the use of the different forms of iron nanoparticles (oxides, oxyhydroxides, phosphates, pyrophosphates and sulfates) as food additives and supplements and to resume the perspectives of legislation regarding the use of these types of nanoparticles in the food industry.
Keywords: Nanoparticles, Food industry, Fortification, Supplements, Legislation
Introduction
Nanotechnology is the science that studies the manipulation and control of various materials, that have at least one characteristic dimension between 1 and 100 nm (Sahoo et al., 2021). Among these materials, iron nanoparticles have recently been studied and applied in different fields due to their chemical, physical, and structural properties (Patra et al., 2018; Vargas-Ortiz et al., 2022). Some of these fields include biomedicine, pharmacology (Ferreira et al., 2022), environmental bioremediation (Flores-Cano et al., 2022), chemistry, and food (Rayamajhi et al., 2021), among others. The main forms used in food science research include oxides, phosphates, and sulfates (Ghibaudo et al., 2021; Von-Moos et al., 2017). The oxo-hydroxides and pyrophosphates have also been proposed as alternatives to the use of iron sulfate in food fortification (Fernández-Menéndez et al., 2018; Srinivasu et al., 2015). Iron nanoparticles have remarkable properties such as high surface area, porosity, tunable hydrophobic-hydrophilic balance, excellent dispersibility in suspensions, higher iron bioavailability and iron taste masking. Some of them exhibit magnetic properties, such as iron oxides, which allow them to be manipulated by external magnetic fields (Ajinkya et al., 2020).
Several papers have been published on the potential application of iron nanoparticles as food additives and supplements (Ghibaudo et al., 2021; Mahmoud and Helmy, 2014; Mohammed et al., 2023; Razack et al., 2020; Santillán-Urquiza et al., 2017). The application of iron nanoparticles in the food industry is still at a very early stage, but the food colorant E172, which consists of iron oxide and hydroxide particles of different sizes, has been used since the early 1970s, when the Joint FAO/WHO Expert Committee on Food Additives approved its use as a food additive (EFSA, 2016), without knowing the presence of nanoparticles in this food colorant.
On the other hand, some supplements based on iron nanoparticles coated with vitamins for the treatment of iron deficiency anemia are available to consumers in the market (Mahmoud and Helmy, 2014). The commercialization of these products is forcing global regulatory organizations and agencies to develop new standards and test methods and to issue new recommendations for safety regulations regarding human health and environmental safety, derived from the use and application of nanoparticles (He and Hwang, 2016; Saldívar-Tanaka, 2019). These actions have also been addressed to mitigate emerging social and legal concerns around the world (Grieger et al., 2016). Therefore, the aim of this work was to summarize the use of the different forms of iron nanoparticles as food additives and supplements and to resume the perspectives on the legislation regarding the use of these types of nanoparticles in the food industry.
Iron nanoparticles used in food
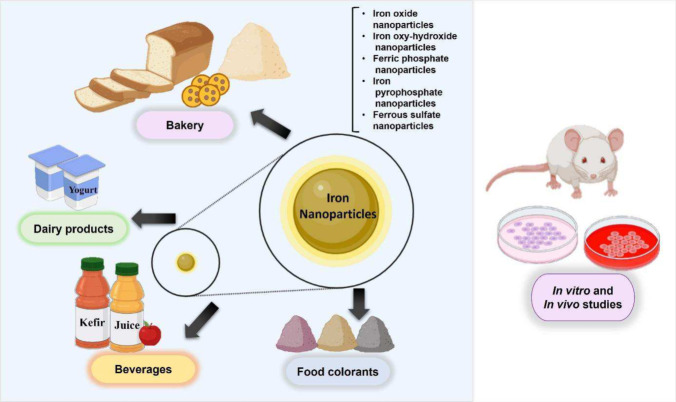
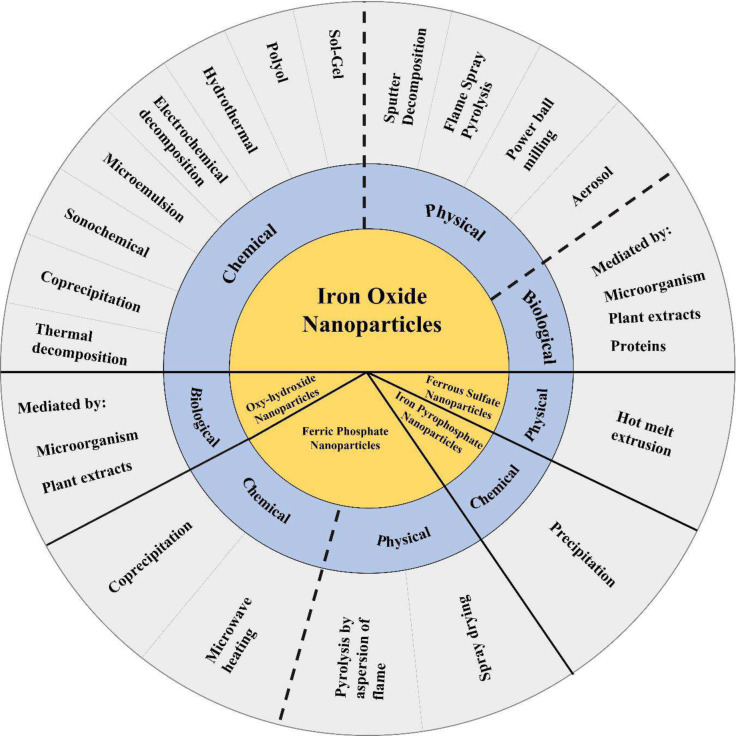
In recent years, there has been increased interest in researching the use of iron nanoparticles in foods. Potential applications of iron nanoparticles in foods include their use as iron fortifiers (Couto and Almeida, 2022), colorants (Voss et al., 2020), and antimicrobial agents (Gudkov et al., 2021). Some specific foods that have been proposed as matrices for the addition of iron nanoparticles include bakery products, dairy products, juices and fermented beverages, either as iron fortifiers or as antimicrobial agents; however, these proposals have only been applied experimentally either in vivo or in vitro at the laboratory level. These examples, together with the presence of iron nanoparticles in food colorants, are illustrated in Fig. 1. However, to date, no commercially available food products are based on this technology, and only iron nanoparticle food supplements are on the market (Mahmoud and Helmy, 2014). The types of iron nanoparticles that have been studied as potential food ingredients include iron oxide nanoparticles (IONPs) (Góral et al., 2023; Razack et al., 2020), iron oxyhydroxide, iron phosphate (Von-Moos et al., 2017), iron pyrophosphate, and iron sulfate nanoparticles (Bonyadian et al., 2022). These nanoparticles have been studied either alone or functionalized with other compounds such as minerals, vitamins (Mahmoud and Helmy, 2014), antibiotics (Song et al., 2019), and other bioactive compounds. Figure 2 shows some of the methods of synthesis for these types of iron nanoparticles which will be discussed in this work. The following sections provide a comprehensive review of the types of nanoparticles with potential applications in food, including their use as dietary supplements and parenteral applications.
Fig. 1.
