Abstract
Background
Preliminary studies have suggested that soluble programmed death-1 (sPD-1) and soluble programmed cell death ligand-1 (sPD-L1) have prognostic implications in many malignant tumors. However, the correlation between sPD-1/sPD-L1 level and prognosis of hepatocellular carcinoma (HCC) is still unclear.
Methods
We searched several electronic databases from database inception to October 7, 2021. Meta-analyses were performed separately for overall survival (OS), disease-free survival (DFS), recurrence-free survival (RFS), time to progression (TTP), and tumor-free survival (TFS). Random effects were introduced to this meta-analysis. The correlation between sPD-1/sPD-L1 level and prognosis was evaluated using hazard ratios (HRs) with 95% confidence intervals (95%CIs).
Results
A total of 11 studies (1291 patients) were incorporated into this meta-analysis, including seven on sPD-L1, two on sPD-1, and two about both factors. The pooled results showed that high sPD-L1 level was associated with worse OS (HR = 2.46, 95%CI 1.74–3.49, P < 0.001; I2 = 31.4, P = 0.177) and poorer DFS/RFS/TTP/TFS of patients with HCC (HR = 2.22, 95%CI 1.47–3.35, P < 0.001; I2 = 66.1, P = 0.011), irrespective of method of detection, study type, treatment, cut-off value and follow-up time. In contrast, the level of sPD-1 was not correlated to the OS (HR = 1.19, 95%CI 0.55–2.56, P = 0.657) and DFS/TFS of patients with HCC (HR = 0.94, 95%CI 0.36–2.49, P = 0.906).
Conclusion
sPD-L1 rather than sPD-1 could be a good predictor for recurrence and survival after treatment for HCC. More high-quality prospective studies are warranted to assess the prognostic value of sPD-1 or sPD-L1 for HCC.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00262-021-03103-2.
Keyword: sPD-1, sPD-L1, Hepatocellular carcinoma, Prognosis, Meta-analysis
Introduction
Hepatocellular carcinoma (HCC), the 6th most common cancer in the world, continues to threaten the health of people [1]. Currently, there are many treatment options for HCC, including radical surgical resection, liver transplantation, transhepatic arterial chemoembolization (TACE), and ablation. However, the long-term survival rate of patients is still unsatisfied and the recurrence rate is also high [2]. Therefore, it is imperative to identify novel predictors that can predict treatment efficacy and help to select the subpopulation of patients who will be most likely benefit from the treatment methods mentioned above.
Immune-based therapies, such as immune checkpoint inhibitors (ICIs), have emerged as novel pillars of cancer therapy [3]. In the study of Tian et al., activated CD4 + type 1 helper T cells played a crucial role in normalizing tumor vasculature, suggesting a reciprocal regulation of vascular normalization and immune response [4]. Therefore, combining programmed cell death-1 (PD-1) blockers with anti-angiogenesis promises to be a novel treatment for HCC [5]. In addition, antibody-based blockage of inhibitory checkpoint molecules on cytotoxic T cells, such as PD-1 and cytotoxic T lymphocyte antigen-4 (CTLA-4), or their counterparts on antigen-presenting cells (APCs) and tumor cells, such as programmed cell death ligand-1 (PD-L1), have been shown to be an effective and well-tolerated treatment option for many cancers [3].
PD-1 is mainly present on the surface of T cells (CD 4 + and CD 8 + T cells), but is also expressed on other immune cells such as B cells, NK cells, and NKT cells [6, 7]. PD-L1 is mainly present on the surface of tumor cells, APCs, and dendritic cells (DCs) [8, 9]. PD-L1 binding to PD-1 on the surface of T cells can maintain immune tolerance and make tumor cells evade immune surveillance by blocking PD-1/PD-L1 interaction [10]. Besides, in some inflammatory responses or immune system diseases, PD-1/PD-L1 is also altered and plays a valuable role in the development of the disease [11, 12]. Recently, PD-1/PD-L1 is found to have a soluble state, which can be detected by enzyme-linked immunosorbent assay (ELASA) or other methods [13].
Soluble programmed cell death-1 (sPD-1) is present in the circulation, and is produced either by shedding the membrane form or by alternatively splicing variants. There are four main distinct forms, and the major role is played by PD-1 Δx3, which retains the extracellular structural domain and can encode the soluble form of PD-1 [13]. sPD-1 can bind to PD-L1 competitively on the surface of tumor cell membranes to promote T cells proliferation and activation, and plays a role similar to that of PD-L1 monoclonal antibody [14]. Similarly, soluble programmed cell death ligand-1 (sPD-L1) is thought to be generated primarily from an alternative splicing of PD-L1 pre-mRNA or protease-produced membrane-bound PD-L1, which exerts a widespread inhibitory effect by interacting with cell surface receptors such as membrane-bound PD-1 [15, 16]. The soluble PD-1/PD-L1 strives to strike a balance to maintain immune homeostasis.
In HCC, the correlation between sPD-1/sPD-L1 level and prognosis of HCC remains controversial. Finkelmeier et al. suggested that high sPD-L1 level could be used as a poor prognostic biomarker of HCC [17], and the conclusion was confirmed by Chang et al., Mocan et al. and, Itoh et al. [18–20]. Meanwhile, contradictory results have also been published. High levels of sPD-L1 were reported to be associated with good prognosis of HCC in Sideras’s study [21]. Therefore, whether sPD-L1/sPD-1 plays a part in the prognosis of HCC is worthy of an in-depth study. The purpose of this meta-analysis is to investigate the value of sPD-1/sPD-L1 level in predicting the prognosis of HCC after treatment.
Materials and methods
Search strategy
This meta-analysis was carried out according to the standards set by the preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement guideline (supplementary PRISMA Checklist) [22]. We searched PubMed, Embase, Web of Science, and the Cochrane Library databases from its inception until October 7, 2021, using the keywords (“hepatocellular carcinoma” OR “hepatocellular, carcinoma” OR “hepatomas” OR “liver carcinoma” OR “liver cell carcinoma” OR “HCC” OR “liver cancer”) AND (“soluble programmed cell death-ligand 1” OR “sPD-L1” OR “soluble programmed cell death 1” OR “sPD-1” OR “sB7-H1”). The detailed search strategy was described in supplementary table 1. Additional papers were identified by a manual search of the references from the eligible articles.
Selection criteria
Studies satisfying the following criteria were included: (1) all patients were confirmed to have HCC; (2) studies revealed the expression of sPD-1/sPD-L1 and obtained its level by assaying; (3) enough data were available to analyze the relationship between sPD-1/sPD-L1 expression, clinical characteristics, and the prognostic indexes such as overall survival (OS), disease-free survival (DFS), recurrence-free survival (RFS), time to progression (TTP), and tumor-free survival (TFS); and (4) studies were published in English. The exclusion criteria were as follows: (1) case report, review, meta-analysis, and ongoing research; (2) animal experiments on HCC and research unrelated to the sPD-1/sPD-L1; (3) studies provided insufficient information to assess the relationship between sPD-1/sPD-L1 expression and prognosis. For re-published studies, only the latest literature and relevant data can be collected; alternatively, the study with the largest sample size was selected.
