Abstract
Immunotherapy (ITH) holds the possibility of tumor burden decrease after initial RECIST 1.1 defined progression. The clinical concept of treating selected patients (pts) beyond disease progression (PD) is supported by so-called pseudoprogression phenomenon. The aim of this study was to evaluate real-life practice and outcomes related to treatment beyond (RECIST) progression (TBP) in advanced melanoma patients. Of 584 subsequent melanoma pts analyzed 77 (13.2%) received TBP. In this cohort, the median time to first PD (TTFP) was 5.29 months (m), while time to second PD (TTSP)—8.02 m. On TBP 23.4% pts achieved an objective response (OR), and next 42.9%—stabilization of the disease (SD). 1st PD was reported most often as the development of a new lesion or increase (> 20%) of the diameter of three or more targets. In about 50% second PD was observed as an increase in the diameter of different targets that in 1st PD. Multimodal treatment resulted in 9.82 m TTSP, while ITH alone—4.93 m (p = 0.128). An oligoprogressive pattern of first PD was associated with longer TTSP (HR 0.55, 95% CI: 0.32–0.94). Median OS after first PD was 28.75 months and correlated with OR during TBP (HR 0.18, 95% CI: 0.004–0.76). Selected clinically fit melanoma patients, despite evidence of first radiographic progression, may benefit from continued treatment with PD-1 checkpoint inhibitors, but the findings should be validated in larger prospective trials. Multidisciplinary treatment should be offered to advanced melanoma patients, including radiosurgery or stereotactic radiotherapy of single loci progressing during immunotherapy.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00262-021-03132-x.
Keywords: Melanoma, Immunotherapy, Disease progression, RECIST, Treatment beyond progression
Introduction
Immune checkpoint inhibitors (ICIs) development is one of the most successful and vast research areas in oncology and drug development. However, several uncertainties remain, including the optimal use of these drugs in routine clinical practice. Other important remaining unknown areas are the prediction of patients benefit, optimal dosing, scheduling, sequencing, and the optimal duration of treatment [1]. These questions are particularly open in the field of advanced/metastatic melanoma [2–4], as multiple anti-PD1 (nivolumab and pembrolizumab), anti-CTLA4 (ipilimumab) as well combination (nivolumab with ipilimumab) immunotherapies are currently in routine use along with targeted therapies. Moreover, patients who receive immunotherapy may develop an atypical response that initially may be classified as a conventionally defined progressive disease, but later lesions decrease. Such bias in tumor progression pattern is resultant from the mechanism of action of immunotherapy since although some metastases may shrink in response to treatment, others could be stable or increase, and, in some cases, new metastases become detectable [5]. In fact, the anti-PD-1 therapy effect is related to infiltration of intratumoral T cells that execute anti-cancer effects. A high load of CD8+ T cells within the tumor and induced T cell proliferation are associated with prolonged overall survival (OS) [6]. Moreover, the time required to establish an effective immune response by immunotherapy may exceed what is known for typical response times to chemotherapy or targeted therapies [7]. Late responses and mechanisms of immunotherapy activity prompt further investigation into the potential benefits and risks for patients who continue immunotherapy beyond disease progression defined by the Response Evaluation Criteria in Solid Tumors (RECIST) 1.1.—treatment beyond progression (TBP).
PD-1 monotherapy treatment beyond progression is primarily supported by the concept of pseudoprogression—where patients receiving immunotherapy initially meet the RECIST criteria for progression (PD) but later demonstrate significant decreases in tumor burden on subsequent imaging studies. Based on the iRECIST definition, pseudoprogression (psPD) is defined as progressive disease on RECIST1.1, subsequently resolving to non-PD [8, 9]. psPD during immunotherapy was first characterized in a phase II trial of ipilimumab in advanced melanoma [10] and later described not only in melanoma but also in renal cell carcinoma (RCC) and non-small cell lung cancer patients (NSCLC) [11]. It is expected that psPD develops due to an initial delay in the anti-tumor response to the immunotherapy or due to an increase in target lesions by an inflammatory infiltrate volume [12]. Given the uncertainty on treatment discontinuation based on disease progression per RECIST 1.1 being premature in the context of immunotherapy, recent clinical trials commonly allow for treatment beyond RECIST-defined progressive disease (PD). Moreover, immunotherapy, including anti-PD1 nivolumab or pembrolizumab, may alter tumor biology so that treatment effects are not concordant with radiographic measurements. This phenomenon explains the discordance between relatively low response rate or progression-free survival and prolonged overall survival [13].
Until now, it remains a challenge to assess the actual clinical benefit of TBP [12], as clinical trials rarely allow for the continuation of treatment beyond progression. Moreover, in studies with TBP, the number of patients who developed significant objective tumor responses after initial PD is generally small, and association analyses of TBP and overall survival previously reported in patients with melanoma did not include control arm where TBP was also permitted. TBP analyses seem important for researchers working on recommendations on regulatory endpoints and clinical trial protocols and for practicians considering continuing therapy after disease progression by conventional criteria. Although immunotherapy is currently used in routine clinical practice after approval from regulatory agencies worldwide, clinical trials have used immune-related Response Criteria (irRC) or irRECIST for evaluating the clinical significance of TBP only in the research setting. TBP in the product characteristics of immunotherapeutic agents has not been recommended as the clinical benefit still needs to be proven [12]. From this perspective, we aimed to evaluate TBP's impact on patient outcomes in advanced/metastatic melanoma patients treated with immunotherapy in the real-world setting. We aimed to investigate the impact of TBP in long-term melanoma treatment and characterize melanoma patients who may benefit from TBP when qualified to continue therapy after RECIST defined PD due to the high risk of pseudoprogression. To our knowledge, this is the first report on overall survival in melanoma patients on TBP in a regular clinical practice.