Examples of foods fortified with iron nanoparticles for fortifying and supplementing purposes in in vitro and in vivo studies (Created with Biorender)
Fig. 2.
Chemical, physical and biological methods to synthesize the different types of iron nanoparticles proposed in food research
Iron oxide nanoparticles (IONPs)
Iron oxide nanoparticles (IONPs) are the most studied type of iron nanoparticle with food science applications. They include magnetite (Fe3O4), maghemite (γ-Fe2O3), and hematite (α-Fe2O3) as the three main forms used in food research (Ferreira et al., 2022). They exhibit interesting physicochemical properties such as magnetism, chemical stability, biocompatibility, low production cost, high surface-to-volume ratio, high porosity, and high dispersibility (Bustamante-Torres et al., 2022), some of which are exploited at sizes below 20 nm (Ajinkya et al., 2020). The IONPs can be obtained with or without a coating protecting their crystalline core. The latter show relatively lower chemical stability, lower dispersibility, and higher precipitation in water (Bustamante-Torres et al., 2022; Ansari et al., 2019). The application of coatings provides higher colloidal stability depending on the surface charge, particle size, and polarity of the suspension. In addition, the coating can also help to maintain the magnetic properties in those IONPs that exhibit them (Ferreira et al., 2022).
The IONPs can be synthesized by chemical (thermal decomposition, coprecipitation, sonochemical, microemulsion, electrochemical decomposition, hydrothermal, polyol, and sol–gel), physical (sputter deposition, flame spray pyrolysis, aerosol, power ball milling), and biological methods (Ajinkya et al., 2020; Ali et al., 2016; Ansari et al., 2019). The choice of synthesis method should depend on the desired properties and the required application of the nanoparticles (Ansari et al., 2019). The reviews by Ajinkya et al. (2020), Ansari et al. (2019), and Ali et al. (2016), provide a good description of the main methods to produce IONPs.
Iron oxides have been used as food colorants for at least five decades and are coded as E172 in the European Union (EFSA, 2015; EFSA, 2016). Depending on the type of oxide, these colorants can provide red (hematite; α-Fe2O3), brown (maghemite; γ-Fe2O3), and black (magnetite; Fe3O4) colors. Of these, magnetite has higher magnetism and lower stability against agglomeration (Voss et al., 2020). They were considered harmless due to their chemical composition, but the presence of nanoparticles in this food additive was recognized in 2019, when the French National Agency for Food Safety and Health (ANSES) considered that the colorant E172 contains a certain percentage of IONPs (OCU, 2023). This information was corroborated by Voss et al. (2020), who determined the presence of nanoparticles in E172 using small-angle X-ray scattering (SAXS) and transmission electron microscopy (TEM) techniques and found nanoparticle contents above 50%. These findings naturally raised concerns about the presence of these nanoparticles in E172, and the same researcher conducted studies on their toxicity. According to the results of in vitro experiments performed by Voss et al. (2020), E172 can be considered safe and with low toxicity in human intestinal cells. Therefore, IONPs have been unintentionally added to foods when using these colorants and further in vitro and in vivo research is needed to confirm their harmlessness.
Iron oxide nanoparticles have been proposed to be incorporated into various food products (Ghibaudo et al., 2021; Razack et al., 2020; Santillán-Urquiza et al., 2017; Song et al., 2019), without affecting their taste, texture, and appearance. Briefly, Razack et al. (2020) proposed their addition in cookies made of wheat flour, these IONPs were green synthesized using an aqueous extract of Hibiscus rosa-sinensis petals. The addition of IONPs in cookies increased the iron uptake from 0.039 to 1.5 mg of iron per gram of cookie and delayed the growth of mesophilic microorganisms. This research highlights the potential use of IONPs either as iron fortifiers or as preservatives in foods.
Santillan et al. (2017) added inulin-coated IONPs to yogurt, highlighting that adding these nanoparticles increased iron solubility compared to micrometric particles. In addition, the syneresis of yogurt with added nanoparticles was lower compared to the control.
Fruit juices and fermented beverages have also been highlighted as products of regular consumption in which the use of IONPs has been investigated. Ghibaudo et al. (2021) prepared pectin-coated magnetite nanoparticles and incorporated them into a non-dairy, fermented beverage composed of insoluble dextran granules and various strains of yeast and bacteria (Saccharomyces, Kloeckera, Zygotorulaspora, Brettanomyces, Zygosaccharomyces, Meyerozyma) known as water kefir. Upon the addition of IONPs at a concentration of 14.5 µg/mL, the basal level of ferritin in CaCo-2/TC7 intestinal cells doubled. The experiments analyzed the toxicological effects of various concentrations of IONPs on Artemia salina as an animal model and showed very promising results. The IONPs were non-toxic up to 500 µg/mL, the highest concentration tested in this study, while other common iron fortifiers like bulk FeSO4 had a median lethal dose (LD50) of 304.08 µg/mL. Based on the hazard compound classification of the WHO and these results, it can be concluded that the IONPs are “practically non-toxic” (WHO, 2010). Consequently, the application of IONPs in a multi-functional beverage would result in a product that not only provides prebiotics, probiotics, and postbiotics, but also serves as an excellent source of iron that is safe for individuals with iron deficiency anemia. On the other hand, Song et al. (2019) used IONPs functionalized with polydopamine-nisin composites as an antimicrobial agent to inactivate Alicyclobacillus acidoterrestris in apple juice. The addition of these functionalized IONPs was successful, reducing the concentration of bacterial survivors from 106 to 10 UFC/mL in 10 min using 20 mg of nanoparticles per mL of juice. Therefore, the IONPs could be designed for different purposes as explained above, and functionalized with different materials coating the IONPs, making these nanoparticles promising as multifunctional materials.
Iron oxy-hydroxide nanoparticles (FeOOH)
The most common forms of iron oxy-hydroxide nanoparticles include goethite (α-FeOOH), akageneite (β-FeOOH), lepidocrocite (γ-FeOOH), and feroxihite (δ-FeOOH), which can be synthesized chemically at high temperatures or biologically at lower temperatures (Ebrahiminezhad et al., 2018; Pereira et al., 2014). The biological method allows for better control of the size of the iron oxyhydroxide nanoparticles obtained, as demonstrated by the research of Ghanbariasad et al. (2019), who used the secretory compounds of the microalga Chlorella vulgaris as a shape and size controller for the synthesis reaction. The secretory compounds were dissolved in an aqueous solution of ferric chloride hexahydrate (FeCl3 6H2O) and sodium hydroxide as a precipitant, yielding spherical nanoparticles with a relatively narrow size distribution ranging from 8 to 17 nm.
Iron oxy-hydroxide nanoparticles have been proposed as a good alternative for food fortification to increase the bioavailability of iron, since their chemical structure is very close to the form of iron stored in ferritin, making them more absorbable without causing adverse sensory changes (Alves-Peixoto et al., 2019). The experiments of this research were carried out in lactating rats, but it opens the way to the development of new foods fortified with iron oxy-hydroxide nanoparticles, taking advantage of their demonstrated bioavailability. Iron oxy-hydroxide nanoparticles can also be administered intravenously to people who cannot take them orally. This form of nanoparticle administration will be the subject of discussion in section Intravenous supplements: parenteral formulas.
