Abstract
Backgound
Literature reports suggest that the host immune system may control Malignant Pleural Mesothelioma (MPM) growth, although its activity is limited by regulatory mechanisms. In this retrospective study, we analyzed the levels of pro-inflammatory (IL-1, IL-6, TNF), immune-regulatory (IL-10) and Th1/CTL-related cytokines (IL-12p70, IFN-γ) in the pleural exudate and their relationship with overall survival (OS) in MPM.
Methods
Cytokines were quantified by multiplexed immunoassay. Concentrations were dichotomized with respect to the median value. Correlation between cytokine level and OS was assessed using univariate (Kaplan–Meier curves) and multivariate (Cox regression) analyses.
Results
Regarding outcome, tumor histology, therapies undergone and IFN-γ were independent prognostic factors of OS in a 72 MPM training cohort. Notably, high concentrations of IFN-γ halved death probability (HR of high vs low IFN-γ concentration = 0.491, 95%CI 0.3–0.8, p = 0.007). Also in patients with epithelioid histology and those receiving at least one line of therapy, high IFN-γ level was an independent factor predictive of OS (HR of high vs low IFN-γ concentration were 0.497, p = 0.007 and 0.324, p = 0.006, respectively). However, these data were not confirmed in a 77 MPM validation cohort, possibly due to the low IFN-γ levels encountered in this population, and the heterogeneous distribution of disease stages between the training and the validation cohorts. None of the other cytokines showed any effect on survival.
Conclusions
High level of IFN-γ in pleural effusion may be associated with better survival in MPM patients and potentially serve as a prognostic biomarker. Larger prospective studies are needed to ascertain this hypothesis.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00262-021-02965-w.
Keywords: Mesothelioma, Interferon-gamma, Pleural effusion, Biomarker, Outcome, Immune response
Introduction
Malignant Pleural Mesothelioma (MPM) is a rare type of cancer that arises from the mesothelial membranes of the pleural cavities and is causally associated with exposure to asbestos. Asbestos causes a chronic inflammatory process related to the release of the damage-associated molecular pattern HMGB1 and several cytokines such as IL-1, IL-6 and TNF [1]. MPM prognosis is still poor as the median survival is about 17–25 months for resectable MPM and 9–12 months for unresectable MPM. Immunotherapy is currently being addressed in several clinical studies in MPM (reviewed in [2]). The anti-CTLA4 mAb tremelimumab showed some promising results in phase II studies but had no impact on MPM patient survival in a large randomized trial in relapsing MPM [3]. In phase II studies, responses to the anti-PD-1 mAbs pembrolizumab or nivolumab were seen in about 10–30% of MPM patients [2, 4, 5], but pembrolizumab was not superior to chemotherapy in a randomized study in pretreated patients [2]. Also, the combination of anti-PD-L1 blocking agent and anti-CTLA4 mAbs showed promising results [6]. In addition, studies of Dendritic Cell vaccines showed interesting results in MPM [7–9]. Phase III studies of first line immunotherapy or studies of different drug and immunotherapy combinations are in progress [2]. On the whole, all these observations suggest that a subgroup of MPM patients may respond to immunotherapy, and consequently, the identification of new biomarkers to select optimal candidates for immunotherapy is an urgent need.
Previously, spontaneous regressions of MPM have been related to an anti-tumor immune response [10], and the presence of cytotoxic CD8 + tumor-infiltrating lymphocytes has been considered as a good prognostic marker in MPM [11, 12]. A more recent study showed that the intra-tumor T-regulatory and myeloid-derived suppressor cells correlated with progression-free survival and overall survival (OS), while PD-1 + /LAG-3 + /TIM-3 + /CD4 + tumor-infiltrating lymphocytes correlated with OS [13]. Overall, these data argue that the immune system may control MPM growth, although its activity can be inhibited by multiple regulatory mechanisms.
On these bases, the present retrospective study was aimed at assessing the concentration of a panel of cytokines in pleural exudates (PE) of MPM patients and their correlation with patient outcome. This panel included cytokines that are predominantly related to inflammation (TNF, IL-1, IL-6), immune-regulation (IL-10) or Th1/CTL immune response (IL-12p70 and IFN-γ). Our data suggest that among these cytokines, IFN-γ should be further analyzed as prognostic factor of OS in larger prospective cohorts of MPM patients.