Materials and methods
Data extraction
Adult patients with histologically confirmed stage III unresectable or stage IV melanoma patients, with known BRAFV600 mutation status, treated in the Department of Soft Tissue/Bone Sarcoma and Melanoma of the Maria Sklodowska-Curie National Research Institute of Oncology were screened for study enrolment. Other eligibility criteria included measurable disease as assessed by means of computed tomography (CT) or magnetic resonance imaging (MRI) according to Response Evaluation Criteria in Solid Tumors (RECIST), version 1.1. Electronic medical history recorded in the CGM CLININET HIS system (CompuGroup Medical Poland Ltd.) were screened with MedStream Designer (MSD) software (Transition Technologies, Poland). Corresponding 10th revision of the International Statistical Classification of Diseases and Related Health Problems (ICD) C43 code and the keyword 'melanoma' and 'nivolumab' or 'pembrolizumab' or 'immunotherapy' were used. Data were reviewed independently by two researchers. Data on the date of death were confirmed in the Polish National Cancer Registry at the Department of Epidemiology & Cancer Prevention via the personal identification number of all patients.
All consecutive patients who received the first dose of anti-PD-1 antibody monotherapy with nivolumab or pembrolizumab between December 1st, 2015 and December 31st, 2019, experienced disease progression according to RECIST v.1.1 [14] and were treated beyond progression were considered eligible. Patients were enrolled in the study and defined as being treated beyond progression (TBP) if the date of last drug exposure was recorded after RECIST 1.1 progression date. Patients were enrolled in the study only if they had undergone at least one imaging (CT/MRI) or died during TBP. Patients treated within clinical trials or receiving a combination of anti-PD-1 antibodies with other agents were excluded from the study.
Treatment
All patients provided written informed consent for the treatment. Patients were treated, and drugs were dosed according to the Summary of Product Characteristics. According to the national drug reimbursement regulations patients underwent regular response assessment with computed tomography or magnetic resonance imaging every 10–12 weeks according to requirements of the Polish Ministry of Health (https://www.gov.pl/web/zdrowie/choroby-onkologiczne). Additionally, clinical progression was confirmed by CT scan as per the decision of the attending physician. RECIST v1.1 was used for response assessment [14]. Radiological progression was defined by RECIST 1.1 criteria determined by one or more of the following: (1) an increase in target tumor lesions size sum of ≥ 20% from nadir along with a relative increase of at least 5 mm in tumor burden; (2) unequivocal progression in non-target lesions; or (3) the appearance of a new lesion [14]. Treatment beyond the first progression was allowed upon multidisciplinary team (MDT) decision for patients in good performance status, without substantial adverse events, and those who exhibited clinical benefit from treatment according to attending physician assessment.
Statistical analysis
Descriptive statistics were used to characterize demographic features, tumor characteristics, disease stage, number of organ sites with metastasis, progression, treatment duration, best response. Discrete variables were summarized as numbers and percentages while continuous ones with mean and range in the case of a normal distribution or median and interquartile range when distribution was skewed. Kaplan–Meier method was used to estimate survival data [15]. Time-to-first-progression (TTFP) was defined as the time from the first administration of ICI to the date of the first radiological progression. Time-to-second progression (TTSP) was defined as the time from the date of first to the date of second radiological progression or last follow-up, whichever occurred first. Overall survival (OS) was defined as time from the date of first ICI administration to death or last follow-up, while post-PD OS was the time from the date of first radiological progression to death or last follow-up, whichever occurred first. The duration of TBP was defined as the time from RECIST PD date to the date of the last dose of anti-PD-1 therapy administration or death, whichever occurred first. Patients achieving at least a 30% decrease in tumor burden—relative to baseline—during TBP were considered responders. Oligoprogression was defined as up to 3 progressive or new lesions in a single organ as defined before [16].
Two-sided 95% confidence intervals (CI) for median TTFP, TTSP, OS, and treatment duration were computed by the Brookmeyer and Crowley method [17]. Log-rank, chi-square, and Fisher exact tests were used for comparison between groups. All analyses and figures were prepared with the IBM® SPSS® software platform for Windows version 26 (International Business Machines Corporation, Armonk, New York). The differences were considered statistically significant if the p values were < 0.05. Data cut-off was May 31st 2021 (Figs. 1, 2, 3).
Fig. 1.
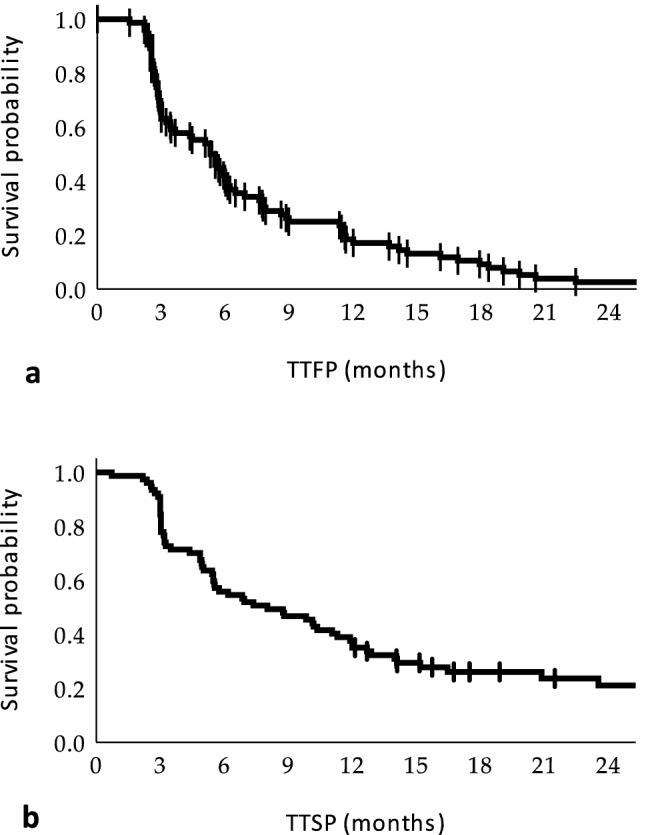
Time to first (a) and second (b) progression in the study population
Fig. 2.