As with IONPs, iron oxy-hydroxide nanoparticles have been found in the food colorant E172, specifically in the yellow colorant whose component is goethite (α-FeOOH) (Voss et al., 2020). As discussed above, the presence of iron nanoparticles in E172 has shown only low toxicity, but more in-depth studies are needed to obtain conclusive results.
Ferric phosphate nanoparticles (NP-FePO4)
Ferric phosphate is a compound that is difficult to absorb in humans due to its low solubility in water and its large particle size in its commercial form (Baumgartner et al., 2022). However, when it is used on a nanometric scale as ferric phosphate nanoparticles (NP-FePO4), a higher surface area is achieved, which improves its absorption by increasing bioavailability and bioaccessibility (Baumgartner et al., 2022; Rayamajhi et al., 2021; Von-Moos et al., 2017). It has also been reported that the morphology of ferric phosphate plays a critical role in bioavailability, with the amorphous structures being better absorbed than the crystalline ones (Ghosh and Arcot, 2022).
The most common method used to obtain NP-FePO4 is pyrolysis by aspersion of flame, using as precursors a solution of iron nitrate nonahydrate and tributyl phosphate in ethanol and ethyl ethanoic acid. This method of synthesis has been shown to improve the solubility of the resulting nanoparticles under gastric conditions (Baumgartner et al., 2022; Von-Moos et al., 2017). Other methods include co-precipitation, which allows for obtaining amorphous nanoparticles between 15 and 100 nm, microwave heating (Zhang et al., 2020), and spray drying (Yang et al., 2017).
The use of NP-FePO4 as a food fortifier looks promising. These nanoparticles have been used in in vivo studies with rats and humans. Toxicity and potential side effects were assessed using the same doses commonly used for ferrous sulfate and showed no cellular toxicity, oxidative damage, or excessive tissular iron accumulation even at doses as high as 25 mg Fe/kg, suggesting that their intake is safe (Von-Moos et al., 2017).
Baumgartner et al. (2022) added 2 mg NP-FePO4 to the diet (rice and vegetables) of iron-deficient anemic women in a randomized crossover trial and evaluated the bioavailability of NP-FePO4 using stable isotopes to measure 57Fe incorporation into erythrocytes after 14 days of intake. The bioavailability was as high as 72% when compared to ferrous sulfate as a positive control and 5.4 times when compared to bulk ferrous phosphate as a negative control, suggesting that NP-FePO4 may have an efficient use in nutritional applications.
Iron pyrophosphate nanoparticles (NP-Fe4(P2O7)3)
It has been reported that iron pyrophosphate is a type of iron salt with low bioavailability due to its poor solubility in water (Srinivasu et al., 2015). In the food industry, iron pyrophosphate has been used to formulate supplements for parenteral administration and fortified foods (Fishbane et al., 2022). Due to its white color, iron pyrophosphate has been used to fortify infant cereals and chocolate drinks, but its particle size is still micrometric (Shukla et al., 2017; Srinivasu et al., 2015).
Recently, research has focused on obtaining iron pyrophosphate nanoparticles, mainly because they have a higher specific surface area, which improves the dispersibility and uptake of the iron compound due to easier penetration into the biological barriers and subcellular compartments (Srinivasu et al., 2015).
The synthesis method of NP-Fe4(P2O7)3 reported in food fortification research is through a precipitation reaction from a mixture of chemical compounds, such as sodium pyrophosphate decahydrate and ferric chloride hexahydrate (Rossi et al., 2014). Although this reported method of synthesis is relatively inexpensive and easy to perform, no further research has been conducted on this iron compound for use in food, opening an opportunity for further research on this topic. While it is known that iron pyrophosphate can increase its bioavailability when used at the nanoscale, there is limited information on the use of NP-Fe4(P2O7)3 in the scientific literature. Srinivasu et al. (2015) used NP-Fe4(P2O7)3 to enhance the diets of rats, which resulted in higher bioavailability without any toxic effects. They reported a No Observed Adverse Effect Level (NOAEL) of 1000 mg/kg bw and an LD50 value of 2000 mg/kg bw for male and female rats. Kim et al. (2017) assessed the potential toxicity of ferric pyrophosphate nanoparticles with and without coating in dextrin and glycerides on human intestinal INT-407 cells. After 24 h of exposure at the highest concentration (1000 µg/mL), both coated and uncoated nanoparticles did not impact cell proliferation or bioavailability.
Ferrous sulfate nanoparticles (FSNPs)
Ferrous sulfate is a water-soluble iron salt used as a gold standard in food fortification due to its high bioavailability at the cellular level. However, it has been reported to cause changes in the sensory properties of foods, such as a bitter taste, and mainly changes in coloration (Baumgartner et al., 2022; Perfecto et al., 2017). Therefore, alternatives have been sought, such as the incorporation of ferrous sulfate nanoparticles (FSNPs), to mitigate these undesirable changes.
Some physical methods, such as hot melt extrusion, can be used to synthesize ferrous sulfate nanoparticles (Kim et al., 2022; Koo et al., 2019). Bonyadian et al. (2022) synthesized ferrous sulfate nanoparticles and added them to flour for bread production and subsequently administered them to Wistar rats, reporting that at a dose of 1.57 mM, it did not cause damage to healthy cells (fibroblasts) or histopathological lesions in rats. Kim et al. (2022) used ferrous sulfate nanoparticles to supplement broiler chickens to increase their growth. As a result, the nanoparticles reduced the amount of iron excreted and improved the digestibility, absorption, and concentration of red blood cells and hemoglobin in the blood compared to ferrous salt supplementation, making the treatment with nanoparticles an alternative in the supplementation of broilers.
Iron nanoparticles used in nutritional supplements
Iron nanoparticles as a supplement for the treatment of iron deficiency anemia
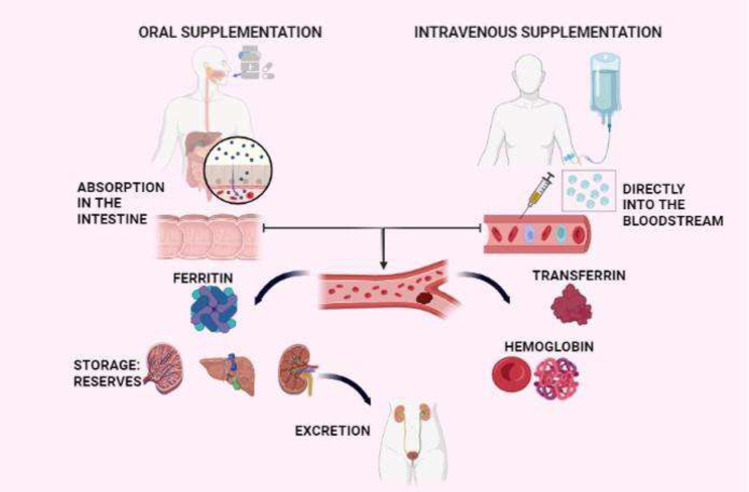
Anemia is defined as a condition in which the concentration of hemoglobin (Hb) is below normal levels and/or there is a decrease in the number of erythrocytes (red blood cells) relative to sex and age (Kumar et al., 2022; Sundararajan and Rabe, 2021). The goal of supplementation is to provide sufficient iron to restore normal levels of stored iron or to restore hemoglobin levels in the blood. Iron supplementation is indicated when diet alone is unable to restore iron levels to optimal levels within a reasonable time (Elsayed et al., 2014). Clinical treatment of iron deficiency anemia may be by oral supplementation in the form of capsules, tablets, and powders, or intravenously in the form of iron-based parenteral formulations (iron salts or iron nanoparticles). The routes of administration and pharmacokinetics of iron nanoparticle supplementation are illustrated in Fig. 3.
