Abstract
Disruption of the tumor extracellular matrix (ECM) may alter immune cell infiltration into the tumor and antitumor T cell priming in the tumor-draining lymph nodes (tdLNs). Here, we explore how intratumoral enzyme treatment (ET) of B16 melanoma tumors with ECM-depleting enzyme hyaluronidase alters adaptive and innate immune populations, including T cells, DCs, and macrophages, in the tumors and tdLNs. ET increased CD103+ DC abundance in the tdLNs, as well as antigen presentation of a model tumor antigen ovalbumin (OVA), eliciting local OVA-specific CD8+ T cell responses. Delivered in combination with a distant cryogel-based cancer vaccine, ET increased the systemic antigen-specific CD8+ T cell response. By enhancing activity within the tdLN, ET may broadly support immunotherapies in generating tumor-specific immunity.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00262-022-03212-6.
Keywords: Tumor-draining lymph nodes, Tumor extracellular matrix, Cancer vaccine, Adaptive immunity
Introduction
Tumor resident cells can facilitate pro-tumorigenic signaling, migration, and metastasis by remodeling the surrounding extracellular matrix (ECM) [1–3]. Tumor ECM can also serve as a barrier to drug delivery and immunotherapy by impeding diffusion, increasing solid stress and interstitial fluid pressure (IFP), and compressing blood and lymphatic vessels [4]. For example, physical restraints can hinder cytotoxic T cell entry into solid tumors, resulting in an immune-excluded or “cold” tumor mass with T cells confined to the outer margins [5–7].
Hyaluronic acid (HA) and collagen are two key ECM subtypes found in healthy and malignant tissues [8, 9]. HA is a glycosaminoglycan involved in homeostasis and aging, and can provide cells with both physical support and signaling cues [8]. However, HA can be overexpressed in tumors compared to healthy tissues, and heightened HA levels correlate with malignancy and worse prognosis in certain cancers [2, 8]. Additionally, HA’s chemical structure facilitates retention of water molecules, which can serve to raise IFP and resist drug delivery into tumors [4, 10]. Similarly, collagen serves as a major structural component of the tumor microenvironment and can support cancer progression, fibrosis, and therapeutic resistance [11].
Various approaches have been employed to target tumor HA and collagen to improve the delivery and efficacy of anticancer therapies. For instance, intratumoral hyaluronidase (HAase) injection reduced IFP of osteosarcoma and prostate cancer xenografts and improved penetration of chemotherapeutic agents [12, 13]. Likewise, intratumoral collagenase injection reduced IFP in several tumor models and increased access of drugs including nanoparticles and monoclonal antibodies [14–17]. Alternatively, vascular infusion of a recombinant PEGylated hyaluronidase (PEGPH20) led to ablation of intratumoral HA, reduced IFP in mouse models of pancreatic ductal adenocarcinoma (PDAC) and prostate cancer, and improved chemotherapy access and therapeutic response [18–20]. PEGPH20 has now been tested in human PDAC, which features a notoriously ECM-dense tumor microenvironment, and has demonstrated safety but variable efficacy [21, 22]. However, these strategies have primarily been employed for drug delivery, and their effects on local lymphoid organs remain largely unexplored.
Lymph nodes play an essential role in antitumor immunity, coordinating antigen presentation and T cell priming in the steady state and during immunotherapy [23]. In particular, tumor-draining lymph nodes (tdLNs), which receive tumor-derived antigens and cells, have been explored as immunotherapy targets to initiate adaptive tumor-specific immunity [24, 25].
Here we hypothesized that intratumoral enzyme treatment (ET) targeting HA and collagen could alter immune cell infiltration in tumors and improve antitumor T cell priming in tdLNs. We administered ET alone or in concert with two immunotherapies, namely adoptive T cell transfer and a biomaterial-based cancer vaccine [26]. Following ET, we assessed T cell infiltration in tumors, and immune cell populations, antigen presentation, and T cell responses in tdLNs. Although no differences were detected in T cell tumor infiltration, ET significantly expanded various immune cell types (e.g., antigen-presenting cells, antigen-specific CD8+ T cells) within tdLNs, and in combination with a cryogel vaccine-supported systemic T cell responses against a model tumor antigen.
Materials and methods
Materials
MVG ultrapure sodium alginate was purchased from NovaMatrix (Sandvika, Norway). 2-(N-Morpholino)ethanesulfonic acid hydrate (MES), N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (EDC), N-hydroxysuccinimide (NHS), N,N,N′,N′- tetramethylethylenediamine (TEMED), ammonium persulfate (APS), and hydroquinone were purchased from Sigma-Aldrich (Atlanta, GA). Hyaluronidase from bovine testes and collagenase from Clostridium histolyticum were purchased from MP Biomedicals (Irvine, CA) and Sigma-Aldrich (Atlanta, GA). Hyaluronidase and collagenase were tested for endotoxin (each < 0.1 EU/dose). 2-Aminoethyl methacrylate hydrochloride (AEMA) was purchased from Polysciences (Warrington, PA). GM-CSF was purchased from PeproTech (Rocky Hill, NJ). CpG-ODN 1826 5′-TCCATGACGTTCCTGACGTT-3′ was synthesized by Integrated DNA Technologies (Chicago, IL). OVA was purchased from InvivoGen (InvivoGen vac-pova, San Diego, CA).
Cell lines and animals
The B16-OVA cell line was obtained from Professor Glenn Dranoff’s Laboratory, Dana-Farber Cancer Institute, Boston, Massachusetts. B16-OVA and EG7-OVA cells were cultured in RPMI (Lonza) containing 10% FBS (Gibco) and 1 mg/mL geneticin (G418 antibiotic, Gibco). TUBO cells were cultured in DMEM containing 10% FBS (Gibco). C57BL/6 J or BALB/cJ female mice, aged 6–8 weeks to begin each experiment, were purchased from Jackson Laboratory (Bar Harbor, ME). OT1 mice (C57BL/6-Tg(TcraTcrb)1100Mjb/J) were purchased from Jackson Laboratory. Animal procedures were compliant with National Institutes of Health and Institutional guidelines and approved by Harvard University’s Institutional Animal Care and Use Committee.
