Abstract
Neutrophil-to-lymphocyte ratio (NLR) was reported to be associated with prognosis of urothelial cancer (UC) patients receiving systemic chemotherapy or immunotherapy. However, it has not been elucidated how preceding first-line chemotherapy affects NLR and subsequent second-line pembrolizumab treatment. This multicenter study analyzed 458 patients with metastatic UC who received first-line chemotherapy and second-line pembrolizumab with regard to pre-chemotherapy and pre-pembrolizumab NLR in association with the efficacy of chemotherapy and pembrolizumab treatment. NLR was increased in 47% while decreased in 53% of patients before and after first-line chemotherapy. High pre-chemotherapy NLR (≥ 3) was significantly associated with unfavorable overall (OS, P = 0.0001) and progression-free (P < 0.0001) survivals after first-line chemotherapy. However, pre-chemotherapy NLR showed only modest influence on radiological response and survival after second-line pembrolizumab treatment, whereas pre-pembrolizumab NLR showed higher association. NLR decrease was associated with partial response or greater objective response by first-line chemotherapy, while NLR increase was associated with higher patient age. In conclusion, immediate pre-chemotherapy and pre-pembrolizumab NLR was significantly associated with efficacy of the following treatment, respectively. However, even patients with high pre-chemotherapy NLR achieved favorable OS if they had their NLR reduced by chemotherapy, whereas those with high pre-chemotherapy NLR yielded unfavorable OS if they had their NLR remained high after chemotherapy, suggesting that chemotherapy may have differential effect on the efficacy of subsequent pembrolizumab treatment in UC patients.
Keywords: Metastatic urothelial cancer, Overall survival, Progression-free survival, Chemotherapy, Pembrolizumab
Introduction
There have long been limited therapeutic agents that prolong the prognosis of patients with metastatic or surgically unresectable urothelial cancer (mUC). Recently, some immune checkpoint inhibitors (ICIs) have been shown to be effective for mUC. Pembrolizumab has been reported to prolong overall survival in patients with chemot-resistant mUC [1]. Avelumab has also shown to improve overall survival of mUC patients when administered after first-line chemotherapy yielding stable disease, partial response or complete response, compared with best supportive care [2].
Since first-line treatment with ICI alone or in combination with chemotherapy has not shown effectiveness enough to replace first-line chemotherapy [3, 4], second-line or sequential use of ICI following first-line chemotherapy is likely to remain standard treatment for some time. In such era, it is a clinically important issue when to switch chemotherapy to ICI to yield best effectiveness of the two therapeutic modalities as a series of treatments. In this regard, little is known about how first-line chemotherapy affects the efficacy of subsequent ICI treatment in the management of mUC.
Neutrophil-to-lymphocyte ratio (NLR) has been reported to be associated with survival of mUC patients receiving ICI [5–8]; higher NLR indicates unfavorable survival. It was reported that NLR tended to increase as time on chemotherapy was prolonged [9], suggesting that first-line chemotherapy should not be given so long when we consider the efficacy of subsequent pembrolizumab treatment. Herein, we have investigated the effect of first-line chemotherapy on NLR and the impact of pre-chemotherapy and pre-pembrolizumab NLR values on the efficacy of subsequent pembrolizumab treatment using data of 458 patients with mUC who received second-line pembrolizumab treatment after first-line systemic chemotherapy.
Materials and methods
Study cohorts
In a multicenter retrospective study conducted under the Japan Urological Oncology Group framework, which was approved by Institutional Review Board (IRB) at Kyoto University Graduate School of Medicine (approval number R1783) and local IRB at each participating institute, the clinicopathological data of patients with surgically unresectable, chemoresistant UC who received pembrolizumab were collected from 59 institutions across Japan (Appendix) [8]. After excluding patients with missing data or those who received pembrolizumab treatment as the third-line treatment or later, 458 patients who received second-line pembrolizumab treatment following the first-line systemic chemotherapy were included in the analysis.
Data collection
The cutoff date for data inclusion was January 24, 2020. The following data were collected using a standardized case report form: age; sex; smoking history; first-line chemotherapy regimen; number of courses of first-line chemotherapy; pre-chemotherapy and pre-pembrolizumab NLR, hemoglobin (Hb) and Eastern Cooperative Oncology Group (ECOG) performance status (PS); best objective response to pembrolizumab treatment according to Response Evaluation Criteria in Solid Tumors version 1.1. Blood test data within 2 weeks before the initiation of chemotherapy and pembrolizumab treatment were collected. The NLR was calculated by dividing the number of neutrophils by that of lymphocytes.
Statistical analysis
OS was defined as the time from the initiation of chemotherapy or pembrolizumab treatment to death from any cause and estimated using Kaplan–Meier analysis with the log-rank test. Progression-free survival (PFS) was defined as the time from the initiation of chemotherapy to any of clinical and radiological disease progression.
Univariate and multivariate Cox proportional hazards models were used to examine the associations of OS with the clinical variables. Univariate and multivariate logistic regression models were used to examine the associations of NLR changes with the clinical variables. The optimal cutoffs for Hb (g/dL) and NLR were screened via time-dependent receiver-operating characteristic (ROC) curve analysis [10]. The association between NLR change and the best objective response was evaluated using patients for whom data for objective responses to pembrolizumab were available. The results were depicted using mosaic (Marimekko) plots and statistically examined using the chi-squared test.
All statistical analyses were performed using JMP Pro 15.1.0 (SAS Institute, Cary, NC, USA), Prism 6 version 6.0 h (GraphPad Software, Inc., San Diego, CA, USA).