Impact of the objective response (ORR) before first progression (a) and pattern of progression (b) on time to the second progression (TTSP)
Fig. 3.
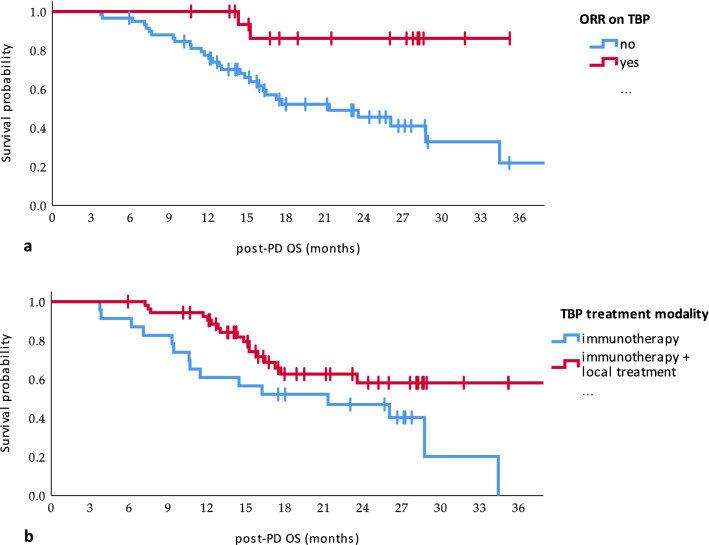
Impact of the objective response (ORR) on treatment beyond progression (TBP) (a) and treatment modality after progression (b) on overall survival of the patients after the first disease progression (post-PD OS)
Results
Patients characteristics
Overall, among all 584 patients with melanoma who started treatment with nivolumab or pembrolizumab, 77 (13.2%) received an anti-PD-1 antibody beyond RECIST 1.1 PD and were enrolled in the study. Patients enrolled in the study were of median age of 62 years at the start of treatment (range 29–85), 53.2% were men, and 39.0% had BRAF-mutant melanoma (Table 1). All patients with BRAF-wild-type melanoma were treated in the first line, while 66.7% of BRAF(+) patients received BRAF-MEK inhibitors before anti-PD-1 therapy. The median follow-up time was 25.9 months (95% CI: 22.8–29.1).
Table 1.
Baseline patients characteristics at the start of anti-PD-1 therapy
| Characteristics | N (%) | |
|---|---|---|
| Gender | Female | 36 (46.8) |
| Male | 41 (53.2) | |
| Age | Median (range) | 62 (29–85) |
| ECOG status | 0 | 36 (46.8) |
| 1 | 41 (53.2) | |
| Primary tumor location | Skin | 60 (77.9) |
| Mucous | 4 (5.2) | |
| UPM | 13 (16.9) | |
| BRAF mutation | Positive | 30 (39.0) |
| Negative | 47 (61.0) | |
| Disease stage (TNM 8.0) | III | 1 (2.6) |
| M1a | 18 (23.4) | |
| M1b | 14 (18.2) | |
| M1c | 24 (31.2) | |
| M1d | 19 (24.7) | |
| Number of organ sites with metastasis* | 1–3 organs | 66 (85.7) |
| > 3 organs | 11 (14.3) | |
| Line of treatment | 1 | 57 (74.0) |
| 2 | 20 (26.0) | |
| Drug used | Nivolumab | 32 (41.6) |
| Pembrolizumab | 45 (58.4) | |
UPM unknown primary melanoma
*Multiple metastases in a single organ are considered as one organ involvement
Treatment outcomes before the first progression
The median time to first PD (TTFP) was 5.29 months (95% CI: 3.96–6.62). Before first PD patients achieved objective response (OR) in 22.1% (17 cases), including 3.9% (3 cases)—with complete response (CR) and 18.2% (14 cases) with partial response (PR); among other patients 37.7% (29 cases) achieved stable disease (SD), while 40.3% (31 cases) progressed (PD) as their best response. In particular, 40.3% of patients progressed at the first assessment after treatment initiation (week 1–12). Most patients progressed due to the development of a new lesion or cumulative increase in the diameter of three or more metastases (Supp. Tab. 1).
Treatment beyond-progression outcomes
After first PD, 23 patients (29.9%) continued immunotherapy-only treatment, while in 54 cases (70.1%), a multidisciplinary treatment was implemented, including local treatment—radiotherapy in 46 patients (59.7%), surgery in 4 (5.2%), or both radiotherapy and surgery in 4 (5.2%); in addition to immunotherapy. During TBP 23.4% patients (18 cases) achieved objective response, including 6.5% (5 cases) with CR and 16.9% (13 cases) with PR; among other patients 42.9% (33 cases) achieved SD, while 33.8% (26 cases) progressed further. Among patients with immunotherapy alone as TBP, ORR was 13.0% (3/23 patients) while in patients with concurrent surgery and/or radiotherapy—27.8% (15/54 patients) (p = 0.241) (Supp. Tab. 2). Disease control rates (DCR), that is CR, PR, and SD, in this subgroups were 47.8 (11/23 patients) vs. 74.1% (40/54 patients), respectively. Patients with an objective response before the first PD had a higher ORR on TBP. Patients did not suffer from unexpected toxicities neither from immunotherapy nor from local therapies.