Immunohistochemistry
Tumors and tdLNs were harvested, fixed in 1% paraformaldehyde, equilibrated with 30% sucrose and Tissue-Tek OCT (VWR), and frozen in OCT blocks. 15–20-μm sections were prepared using a cryostat (Leica CM1950) and stained using standard immunohistochemistry protocols. For imaging extracellular matrix, samples were blocked in 10% normal goat serum (Fitzgerald) and 1% bovine serum albumin in PBS and then stained with biotin-conjugated hyaluronic acid binding protein (1:50, EMD Millipore) and rabbit anti-mouse collagen I antibody (1:40, EMD Millipore) overnight. Sections were then washed and incubated with streptavidin-AF594 and donkey anti-rabbit AF594 (both 1:100, ThermoFisher). Stained sections were mounted with ProLong Gold Antifade Mountant with DAPI (Invitrogen) and imaged on a Zeiss 710 Confocal System. Hyaluronic acid and collagen signal intensities in tumor sections were quantified using ImageJ software. For imaging T cell and antigen-presenting cell infiltration, samples were blocked in 3% normal goat serum and 1% bovine serum albumin in PBS and then stained with AF647 anti-mouse CD3 (BioLegend) or AF488 anti-mouse I-A/I-E (MHCII, BioLegend) overnight. Sections were then washed and incubated with Hoechst 33342 (1:500, Thermo Scientific) and mounted with ProLong Gold Antifade reagent (Invitrogen). For lymph node ECM analysis, collagen VI was detected using the ER-TR7 antibody [27].
Rheological measurements
Rheological characterization was performed on a stress-controlled rheometer (Discovery HR-3 Hybrid Rheometer, TA Instruments) equipped with an 8-mm parallel plate. Tumor samples were punched by 7-mm biopsy punch before loading onto the rheometer. The sample gap was set between 800 and 2000 μm depending on the sample thickness. The storage modulus (Gʹ) of the tumor was measured by time sweep tests performed at 1 Hz and 1% constant strain at 25 °C [note: stiffness data were obtained and reported as storage modulus (Gʹ) instead of elastic modulus (E)].
Cryogel preparation and injection
MA-alginate was synthesized as previously described [28]. Cryogels were formed by dissolving MA-alginate to 15 mg/mL in DI water. When relevant, the liquid MA-alginate was added to lyophilized solutions of GM-CSF, CpG, and/or OVA, to load 1.8 μg GM-CSF, 70 μg CpG, and 450 μg OVA per gel. This solution was mixed with TEMED (0.5% wt/vol) and stored at 4 °C for 10 min before adding APS (0.25% wt/vol). The alginate solution was rapidly pipetted into cold (− 20 °C) Teflon molds and placed in a − 20 °C freezer overnight. The next day, cryogels were thawed, placed into the head of a 16G needle, and attached to a 1-mL syringe loaded with 250 μL saline (0.9% NaCl in DI water) for injection. Mice were injected with two gels in the inguinal region above the hindlimb.
Tissue preparation for flow cytometry
Harvested lymph nodes were mechanically digested in RMPI containing 250 U/mL collagenase and filtered to obtain a single-cell suspension. Spleens were disrupted manually over a 70-μm filter while washing with PBS, and red blood cells were lysed using ACK lysis buffer (Lonza). Tumors were harvested and mechanically digested in RMPI containing 150 U/mL collagenase IV (Worthington), 50 U/mL hyaluronidase (MP Biomedicals), 20 U/mL DNase I (Sigma), and 2% FBS (Gibco). Red blood cells were lysed and the solution filtered to obtain a single-cell suspension.
OT1 adoptive transfer experiments
4 × 105 B16-OVA or 1 × 106 EG7-OVA cells in cold PBS were injected subcutaneously into C57BL/6J mice. 13 or 8 days later, respectively, once tumors measured ~ 5 mm in both dimensions, 107 CFSE-labeled OT1 cells were injected intravenously via the tail vein. OT1 CD8+ T cells were isolated from the spleens of OT1 mice using a CD8a mouse MACS isolation kit (Miltenyi Biotec), cultured in T cell media (RMPI 1640 (Lonza) containing 2 mM L-glutamine, 10% FBS, 1% penicillin/ streptomycin, 55 μm beta-mercaptoethanol, 1× non-essential amino acids, 1 mM sodium pyruvate, and 10 mL HEPES free acid supplemented with murine IL-2 (BioLegend), and expanded on APC-mimetic scaffolds as previously reported [29, 30]. Isolated and cultured OT1 cells remained highly viable (> 99%) and pure (> 99% CD3+ and CD8+) after 11 days of culture prior to injection. For HAase treatment, 780 U hyaluronidase was injected intratumorally in 100 μL PBS (timeline in Supplementary Fig. 4a, b). At endpoint, tumors and tumor-draining lymph nodes were collected and processed to obtain a single-cell suspension. Cells were then stained for flow cytometry.
Enzyme treatment experiments in B16 models
3 × 105 B16-OVA or B16-mCherry cells in 100 μL cold PBS were injected subcutaneously into mice. When relevant, cryogel vaccines containing GM-CSF and CpG with or without OVA were injected subcutaneously at the inguinal region (2×/mouse) above the hindlimb on day 6. At day 13, once tumors measured ~ 5 mm in both dimensions, mice were injected intratumorally with PBS or a mixture of ~ 1800 U hyaluronidase and 0.25 mg collagenase (~ 41 U) in 50μL PBS. Mice were injected twice more on days 14 and 15, and tumors were harvested on day 16 (timeline depicted in Fig. 2e, Supplementary Fig. 4a).
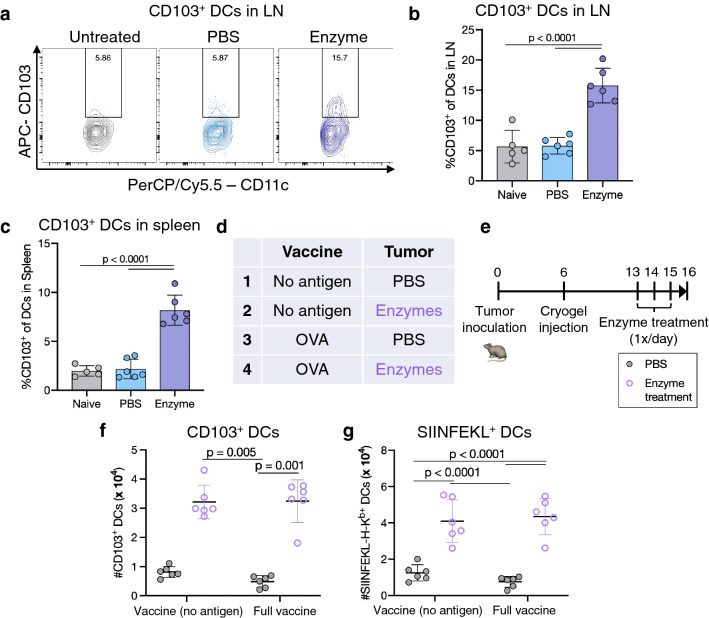
Fig. 2.