Materials and methods
Patients
The MPM patients of the training cohort were recruited from the Division of Pneumology (Azienda Sanitaria Locale n.5 La Spezia; Italy) between June 2008 and May 2013 and underwent thoracentesis and thoracoscopy. During these procedures, a sample of pleural exudate was collected. Debris and cells were removed by centrifugation at 1500 × g, and fluids were stored in aliquots at −80 °C until use. MPM diagnosis was based on imaging data, cytology of pleural effusion and histology of pleural biopsies. The local Ethical Committee approved the study (P.R. 207REG2014) and all patients signed an informed consent. The validation cohort was obtained from the “Alessandria Biobank-Centro Raccolta Materiali Biologici”, Department of Integrated Activities Research and Innovation, Azienda Ospedaliera SS. Antonio e Biagio e Cesare Arrigo, Alessandria, Italy.
Cytokine dosage
Cytokine levels in MPM PE were measured with a bead-based multiplexed immunoassay and Luminex technology (MILLIPLEX MAP Human TH17 Magnetic Bead Panel, customized HTH17MAG-8 K, Merck Millipore, Darmstadt, Germany), following the manufacturer’s instructions. Samples were tested both at 1:2 and 1:10 dilution in Assay Buffer. The detection limits and sensitivities of the immunoassay are summarized in Supplementary Table 1.
Statistical analysis
Continuous variables (patient age and cytokine concentrations) were dichotomized with respect to their median value. Kaplan–Meier curves were used to evaluate OS, as estimated from the date of PE sample withdrawal to the date of last contact or death from any cause. Differences between curves were estimated by the log-rank test. Cox regression model was used for multivariate analyses to assess the independent effect of each prognostic factor on OS, while adjusting for the effects of the other factors. The multivariate analyses started from the full model, with all covariates included. Factors not significantly associated with survival were removed from the model by means of a step-down procedure based on the likelihood ratio test.
All statistical analyses were two-sided and were carried out using the SPSS software package (version 21.0 for Windows). Significance was accepted for any p value < 0.05.
Results
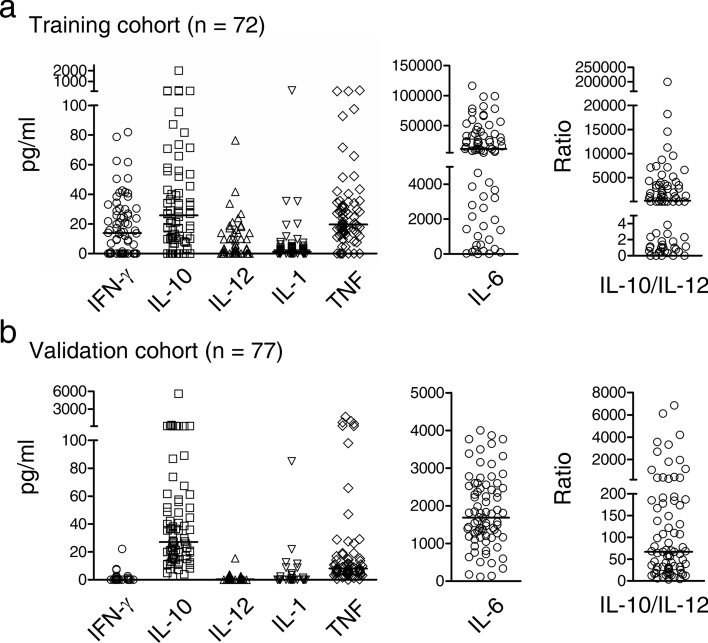
Seventy-two MPM patients were included in the training cohort, enrolled in an Italian area of high MPM incidence. The distribution of the patients within the strata of each clinico-pathological prognostic factor is shown in Table 1. By the end of the observation period, all patients but 2 had died. Overall median OS was 13.5 months (95%CI 11.3–15.6). The levels of each cytokine in MPM pleural effusion are shown in Fig. 1a.
Table 1.