Results
Baseline demographic characteristics of the 458 study patients are shown in Table 1. At the data cutoff date, the median follow-up (interquartile range) periods from the initiation of first-line chemotherapy was 13.6 months (8.7–22.2), while that from the initiation of second-line treatment was 6.7 months (3.4–11.2). For censored cases, the median follow-up (interquartile range) periods from the initiation of first-line chemotherapy was 20.4 months (12.1–26.6), while that from the initiation of second-line treatment was 10.8 months (6.6–17.0). Progression-free survival for the first-line chemotherapy was 5.7 months (3.0–10.6).
Table 1.
Demographic and disease characteristics of the 458 study patients
| Age, years, median (range) | 72 | (31–91) | |
|---|---|---|---|
| Sex, N (%) |
Male Female |
324 124 |
(70.7) (29.3) |
| Smoking history, N (%) |
Yes No Unknown |
260 192 6 |
(56.8) (41.9) (1.3) |
| Primary site, N (%) |
Bladder/urethra Upper tract Both Unknown |
213 208 34 2 |
(46.5) (45.6) (7.4) (0.4) |
| Metastasis site, N (%) |
LN only Other organs Liver |
166 204 88 |
(36.2) (44.5) (19.2) |
| First-line chemotherapy regimen, N (%) |
GC G-CBDCA M-VAC Others |
259 132 6 61 |
(56.6) (28.8) (1.3) (13.3) |
| Number of courses of first-line chemotherapy, median (range) | 4 | (1–42) | |
| Pretreatment NLR, median (range) | 3.36 | (0.13–92) | |
| Pretreatment Hb, median (range) | 11.9 | (6.6–16.8) | |
| Pretreatment ECOG PS, N (%) |
0 1 ≥ 2 |
303 110 45 |
66.2 24.0 9.8 |
| Pre-pembrolizumab NLR, median (range) | 3.44 | (0.31–69) | |
| Pre-pembrolizumab Hb, median (range) | 10.4 | (5.9–15.2) | |
| Pre-pembrolizumab ECOG PS, N (%) |
0 1 ≥ 2 |
210 152 96 |
45.9 33.2 21.0 |
GC, gemcitabine, cisplatin, G-CBDCA, gemcitabine, carboplatin, MVAC, methotrexate, vinblastine, adriamycin, cisplatin, NLR, Neutrophil-to-lymphocyte ratio, Hb, hemoglobin, ECOG PS, Eastern Cooperative Oncology Group performance status
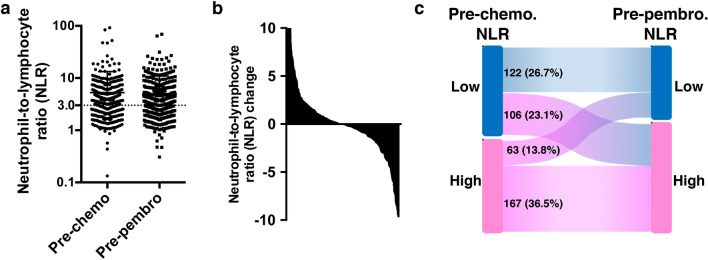
The median NLR (interquartile range) at the initiation of first-line chemotherapy was 3.36 (2.32–4.89), while that at the initiation of second-line pembrolizumab treatment was 3.44 (2.32–5.29) (Fig. 1a). The difference between the two was not statistically significant (P = 0.164, Wilcoxon matched pairs signed rank test). NLR was increased in 47% while decreased in 53% of patients before and after first-line chemotherapy (Fig. 1b). NLR was lower than 3.0 in 228 (49.8%) of patients at pre-chemotherapy settings. Of those, 106 (23.1%) had their NLR increased after chemotherapy to 3.0 or higher (Fig. 1c). On the other hand, of 230 (50.2%) patients with pre-chemotherapy NLR of 3.0 or higher, 63 (13.8%) had their NLR decreased after chemotherapy to less than 3.0.
Fig. 1.
a Chart of NLR values at the pre-chemotherapy (pre-chemo) and pre-pembrolizumab (pre-pembro) settings. b Histogram chart of change in neutrophil-to-lymphocyte ratio (NLR). c Sankey diagraMDepicting change in NLR from pre-chemotherapy to pre-pembrolizumab settings. High NLR was defined as ≥ 3
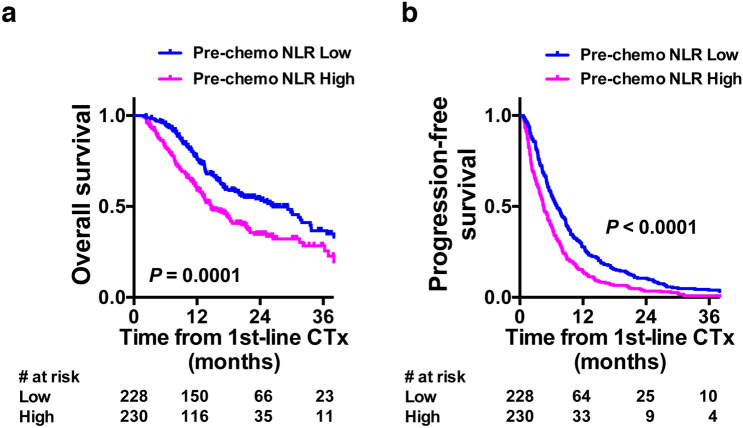
OS and PFS after first-line chemotherapy for overall study patients were 18.8 and 5.7 months, respectively. OS after the initiation of pembrolizumab treatment was 10.5 months, while PFS after pembrolizumab treatment was not available in the present study. Post-chemotherapy OS (median 14.7 vs 26.4 months, hazard ratio [HR] 1.655, 95%CI 1.284–2.133, P = 0.0001, log-rank test, Fig. 2a) and PFS (median 4.5 vs 7.1 months, HR 1.541, 95%CI 1.312–1.908, P < 0.0001, log-rank test, Fig. 2b) were significantly poorer for those with pre-chemotherapy NLR ≥ 3.0 compared with those with pre-chemotherapy NLR < 3.0.