Enzyme treatment enhances tumor antigen presentation in secondary lymphoid organs. a Representative flow cytometry plots of CD103 expression on DCs (CD11c+MHCII+) in tdLNs. b Percentage of DCs expressing CD103 in tdLNs. c Percentage of DCs expressing CD103 in spleens. d Overview of experimental conditions and e timeline. f Number of CD11c+ DCs expressing CD103 in tdLNs. g Number of SIINFEKL-presenting CD11c+ DCs in tdLNs. In a–c, tdLNs and spleens were collected from mice bearing B16-mCherry tumors treated with ET or PBS, or naïve mice without tumors (non-tdLN). In d–g, B16-OVA tumor-bearing mice were injected with vaccine cryogels with or without OVA antigen, administered intratumoral enzyme or PBS treatment, and tdLNs and spleens collected. Data are depicted mean ± SD. For b, c, n = 5 (untreated) or 6 (PBS or enzyme-treated) biologically independent animals per group; statistical analysis was performed using ANOVA with Tukey’s post hoc test. For f, g, n = 6 biologically independent animals per group; statistical analysis was performed using a Kruskal–Wallis test with Dunn’s post hoc test (f) or ANOVA with Tukey’s post hoc test (g)
Assessment of T cell polyfunctionality
Single-cell suspensions from lymph nodes and spleens were incubated with 2ug/mL OVA257–264 SIINFEKL peptide for 1.5 h at 37 °C before adding GolgiStop (BD Biosciences) and incubating for another 4 h at 37 °C. Samples were stained with fixable viability dye and anti-mouse CD3 and CD8 antibodies before fixation and permeabilization using a Cytofix/Cytoperm kit (BD Biosciences) and intracellular staining with anti-mouse IFN-γ, IL-2, and TNF-α antibodies. Samples were analyzed using flow cytometry to identify populations of cells expressing or co-expressing these intracellular cytokines.
Flow cytometry
Primary antibodies included PerCP/Cy5.5-conjugated anti-CD11c, allophycocyanin (APC)-conjugated anti-CD11c, APC-conjugated anti-CD103, Pacific Blue-conjugated anti-CD103, fluorescein isothiocyanate (FITC)-conjugated anti-MHCII, PE/Cy7-conjugated anti-MHCII, Pacific Blue-conjugated anti-CD11b, FITC-conjugated anti-CD11b, Pacific Blue-conjugated anti-CD3, FITC-conjugated anti-CD8α, PerCP/Cy5.5-conjugated anti-PD-1, APC-conjugated anti-PD-1, PerCP/Cy5.5-conjugated anti-PD-L1, phycoerythrin (PE)-conjugated anti-LAG-3, APC-conjugated anti-IFN-γ, PE-conjugated anti-IL-2, PE/Cy7-conjugated anti-TNF-α, PE/Cy7-conjugated anti-CD45, PE-conjugated anti-SIINFEKL-H-2Kb, PerCP/Cy5.5-conjugated anti-F4/80, PE-conjugated anti-F4/80, PE/Cy7-conjugated anti-CD4, PE-conjugated anti-CD86, PE-conjugated anti-FoxP3, PerCP/Cy5.5-conjugated anti-CD25, APC-conjugated anti-Gr-1, all purchased from BioLegend (San Diego, CA), and diluted per manufacturer recommendations. Cell viability was assessed through eBioscience Fixable Viability Dye eFluor 780 (ThermoFisher) or 7AAD stain (Invitrogen). APC-conjugated H-2Kb-SIINFEKL tetramer was obtained from the National Institutes of Health tetramer core facility (Emory University). Samples were run on BD LSRII or LSR Fortessa (San Jose, CA) flow cytometers and analyzed on FlowJo v7 and v10 software.
Statistical analyses
Statistical analyses were performed using GraphPad Prism v8 software. For normally distributed samples, the significance between two groups was analyzed by a two-tailed Student’s t test, or a Mann–Whitney U test otherwise. For multiple comparisons, normally distributed samples were compared using a one-way analysis of variance (ANOVA) with Tukey’s post hoc test or a Kruskal–Wallis test with Dunn’s post hoc test otherwise. All data are depicted as mean ± SD. Sample sizes of 4–6 biologically independent animals per group were used for in vivo studies, determined empirically based on results from prior publications along with approval from Harvard University’s Institutional Animal Care and Use Committee.
Results
Enzyme treatment does not alter tumor T cell infiltration
We first explored whether disrupting tumor HA could support T cell entry in concert with immunotherapy, potentially by ameliorating physical barriers to infiltration. B16-OVA tumors display a heterogeneous ECM architecture, and intratumoral injection of the ECM-depleting enzymes HAase and collagenase altered the matrix distribution, with HA significantly depleted, but not collagen (Supplementary Fig. 1a–d). Bulk tumor stiffness was variable among PBS-treated control tumors and was not significantly altered by enzyme treatment (Supplementary Fig. 1e). TUBO breast tumors also displayed heterogeneous ECM and, when treated with HAase, demonstrated changes in matrix density and distribution compared to a PBS injection (Supplementary Fig. 2a, b).
ECM disruption was next performed in the contexts of adoptive cell transfer and cancer vaccination to explore its impact on cell infiltration into the tumor. Two tumor models expressing ovalbumin (OVA) were treated with intratumoral HAase or PBS followed by adoptive transfer of CFSE-labeled OT-1 (OVA-specific CD8+) T cells (Supplementary Fig. 3a, b). At endpoint, no differences in total T cell, CD8+ T cell, or CFSE+ T cell numbers were observed with HAase treatment relative to PBS in either B16-OVA or EG7-OVA tumors (Supplementary Fig. 3c). Over the short treatment course, HAase appeared to support OT1 transfer in regressing B16-OVA but not EG7-OVA tumors prior to the experimental endpoint, although the longevity of this response was not tracked (Supplementary Fig. 3d, e). Because collagen is another key tumor ECM molecule that could affect T cell infiltration, the enzyme treatment (ET) was next expanded to include both HAase and collagenase in the B16-OVA model. To elicit OVA-specific T cells, animals were treated with a cryogel vaccine containing granulocyte–macrophage colony-stimulating factor (GM-CSF), CpG-ODN (CpG), and OVA antigen and administered ET (Supplementary Fig. 4a). Cryogel-based vaccines have previously been employed as scaffolds to deliver immunomodulatory components and provide a niche for immune cell activation, thus generating robust T cell responses [26, 31]. Again, no differences in tumor T cell infiltration were observed compared to intratumoral injection of PBS (Supplementary Fig. 4b, c). Enzyme treatment did not noticeably alter intratumoral distribution of T cells or antigen-presenting cells, which were mainly found on the tumor periphery, although improved localization of MHCII+ antigen-presenting cells in the tumor interior was observed in some cases (Supplementary Fig. 5).