Overall at the time of analysis, 58 patients (75.3%) suffered from a second progression. Most patients (50.0%) progressed within different loci (metastases) than during the first progression and progressed by diameter increases in three or more targets (Supp. Tab. 3). The median time to second progression (TTSP) was 8.02 months (95% CI: 3.84–12.20). The median duration of TBP was 9.66 months (m) (95% CI: 5.83–13.49), while the median overall treatment duration with anti-PD-1 antibody was 16.95 (95% CI: 9.58–24.33) months.
TTSP was not correlated with gender, ECOG status, primary tumor location, BRAF mutation status, TNM at treatment initiation, or a number of metastatic loci. Trend toward longer TTSP in patients with TTFP > 6 months and in patients treated with local therapy was recorded; however, statistical significance was not reached in the univariate model. The only factors significantly correlated with TTSP were objective response before the first PD (median TTSP 16.46 vs. 5.75 months) and oligoprogression as a pattern of PD (10.35 vs. 5.03 months). In the multivariate model, only an oligoprogression was a favorable prognostic factor for TTSP (HR 0.55, 95% CI: 0.32–0.94, p = 0.029) (Table 2).
Table 2.
Correlation of patients characteristics with time-to-second progression—a univariate and multivariate analysis
| Factor | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| TTSP [months (95% CI) | p | HR (95% CI) | p | ||
| Gender | Female | 8.81 (0.82–16.79) | 0.08 | ||
| Male | 6.18 (2.01–10.26) | ||||
| ECOG | 0 | 10.12 (4.08–16.16) | 0.336 | ||
| 1 | 6.18 (2.20–10.13) | ||||
| Primary tumor location | Skin | 8.02 (3.86–12.17) | 0.526 | ||
| Mucous | 3.48 (0.00–10.70) | ||||
| FPI | 3.02 (0.00–9.85) | ||||
| BRAF mutation | Positive | 6.93 (0.76–13.11) | 0.357 | ||
| Negative | 8.02 (3.91–12.12) | ||||
| TNM stage | III | NR (NR-NR) | 0.509 | ||
| M1a | 8.74 (1.51–15.97) | ||||
| M1b | 6.93 (0.00–19.48) | ||||
| M1c | 4.96 (3.52–6.40) | ||||
| M1d | 7.36 (3.03–11.69) | ||||
| Line of treatment | 1 | 7.36 (4.13–10.59) | 0.490 | ||
| 2 | 9.82 (0.00–20.48) | ||||
| Number of organ sites with metastasis | ≤ 3 | 8.74 (4.75–12.73) | 0.861 | ||
| 3+ | 4.86 (0.00–10.25) | ||||
| Objective response before first PD | Yes | 16.46 (6.47–26.45) | 0.034 | 0.59 (0.29–1.20) | 0.143 |
| No | 5.75 (3.92–7.58) | 1 | |||
| TTFP | < 6 m | 5.55 (3.99–7.12) | 0.110 | ||
| > 6 m | 11.89 (4.90–18.89) | ||||
| Pattern of progression | Oligoprogression | 10.35 (5.95–14.75) | 0.005 | 0.55 (0.32–0.94) | 0.029 |
| Other | 5.03 (2.77–7.28) | 1 | |||
| TBP modality | ICI | 4.93 (1.89–7.96) | 0.128 | ||
| ICI + local therapy | 9.82 (6.20–13.45) | ||||
TTFP time to first progression, TTSP time to the second progression, TBP treatment beyond progression, ICI immunotherapy with checkpoint inhibitors
Median OS from the first anti-PD1 treatment dose for this population was 34.79 months (95% CI: 25.11–44.47), and 32 patients died at the time of analysis. Median post-PD OS was 28.75 months (95% CI: 20.01–37.40). In the subgroup of patients who experienced second disease progression, the median follow-up was 8.2 months.
In a univariate analysis, objective response on TBP and combination of immunotherapy and local treatment were significantly associated with post-PD OS. In a multivariate model, only the objective response on TBP was significantly correlated with post-PD OS (HR 0.18, 95% CI: 0.04–0.76, p = 0.02) (Table 3).
Table 3.