Fig. 2.
Kaplan–Meier plots displaying survival of 458 patients. a, b Overall a and progression-free b survivals from the initiation of first-line chemotherapy (CTx) with regard to pre-chemotherapy NLR
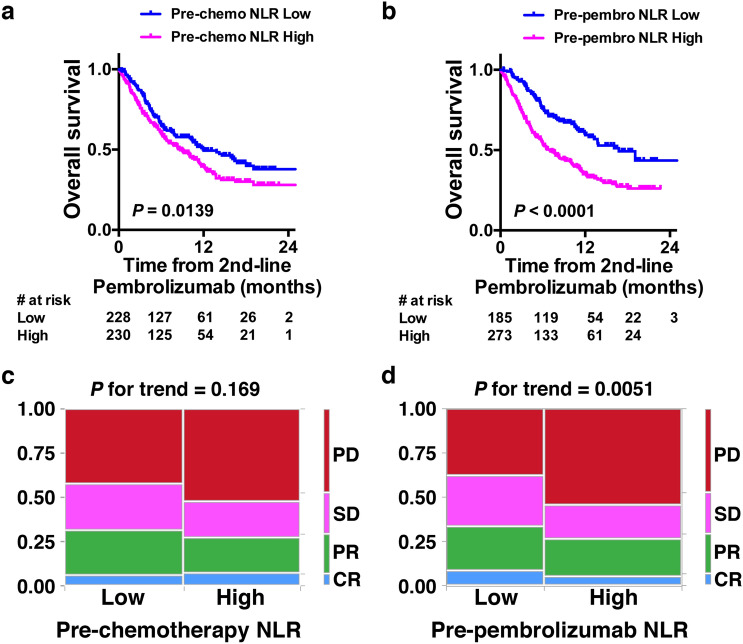
High pre-chemotherapy NLR was also significantly associated with poorer survival after the initiation of second-line pembrolizumab treatment (median 8.7 vs 12.0 months, HR 1.371, 95%CI 1.067–1.765, P = 0.0139, log-rank test, Fig. 3a), whereas high pre-pembrolizumab NLR showed stronger association (median 6.7 vs 16.8 months, HR 2.062, 95%CI 1.526–2.529, P < 0.0001, log-rank test, Fig. 3b). Radiological response to second-line pembrolizumab treatment was evaluable in 416 (90.8%) of 458 patients. Pre-chemotherapy NLR did not show a statistically significant association with radiological response to pembrolizumab treatment (P for trend = 0.169, Fig. 3c), whereas Pre-pembrolizumab NLR showed a statistically significant association with radiological response to pembrolizumab treatment (P for trend = 0.0051, Fig. 3d).
Fig. 3.
a Overall survival from the initiation of second-line pembrolizumab with regard to pre-chemotherapy NLR. High NLR was defined as ≥ 3. b Overall survival from the initiation of second-line pembrolizumab with regard to pre-pembrolizumab NLR. c, d Mosaic charts for the proportion of best objective response to pembrolizumab treatment with regard to pre-chemotherapy c and pre-pembrolizumab d NLR. High NLR was defined as ≥ 3
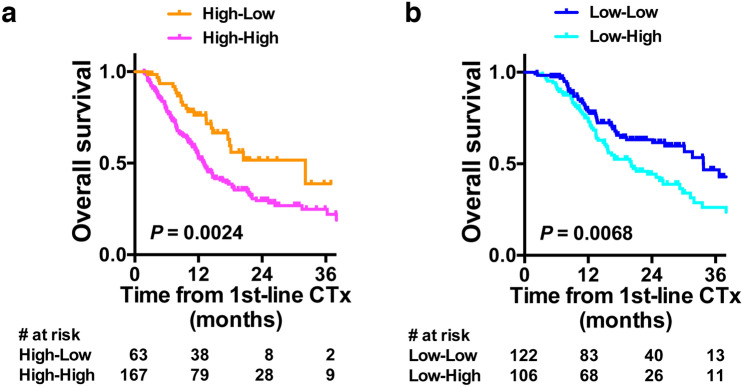
In 230 patients with high pre-chemotherapy NLR, OS was significantly better if NLR was decreased to < 3.0 after first-line chemotherapy (median 32.2 vs 13.0 months, HR 0.405, 95%CI 0.258–0.634, P = 0.0024, log-rank test, Fig. 4a). Objective response of progressive disease (PD) by first-line chemotherapy was significantly associated with non-improvement in NLR in uni- and multi-variate logistic regression analyses (Table 2 left). In 228 patients with low pre-chemotherapy NLR, OS was significantly poorer if NLR was increased to ≥ 3.0 after first-line chemotherapy (median 20.1 vs 33.7 months, HR 1.679, 95%CI 1.146–2.458, P = 0.0068, log-rank test, Fig. 4b). Age of 75 years or older was significantly associated with the NLR increase in uni- and multi-variate logistic regression analyses (Table 2 right). Objective response of progressive disease (PD) by first-line chemotherapy showed similar tendency toward deterioration in NLR, although it did not reach statistical significance.
Fig. 4.
a Kaplan–Meier plots displaying overall survival of 230 patients with pre-chemotherapy NLR ≥ 3 from the initiation of first-line chemotherapy (CTx) with regard to pre-pembrolizumab NLR. b Kaplan–Meier plots displaying overall survival of 228 patients with pre-chemotherapy NLR < 3 from the initiation of first-line chemotherapy (CTx) with regard to pre-pembrolizumab NLR. High NLR was defined as ≥ 3
Table 2.