Enzyme treatment expands immune populations in tumor-draining lymph nodes
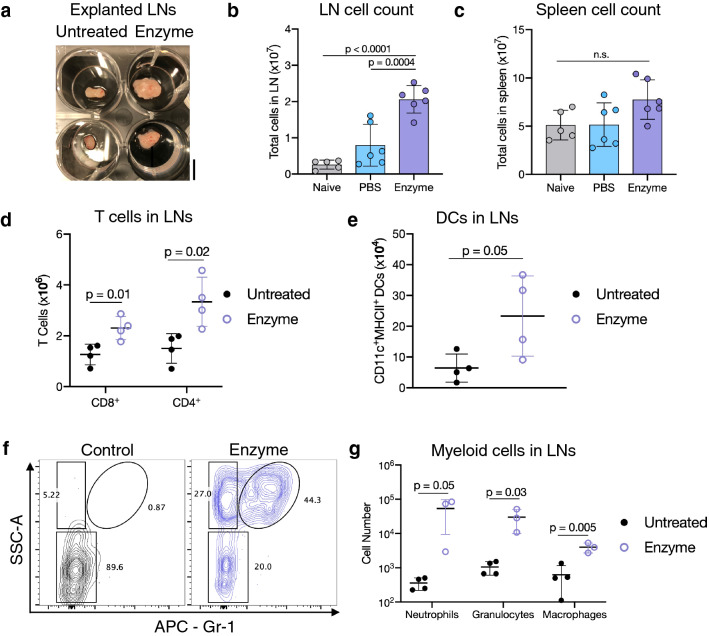
The effects of enzyme delivery on the tdLNs were next explored. TdLNs isolated from enzyme-treated mice increased in volume (Fig. 1a). Compared to naïve (tumor-free) mice or tumor-bearing mice injected with PBS, intratumoral ET increased B16-mCherry tdLN cell counts but did not significantly alter spleen cell count (Fig. 1b, c). Flow cytometric analysis revealed the total CD8+ and CD4+ T cell counts in B16-OVA tdLNs from the ACT study were significantly increased with ET, as were dendritic cells (DCs, Fig. 1d, e). Myeloid cell populations in tdLNs were also affected, with increases in macrophages, neutrophils, and other granulocytes (Fig. 1f, g). These broad changes were mimicked in tdLNs from the EG7-OVA ACT study, which also displayed increased numbers of tdLN total cells, CD8+ T cells, DCs, and myeloid cells (Supplementary Fig. 6a–d).
Fig. 1.
Tumor enzyme treatment expands immune cell populations in melanoma tumor-draining lymph nodes. a Image of untreated or enzyme-treated tdLNs; scale bar = 5 mm. b Total cell count in tdLNs. c Total cell count in spleens. No significant differences were found between groups. d CD8+ and CD4+ T cell numbers in tdLNs. e DC numbers (CD11c+MHCII+) in tdLNs. f Representative flow cytometry plots of myeloid cells within tdLNs, gated on CD45+CD11b+ live cells. g Numbers of neutrophils (CD11b+Gr-1+SSC-Ahi), granulocytes (CD11b+Gr-1−SSC-Ahi), and macrophages (CD11b+F4/80+Gr-1−) within tdLNs. In a and d–g, mice bearing B16-OVA tumors received intratumoral hyaluronidase (enzyme) or no injection (untreated) along with adoptive transfer of OT1 cells, and tdLNs were collected. In b, c, tdLNs and spleens were collected from mice bearing B16-mCherry tumors treated with ET or PBS, or naïve mice without tumors (non-tdLN). Data are depicted mean ± SD. For b, c, n = 5 (untreated) or 6 (PBS or enzyme-treated) biologically independent animals per group; statistical analysis was performed using analysis of variance (ANOVA) with Tukey’s post hoc test. For d, e and g, n = 3–4 biologically independent animals per group; statistical analyses were performed using two-tailed t tests
To determine whether intratumoral ET affected ECM or lymphocyte distribution within the lymph nodes, tdLNs were collected and imaged (Supplementary Fig. 7a). Consistent with increased cell counts, tdLNs were significantly enlarged in mice receiving ET (Supplementary Fig. 7b). ET did not affect patterns of collagen I or VI distribution within tdLNs compared to PBS treatment, although the HA signal was more visible in the ET group (Supplementary Fig. 7c, d). Lymphocyte organization within tdLNs was maintained after ET (Supplementary Fig. 8).
Enzyme treatment supports tumor antigen presentation in lymphoid organs
Studies were next performed to determine whether intratumoral ET enhances the drainage and/or trafficking of tumor antigens to the tdLN to improve antitumor T cell priming. CD103-expressing migratory conventional DCs play a critical role in tumor-to-tdLN antigen trafficking [32, 33], and both the proportion and number of CD103+ DCs were significantly increased in tdLNs of B16-mCherry-bearing mice receiving ET, relative to PBS-treated mice or naïve, tumor-free mice (Fig. 2a, b, Supplementary Fig. 9a). A similar effect was observed in the spleens, although total spleen cell numbers were not affected (Fig. 2c, Supplementary Fig. 9b, c). Importantly, the number of mCherry-expressing cells in tdLNs was consistent between conditions, suggesting that ET did not facilitate tumor cell metastasis to the sentinel lymph node (Supplementary Fig. 9d).
To probe presentation of a specific tumor-expressed antigen, we assessed MHC-I presentation of the ovalbumin SIINFEKL epitope in tdLNs downstream of B16-OVA tumors. We also tested whether cryogel vaccination against OVA at a distant site (i.e., not draining to the tdLN) could affect OVA presentation in the tdLN and/or synergize with ET (Supplementary Fig. 10). Mice were immunized with cryogel vaccines containing GM-CSF, CpG, and either OVA or no antigen (OVA would derive solely from the tumor in the latter case) and treated with intratumoral ET or PBS beginning one week later (Fig. 2d, e). As previously observed, enzyme treatment expanded CD103+ DCs in the tdLN (Fig. 2f). Interestingly, SIINFEKL-expressing DCs were significantly expanded in tdLNs of enzyme-treated tumors, regardless of whether the distant cryogel vaccine contained OVA (Fig. 2g). TdLN DCs also exhibited an increase in PD-L1 expression (Supplementary Fig. 11).
