Abstract
Background
The clinical implications of the third dose of coronavirus disease 2019 (COVID-19) vaccines in patients receiving immune checkpoint inhibitors are currently unknown. We performed a prospective analysis of the Vax-On-Third study to investigate the effects of antibody response on immune-related adverse events (irAEs) and disease outcomes.
Methods
Recipients of the booster dose of SARS-CoV-2 mRNA-BNT162b2 vaccine who had received at least one course of an anti-PD-1/PD-L1 treatment before vaccination for an advanced solid malignancy were eligible.
Results
The current analysis included 56 patients with metastatic disease (median age: 66 years; male: 71%), most of whom had a lung cancer diagnosis and were being treated with pembrolizumab- or nivolumab-based regimens. The optimal cut-point antibody titer of 486 BAU/mL allowed a dichotomization of recipients into low-responders (Low-R, < 486 BAU/mL) or high-responders (High-R, ≥ 486 BAU/mL). After a median follow-up time of 226 days, 21.4% of patients experienced moderate to severe irAEs without any recrudescence of immune toxicities preceding the booster dose. The frequencies of irAE before and after the third dose did not differ, but an increase in the cumulative incidence of immuno-related thyroiditis was observed within the High-R subgroup. On multivariate analysis, an enhanced humoral response correlated with a better outcome in terms of durable clinical benefit, which resulted in a significant reduction in the risk of disease control loss but not mortality.
Conclusions
Our findings would strengthen the recommendation not to change anti-PD-1/PD-L1 treatment plans based on current or future immunization schedules, implying that all these patients should be closely monitored.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00262-023-03489-1.
Keywords: COVID-19 vaccination, Antibody response, Cancer patients, Immune-checkpoint inhibitors, Immune-related adverse events, Disease outcomes
Introduction
The global increase in SARS-CoV-2 variants of concern (VOC) breakthrough infections among fully vaccinated cancer patients has prompted the need for additional interventions, including supplemental vaccine dosing [1, 2]. A third dose of mRNA-BNT162b2 vaccine (tozinameran) is able to elicit a stronger antibody response than the initial two-dose series in most patients with solid malignancies receiving active treatments [3]. A viable recall of T cell-mediated response is thought to underlie the enhancement of humoral immunogenicity [4]. While the intensification of adaptive immunity induced by booster dosing would confer protection against symptomatic COVID-19 even in recipients with no or waning response [5–8], it renews concerns about clinical interactions with immune checkpoint inhibitors (ICIs) in terms of safety and efficacy [9]. The evidence of an unincreased risk of immune-related adverse events (irAEs) following immunization supports the short-term safety of COVID-19 vaccines in patients with cancer receiving ICIs [10]. However, the effects of booster dosing on new or worsening pre-existing irAEs and their potential interactions with the magnitude of the immune response have not been thoroughly elucidated [11]. In general, any vaccination can induce increased cytokine release, which is responsible for systemic symptoms in some recipients. This reaction could theoretically trigger new immune responses or enhance an ongoing inflammatory process. Specifically, COVID-19 mRNA-based vaccines encode a SARS-CoV-2 transmembrane spike protein, whose shedding and binding to angiotensin-converting enzyme 2 (ACE2) in human tissues may exert proinflammatory effects [12]. On the other hand, immune checkpoint therapies that block the co-inhibitory pathway of programmed cell death protein-1 (PD-1) and its cognate ligand-1 (PD-L1) induce reactivation of exhausted T cells. It is conceivable that an enhanced adaptive response against tumor antigens may also involve the reactivation of SARS-CoV-2 spike protein-specific T cells, leading to increased cytokine release and subsequent clinical events [13]. The issue of whether the enhanced effects of the booster dose have a functional impact on the activity of ICI treatment arises and remains further unaddressed [14]. We therefore performed a pre-planned subgroup analysis of the Vax-On-Third study to investigate whether the third dose of tozinameran affects the clinical outcomes of patients with advanced solid malignancies receiving monoclonal antibodies targeting the PD-1/PD-L1 axis.
Materials and methods
Study design and participants
The Vax-On-Third was a prospective, observational, cohort study, whose design and primary results were previously reported (clinical study identifier: EudraCT number 2021-002611-54) [15]. The study protocol follows the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) standards. The referring Ethics Committee approved the study, and all patients provided written informed consent (protocol number: 1407/CE Lazio1). Participants who were eligible for the current analysis met the following inclusion criteria: histologically confirmed diagnosis of solid malignancy, locally advanced or metastatic extent of disease, at least one dose of an anti-PD-1 (nivolumab, pembrolizumab, or cemiplimab) or anti-PD-L1 (atezolizumab, durvalumab, or avelumab) agent received before the third dose of tozinameran, no evidence of progressive disease (PD) on restaging performed within 8 weeks before the third dose, subsequent disease reassessment carried out within 6 months of the third dose, and availability of IgG antibody titer against receptor binding domain of SARS-CoV-2 Spike protein (RBD-S1) tested 4 weeks after the third dose. The SARS-CoV-2 IgG II Quant assay on the ARCHITECT i2000sr automated platform (Abbott Laboratories, Diagnostics Division, Sligo, Ireland) was used to detect anti-RBD-S1 IgG antibodies according to the manufacturer's instructions [16]. The results were provided as arbitrary units per milliliter (AU/mL) within a linear range that expanded to 80,000 with an automated dilution. The serological titers obtained were converted from AU to binding antibody units (BAU) after WHO International Standards for anti-SARS-CoV-2 immunoglobulin testing were released (1 Abbott AU corresponds to 0.142 WHO BAU) [17]. The primary end points were safety and disease outcomes based on anti-RDB-S1 IgG titer levels. The National Cancer Institute’s Common Terminology Criteria for Adverse Events, version 5.0, were used to classify treatment-related toxicities. We reviewed all patient imaging to determine response rates as per Response Evaluation Criteria in Solid Tumors (RECIST 1.1) [18]. In addition to objective responses defined by RECIST, we also decided to aggregate patients with complete (CR) or partial (PR) responses to those showing a stable disease (SD) lasting more than 6 months. This group (DCB, durable clinical benefit) was therefore compared to those who did not demonstrate a durable clinical benefit (NCB group, PD or SD lasting less than 6 months). The outcomes of survival were investigated in terms of vaccine-related time-to-treatment failure (V-TTF, which referred to the time elapsed from the treatment with an anti-PD-1/PD-L1 agent immediately preceding the booster dose to its permanent discontinuation for any reason) and overall survival (OS, which referred to the time elapsed from initiation of an anti-PD-1/PD-L1 treatment to death for any reason). Patients who did not progress or die as of the last interim analysis were censored (April 30, 2023).