Correlation of patients characteristics with overall survival after the first progression (post-PD OS)—a univariate and multivariate analysis
| Factor | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| Post-PD OS [months (95% CI) | P | HR (95% CI) | p | ||
| Gender | Female | 28.75 (17.17–41.33) | 0.175 | ||
| Male | 23.56 (10.98–36.14) | ||||
| ECOG | 0 | 28.75 (23.41–34.09) | 0.370 | ||
| 1 | 21.32 (12.13–30.52) | ||||
| Primary tumor location | Skin | 28.75 (20.42–37.08) | 0.637 | ||
| Mucous | NR | ||||
| UPM | NR | ||||
| BRAF mutation | Positive | 26.05 (14.41–37.70) | 0.280 | ||
| Negative | NR | ||||
| TNM stage at PD | III | NR | 0.130 | ||
| M1a | NR | ||||
| M1b | 16.46 (13.57–19.35) | ||||
| M1c | 34.43 (NR-NR) | ||||
| M1d | 17.25 (10.41–24.08) | ||||
| Line of treatment | 1 | 28.75 (11.31–46.18) | 0.783 | ||
| 2 | 23.56 (NR-NR) | ||||
| Number of metastatic sites | ≤ 3 | 28.75 (21.82–35.67) | 0.557 | ||
| 3+ | 17.68 (8.85–26.50) | ||||
| Objective response before the first PD | Yes | NR | 0.360 | ||
| No | 28.75 (19.39–38.11) | ||||
| TTFP | < 6 m | 28.75 (21.44–36.06) | 0.697 | ||
| > 6 m | 34.43 (12.16–56.70) | ||||
| Pattern of disease progression | Oligoprogression | 34.43 (NR-NR) | 0.099 | ||
| Other | 23.56 (14.16–32.96) | ||||
| TBP modality | ICI | 21.32 (6.51–26.13) | 0.024 | 1 | 0.076 |
| ICI + local therapy | NR | 0.53 (0.26–1.07) | |||
| Objective response on TBP | Yes | NR | 0.005 | 0.18 (0.04–0.76) | 0.02 |
| No | 21.32 (12.67–29.97) | 1 | |||
UPM unknown primary melanoma, ICI immunotherapy with checkpoint inhibitors, TTFP time to the first progression, TBP treatment beyond progression
Discussion
In a systemic review, based on 5334 patients treated in 36 immunotherapy trials, 30.2% of patients received treatment beyond progressive disease, and 19.7% of patients achieved response during TBP [18]. In an analysis of individual melanoma patient data pooled from 8 multicenter clinical trials, 19% of patients were treated beyond initially documented RECIST progression, consistent with data reported by us [12]. More detailed analysis of 2 phase III nivolumab trials analysis revealed that 28% of the TBP patients achieve greater than 30% target lesion reduction. In the trial, the median time from progression to last dose of treatment was 4.7 (1.4–25.8) months for TBP patients with 7.6 (2.4–19.4) months for TBP patients who achieved subsequent PR/CR, which is similar to TTSP reported by us. Median time from PD to secondary tumor reduction was 1.4 (0.2–7.0) months in the trials [19] (Supp. Tab. 4).
In the clinical-trial driven data, the median OS in patients who did receive TBP was 24.4 months compared to 11.2 months in those who did not receive such treatment [12]. OS reported by us in the TBP population is consistent with that reported from clinical trials and significantly longer than reported by us previously for the regular treatment beyond clinical trials in our department [4]. Moreover, the time-to-second progression reported by us in this study is more prolonged than PFS on ipilimumab in the second line of treatment (2.8 months) [3]. Overall survival, as well as time to the second progression reported in this study, are also longer than median overall survival (19.9 months) and progression-free survival (7.9 months) reported by us for anti-PD1 treatment in the first line in regular clinical practice in Poland [2].
The efficacy of TBP reported by us in advanced/metastatic melanoma is similar to that reported for lung cancer—where 27% of patients had a progression-free interval over six months after receiving TBP, and a total of 36% achieved a clinical benefit (Supp. Tab. 4) [20]. Other reports also confirmed that in patients who demonstrated RECIST1.1 PD, median OS is significantly longer in patients with TBP (17.2 months) than in those patients without TBP (7.4 months, p < 0.001). Moreover, it was also indicated that the median PFS is significantly longer in patients with psPD (p = 0.02) [8]. In advanced NSCLC, patients treated with immunotherapy for more than six weeks after the initial progression had longer overall survival (median OS 26.6 vs. 9.5 months; HR, 0.40, p < 0.001) and progression-free survival (median PFS, 8.9 vs. 4.1 months; HR, 0.41, p < 0.001), when compared to those not treated. In a more detailed analysis, despite no significant difference in ORR, the DCR was significantly higher in the TBP group [21], which is concordant with our data. Also, data from advanced renal cell carcinoma treated in the expanded access program confirm that selected patients who are offered to continue treatment beyond PD may achieve reduction (CR/PR) or stabilization (SD) of tumor burden, with an acceptable safety profile. In the RCC report, 28% (28/100) patients treated beyond PD achieved clinical benefit—subsequent tumor reduction or stabilization and a median follow-up of 9.2 months with 1-year overall survival of 73.5% [22]. In the phase 2 dose-ranging nivolumab RCC trial, 69% of patients treated more than six weeks beyond progression experienced subsequent tumor reduction or stabilization [7]. In general, it may be suggested that anti-PD1 treatment beyond progression disease may be associated with improved survival in patients with melanoma, renal cell carcinoma, and NSCLC. Post-PD OS tends to be longer in patients continuing immunotherapy beyond PD than in patients switching to other anti-melanoma/anti-cancer therapy [23]. We have also confirmed in melanoma patients that, as reported for NSCLS, local radiotherapy with continued immunotherapy beyond oligoprogression in initial responders leads to prolonged TTSP and OS [24].
It should be remembered that the RECIST scale was developed to improve reproducibility among observers and reduce measurement error, mainly in clinical trials. RECIST measures are not based or correlated to the mechanistic understanding of response to therapy, especially immunotherapy. Therefore the use of immune RECIST (iRECIST) or immune-related RECIST (irRECIST) is expected to be superior to RECIST in the case of immunotherapy. The iRECIST is based on RECIST 1.1, with the major change being the resetting the level for progression if tumor progression in current time point is followed by response or stable disease in follow-up scan [25]. The irRECIST is to be more reliable in determining whether patients benefit from immunotherapy. It must be remembered that data and literature analysis of immune-related RECIST responses can't be compared directly with RECIST responses because they tend to be numerically higher [5]. In fact, PR or CR classically requires confirmation one month later in RECIST 1.1, while ir-RECIST requires confirmation of PD 4–6 weeks later and what is more, the definition of confirmed PD vastly differs between trials and sponsors [26]. In the case of melanoma, in a phase Ib KEYNOTE-001 trial of the 592 patients who survived ≥ 12 weeks, 84 (14%) experienced PD per RECIST v1.1 but not per irRC. It was concluded by the authors that modified criteria prevent premature cessation of treatment and that conventional RECIST analysis underestimates the benefit of pembrolizumab [27]. Moreover, already in selected immunotherapy clinical trials, despite repeated imaging confirming progression after 4–6 weeks, treatment is currently continued based on the assessment by the investigator and medical monitor as long as there is a clinically meaningful benefit [25].
