Abstract
Background
Immunotherapy has largely improved clinical outcome of patients with esophageal squamous cell carcinoma (ESCC). However, a proportion of patients still fail to benefit. Thus, biomarkers predicting therapeutic resistance and underlying mechanism needs to be investigated.
Methods
Transcriptomic profiling was applied in FFPE tissues from 103 ESCC patients, including surgical samples from 66 treatment-naïve patients with long-term follow-up, and endoscopic biopsies from 37 local advanced ESCC cases receiving neoadjuvant immunotherapy plus chemotherapy. Unsupervised clustering indicated an aggressive phenotype with mesenchymal character in 66 treatment-naïve samples. Univariant logistic regression was applied to identify candidate biomarkers potentially predicted resistance to neoadjuvant immunotherapy within the range of mesenchymal phenotype enriched genes. These biomarkers were further validated by immunohistochemistry. Putative mechanisms mediating immunotherapy resistance, as indicated by microenvironment and immune cell infiltration, were evaluated by transcriptomic data, and validated by multiplex immunofluorescence.
Results
PLEK2 and IFI6, highly expressed in mesenchymal phenotype, were identified as novel biomarkers relating to non-MPR in neoadjuvant immunotherapy cohort [PLEK2high, OR (95% CI): 2.15 (1.07–4.33), P = 0.032; IFI6high, OR (95% CI): 2.21 (1.16–4.23), P = 0.016). PLEK2high and IFI6 high ESCC patients (versus low expressed patients) further exhibit higher chance of non-major pathological remissions (90%, P = 0.004) in neoadjuvant immunotherapy cohort and high mortality (78.9%, P = 0.05), poor prognosis in retrospective cohort. PLEK2high/IFI6high ESCC recapitulated mesenchymal phenotype, characterized by extracellular matrix composition and matrix remodeling. In addition, PLEK2high or IFI6high ESCC displayed an immune-unfavored microenvironment, represented by positive correlating with regulatory T cells, Helper 2 T cell as well as less infiltration of B cells, effector T cells and mast cells.
Conclusions
PLEK2 and IFI6 was discovered of first time to identify a distinct ESCC subpopulation cannot be benefited from neoadjuvant immunotherapy and present a poor survival, which putatively associated with mesenchymal and immune-suppressive microenvironment.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00262-022-03288-0.
Keywords: Neoadjuvant immunotherapy, Biomarker, Esophageal squamous cell carcinoma, Tumor microenvironment
Introduction
Esophageal squamous cell carcinoma (ESCC), the predominant form of esophageal cancer (EC), accounts for approximately 90% of patients in East Asia [1]. Even with great efforts and significant achievements regarding early diagnosis and multimodality treatments, ESCC is still a serious medical issue owing to its high morbidity and mortality [2]. It is well known that neoadjuvant chemoradiotherapy (NCRT) has been recommended as the golden standard for locally advanced ESCC by the National Comprehensive Cancer Network and European Society for Medical Oncology guidelines [3, 4], whereas the clinical outcomes of patients remain unfavorable because of postoperative complications caused by NCRT-associated toxicity. Fortunately, this situation was revolutionized due to the application of anti-programmed death 1 (PD-1)/programmed death-ligand 1 (PD-L1) antibodies into clinical practice of ESCC from advanced stage to peri-operative stage, which represented a vital milestone in precision medicine [5–7].
Recently, neoadjuvant chemotherapy plus immune checkpoint inhibitors (ICIs) in ESCC patients with locally advanced disease demonstrated remarkable clinical efficacy with a major pathological remission (MPR) rate of around 50.0% and pathological complete response (pCR) of approximate 30% [8]. However, the treatment findings remained heterogeneous. For those who were intrinsically resistant to immunotherapy, this regimen may delay the patients’ condition, miss the opportunity of radical surgery, and ultimately lead to a worse prognosis. Thus, investigating resistant biomarkers, rather than responsive biomarkers, sought to be more clinically urgent.
Previous investigations on resistant mechanisms of immunotherapy implied the essential roles of tumor microenvironment (TME). Infiltration of immune-suppressive cells including regulatory T cells [9], M2-like monocyte/macrophages and myeloid-derived suppressive cells may contribute to defect immune-surveillance resulting the failure of ICIs treatment [10]. Meanwhile, identification of the presence of massive fibrosis and deposition of extracellular matrix, indicated an immune exclusive phenotype in tumors resistant to ICIs [11]. In ESCC, the chromosome 11q13 amplification, as recently reported, was considered as a putatively negative predictor for immunotherapy in advanced ESCC patients [12]. For locally advanced ESCC patients received with neoadjuvant immunotherapy (NAI), the resistant mechanisms and predictive biomarkers were seldom studied. Most recently, a study reported that combination of low PD-L1 expression and low tumor mutation burden (TMB) can identify potential non-responders [13]. However, the interpretation of TMB remained controversial to date. To address this key problem, we integrated two independent cohorts for identifying potential biomarkers to predict resistance to NAI.
Herein, based on recognition of the biological characters of ESCC in Chinese population, we explored distinct subtypes and associated features of TME by analysis of transcriptomic data. Novel biomarkers, PLEK2 and IFI6, which specifically enriched in mesenchymal subtype, were found to have the potentials for predicting intrinsic resistance to immunotherapy, and patients with overexpression of both genes were more aggressive in nature. Altogether, we successfully established a pre-treatment prediction model for locally advanced ESCC patients, which helped to develop individual treatment strategies.
Materials and methods
Participants and sample collection
103 formalin-fixed paraffin-embedded (FFPE) ESCC samples at Guangdong Provincial People’s Hospital between 2015 and 2021 were collected. Among them, 66 treatment-naive samples were retrospectively collected, and samples from 37 patients received neoadjuvant immunotherapy plus chemotherapy were prospectively collected. The clinicopathological characteristics were listed in Supplementary Table 1/2/3. The neoadjuvant regimen was nab-paclitaxel (250 mg/m2, day 1) and cisplatin (75 mg/m2, day 1) combined with Tirelizumab (200 mg, day 1, 29 cases)/Pembrolizumab (2 mg/kg, 8 cases) in each 21-day cycle. All patients received three cycles of drug treatment before surgical resection. The Response Evaluation Criteria in Solid Tumors, version 1.1 was applied to define time to progression and tumor response in individual patients. The cutoff date for the last follow-up was February 2022. This study was carried out with approval from the Medical Ethics Committee of Guangdong Provincial People’s Hospital.
RNA extraction and library preparation
Total RNA was extracted from FFPE tumor specimens with an AmoyDx® FFPE RNA Extraction Kit. After quantifying RNA concentration, it was fragmented based on the DV200 value estimated by an Agilent 2100 Bioanalyzer System. Subsequently, fragment length was assessed, RNA fragments underwent reverse transcription, complementary DNA synthesis and strand-specific library preparation using the NEBNext® Ultra™ II Directional RNA Library Prep Kit for Illumina®.