Statistical analysis
A mean with standard deviation was used to describe normally distributed variables, while a median with a 95% confidence interval (CI) or interquartile range (IQR) was reported for skewed variables. Comparative assessments were performed by applying Pearson’s χ2 test for categorical data and Mann–Whitney U test for continuous variables. The Wilcoxon signed-rank and McNemar tests were used for pairwise comparisons. A receiver operating characteristic (ROC) curve was calculated to determine the sensitivity and specificity of anti-RBD-S1 titers related to clinical benefit outcomes. The Youden index was applied to identify the optimal cut-point. A Fisher's exact test was used to perform a univariate analysis of the correlation between clinical variables and DCB. We applied the Holm-Bonferroni method [19] to adjust p values while keeping α (0.05) constant for multiple comparisons of clinical-pathological and immune parameters with disease outcomes and immune-related toxicities. A multivariate logistic regression model was performed to estimate the odds ratio (OR) of DCB with a 95% CI as a function of significant variables at univariate analysis. A Mantel-Cox log-rank test was used to compare the survival outcomes of different patient subgroups according to significant variables. Survival curves were visualized through the Kaplan–Meier method. A multivariate Cox regression model was applied to estimate the hazard ratio (HR) with a 95% CI of confirmed significant variables. The Spearman method was used to assess the correlation between anti-RBD-S1 IgG titer log values and duration of both V-TTF and OS. All tests performed were two-sided, and a p value < 0.05 was considered significant. SPSS (IBM SPSS Statistics for Windows, version 23.0, Armonk, NY) and Prism (GraphPad, version 9) software were used for statistical evaluations and figure rendering, respectively.
Results
Patient characteristics and general outcomes
The present analysis included 56 eligible patients who had received their third dose of tozinameran between September 23 and October 7, 2021. The median age was 66 years, and the majority of patients were male (71%). Non-small-cell lung cancer (NSCLC) was the most frequent diagnosis (57%), and all participants had a metastatic extent of disease with an Eastern Cooperative Oncology Group Performance Status (ECOG PS) 0–1. The most common immune checkpoint therapies with PD-1/PD-L1 blocking agents were pembrolizumab (45%) and nivolumab (37%). The median duration of active treatment before and after the booster dose was 8.4 and 4.6 months, respectively. Supplementary Table 1 details the baseline characteristics of the patients. As of the last interim analysis, 11 patients (20%) were still receiving treatment, 45 (80%) have been discontinued, and 19 (34%) have been censored due to no event relevant to survival outcome. We observed 12 PR (21%), 23 SD (41%), 14 (25%) of which lasted more than 6 months, and 21 PD (37%). As a result, DCB and NCB were reported in 26 (46%) and 30 (54%) cases, respectively. The median OS was 26.2 months (95% CI 17.7–34.7) after a median follow-up time of 32.3 months (95% CI 27.4–31.7).
Antibody responses
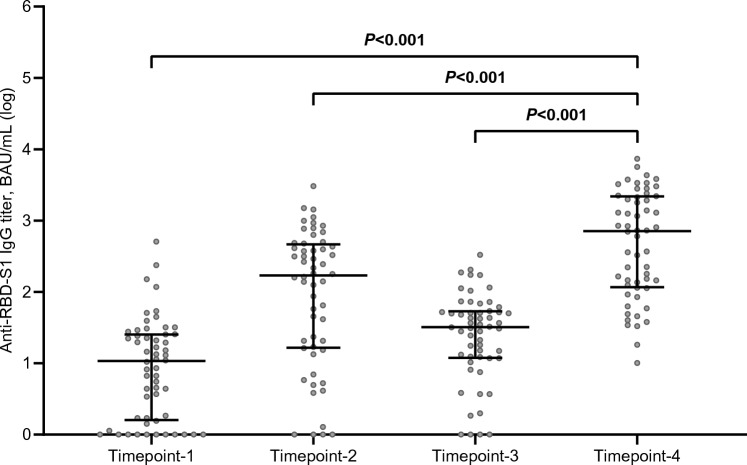
The third dose of tozinameran resulted in an exponential increase in anti-RBD-S1 IgG titer, the median value of which (714 BAU/mL, 95% CI 179–1271) was significantly higher than the same figures obtained 3 weeks after the first dose (11 BAU/mL, 95% CI 5–19), 8 weeks after the second dose (170 BAU/mL, 95% CI 61–314), and shortly before the booster dosing (32 BAU/mL, 95% CI 21–46; Fig. 1). A ROC curve was calculated to determine the relationship between anti-RBD-S1 IgG titers after the booster dose and DCB. The relative AUC value [0.76 (95% CI 0.63–0.89), p = 0.001] was considered valuable in predicting the likelihood of a positive outcome (Fig. 2). The IgG titer cut-point of 486 BAU/mL was associated with a sensitivity of 0.84 and a specificity of 0.74, allowing a dichotomization of recipients into low-responder (Low-R, < 486 BAU/mL) and high-responder (High-R, ≥ 486 AU/mL) subgroups. Of note, after a median follow-up of 226 days (95% CI 125–525), 13 patients in the Low-R subgroup (50%) and 7 in the High-R subgroup (23.3%, p = 0.037) reported contracting SARS-CoV-2 infections, none of which was clinically severe. Computation of the ROC curve in the subgroup of patients with lung cancer confirmed a statistically significant association with DCB [AUC 0.73 (95% CI 0.56–0.90), p = 0.021, Supplementary Fig. 1]. Compared to the general population, the same antibody titer (486 BAU/mL) was identified as the optimal cut-point with higher sensitivity (0.91) and specificity (0.77).
Fig. 1.
Longitudinal comparison of scatter plot distributions and medians of antibody titers. RBD-S1 receptor-binding domain (RBD) of the SARS-CoV-2 Spike protein (S1), BAU binding antibody unit; log, logarithmic values. Bars represent median values with interquartile range; timepoint-1 denotes assessment 3 weeks after the first dose of tozinameran; timepoint-2 denotes assessment 8 weeks after the second dose of tozinameran; timepoint-3 denotes assessment before the third dose of tozinameran; timepoint-4 denotes assessment 4 weeks after the third dose of tozinameran
Fig. 2.
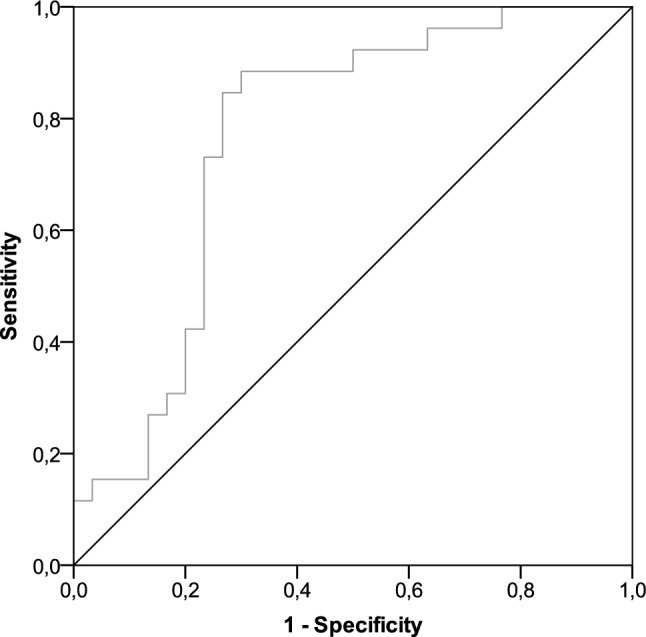
ROC curve analysis of anti-RBD-S1 IgG titers on DCB. AUC relative value: 0.76 (95% CI 0.63–0.89), p = 0.001. ROC receiver operating characteristic, RBD-S1 receptor-binding domain of the SARS-CoV-2 Spike protein, DCB durable clinical benefit, AUC area under the curve, CI confidence interval
Safety
The median interval between the administration of anti-PD-1/PD-L1 agents immediately preceding and following the third dose of tozinameran was 8.5 days (IQR 6–15) and 9.5 days (IQR 6–15), respectively. Before booster vaccination, 18 patients (32%) reported irAEs. Since six patients developed two separate toxicities, we described 24 irAEs, none of which was higher than grade 2. The median time to the first onset of any irAE after the initiation of ICI was 87 days (IQR 29–523). After a median follow-up time of 226 days (IQR 89–565) from booster dosing, irAEs were observed in 13 patients (23%), two of whom experienced severe toxicity (namely, grade 3 thyroiditis and grade 3 vasculitis). Two patients who had previously presented with an irAE developed additional toxicity (grade 2 colitis after grade 2 skin rash and grade 2 arthritis after grade 2 thyroiditis), but we did not observe any flare of irAEs preceding the third dose of tozinameran. A median of 39 days (IQR 21–164) elapsed between the booster dose and the onset of immune-mediated toxicities. On univariate comparison, the overall frequency of irAEs did not differ significantly between ICI exposure before and after the booster dose. Interestingly, the High-R subgroup experienced a significant increase in the cumulative incidence of immuno-related thyroiditis (p = 0.036, Table 1). Statistical significance, however, was not maintained after applying correction for multiple testing.