Patient characteristics (n = 72)
| n (%) | |
|---|---|
| Age (around median) | |
| ≤ 74y | 35 (48.6) |
| > 74y | 37 (51.4) |
| Gender | |
| Male | 60 (83.3) |
| Female | 12 (16.7) |
| Histology | |
| Epithelioid | 47 (65.3) |
| Sarcomatoid | 13 (18.1) |
| Other | 12 (16.6) |
| Stage | |
| I | 41 (56.9) |
| II | 19 (26.4) |
| III-IV | 11 (15.3) |
| Unknown | 1 (1.4) |
| ECOG | |
| 0 | 26 (36.1) |
| 1 | 29 (40.3) |
| ≥ 2 | 17 (23.6) |
| Exposure to asbestos | |
| No | 26 (36.1) |
| Yes | 44 (61.1) |
| Unknown | 2 (2.8) |
| Smoking history | |
| Never smoked | 28 (38.9) |
| Current smoker | 12 (16.7) |
| Ex smoker | 30 (41.7) |
| Unknown | 2 (2.8) |
| Therapy | |
| No | 16 (22.2) |
| At least 1 line | 37 (51.4) |
| Unknown | 19 (26.4) |
Fig. 1.
Levels of each cytokine in pleural effusion. The concentrations, in pg/ml, of IFN-γ, IL-10, IL-12p70, IL-1, TNF and IL-6 in pleural effusions of MPM patients are shown. Both training a and validation cohorts b are displayed. Right panels show the ratio values of IL-10/IL-12, used to define regulatory macrophages. Bars indicate median values
In univariate analyses, among all the cytokines we evaluated, only IFN-γ was significantly associated with OS (Fig. 2). Overall survival at thirty-month was 27.3% (95%CI 15.6–39.0) in the group of patients with high IFN-γ concentrations as compared to 5.6% in the group with lower IFN-γ concentrations. Median survival for these two groups was 14.7 and 12.8 months, respectively (log rank p = 0.050, Table 2). The concentrations of the others cytokines had no effect on survival (log rank p = 0.963 for TNF, p = 0.835 for IL-6, p = 0.800 for IL-10, p = 0.869 for IL-12p70 and p = 0.546 for IL-1β). Although the IL-10/IL-12 ratio, which is used to identify regulatory macrophages [reviewed in 16], showed no relationship with survival (p = 0.728, data not shown), it should be noted that IL-12 levels were in most instances below the detection limits (Fig. 1a). This observation together with the high IL-10 levels supports the presence of regulatory macrophages in the MPM microenvironment.
Fig. 2.
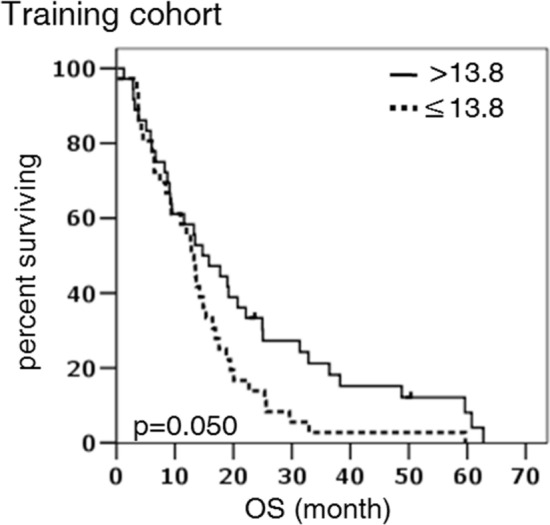
Kaplan–Meier survival curves according to IFN-γ concentration (as dichotomized around the median value of 13.8 pg/ml) in the whole population
Table 2.