Hybrid capture and sequencing
Libraries were captured for RNA expression detection. Library size was assessed using an Agilent 2100 Bioanalyzer. After pooling, libraries were sequenced on an Illumina NovaSeq 6000 instrument (Illumina) with 2 × 150 bp paired end reads. The corresponding experimental operations were performed according to a set of established experimental and data quality control parameters. Then, sequencing data were analyzed and annotated through an in-house developed pipeline.
Gene expression estimation
We mapped RNA-seq paired-end reads to the Homo sapiens genome assembly GRCh37 (hg19) using STAR32 (version 020,201) with transcriptome annotation (Genecode version 20) and gene quantification was performed using RSEM 33 (version v1.2.28). Taking different kinds of library preparation into consideration, we counted coding region reads to calculate transcripts per million (TPM) at the gene level.
Consensus clustering
Consensus Cluster Plus was utilized to identify different subtypes based on the comparison of gene expression profile [14]. Measured by median absolute deviation (> 1), 5000 genes with highly variable expression across samples were used for subsequent clustering. The cumulative distribution function (CDF) was constructed for a range from 2 to 10 consensus clusters. CDF and consensus matrices were used to determine the optimal number of clusters.
Differential gene expression and pathway analysis
Differential expressed genes (DEGs) in each subtype was selected based on following criteria (Specific subtype vs other three subtypes, genes with Log2FC ≥ 1 and adjusted p-values (padj) < 0.1 were defined as DEG) followed by enrichment analysis of Gene Ontology (GO), Kyoto Encyclopedia of Genes and Genomes (KEGG), Hallmark. ESTIMATE enrichment analyses were conducted to clarify the functional roles of subtypes.
Evaluation of infiltrating immune cells in the TME
Single-sample gene set enrichment analysis (ssGSEA) algorithm was used to evaluate the relative abundance of infiltration immune cells in the TME of ESCC. The marker gene set for TME infiltration immune cell type was obtained from Bindea et al. [15]. The enrichment scores calculated by ssGSEA were used to represent the relative abundance of each TME infiltrating cell in ESCC.
Immunohistochemistry (IHC) and multiplex immunofluorescence (mIF)
Both assays were performed as previously described [16]. FFPE tumor samples were analyzed by IHC using antibodies against Ki-67, PD-L1, PLEK2 and IFI6. TME characteristics were further verified with the Akoya OpalTM five-color fluorescent platform. All slides were stained by the Opal Polaris 5 Color Automation IHC Detection Kit for the simultaneous detection and quantification of FCER1A, CD27, CD20, CD8 and DAPI. The degree of immunostaining was scored by two independent pathologists.
Statistical analysis
Unsupervised clustering was performed by R package “Consensus Cluster Profiler”. Differential gene expression was analyzed with R package “DESeq2”. (Single sample) gene set enrichment was conducted by R package “GSVA” and “clusterProfiler” respectively. The P value was calculated via a hypergeometric test and corrected using the Benjamin-Hochberg method. Boxplot was generated by R package “ggplot2.” P values of differential expression and distribution comparisons were obtained with a two-sided Mann–Whitney U test. The “survival” and “survminer” packages in R were used to perform Kaplan–Meier analysis. The significance of disease-free survival (DFS) or overall survival (OS) of patients was tested by a two-sided log-rank test. Receiver operator characteristic (ROC)-area under the curve (AUC) analysis was performed by R package “pROC”. Subtype prediction model was constructed trough R package “randomForest”. In this study, R software (V4.0.5) was applied.
Results
Baseline characteristics
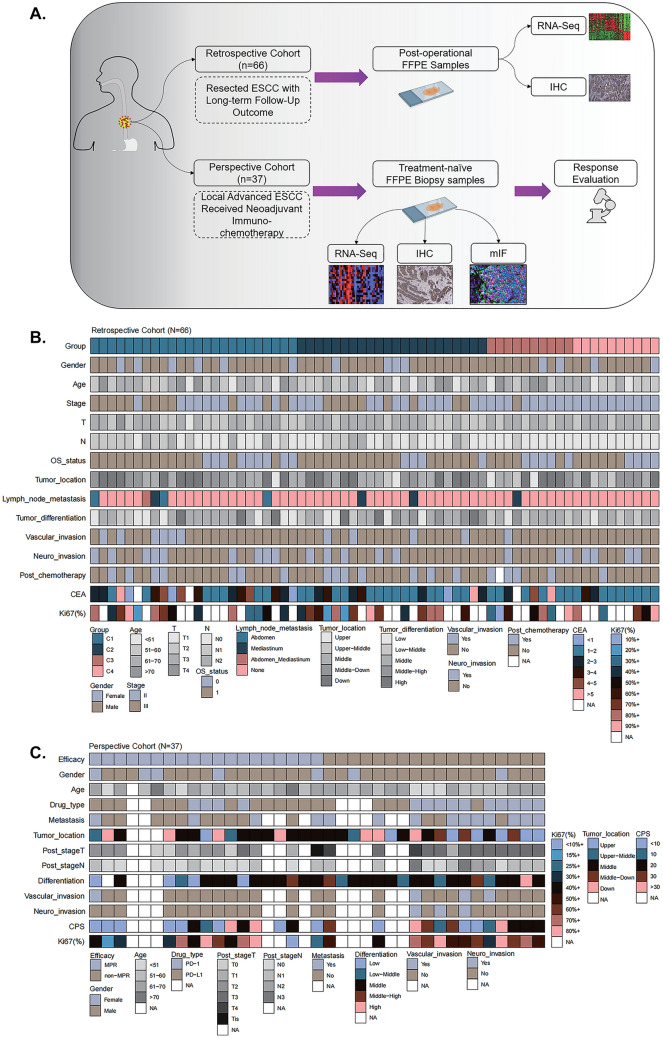
In total, 103 patients with ESCC were included in this study (Fig. 1A), with 66 patients in retrospective cohort, and 37 patients in perspective cohort.
Fig. 1.
Schematic illustration of study design and the baseline characteristics of patients. A Total of 103 patients from Guangdong Provincial People’s Hospital between 2015 and 2021 were included and all formalin-fixed paraffin-embedded (FFPE) ESCC samples subjected to RNA expression profiling via RNA sequencing. Patients were divided into retrospective cohort (Resected ESCC with long-term follow-up outcome; N = 66) and prospective cohort (Received neoadjuvant immuno-chemotherapy; N = 37) B–C. Heatmaps based on baseline characteristics of retrospective cohort and prospective cohort
In the retrospective cohort, the median age of the patients was 60.8 (range, 45.0–83.2) years, and 21.2% were women. All patients had undergone surgery and long-term follow-up data were available. At the end of follow-up period, 66.7% (44/66) of patients were alive with 44 cases in stage II and 22 in stage III. Among these, 14 patients (21.2%) received adjuvant chemotherapy, and 5 cases (7.6%) received adjuvant CRT (Fig. 1B and Table S1).