Fig. 3.
Iron nanoparticle supplementation routes of administration and pharmacokinetics (Created with Biorender)
Nanoparticles in oral supplements
Oral administration is the first and most widely used route of treatment for iron deficiency anemia because it is easy to self-administer, easy to follow, and more feasible for use in ambulatory patients (Garcia-Fernandez et al., 2020). Most treatments use iron salts, specifically ferrous sulfate, gluconate, and fumarate, which, as mentioned repeatedly, are associated with gastrointestinal side effects such as diarrhea, constipation, and nausea in more than 60% of patients (Fernández et al., 2018; Perfecto et al., 2017). In recent years, the incorporation of nanocomposites has played an important role in the development of dietary supplements. Their nanometer size allows better cellular uptake and absorption, as well as a more efficient clearance/elimination mechanism by the kidney for nanoparticles smaller than 10 nm or by phagocytosis (mononuclear phagocytes) for larger nanoparticles (Garcia-Fernandez et al., 2020). Their larger surface area also improves their solubility at the pH of gastric juice and increases absorption after oral intake (Singh et al., 2022). Some of the proposals are based on the use of compounds that simulate the ferritin model. For example, Mohammed et al. (2023) tested a new iron nanoparticle supplement based on iron hydroxide adipate tartrate (IHAT) for the treatment of iron deficiency in children aged 6 to 35 months for 85 days. They evaluated 3 groups: the first group received a dose of 20 mg Fe/day of IHAT, the second group received 12.5 mg Fe/day of bulk iron sulfate (FeSO4), and the third group received a placebo only. All treatments were administered in the form of capsules with the same appearance. The results showed higher efficacy and correction of anemia with IHAT (28%) than with FeSO4 (22.15%) and placebo (1.1%). The incidence of diarrhea was lower in the IHAT group than in the FeSO4 group. This iron nanoparticle supplement is cheaper and was the first nano-iron approved by the European Commission (2022/1373 August 5, 2022), and represents a viable and accessible option for vulnerable low-income populations (Mohammed et al., 2023).
Other supplements on the market and patents that have been tested in animals are based on magnetite and bioactive compounds, such as the patent by El-Din et al. (2010), who administered magnetite nanoparticles coated with ascorbic acid in a single dose with the effect of increasing hemoglobin levels from 7 to 14 g/dL in only 10 days, being able to stimulate erythropoiesis, with no apparent toxicity. Mahmoud and Helmy et al. (2014) developed patent WO2014135170A1 based on iron oxide nanoparticles functionalized with vitamins B9, B3, and C as a treatment for iron deficiency anemia at a dose of 25 mg in a gel capsule or aqueous solution presentations. This dose was sufficient to increase hemoglobin levels from 4.4 to 14.6 g/dL in a short period of 4 days.
Intravenous supplements: parenteral formulas
The use of iron oxyhydroxide nanoparticles, specifically akaganeite, bound to carbohydrates (sucrose and gluconate) to form complexes (iron-carbohydrate complexes) has been reported (Di Francesco and Borchard, 2018; Fütterer et al., 2013). These complexes have been used commercially since 1949, long before the term nanomedicine was coined. The product Venofer (iron-sucrose) has been used in Switzerland since 1949 but was not approved by the FDA until 2000. It was the first product based on nano-sized colloids to be introduced to replace oral supplements (Macdougall et al., 2020; Nikravesh et al., 2020). Carbohydrate-coated iron nanoparticles administered intravenously have reduced the toxicity generally associated with the administration of free iron in the body. These iron-carbohydrate complexes are nanoparticles with a polynuclear ferric oxyhydroxide/ferric oxide core coated with carbohydrates, with a spheroidal shape and sizes between 8 and 30 nm (Nikravesh et al., 2020). They have certain advantages, such as they do not cause gastric intolerance or alterations in iron absorption, and the replenishment time of iron stores is shorter (Nikravesh et al., 2020). For these reasons, they have been widely used as additives in the preparation of parenteral formulas for the treatment of iron deficiency in susceptible individuals or those with absorption problems or low iron stores, such as dialyzed patients due to chronic renal failure (Pai, 2017), with gastrointestinal disorders, postoperative patients, among others (Macdougall et al., 2020).
The bioavailability of these complexes is high because they enter the bloodstream and are taken up by the reticuloendothelial system, which then degrades the carbohydrate shell and releases iron from the core. Ferritin is responsible for storing circulating iron in iron stores and transferrin for transporting it to extracellular proteins (Nikravesh et al., 2020). However, a disadvantage of its use is that it has been associated with increased production of reactive oxygen species (ROS) due to the high levels of free iron released into the bloodstream within a short time after administration (Singh et al., 2022). Certification and approval for the use of commercial intravenous nanoparticle-based supplements are shown in Table 1.
Table 1.
Iron nanoparticle-based dietary supplements approved by the FDA for the intravenous treatment of iron deficiency anemia
| Supplement | Composition | Dosage (maximum individual) | Recommendation | Year of approval | References |
|---|---|---|---|---|---|
| CosmoFer® | Iron-dextran colloid | 20 mg/kg | Anemia inflammatory bowel disease, non-myeloid malignancies, pregnancy | 1992 | Anselmo and Mitragotri (2016); Huang et al. (2020); Schaefer et al. (2020) |
| Ferrlecit® (sanofi) | Sodium ferric gluconate | 125 mg | Anemia treatment in patients with chronic kidney disease | 1999 | Anselmo and Mitragotri (2016); Schaefer et al. (2020) |
| Venofer® (American Reagent) | Iron-Sucrose colloid | 200 mg | Anemia in chronic kidney disease, chronic heart failure, inflammatory bowel disease, renal failure, colorectal neoplasm, critical Illness, gynecologic cancer, surgical intervention | 2000 | Huang et al. (2020); Schaefer et al. (2020) |
| Ferinject® (Vifor Pharma) | Iron-Carboxymaltose colloid | 750 mg | Iron deficiency anemia in pancreatic or colorectal cancer, inflammatory bowel disease, cardiac surgery, endothelial dysfunction | 2013 | Anselmo and Mitragotri (2016); Huang et al. (2020); Schaefer et al. (2020) |
| Monofer® (Pharmacosmos) | Iron-derisomaltose colloid | 20 mg/kg | Iron deficiency anemia in inflammatory bowel disease, gastrectomy, gastric, esophageal, or colorectal cancer, severe aortic stenosis, or heart surgery | 2020 | Huang et al. (2020); Schaefer et al. (2020); Shepherd et al. (2021) |
Perspectives of regulation and legislation of the use of iron nanoparticles in the food industry
The use of nanoparticles in food production still generates much uncertainty about the safety of their consumption by the general population. For this reason, there is a clear need to develop assessment tools and regulations and apply product labeling to ensure quality and safety, as well as to expose the risks associated with the use and consumption of these nanomaterials (Rauscher et al., 2017).