Data extraction and quality assessment
Two investigators (JSX, HL) independently extracted data from eligible studies. Disagreements were resolved by discussion and consensus with a third author (GXM). Baseline characteristics, such as first author, publication year, country, sample size, outcomes, and clinical features, were extracted from the included articles. We chose OS and DFS/RFS/TTP/TFS as our endpoints for meta-analysis. The quality of eligible studies was evaluated by the Newcastle–Ottawa Scale (NOS) criteria [23].
Statistical methods
The relevant data of most studies can be collected directly. However, for those studies in which hazard ratios (HRs) and 95% confidence intervals (95%CIs) were not available, we used Tierney’s method to derive estimates from survival curves [24]. P < 0.05 was considered statistically significant. The HRs and 95% CIs were combined to evaluate the correlation between sPD-1/sPD-L1 level and prognosis of patients with HCC. The heterogeneity among studies was evaluated by Cochran’s Q test and Higgins’ I2 statistic. I2 > 50% and P-value for heterogeneity < 0.10 indicated significant heterogeneity, and the random-effects model was used for analysis. Sensitivity analysis was carried out to test the stability and reliability of the results by removing each study. A subgroup analysis was also conducted to detect the source of heterogeneity. Publication bias among all included studies was tested using Begg’s funnel plots test and Egger’s test. All data in this meta-analysis were analyzed using Stata 16.0 software (College Station).
Results
Literature search
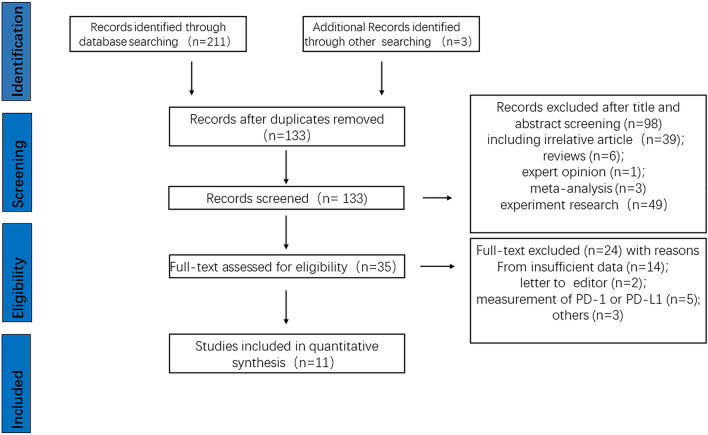
A total of 214 records were identified through initial literature retrieval. After removing duplicates, 133 records remained and were screened by titles and abstracts. A total of 98 studies were excluded, and 35 articles were evaluated by full-text examination. Subsequently, 24 of 35 articles were excluded for providing insufficient data (14) and unrelated subjects (10). Finally, 11 studies were included in our meta-analysis, including seven on sPD-L1, two on sPD-1 and two about both factors. A flow diagram of the literature screening process is shown in Fig. 1.
Fig. 1.
Study flow chart of the data extraction process and selection of studies for meta-analysis
Characteristics of included studies
The main characteristics of included studies were summarized in Table 1. The eligible studies were published from 2011 to 2021. Sample sizes ranged from 53 to 229, with a total number of 1291. The included studies were performed in seven countries: five in China [19, 25–28], two in Korea [29, 30], and one each in Romania [18], Germany [17], the Netherlands [21], and Egypt [31]. A total of nine studies used enzyme-linked immunosorbent assay (ELISA) to detect sPD-1/sPD-L1 levels [17, 18, 21, 25–27, 29–31]. One was analyzed by antibody array assay[19] and the other by flow cytometric analysis [28]. A total of four studies were prospective studies [17, 18, 30, 31], and six were retrospective trials [19, 21, 25, 26, 28, 29]. A total of studies explored the correlation between sPD-L1 levels and OS [17–19, 21, 25, 26, 28, 30, 31]. A total of six studies investigated the relationship between sPD-L1 levels and DFS/RFS/TTP/TFS [18, 19, 21, 25, 26, 28, 31]. The cut-off values ranged from 0.0013 ng/ml to 11,200 ng/ml. According to Cox’s method [32], we removed the maximum and minimum values. The mean of the remaining data was regarded as the threshold for subgroup analysis. The average value was 3.0 ng/ml; therefore, we used the cut-off value of 3.0 ng/ml for subgroup analysis. Similarly, four studies explored the correlation between sPD-1 levels and OS [19, 27–29]. A total of studies investigated the relationship between sPD-1 levels and DFS/TFS [19, 28, 29]. NOS scores of included studies ranged from 4 to 8. A score ≤ 5 is considered low quality, and > 5 can be considered high quality (Supplementary Table 3).
Table 1.