In the perspective cohort, 19 patients (51.4%) achieved MPR. Notably, the median combined positive score (CPS) of non-MPR and MPR patients were 20 (1.0, 60.0) and 4.25 (0, 90.0), respectively (Fig. 1C and Table S2).
PLEK2 and IFI6, as novel mesenchymal subtype-specific genes, associated with response to NAI
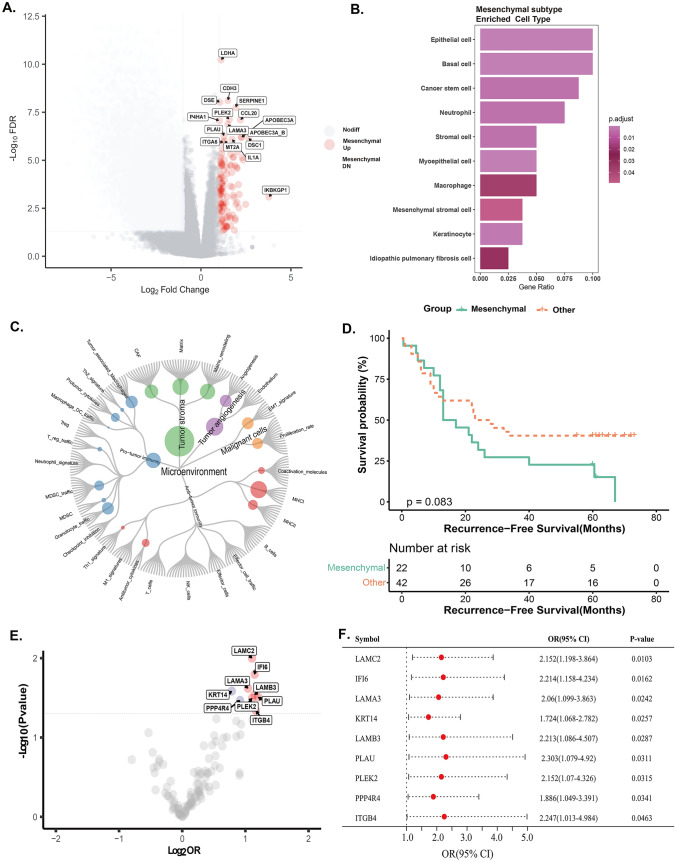
Mesenchymal subtype was identified via unsupervised clustering of transcriptome data from 66 patients (Fig. S1A-E). As exhibiting higher stromal score, enrichment of extracellular matrix features (Fig. S2) and stromal, mesenchymal cell types and microenvironment (Fig. 2A-C), patients of mesenchymal subtype showed worse recurrence-free survival (RFS) than those in other groups (P = 0.0843) (Fig. 2D). Mesenchymal subtype-specific upregulated genes including PLEK2 and IFI6 were significantly associated with the therapeutic efficacy of NAI (P < 0.05) in perspective cohort (Fig. 2E-F). Due to being closely related to cell apoptosis and invasion in ESCC, but seldom investigated in immunotherapeutic response, PLEK2 and IFI6 were further investigated.
Fig. 2.
Identifying the differential genes in retrospective cohort and prospective cohort. A Differential gene expression analysis according to major pathologic responders (MPR) (log2FC > 1, padj < 0.1). Red points represent highly expressed genes in mesenchymal subtype. B GSEA analysis of differentially expressed genes based on the GO-CC biological process enrichment. C Molecular functional portrait (potential targetable genes, signaling pathways, and cellular processes related to each of 29 TME gene expression signatures created by Bagaev et al. [47]) of the mesenchymal subtype. D Comparison of recurrence-free survival between mesenchymal and non-mesenchymal subtypes in retrospective cohort. E Univariate logistic regression analysis was performed and further identified 9 prognostic genes. F Forest plot for association between 9 genes and major pathological remission
PLEK2high or IFI6high expression predicts poor prognosis and NAI efficacy
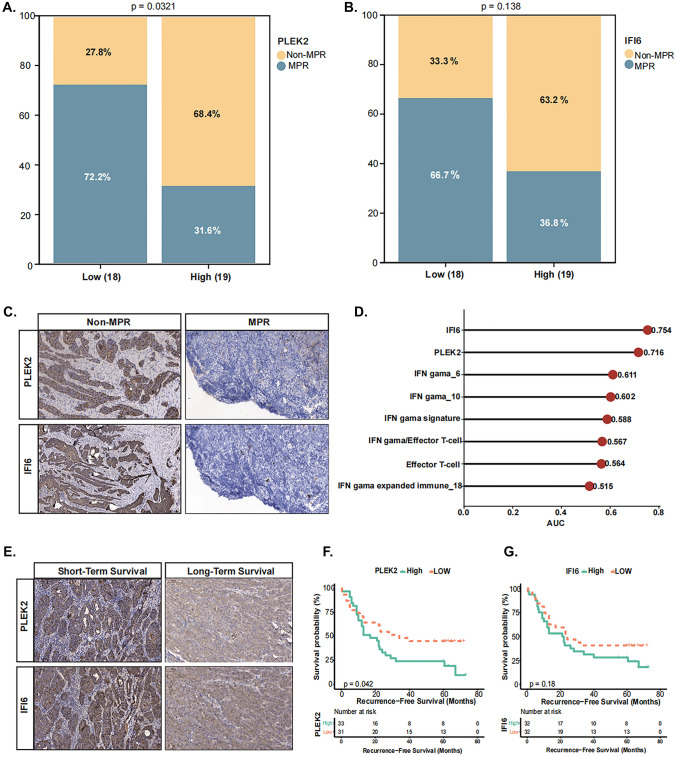
Median expressional level of these two genes in each cohort defined PLEK2high/PLEK2low or IFI6high/IFI6low groups. In perspective cohort, non-MPR rate was remarkably higher in PLEK2high patients (13/19, 68.4%) than PLEK2low patients (10/18, 27.8%) (P = 0.0321) (Fig. 3A). Similarly, IFI6high patients had more probability of non-MPR (12/19, 63.2%) than IFI6 low patients (6/18, 33.3%), although it was not statistically significant (P = 0.138) (Fig. 3B). Moreover, at protein level, IFI6 or PLEK2 was highly expressed in non-MPR ESCC samples (Fig. 3C), and the AUC value of the two genes were 0.754 and 0.716 respectively, obviously superior to those of IFN-γ and Effector T cell related models reported previously (Fig. 3D). These data indicated that patients with PLEK2high or IFI6high expression presented poor immunotherapy response. In the retrospective cohort, PLEK2 high and IFI6 high patients displayed short-term survival (Fig. 3E). The Kaplan–Meier survival analysis further revealed that PLEK2 high was significantly associated with shorter RFS (P = 0.042), and a similar trend, but not significant, can be observed in IFI6 high group (P = 0.18) (Fig. 3F, G).
Fig. 3.