Enzyme treatment improves antigen-specific T cell response in the tdLN
We next assessed whether antigen-specific T cell responses were impacted by ET, using the same experimental setup as the antigen presentation study (Fig. 2d, e). We observed increased total numbers of CD8+ T cells in tdLNs of enzyme-treated tumors (Fig. 3a). Notably, the number of OVA-tetramer+ CD8+ T cells was significantly increased with ET, independent of the presence of OVA in the distant cryogel vaccine (Fig. 3b). To further assess CD8+ T cell antigen specificity and functionality, we investigated intracellular expression of the cytokines IFN-γ, IL-2, and TNF-α after restimulation of tdLN-derived cells with the OVA SIINFEKL peptide. These cytokines are involved in CD8+ T cell cytolysis and proliferation, and their expression has previously been used to indicate T cell polyfunctionality [34, 35]. CD8+ T cells from tdLNs of enzyme-treated mice demonstrated increased expression of IFN-γ, IL-2, and TNF-α both individually and through co-expression, indicating increased polyfunctionality (Fig. 3c, d, Supplementary Fig. 12a–c). Around 3.5% of CD8+ T cells in ET tdLNs expressed at least one of these cytokines, compared to 1.3% with PBS-injected tumors. The proportion of CD8+ T cells expressing multiple (at least two) cytokines was also significantly enhanced with enzyme treatment, irrespective of the presence of antigen in the vaccine. No difference in PD-1 expression on CD8+ T cells in the tdLN was found between groups, although a trend toward an increase in LAG-3 expression was observed with ET (Supplementary Fig. 13a, b).
Fig. 3.
Enzyme treatment improves antigen-specific T cell responses in the tumor-draining lymph node. B16-OVA tumor-bearing mice were injected with vaccine cryogels with or without OVA antigen, administered intratumoral enzyme or PBS treatment, and tdLNs collected. a Number of CD8+ T cells in tdLNs. b Number of OVA-tetramer+ CD8+ T cells in tdLNs. c Pie charts depicting the percentage of CD8+ T cells expressing 0–3 of the cytokines IFN-γ, IL-2, or TNF-α in each group following restimulation with SIINFEKL peptide. Blue represents T cells with no detectable cytokine expression, orange represents cells expressing a single cytokine, gray represents two cytokines, and yellow represents cells expressing all three cytokines. Higher-magnification view of the cytokine positive cells shown to the right of each pie chart. d Quantification of T cell polyfunctionality through the percentage of CD8+ T cells expressing two or more of the cytokines IFN-γ, IL-2, or TNF-α. Data are depicted mean ± SD; n = 6 biologically independent animals per group. Statistical analysis was performed using a Kruskal–Wallis test with Dunn’s post hoc test (a, b) or ANOVA with Tukey’s post hoc test (d)
Enzyme treatment synergizes with cryogel vaccine for systemic antigen-specific immune responses
Finally, systemic antigen-specific T cell responses were examined via analysis of the T cell compartment in the spleen. The proportion of SIINFEKL-tetramer+ CD8+ T cells was elevated only with the combination of ET and the full cryogel vaccine (Fig. 4a). This result was consistent with cytokine secretion after T cell restimulation with SIINFEKL peptide, as only the ET group with the OVA-containing vaccine cryogel increased IFN-γ and IL-2-expressing cells (Fig. 4b–d). Expression of TNF-α was not significantly increased in any group (Supplementary Fig. 14). Approximately 4.4% of CD8+ T cells in the ET + full vaccine group expressed at least one cytokine (among IFN-γ, IL-2, and TNF-α), compared to 1.6–2.2% in the other groups (Fig. 4e). A significantly elevated proportion of CD8+ T cells in the combination group also expressed two or more of these cytokines (Fig. 4f). CD8+ T cells from spleens of these mice also increased in PD-1 expression (Fig. 4g). Consistent with systemic immune activation, splenic macrophages increased in inflammatory phenotype (CD86 expression) only with the combination of the full cryogel vaccine with ET (Supplementary Fig. 15a, b). Broadly, no differences in tumor immune cell populations (all leukocytes or myeloid, NK, or dendritic cells) were observed (Supplementary Fig. 16).
Fig. 4.
Enzyme treatment synergizes with cryogel vaccination to elicit systemic antigen-specific immune responses. B16-OVA tumor-bearing mice were injected with vaccine cryogels with or without OVA antigen, administered intratumoral enzyme or PBS treatment, and spleens collected. a Percentage of CD8+ T cells binding OVA tetramer in spleens. b Representative flow cytometry plots of IFN-γ (top) and IL-2 expression (bottom) in CD8+ T cells in spleens following OVA peptide restimulation. c Percentage of CD8+ T cells expressing IFN-γ in spleens. d Percentage of CD8+ T cells expressing IL-2 in spleens. e Pie charts depicting the percentage of CD8+ T cells expressing 0–3 of the cytokines IFN-γ, IL-2, or TNF-α in each group following OVA peptide restimulation. Blue represents T cells with no detectable cytokine expression, orange represents cells expressing a single cytokine, gray represents two cytokines, and yellow represents cells expressing all three cytokines. Higher-magnification view of the cytokine positive cells shown to the right of each pie chart. f Quantification of T cell polyfunctionality through the percentage of CD8+ T cells expressing two or more of the cytokines IFN-γ, IL-2, or TNF-α. g Percentage of CD8+ T cells expressing PD-1 in spleens. Data are depicted mean ± SD; n = 6 biologically independent animals per group. Statistical analysis was performed using a Kruskal–Wallis test with Dunn’s post hoc test (a, c, d, f) or ANOVA with Tukey’s post hoc test (g)
Discussion
HA and collagen were detected heterogeneously in B16-OVA tumors and displayed changes in content and localization after ET. Previously, the B16 melanoma line was found to form tumors with low HA content relative to other tumor types, which may have contributed to our observed variability [10]. However, ECM targeting strategies were effective in treating syngeneic and xenograft mouse melanoma models, including the B16 line, supporting its use in our work [36–39]. In our studies, tumor stiffness was not altered by enzyme treatment; however, treated tumors presented a more uniform stiffness distribution. In future studies of ET, the choice of cancer model will likely be important, as would proposed clinical settings. For example, ECM targeting may be relevant to desmoplastic melanoma, a subtype featuring fibrosis and collagen deposition [40]. The melanoma tumors explored here are relatively soft (stiffness mean ~ 0.3 kPa) and heterogeneous, and it is possible that stiffer tumor models such as breast cancer (reported stiffness ~ 1–10 kPa) would demonstrate more apparent changes with ET [41, 42]. Relative to the bulk rheology employed here, techniques such as nanoindentation [41] or atomic force microscopy [42] could allow for a more granular understanding of tissue mechanics after HA degradation. Additional strategies to assess the extent of tumor ECM disruption in different tumor models such as by characterizing mesh size, fiber thickness, and collagen crosslinking would support the development of this approach.