In order to select patients who could benefit from TBP, watchful clinical consideration or tumor board decisions are needed. Additional biomarkers, including NLR or PLT, as well as tumor-related markers, could help in the stratification of patients for such therapy [28]. For RCC nivolumab TBP treatment, it was shown that patients with better Karnofsky performance status, less deterioration in Karnofsky performance status, shorter time to first objective response, lower incidence of new bone lesions, and improved quality of life are correlated with benefit from TBP [29]. In NSCLC, numerically more women, fewer prior therapies, and higher PD-L1 expression were reported in patients TBP with atezolizumab. Moreover, these patients had a higher incidence of multiple brain metastases and a lower incidence of worsened ECOG performance status [30]. Clinical biomarkers that should be used to stratify patients to TBP in melanoma require further analysis. We have found that patients who experienced objective tumor response before and presented with oligoprogressive patterns of progression have longer TTSP and OS. We also observed that performance status might have some impact; however, we did not prove its statistical significance. Due to a small cohort of patients, these markers need to be further validated in future prospective studies and confirmed in a meta-analysis.
Further randomized studies are expected to confirm and fully characterize the potential benefit of treatment beyond progression. The molecular/cellular mechanism by which some patients who experience PD after initial immunotherapy treatment subsequently benefit from TBP immunotherapy is still under investigation. Our and other published data suggest that selected fit patients who are clinically stable despite radiographically progressive disease defined as per RECIST criteria may benefit from continuing anti—PD-1 therapy in melanoma. The reported reduction in tumor burden after initial RECIST-defined progression suggests that selected patients benefit from immunotherapy beyond time point when traditionally treatment would be discontinued. Future investigations are warranted to better define melanoma patients who can benefit from immunotherapy treatment beyond PD, and a prospective trial could include an arm evaluating TBP.
Another unsolved but important issue remains the true benefit of adding local therapy, mainly radiotherapy, to immunotherapy while continuing treatment beyond RECIST progression. We have found that combining immunotherapy with local treatment improves outcomes compared to immunotherapy alone, especially for overall survival. It has not been confirmed in a multivariate model, probably due to the small sample size. A similar observation was made in a large multicenter analysis of patients with solitary progression of melanoma, where TTSP, but not OS, was higher for ICI recommencement plus local therapy than local therapy or ICI recommencement alone [31]. Several studies report the use of irradiation as a "vaccine" to overcome immunotherapy resistance [32–34]. However, the available evidence does not allow for routine use of this approach in clinical practice. Interestingly, the optimal fractionation of stereotactic radiotherapy with concomitant immunotherapy may differ from ablative regimens commonly used in oligometastatic and oligoprogressive stages of other cancers [35, 36]. Further validation of stereotactic radiotherapy in TBP in prospective clinical trials is warranted.
Limitation of this study is its retrospective design. Overall, the findings of our study should be considered with caution and possibly could be evaluated in larger, prospective, trials. Within the limitations of our analysis, this study suggests anti-PD-1 treatment beyond PD may be efficient and safe in selected subgroups of patients with advanced melanoma. As such in selected advanced melanoma patients, local treatment approach, including radiotherapy, contribute to long-term control of oligoprogressive disease, and may enable continued systemic treatment with anti-PD1 therapy. Such multidisciplinary approach may prolong overall survival in selected patients. In general patients who achieved objective responses before first progression, as well as patients with oligoprogression benefit the most from anti-PD1 treatment beyond first RECIST 1.1—defined progression. Multidisciplinary tumor board should evaluate such patients, and radiosurgery or stereotactic radiotherapy of progressing loci should be considered as active treatment option concordant to ani-PD1 treatment. Watchful clinical assessment seems essential when switching therapy in advanced melanoma patients. Molecular biomarkers of TTSP should be analyzed in prospective studies.
Supplementary Information
Below is the link to the electronic supplementary material.
Abbreviations
- CI
Confidence interval
- CR
Complete response
- ECOG
Eastern cooperative oncology group
- FPI
Focus primaries ignotus
- HR
Hazard ratio
- ICIs
Immune checkpoint inhibitors
- irRC
Immune-related response criteria
- NSCLC
Non-small cell lung cancer patients
- OS
Overall survival
- ORR
Objective response rate
- post-PD OS
Overall survival after first progression
- PD
Progressive disease
- PR
Partial response
- psPD
Pseudoprogression
- RCC
Renal cell carcinoma
- RECIST
Response evaluation criteria in solid tumors
- SD
Stable disease
- TBP
Treatment beyond progression
- TNM
The TNM classification of malignant tumors
- TTFP
Time-to-first progression
- TTSP
Time-to-second progression
Authors' contributions
Conceptualization: Anna M. Czarnecka, Paweł Sobczuk; Methodology: Anna M. Czarnecka, Paweł Sobczuk; Formal analysis, treatment and investigation: Anna M. Czarnecka, Pawel Sobczuk, Tomasz Switaj, Pawel Rogala, Mateusz Spalek, Pawel Teterycz, Monika Dudzisz-Śledź, Joanna Placzke, Katarzyna Kozak, Aneta Borkowska, Piotr Rutkowski; Writing—original draft preparation: Anna M. Czarnecka, Paweł Sobczuk; Writing—review and editing: all authors; Supervision: Anna M. Czarnecka, Piotr Rutkowski.