There is little information on what and how much consumers are exposed to when they consume products containing nanoparticles. The lack of information makes it difficult to quantitatively and qualitatively assess these nanoproducts (Hansen et al., 2016). Current legislation and regulations on the use of iron nanoparticles in food are being developed by international governmental regulatory agencies. The European Food Safety Authority (EFSA) and the Food and Drug Administration (FDA) regulate the Acceptable Daily Intake (ADI) of nanoparticles, whether consumed directly or indirectly (Kumar et al., 2020). They also provide information for risk assessment of the use of nanoparticles in food additives and contact materials and guide applicants on the physicochemical characterization and identification of emerging hazards of nanomaterials. In 2010, EFSA launched the EFSA Nanonetwork, a risk assessment network to facilitate assessment methodologies and the generation and exchange of data and information between members in the European Union (EFSA et al., 2021). Other actively involved agencies include the Organization for Economic Cooperation and Development (OECD), the International Organization for Standardization (ISO), the European Parliament, and some nongovernmental organizations (Amenta et al., 2015).
International organizations such as the Joint FAO/WHO Expert Committee on Food Additives (JECFA) have been evaluating the safety of food additives since 1956 (FAO, 2023). The iron oxides and hydroxides present in color additives are the only forms of iron on the list of food additives exempt from certification, labeled E172 (U.S. Code of Federal Regulations, 2023). The color additive E172 contains iron oxides and hydroxides with a varied particle size distribution, on the one hand, the presence of larger particles between 318 and 1677 nm and, on the other hand, the content of small particles with one or more dimensions below 100 nm (nanoparticles). The presence of this nano-fraction could have different effects on absorption and distribution at the physiological level and should also be evaluated (EFSA, 2015). The JECFA in 1980 established an Acceptable Daily Intake (ADI) (according to EFSA, the ADI refers to the amount of a substance present in a food that can be consumed per day without risk to health) for iron oxide consumption of 0–0.5 mg/kg bw/d as safe and with low toxicity (Voss et al., 2020). They also report a NOAEL for red iron oxide (Fe2O3) of 1000 mg/kg bw/d (EFSA, 2015). These data support the notion that the intake of these food additives is safe to date; however, their use as food colorants in the US is very limited, up to 0.1% w/w in sausages as part of the casing (McClements and Xiao, 2017).
Toxicity is undoubtedly the key factor in the approval of any product for commercial use. For the use of nanoparticles specifically of iron, studies have been conducted to determine if there are toxicological signs or data. Toxicological aspects at the gastrointestinal level can be evaluated in vitro through the impact on cell viability and ROS formation, for example, Garcia-Fernandez et al. (2020) evaluated Fe2O3 iron nanoparticles functionalized with tartaric/adipic acid with a size of only 4 nm, using an oral iron supplement. The cell viability of Caco-2 and HT-29 cells was not affected, and the solubility of the iron nanoparticles (NPFe) was higher than 79%, higher than other traditional treatments. The doses administered were 20 mL of a 35 mg NPFe/L solution, equivalent to 230 mgFe/d for adults. As for ROS generation, they observed only a small increase, which was attributed to the slow dissolution of iron from the nanoparticles. Bonfanti et al. (2020) proposed frog embryos as a suitable model to investigate the toxicity of Fe3O4 iron nanoparticles, demonstrating that this kind of IONPs do not cause acute toxicity or teratogenicity at concentrations at which iron salts induce adverse effects, even if the IONPs accumulate massively in the embryo gut. It has been reported that the toxicity of iron oxide nanoparticles can be modified by their shape, size, and crystalline structure (Patil et al., 2015). It is therefore clear that both the standardization of the synthesis and the precise characterization of iron nanoparticles are key factors in determining their toxicity both in vitro and in vivo. Results that will provide the basis for progress in their regulation and legislation.
In conclusion, the iron nanoparticles, in addition to their multiple properties, have a double advantage: on the one hand, when degraded, the iron released is bioavailable to the organism, representing a good source of iron for the treatment of anemia; on the other hand, they can be coated with a wide variety of active compounds with independent properties and benefits that can enhance the use of these nanomaterials. However, there is a point at which the synthesis of nanomaterials has outpaced the analytical capacity. At present, the benefits of nanomaterial applications are great; however, their increasing use in commercial products has raised troubling questions about their toxicological safety. The impact of the use of nanomaterials in food and dietary supplements for human consumption and intravenous administration must be addressed through legislation and regulation worldwide; however, for the use of iron nanoparticles, information is scarce, and legislation is still at a very early stage. In this sense, to guarantee the chemical and biological safety of nanomaterials, it is essential to standardize the synthesis conditions, as well as to carry out complete studies of their physicochemical properties to relate them to their respective activities or biological properties and the cellular responses that they may induce. In vitro and in vivo studies allow us to have an idea of the biosafety of the use of these nanoparticles, as well as the doses that do not produce a toxic effect on the organism. The toxicity data of various forms of iron nanoparticles, presented here, supports the potential value of iron nanoparticles for use in food products. Based on these data, organizations such as the Food and Drug Administration, the World Health Organization, and the European Food Safety Authority, among others, establish the Acceptable Daily Intake, the Tolerable Daily Intake, and the No Observed Adverse Effect Level, specific to each situation, and issue certificates authorizing their use only in cases where the safety of the product has been proven.
Acknowledgements
The authors thank the Consejo Nacional de Humanidades, Ciencia y Tecnología (CONAHCYT/grant no. 797746) for the grant awarded for this research.
Declarations
Competing interests
On behalf of all authors, the corresponding author states that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Rubén Jiménez-Alvarado, Email: ruben_jimenez@uaeh.edu.mx.
Raquel Cariño-Cortés, Email: raquel_carino4897@uaeh.edu.mx.