PLEK2high and IFI6high expression predicts therapeutic efficacy and resistance to neoadjuvant immunotherapy in ESCC. A–B. In perspective cohort, stacked column graph showing the distribution of non-MPR and MPR patients in groups with different levels of PLEK2 and IFI6 expression. C Patients who received neoadjuvant immunotherapy were analyzed using immunohistochemistry for PLEK2 and IFI6. D AUC values for different genes are shown. E PLEK2 and IFI6 expression in retrospective cohort. F-G Kaplan–Meier analyses of RFS stratified by the expression of PLEK2 and IFI6 in retrospective cohort. Data were analyzed by log-rank test. MPR: major pathological remission
PLEK2high or IFI6high ESCC associated with immunosuppressive context of tumor microenvironment
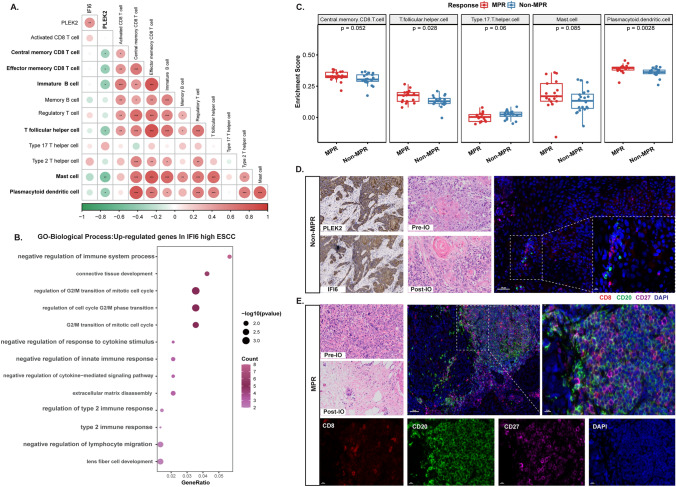
PLEK2high expression reversely correlated with immune-activated features like memory CD8 + T cell, mast cell, (immature/activated) B cell and plasmacytoid dendritic cell (Fig. 4A, Fig. S3). And positive association with immune-suppressive T cells (Regulatory T cells and Th2 cells) as well as mesenchymal phenotypes (Fig. S4). IFI6high group was associated with higher levels of immune suppressive context characterized by negative regulation of immune function (Fig. 4B) and positive association with immune-suppressive T cell subtypes (T helper 2 and 17 cells) and production of related interleukins (Fig. S5A). IFI6 upregulation also associated as enrichment of type I/II interferon response (Fig. S5B). IFI6 high patients also showed elevated activated CD4+ T cells and positively correlated with Regulatory T cells (Fig. S6A, B and E). In addition, IFI6 high recapitulated the phenotype of mesenchymal by elevated level of “Matrix” and “Matrix remodeling” (Fig. S6C, D and F).
Fig. 4.
Characterization of the immune microenvironment of ESCC patients with PLEK2high and IFI6high expression. A Correlation map and heatmaps analyzing the association of PLEK2 and IFI6 genes to immune cell gene signatures in the 28 Immune Cells (Prospective cohort). Dot color represents the pearson correlation coefficient, dot size represents the absolute value of the correlation coefficient. *, **, and *** represent P < 0.05, P < 0.01, and P < 0.001, respectively. B The results of GO-BP biological process enrichment in IFI6 highly expressed samples. C Boxplots indicating the fraction of 28 immune cells in non-MPR and MPR group in 37 samples. D–E Representative H&E, immunohistochemistry, and multiplex immunofluorescence staining results of the patients from mesenchymal subtype. nuclei (DAPI, blue), CD8 (red), CD20 (green) and CD27 (purple). IO: Immunotherapy, MPR: major pathological remission
Relative abundance of the 28 immune cell populations in each sample using ssGSEA algorithm was estimated. Th17 cells shows significant enrichment in No-MPR subgroup. We also found that, other immune cell types including CD8 T cells (P = 0.052), T follicular helper cells (P = 0.028), mast cells (P = 0.085) and plasmacytoid dendritic cells (P = 0.0028) were also enriched in MPR subgroup (Fig. 4C).
Validation of distinct therapeutic response related TME features by mIF, and results showed that limited CD20+/CD27±, CD8+/CD27±, and almost no Fc-epsilon+ cells in non-MPR patients (Fig. S7A). Similarly, patients with enhanced PLEK2 and IFI6 had less infiltration of immature/activated B cells (CD20+/CD27− and CD20+/CD27+), and (memory) effector CD8+ T cells (CD8+/CD27±) (Fig. 4D). In contrast, patients with MPR to NAI displayed higher infiltration of immature/activated B cells, (memory) effector CD8+ T cells and Fc-epsilon+ mast cells (Fig. 4E, Fig. S7B).
Combination of PLEK2high and IFI6high expression predicts poor resistance to NAI and poor prognosis in ESCC
Due to significant co-expression of PLEK2 and IFI6, it was hypothesized that whether combination of PLEK2high and IFI6high expression would improve the accuracy of prediction of NAI efficacy and survival outcomes compared with either single biomarker.
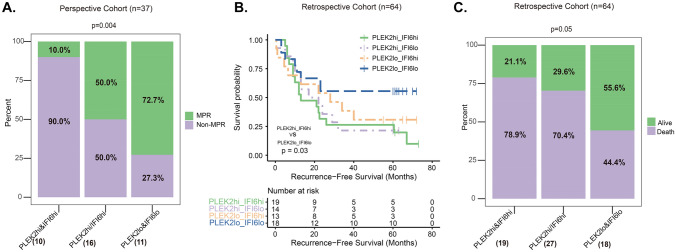
Patients in perspective cohort were divided into 3 groups according to different PLEK2 and IFI6 status: PLEK2high and IFI6high group, PLEK2high or IFI6high group and PLEK2low and IFI6low group. In perspective cohort, PLEK2high and IFI6high patients (10/37, 27.0%) had significantly higher non-MPR events (non-MPR 90% [9/10] vs MPR 10% [1/10], P = 0.004) (Fig. 5 A). PLEK2high or IFI6high patients (43.2%) had moderate non-MPR events (non-MPR 50% [8/16] vs MPR 50% [8/16]). PLEK2low and IFI6low patients (11/37, 29.7%) benefited more from NAI (non-MPR 27.3% [3/11] vs MPR 72.7% [8/11]).
Fig. 5.