Correlation between prognostic factors and overall survival: univariate and multivariate analyses
| Prognostic factor | Median OS month (95%CI) | HR (95% CI) univariate | P value b | HR (95%CI) multivariate | P value c |
|---|---|---|---|---|---|
| Age | |||||
| ≤ 74ya | 14.8 (11.4–18.3) | 1 (ref) | 0.647 | – | – |
| > 74y | 13.3 (8.4–18.2) | 1.12 (0.7–1.8) | |||
| Gender | |||||
| Male | 13.3 (12.0–14.5) | 1 (ref) | 0.083 | 1 (ref) | 0.377 |
| Female | 16.9 (4.1–29.8) | 0.59 (0.3–1.1) | 0.70 (0.3–1.5) | ||
| Histology | |||||
| Epithelioid | 15.3 (10.8–19.3) | 1 (ref) | ≤ 0.001 | 1 (ref) | 0.001 |
| Sarcomatoid | 6.5 (3.1–9.9) | 3.79 (1.9–7.4) | 4.28 (2.0–9.1) | ||
| Other | 9.4 (2.2–16.6) | 1.71 (0.9–3.4) | 1.64 (0.8–3.4) | ||
| Stage | |||||
| I | 13.7 (11.7–15.6) | 1 (ref) | 0.060 | 1 (ref) | 0.060 |
| II | 17.7 (11.6–23.8) | 0.82 (0.5–1.4) | 1.29 (0.7–2.4) | ||
| III-IV | 5.9 (1.3–10.4) | 1.98 (1.0–3.9) | 2.37 (1.2–4.8) | ||
| ECOG | |||||
| 0 | 14.2 (7.9–20.5) | 1 (ref) | 0.040 | 1 (ref) | 0.545 |
| 1 | 15.3 (13.4–17.2) | 0.99 (0.6–1.7) | 0.79 (0.4–1.7) | ||
| ≥ 2 | 9.2 (2.6–15.8) | 1.13 | 1.13 (0.5–2.6) | ||
| Exposure to asbestos | |||||
| No | 15.8 (7.9–23.7) | 1 (ref) | 0.176 | – | – |
| Yes | 13.5 (11.1 –15.8) | 1.41 (0.8–2.4) | |||
| Smoking history | |||||
| Never smoked | 14.7 (7.0–22.5) | 1 (ref) | 0.323 | – | – |
| Current smoker | 13.7 (5.4–21.9 | 1.72 (0.8–3.5) | |||
| Ex smoker | 13.3 (10.7–15.9) | 1.19 (0.7–2.0) | |||
| Therapy | |||||
| No | 4.5 (0.2–8.9) | 1 (ref) | ≤ 0.001 | 1 (ref) | 0.001 |
| At least 1 line | 14.7 (10.9–18.5) | 0.35 (0.2–0.6) | 0.30 (0.2–0.6) | ||
| Unknown | 16.9 (8.3–25.5) | 0.26 (0.1–0.5) | 0.27 (0.1–0.7) | ||
| IFN-γ (pg/ml) | |||||
| ≤ 13.8 d | 12.8 (10.6– 15.1) | 1 (ref) | 0.050 | 1 (ref) | 0.007 |
| > 13.8 | 14.7 (8.2–21.3) | 0.61 (0.4–1.0) | 0.49 (0.3–0.8) | ||
CI confidence interval; HR hazard ratio; ref reference stratum; IFN-γ interferon gamma
a median age
b from log rank test
c from multivariate Cox regression model
d median IFN-γ concentration
Other clinico-pathological factors significantly associated with OS were tumor histotype, performance status (ECOG European Cooperative Oncology Group) and number of therapies undergone (Table 2). Median OS was higher in patients with epithelioid histotype as compared to patients with sarcomatoid histotype (15.3 vs 6.5 months, log rank p ≤ 0.001) and in patients with ECOG score 0 or 1 as compared to those with ECOG score ≥ 2 (14.2 and 15.3 vs 9.2 months, p = 0.040). Likewise, undergoing at least 1 line of therapy determined a significant improvement in OS as compared to not undergoing any therapy (14.7 vs 4.5 months, p ≤ 0.001). Correlation between OS and gender or tumor stage was borderline (log rank p = 0.083 and 0.060, respectively). Patient age, exposure to asbestos and smoking history had no effect on survival (log rank p = 0.647, 0.176 and 0.323, respectively).
The variables that were statistically significant or close to significance in univariate analyses were then included in a Cox regression model. The model thus included gender, tumor histology, tumor stage, ECOG score, therapy undergone and IFN-γ concentration. This multivariate analysis showed that histology, therapy undergone and IFN-γ were independent prognostic factors of survival. Risk of death was more than 4 times higher in sarcomatoid vs epithelioid histotype (HR = 4.28, 95%CI 2.0–9.1, p = 0.001, Table 2). Undergoing at least 1 line of therapy decreased the risk of death by 70% (HR = 0.30, 95%CI 0.2–0.6, p = 0.001). Most interestingly, high concentrations of IFN-γ halved death probability (HR = 0.49, 95%CI 0.3–0.8, p = 0.007).