References
- Ajinkya N, Yu X, Kaithal P, Luo H, Somani P, Ramakrishna S. Magnetic iron oxide nanoparticle (IONP) synthesis to applications: present and future. Materials. 2020;13:4644. doi: 10.3390/ma13204644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali A, Zafar H, Zia M, Haq I, Phull AR, Ali JS, Hussain A. Synthesis, characterization, applications, and challenges of iron oxide nanoparticles. Nanotechnology, Science and Applications. 2016;9:49–67. doi: 10.2147/NSA.S99986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves-Peixoto RR, Fernández-Menéndez S, Fernández-Colomer B, Cadore S, Sanz-Medel A, Fernández-Sánchez ML. Quantitative speciation analysis for the in vivo study of iron metabolism and bioavailability from formula milk fortified with stable isotope enriched iron oxo-hydroxide nanoparticles. Journal Analytical Atomic Spectrometry. 2019;34:774–781. doi: 10.1039/C8JA00364E. [DOI] [Google Scholar]
- Amenta V, Aschberger K, Arena M, Bouwmeester H, Moniz FB, Brandhoff P, Gottardo S, Marvin HJP, Mech A, Pesudo LQ, Rauscher H, Schoonjans R, Vettori MV, Weigel S, Peters RJ. Regulatory aspects of nanotechnology in the agri/feed/food sector in EU and non-EU countries. Regulatory Toxicology and Pharmacology. 2015;73:463–476. doi: 10.1016/j.yrtph.2015.06.016. [DOI] [PubMed] [Google Scholar]
- Ansari SAMK, Ficiarà E, Ruffinatti FA, Stura L, Argenziano M, Abollino O, Cavalli R, Guiot C, D’Agata F. Magnetic Iron oxide nanoparticles: synthesis, characterization and functionalization for biomedical applications in the central nervous system. Materials. 2019;12:465. doi: 10.3390/ma12030465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anselmo AC, Mitragotri S. Nanoparticles in the clinic. Bioengineering & Translational Medicine. 2016;1:10–29. doi: 10.1002/btm2.10003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgartner J, Winkler HC, Zandberg L, Tuntipopipat S, Mankong P, Bester C, Hilty F, Zeevaart JR, Gowachirapant S, Zimmermann MB. Iron from nanostructured ferric phosphate: absorption and biodistribution in mice and bioavailability in iron deficient Anemic Women. Scientific Reports. 2022;12:2792. doi: 10.1038/s41598-022-06701-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonfanti P, Colombo A, Saibene M, Fiandra L, Armenia I, Gamberoni F, Gornati R, Bernardini G, Mantecca P. Iron nanoparticle bio-interactions evaluated in Xenopus laevis embryos, a model for studying the safety of ingested nanoparticles. Nanotoxicology. 2020;14:196–213. doi: 10.1080/17435390.2019.1685695. [DOI] [PubMed] [Google Scholar]
- Bonyadian M, Moeini E, Ebrahimnejad H, Askari N, Karimi I. The effect of iron sulfate nanoparticles and their fortified bread on Wistar rats and human cell lines. Journal of Trace Elements in Medicine and Biology. 2022;73:27005. doi: 10.1016/j.jtemb.2022.127005. [DOI] [PubMed] [Google Scholar]
- Bustamante-Torres M, Romero-Fierro D, Estrella-Nuñez J, Arcentales-Vera B, Chichande-Proaño E, Bucio E. Polymeric composite of magnetite iron oxide nanoparticles and their application in biomedicine: a review. Polymers. 2022;14:752. doi: 10.3390/polym14040752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couto C, Almeida A. Metallic nanoparticles in the food sector: a mini-review. Foods. 2022;11:402. doi: 10.3390/foods11030402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Francesco T, Borchard G. A robust and easily reproducible protocol for the determination of size and size distribution of iron sucrose using dynamic light scattering. Journal Pharmaceutical and Biomedical Analysis. 2018;152:89–93. doi: 10.1016/j.jpba.2018.01.029. [DOI] [PubMed] [Google Scholar]
- Ebrahiminezhad A, Zare-Hoseinabadi A, Sarmah AK, Taghizadeh S, Ghasemi Y, Berenjian A. Plant-mediated synthesis and applications of iron nanoparticles. Molecular Biotechnology. 2018;60:154–168. doi: 10.1007/s12033-017-0053-4. [DOI] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) Schoonjans R, Tarazona J. Annual report of the EFSA scientific network of risk assessment of nanotechnologies in food and feed for 2020. EFSA Journal. 2021;18:EN-6502. [Google Scholar]
- EFSA Feedap Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed) Safety and efficacy of iron oxide black, red and yellow for all animal species. EFSA Journal. 2016;14:4482. [Google Scholar]
- EFSA Panel on Food Additives and Nutrient Sources added to Food Scientific opinion on the re-evaluation of iron oxides and hydroxides (E 172) as food additives. EFSA Journal. 2015;13:4317. [Google Scholar]
- El-Din TAS, Mohamed MB, Kamel HM, Kader MA. (2010). Magnetite nanoparticles as a single dose treatment for iron deficiency anemia. Egypt. Patent WO2010034319A1
- Elsayed HH, Al-Sherbini ASAM, Abd-Elhady EE, Ahmed KAEA. Treatment of anemia progression via magnetite and folate nanoparticles in vivo. International Scholarly Research Notices. 2014;2014:287575. [Google Scholar]
- Fernández JG, Sánchez-González C, Bettmer J, Llopis J, Jakubowski N, Panne U, Montes-Bayón M. Quantitative assessment of the metabolic products of iron oxide nanoparticles to be used as iron supplements in cell cultures. Analytica Chimica Acta. 2018;1039:24–30. doi: 10.1016/j.aca.2018.08.003. [DOI] [PubMed] [Google Scholar]
- Fernández-Menéndez S, Peixoto RRA, Fernández-Colomer B, Romero MC, Sanz-Medel A, Fernández-Sánchez ML. Searching for enhanced iron fortification of formula milk via nanoparticles and isotope pattern deconvolution. Spectrochimica Acta Part B: Atomic Spectroscopy. 2018;148:165–171. doi: 10.1016/j.sab.2018.06.017. [DOI] [Google Scholar]
- Ferreira LP, Reis CP, Robalo TT, Jorge MEM, Ferreira P, Gonçalves J, Hajalilou A, Cruz MM. Assisted synthesis of coated iron oxide nanoparticles for magnetic hyperthermia. Nanomaterials. 2022;12:1870. doi: 10.3390/nano12111870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishbane S, Ganz T, Pratt RD. Ferric pyrophosphate citrate for parenteral administration of maintenance iron: structure, mechanism of action, clinical efficacy and safety. Current Medical Research and Opinion. 2022;38:1417–1429. doi: 10.1080/03007995.2022.2092373. [DOI] [PubMed] [Google Scholar]
- Flores-Cano DA, Checca-Huaman NR, Castro-Merino IL, Pinotti CN, Passamani EC, Litterst FJ, Ramos-Guivar JA. Progress toward room-temperature synthesis and functionalization of iron-oxide nanoparticles. International Journal of Molecular Sciences. 2022;23:8279. doi: 10.3390/ijms23158279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Food and Agriculture Organization of the United Nations (FAO). Food safety and quality-Joint FAO/WHO expert committee on food additives (JECFA). Available at: https://www.fao.org/food-safety/scientific-advice/jecfa/en/#:~:text=JECFA%20is%20an%20international%20scientific,of%20veterinary%20drugs%20in%20food. Accessed June 06, 2023