Funding
This work has been supported by Maria Sklodowska-Curie National Research Institute of Oncology statutory funding (Ministry of Education and Science subsidy).
Availability of data and materials
Data are available from the corresponding author upon research-based request and DTA.
Declarations
Conflict of interest
Paweł Sobczuk received travel grants from MSD, Roche, Novartis, and Pierre Fabre, honoraria for lectures from Swixx BioPharma and BMS; he is a stockholder of CelonPharma and has a non-financial interest as ESMO Officer and Member of the Board of Polish Society of Clinical Oncology. Anna M. Czarnecka, Katarzyna Kozak, Paweł Rogala, and Tomasz Świtaj received travel grants from BMS, MSD, Novartis, and Pierre Fabre, honoraria for lectures from BMS, MSD, Pierre Fabre, Novartis, and Pfizer. Paweł Teterycz, Joanna Placzke, and Anna Mariuk-Jarema received travel grants from MSD and BMS. Monika Dudzisz-Śledź received honoraria for lectures from Pierre Fabre, Merck KGaA, Sanofi Aventis, Novartis, and BMS, honoraria for participation in advisory meetings from Merck KGaA and Novartis, and financial support for participation in conferences from Novartis. Aneta Borkowska declare no conflict of interest. Mateusz Spałek received travel grants from BMS, honoraria for lectures from BMS, Novartis, and Roche, and has non-financial interests include being a member of the Young ESTRO Committee, ESTRO Education Council, and Co-president of the Young Section of the Polish Society of Radiation Oncology. Piotr Rutkowski received honoraria for lectures from BMS, Merck, MSD, Novartis, Pierre Fabre, and Sanofi, honoraria for participation in advisory meetings from Blueprint Medicines, BMS, Merck, MSD, Novartis, Philogen, Pierre Fabre and Sanofi, and his non-financial interests include being an ASCO Officer and Member of Board of Directors Polish Society of Surgical Oncology.
Ethics approval
The study has been approved by the local bioethics committee at Maria Sklodowska-Curie National Research Institute of Oncology in Warsaw.
Footnotes
Anna Małgorzata Czarnecka and Paweł Sobczuk are equally contributed to the work.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Blumenthal GM, Theoret MR, Pazdur R. Treatment beyond progression with immune checkpoint inhibitors—known unknowns. JAMA Oncol. 2017;3(11):1473–1474. doi: 10.1001/jamaoncol.2017.1819. [DOI] [PubMed] [Google Scholar]
- 2.Cybulska-Stopa B, et al. First-line treatment of advanced/metastatic melanoma with anti-PD-1 antibodies: multicenter experience in Poland. Immunotherapy. 2021;13(4):297–307. doi: 10.2217/imt-2020-0217. [DOI] [PubMed] [Google Scholar]
- 3.Cybulska-Stopa B, et al. Efficacy of ipilimumab after anti-PD-1 therapy in sequential treatment of metastatic melanoma patients—real world evidence. Adv Med Sci. 2020;65(2):316–323. doi: 10.1016/j.advms.2020.05.005. [DOI] [PubMed] [Google Scholar]
- 4.Czarnecka AM, et al. Treatment sequencing and clinical outcomes in BRAF-positive and BRAF-negative unresectable and metastatic melanoma patients treated with new systemic therapies in routine practice. Target Oncol. 2019;14(6):729–742. doi: 10.1007/s11523-019-00688-8. [DOI] [PubMed] [Google Scholar]
- 5.Daud AI. Revisiting RECIST: the case of treatment beyond progression. Lancet Oncol. 2018;19(2):157–159. doi: 10.1016/S1470-2045(18)30007-X. [DOI] [PubMed] [Google Scholar]
- 6.Ren D, et al. Predictive biomarkers and mechanisms underlying resistance to PD1/PD-L1 blockade cancer immunotherapy. Mol Cancer. 2020;19(1):19. doi: 10.1186/s12943-020-1144-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.George S, et al. Safety and efficacy of nivolumab in patients with metastatic renal cell carcinoma treated beyond progression. JAMA Oncol. 2016;2(9):1179–1186. doi: 10.1001/jamaoncol.2016.0775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Won SE, et al. Impact of pseudoprogression and treatment beyond progression on outcome in patients with non-small cell lung cancer treated with immune checkpoint inhibitors. Oncoimmunology. 2020;9(1):1776058. doi: 10.1080/2162402X.2020.1776058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seymour L, et al. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. 2017;18(3):e143–e152. doi: 10.1016/S1470-2045(17)30074-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Di Giacomo AM, et al. Therapeutic efficacy of ipilimumab, an anti-CTLA-4 monoclonal antibody, in patients with metastatic melanoma unresponsive to prior systemic treatments: clinical and immunological evidence from three patient cases. Cancer Immunol Immunother. 2009;58(8):1297–1306. doi: 10.1007/s00262-008-0642-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ma Y, et al. How to differentiate pseudoprogression from true progression in cancer patients treated with immunotherapy. Am J Cancer Res. 2019;9(8):1546–1553. [PMC free article] [PubMed] [Google Scholar]
- 12.Beaver JA, et al. Patients with melanoma treated with an anti-PD-1 antibody beyond RECIST progression: a US Food and Drug Administration pooled analysis. Lancet Oncol. 2018;19(2):229–239. doi: 10.1016/S1470-2045(17)30846-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gandara D. GR02.04 immunotherapy: hyperprogression and treatment beyond progression. J Thorac Oncol. 2019;14(10):S85. doi: 10.1016/j.jtho.2019.08.195. [DOI] [Google Scholar]