The characteristics of all included eligible studies
| Study | Year | Country | Study style | Sample size | The indicators | Method of detection | Treatment | Case number (Low/High) | Value of cutoff | Outcome | Follow-up times | NOS score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mocan et al | 2021 | Romania | Prospective | 121 | sPD-L1 | ELISA | Mixed | 50/71 | 96 pg/ml | OS; DFS | Median time 29.23 months | 8 |
| Ma et al | 2020 | China | Retrospective | 114 | sPD-L1 | ELISA | TACE | 57/57 | 607.25 pg/ml | OS; TTP | Up to date:2016.12 | 6 |
| Han et al | 2019 | China | Retrospective | 81 | sPD-L1 | ELISA | Curative resection/ablation | 59/22 | 2.825 ng/ml | OS; DFS | Median time 39.5 months | 8 |
| Chang et al | 2019 | China | Retrospective | 120 | sPD-L1; sPD-1 | Antibody array assay | Curative resection | 85/35 | 11.2 μg/ml;33.0 μg/ml | OS; DFS | N/A | 8 |
| Finkelmeier et al | 2016 | Germany | Prospective | 215 | sPD-L1 | ELISA | Mixed | 151/64 | 0.8 ng/ml | OS | Median time 10 months | 6 |
| Kim et al | 2018 | Korea | Prospective | 53 | sPD-L1 | ELISA | Radiation therapy | 10/43 | 1.315 pg/ml | OS | Median time 21.3 months | 6 |
| Zeng et al | 2011 | China | Retrospective | 109 | sPD-L1; sPD-1 | Flow cytometric analysis | Ablation | 54/55 | The median | OS; DFS | Median time 23 months | 5 |
| Sideras et al | 2019 | Netherlands | Retrospective | 81 | sPD-L1 | ELISA | Curative resection/Liver transplantation | 55/24 | 700 pg/ml | OS; RFS | N/A | 4 |
| El-Gebaly et al | 2019 | Egypt | Prospective | 85 | sPD-L1 | ELISA | N/A | N/A | 7.42 ng/ml | OS | Over-all 24 months | 5 |
| Na et al | 2021 | Korea | Retrospective | 229 | sPD-1 | ELISA | Liver transplantation | 216/13 | 300 pg/ml | DFS | Median time 64.5 months | 7 |
| Li et al | 2017 | China | N/A | 83 | sPD-1 | ELISA | N/A | 42/41 | 10 ng/ml | OS | Median time 36 months | 6 |
sPD-1, Soluble programmed cell death-1; sPD-L1, Soluble programmed cell death ligand-1; ELISA, Enzyme-linked immunosorbent assay; Mixed, Greater than 2 types of treatment; TACE, Transcatheter arterial chemoembolization; OS, overall survival; DFS, disease-free survival; RFS, Recurrence-free survival; TTP, Time to progression; TFS, tumor-free survival; NOS, Newcastle–Ottawa Scale
Correlation of sPD-L1 with OS and DFS/RFS/TTP/TFS
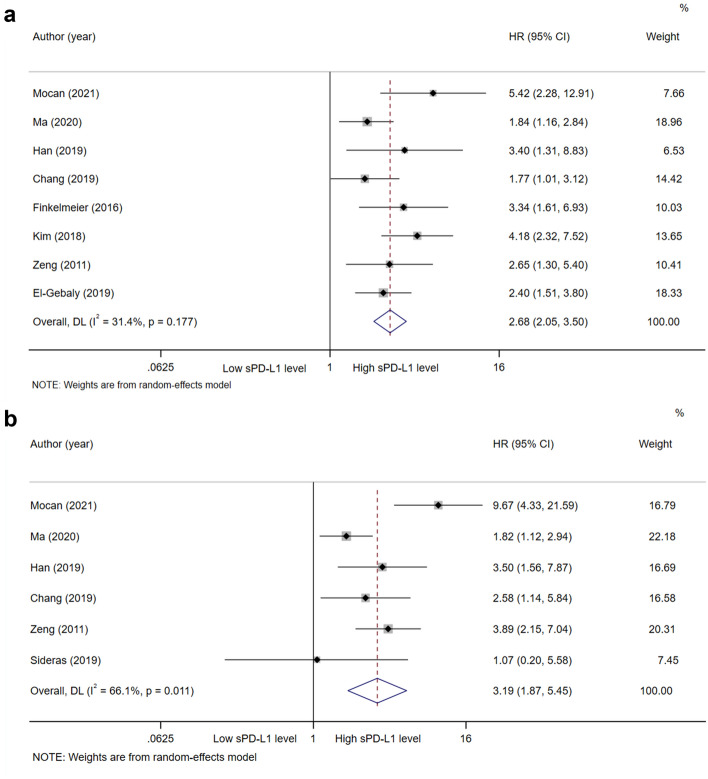
A total of nine studies, with 979 patients, explored the prognostic value of sPD-L1 for OS. A random-effects model was applied. The pooled HR (95%CI) from nine comparative studies was 2.19 (1.35–3.55, P = 0.001), but the data were heterogeneous (I2 = 79.7%, P < 0.001; supplementary Fig. 1). We further performed subgroup analysis and sensitivity analysis to explore the source of heterogeneity. The subgroup analysis was stratified by ethnicity, study type, detection methods, treatment, cut-off value of sPD-L1, and follow-up time (supplementary Table 2). By sensitivity analysis and publication bias (supplementary Fig. 2 and 3), we found that Sideras’s study may be the source of heterogeneity. After excluding Sideras’s study, pooled results showed that high sPD-L1 level was associated with worse OS (HR = 2.68, 95%CI 2.05–3.50, P < 0.001; I2 = 31.4%, P = 0.177; Fig. 2a). A funnel plot was shown in Fig. 3a and no bias was observed. Therefore, we can conclude with certainty that high sPD-L1 level was significantly related to worse OS of HCC. The same results were obtained in a pan-cancer analysis in prior meta-analyses [33, 34].
Fig. 2.
Forest plot of sPD-L1 in hepatocellular carcinoma. a Forest plot of sPD-L1 and OS in hepatocellular carcinoma. b Forest plot of sPD-L1 and DFS/RFS/TTP/TFS in hepatocellular carcinoma. CI, Confidence interval; HR, Hazard ratio; OS, Overall survival; sPD-L1, Soluble programmed cell death ligand-1; DFS, Disease-free survival; RFS, Recurrence-free survival; TTP, Time to progression; TFS, Tumor-free survival
Fig. 3.
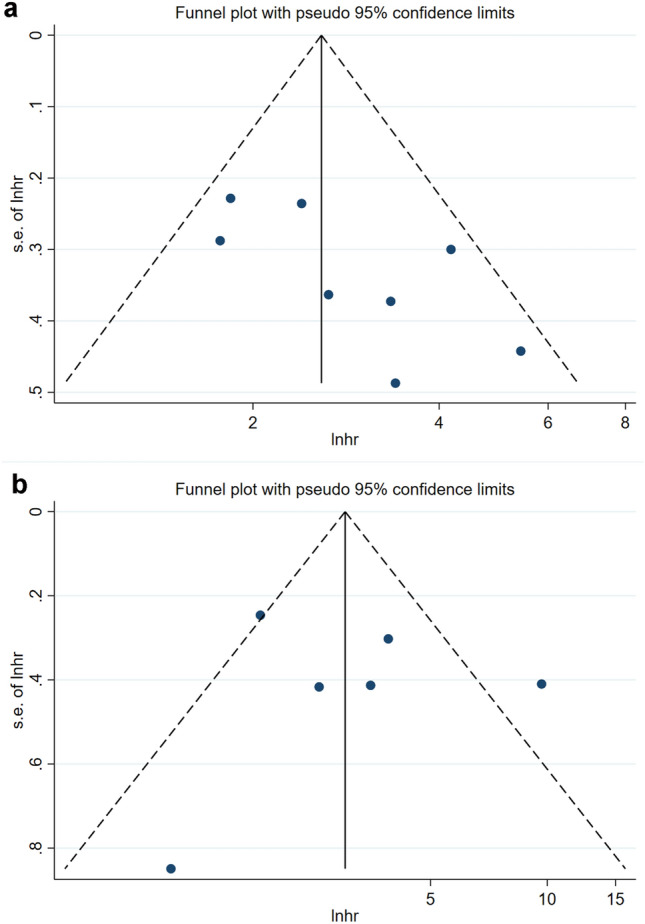
Funnel plots of sPD-L1 in hepatocellular carcinoma. a Funnel plots of HR for OS of sPD-L1. b Funnel plots of HR for DFS/RFS/TTP/TFS of sPD-L1. CI, Confidence interval; HR, Hazard ratio; OS, Overall survival; sPD-L1, Soluble programmed cell death ligand-1; DFS, Disease-free survival; RFS, Recurrence-free survival; TTP, Time to progression; TFS, Tumor-free survival
Similarly, a total of six studies, with 626 patients, explored the prognostic value of sPD-L1 for DFS/RFS/TTP/TFS. A random-effects model was applied. The pooled HR (95% CI) from six comparative studies was 3.19 (1.87–5.45, P < 0.001), but the data were heterogeneous (I2 = 66.1%, P = 0.011; Fig. 2b). A funnel plot was shown in Fig. 3b. We further explored the heterogeneity through subgroup analysis. In subgroup analysis, high sPD-L1 levels were associated with poorer DFS/RFS/TTP/TFS of Asian patients with HCC (HR = 2.70, 95% CI 1.82–4.00, P < 0.001), but not associated with that of non-Asian patients (HR = 3.65, 95% CI 0.43–31.10, P = 0.237). Moreover, high sPD-L1 level was consistently correlated with poor DFS/RFS/TTP/TFS of HCC, irrespective of method of detection, study type, treatment, greater or less than average value of cut-off, and follow-up time (Table 2).