Univariate and multivariate step-wise logistic regression analyses. Left: For reduction of NLR after 1L chemotherapy in 230 patients with pre-chemotherapy NLR ≥3. Right: For elevation of NLR after 1L chemotherapy in 228 patients with pre-chemotherapy NLR <3
| Univariate | Multivariate | Univariate | Multivariate | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | Lower 95% | Upper 95% | P value | HR | Lower 95% | Upper 95% | P value | HR | Lower 95% | Upper 95% | P value | HR | Lower 95% | Upper 95% | P value | |
| ≥75 years | 0.949 | 0.521 | 1.731 | 0.8650 | 0.957 | 0.506 | 1.813 | 0.8939 | 1.695 | 0.988 | 2.909 | 0.0554 | 1.969 | 1.102 | 3.519 | 0.0221* |
| <75 years | Ref. | Ref. | Ref. | Ref. | ||||||||||||
| Male | 0.922 | 0.484 | 1.757 | 0.8053 | 1.058 | 0.501 | 2.233 | 0.8824 | 0.944 | 0.525 | 1.699 | 0.8477 | 1.439 | 0.716 | 2.893 | 0.3072 |
| Female | Ref. | Ref. | Ref. | Ref. | ||||||||||||
| Yes | 0.924 | 0.516 | 1.658 | 0.7921 | 0.883 | 0.443 | 1.759 | 0.7234 | 0.593 | 0.346 | 1.015 | 0.0568 | 0.554 | 0.296 | 1.039 | 0.0655 |
| No | Ref. | Ref. | Ref. | Ref. | ||||||||||||
| LN only | Ref. | Ref. | Ref. | Ref. | ||||||||||||
| Other organs | 1.502 | 0.791 | 2.852 | 0.2142 | 1.533 | 0.781 | 3.007 | 0.2142 | 1.132 | 0.629 | 2.036 | 0.6787 | 1.175 | 0.631 | 2.188 | 0.6111 |
| Liver | 0.717 | 0.287 | 1.879 | 0.4760 | 0.654 | 0.252 | 1.697 | 0.3827 | 1.146 | 0.555 | 2.368 | 0.7132 | 1.072 | 0.500 | 2.296 | 0.8581 |
| GC | 1.678 | 0.925 | 3.045 | 0.0888 | 1.968 | 1.047 | 3.700 | 0.0356* | 1.310 | 0.771 | 2.228 | 0.3184 | 1.682 | 0.947 | 2.989 | 0.0763 |
| Others | Ref. | Ref. | Ref. | Ref. | ||||||||||||
| ≤6 | Ref. | Ref. | Ref. | Ref. | ||||||||||||
| ≥7 | 0.867 | 0.367 | 2.045 | 0.7439 | 0.600 | 0.237 | 1.520 | 0.2816 | 1.399 | 0.742 | 2.636 | 0.2992 | 1.656 | 0.836 | 3.281 | 0.1480 |
| PD | 0.541 | 0.289 | 1.012 | 0.0456* | 0.428 | 0.218 | 0.841 | 0.0138* | 0.451 | 0.803 | 2.623 | 0.217 | 1.611 | 0.852 | 3.044 | 0.1423 |
| SD / PR / CR | Ref. | Ref. | Ref. | Ref. | ||||||||||||
NLR, Neutrophil-to-lymphocyte ratio, 1L, first-line, PD, Progressive disease, SD, Stable disease, PR, Partial response, CR, Complete response, *statistically significant
Cox proportional hazard analyses showed that pre-chemotherapy NLR was not significantly associated with OS from the initiation of 1st-line chemotherapy or 2nd-line pembrolizumab, whereas pre-pembrolizumab NLR was associated with both (Table 3).
Table 3.
Multivariate Cox proportional hazard analyses for overall survival after the initiation of 1st-line chemotherapy (top) and 2nd-line pembrolizumab (bottom)
| HR | Lower 95% | Upper 95% | P value | ||
|---|---|---|---|---|---|
| ECOG PS | 0 | Ref | |||
| 1 | 1.4535 | 1.0580 | 1.9970 | 0.0210* | |
| 2 ≤ | 2.6027 | 1.8448 | 3.6720 | < .0001* | |
| Metastasis site | LN only | Ref | |||
| Other organs | 1.5861 | 1.1585 | 2.1714 | 0.0040* | |
| Liver | 2.2134 | 1.5418 | 3.1777 | < .0001* | |
| Pre-pembrolizumab NLR | 3 > | Ref | |||
| 3 ≤ | 1.6230 | 1.2013 | 2.1927 | 0.0016* | |
| Hb | 11 g/dL ≤ | Ref | |||
| 11 g/dL > | 1.7076 | 1.2693 | 2.2973 | 0.0004 | |
| ORR by1st-line chemotherapy | SD / PR / CR | Ref | |||
| PD | 1.4135 | 1.0717 | 1.8643 | 0.0143* | |
| Pre-chemotherapy NLR | 3 > | Ref | |||
| 3 ≤ | 1.0359 | 0.7922 | 1.3545 | 0.7966 | |
| Smoking history | Yes | 1.1806 | 0.8644 | 1.6125 | 0.2966 |
| No | Ref | ||||
| Sex | Male | Ref | |||
| Female | 1.1443 | 0.8191 | 1.5987 | 0.4295 | |
| Age | 75 years > | 1.1223 | 0.8543 | 1.4744 | 0.4071 |
| 75 years ≤ | Ref | ||||
| 1st-line chemo-regimen | GC | Ref | |||
| Others | 1.2766 | 0.9777 | 1.6669 | 0.0728 | |
| # of 1st-line chemotherapy courses | 7 > | 1.9920 | 1.3637 | 2.9097 | 0.0004* |
| 7 ≤ | Ref | ||||
| ECOG PS | 0 | Ref | |||
| 1 | 1.5280 | 1.1110 | 2.1018 | 0.0091* | |
| 2 ≤ | 3.2775 | 2.3352 | 4.6003 | < .0001* | |
| Metastasis site | LN only | Ref | |||
| Other organs | 1.5571 | 1.1416 | 2.1237 | 0.0052* | |
| Liver | 2.4522 | 1.7078 | 3.5212 | < .0001* | |
| Pre-pembrolizumab NLR | 3 > | Ref | |||
| 3 ≤ | 1.7395 | 1.2931 | 2.3400 | 0.0003* | |
| Hb | 11 g/dL ≤ | Ref | |||
| 11 g/dL > | 1.5855 | 1.1778 | 2.1326 | 0.0023* | |
| ORR by1st-line chemotherapy | SD / PR / CR | Ref | |||
| PD | 1.3115 | 0.9976 | 1.7347 | 0.0520 | |
| Pre-chemotherapy NLR | 3 > | Ref | |||