Combination of PLEK2high and IFI6high expression predicts poor prognosis and resistance to neoadjuvant immunotherapy in ESCC. A In perspective cohort, the distribution of non-MPR and MPR patients among three different combination groups. B Kaplan–Meier survival analyses of RFS for the four groups in retrospective cohort (PLEK2high and IFI6high expression group, PLEK2high and IFI6low expression group, PLEK2low and IFI6high expression group, and PLEK2low and IFI6low expression group). C In retrospective cohort, the distribution of alive and dead patients. MPR: major pathological remission
In retrospective cohort (N = 64, 2 patients with double primary malignant tumors were excluded), the Kaplan–Meier survival analysis revealed that patients with PLEK2high and IFI6high patients (27/64, 42.2%) had shorter RFS than those with PLEK2low and IFI6low expression (18/64, 28.1%) (P = 0.03), and a similar but not statistically significant (P = 0.10) outcome was observed in PLEK2high or IFI6high patients (19/64, 29.7%) (Fig. 5B). Mortality of PLEK2high and/or IFI6high patients was significantly higher (P = 0.05), 78.9% (15/19) and 70.4% (19/27) in PLEK2high and IFI6high and PLEK2high or IFI6high patients respectively, than that of PLEK2low and IFI6low patients, 44.4% (8/18) (Fig. 5C). Recurrence and mortality rates in four different sub-groups were also similar (Fig.S8A and S8B).
Discussion
The conclusive evidence from neoadjuvant studies have confirmed that preoperative application of immunotherapy largely shrinks the tumor size in most patients with ESCC at localized advanced stage, which greatly improves the success rate of radical surgery [13, 17]. Unfortunately, part of patients is resistant to immunotherapy by either intrinsic or adapted mechanisms. Accordingly, rather than selecting patients who may benefit from ICIs, predicting patients potentially resistant to ICIs are more clinically valuable. In this study, a systematic series of analyses and experiments were undertaken to screen a panel of candidate biomarkers for indicating therapeutic response, especially non-MPR in locally advanced ESCC patients.
Herein, a ‘biology-centric’ strategy was applied to precisely target potential biomarkers. As indicated by unsupervised clustering using RNA-sequencing data generated from retrospective cohort, a significant inter-tumoral heterogeneity was discovered. Notably, mesenchymal, and immune-enriched subtypes displayed the worst and the best prognosis respectively, which were in parallel with previous reported studies by bulk and single-cell RNA sequencing [18–20].
Due to the immune-suppressive features of mesenchymal subtype, subtype-specific genes were selected as candidate “resistance” biomarkers. Among them, nine genes were remarkably associated with non-MPR in NAI cohort (N = 37). Specifically, because of being seldom reported in ESCC, PLEK2 and IFI6 were selected and validated. In NAI cohort, as defined by IHC, cancer cell expressed both PLEK2 and IFI6 displayed higher accuracy predicting therapeutic response than widely used IFN-γand Teff signatures [21]. Upregulation of the two genes could recapitulate the mesenchymal features of “connective tissue development”, “Matrix” and “Matrix Remodeling”. Moreover, overexpression of IFI6 may increase cell cycle activity and induce an immunosuppressive microenvironment. As illustrated by Liu et al. [22], high level of IFI6 expression was correlated with aggressive ESCC phenotype and reduced apoptosis of cancer cells. Accumulated deposition of IFI6 in mitochondrial inner membrane of malignant cells can contribute to decreased production of mitochondrial Reactive Oxygen Specials (mtROS) via interrupting mitochondria calcium influx. It is well known that ROS may trigger and play vital roles in anti-tumor immunity such as activating M1-macrophages, Th1 cells, B cells and cytotoxic T cells [23, 24]. Accord with lately released data, addition to significantly associated with regulatory T cells which was a typical cell type mediating immune tolerance [25], increased IFI6 was also related with induction of a type 2/type 17 helper T cell skewing and production of immune-suppressive type 2 and 17 related cytokines [26] like IL4, IL13 [27] and IL23 [28, 29]. These results indicated multifaceted suppressive roles of IFI6 in modulating TME of ESCC. As supported, a recent released biomarker analysis of a phase I/II immunotherapy study in advanced ESCC suggested Th17 signature was associated with poor therapeutic response [30].
Also, PLEK2 overexpression decreased the mtROS production through preventing translocation of cofilin induced ROS production [31]. In this study, negative correlation of PLEK2 upregulation with pro-inflammatory immune cell infiltrations (B cell, Follicular helper T cells, CD8 positive T cells) were observed. As shown in diverse types of cancers including ESCC [32, 33], the presence of tertiary lymphoid structures (TLS) consisting of B cells, follicular helper T cells etc. might aid the maturation of cytotoxic T cells and predict response to immunotherapy. Our outcomes confided this phenomenon, and showed depleted presence of effector/memorial B cells (CD20, CD27) [34, 35] and activated cytotoxic T cells (CD8, CD27) [36, 37] in PLEK2 overexpressed ESCC samples. As internally validated, B cell and cytotoxic T cells was also enriched in patients with major pathological response. PLEK2 was also observed to be an indicator for poor prognosis, which was in line with previous finding from Wang F et al. [38]. In addition, a recent study indicated that PLEK2 was induced by TGF-βsignature through Smad2/3 manners[38]. Together with its downstream, LCN2, a secreted glycoprotein potentially heterodimerized with MMP-9 [39], PLEK2 may activate PI3K/AKT pathway promoting cell survival and progression [40, 41]. Particularly, an unexpected, but interesting finding in our study is the potential contribution of tumor infiltrating mast cells to the response to ICIs, which was never reported before. While for the prognostic relevance of mast cell was controversial [42–44]. Mast cell infiltration has been observed in pre-treated ESCC samples with MPR outcome, and its activation recruited effector T cells by secretion of CXCL10, predicting an good response to ICIs [45]. A study in gastric cancer also implicated that tumor infiltrated mast cell enhanced PD-L1 expression in cancer cells, implying the sensibility to anti-PD-L1 antibodies [46]. Here, our data showed PLEK2 was negatively correlated with mast cell in NAI cohort.
In short, all above evidence have corroborated the predictive value of PLEK2 or IFI6 gene-expression for the diagnosis and treatment of ESCC. However, when two genes were co-expressed, a greater pro-tumor effect was founded, suggesting a synergistic interaction between the two genes. While no studies have shown cooperativity between PLEK2 and IFI6 gene, this is certainly a possibility. Further studies are needed to determine the precise mechanism of the synergistic effect.
Nevertheless, several limitations should be taken into consideration. Firstly, the prospective cohort was composed of real-world patients which were eligible for NAI, and the therapeutic regimens were not uniformed including different anti-PD-1 disciplines. Secondly, sample size of both cohorts was limited. Typically, large sample size is often required to perform unsupervised clustering, and small samples were only eligible for analysis. Inspiringly, in this study, separation of four subtypes with distinct biological characteristics indicated the accuracy of the clustering even limited sample size was applied. Thirdly, owing to the complexity of host-immune microenvironment, functional experiments disclosing molecular mechanisms and cellular interactions of our findings are needed.