Table 2.
Subgroup meta-analysis of prognostic role of sPD-L1 for DFS/RFS/TTP/TFS in HCC after treatment
| Factor | No. of study | No. of patients | HR (95%CI) | P-value | Heterogeneity | |
|---|---|---|---|---|---|---|
| I2(%) | P-value | |||||
| DFS/RFS/TTP/TFS | ||||||
| Total | 6 | 626 | 3.19 (1.87–5.45) | < 0.001 | 66.1 | 0.011 |
| Ethnicity | ||||||
| Asian | 4 | 424 | 2.70 (1.82–4.00) | < 0.001 | 31.7 | 0.222 |
| Non-Asian | 2 | 202 | 3.65 (0.43–31.10) | 0.237 | 81.7 | 0.020 |
| Study style | ||||||
| Prospective study | 1 | 121 | 9.67 (4.33–21.59) | < 0.001 | / | / |
| Retrospective study | 5 | 505 | 2.58 (1.76–3.78) | < 0.001 | 26.8 | 0.243 |
| Treatment | ||||||
| Multiple therapies | 3 | 283 | 3.98 (1.37–11.56) | 0.011 | 69.8 | 0.036 |
| Monotherapy | 3 | 343 | 2.57 (1.58–4.19) | < 0.001 | 47.4 | 0.149 |
| Method of detection | ||||||
| ELISA | 4 | 397 | 3.12 (1.29–7.56) | 0.012 | 78.2 | 0.003 |
| Other methods | 2 | 229 | 3.38 (2.09–5.46) | < 0.001 | 0.0 | 0.425 |
| Cut-off value of sPD-L1 | ||||||
| Less than average | 4 | 397 | 3.12 (1.29–7.56) | 0.012 | 78.2 | 0.003 |
| Greater than average | 2 | 229 | 3.38 (2.09–5.46) | < 0.001 | 0.0 | 0.425 |
| Follow-up times | ||||||
| 2 years or longer | 2 | 202 | 5.83 (2.15–15.76) | 0.001 | 67.2 | 0.081 |
| Less than 2 years or NR | 4 | 424 | 2.42 (1.53–3.82) | < 0.001 | 36.8 | 0.191 |
HCC, Hepatocellular carcinoma; CI, confidence interval; DFS , disease-free survival; HR, hazard ratio; TTP, time to progression; Ph P value for heterogeneity; RFS, recurrence-free survival; TFS, tumor-free survival; sPD-L1 , Soluble programmed cell death ligand-1
Correlation of sPD-1 with OS and DFS/TFS
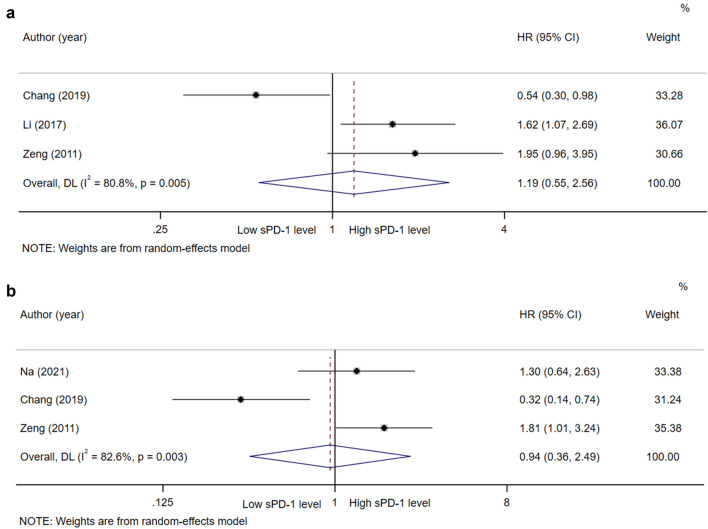
In the studies of sPD-1, a total of four articles were included for analysis. According to the pooled results, it was concluded that the level of sPD-1 was not correlated to the OS and DFS/TFS of patients with HCC (HR = 1.19, 95% CI 0.55–2.56, P = 0.657, Fig. 4a; HR = 0.94, 95% CI 0.36–2.49, P = 0.906, Fig. 4b).
Fig. 4.
Forest plot of sPD-1 in hepatocellular carcinoma. a Forest plot of sPD-1 and OS in hepatocellular carcinoma. b Forest plot of sPD-1 and DFS/TFS in hepatocellular carcinoma. CI, Confidence interval; HR, Hazard ratio; OS, Overall survival; sPD-L1, Soluble programmed cell death ligand-1; DFS, Disease-free survival; RFS, Recurrence-free survival; TTP, Time to progression; TFS, Tumor-free survival
Sensitivity analysis and publication bias
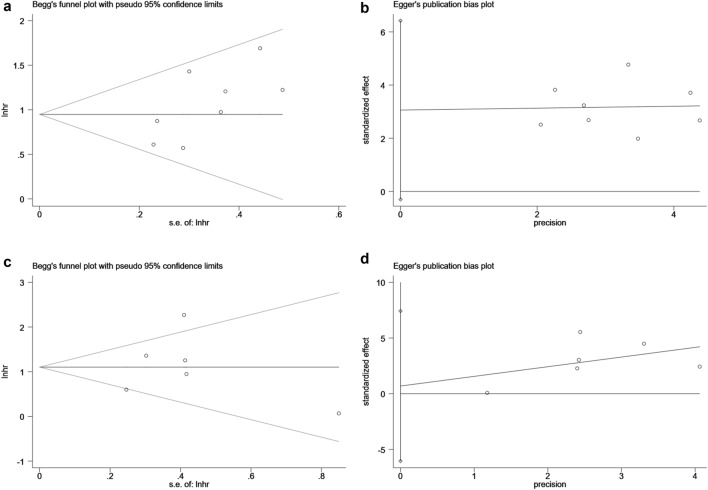
Sensitivity analysis was performed to estimate the stability of sPD-L1 for predicting OS and DFS/RFS/TTP/TFS. No significant differences beyond the limits of 95% CI of combined results were found (Fig. 5a, b). Potential publication bias in this meta-analysis was evaluated using Begg’s test and Egger’s linear regression test. No evidence for apparent publication bias for the analysis between sPD-L1 and OS was found (Begg’s test: P = 0.063, Fig. 6a; Egger’s test: P = 0.068, Fig. 6b). Likewise, no apparent publication bias was found for DFS/RFS/TTP/TFS analysis (Begg’s test: P = 1.000; Fig. 6c Egger’s test: P = 0.231, Fig. 6d).