| 3 ≤ | 0.9002 | 0.6857 | 1.1818 | 0.4490 | |
| Smoking history | Yes | 1.1204 | 0.8245 | 1.5226 | 0.4675 |
| No | Ref | ||||
| Sex | Male | Ref | |||
| Female | 1.0919 | 0.7834 | 1.5220 | 0.6038 | |
| Age | 75 years > | 1.0722 | 0.8185 | 1.4046 | 0.6127 |
| 75 years ≤ | Ref | ||||
| 1st-line chemo-regimen | GC | Ref | |||
| Others | 1.0510 | 0.8057 | 1.3709 | 0.7138 | |
| # of 1st-line chemotherapy courses | 7 > | 1.044 | 0.7174 | 1.5213 | 0.8195 |
| 7 ≤ | Ref |
Discussion
The present study showed that pre-chemotherapy NLR was associated with the efficacy of chemotherapy whereas pre-pembrolizumab was associated with the efficacy of 2nd-line pembrolizumab in mUC patients. Our findings are consistent with previous reports showing that pre-pembrolizumab NLR is associated with survival after pembrolizumab treatment [5–8]. NLR is one of the biomarkers that can change over the course of treatment. Indeed, some of the previous reports on NLR [5–7] as well as C-reactive protein [11] showed that the improvement in the biomarkers after pembrolizumab treatment was associated with longer survival. However, effect of NLR before and after first-line chemotherapy on the treatment outcomes of subsequent pembrolizumab has not been reported.
A previous study reported that multiple courses of systemic chemotherapy increases NLR in mUC patients. However, the present study demonstrated that a subset (13.8% of overall or 27.5% of those with high pre-chemotherapy NLR in our cohort) of patients have their NLR decreased from ≥ 3 to < 3 during the first-line chemotherapy. Additionally, those patients can expect longer survival after second-line pembrolizumab treatment than those with their NLR remained high. Our findings indicate that first-line chemotherapy may show differential effects on NLR and efficacy of subsequent pembrolizumab treatment.
Longer OS after pembrolizumab treatment in patients who had their NLR decreased by first-line chemotherapy indicates that those patients were benefited from the first-line chemotherapy. The NLR change during 1st-line chemotherapy could reflect the efficacy of chemotherapy. The current results suggest that the efficacy of 1st-line chemotherapy could be associated with the efficacy of 2nd-line pembrolizumab. Indeed, our analysis showed NLR change was associated with the efficacy of 1st-line chemotherapy. Post-chemotherapy reduction of NLR in the 230 patients with high pre-chemotherapy NLR was associated with objective response by first-line chemotherapy (PD). Poor objective response showed similar impact on post-chemotherapy NLR deterioration in those with low pre-chemotherapy NLR. Furthermore, we may expect a greater benefit from pembrolizumab treatment if it is used when NLR remains low during on-going response of chemotherapy. In this regard, our results partly verify the main eligibility criteria of JAVELIN Bladder 100 trial; ‘those who had disease that had not progressed with first-line chemotherapy’ [2]. When the first-line chemotherapy and maintenance immunotherapy were used sequentially, the first-line chemotherapy functions as a kind of ‘induction’ therapy that enhances the efficacy of the subsequent ICI treatment. Although avelumab at the maintenance treatment setting and pembrolizumab at the second-line treatment setting should be considered separately, our findings suggest that, if NLR was kept low, the effect of first-line chemotherapy as the ‘induction’ can be considered to be remained even at the setting of treatment failure.
On the other hand, the present study demonstrated that another subset (23.1% of overall or 46.4% of those with low pre-chemotherapy NLR in our cohort) of patients have their NLR increased from < 3 to ≥ 3 during the first-line chemotherapy. Those patients showed shorter OS after second-line pembrolizumab treatment than those with their NLR remained low. In this regard, previous reports showed that longer time on chemotherapy was associated with an increase in NLR [9] but not with prolonged OS [12]. Our multivariate logistic regression analysis showed that older age (≥ 75 years) was independently associated with NLR increase to ≥ 3.0. Thus, it seems better to switch chemotherapy to ICI to avoid NLR increase as well as unnecessary cumulative toxicity, particularly in the elderly patients.