The effect of IFN-γ was further assessed in the 2 subgroups significantly associated with OS, i.e., patients with epithelioid histotype and patients undergoing at least one line of therapy. In univariate analyses, OS was significantly favored by high concentrations of IFN-γ in both subgroups. In the epithelioid histotype subgroup, median OS was 20.7 months if IFN-γ concentration was > 13.8 pg/ml as compared to 13.7 months if IFN-γ concentration was ≤ 13.8 pg/ml (p = 0.010, Fig. 3a). Similarly, median OS in patients undergoing therapy was 19.2 months in presence of high IFN-γ concentration as compared to 12.8 months if IFN-γ concentration was lower (p = 0.006, Fig. 3b). Also in these 2 subgroups, IFN-γ was an independent factor predictive of survival, as determined in multivariate analyses. The HR observed for the ratio > 13.8 pg/ml vs ≤ 13.8 pg/ml were 0.497 and 0.324 in the epithelioid histology and the therapy undergone subgroups, respectively (Fig. 3a, b, insets). In both subgroups, this association between high IFN-γ concentration and better survival was highly significant, the p values being 0.007 and 0.006, respectively.
Fig. 3.

Kaplan–Meier survival curves according to IFN-γ concentration (as dichotomized around the median value of 13.8 pg/ml) in the subgroup of patients with epithelioid histotype a and the subgroup of patients undergoing at least one line of therapy b. The insets report the HR values for IFN-γ (as > 13.8 pg/ml vs ≤ 13.8 pg/ml) obtained in multivariate Cox regressions including in a gender, tumor stage, ECOG score, therapy undergone and IFN-γ concentration; in b gender, histology, tumor stage, ECOG score and IFN-γ concentration
In the attempt to validate the data, we examined a second cohort of 77 MPM pleural fluids with similar histotype distribution (Supplementary Table 2), obtained from a different geographic area in Italy where MPM incidence is also high. Unfortunately, the IFN-γ levels showed no relationship with survival in this second cohort (data not shown). However, it should be noted that overall the IFN-γ levels detected in this cohort were extremely low or undetectable (Fig. 1b), thus potentially limiting data reliability.
Discussion
The present finding that high PE IFN-γ levels are associated with enhanced survival in a cohort of MPM patients suggests that the endogenous immune response might control MPM growth. However, we were unable to validate this finding on a second, independent cohort of MPM patients. Although the PE samples were similarly processed, differences in manipulation conditions from collection to storage may account for these discrepant results. As a matter of fact, the concentrations of IFN-γ were much lower in the validation cohort as compared to the training cohort. It should be also noted that the tumor stage distribution was unbalanced between the two cohorts, with a very limited number of stage I-II patients in the validation cohort. By contrast, the training cohort was composed largely by stage I-II cases and when OS was evaluated in this subgroup, the association between high IFN-γ concentration and better survival was quite significant (p = 0.029, Supplementary Fig. 1).
It is conceivable that cytokines present in the PE may reflect cellular interactions in the MPM microenvironment more closely than those present in peripheral blood. IFN-γ is typically related to Th1 responses and is released by activated CTLs, which represent main effector cells in controlling tumor growth. Accordingly, previous studies showed that the CD8 + T cell counts correlate with improved survival in several tumors, including MPM [11, 12]. Besides CTLs, activated NK cells release IFN-γ and may play a role in a subset of MPM predominantly with epithelioid features, as reported in a recent molecular profiling study [14]. In addition, a recent study showed that NK cells infiltrate MPM, and that they can recognize and kill mesothelioma cells [15]. Differently, sarcomatoid variants, which more frequently respond to PD-1 blockade are mainly infiltrated by exhausted CD8 + T cells.