Table 1.
Immune-related adverse events
| Adverse event | Before third vaccine dose, N = 56 (100%) | After third vaccine dose, N = 56 (100%) | P value | Low-Ra, N = 26 (100%) | High-Rb, N = 30 (100%) | P value | ||
|---|---|---|---|---|---|---|---|---|
| N (%) | CTCAE Grading | N (%) | CTCAE Grading | N (%) | N (%) | |||
| Thyroiditis | 8 (14.3%) | 1–2 | 3 (5.3%) | 2–3 | 0.13 | 2 (7.7%) | 9 (30%) | 0.036† |
| Skin rash | 4 (7.1%) | 1–2 | 1 (1.8%) | 2 | 0.17 | 2 (7.7%) | 3 (10%) | 0.76 |
| Pancreatitis | 3 (5.3%) | 2 | 1 (1.8%) | 2 | 0.31 | 3 (11.5%) | 1 (3.3%) | 0.23 |
| Colitis | 3 (5.3%) | 2 | 1 (1.8%) | 2 | 0.31 | 1 (3.8%) | 3 (10%) | 0.37 |
| Hepatitis | 2 (3.5%) | 2 | 1 (1.8%) | 2 | 0.56 | 1 (3.8%) | 2 (6.6%) | 0.63 |
| Pneumonitis | 2 (3.5%) | 2 | – | – | 0.15 | – | 2 (6.6%) | 0.17 |
| Arthritis | 2 (3.5%) | 1–2 | 2 (3.5%) | 2 | 1.00 | 2 (7.7%) | 2 (6.6%) | 0.88 |
| Myositis | – | – | 1 (1.8%) | 2 | 0.31 | – | 1 (3.3%) | 0.34 |
| Vasculitis | – | – | 1 (1.8%) | 3 | 0.31 | 1 (3.8%) | – | 0.27 |
| Uveitis | – | – | 1 (1.8%) | 2 | 0.31 | – | 1 (3.3%) | 0.34 |
CTCAE common terminology criteria for adverse events, version 5.0
aLow-R indicates the subgroup of patients with an anti-RBD-S1 IgG titer < 486 BAU/mL after the third dose of vaccine
bHigh-R indicates the subgroup of patients with an anti-RBD-S1 IgG titer ≥ 486 BAU/mL after the third dose of vaccine,
†Statistical significance not maintained after Holm-Bonferroni p value correction for multiple comparisons
Disease outcomes
In univariate analyses, a diagnosis other than lung cancer (p = 0.005), a limited disease burden with a single metastatic site (p < 0.001), absence of bone (p = 0.008) or liver (p = 0.025) involvement, a weight loss ≤ 5% from baseline (p = 0.001), and any positive PD-L1 expression (p < 0.001) were found to be significantly associated with DCB. The same comparison also revealed a better outcome in favor of the High-R subgroup of patients (p < 0.001). In multivariate analysis, the type of cancer diagnosis, extent of disease, PD-L1 expression, and antibody response level after the booster dose retained their predictive significance (Table 2).
Table 2.
Correlation between clinical-pathological variables and clinical benefit outcome
| Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|
| NCB N = 30 (100%) |
DCB N = 26 (100%) |
P value | OR (95% CI) | P value | |
| Age | 0.99 | – | – | ||
| ≤ 70 years (N = 32) | 17 (56.7%) | 15 (57.7%) | |||
| > 70 years (N = 24) | 13 (43.3%) | 11 (43.3%) | |||
| Sex | 0.55 | – | – | ||
| Female (N = 16) | 10 (33.3%) | 6 (23.1%) | |||
| Male (N = 40) | 20 (66.7%) | 20 (76.9%) | |||
| ECOG PS | 0.25 | – | – | ||
| 0 (N = 17) | 7 (23.3%) | 10 (38.5%) | |||
| 1 (N = 39) | 23 (76.7%) | 16 (61.5%) | |||
| Cancer type | 0.005† | 0.021 | |||
| Others (N = 19) | 5 (16.7%) | 14 (53.8%) | 1.00 | ||
| Lung (N = 37) | 25 (83.3%) | 12 (46.2%) | 0.04 (0.01–0.61) | ||
| Number of metastatic sites | < 0.001† | 0.038 | |||
| 1 (N = 23) | 4 (13.3%) | 19 (73.1%) | 1.00 | ||
| ≥ 2 (N = 33) | 26 (86.7%) | 7 (26.9%) | 0.08 (0.01–0.86) | ||
| CNS metastases | 0.34 | – | – | ||
| No (N = 44) | 22 (73.3%) | 22 (84.6%) | |||
| Yes (N = 12) | 8 (26.7%) | 4 (15.4%) | |||
| Bone metastases | 0.008† | 0.536 | |||
| No (N = 39) | 16 (53.3%) | 23 (88.5%) | 1.00 | ||
| Yes (N = 17) | 14 (46.7%) | 3 (11.5%) | 0.43 (0.03–5.99) | ||
| Liver metastases | 0.025 | – | – | ||