- Fütterer S, Andrusenko I, Kolb U, Hofmeister W, Langguth P. Structural characterization of iron oxide/hydroxide nanoparticles in nine different parenteral drugs for the treatment of iron deficiency anaemia by electron diffraction (ED) and X-ray powder diffraction (XRPD) Journal of Pharmaceutical and Biomedical Analysis. 2013;86:151–160. doi: 10.1016/j.jpba.2013.08.005. [DOI] [PubMed] [Google Scholar]
- Garcia-Fernandez J, Turiel D, Bettmer J, Jakubowski N, Panne U, Rivas LG, Llopis J, González CS, Montes-Bayón M. In vitro and in situ experiments to evaluate the biodistribution and cellular toxicity of ultrasmall iron oxide nanoparticles potentially used as oral iron supplements. Nanotoxicology. 2020;14:388–403. doi: 10.1080/17435390.2019.1710613. [DOI] [PubMed] [Google Scholar]
- Ghanbariasad A, Taghizadeh SM, Show PL, Nomanbhay S, Berenjian A, Ghasemi Y, Ebrahiminezhad A. Controlled synthesis of iron oxyhydroxide (FeOOH) nanoparticles using secretory compounds from Chlorella vulgaris microalgae. Bioengineered. 2019;10:390–396. doi: 10.1080/21655979.2019.1661692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghibaudo F, Gerbino E, Hugo AA, Dall’Orto VC, Gomez-Zavaglia A. Fortification of water kefir with magnetite nanoparticles. Food Research International. 2021;149:110650. doi: 10.1016/j.foodres.2021.110650. [DOI] [PubMed] [Google Scholar]
- Ghosh R, Arcot J. Fortification of foods with nano-iron: its uptake and potential toxicity: current evidence, controversies, and research gaps. Nutrition Reviews. 2022;80:1974–1984. doi: 10.1093/nutrit/nuac011. [DOI] [PubMed] [Google Scholar]
- Góral D, Marczuk A, Góral-Kowalczyk M, Koval I, Andrejko D. Application of iron nanoparticle-based materials in the food industry. Materials. 2023;16:780. doi: 10.3390/ma16020780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieger KD, Hansen SF, Mortensen NP, Cates S, Kowalcyk B. International implications of labeling foods containing engineered nanomaterials. Journal of Food Protection. 2016;79:830–842. doi: 10.4315/0362-028X.JFP-15-335. [DOI] [PubMed] [Google Scholar]
- Gudkov SV, Burmistrov DE, Serov DA, Rebezov MB, Semenova AA, Lisitsyn AB. Do iron oxide nanoparticles have significant antibacterial properties? Antibiotics. 2021;10:884. doi: 10.3390/antibiotics10070884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen SF, Heggelund LR, Besora PR, Mackevica A, Boldrin A, Baun A. Nanoproducts–what is actually available to European consumers? Environmental Science: Nano. 2016;3:169–180. [Google Scholar]
- He X, Hwang HM. Nanotechnology in food science: functionality, applicability, and safety assessment. Journal of Food and Drug Analysis. 2016;24:671–681. doi: 10.1016/j.jfda.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Feng W, Chen Y, Shi J. Inorganic nanoparticles in clinical trials and translations. Nano Today. 2020;35:100972. doi: 10.1016/j.nantod.2020.100972. [DOI] [Google Scholar]
- Kim HJ, Bae SH, Kim HJ, Kim KM, Song JH, Go MR, Yu J, Oh JM, Choi SJ. Cytotoxicity, intestinal transport, and bioavailability of dispersible iron and zinc supplements. Frontiers in Microbiology. 2017;8:749. doi: 10.3389/fmicb.2017.00749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KY, Lee JH, Hosseindoust A, Kim MJ, Mun JY, Moturi J, Tajudeen H, Kim TG, Chae BJ. Enhancement of ferrous sulfate absorption using nano-technology in broiler chickens. Livestock Science. 2022;260:104869. doi: 10.1016/j.livsci.2022.104869. [DOI] [Google Scholar]
- Koo JS, Lee SY, Azad MOK, Kim M, Hwang SJ, Nam S, Kim S, Chae BJ, Kang WS, Cho HJ. Development of iron (II) sulfate nanoparticles produced by hot-melt extrusion and their therapeutic potentials for colon cancer. International Journal of Pharmaceutics. 2019;558:388–395. doi: 10.1016/j.ijpharm.2019.01.018. [DOI] [PubMed] [Google Scholar]
- Kumar P, Mahajan P, Kaur R, Gautam S. Nanotechnology and its challenges in the food sector: a review. Materials Today Chemistry. 2020;17:100332. doi: 10.1016/j.mtchem.2020.100332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar SB, Arnipalli SR, Mehta P, Carrau S, Ziouzenkova O. Iron deficiency anemia: efficacy and limitations of nutritional and comprehensive mitigation strategies. Nutrients. 2022;14:2976. doi: 10.3390/nu14142976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdougall IC, Comin-Colet J, Breymann C, Spahn DR, Koutroubakis IE. Iron sucrose: a wealth of experience in treating iron deficiency. Advances in Therapy. 2020;37:1960–2002. doi: 10.1007/s12325-020-01323-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoud MBM, Helmy SHA. (2014). Novel formula of iron-based nanocomposites for rapid and efficient treatment of iron deficiency anemia. Egypt. Patent WO2014/135170A1
- McClements DJ, Xiao H. Is nano safe in foods? Establishing the factors impacting the gastrointestinal fate and toxicity of organic and inorganic food-grade nanoparticles. NPJ Science of Food. 2017;1:6. doi: 10.1038/s41538-017-0005-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammed NI, Wason J, Mendy T, Nass SA, Ofordile O, Camara F, Baldeh B, Sanyang C, Jallow AT, Hossain I, Faria N, Powell JJ, Prentice AM, Pereira DIA. A novel nano-iron supplement versus standard treatment for iron deficiency anaemias in children 6–35 months (IHAT-GUT trial): a double-blind, randomised, placebo-controlled non inferiority phase II trial in the Gambia. EClinicalMedicine. 2023;56:101853. doi: 10.1016/j.eclinm.2023.101853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikravesh N, Borchard G, Hofmann H, Philipp E, Flühmann B, Wick P. Factors influencing safety and efficacy of intravenous iron-carbohydrate nanomedicines: from production to clinical practice. Nanomedicine: Nanotechnology, Biology and Medicine. 2020;26:102178. doi: 10.1016/j.nano.2020.102178. [DOI] [PubMed] [Google Scholar]
- OCU (Organización de Consumidores y Usuarios de España). E172 Óxidos e hidróxidos de hierro. Available at: https://www.ocu.org/alimentacion/seguridad-alimentaria/calculadora/aditivos/172. Accessed May 08, 2023
- Pai AB. Complexity of intravenous iron nanoparticle formulations: implications for bioequivalence evaluation. Annals of the New York Academy of Sciences. 2017;1407:17–25. doi: 10.1111/nyas.13461. [DOI] [PubMed] [Google Scholar]