Conclusions
Altogether, PLEK2 and IFI6, for the first time, acted as a combinatorial biomarker defined a subpopulation of ESCC patients with immunotherapeutic resistance and poor survival because of modulating mesenchymal and immune-suppressive microenvironment by molecular-specific mechanisms. Patients with PLEK2high and IFI6high expression may require closer follow-up and minimal/molecular residual disease (MRD) monitoring for disease progression and relapse. Validation by perspective, multi-center investigation with larger sample size is warranted and is under designing.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
This work was supported by the Key Lab System Project of Guangdong Science and Technology Department, Guangdong Provincial Key Lab of Translational Medicine in Lung Cancer (Grant No. 2017B030314120), Guangdong Provincial People's Hospital Scientific Research Funds for Leading Medical Talents in Guangdong Province (Grant No. KJ012019426), and the Research Start-up Funds for National Natural Science Foundation of China (Grant No. 8200110771, 8190110562). We thank the members of Qian Li, Zhan Huang, and Jing Wang for discussion and helpful comments on the article.
Abbreviations
- AUC
Area under curve
- CPS
Combined positive score
- CDF
Cumulative distribution function
- DEG
Differential gene expression
- DFS
Disease-free survival
- ESCC
Esophageal squamous cell carcinoma
- EC
Esophageal cancer
- FFPE
Formalin-fixed paraffin-embedded
- GO
Gene ontology
- ICIs
Immune checkpoint inhibitors
- IHC
Immunohistochemistry
- KEGG
Kyoto encyclopedia of genes and genomes
- MPR
Major pathological remission
- MRD
Minimal/molecular residual disease
- mIF
Multiplex immunofluorescence
- NCRT
Neoadjuvant chemoradiotherapy
- NAI
Neoadjuvant immunotherapy
- OS
Overall survival
- PD-1
Programmed death 1
- PD-L1
Programmed death-ligand 1
- PFS
Progression-free survival
- ROC
Receiver operator characteristic
- RFS
Recurrence-free survival
- ROS
Reactive oxygen specials
- ssGSEA
Single-sample gene set enrichment analysis
- TME
Tumor microenvironment
- TMB
Tumor mutation load
- TPM
Transcripts per million
- TLS
Tertiary lymphoid structures
Authors contributions
Conceptualization, Y-LW; writing-review and editing, Y-LW, JT and DM; writing-original draft and data curation, J-H L; Funding acquisition, HC; methodology and software, C-B Z, J-T Z and S-T Z; resources, G-B Q, J-M T and YC; formal analysis, C-B Z, W-W L and HC; visualization, W-W L; investigation, Z-H C and S-Y W; validation, `H–X T, Z-H C and S-Y W; supervision, Y-LW, JT and G-B Q. All authors have read and agreed to the published version of the manuscript.
Availability of data and materials
The data and materials that supports the findings of this study are available from the corresponding author upon reasonable request.
Declarations
Conflict of interest
Yi-Long Wu declares speaker fees from AstraZeneca, Eli Lilly, Pfizer, Roche, and Sanofi. Weiwei Li and Changbin Zhu were employed by Amoy Diagnostics Co., Ltd. None of the other authors have any conflicts of interest to declare.
Ethics approval and consent to participate
The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Research Ethics Committee of Guangdong Provincial People's Hospital (No. KY-Z-2022–006-02). All subjects gave their informed consent for inclusion before they participated in this study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jianhua Liu, Hao Chen and Guibin Qiao these authors contributed to the work equally.
Contributor Information
Dong Ma, Email: dr_madong@163.com.
Jie Tian, Email: jie.tian@ia.ac.cn.
Yi-Long Wu, Email: syylwu@live.cn.
References
- 1.Lin Y, Totsuka Y, He Y, Kikuchi S, Qiao Y, Ueda J, Wei W, Inoue M, Tanaka H. Epidemiology of esophageal cancer in Japan and China. J Epidemiol. 2013;23:233–242. doi: 10.2188/jea.JE20120162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shao Y, Geng Y, Gu W, Ning Z, Huang J, Pei H, Jiang J. Assessment of lymph node ratio to replace the pN categories system of classification of the TNM system in esophageal squamous cell Carcinoma. J Thorac Oncol. 2016;11:1774–1784. doi: 10.1016/j.jtho.2016.06.019. [DOI] [PubMed] [Google Scholar]
- 3.Yoon YC, Lee KS, Shim YM, Kim BT, Kim K, Kim TS. Metastasis to regional lymph nodes in patients with esophageal squamous cell carcinoma: CT versus FDG PET for presurgical detection prospective study. Radiology. 2003;227:764–770. doi: 10.1148/radiol.2281020423. [DOI] [PubMed] [Google Scholar]
- 4.Ajani JA, D'Amico TA, Bentrem DJ, Chao J, Corvera C, Das P, Denlinger CS, Enzinger PC, Fanta P, Farjah F, et al. Esophageal and esophagogastric junction cancers, version 2.2019, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2019;17:855–883. doi: 10.6004/jnccn.2019.0033. [DOI] [PubMed] [Google Scholar]
- 5.Wang ZX, Cui C, Yao J, Zhang Y, Li M, Feng J, Yang S, Fan Y, Shi J, Zhang X, et al. Toripalimab plus chemotherapy in treatment-naive, advanced esophageal squamous cell carcinoma (JUPITER-06): a multi-center phase 3 trial. Cancer Cell. 2022;40(277–288):e273. doi: 10.1016/j.ccell.2022.02.007. [DOI] [PubMed] [Google Scholar]
- 6.Sun JM, Shen L, Shah MA, Enzinger P, Adenis A, Doi T, Kojima T, Metges JP, Li Z, Kim SB, et al. Pembrolizumab plus chemotherapy versus chemotherapy alone for first-line treatment of advanced oesophageal cancer (KEYNOTE-590): a randomised placebo-controlled phase 3 study. Lancet. 2021;398:759–771. doi: 10.1016/S0140-6736(21)01234-4. [DOI] [PubMed] [Google Scholar]
- 7.Luo H, Lu J, Bai Y, Mao T, Wang J, Fan Q, Zhang Y, Zhao K, Chen Z, Gao S, et al. Effect of camrelizumab vs placebo added to chemotherapy on survival and progression-free survival in patients with advanced or metastatic esophageal squamous cell carcinoma: the ESCORT-1st randomized clinical trial. JAMA. 2021;326:916–925. doi: 10.1001/jama.2021.12836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shang X, Zhao G, Liang F, Zhang C, Zhang W, Liu L, Li R, Duan X, Ma Z, Yue J, et al. Safety and effectiveness of pembrolizumab combined with paclitaxel and cisplatin as neoadjuvant therapy followed by surgery for locally advanced resectable (stage III) esophageal squamous cell carcinoma: a study protocol for a prospective, single-arm, single-center, open-label, phase-II trial (Keystone-001) Ann Transl Med. 2022;10:229. doi: 10.21037/atm-22-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ellis GI, Riley JL. How to kill Treg cells for immunotherapy. Nat Cancer. 2020;1:1134–1135. doi: 10.1038/s43018-020-00155-8. [DOI] [PubMed] [Google Scholar]