We found that ET, as administered here, did not increase intratumoral T cell infiltration. Adoptively transferred OT1 cell numbers did not differ between ET or PBS-treated B16-OVA or EG7-OVA tumors, and T cell levels in B16-OVA tumors were comparable after ET or PBS with an OVA-containing cryogel vaccine. It is possible that while the total numbers of T cells within tumors did not change, their relative localization (i.e., tumor center versus periphery) could have been altered, a potentially meaningful alteration in immune-excluded tumors [5]. However, immunohistochemistry/IHC analysis did not reveal consistent improvements in intratumoral T cell distribution after enzyme treatment. Flow cytometry and IHC analysis of myeloid and antigen-presenting cells in B16-OVA tumors also did not demonstrate consistent alterations. Alternative strategies to improve T cell infiltration, such as engineering CAR-T cells or oncolytic viruses to secrete HAase, may prove more effective at enhancing infiltration at a cellular level [43, 44]. Furthermore, adjusting the relative timing of ET and immunotherapy could affect T cell infiltration. Broadly, we conclude that targeting tumor HA through our methodology did not facilitate T cell tumor entry.
In the tdLN, ET elicited robust expansion of both adaptive and innate immune cells, including T cells, DCs, and macrophages. ET expanded CD8+ T cells without altering their PD-1 expression, but also expanded CD11b+Gr-1+ cells, associated with a myeloid-derived suppressor cell phenotype, and increased PD-L1 expression on DCs. These results are consistent with prior reports that tumor-derived inflammatory factors can play competing activating or suppressive roles in the tdLN [25, 45, 46]. Additionally, HA degradation products (i.e., low molecular weight HA) are associated with pro-inflammatory signaling and immune cell activation, can signal LN-resident immune or stromal cells, and could prompt immune expansion in the tdLN [8, 47, 48]. A trend toward a higher HA signal in enzyme-treated tdLNs could imply enhanced drainage of tumor-derived HA to the tdLN, where it could signal resident cells. However, the contribution of HA degradation products to the observed LN response remains to be elucidated. Targeting alternative molecules (e.g., laminin, fibronectin) using other enzymes (e.g., various matrix metalloproteinases) could uncover whether the observed response to ET is HA-specific or could be reproduced by disrupting the tumor ECM in an alternative manner. It is also possible that ET enzymes themselves drained to the tdLN. We found that tdLN collagen networks were maintained after enzyme treatment, important for efficient antigen trafficking. Similarly, T and B cell organization was maintained in the typical pattern in tdLNs after enzyme treatment, suggesting that ET did not disrupt tdLN cellular localization. Broadly, the observation that ET increased tdLN but not spleen cell numbers indicates that a local mechanism, likely based on the tumor-draining lymphatics, was responsible for the increases in immune cell numbers observed.
ET played an important role in generating both local and systemic immune responses to a model tumor antigen (OVA), in combination with a distant cryogel vaccine. ET mobilized CD103+ DCs trafficking to the tdLN and spleen, and expanded SIINFEKL-expressing DCs and OVA-specific CD8+ T cells in B16-OVA tdLNs. These changes occurred irrespective of the presence of antigen in the distant vaccine, likely because minimal vaccine-site drainage reached the tdLN, suggesting that ET induced drainage or trafficking of tumor antigens from the tumor to local lymphoid organs. Thus, we expect that ET itself, not the vaccine, was responsible for increasing antigen-specific CD8+ T cells locally in the tdLN. Because cryogel vaccines have previously been shown to elicit T cell responses in local LNs, we predict that an examination of the vaccine-draining LNs, which were distant from the tumor site and not considered in these studies, would likely demonstrate the opposite result [26]. Systemically, only the combination of ET with the full cryogel vaccine elevated splenic OVA-specific CD8+ T cell responses and activated macrophages (CD86 expression). These results are consistent with prior reports of splenic antigen-presenting cell activation by cryogel-based cancer vaccines, although here the T cell response initiated by the cryogel vaccine alone was weaker than previously observed [26, 31]. Taken together, these results highlight the location specificity of draining LNs and the importance of combination therapies in generating systemic antitumor immunity.
Importantly, tumor ECM depletion through ET did not facilitate sentinel LN metastasis or tumor progression in these studies. In experiments where tumor size was measured, ET either slowed or did not affect tumor growth. Furthermore, the use of an mCherry-expressing B16 model confirmed that ET did not alter the number of tdLN mCherry+ (tumor) cells. During tumor progression, cancer cells remodel their surrounding ECM to support migration and metastasis, and previous therapeutic interventions targeting tumor stroma (e.g., sonic hedgehog signaling, myofibroblasts) actually increased PDA invasiveness [1, 3, 49, 50]. However, our results suggest that the therapeutic regimen used for ET here did not promote cancer progression in the tumor models and timescale investigated.
In summary, intratumoral injection of ECM-depleting enzymes expanded various immune cell subsets within the tdLN, increased presentation of a model tumor antigen, and heightened antigen-specific T cell responses both locally and, in combination with a cryogel-based cancer vaccine, systemically. These findings support the relevance of targeting architectural barriers in the tumor microenvironment to support cancer immunotherapy and drug delivery.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contributions
AJN, TS, DKYZ, JL, MOD, KAB, and DJM designed the experiments. AJN, TS, DKYZ, JL, MCS, and HW performed the experiments. AJN and DJM analyzed the data and wrote the manuscript.
Funding
This work is supported by the National Institutes of Health (R01 EB015498, R01 DK098055, and R01 CA223255) and the Wyss Institute for Biologically Inspired Engineering. A.J.N. acknowledges a Graduate Research Fellowship from the National Science Foundation.
Data availability
All data represented in this manuscript will be made available from the corresponding authors upon reasonable request.
Code availability
Not applicable.
Declarations
Conflict of interest
D.J.M. declares the following competing interests: Novartis, sponsored research, licensed IP; Immulus, equity; IVIVA, SAB; Attivare, SAB, equity; Lyell, licensed IP, equity. The other authors declare no competing interests.