Several prognostic blood- or serum-based biomarkers have been reported in mUC including, CRP, LDH, platelet-to-lymphocyte ratio (PLR), lymphocyte-to-monocyte ratio (LMR), absolute monocyte count, or absolute eosinophils count. Among those, the present study focused on NLR since it has shown the consistent ability as a prognosticator in a variety of settings, including intravesical recurrence and muscle-invasive or metastatic progression non-muscle-invasive disease [13–15], adverse outcomes of radical cystectomy for non-metastatic muscle-invasive disease (MIBC) [16–26]. In addition to surgical treatment, high NLR has been reported to be associated with unfavorable oncological outcomes in patients with surgically unresectable metastatic UC receiving first- [9] or second-line [27–29] chemotherapy. However, the results of the present study suggest that pre-treatment NLR was no longer associated with patient survival after adjusting other prognostic factors including post-chemotherapy NLR in the immune checkpoint inhibitor era. High NLR or lymphocytopenia has been reported to be associated with higher mortality rates in patients with various malignant [30] and non-malignant [31–35] diseases, as well as the general population [36], indicating that NLR is not a specific biomarker for UC patients. However, a previous study [8] and the present study showed significant correlations between NLR and objective response to pembrolizumab treatment, indicating the association of NLR with survival of patients through affecting efficacy of pembrolizumab treatment.
There are several limitations of this study that should be acknowledged. Multiple values on NLR during chemotherapy and pembrolizumab treatment were not available in the study. I this regard, previous studies suggest that NLR evaluation at earlier timepoints might have a certain impact on the clinical outcomes [5, 7]. Objective response or pathological diagnosis was determined in each study institute, and thus, the findings were not centralized. The chemotherapy and pembrolizumab treatment, particularly when to switch or discontinue treatments, were not standardized throughout the study. Post-chemotherapy NLR should depend on several factors such as the time from chemotherapy agent administration and presence of infection, which were not incorporated in the analyses of the present study. The present study lacks data for PFS from 2nd-line pembrolizumab treatment and NLR after pembrolizumab treatment CRP, LDH and absolute eosinophil count were not available in our database. This study did not include those who received only first-line chemotherapy but not second-line pembrolizumab for certain reasons including rapid progression or deteriorated general status. Nonetheless, this was the largest study to analyze the effect of first-line chemotherapy on the efficacy subsequent second-line pembrolizumab treatment, which is relevant to clinical decision-making in the management of metastatic UC.
In summary, fist-line chemotherapy affects NLR of a subset of patients with mUC. Even patients with high pre-chemotherapy NLR may expect favorable OS if they have their NLR reduced or remained low on chemotherapy. On the contrary, patients with their NLR remained high or increased on chemotherapy will have unfavorable OS. Improvement in NLR by first-line chemotherapy was associated with objective response to the chemotherapy, which benefit patients receiving subsequent pembrolizumab treatment.
Acknowledgements
We thank Joe Barber Jr., PhD, from Edanz Group (https://en-author-services.edanzgroup.com/) for editing a draft of this manuscript.
Appendix
Study institutes
Osaka City University, Akita University, Hirosaki University, National Cancer Center Hospital, Hamamatsu University School of Medicine, Yamagata University Faculty of Medicine, Kyoto University, University of Tsukuba, Nara Medical University, Shizuoka General Hospital, University of the Ryukyus, Iwate Medical University, Oita University, Hiroshima University, Shimane University, Kansai Medical University, Osaka University, Kagawa University, University of Yamanashi, Japanese Red Cross Wakayama Medical Center, Kyoto Prefectural University of Medicine, Kobe City Nishi-Kobe Medical Center, Japanese Red Cross Osaka Hospital, Nagoya University, Harasanshin Hospital, Hokkaido University, Japanese Red Cross Otsu Hospital, Kagoshima University, Kyushu University, Shikoku Cancer Center, Tenri Hospital, Hakodate Goryoukaku Hospital, Kitasato University, Kyoto Katsura Hospital, National Hospital Organization Kyoto Medical Center, Kumamoto University, National Hospital Organization Himeji Medical Center, Tazuke Kofukai Medical Research Institute, Kitano Hospital, Toyooka Hospital, Hokkaido Cancer Center, University of Miyazaki, Hitachi General Hospital, The Jikei University Kashiwa Hospital, Shimada Municipal Hospital, Mie University, Yamaguchi University, Ibaraki Prefectural Central Hospital, Kyoto City Hospital, Kochi School of Medicine, Ijinkai Takeda General Hospital, University of Toyama, Otsu City Hospital, Sapporo Medical University, Kansai Electric Power Hospital, Kurume University, Hyogo College of Medicine, Hirakata Kohsai Hospital, Rakuwakai Otowa Hospital.