- 10.Fares CM, Van Allen EM, Drake CG, Allison JP, Hu-Lieskovan S. Mechanisms of resistance to immune checkpoint blockade: why does checkpoint inhibitor immunotherapy not work for all patients? Am Soc Clin Oncol Educ Book. 2019;39:147–164. doi: 10.1200/EDBK_240837. [DOI] [PubMed] [Google Scholar]
- 11.Galbo PM, Jr, Zang X, Zheng D. Molecular features of cancer-associated fibroblast subtypes and their implication on cancer pathogenesis, prognosis, and immunotherapy resistance. Clin Cancer Res. 2021;27:2636–2647. doi: 10.1158/1078-0432.CCR-20-4226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feng Wang CR, QZ, Nong Xu, Lin Shen, Guanghai Dai, Xianglin Yuan, Ye Chen, Shujun Yang, Jianhua Shi, Xichun Hu, Xiaoyan Lin, Qingyuan Zhang, Jifeng Feng, Yi Ba, Yunpeng Liu, Wei Li, Yongqian Shu, Fenghua Wang, Rui-hua Xu. Association of frequent amplification of chromosome 11q13 in esophageal squamous cell cancer with clinical benefit to immune check point blockade. J Clin Oncol. 2019;37:4036. doi: 10.1200/JCO.2019.37.15_suppl.4036. [DOI] [Google Scholar]
- 13.Yang W, Xing X, Yeung SJ, Wang S, Chen W, Bao Y, Wang F, Feng S, Peng F, Wang X, et al. Neoadjuvant programmed cell death 1 blockade combined with chemotherapy for resectable esophageal squamous cell carcinoma. J Immunother Cancer. 2022;10(1):3049. doi: 10.1136/jitc-2021-003497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamamoto S, Kato K, Daiko H, Kojima T, Hara H, Abe T, Tsubosa Y, Nagashima K, Aoki K, Mizoguchi Y, et al. Feasibility study of nivolumab as neoadjuvant chemotherapy for locally esophageal carcinoma: FRONTiER (JCOG1804E) Future Oncol. 2020;16:1351–1357. doi: 10.2217/fon-2020-0189. [DOI] [PubMed] [Google Scholar]
- 15.Bindea G, Mlecnik B, Tosolini M, Kirilovsky A, Waldner M, Obenauf AC, Angell H, Fredriksen T, Lafontaine L, Berger A, et al. Spatiotemporal dynamics of intratumoral immune cells reveal the immune landscape in human cancer. Immunity. 2013;39:782–795. doi: 10.1016/j.immuni.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 16.Taube JM, Akturk G, Angelo M, Engle EL, Gnjatic S, Greenbaum S, Greenwald NF, Hedvat CV, Hollmann TJ, Juco J, Parra ER, Rebelatto MC, Rimm DL, Rodriguez-Canales J, Schalper KA, Stack EC, Ferreira CS, Korski K, Lako A, Rodig SJ, Schenck E, Steele KE, Surace MJ, Tetzlaff MT, von Loga K, Wistuba II, Bifulco CB. The Society for Immunotherapy of Cancer statement on best practices for multiplex immunohistochemistry (IHC) and immunofluorescence (IF) staining and validation. J Immunother Cancer. 2020;8(1):e000155. doi: 10.1136/jitc-2019-000155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu J, Li J, Lin W, Shao D, Depypere L, Zhang Z, Li Z, Cui F, Du Z, Zeng Y, et al. Neoadjuvant camrelizumab plus chemotherapy for resectable, locally advanced esophageal squamous cell carcinoma (NIC-ESCC2019): a multicenter, phase 2 study. Int J Cancer. 2022;151:128–137. doi: 10.1002/ijc.33976. [DOI] [PubMed] [Google Scholar]
- 18.Wang F, Yan Z, Lv J, Xin J, Dang Y, Sun X, An Y, Qi Y, Jiang Q, Zhu W, et al. Gene expression profiling reveals distinct molecular subtypes of esophageal squamous cell carcinoma in Asian populations. Neoplasia. 2019;21:571–581. doi: 10.1016/j.neo.2019.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zheng Y, Chen Z, Han Y, Han L, Zou X, Zhou B, Hu R, Hao J, Bai S, Xiao H, et al. Immune suppressive landscape in the human esophageal squamous cell carcinoma microenvironment. Nat Commun. 2020;11:6268. doi: 10.1038/s41467-020-20019-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dinh HQ, Pan F, Wang G, Huang QF, Olingy CE, Wu ZY, Wang SH, Xu X, Xu XE, He JZ, et al. Integrated single-cell transcriptome analysis reveals heterogeneity of esophageal squamous cell carcinoma microenvironment. Nat Commun. 2021;12:7335. doi: 10.1038/s41467-021-27599-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cristescu R, Nebozhyn M, Zhang C, Albright A, Kobie J, Huang L, Zhao Q, Wang A, Ma H, Alexander Cao Z, et al. Transcriptomic determinants of response to pembrolizumab monotherapy across solid tumor types. Clin Cancer Res. 2022;28:1680–1689. doi: 10.1158/1078-0432.CCR-21-3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu Z, Gu S, Lu T, Wu K, Li L, Dong C, Zhou Y. IFI6 depletion inhibits esophageal squamous cell carcinoma progression through reactive oxygen species accumulation via mitochondrial dysfunction and endoplasmic reticulum stress. J Exp Clin Cancer Res. 2020;39:144. doi: 10.1186/s13046-020-01646-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nathan C, Cunningham-Bussel A. Beyond oxidative stress: an immunologist's guide to reactive oxygen species. Nat Rev Immunol. 2013;13:349–361. doi: 10.1038/nri3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lloberas J, Munoz JP, Hernandez-Alvarez MI, Cardona PJ, Zorzano A, Celada A. Macrophage mitochondrial MFN2 (mitofusin 2) links immune stress and immune response through reactive oxygen species (ROS) production. Autophagy. 2020;16:2307–2309. doi: 10.1080/15548627.2020.1839191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mair F, Erickson JR, Frutoso M, Konecny AJ, Greene E, Voillet V, Maurice NJ, Rongvaux A, Dixon D, Barber B, et al. Extricating human tumour immune alterations from tissue inflammation. Nature. 2022;605:728–735. doi: 10.1038/s41586-022-04718-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Munoz M, Hegazy AN, Brunner TM, Holecska V, Marek RM, Frohlich A, Lohning M (2021) Th2 cells lacking T-bet suppress naive and memory T cell responses via IL-10. Proc Natl Acad Sci USA 118. [DOI] [PMC free article] [PubMed]
- 27.Wynn TA. Type 2 cytokines: mechanisms and therapeutic strategies. Nat Rev Immunol. 2015;15:271–282. doi: 10.1038/nri3831. [DOI] [PubMed] [Google Scholar]