Fig. 5.
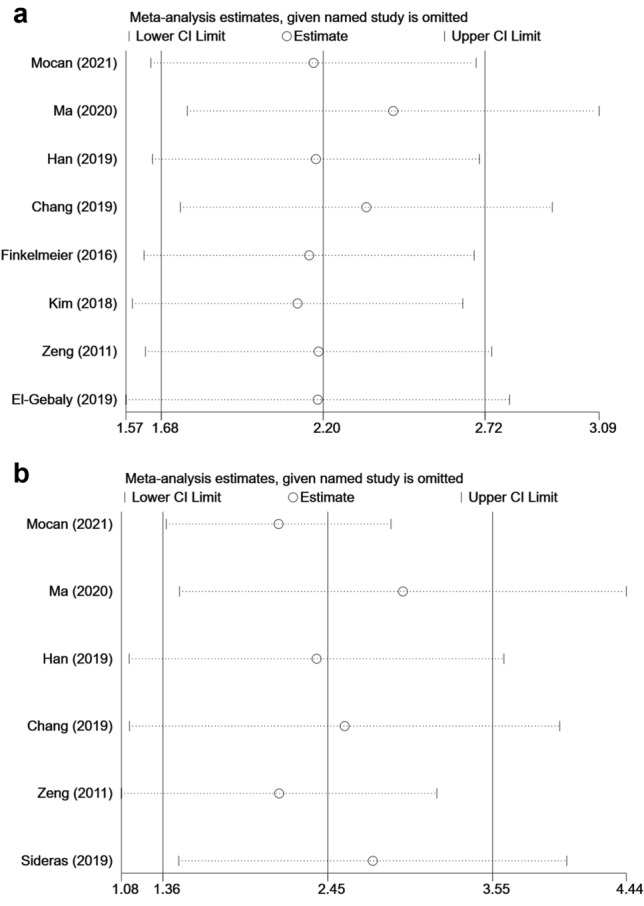
Sensitivity analysis of this meta-analysis. a Sensitivity analysis of OS. b Sensitivity analysis of DFS/RFS/TTP/TFS. CI, Confidence interval; HR, Hazard ratio; OS, Overall survival; sPD-L1, Soluble programmed cell death ligand-1; DFS, Disease-free survival; RFS, Recurrence-free survival; TTP, Time to progression; TFS, Tumor-free survival
Fig. 6.
Assessment of publication bias for this meta-analysis using Begg’s test and Egger’s test. a Begg’s test for OS, P = 0.063. b Egger’s test for OS, P = 0.068. c Begg’s test for DFS/RFS/TTP/TFS, P = 1.000. d Egger’s test for DFS/RFS/TTP/TFS, P = 0.231. lnhr the ln of HR; s.e. standard error, OS, Overall survival, DFS, Disease-free survival; RFS, Recurrence-free survival; TTP, Time to progression; TFS, Tumor-free survival
Discussion
The discovery of PD-1/PD-L1 has been a major breakthrough in immuno-oncology in the last decade. PD-1, including an immunoreceptor tyrosine-based inhibitory motif and an immunoreceptor tyrosine-based switch motif (ITSM), ligates with PD-L1 to cause phosphorylation of tyrosines within these motifs, which leads to the inactivation of downstream molecules such as phospholipase C-γ2 (PLCγ2), phosphatidylinositol 3 kinase (PI3K) and extracellular-signal-regulated kinase (ERK) in B cells and related kinases in T cells. In a soluble state, sPD-1 and sPD-L1 had been confirmed to have a proportional relationship, and played a specific role in the immune regulation and development of HCC [7, 35]. Zeng et al. concluded that upregulation of circulating PD-1/PD-L1 could serve as novel valid immunological markers in predicting disease progression of HCC after cryoablation treatment [28]. The possible reason might be that the body produced substantial sPD-1/sPD-L1 in order to maintain peripheral self-tolerance and immune evasion, which may shift immune balance and predict worse prognosis [14].
Elevated levels of sPD-L1 have been correlated with worse prognosis of several cancers and have displayed a persistent outlook [27, 28], however, it should be noted that different types of tumor may have enormously different immunobiology. Previous studies have demonstrated that elevated levels of sPD-L1 in different types of cancer can predict the effects of various therapies, such as surgery, chemotherapy, radiotherapy, and immunotherapy [20, 30, 36–38]. In contrast, the levels of sPD-1 are inconsistent in their predictive and prognostic ability, and the prognostic value of sPD-1 remains controversial [19, 27, 28, 39]. In this study, we also found that sPD-L1 rather than sPD-1 could be a good predictor for recurrence and survival after HCC treatment. There is currently no clinically accepted method for sPD-1/sPD-L1 measurement. In most included studies, ELASA was widely used for the detection of sPD-1/sPD-L1. Although flow cytometric analysis and antibody array assay were also used to detect the level of sPD-1/sPD-L1, the levels of sPD-1/sPD-L1 measured were significantly different among them [19, 21, 28]. ELASA seems to be more promising due to high sensitivity and specificity, however, the cut-off values of sPD-1/sPD-L1 measured in different studies are highly variable, and some form of cross-assay comparison and validation is still necessary [40]. Therefore, a reliable sPD-1/sPD-L1 detection method is urgently needed.
Mechanically, sPD-L1 is able to diminish cell cycle protein cyclin A and cell survival signaling molecules phosphorylated-ERK (p-ERK) and p-Akt, reducing adenosine triphosphate production, and attenuating the respiratory capacity of T cells. The respiratory capacity is a major factor regulating the memory development of CD8 T cells and associated with improved T-cell longevity and supports antigen-specific T-cell activation [41]. sPD-L1 can bind to PD-1 on the surface of T cells and inhibit their anti-tumor function, inducing T cells apoptosis and allowing tumor cells to evade immunity [13]. In addition, sPD-L1 is structurally similar to PD-L1, which binds to PD-L1 monoclonal antibodies, rendering them ineffective and clinically manifesting as PD-1/PD-L1 monoclonal resistance [14]. However, Sideras et al. described that high levels of circulating PD-L1 were associated with delayed relapse and better survival in patients with HCC. PD-L1 is not only produced by tumor cells but also by other cells in the body, such as DCs and macrophages, and differences regarding the inclusion of national populations and geographic regions may be also among the sources of their different results [9, 42].