Ethics approval
Animal procedures were compliant with National Institutes of Health and Institutional guidelines and approved by Harvard University’s Institutional Animal Care and Use Committee (protocol 24-16).
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Winkler J, Abisoye-Ogunniyan A, Metcalf KJ, Werb Z. Concepts of extracellular matrix remodelling in tumour progression and metastasis. Nat Commun. 2020;11:1–19. doi: 10.1038/s41467-020-18794-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Toole BP. Hyaluronan: from extracellular glue to pericellular cue. Nat Rev Cancer. 2004;4:528–539. doi: 10.1038/nrc1391. [DOI] [PubMed] [Google Scholar]
- 3.Leight JL, Drain AP, Weaver VM. Extracellular matrix remodeling and stiffening modulate tumor phenotype and treatment response. Annu Rev Cancer Biol. 2017;1:313–334. doi: 10.1146/annurev-cancerbio-050216-034431. [DOI] [Google Scholar]
- 4.Jain RK, Martin JD, Stylianopoulos T. The role of mechanical forces in tumor growth and therapy. Annu Rev Biomed Eng. 2014;16:321–346. doi: 10.1146/annurev-bioeng-071813-105259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Binnewies M, Roberts EW, Kersten K, et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat Med. 2018;24:541–550. doi: 10.1038/s41591-018-0014-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hegde PS, Chen DS. Top 10 challenges in cancer immunotherapy. Immunity. 2020;52:17–35. doi: 10.1016/j.immuni.2019.12.011. [DOI] [PubMed] [Google Scholar]
- 7.Rafiq S, Hackett CS, Brentjens RJ. Engineering strategies to overcome the current roadblocks in CAR T cell therapy. Nat Rev Clin Oncol. 2020;17:147–167. doi: 10.1038/s41571-019-0297-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bohaumilitzky L, Huber AK, Stork EM, et al. A trickster in disguise: Hyaluronan’s ambivalent roles in the matrix. Front Oncol. 2017 doi: 10.3389/fonc.2017.00242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee-Sayer SSM, Dong Y, Arif AA, et al. The where, when, how and why of hyaluronan binding by immune cells. Front Immunol. 2015;6:150. doi: 10.3389/fimmu.2015.00150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dufort CC, DelGiorno KE, Carlson MA, et al. Interstitial pressure in pancreatic ductal adenocarcinoma is dominated by a gel-fluid phase. Biophys J. 2016;110:2106–2119. doi: 10.1016/j.bpj.2016.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu S, Xu H, Wang W, et al. The role of collagen in cancer: from bench to bedside. J Transl Med. 2019;17:1–22. doi: 10.1186/s12967-019-2058-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brekken C, De Lange DC. Hyaluronidase reduces the interstitial fluid pressure in solid tumours in a non-linear concentration-dependent manner. Cancer Lett. 1998;131:65–70. doi: 10.1016/S0304-3835(98)00202-X. [DOI] [PubMed] [Google Scholar]
- 13.Eikenes L, Tari M, Tufto I, et al. Hyaluronidase induces a transcapillary pressure gradient and improves the distribution and uptake of liposomal doxorubicin (Caelyx™) in human osteosarcoma xenografts. Br J Cancer. 2005;93:81–88. doi: 10.1038/sj.bjc.6602626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eikenes L, Bruland ØS, Brekken C, De Lange DC. Collagenase increases the transcapillary pressure gradient and improves the uptake and distribution of monoclonal antibodies in human osteosarcoma xenografts. Cancer Res. 2004;64:4768–4773. doi: 10.1158/0008-5472.CAN-03-1472. [DOI] [PubMed] [Google Scholar]
- 15.Kato M, Hattori Y, Kubo M, Maitani Y. Collagenase-1 injection improved tumor distribution and gene expression of cationic lipoplex. Int J Pharm. 2012;423:428–434. doi: 10.1016/j.ijpharm.2011.12.015. [DOI] [PubMed] [Google Scholar]
- 16.Lee S, Han H, Koo H, et al. Extracellular matrix remodeling in vivo for enhancing tumor-targeting efficiency of nanoparticle drug carriers using the pulsed high intensity focused ultrasound. J Control Release. 2017;263:68–78. doi: 10.1016/j.jconrel.2017.02.035. [DOI] [PubMed] [Google Scholar]
- 17.Zheng X, Goins BA, Cameron IL, et al. Ultrasound-guided intratumoral administration of collagenase-2 improved liposome drug accumulation in solid tumor xenografts. Cancer Chemother Pharmacol. 2011;67:173–182. doi: 10.1007/s00280-010-1305-1. [DOI] [PubMed] [Google Scholar]
- 18.Provenzano PP, Cuevas C, Chang AE, et al. Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell. 2012;21:418–429. doi: 10.1016/j.ccr.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thompson CB, Shepard HM, O’Connor PM, et al. Enzymatic depletion of tumor hyaluronan induces antitumor responses in preclinical animal models. Mol Cancer Ther. 2010;9:3052–3064. doi: 10.1158/1535-7163.MCT-10-0470. [DOI] [PubMed] [Google Scholar]
- 20.Jacobetz MA, Chan DS, Neesse A, et al. Hyaluronan impairs vascular function and drug delivery in a mouse model of pancreatic cancer. Gut. 2013;62:112–120. doi: 10.1136/gutjnl-2012-302529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hingorani SR, Zheng L, Bullock AJ, et al. HALO 202: randomized phase II study of PEGPH20 plus nab-paclitaxel/gemcitabine versus nab-paclitaxel/gemcitabine in patients with untreated, metastatic pancreatic ductal adenocarcinoma. J Clin Oncol. 2018;36:359–366. doi: 10.1200/JCO.2017.74.9564. [DOI] [PubMed] [Google Scholar]
- 22.Van Cutsem E, Tempero MA, Sigal D, et al. Randomized phase III trial of pegvorhyaluronidase alfa with nab-paclitaxel plus gemcitabine for patients with hyaluronan-high metastatic pancreatic adenocarcinoma. J Clin Oncol. 2020;38:3185–3194. doi: 10.1200/JCO.20.00590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39:1–10. doi: 10.1016/j.immuni.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 24.Najibi AJ, Mooney DJ. Cell and tissue engineering in lymph nodes for cancer immunotherapy. Adv Drug Deliv Rev. 2020;161–162:42–62. doi: 10.1016/j.addr.2020.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rotman J, Koster BD, Jordanova ES, et al. Unlocking the therapeutic potential of primary tumor-draining lymph nodes. Cancer Immunol Immunother. 2019;68:1681–1688. doi: 10.1007/s00262-019-02330-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bencherif SA, Sands RW, Ali OA, et al. Injectable cryogel-based whole-cell cancer vaccines. Nat Commun. 2015;6:1–13. doi: 10.1038/ncomms8556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schiavinato A, Przyklenk M, Kobbe B, et al. Collagen type VI is the antigen recognized by the ER-TR7 antibody. Eur J Immunol. 2021;51:2345–2347. doi: 10.1002/eji.202149263. [DOI] [PubMed] [Google Scholar]