- 28.Mikami Y, Philips RL, Sciume G, Petermann F, Meylan F, Nagashima H, Yao C, Davis FP, Brooks SR, Sun HW, et al. MicroRNA-221 and -222 modulate intestinal inflammatory Th17 cell response as negative feedback regulators downstream of interleukin-23. Immunity. 2021;54(514–525):e516. doi: 10.1016/j.immuni.2021.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Walch-Ruckheim B, Stroder R, Theobald L, Pahne-Zeppenfeld J, Hegde S, Kim YJ, Bohle RM, Juhasz-Boss I, Solomayer EF, Smola S. Cervical cancer-instructed stromal fibroblasts enhance IL23 expression in dendritic cells to support expansion of Th17 cells. Cancer Res. 2019;79:1573–1586. doi: 10.1158/0008-5472.CAN-18-1913. [DOI] [PubMed] [Google Scholar]
- 30.Jianming Xu NX, Bai Y, Lin C-C, Millward M, Shi J, Zhang Y, Ma X, Shen Z, Huang R, Huang W, Shen L. 79 Tumor-immune signatures associated with response or resistance to tislelizumab (Anti-PD-1) in esophageal squamous cell carcinoma (ESCC) J Immunother Cancer. 2020;8(Suppl 3):A1–A559. [Google Scholar]
- 31.Chua BT, Volbracht C, Tan KO, Li R, Yu VC, Li P. Mitochondrial translocation of cofilin is an early step in apoptosis induction. Nat Cell Biol. 2003;5:1083–1089. doi: 10.1038/ncb1070. [DOI] [PubMed] [Google Scholar]
- 32.Barros LRC, Souza-Santos PT, Pretti MAM, Vieira GF, Bragatte MAS, Mendes MFA, De Freitas MV, Scherer NM, De Oliveira IM, Rapozo DCM, et al. High infiltration of B cells in tertiary lymphoid structures, TCR oligoclonality, and neoantigens are part of esophageal squamous cell carcinoma microenvironment. J Leukoc Biol. 2020;108:1307–1318. doi: 10.1002/JLB.5MA0720-710RRR. [DOI] [PubMed] [Google Scholar]
- 33.Helmink BA, Reddy SM, Gao J, Zhang S, Basar R, Thakur R, Yizhak K, Sade-Feldman M, Blando J, Han G, et al. B cells and tertiary lymphoid structures promote immunotherapy response. Nature. 2020;577:549–555. doi: 10.1038/s41586-019-1922-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harris RJ, Cheung A, Ng JCF, Laddach R, Chenoweth AM, Crescioli S, Fittall M, Dominguez-Rodriguez D, Roberts J, Levi D, et al. Tumor-infiltrating B lymphocyte profiling identifies IgG-Biased, clonally expanded prognostic phenotypes in triple-negative breast cancer. Cancer Res. 2021;81:4290–4304. doi: 10.1158/0008-5472.CAN-20-3773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nielsen JS, Sahota RA, Milne K, Kost SE, Nesslinger NJ, Watson PH, Nelson BH. CD20+ tumor-infiltrating lymphocytes have an atypical CD27- memory phenotype and together with CD8+ T cells promote favorable prognosis in ovarian cancer. Clin Cancer Res. 2012;18:3281–3292. doi: 10.1158/1078-0432.CCR-12-0234. [DOI] [PubMed] [Google Scholar]
- 36.Reithofer M, Rosskopf S, Leitner J, Battin C, Bohle B, Steinberger P, Jahn-Schmid B. 4–1BB costimulation promotes bystander activation of human CD8 T cells. Eur J Immunol. 2021;51:721–733. doi: 10.1002/eji.202048762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bai L, Peng H, Hao X, Tang L, Sun C, Zheng M, Liu F, Lian Z, Bai L, Wei H, et al. CD8(+) T cells promote maturation of liver-resident NK cells through the CD70-CD27 axis. Hepatology. 2019;70:1804–1815. doi: 10.1002/hep.30757. [DOI] [PubMed] [Google Scholar]
- 38.Wang F, Zhang C, Cheng H, Liu C, Lu Z, Zheng S, Wang S, Sun N, He J. TGF-beta-induced PLEK2 promotes metastasis and chemoresistance in oesophageal squamous cell carcinoma by regulating LCN2. Cell Death Dis. 2021;12:901. doi: 10.1038/s41419-021-04155-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goldberg GI, Strongin A, Collier IE, Genrich LT, Marmer BL. Interaction of 92-kDa type IV collagenase with the tissue inhibitor of metalloproteinases prevents dimerization, complex formation with interstitial collagenase, and activation of the proenzyme with stromelysin. J Biol Chem. 1992;267:4583–4591. doi: 10.1016/S0021-9258(18)42873-6. [DOI] [PubMed] [Google Scholar]
- 40.Wang G, Zhou Q, Xu Y, Zhao B. Emerging roles of pleckstrin-2 beyond cell spreading. Front Cell Dev Biol. 2021;9:768238. doi: 10.3389/fcell.2021.768238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee EK, Kim HJ, Lee KJ, Lee HJ, Lee JS, Kim DG, Hong SW, Yoon Y, Kim JS. Inhibition of the proliferation and invasion of hepatocellular carcinoma cells by lipocalin 2 through blockade of JNK and PI3K/Akt signaling. Int J Oncol. 2011;38:325–333. doi: 10.3892/ijo.2010.854. [DOI] [PubMed] [Google Scholar]
- 42.Wang B, Li L, Liao Y, Li J, Yu X, Zhang Y, Xu J, Rao H, Chen S, Zhang L, et al. Mast cells expressing interleukin 17 in the muscularis propria predict a favorable prognosis in esophageal squamous cell carcinoma. Cancer Immunol Immunother. 2013;62:1575–1585. doi: 10.1007/s00262-013-1460-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yin H, Wang X, Jin N, Ling X, Leng X, Wang Y, Ma K, Jiang X, Zhu J, Ma J. Integrated analysis of immune infiltration in esophageal carcinoma as prognostic biomarkers. Ann Transl Med. 2021;9:1697. doi: 10.21037/atm-21-5881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lichterman JN, Reddy SM. Mast cells: a new frontier for cancer immunotherapy. Cells. 2021;10(6):1270. doi: 10.3390/cells10061270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kaesler S, Wolbing F, Kempf WE, Skabytska Y, Koberle M, Volz T, Sinnberg T, Amaral T, Mockel S, Yazdi A, et al. Targeting tumor-resident mast cells for effective anti-melanoma immune responses. JCI Insight. 2019;4:19. doi: 10.1172/jci.insight.125057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lv Y, Zhao Y, Wang X, Chen N, Mao F, Teng Y, Wang T, Peng L, Zhang J, Cheng P, et al. Increased intratumoral mast cells foster immune suppression and gastric cancer progression through TNF-alpha-PD-L1 pathway. J Immunother Cancer. 2019;7:54. doi: 10.1186/s40425-019-0530-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bagaev A, Kotlov N, Nomie K, Svekolkin V, Gafurov A, Isaeva O, Osokin N, Kozlov I, Frenkel F, Gancharova O, et al. Conserved pan-cancer microenvironment subtypes predict response to immunotherapy. Cancer Cell. 2021;39(845–865):e847. doi: 10.1016/j.ccell.2021.04.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data and materials that supports the findings of this study are available from the corresponding author upon reasonable request.