In some animal studies, sPD-1 had been used as a vector to combine with tumor virus vaccines or other cytokines, including interferon(IFN)-α, IFN-λ, interleukin(IL)-2, IL-7, IL-12, IL-15, IL-21, and granulocyte–macrophage colony-stimulating factor (GM-CDF), for the treatment of several malignancies, even if efficacy was modest [43–45]. sPD-1 expressing senescent tumor cell vaccine delayed tumorigenesis and inhibited tumor growth in a triple-negative breast cancer mouse model [46]. However, the value of sPD-1 in clinical studies still remains controversial. Vajavaara et al. also found that high sPD-1 level before treatment was correlated with the quantities of tumor-infiltrating PD-1 + T cells, and predicted poor outcomes in patients with high-risk diffuse large B-Cell lymphoma [47].
In contrast, in the study of Chang et al., a different result was obtained that high sPD-1 level was associated with better OS and DFS in HCC [19]. The effect of sPD-1 is known as anti-PD-1-like or anti-PD-L1 monoclonal antibody [48, 49]. On the one hand, sPD-1 can bind to PD-1 receptors on the surface of tumor cells, affecting the binding of PD-1 on the surface of CD4 + T cells and inhibiting signal transmission, thus allowing CD4 + T cells to perform their normal immune function. On the other hand, sPD-1 may interfere with circulating PD-L1, thereby reducing its inhibitory effect and enhancing the potency of anti-PD-1 monoclonal antibodies [50]. In addition, the receptor is also present on the surface of DC cells. sPD-1 binding to it may promote the maturation of DC cells by upregulating B7-2 and CD40 co-stimulatory molecules, thus exerting an indirect anti-tumor effect [51, 52]. However, in our analysis, the pooled results showed no significant correlation between sPD-1 level and prognosis of patients with HCC, and these non-significant results were driven by only one study from Chang’s. The prognostic value of sPD-1 may be clarified when more investigations are carried out. Therefore, more high-quality prospective studies are warranted to explore the prognostic value of sPD-1 in HCC.
In the present analysis, we have certain limitations. First, the disease stage and the cut-off values of sPD-1/sPD-L1 for HCC in the included studies were different, which may be sources of heterogeneity [53]. Significantly, the cut-off values of sPD-1/sPD-L1 ranged from 0.0013 to 11,200 ng/ml. The range of cut-off values was relatively large and the rational method had been taken to resolve it. Second, studies on sPD-1/sPD-L1 could not reach a large sample size in the included articles. Sample sizes only ranged from 53 to 229, with a total number of 1291. Third, there were different etiologies regarding the causes of HCC, including HBV infection, HCV infection, and alcoholic liver disease. Fourth, the numbers of included studies for DFS, RFS, TTP, and TFS were relatively small. In addition, there were also significant differences in treatment, including curative resection, liver transplantation, radiation therapy, and ablation, etc.
Conclusions
In summary, we concluded that sPD-L1 rather than sPD-1 could be a good predictor for recurrence and survival after HCC treatment. A reliable sPD-1/sPD-L1 detection method is urgently needed, and more high-quality prospective studies are warranted to assess the prognostic value of sPD-1 or sPD-L1 for HCC.
Supplementary Information
Below is the link to the electronic supplementary material.
Abbreviations
- APCs
Antigen-presenting cells
- CI
Confidence interval
- CTLA-4
Cytotoxic T lymphocyte antigen 4
- DCs
Dendritic cells
- DFS
Disease-free survival
- ELISA
Enzyme-linked immunosorbent assay
- ERK
Extracellular-signal regulated kinase
- GM-CDF
Granulocyte–macrophage colony-stimulating factor
- HR
Hazard ratio
- HCC
Hepatocellular carcinoma
- ICIs
Immune checkpoint inhibitors
- IFN
Interferon
- IL
Interleukin
- ITSM
Immunoreceptor tyrosine-based switch motif
- NK cells
Natural killer cells
- NKT cells
Natural killer T cells
- NOS
Newcastle–ottawa scale
- OS
Overall survival
- PD-1
Programmed cell death-1
- PD-L1
Programmed cell death ligand-1
- PLCγ2
Phospholipase C-γ2
- PI3K
Phosphatidylinositol 3 kinase
- RFS
Recurrence-free survival
- sPD-1
Soluble programmed cell death-1
- sPD-L1
Soluble programmed cell death ligand-1
- TACE
Transcatheter arterial chemoembolization
- TFS
Tumor-free survival
- TTP
Time to progression
Author contributions
JSX and TL designed the study. JSX, HL, and ZRD performed the systematic search. JSX, HL, GXM, ZND, LJY, SYY, HCL, JGH, ZRD, and ZQC selected eligible articles and conducted the quality assessment. JSX and HL analyzed, interpreted the data, and drafted the manuscript. TL revised the manuscript. All authors have read and approved the final version of the manuscript.
Funding
This work was supported by the Taishan Scholars Program for Young Expert of Shandong Province (tsqn20161064), the National Natural Science Foundation of China (81874178 & 82073200), and founds for Independent Cultivation of Innovative Team from Universities in Jinan (Grant No.2020GXRC023).
Data availability
All data and material analyzed during this study are included in this article.