| No (N = 50) | 24 (80.0%) | 26 (100%) | |||
| Yes (N = 6) | 6 (20.0%) | – | |||
| Weight loss | 0.001† | 0.254 | |||
| < 5% (N = 39) | 15 (50.0%) | 24 (93.2%) | 1.00 | ||
| ≥ 5% (N = 17) | 15 (50.0%) | 2 (7.7%) | 0.19 (0.01–3.29) | ||
| PD-L1 TPS | < 0.001† | 0.004 | |||
| < 1% or unknown (N = 22) | 20 (66.7%) | 2 (7.7%) | 1.00 | ||
| ≥ 1% (N = 34) | 10 (33.3%) | 24 (93.3%) | 49.65 (3.49– > 100) | ||
| Treatment setting | 0.26 | – | – | ||
| First line (N = 36) | 17 (56.7%) | 19 (73.1%) | |||
| Second or later line (N = 20) | 13 (43.3%) | 7 (26.9%) | |||
| Corticosteroid therapya | 0.34 | – | – | ||
| No (N = 44) | 22 (73.3%) | 22 (84.6%) | |||
| Yes (N = 12) | 8 (26.7%) | 4 (15.4%) | |||
| ICI therapy | 0.48 | – | – | ||
| Anti-PD-1 (N = 47) | 24 (80.0%) | 23 (88.5%) | |||
| Anti-PD-L1 (N = 9) | 6 (20.0%) | 3 (11.5%) | |||
| Antibody responseb | < 0.001† | 0.005 | |||
| Low-R (N = 26) | 22 (73.3%) | 4 (15.4%) | 1.00 | ||
| High-R (N = 30) | 8 (26.7%) | 22 (84.6%) | 49.45 (3.27– > 100) | ||
NCB no clinical benefit, DCB durable clinical benefit, OR odds ratio, CI confidence interval, ECOG PS eastern cooperative oncology group performance status, CNS central nervous system, PD-L1 TPS programmed cell death-ligand 1 tumor proportion score, ICI immune checkpoint inhibitor
acorticosteroid therapy indicates ≥ 10 mg prednisone equivalent daily for at least 7 days in the 28 days preceding the third dose of vaccine
bLow-R indicates the subgroup of patients with an anti-RBD-S1 IgG titer < 486 BAU/mL after the third dose of vaccine, High-R indicates the subgroup of patients with an anti-RBD-S1 IgG titer ≥ 486 BAU/mL after the third dose of vaccine
†Statistical significance maintained after Holm-Bonferroni p value correction for multiple comparisons
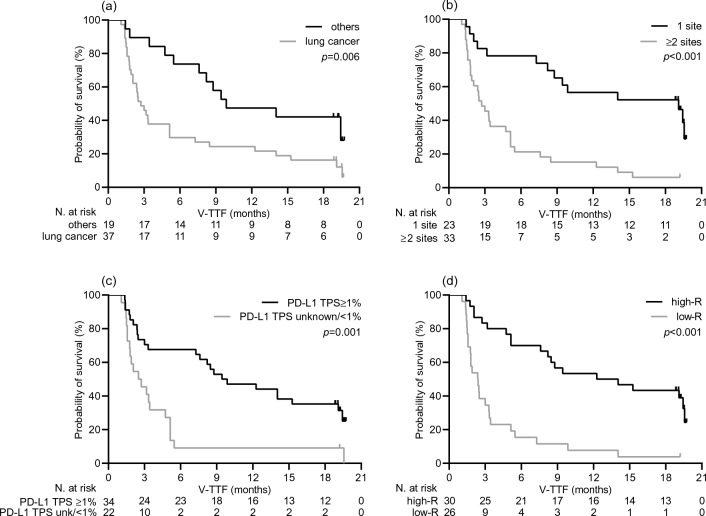
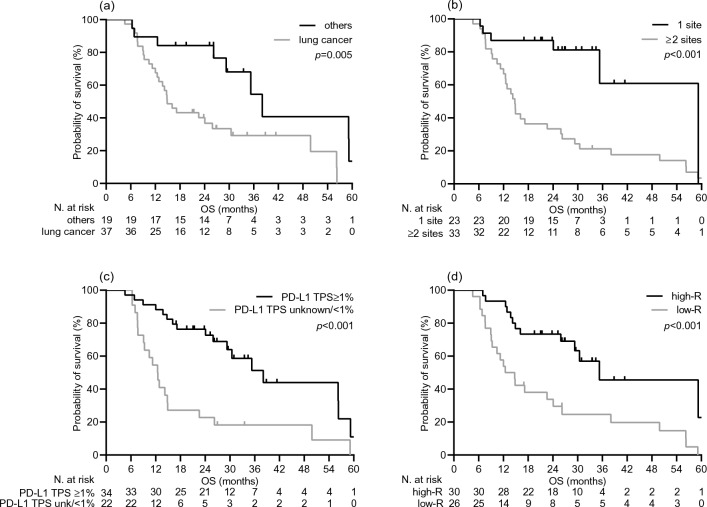
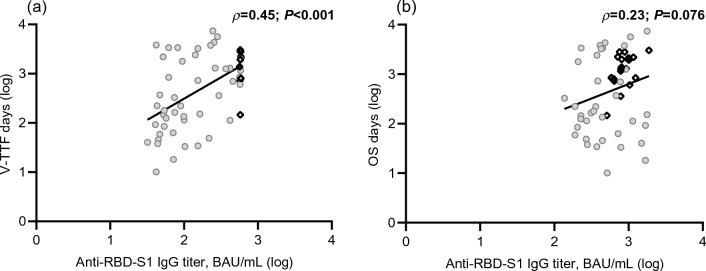
As expected, the DCB resulted in a statistically significant improvement in median V-TTF [19.1 months (95% CI 12.8–25.3) vs. 2.0 months (95% CI 1.2–2.8), p < 0.001; Supplementary Fig. 2A] and median OS [38.0 months (95% CI 31.5–44.5) vs. 12.5 months (95% CI 10.1–14.9), p < 0.001; Supplementary Fig. 2B]. Consistently, univariate survival testing confirmed the variables that significantly correlated with DCB in the multivariate analysis to be significant survival predictors, as all were associated with a lower risk of treatment failure (Table 3, Fig. 3) and mortality (Table 3, Fig. 4). However, only the diagnosis other than lung cancer, burden of disease, and increased humoral response retained their significant potential for V-TTF in multivariate analysis. Cancer type and PD-L1 TPS had an independent impact on OS (Table 3). Furthermore, when these outcomes were evaluated as a function of log anti-RBD-S1 IgG titers, a significant positive linear correlation was found for V-TFF [ρ = 0.45 (95% CI 0.21–0.63), p < 0.001; Fig. 5A] but not for OS [ρ = 0.23 (95% CI − 0.05–0.50), p = 0.076; Fig. 5B]. The univariate analysis within the subgroup of patients with lung cancer showed a significant benefit in favor of high-responder recipients not only in terms of V-TTF [8.4 months (95% CI 3.1–23.2) vs. 1.9 months (95% CI 1.0–3.7), p < 0.001; Supplementary Fig. 3A] but also OS [30.4 months (95% CI 9.3–51.5) vs. 12.0 months (95% CI 9.1–14.8), p = 0.014; Supplementary Fig. 3B].
Table 3.