Funding
No funding exists.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bellmunt J, de Wit R, Vaughn DJ, et al. Pembrolizumab as Second-Line Therapy for Advanced Urothelial Carcinoma. N Engl J Med. 2017;376:1015–1026. doi: 10.1056/NEJMoa1613683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Powles T, Park SH, Voog E, et al. Avelumab Maintenance Therapy for Advanced or Metastatic Urothelial Carcinoma. N Engl J Med. 2020;383:1218–1230. doi: 10.1056/NEJMoa2002788. [DOI] [PubMed] [Google Scholar]
- 3.Galsky MD, Arija JAA, Bamias A, et al. Atezolizumab with or without chemotherapy in metastatic urothelial cancer (IMvigor130): a multicentre, randomised, placebo-controlled phase 3 trial. Lancet. 2020;395:1547–1557. doi: 10.1016/S0140-6736(20)30230-0. [DOI] [PubMed] [Google Scholar]
- 4.Powles T, van der Heijden MS, Castellano D, et al. Durvalumab alone and durvalumab plus tremelimumab versus chemotherapy in previously untreated patients with unresectable, locally advanced or metastatic urothelial carcinoma (DANUBE): a randomised, open-label, multicentre, phase 3 trial. Lancet Oncol. 2020 doi: 10.1016/S1470-2045(20)30541-6. [DOI] [PubMed] [Google Scholar]
- 5.Ogihara K, Kikuchi E, Shigeta K, et al. The pretreatment neutrophil-to-lymphocyte ratio is a novel biomarker for predicting clinical responses to pembrolizumab in platinum-resistant metastatic urothelial carcinoma patients. Urol Oncol. 2020;38(602):e1–e10. doi: 10.1016/j.urolonc.2020.02.005. [DOI] [PubMed] [Google Scholar]
- 6.Tamura D, Jinnouchi N, Abe M, et al. Prognostic outcomes and safety in patients treated with pembrolizumab for advanced urothelial carcinoma: experience in real-world clinical practice. Int J Clin Oncol. 2020;25:899–905. doi: 10.1007/s10147-019-01613-9. [DOI] [PubMed] [Google Scholar]
- 7.Yamamoto Y, Yatsuda J, Shimokawa M, et al. Prognostic value of pre-treatment risk stratification and post-treatment neutrophil/lymphocyte ratio change for pembrolizumab in patients with advanced urothelial carcinoma. Int J Clin Oncol. 2020 doi: 10.1007/s10147-020-01784-w. [DOI] [PubMed] [Google Scholar]
- 8.Kobayashi T, Ito K, Kojima T, et al. Risk stratification for the prognosis of patients with chemoresistant urothelial cancer treated with pembrolizumab. Cancer Sci. 2020 doi: 10.1111/cas.14762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Santoni M, Crabb SJ, Conti A, et al. Conditional survival of patients treated with first-line chemotherapy for metastatic urothelial cancer. Clin Genitourin Cancer. 2015;13:244–249. doi: 10.1016/j.clgc.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 10.Heagerty PJ, Lumley T, Pepe MS. Time-dependent ROC curves for censored survival data and a diagnostic marker. Biometrics. 2000;56:337–344. doi: 10.1111/j.0006-341x.2000.00337.x. [DOI] [PubMed] [Google Scholar]
- 11.Kijima T, Yamamoto H, Saito K, et al. Early C-reactive protein kinetics predict survival of patients with advanced urothelial cancer treated with pembrolizumab. Cancer Immunol Immunother. 2020 doi: 10.1007/s00262-020-02709-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sonpavde GP, Mariani L, Lo Vullo S, et al. Impact of the number of cycles of platinum based first line chemotherapy for advanced urothelial carcinoma. J Urol. 2018;200:1207–1214. doi: 10.1016/j.juro.2018.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.D'Andrea D, Moschini M, Gust K, et al. Prognostic role of neutrophil-to-lymphocyte ratio in primary non-muscle-invasive bladder cancer. Clin Genitourin Cancer. 2017;15:e755–e764. doi: 10.1016/j.clgc.2017.03.007. [DOI] [PubMed] [Google Scholar]
- 14.Mano R, Baniel J, Shoshany O, Margel D, Bar-On T, Nativ O, Rubinstein J, Halachmi S. Neutrophil-to-lymphocyte ratio predicts progression and recurrence of non-muscle-invasive bladder cancer. Urol Oncol. 2015;33(67):e1–7. doi: 10.1016/j.urolonc.2014.06.010. [DOI] [PubMed] [Google Scholar]
- 15.Racioppi M, Di Gianfrancesco L, Ragonese M, Palermo G, Sacco E, Bassi PF. Can Neutrophil-to-Lymphocyte ratio predict the response to BCG in high-risk non muscle invasive bladder cancer? Int Braz J Urol. 2019;45:315–324. doi: 10.1590/S1677-5538.IBJU.2018.0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.D'Andrea D, Moschini M, Gust KM, et al. Lymphocyte-to-monocyte ratio and neutrophil-to-lymphocyte ratio as biomarkers for predicting lymph node metastasis and survival in patients treated with radical cystectomy. J Surg Oncol. 2017;115:455–461. doi: 10.1002/jso.24521. [DOI] [PubMed] [Google Scholar]
- 17.Gondo T, Nakashima J, Ohno Y, et al. Prognostic value of neutrophil-to-lymphocyte ratio and establishment of novel preoperative risk stratification model in bladder cancer patients treated with radical cystectomy. Urology. 2012;79:1085–1091. doi: 10.1016/j.urology.2011.11.070. [DOI] [PubMed] [Google Scholar]