- 14.Eisenhauer EA, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45(2):228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 15.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53(282):457–481. doi: 10.1080/01621459.1958.10501452. [DOI] [Google Scholar]
- 16.Patel PH, et al. The dandelion dilemma revisited for oligoprogression: treat the whole lawn or weed selectively? Clin Oncol (R Coll Radiol) 2019;31(12):824–833. doi: 10.1016/j.clon.2019.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brookmeyer R, Crowley J. A confidence interval for the median survival time. Biometrics. 1982;38(1):29–41. doi: 10.2307/2530286. [DOI] [Google Scholar]
- 18.Spagnolo F, et al. Treatment beyond progression with anti-PD-1/PD-L1 based regimens in advanced solid tumors: a systematic review. BMC Cancer. 2021;21(1):425. doi: 10.1186/s12885-021-08165-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Long GV, et al. Nivolumab for patients with advanced melanoma treated beyond progression. JAMA Oncol. 2017;3(11):1511–1519. doi: 10.1001/jamaoncol.2017.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reinhorn D, et al. Treatment beyond progression with immune checkpoint inhibitors in non-small-cell lung cancer. Immunotherapy. 2020;12(4):235–243. doi: 10.2217/imt-2019-0131. [DOI] [PubMed] [Google Scholar]
- 21.Ge X, et al. Immunotherapy beyond progression in patients with advanced non-small cell lung cancer. Transl Lung Cancer Res. 2020;9(6):2391–2400. doi: 10.21037/tlcr-20-1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cortesi E, et al. Treatment beyond progression in patients with advanced RCC participating in the expanded access programme (EAP) Ann Oncol. 2017;28:v316. doi: 10.1093/annonc/mdx371.046. [DOI] [Google Scholar]
- 23.Enomoto T, et al. Nivolumab treatment beyond progression disease in advanced non-small cell lung cancer. Ann Oncol. 2019;30:xi28. doi: 10.1093/annonc/mdz449.033. [DOI] [Google Scholar]
- 24.Xu Y, Li H, Fan Y. Progression patterns, treatment, and prognosis beyond resistance of responders to immunotherapy in advanced non-small cell lung cancer. Front Oncol. 2021;11:642883. doi: 10.3389/fonc.2021.642883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Somarouthu B, et al. Immune-related tumour response assessment criteria: a comprehensive review. Br J Radiol. 2018;91(1084):20170457. doi: 10.1259/bjr.20170457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Le Lay J, et al. irRECIST and iRECIST: the devil is in the details. Ann Oncol. 2017;28(7):1676–1678. doi: 10.1093/annonc/mdx168. [DOI] [PubMed] [Google Scholar]
- 27.Hodi FS, et al. Evaluation of immune-related response criteria and RECIST v1.1 in patients with advanced melanoma treated with pembrolizumab. J Clin Oncol. 2016;34(13):1510–1517. doi: 10.1200/JCO.2015.64.0391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haddad R, et al. Nivolumab treatment beyond RECIST-defined progression in recurrent or metastatic squamous cell carcinoma of the head and neck in CheckMate 141: a subgroup analysis of a randomized phase 3 clinical trial. Cancer. 2019;125(18):3208–3218. doi: 10.1002/cncr.32190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Escudier B, et al. treatment beyond progression in patients with advanced renal cell carcinoma treated with nivolumab in CheckMate 025. Eur Urol. 2017;72(3):368–376. doi: 10.1016/j.eururo.2017.03.037. [DOI] [PubMed] [Google Scholar]
- 30.Gandara DR, et al. Atezolizumab treatment beyond progression in advanced NSCLC: results from the randomized, phase III OAK study. J Thorac Oncol. 2018;13(12):1906–1918. doi: 10.1016/j.jtho.2018.08.2027. [DOI] [PubMed] [Google Scholar]
- 31.Versluis JM, et al. The role of local therapy in the treatment of solitary melanoma progression on immune checkpoint inhibition: a multicentre retrospective analysis. Eur J Cancer. 2021;151:72–83. doi: 10.1016/j.ejca.2021.04.003. [DOI] [PubMed] [Google Scholar]
- 32.Vanpouille-Box C, et al. In situ vaccination by radiotherapy to improve responses to anti-CTLA-4 treatment. Vaccine. 2015;33(51):7415–7422. doi: 10.1016/j.vaccine.2015.05.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kabiljo J, et al. Radiotherapy as a backbone for novel concepts in cancer immunotherapy. Cancers (Basel) 2019;12(1):79. doi: 10.3390/cancers12010079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chicas-Sett R, et al. Combining radiotherapy and ipilimumab induces clinically relevant radiation-induced abscopal effects in metastatic melanoma patients: a systematic review. Clin Transl Radiat Oncol. 2018;9:5–11. doi: 10.1016/j.ctro.2017.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rogers SJ, et al. Radiotherapy for melanoma: more than DNA damage. Dermatol Res Pract. 2019;2019:9435389. doi: 10.1155/2019/9435389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grapin M, et al. Optimized fractionated radiotherapy with anti-PD-L1 and anti-TIGIT: a promising new combination. J Immunother Cancer. 2019;7(1):160. doi: 10.1186/s40425-019-0634-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available from the corresponding author upon research-based request and DTA.