Declarations
Conflict of interest
The authors declare that they do not have any conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jun-shuai Xue and Hui Liu contributed equally to this paper.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Llovet JM, Zucman-Rossi J, Pikarsky E, Sangro B, Schwartz M, Sherman M, et al. Hepatocellular carcinoma Nat Rev Dis Primers. 2016;2:16018. doi: 10.1038/nrdp.2016.18. [DOI] [PubMed] [Google Scholar]
- 3.Ruf B, Heinrich B, Greten TF. Immunobiology and immunotherapy of HCC: spotlight on innate and innate-like immune cells. Cell Mol Immunol. 2021;18:112–127. doi: 10.1038/s41423-020-00572-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tian L, Goldstein A, Wang H, Ching Lo H, Sun Kim I, Welte T, et al. Mutual regulation of tumour vessel normalization and immunostimulatory reprogramming. Nature. 2017;544:250–254. doi: 10.1038/nature21724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shigeta K, Datta M, Hato T, Kitahara S, Chen IX, Matsui A, et al. Dual Programmed Death Receptor-1 and Vascular Endothelial Growth Factor Receptor-2 Blockade Promotes Vascular Normalization and Enhances Antitumor Immune Responses in Hepatocellular Carcinoma. Hepatology. 2020;71:1247–1261. doi: 10.1002/hep.30889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schildberg FA, Klein SR, Freeman GJ, Sharpe AH. Coinhibitory Pathways in the B7-CD28 Ligand-Receptor Family. Immunity. 2016;44:955–972. doi: 10.1016/j.immuni.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sharpe AH, Pauken KE. The diverse functions of the PD1 inhibitory pathway. Nat Rev Immunol. 2018;18:153–167. doi: 10.1038/nri.2017.108. [DOI] [PubMed] [Google Scholar]
- 8.Sun C, Mezzadra R, Schumacher TN. Regulation and Function of the PD-L1 Checkpoint. Immunity. 2018;48:434–452. doi: 10.1016/j.immuni.2018.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bailly C, Thuru X, Quesnel B. Soluble Programmed Death Ligand-1 (sPD-L1): A Pool of Circulating Proteins Implicated in Health and Diseases. Cancers (Basel) 2021 doi: 10.3390/cancers13123034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu Z, Lin Y, Zhang J, Zhang Y, Li Y, Liu Z, et al. Molecular targeted and immune checkpoint therapy for advanced hepatocellular carcinoma. J Exp Clin Cancer Res. 2019;38:447. doi: 10.1186/s13046-019-1412-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qin W, Hu L, Zhang X, Jiang S, Li J, Zhang Z, et al. The Diverse Function of PD-1/PD-L Pathway Beyond Cancer. Front Immunol. 2019;10:2298. doi: 10.3389/fimmu.2019.02298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pauken KE, Wherry EJ. Overcoming T cell exhaustion in infection and cancer. Trends Immunol. 2015;36:265–276. doi: 10.1016/j.it.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gu D, Ao X, Yang Y, Chen Z, Xu X. Soluble immune checkpoints in cancer: production, function and biological significance. J Immunother Cancer. 2018;6:132. doi: 10.1186/s40425-018-0449-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khan M, Zhao Z, Arooj S, Fu Y, Liao G. Soluble PD-1: Predictive, Prognostic, and Therapeutic Value for Cancer Immunotherapy. Front Immunol. 2020;11:587460. doi: 10.3389/fimmu.2020.587460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Romero Y, Wise R, Zolkiewska A. Proteolytic processing of PD-L1 by ADAM proteases in breast cancer cells. Cancer Immunol Immunother. 2020;69:43–55. doi: 10.1007/s00262-019-02437-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Orme JJ, Jazieh KA, Xie T, Harrington S, Liu X, Ball M, et al. ADAM10 and ADAM17 cleave PD-L1 to mediate PD-(L)1 inhibitor resistance. Oncoimmunology. 2020;9:1744980. doi: 10.1080/2162402x.2020.1744980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Finkelmeier F, Canli Ö, Tal A, Pleli T, Trojan J, Schmidt M, et al. High levels of the soluble programmed death-ligand (sPD-L1) identify hepatocellular carcinoma patients with a poor prognosis. Eur J Cancer. 2016;59:152–159. doi: 10.1016/j.ejca.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 18.Mocan T, Ilies M, Nenu I, Craciun R, Horhat A, Susa R, et al. Serum levels of soluble programmed death-ligand 1 (sPD-L1): A possible biomarker in predicting post-treatment outcomes in patients with early hepatocellular carcinoma. Int Immunopharmacol. 2021;94:107467. doi: 10.1016/j.intimp.2021.107467. [DOI] [PubMed] [Google Scholar]
- 19.Chang B, Huang T, Wei H, Shen L, Zhu D, He W, et al. The correlation and prognostic value of serum levels of soluble programmed death protein 1 (sPD-1) and soluble programmed death-ligand 1 (sPD-L1) in patients with hepatocellular carcinoma. Cancer Immunol Immunother. 2019;68:353–363. doi: 10.1007/s00262-018-2271-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Itoh S, Yoshizumi T, Yugawa K, Imai D, Yoshiya S, Takeishi K, et al. Impact of Immune Response on Outcomes in Hepatocellular Carcinoma: Association With Vascular Formation. Hepatology. 2020;72:1987–1999. doi: 10.1002/hep.31206. [DOI] [PubMed] [Google Scholar]
- 21.Sideras K, de Man RA, Harrington SM, Polak WG, Zhou G, Schutz HM, et al. Circulating levels of PD-L1 and Galectin-9 are associated with patient survival in surgically treated Hepatocellular Carcinoma independent of their intra-tumoral expression levels. Sci Rep. 2019;9:10677. doi: 10.1038/s41598-019-47235-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;350:g7647. doi: 10.1136/bmj.g7647. [DOI] [PubMed] [Google Scholar]
- 23.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 24.Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. doi: 10.1186/1745-6215-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ma XL, Qu XD, Yang WJ, Wang BL, Shen MN, Zhou Y, et al. Elevated soluble programmed death-ligand 1 levels indicate immunosuppression and poor prognosis in hepatocellular carcinoma patients undergoing transcatheter arterial chemoembolization. Clin Chim Acta. 2020;511:67–74. doi: 10.1016/j.cca.2020.09.026. [DOI] [PubMed] [Google Scholar]
- 26.Han X, Gu YK, Li SL, Chen H, Chen MS, Cai QQ, et al. Pre-treatment serum levels of soluble programmed cell death-ligand 1 predict prognosis in patients with hepatitis B-related hepatocellular carcinoma. J Cancer Res Clin Oncol. 2019;145:303–312. doi: 10.1007/s00432-018-2758-6. [DOI] [PubMed] [Google Scholar]
- 27.Li N, Zhou Z, Li F, Sang J, Han Q, Lv Y, et al. Circulating soluble programmed death-1 levels may differentiate immune-tolerant phase from other phases and hepatocellular carcinoma from other clinical diseases in chronic hepatitis B virus infection. Oncotarget. 2017;8:46020–46033. doi: 10.18632/oncotarget.17546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zeng Z, Shi F, Zhou L, Zhang MN, Chen Y, Chang XJ, et al. Upregulation of circulating PD-L1/PD-1 is associated with poor post-cryoablation prognosis in patients with HBV-related hepatocellular carcinoma. PLoS ONE. 2011;6:e23621. doi: 10.1371/journal.pone.0023621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Na BG, Kim YK, Hwang S, Lee KJ, Park GC, Ahn CS, et al. Absence of association between pretransplant serum soluble programmed death protein-1 level and prognosis following living donor liver transplantation in patients with hepatocellular carcinoma. Medicine (Baltimore) 2021;100:e25640. doi: 10.1097/md.0000000000025640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim HJ, Park S, Kim KJ, Seong J. Clinical significance of soluble programmed cell death ligand-1 (sPD-L1) in hepatocellular carcinoma patients treated with radiotherapy. Radiother Oncol. 2018;129:130–135. doi: 10.1016/j.radonc.2017.11.027. [DOI] [PubMed] [Google Scholar]
- 31.El-Gebaly F, Abou-Saif S, Elkadeem M, Helmy A, Abd-Elsalam S, Yousef M, et al. Study of Serum Soluble Programmed Death Ligand 1 as a Prognostic Factor in Hepatocellular Carcinoma in Egyptian Patients. Curr Cancer Drug Targets. 2019;19:896–905. doi: 10.2174/1568009619666190718141647. [DOI] [PubMed] [Google Scholar]
- 32.Cox NJ (2013) TRIMMEAN: Stata module for trimmed means as descriptive or inferential statistics. Statistical Software Components.