- 18.Hermanns T, Bhindi B, Wei Y, et al. Pre-treatment neutrophil-to-lymphocyte ratio as predictor of adverse outcomes in patients undergoing radical cystectomy for urothelial carcinoma of the bladder. Br J Cancer. 2014;111:444–451. doi: 10.1038/bjc.2014.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lucca I, Jichlinski P, Shariat SF, et al. The neutrophil-to-lymphocyte ratio as a prognostic factor for patients with urothelial carcinoma of the bladder following radical cystectomy: validation and meta-analysis. Eur Urol Focus. 2016;2:79–85. doi: 10.1016/j.euf.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 20.Tan YG, Eu E, Lau Kam On W, Huang HH. Pretreatment neutrophil-to-lymphocyte ratio predicts worse survival outcomes and advanced tumor staging in patients undergoing radical cystectomy for bladder cancer. Asian J Urol. 2017;4:239–246. doi: 10.1016/j.ajur.2017.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Viers BR, Boorjian SA, Frank I, Tarrell RF, Thapa P, Karnes RJ, Thompson RH, Tollefson MK. Pretreatment neutrophil-to-lymphocyte ratio is associated with advanced pathologic tumor stage and increased cancer-specific mortality among patients with urothelial carcinoma of the bladder undergoing radical cystectomy. Eur Urol. 2014;66:1157–1164. doi: 10.1016/j.eururo.2014.02.042. [DOI] [PubMed] [Google Scholar]
- 22.Black AJ, Zargar H, Zargar-Shoshtari K, et al. The prognostic value of the neutrophil-to-lymphocyte ratio in patients with muscle-invasive bladder cancer treated with neoadjuvant chemotherapy and radical cystectomy. Urol Oncol. 2020 doi: 10.1016/j.urolonc.2019.09.023. [DOI] [PubMed] [Google Scholar]
- 23.Buisan O, Orsola A, Areal J, Font A, Oliveira M, Martinez R, Ibarz L. Low pretreatment neutrophil-to-lymphocyte ratio predicts for good outcomes in patients receiving neoadjuvant chemotherapy before radical cystectomy for muscle invasive bladder cancer. Clin Genitourin Cancer. 2017;15(145–51):e2. doi: 10.1016/j.clgc.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 24.van Kessel KE, de Haan LM, Fransen van de Putte EE, van Rhijn BW, de Wit R, van der Heijden MS, Zwarthoff EC, Boormans JL. Elevated derived neutrophil-to-lymphocyte ratio corresponds with poor outcome in patients undergoing pre-operative chemotherapy in muscle-invasive bladder cancer. Bladder Cancer. 2016;2:351–360. doi: 10.3233/BLC-160055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kang M, Jeong CW, Kwak C, Kim HH, Ku JH. The Prognostic significance of the early postoperative neutrophil-to-lymphocyte ratio in patients with urothelial carcinoma of the bladder undergoing radical cystectomy. Ann Surg Oncol. 2016;23:335–342. doi: 10.1245/s10434-015-4708-8. [DOI] [PubMed] [Google Scholar]
- 26.Seah JA, Leibowitz-Amit R, Atenafu EG, Alimohamed N, Knox JJ, Joshua AM, Sridhar SS. Neutrophil-lymphocyte ratio and pathological response to neoadjuvant chemotherapy in patients with muscle-invasive bladder cancer. Clin Genitourin Cancer. 2015;13:e229–e233. doi: 10.1016/j.clgc.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 27.Bellmunt J, Choueiri TK, Fougeray R, et al. Prognostic factors in patients with advanced transitional cell carcinoma of the urothelial tract experiencing treatment failure with platinum-containing regimens. J Clin Oncol. 2010;28:1850–1855. doi: 10.1200/JCO.2009.25.4599. [DOI] [PubMed] [Google Scholar]
- 28.Matsumoto R, Abe T, Ishizaki J, et al. Outcome and prognostic factors in metastatic urothelial carcinoma patients receiving second-line chemotherapy: an analysis of real-world clinical practice data in Japan. Jpn J Clin Oncol. 2018;48:771–776. doi: 10.1093/jjco/hyy094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rossi L, Santoni M, Crabb SJ, et al. High neutrophil-to-lymphocyte ratio persistent during first-line chemotherapy predicts poor clinical outcome in patients with advanced urothelial cancer. Ann Surg Oncol. 2015;22:1377–1384. doi: 10.1245/s10434-014-4097-4. [DOI] [PubMed] [Google Scholar]
- 30.Templeton AJ, McNamara MG, Seruga B, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst. 2014 doi: 10.1093/jnci/dju124. [DOI] [PubMed] [Google Scholar]
- 31.Dong CH, Wang ZM, Chen SY. Neutrophil to lymphocyte ratio predict mortality and major adverse cardiac events in acute coronary syndrome: a systematic review and meta-analysis. Clin Biochem. 2018;52:131–136. doi: 10.1016/j.clinbiochem.2017.11.008. [DOI] [PubMed] [Google Scholar]
- 32.Gharebaghi N, Valizade Hasanloei MA, Medizadeh Khalifani A, Pakzad S, Lahooti D. Neutrophil-to-lymphocyte ratio in patients with gram-negative sepsis admitted to intensive care unit. Anaesthesiol Intensive Ther. 2019;51:11–16. doi: 10.5603/AIT.a2019.0009. [DOI] [PubMed] [Google Scholar]
- 33.Kim S, Eliot M, Koestler DC, Wu WC, Kelsey KT. Association of neutrophil-to-lymphocyte ratio with mortality and cardiovascular disease in the jackson heart study and modification by the duffy antigen variant. JAMA Cardiol. 2018;3:455–462. doi: 10.1001/jamacardio.2018.1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Song H, Kim HJ, Park KN, Kim SH, Oh SH, Youn CS. Neutrophil to lymphocyte ratio is associated with in-hospital mortality in older adults admitted to the emergency department. Am J Emerg Med. 2020 doi: 10.1016/j.ajem.2020.01.044. [DOI] [PubMed] [Google Scholar]
- 35.Soulaiman SE, Dopa D, Raad AT, Hasan W, Ibrahim N, Hasan AY, Sulaiman HA, Darwich M. Cohort retrospective study: the neutrophil to lymphocyte ratio as an independent predictor of outcomes at the presentation of the multi-trauma patient. Int J Emerg Med. 2020;13:5. doi: 10.1186/s12245-020-0266-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zidar DA, Al-Kindi SG, Liu Y, et al. Association of lymphopenia with risk of mortality among adults in the US general population. JAMA Netw Open. 2019;2:e1916526. doi: 10.1001/jamanetworkopen.2019.16526. [DOI] [PMC free article] [PubMed] [Google Scholar]