Analysis of survival
| Variable | Vaccine-related time-to-treatment failure | Overall survival | ||||||
|---|---|---|---|---|---|---|---|---|
| Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | |||||
| Median V-TTF (95% CI), months | P value | HR (95% CI) | P value | Median OS (95% CI), months | P value | HR (95% CI) | P value | |
| Cancer type | 0.006 | 0.005 | 0.005 | 0.019 | ||||
| Others | 9.8 (2.3–17.3) | 1.00 | 38.0 (27.5–48.5) | 1.00 | ||||
| Lung | 2.7 (1.8–3.6) | 2.77 (1.36–5.64) | 14.9 (11.4–18.3) | 2.79 (1.18–6.62) | ||||
| Number of metastatic sites | < 0.001 | 0.026 | < 0.001 | 0.121 | ||||
| − 1 | 19.1 (7.8–30.3) | 1.00 | 26.2 (23.8–NR) | 1.00 | ||||
| ≥ 2 | 2.7 (1.7–3.7) | 2.44 (1.11–5.31) | 14.7 (12.4–17.0) | 2.27 (0.80–6.39) | ||||
| PD-L1 TPS | 0.001 | 0.167 | < 0.001 | 0.04 | ||||
| < 1% or unknown | 9.4 (4.0–14.8) | 1.00 | 12.4 (9.6–15.2) | 1.00 | ||||
| ≥ 1% | 2.5 (1.0–3.9) | 0.62 (0.31–1.22) | 38.0 (26.3–49.8) | 0.46 (0.22–0.96) | ||||
| Antibody responsea | < 0.001 | < 0.001 | < 0.001 | 0.261 | ||||
| Low-R | 2.3 (1.5–3.2) | 1.00 | 12.4 (8.0–16.9) | 1.00 | ||||
| High-R | 12.2 (3.5–20.9) | 0.25 (0.12–0.52) | 35.3 (13.4–57.1) | 0.62 (0.28–1.41) | ||||
HR hazard ratio, CI confidence interval, PD-L1 TPS, programmed cell death-ligand 1 tumor proportion score, NR not reached
aLow-R indicates the subgroup of patients with an anti-RBD-S1 IgG titer < 486 BAU/mL after the third dose of vaccine, High-R indicates the subgroup of patients with an anti-RBD-S1 IgG titer ≥ 486 BAU/mL after the third dose of vaccine
Fig. 3.
Vaccine-related time-to-treatment failure depending on significant clinical variables. a type of cancer diagnosis: others versus lung cancer; b metastatic extent of disease: 1 site versus ≥ 2 sites; c programmed cell death-ligand 1 tumor proportion score (PD-L1 TPS): ≥ 1% versus unknown or < 1%; d level of antibody response: Low-R (subgroup of patients with an anti-RBD-S1 IgG titer < 486 BAU/mL after the third dose of vaccine) versus High-R (subgroup of patients with an anti-RBD-S1 IgG titer ≥ 486 BAU/mL after the third dose of vaccine)
Fig. 4.
Overall survival depending on significant clinical variables. a type of cancer diagnosis: others versus lung cancer; b metastatic extent of disease: 1 site versus ≥ 2 sites; c programmed cell death-ligand 1 tumor proportion score (PD-L1 TPS): ≥ 1% versus unknown or < 1%; d level of antibody response: Low-R (subgroup of patients with an anti-RBD-S1 IgG titer < 486 BAU/mL after the third dose of vaccine) versus High-R (subgroup of patients with an anti-RBD-S1 IgG titer ≥ 486 BAU/mL after the third dose of vaccine)
Fig. 5.
Scatter plot of survival by anti-RBD-S1 IgG titers. a Vaccine-related time-to-treatment failure and b overall survival. Abbreviations: RBD-S1, receptor-binding domain (RBD) of the SARS-CoV-2 Spike protein (S1); BAU, Binding Antibody Unit; log, logarithmic values. Circle-shaped marks indicate cases that experienced survival-relevant events; rhombus-shaped marks indicate censored cases
Discussion
The extensive deployment of COVID-19 vaccination has raised new concerns about clinical interactions between active antiviral immunization and immunologic therapies [20]. Since the third dose of tozinameran is expected to maximize the humoral and cellular immune responses [21, 22], its impact on cancer patients receiving ICI-based treatments warrants special attention. In this research, we investigated the specific safety and disease outcomes of patients treated with agents targeting the immunoregulatory PD-1/PD-L1 axis for a wide range of advanced solid malignancies over a 18-month period following booster dosing. Although longitudinal assessment of humoral responses confirmed a significant increase in antibody titers after the third dose, we observed no difference in the frequency of irAEs. However, it is noteworthy that recipients who demonstrated a more sustained antibody response had an increased incidence of moderate to severe immune-related thyroiditis. The same patients also appear to benefit from improved time-to-event outcomes as a result of a lower risk of loss of disease control and death. The fact that, to the best of our knowledge, similar findings have not been previously reported requires a critical assessment of their clinical relevance.
Because COVID-19 vaccination might elicit hyperstimulation of T-cell and dendritic cell functions [23] and increased cytokine release [13, 24], concomitant treatment with ICIs has been considered a risk factor for developing severe irAE. After initial evidence of a favorable short-term safety profile in this group of patients [10], a subsequent prospective study confirmed a low rate of severe irAEs in mRNA-1273 vaccine recipients receiving ICIs with or without cytotoxic chemotherapy [25]. Our prospective assessment indicates an incidence of any grade immune-related toxicities within the expected range [26], with no increase after the booster dose of the vaccine. In addition, the median time to irAE development and lack of exacerbation of previous toxicities are consistent with the results of several retrospective series addressing these aspects of ICI treatment after the second or third dose [27–31]. The apparent increase in the frequency of thyroiditis was relatively unexpected because it has already been described as an autoimmune disorder associated with SARS-CoV-2 infections [32]. Although not confirmed by statistical testing for multiple comparisons, we observed an associative trend between immune-related thyroiditis and the magnitude of antibody response. The evidence on thyroid dysfunction resulting from COVID-19 vaccination is still controversial, as it mainly relies on low-rated meta-analytic appraisals of data from non-cancer patients [33, 34]. Our findings suggest a potential interaction between vaccination and treatment with anti-PD-1/PD-L1 agents. The unavailability of measuring anti-thyroid antibodies before and after the third immunization, as well as the impossibility of comparison with a control group of unvaccinated patients, does not allow further insights to be drawn [35].
The clinical effects of SARS-CoV-2 vaccination on the efficacy of ICI-based therapies are currently unknown. However, the complex relationship between vaccine adaptive responses and immune checkpoint blockade has raised concerns about a potential increase in tumor hyper-progression [36]. This paradoxical phenomenon has been related to the diffuse infiltration of metastatic sites by activated lymphocytes as a result of enhanced effects of the vaccination itself [37]. In this regard, two retrospective studies reported that influenza vaccination could even provide an advantage in terms of overall survival, ruling out the generic risk of hyper-progression associated with active antiviral immunization during ICI exposure [38, 39]. Similarly, a retrospective review of advanced cancer patients on PD-1 blockade who received seasonal flu vaccine reported no increase in incidence or severity of irAEs within 2 months of treatment [40]. Nonetheless, because COVID-19 vaccination may elicit less predictable immune responses than influenza vaccines, the threat of a detrimental effect on disease outcome cannot be overlooked. A large retrospective study showed that the clinical benefit of the anti-PD-1 agent camrelizumab has improved in the subgroup of patients who received the SARS-CoV-2 BBIPB-CorV vaccine compared with unvaccinated counterparts [41]. Despite significant differences in methodology, our results appear to be consistent with the described evidence, implying that COVID-19 vaccination does not reduce the clinical efficacy of PD-1/PD-L1 targeting agents. Similar to previous studies, we did not observe any cases of hyper-progression nor did the rate of loss of disease control differ from what was expected before the third dose of tozinameran. Although the unavoidability of SARS-CoV-2 vaccination during COVID-19 outbreaks precluded a direct comparison with unvaccinated patients, we observed a significant survival advantage in favor of high-level responders. Furthermore, the survival benefit observed in the subgroup of patients with advanced lung cancer who exhibited an enhanced antibody response seems to confirm previous insights.