- 33.Li X, Zheng Y, Yue F. Prognostic Value of Soluble Programmed Cell Death Ligand-1 (sPD-L1) in Various Cancers: A Meta-analysis. Target Oncol. 2021;16:13–26. doi: 10.1007/s11523-020-00763-5. [DOI] [PubMed] [Google Scholar]
- 34.Ding Y, Sun C, Li J, Hu L, Li M, Liu J, et al. The Prognostic Significance of Soluble Programmed Death Ligand 1 Expression in Cancers: A Systematic Review and Meta-analysis. Scand J Immunol. 2017;86:361–367. doi: 10.1111/sji.12596. [DOI] [PubMed] [Google Scholar]
- 35.Khairil Anwar NA, Mohd Nazri MN, Murtadha AH, Mohd Adzemi ER, Balakrishnan V, Mustaffa KMF, et al. Prognostic prospect of soluble programmed cell death ligand-1 in cancer management. Acta Biochim Biophys Sin (Shanghai) 2021;53:961–978. doi: 10.1093/abbs/gmab077. [DOI] [PubMed] [Google Scholar]
- 36.Ugurel S, Schadendorf D, Horny K, Sucker A, Schramm S, Utikal J, et al. Elevated baseline serum PD-1 or PD-L1 predicts poor outcome of PD-1 inhibition therapy in metastatic melanoma. Ann Oncol. 2020;31:144–152. doi: 10.1016/j.annonc.2019.09.005. [DOI] [PubMed] [Google Scholar]
- 37.Zhou J, Mahoney KM, Giobbie-Hurder A, Zhao F, Lee S, Liao X, et al. Soluble PD-L1 as a Biomarker in Malignant Melanoma Treated with Checkpoint Blockade. Cancer Immunol Res. 2017;5:480–492. doi: 10.1158/2326-6066.Cir-16-0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ha H, Nam AR, Bang JH, Park JE, Kim TY, Lee KH, et al. Soluble programmed death-ligand 1 (sPDL1) and neutrophil-to-lymphocyte ratio (NLR) predicts survival in advanced biliary tract cancer patients treated with palliative chemotherapy. Oncotarget. 2016;7:76604–76612. doi: 10.18632/oncotarget.12810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sorensen SF, Demuth C, Weber B, Sorensen BS, Meldgaard P. Increase in soluble PD-1 is associated with prolonged survival in patients with advanced EGFR-mutated non-small cell lung cancer treated with erlotinib. Lung Cancer. 2016;100:77–84. doi: 10.1016/j.lungcan.2016.08.001. [DOI] [PubMed] [Google Scholar]
- 40.Li M, Zhao L, Zhou X, Zhang K, Yin P, Liu S, et al. Detection of carcinoma in serous effusions: a review. Am J Cancer Res. 2021;11:43–60. [PMC free article] [PubMed] [Google Scholar]
- 41.Jalali S, Price-Troska T, Paludo J, Villasboas J, Kim HJ, Yang ZZ, et al. Soluble PD-1 ligands regulate T-cell function in Waldenstrom macroglobulinemia. Blood Adv. 2018;2:1985–1997. doi: 10.1182/bloodadvances.2018021113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rich NE, Hester C, Odewole M, Murphy CC, Parikh ND, Marrero JA, et al. Racial and Ethnic Differences in Presentation and Outcomes of Hepatocellular Carcinoma. Clin Gastroenterol Hepatol. 2019;17:551–9.e1. doi: 10.1016/j.cgh.2018.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kinter AL, Godbout EJ, McNally JP, Sereti I, Roby GA, O'Shea MA, et al. The common gamma-chain cytokines IL-2, IL-7, IL-15, and IL-21 induce the expression of programmed death-1 and its ligands. J Immunol. 2008;181:6738–6746. doi: 10.4049/jimmunol.181.10.6738. [DOI] [PubMed] [Google Scholar]
- 44.Terawaki S, Chikuma S, Shibayama S, Hayashi T, Yoshida T, Okazaki T, et al. IFN-α directly promotes programmed cell death-1 transcription and limits the duration of T cell-mediated immunity. J Immunol. 2011;186:2772–2779. doi: 10.4049/jimmunol.1003208. [DOI] [PubMed] [Google Scholar]
- 45.Berraondo P, Sanmamed MF, Ochoa MC, Etxeberria I, Aznar MA, Pérez-Gracia JL, et al. Cytokines in clinical cancer immunotherapy. Br J Cancer. 2019;120:6–15. doi: 10.1038/s41416-018-0328-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen Z, Hu K, Feng L, Su R, Lai N, Yang Z, et al. Senescent cells re-engineered to express soluble programmed death receptor-1 for inhibiting programmed death receptor-1/programmed death ligand-1 as a vaccination approach against breast cancer. Cancer Sci. 2018;109:1753–1763. doi: 10.1111/cas.13618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vajavaara H, Mortensen JB, Leivonen SK, Hansen IM, Ludvigsen M, Holte H, et al. Soluble PD-1 but Not PD-L1 Levels Predict Poor Outcome in Patients with High-Risk Diffuse Large B-Cell Lymphoma. Cancers (Basel) 2021 doi: 10.3390/cancers13030398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.He L, Zhang G, He Y, Zhu H, Zhang H, Feng Z. Blockade of B7–H1 with sPD-1 improves immunity against murine hepatocarcinoma. Anticancer Res. 2005;25:3309–3313. [PubMed] [Google Scholar]
- 49.Abu Hejleh T, Furqan M, Ballas Z, Clamon G. The clinical significance of soluble PD-1 and PD-L1 in lung cancer. Crit Rev Oncol Hematol. 2019;143:148–152. doi: 10.1016/j.critrevonc.2019.08.009. [DOI] [PubMed] [Google Scholar]
- 50.Tiako Meyo M, Jouinot A, Giroux-Leprieur E, Fabre E, Wislez M, Alifano M, et al. Predictive Value of Soluble PD-1, PD-L1, VEGFA, CD40 Ligand and CD44 for Nivolumab Therapy in Advanced Non-Small Cell Lung Cancer: A Case-Control Study. Cancers (Basel) 2020 doi: 10.3390/cancers12020473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Butte MJ, Keir ME, Phamduy TB, Sharpe AH, Freeman GJ. Programmed death-1 ligand 1 interacts specifically with the B7–1 costimulatory molecule to inhibit T cell responses. Immunity. 2007;27:111–122. doi: 10.1016/j.immuni.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Song MY, Park SH, Nam HJ, Choi DH, Sung YC. Enhancement of vaccine-induced primary and memory CD8(+) T-cell responses by soluble PD-1. J Immunother. 2011;34:297–306. doi: 10.1097/CJI.0b013e318210ed0e. [DOI] [PubMed] [Google Scholar]
- 53.Du Y, Nie L, Xu L, Wu X, Zhang S, Xue J. Serum levels of soluble programmed death-1 (sPD-1) and soluble programmed death ligand 1(sPD-L1) in systemic lupus erythematosus: Association with activity and severity. Scand J Immunol. 2020;92:e12884. doi: 10.1111/sji.12884. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data and material analyzed during this study are included in this article.