- Patil US, Adireddy S, Jaiswal A, Mandava S, Lee BR, Chrisey DB. In vitro/in vivo toxicity evaluation and quantification of iron oxide nanoparticles. International Journal of Molecular Sciences. 2015;16:24417–24450. doi: 10.3390/ijms161024417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patra JK, Das G, Fraceto LF, Campos EVR, Rodriguez-Torres MP, Acosta-Torres LS, Diaz-Torres LA, Grillo R, Swamy MK, Sharma S, Habtemariam S, Shin HS. Nano based drug delivery systems: recent developments and future prospects. Journal of Nanobiotechnology. 2018;16:71. doi: 10.1186/s12951-018-0392-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira DI, Bruggraber SF, Faria N, Poots LK, Tagmount MA, Aslam MF, Frazer DM, Vulpe CD, Anderson GJ, Powell JJ. Nanoparticulate iron (III) oxo-hydroxide delivers safe iron that is well absorbed and utilised in humans. Nanomedicine: Nanotechnology, Biology and Medicine. 2014;10:1877–1886. doi: 10.1016/j.nano.2014.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perfecto A, Elgy C, Valsami-Jones E, Sharp P, Hilty F, Fairweather-Tait S. Mechanisms of iron uptake from ferric phosphate nanoparticles in human intestinal caco-2 cells. Nutrients. 2017;9:359. doi: 10.3390/nu9040359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauscher H, Rasmussen K, Sokull-Klüttgen B. Regulatory aspects of nanomaterials in the EU. Chemie Ingenieur Technik. 2017;89:224–231. doi: 10.1002/cite.201600076. [DOI] [Google Scholar]
- Rayamajhi S, Wilson S, Aryal S, DeLong R. Biocompatible FePO4 nanoparticles: drug delivery, RNA stabilization, and functional activity. Nanoscale Research Letters. 2021;16:169. doi: 10.1186/s11671-021-03626-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razack SA, Suresh A, Sriram S, Ramakrishnan G, Sadanandham S, Veerasamy M, Nagalamadaka RB, Sahadevan R. Green synthesis of iron oxide nanoparticles using Hibiscus rosa-sinensis for fortifying wheat biscuits. SN Applied Sciences. 2020;2:898. doi: 10.1007/s42452-020-2477-x. [DOI] [Google Scholar]
- Rossi L, Velikov KP, Philipse AP. Colloidal iron (III) pyrophosphate particles. Food Chemistry. 2014;151:243–247. doi: 10.1016/j.foodchem.2013.11.050. [DOI] [PubMed] [Google Scholar]
- Sahoo M, Vishwakarma S, Panigrahi C, Kumar J. Nanotechnology: current applications and future scope in food. Food Frontiers. 2021;2:3–22. doi: 10.1002/fft2.58. [DOI] [Google Scholar]
- Saldívar-Tanaka L. Regulando la nanotecnología. Mundo Nano Revista Interdisciplinaria En Nanociencias y Nanotecnología. 2019;12:37–57. [Google Scholar]
- Santillán-Urquiza E, Méndez-Rojas MA, Vélez-Ruiz JF. Fortification of yogurt with nano and micro sized calcium, iron and zinc, effect on the physicochemical and rheological properties. LWT-Food Science and Technology. 2017;80:462–469. doi: 10.1016/j.lwt.2017.03.025. [DOI] [Google Scholar]
- Schaefer B, Meindl E, Wagner S, Tilg H, Zoller H. Intravenous iron supplementation therapy. Molecular Aspects of Medicine. 2020;75:100862. doi: 10.1016/j.mam.2020.100862. [DOI] [PubMed] [Google Scholar]
- Shepherd SJ, Issadore D, Mitchell MJ. Microfluidic formulation of nanoparticles for biomedical applications. Biomaterials. 2021;274:120826. doi: 10.1016/j.biomaterials.2021.120826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla A, Dasgupta N, Ranjan S, Singh S, Chidambram R. Nanotechnology towards prevention of anaemia and osteoporosis: from concept to market. Biotechnology & Biotechnological Equipment. 2017;31:863–879. doi: 10.1080/13102818.2017.1335615. [DOI] [Google Scholar]
- Singh K, Chopra DS, Singh D, Singh N. Nano-formulations in treatment of iron deficiency anaemia: an overview. Clinical Nutrition ESPEN. 2022;52:12–19. doi: 10.1016/j.clnesp.2022.08.032. [DOI] [PubMed] [Google Scholar]
- Song Z, Wu H, Niu C, Wei J, Zhang Y, Yue T. Application of iron oxide nanoparticles@ polydopamine-nisin composites to the inactivation of Alicyclobacillus acidoterrestris in apple juice. Food Chemistry. 2019;287:68–75. doi: 10.1016/j.foodchem.2019.02.044. [DOI] [PubMed] [Google Scholar]
- Srinivasu BY, Mitra G, Muralidharan M, Srivastava D, Pinto J, Thankachan P, Suresh S, Shet A, Rao S, Ravikumar G, Thomas TS, Kurpad AV, Mandal AK. Beneficiary effect of nanosizing ferric pyrophosphate as food fortificant in iron deficiency anemia: evaluation of bioavailability, toxicity and plasma biomarker. RSC Advances. 2015;5:61678–61687. doi: 10.1039/C5RA07724A. [DOI] [Google Scholar]
- Sundararajan S, Rabe H. Prevention of iron deficiency anemia in infants and toddlers. Pediatric Research. 2021;89:63–73. doi: 10.1038/s41390-020-0907-5. [DOI] [PubMed] [Google Scholar]
- U.S. Code of Federal Regulations, Title 21, Section 73.1200 Synthetic iron oxide. Available at: https://www.ecfr.gov/current/title-21/chapter-I/subchapter-A/part-73/subpart-B/section-73.1200 Accessed May 23, 2023
- Vargas-Ortiz JR, Gonzalez C, Esquivel K. Magnetic iron nanoparticles: synthesis, surface enhancements, and biological challenges. Processes. 2022;10(11):2282. doi: 10.3390/pr10112282. [DOI] [Google Scholar]
- Von-Moos LM, Schneider M, Hilty FM, Hilbe M, Arnold M, Ziegler N, Mato DS, Winkler H, Tarik M, Ludwing C, Naegeli H, Langhans W, Zimmermann MB, Sturla SJ, Trantakis IA. Iron phosphate nanoparticles for food fortification: biological effects in rats and human cell lines. Nanotoxicology. 2017;11:496–506. doi: 10.1080/17435390.2017.1314035. [DOI] [PubMed] [Google Scholar]
- Voss L, Hsiao IL, Ebisch M, Vidmar J, Dreiack N, Böhmert L, Stock V, Braeuning A, Loeschner K, Laux P, Thünemann AF, Lampen A, Sieg H. The presence of iron oxide nanoparticles in the food pigment E172. Food Chemistry. 2020;327:127000. doi: 10.1016/j.foodchem.2020.127000. [DOI] [PubMed] [Google Scholar]
- WHO World Health Organization. (2010). International programme on chemical safety. Human health risk assessment toolkit: chemical hazards. IPCS harmonization project document; no.8. WHO Press, Geneva, pp 14-30
- Yang F, Zhang H, Shao Y, Song H, Liao S, Ren J. Formic acid as additive for the preparation of high-performance FePO4 materials by spray drying method. Ceramics International. 2017;43:16652–16658. doi: 10.1016/j.ceramint.2017.09.055. [DOI] [Google Scholar]
- Zhang X, Zhang L, Liu H, Cao B, Liu L, Gong W. Structure, morphology, size and application of iron phosphate. Reviews on Advanced Materials Science. 2020;59:538–552. doi: 10.1515/rams-2020-0039. [DOI] [Google Scholar]