The current study acknowledges several shortcomings, including but not limited to the following issues. The Vax-On-Third, like all others of its kind, was designed to enroll large numbers of patients in a short time frame. While the need to address COVID-19-related emergency may explain such an approach, this "all-comers" recruitment did not allow for adequate stratification of participants, making the study prone to selection bias. We included a wide variety of malignancies, implying that different interactions between vaccination and ICIs cannot be ruled out among patients with different types of cancer. The specific design of the present research also has an inherent immortal-time bias, which results from the lapse between initiation of ICI treatment and vaccination, potentially leading to an overestimate of the survival benefit [42]. The lack of a linear correlation between antibody titers and duration of overall survival, which has otherwise been reported for time-to-treatment failure, is consistent with the latter consideration. In addition, we did not provide an independent radiological review or immune-related evaluation of treatment response [43]. These flaws may have led to an incorrect assessment of anti-PD-1/PD-L1 agent activity and V-TTF duration. Finally, despite being overlapped with those of similar studies, the sample size of this series is small, as is the median duration of follow-up, which is relatively short. The last issues emphasize that multivariable statistical comparisons may amplify false-positive results, the significance of which should be therefore considered exploratory.
In conclusion, many areas of uncertainty remain in cancer patients receiving ICIs with regard to the third dose of COVID-19 vaccination. We have investigated the effects of antibody response in this condition for the first time. Our results demonstrate a favorable clinical interaction leading improved disease outcomes. Although the limited sample size of this study, as well as the complexity and variability of mechanisms underlying the development of irAEs [44], does not allow us to rule out rare interactions with tozinameran booster dosing, our findings suggest that the risk of severe immune toxicities on PD-1/PD-L1 blockade is likely small. The increased incidence of specific adverse events associated with both vaccination and treatment, including autoimmune thyroiditis, implies that all these patients should be closely monitored. These results would strengthen the recommendation that anti-PD-1/PD-L1 treatment plans should not be altered depending on immunization schedules [45]. The limitations of the study and the lack of reliable comparisons indicate that these results have hypothesis-generating potential, which should be confirmed in independent prospective cohorts.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contributions
FN and EMR designed the study and wrote the manuscript. DG performed the statistical analysis. AF, JRGB, AV, EM, CF, MS, CS, MGC, and FP enrolled the patients, collected clinical and laboratory data and setup the database. VP, GT, and MAS performed serological testing, and contributed to the interpretation of the data. All authors discussed the results and critically revised the manuscript. All authors read and approved the final manuscript.
Funding
This research did not receive any specific grant from agencies in the public, commercial, or not-for-profit sectors and no sources of funding were used to assist in the preparation of this manuscript.
Data availability
The datasets generated and analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethics approval
This study was approved by the referring Ethics Committee (Comitato Etico Lazio1, Rome, Italy; protocol number: 1407/CE Lazio1). All patients were required to provide written informed consent before participating in this study. All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Daher A, El Zarif T, Friese CR, Griffiths EA, Hawley JE, Hayes-Lattin B, Karivedu V, Latif T, Mavromatis BH, McKay RR, Nagaraj G, Nguyen RH, Panagiotou OA, Portuguese AJ, Puc M, Santos Dutra M, Schroeder BA, Thakkar A, Wulff-Burchfield EM, Mishra S, Farmakiotis D, Shyr Y, Warner JL, Choueiri TK, Cancer Consortium COVID-19 vaccination and breakthrough infections in patients with cancer. Ann Oncol. 2022;33:340–346. doi: 10.1016/j.annonc.2021.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang W, Kaelber DC, Xu R, Berger NA. Breakthrough SARS-CoV-2 infections, hospitalizations, and mortality in vaccinated patients with cancer in the US between December 2020 and November 2021. JAMA Oncol. 2022 doi: 10.1001/jamaoncol.2022.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Al Hajji Y, Taylor H, Starkey T, Lee LYW, Tilby M. Antibody response to a third booster dose of SARS-CoV-2 vaccination in adults with haematological and solid cancer: a systematic review. Br J Cancer. 2022;127:1827–1836. doi: 10.1038/s41416-022-01951-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zeng C, Evans JP, Chakravarthy K, Qu P, Reisinger S, Song NJ, Rubinstein MP, Shields PG, Li Z, Liu SL. COVID-19 mRNA booster vaccines elicit strong protection against SARS-CoV-2 Omicron variant in patients with cancer. Cancer Cell. 2022;40:117–119. doi: 10.1016/j.ccell.2021.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choueiri TK, Labaki C, Bakouny Z, Hsu CY, Schmidt AL, de Lima LG, Hwang C, Singh SRK, Jani C, Weissmann LB, Griffiths EA, Halabi S, Wu U, Berg S, O'Connor TE, Wise-Draper TM, Panagiotou OA, Klein EJ, Joshi M, Yared F, Dutra MS, Gatson NTN, Blau S, Singh H, Nanchal R, McKay RR, Nonato TK, Quinn R, Rubinstein SM, Puc M, Mavromatis BH, Vikas P, Faller B, Zaren HA, Del Prete S, Russell K, Reuben DY, Accordino MK, Singh H, Friese CR, Mishra S, Rivera DR, Shyr Y, Farmakiotis D, Warner JL. Breakthrough SARS-CoV-2 infections among patients with cancer following two and three doses of COVID-19 mRNA vaccines: a retrospective observational study from the COVID-19 and Cancer Consortium. Lancet Reg Health Am. 2023;19:100445. doi: 10.1016/j.lana.2023.100445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gong IY, Vijenthira A, Powis M, Calzavara A, Patrikar A, Sutradhar R, Hicks LK, Wilton D, Singh S, Krzyzanowska MK, Cheung MC. Association of COVID-19 vaccination with breakthrough infections and complications in patients with cancer. JAMA Oncol. 