- 28.Shih TY, Blacklow SO, Li AW, et al. Injectable, tough alginate cryogels as cancer vaccines. Adv Healthc Mater. 2018;7:1–13. doi: 10.1002/adhm.201701469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cheung AS, Zhang DKY, Koshy ST, Mooney DJ. Scaffolds that mimic antigen-presenting cells enable ex vivo expansion of primary T cells. Nat Biotechnol. 2018;36:160–169. doi: 10.1038/nbt.4047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang DKY, Cheung AS, Mooney DJ. Activation and expansion of human T cells using artificial antigen-presenting cell scaffolds. Nat Protoc. 2020 doi: 10.1038/s41596-019-0249-0. [DOI] [PubMed] [Google Scholar]
- 31.Shah NJ, Najibi AJ, Shih TY, et al. A biomaterial-based vaccine eliciting durable tumour-specific responses against acute myeloid leukaemia. Nat Biomed Eng. 2020 doi: 10.1038/s41551-019-0503-3. [DOI] [PubMed] [Google Scholar]
- 32.Roberts EW, Broz ML, Binnewies M, et al. Critical role for CD103+/CD141+ dendritic cells bearing CCR7 for tumor antigen trafficking and priming of T cell immunity in melanoma. Cancer Cell. 2016;30:324–336. doi: 10.1016/j.ccell.2016.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Salmon H, Idoyaga J, Rahman A, et al. Expansion and activation of CD103+ dendritic cell progenitors at the tumor site enhances tumor responses to therapeutic PD-L1 and BRAF inhibition. Immunity. 2016;44:924–938. doi: 10.1016/j.immuni.2016.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Egelston CA, Avalos C, Tu TY, et al. Human breast tumor-infiltrating CD8+ T cells retain polyfunctionality despite PD-1 expression. Nat Commun. 2018;9:1–11. doi: 10.1038/s41467-018-06653-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Larsen M, Sauce D, Arnaud L, et al. Evaluating cellular polyfunctionality with a novel polyfunctionality index. PLoS ONE. 2012;7:1–10. doi: 10.1371/journal.pone.0042403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Magzoub M, Jin S, Verkman AS. Enhanced macromolecule diffusion deep in tumors after enzymatic digestion of extracellular matrix collagen and its associated proteoglycan decorin. FASEB J. 2008;22:276–284. doi: 10.1096/fj.07-9150com. [DOI] [PubMed] [Google Scholar]
- 37.Cemazar M, Golzio M, Sersa G, et al. Hyaluronidase and collagenase increase the transfection efficiency of gene electrotransfer in various murine tumors. Hum Gene Ther. 2012;23:128–137. doi: 10.1089/hum.2011.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Spruß T, Bernhardt G, Schönenberger H, Schiess W. Hyaluronidase significantly enhances the efficacy of regional vinblastine chemotherapy of malignant melanoma. J Cancer Res Clin Oncol. 1995;121:193–202. doi: 10.1007/BF01366962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Muckenschnabel I, Bernhardt G, Spruß T, Buschauer A. Hyaluronidase pretreatment produces selective melphalan enrichment in malignant melanoma implanted in nude mice. Cancer Chemother Pharmacol. 1996;38:88–94. doi: 10.1007/s002800050452. [DOI] [PubMed] [Google Scholar]
- 40.Chen LL, Jaimes N, Barker CA, et al. Desmoplastic melanoma: a review. J Am Acad Dermatol. 2013;68:825–833. doi: 10.1016/j.jaad.2012.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sneider A, Kim JH, Kiemen A, et al. Deep learning identification of stiffness markers in breast cancer. bioRxiv. 2020 doi: 10.1101/2020.12.17.423077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Plodinec M, Loparic M, Monnier CA, et al. The nanomechanical signature of breast cancer. Nat Nanotechnol. 2012;7:757–765. doi: 10.1038/nnano.2012.167. [DOI] [PubMed] [Google Scholar]
- 43.Zhao R, Cui Y, Zheng Y, et al. Human hyaluronidase PH20 potentiates the antitumor activities of mesothelin-specific CAR-T cells against gastric cancer. Front Immunol. 2021;12:1–12. doi: 10.3389/fimmu.2021.660488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Farrera-Sal M, Moreno R, Mato-Berciano A, et al. Hyaluronidase expression within tumors increases virotherapy efficacy and T cell accumulation. Mol Ther Oncolytics. 2021;22:27–35. doi: 10.1016/j.omto.2021.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fankhauser M, Broggi MAS, Potin L, et al. Tumor lymphangiogenesis promotes T cell infiltration and potentiates immunotherapy in melanoma. Sci Transl Med. 2017;9:1–13. doi: 10.1126/scitranslmed.aal4712. [DOI] [PubMed] [Google Scholar]
- 46.Commerford CD, Dieterich LC, He Y, et al. Mechanisms of tumor-induced lymphovascular niche formation in draining lymph nodes. Cell Rep. 2018;25:3554–3563.e4. doi: 10.1016/j.celrep.2018.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dang S, Peng Y, Ye L, et al. Stimulation of TLR4 by LMW-HA induces metastasis in human papillary thyroid carcinoma through CXCR7. Clin Dev Immunol. 2013 doi: 10.1155/2013/712561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cyphert JM, Trempus CS, Garantziotis S. Size matters: molecular weight specificity of hyaluronan effects in cell biology. Int J Cell Biol. 2015 doi: 10.1155/2015/563818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rhim AD, Oberstein PE, Thomas DH, et al. Stromal elements act to restrain, rather than support, pancreatic ductal adenocarcinoma. Cancer Cell. 2014;25:735–747. doi: 10.1016/j.ccr.2014.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Özdemir BC, Pentcheva-Hoang T, Carstens JL, et al. Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell. 2014;25:719–734. doi: 10.1016/j.ccr.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data represented in this manuscript will be made available from the corresponding authors upon reasonable request.
Not applicable.