Pleural exudate IFN-γ level above the median is an independent factor associated with survival in the whole training MPM cohort here studied, and particularly in two subgroups of patients, those with epithelioid histology and those undergoing at least one line of therapy. Conversely, pro-inflammatory cytokines such as IL-1β, TNF and IL-6, which play an important role in MPM genesis and progression showed no correlation with OS. Previous studies supported a role for pleural M2-polarized macrophages, capable of releasing the immune-regulatory cytokine IL-10, in MPM chemotherapy resistance [17] and immune suppression [18, 19]. Although IL-10 showed no association with survival in our MPM cohorts, the high IL-10/IL-12 ratio confirms the presence of immune-suppressive macrophages. In addition to immune suppressive macrophages, molecular profiling studies support a role of different immune regulatory molecules such as OX40L, TGFB1, CD276, OX40 and PD-L2 in sarcomatoid MPM subtype, whereas VISTA expression predominates in the epithelioid MPM subtype [20].
In conclusion, our results suggest that the assessment of PE IFN-γ levels as a prognostic biomarker should be further analyzed in larger prospective cohorts, with the aim at better evaluating the role of the immune response in the MPM microenvironment and possibly identifying patients with more immunogenic tumors. Moreover, such studies should include balanced numbers of stage I-II versus stage III-IV cases to explore the possibility that IFN-γ may act as prognostic factor only at the early stages of disease.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Stefania Martini for excellent technical assistance and the Azienda Ospedaliera di Alessandria Biorepository “Alessandria Biobank” for providing the validation cohort
Authors contributions
Study concept and design, and study supervision: SF, MF, Recruitment of MPM patients: SR, PF, PD, PAC, Acquisition, analysis or interpretation of data: BD, GC, SF, MF, Manuscript drafting: BD, GC, SF, MF, Statistical analyses: BD, Critical revision of the manuscript for important intellectual content: SF, All authors read and approved the final manuscript.
Funding
This work was supported by grants from the Italian Ministry of Health (GR-2013–02356568, Ricerca Corrente and 5 × 1000 Funds).
Declaration
Conflict of interest
The author declares there is no conflict of interest.
Ethical approval
The local Ethical Committee (Azienda Sanitaria Locale n.5 La Spezia) approved the study (P.R. 207REG2014). All procedures performed in the study were in accordance with the Declaration of HELSINKI.
Informed consent
All patients provided a signed informed consent before enrollment.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Beatrice Dozin and Grazia Carbotti have contributed equally.
References
- 1.Carbone M, Adusumilli PS, Alexander HR, et al. Mesothelioma: scientific clues for prevention, diagnosis, and therapy. CA Cancer J Clin. 2019;69:402–429. doi: 10.3322/caac.21572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Gooijer CJ, Borm FJ, Scherpereel A, Baas P. Immunotherapy in malignant pleural mesothelioma. Front Oncol. 2020;10:187. doi: 10.3389/fonc.2020.00187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maio M, Scherpereel A, Calabrò L, et al. Tremelimumab as second-line or third-line treatment in relapsed malignant mesothelioma (DETERMINE): a multicentre, international, randomised, double-blind, placebo-controlled phase 2b trial. Lancet Oncol. 2017;18:1261–1273. doi: 10.1016/S1470-2045(17)30446-1. [DOI] [PubMed] [Google Scholar]
- 4.Scherpereel A, Mazieres J, Greillier L, et al. Nivolumab or nivolumab plus ipilimumab in patients with relapsed malignant pleural mesothelioma (IFCT-1501 MAPS2): a multicentre, open-label, randomised, non-comparative, phase 2 trial. Lancet Oncol. 2019;20:239–253. doi: 10.1016/S1470-2045(18)30765-4. [DOI] [PubMed] [Google Scholar]