2023;9:386–394. doi: 10.1001/jamaoncol.2022.6815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee LYW, Ionescu MC, Starkey T, Little M, Tilby M, Tripathy AR, Mckenzie HS, Al-Hajji Y, Appanna N, Barnard M, Benny L, Burnett A, Cattell EL, Clark JJ, Khan S, Ghafoor Q, Panneerselvam H, Illsley G, Harper-Wynne C, Hattersley RJ, Lee AJ, Lomas O, Liu JK, McCauley A, Pang M, Pascoe JS, Platt JR, Patel G, Patel V, Potter VA, Randle A, Rigg AS, Robinson TM, Roques TW, Roux RL, Rozmanowski S, Taylor H, Tuthill MH, Watts I, Williams S, Beggs A, Iveson T, Lee SM, Middleton G, Middleton M, Protheroe A, Fittall MW, Fowler T, Johnson P, UK Coronavirus Cancer Programme COVID-19: third dose booster vaccine effectiveness against breakthrough coronavirus infection, hospitalisations and death in patients with cancer: a population-based study. Eur J Cancer. 2022;175:1–10. doi: 10.1016/j.ejca.2022.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shapiro LC, Thakkar A, Campbell ST, Forest SK, Pradhan K, Gonzalez-Lugo JD, Quinn R, Bhagat TD, Choudhary GS, McCort M, Sica RA, Goldfinger M, Goel S, Anampa JD, Levitz D, Fromowitz A, Shah AP, Sklow C, Alfieri G, Racine A, Wolgast L, Greenberger L, Verma A, Halmos B. Efficacy of booster doses in augmenting waning immune responses to COVID-19 vaccine in patients with cancer. Cancer Cell. 2022;40:3–5. doi: 10.1016/j.ccell.2021.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Malek AE, Cornejo PP, Daoud N, Alam M. The mRNA COVID-19 vaccine in patients with cancer receiving checkpoint inhibitor therapy: what we know and what we don't. Immunotherapy. 2022;14:91–94. doi: 10.2217/imt-2021-0235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Waissengrin B, Agbarya A, Safadi E, Padova H, Wolf I. Short-term safety of the BNT162b2 mRNA COVID-19 vaccine in patients with cancer treated with immune checkpoint inhibitors. Lancet Oncol. 2021;22:581–583. doi: 10.1016/S1470-2045(21)00155-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luo B, Li J, Hou X, Yang Q, Zhou Y, Ye J, Wu X, Feng Y, Hu T, Xu Z, He Y, Sun J. Indications for and contraindications of immune checkpoint inhibitors in cancer patients with COVID-19 vaccination. Future Oncol. 2021;17:3477–3484. doi: 10.2217/fon-2021-0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Trougakos IP, Terpos E, Alexopoulos H, Politou M, Paraskevis D, Scorilas A, Kastritis E, Andreakos E, Dimopoulos MA. Adverse effects of COVID-19 mRNA vaccines: the spike hypothesis. Trends Mol Med. 2022;28:542–554. doi: 10.1016/j.molmed.2022.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walle T, Bajaj S, Kraske JA, Rösner T, Cussigh CS, Kälber KA, Müller LJ, Strobel SB, Burghaus J, Kallenberger SM, Stein-Thöringer CK, Jenzer M, Schubert A, Kahle S, Williams A, Hoyler B, Zielske L, Skatula R, Sawall S, Leber MF, Kunes RZ, Krisam J, Fremd C, Schneeweiss A, Krauss J, Apostolidis L, Berger AK, Haag GM, Zschäbitz S, Halama N, Springfeld C, Kirsten R, Hassel JC, Jäger D, Investigators NCTANTICIPATE, Ungerechts G. Cytokine release syndrome-like serum responses after COVID-19 vaccination are frequent and clinically inapparent under cancer immunotherapy. Nat Cancer. 2022 doi: 10.1038/s43018-022-00398-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brest P, Mograbi B, Hofman P, Milano G. COVID-19 vaccination and cancer immunotherapy: should they stick together? Br J Cancer. 2022;126:1–3. doi: 10.1038/s41416-021-01618-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nelli F, Giannarelli D, Fabbri A, Silvestri MA, Berrios JRG, Virtuoso A, Marrucci E, Schirripa M, Mazzotta M, Onorato A, Panichi V, Topini G, Pessina G, Natoni F, Signorelli C, Chilelli MG, Primi F, Ruggeri EM. Immunogenicity and early clinical outcome after two or three doses of SARS-CoV-2 mRNA-BNT162b2 vaccine in actively treated cancer patients: results from the prospective observational Vax-On-Third study. Ann Oncol. 2022;33:740–742. doi: 10.1016/j.annonc.2022.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.AdviseDx SARS-CoV-2 IgG II. Package insert. Abbott laboratories; 2021. https://www.fda.gov/media/146371/download/. Accessed 18 May 2023
- 17.Saker K, Escuret V, Pitiot V, Massardier-Pilonchéry A, Paul S, Mokdad B, Langlois-Jacques C, Rabilloud M, Goncalves D, Fabien N, Guibert N, Fassier JB, Bal A, Trouillet-Assant S, Trabaud MA. Evaluation of commercial anti-SARS-CoV-2 antibody assays and comparison of standardized titers in vaccinated health care workers. J Clin Microbiol. 2022;60:e0174621. doi: 10.1128/JCM.01746-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 19.Holm S. A simple sequentially rejective multiple test procedure. Scand J Stat. 1979;6:65–70. [Google Scholar]
- 20.Garassino MC, Ribas A. At the crossroads: COVID-19 and immune-checkpoint blockade for cancer. Cancer Immunol Res. 2021;9:261–264. doi: 10.1158/2326-6066.CIR-21-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shroff RT, Chalasani P, Wei R, Pennington D, Quirk G, Schoenle MV, Peyton KL, Uhrlaub JL, Ripperger TJ, Jergović M, Dalgai S, Wolf A, Whitmer R, Hammad H, Carrier A, Scott AJ, Nikolich-Žugich J, Worobey M, Sprissler R, Dake M, LaFleur BJ, Bhattacharya D. Immune responses to two and three doses of the BNT162b2 mRNA vaccine in adults with solid tumors. Nat Med. 2021;7:2002–2011. doi: 10.1038/s41591-021-01542-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oosting SF, van der Veldt AAM, Fehrmann RSN, GeurtsvanKessel CH, van Binnendijk RS, Dingemans AC, Smit EF, Hiltermann TJN, den Hartog G, Jalving M, Westphal TT, Bhattacharya A, de Wilt F, Boerma A, van Zijl L, Rimmelzwaan GF, Kvistborg P, van Els CACM, Rots NY, van Baarle D, Haanen JBAG, de Vries EGE. Immunogenicity after second and third mRNA-1273 vaccination doses in patients receiving chemotherapy, immunotherapy, or both for solid tumours. Lancet Oncol. 2022;S1470–2045(22):00203. doi: 10.1016/S1470-2045(22)00203-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Donahue RN, Marté JL, Goswami M, Toney NJ, Tsai YT, Gulley JL, Schlom J. Interrogation of the cellular immunome of cancer patients with regard to the COVID-19 pandemic. J Immunother Cancer. 2021;9:e002087. doi: 10.1136/jitc-2020-002087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Au L, Fendler A, Shepherd STC, Rzeniewicz K, Cerrone M, Byrne F, Carlyle E, Edmonds K, Del Rosario L, Shon J, Haynes WA, Ward B, Shum B, Gordon W, Gerard CL, Xie W, Joharatnam-Hogan N, Young K, Pickering L, Furness AJS, Larkin J, Harvey R, Kassiotis G, Gandhi S, Swanton C, Fribbens C, Wilkinson KA, Wilkinson RJ, Lau DK, Banerjee S, Starling N, Chau I, Turajlic S. Cytokine release syndrome in a patient with colorectal cancer after vaccination with BNT162b2. Nat Med. 2021;27:1362–1366. doi: 10.1038/s41591-021-01387-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oosting SF, van der Veldt AAM, GeurtsvanKessel CH, Fehrmann RSN, van Binnendijk RS, Dingemans AC, Smit EF, Hiltermann TJN, den Hartog G, Jalving M, Westphal TT, Bhattacharya A, van der Heiden M, Rimmelzwaan GF, Kvistborg P, Blank CU, Koopmans MPG, Huckriede ALW, van Els CACM, Rots NY, van Baarle D, Haanen JBAG, de Vries EGE. mRNA-1273 COVID-19 vaccination in patients receiving chemotherapy, immunotherapy, or chemoimmunotherapy for solid tumours: a prospective, multicentre, non-inferiority trial. Lancet Oncol. 2021;22:1681–1691. doi: 10.1016/S1470-2045(21)00574-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Song P, Zhang D, Cui X, Zhang L. Meta-analysis of immune-related adverse events of immune checkpoint inhibitor therapy in cancer patients. Thorac Cancer. 2020;11:2406–2430. doi: 10.1111/1759-7714.13541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen YW, Tucker MD, Beckermann KE, Iams WT, Rini BI, Johnson DB. COVID-19 mRNA vaccines and immune-related adverse events in cancer patients treated with immune checkpoint inhibitors. Eur J Cancer. 2021;155:291–293. doi: 10.1016/j.ejca.2021.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Strobel SB, Machiraju D, Kälber KA, Hassel JC. Immune-related adverse events of COVID-19 vaccination in skin cancer patients receiving immune-checkpoint inhibitor treatment. Cancer Immunol Immunother. 2021 doi: 10.1007/s00262-021-03133-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hibino M, Uryu K, Takeda T, Kunimatsu Y, Shiotsu S, Uchino J, Hirai S, Yamada T, Okada A, Hasegawa Y, Hiranuma O, Chihara Y, Kamada R, Tobe S, Maeda K, Horiuchi S, Kondo T, Takayama K. Safety and immunogenicity of mRNA vaccines against severe acute respiratory syndrome coronavirus 2 in patients with lung cancer receiving immune checkpoint inhibitors: a multicenter observational study in Japan. J Thorac Oncol. 2022;S1556–0864(22):00295–297. doi: 10.1016/j.jtho.2022.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gilbert D, Hu J, Medina T, Kessler ER, Lam ET. Safety of COVID-19 vaccines in subjects with solid tumor cancers receiving immune checkpoint inhibitors. Hum Vaccin Immunother. 2023;9:2207438. doi: 10.1080/21645515.2023.2207438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Widman AJ, Cohen B, Park V, McClure T, Wolchok J, Kamboj M. Immune-related adverse events among COVID-19-Vaccinated patients with cancer receiving immune checkpoint blockade. J Natl Compr Canc Netw. 2022;20:1134–1138. doi: 10.6004/jnccn.2022.7048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vahabi M, Ghazanfari T, Sepehrnia S. Molecular mimicry, hyperactive immune system, and SARS-COV-2 are three prerequisites of the autoimmune disease triangle following COVID-19 infection. Int Immunopharmacol. 2022;112:109183. doi: 10.1016/j.intimp.2022.109183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pezzaioli LC, Gatta E, Bambini F, Facondo P, Gava M, Cavadini M, Buoso C, Di Lodovico E, Rotondi M, Ferlin A, Cappelli C. Endocrine system after 2 years of COVID-19 vaccines: a narrative review of the literature. Front Endocrinol (Lausanne) 2022;13:1027047. doi: 10.3389/fendo.2022.1027047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wong CKH, Lui DTW, Xiong X, Chui CSL, Lai FTT, Li X, Wan EYF, Cheung CL, Lee CH, Woo YC, Au ICH, Chung MSH, Cheng FWT, Tan KCB, Wong ICK. Risk of thyroid dysfunction associated with mRNA and inactivated COVID-19 vaccines: a population-based study of 2.3 million vaccine recipients. BMC Med. 2022;20:339. doi: 10.1186/s12916-022-02548-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wright JJ, Powers AC, Johnson DB. Endocrine toxicities of immune checkpoint inhibitors. Nat Rev Endocrinol. 2021;17:389–399. doi: 10.1038/s41574-021-00484-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heine A, Juranek S, Brossart P. Clinical and immunological effects of mRNA vaccines in malignant diseases. Mol Cancer. 2021;20:52. doi: 10.1186/s12943-021-01339-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Denis M, Duruisseaux M, Brevet M, Dumontet C. How can immune checkpoint inhibitors cause hyperprogression in solid tumors? Front Immunol. 2020;11:492. doi: 10.3389/fimmu.2020.00492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bersanelli M, Giannarelli D, Castrignanò P, Fornarini G, Panni S, Mazzoni F, Tiseo M, Rossetti S, Gambale E, Rossi E, Papa A, Cortellini A, Lolli C, Ratta R, Michiara M, Milella M, De Luca E, Sorarù M, Mucciarini C, Atzori F, Banna GL, La Torre L, Vitale MG, Massari F, Rebuzzi SE, Facchini G, Schinzari G, Tomao S, Bui S, Vaccaro V, Procopio G, De Giorgi U, Santoni M, Ficorella C, Sabbatini R, Maestri A, Natoli C, De Tursi M, Di Maio M, Rapacchi E, Pireddu A, Sava T, Lipari H, Comito F, Verzoni E, Leonardi F, Buti S. Influenza vaccine indication during therapy with immune checkpoint inhibitors: a transversal challenge. INVIDIa Study Immunother. 2018;10:1229–1239. doi: 10.2217/imt-2018-0080. [DOI] [PubMed] [Google Scholar]
- 39.Valachis A, Rosén C, Koliadi A, Digkas E, Gustavsson A, Nearchou A, Ullenhag GJ. Improved survival without increased toxicity with influenza vaccination in cancer patients treated with checkpoint inhibitors. Oncoimmunology. 2021;10:1886725. doi: 10.1080/2162402X.2021.1886725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chong CR, Park VJ, Cohen B, Postow MA, Wolchok JD, Kamboj M. Safety of inactivated influenza vaccine in cancer patients receiving immune checkpoint inhibitors. Clin Infect Dis. 2020;70:193–199. doi: 10.1093/cid/ciz202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mei Q, Hu G, Yang Y, Liu B, Yin J, Li M, Huang Q, Tang X, Böhner A, Bryant A, Kurts C, Yuan X, Li J. Impact of COVID-19 vaccination on the use of PD-1 inhibitor in treating patients with cancer: a real-world study. J Immunother Cancer. 2022;10:e004157. doi: 10.1136/jitc-2021-004157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gleiss A, Oberbauer R, Heinze G. An unjustified benefit: immortal time bias in the analysis of time-dependent events. Transpl Int. 2018;31:125–130. doi: 10.1111/tri.13081. [DOI] [PubMed] [Google Scholar]
- 43.Kim HK, Heo MH, Lee HS, Sun JM, Lee SH, Ahn JS, Park K, Ahn MJ. Comparison of RECIST to immune-related response criteria in patients with non-small cell lung cancer treated with immune-checkpoint inhibitors. Cancer Chemother Pharmacol. 2017;80:591–598. doi: 10.1007/s00280-017-3396-4. [DOI] [PubMed] [Google Scholar]
- 44.Sullivan RJ, Weber JS. Immune-related toxicities of checkpoint inhibitors: mechanisms and mitigation strategies. Nat Rev Drug Discov. 2022;21:495–508. doi: 10.1038/s41573-021-00259-5. [DOI] [PubMed] [Google Scholar]
- 45.Gauci ML, Coutzac C, Houot R, Marabelle A, Lebbé C, FITC SARS-CoV-2 vaccines for cancer patients treated with immunotherapies: recommendations from the French society for immunotherapy of cancer (FITC) Eur J Cancer. 2021;148:121–123. doi: 10.1016/j.ejca.2021.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and analysed during the current study are available from the corresponding author on reasonable request.