- 5.Okada M, Kijima T, Aoe K, et al. Clinical efficacy and safety of nivolumab: results of a multicenter, open-label, single-arm, japanese phase ii study in malignant pleural mesothelioma (MERIT) Clin Cancer Res. 2019;25:5485–5492. doi: 10.1158/1078-0432.CCR-19-0103. [DOI] [PubMed] [Google Scholar]
- 6.Disselhorst MJ, Quispel-Janssen J, Lalezari F, et al. Ipilimumab and nivolumab in the treatment of recurrent malignant pleural mesothelioma (INITIATE): results of a prospective, single-arm, phase 2 trial. Lancet Respir Med. 2019;7:260–270. doi: 10.1016/S2213-2600(18)30420-X. [DOI] [PubMed] [Google Scholar]
- 7.Hegmans JP, Veltman JD, Lambers ME, et al. Consolidative dendritic cell-based immunotherapy elicits cytotoxicity against malignant mesothelioma. Am J Respir Crit Care Med. 2010;181:1383–1390. doi: 10.1164/rccm.200909-1465OC. [DOI] [PubMed] [Google Scholar]
- 8.Aerts JGJV, de Goeje PL, Cornelissen R, et al. Autologous dendritic cells pulsed with allogeneic tumor cell lysate in mesothelioma: from mouse to human. Clin Cancer Res. 2018;24:766–776. doi: 10.1158/1078-0432.CCR-17-2522. [DOI] [PubMed] [Google Scholar]
- 9.Cornelissen R, Hegmans JPJJ, Maat APWM, et al. Extended tumor control after dendritic cell vaccination with low-dose cyclophosphamide as adjuvant treatment in patients with malignant pleural mesothelioma. Am J Respir Crit Care Med. 2016;193:1023–1031. doi: 10.1164/rccm.201508-1573OC. [DOI] [PubMed] [Google Scholar]
- 10.Robinson BW, Robinson C, Lake RA. Localised spontaneous regression in mesothelioma–possible immunological mechanism. Lung Cancer. 2001;32:197–201. doi: 10.1016/s0169-5002(00)00217-8. [DOI] [PubMed] [Google Scholar]
- 11.Yamada N, Oizumi S, Kikuchi E, et al. CD8+ tumor-infiltrating lymphocytes predict favorable prognosis in malignant pleural mesothelioma after resection. Cancer Immunol Immunother. 2010;59:1543–1549. doi: 10.1007/s00262-010-0881-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anraku M, Cunningham KS, Yun Z, et al. Impact of tumor-infiltrating T cells on survival in patients with malignant pleural mesothelioma. J Thorac Cardiovasc Surg. 2008;135:823–829. doi: 10.1016/j.jtcvs.2007.10.026. [DOI] [PubMed] [Google Scholar]
- 13.Salaroglio IC, Kopecka J, Napoli F, et al. Potential diagnostic and prognostic role of microenvironment in malignant pleural mesothelioma. J Thorac Oncol. 2019;14:1458–1471. doi: 10.1016/j.jtho.2019.03.029. [DOI] [PubMed] [Google Scholar]
- 14.Blum Y, Meiller C, Quetel L, et al. Dissecting heterogeneity in malignant pleural mesothelioma through histo-molecular gradients for clinical applications. Nat Commun. 2019;10:1333. doi: 10.1038/s41467-019-09307-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sottile R, Tannazi M, Johansson MH, et al. NK- and T-cell subsets in malignant mesothelioma patients: baseline pattern and changes in the context of anti-CTLA-4 therapy. Int J Cancer. 2019;145:2238–2248. doi: 10.1002/ijc.32363. [DOI] [PubMed] [Google Scholar]
- 16.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chéné A-L, d’Almeida S, Blondy T, et al. Pleural effusions from patients with mesothelioma induce recruitment of monocytes and their differentiation into M2 macrophages. J Thorac Oncol. 2016;11:1765–1773. doi: 10.1016/j.jtho.2016.06.022. [DOI] [PubMed] [Google Scholar]
- 18.Lievense LA, Cornelissen R, Bezemer K, et al. Pleural effusion of patients with malignant mesothelioma induces macrophage-mediated t cell suppression. J Thorac Oncol. 2016;11:1755–1764. doi: 10.1016/j.jtho.2016.06.021. [DOI] [PubMed] [Google Scholar]
- 19.Chu GJ, van Zandwijk N, Rasko JEJ. The immune microenvironment in mesothelioma: mechanisms of resistance to immunotherapy. Front Oncol. 2019;9:1366. doi: 10.3389/fonc.2019.01366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hmeljak J, Sanchez-Vega F, Hoadley KA, et al. Integrative molecular characterization of malignant pleural mesothelioma. Cancer Discov. 2018;8:1548–1565. doi: 10.1158/2159-8290.CD-18-0804. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.