Abstract
NK cells, especially FDA-approved NK-92 cells, could be used for TCR engineering owing to their specialized cytotoxicity against tumors, safety profile and potential use as an off-the-shelf cellular therapy. The TCR complex requires assembly of TCR- α/ β chains with CD3 molecules (CD3δ, CD3γ, CD3ε, CD3ζ) to be correctly expressed at the cell membrane, and yet NK cells lack expression of these CD3 subunits besides CD3ζ. Since transmembrane regions of TCR α and β chains are involved in TCR complex assembly, transmembrane regions of TCR replaced by CD28 transmembrane domain could result in the expression of TCR independent of its companion CD3 subunits. However, since the absence of CD3 signaling components can influence the transmission of TCR signals to NK cells, it is necessary to add the signaling molecules of NK cells followed by CD28 transmembrane domain. Both CD3ζ and DAP10 play an important role in the activation and cytotoxicity of NK cells; moreover, 2B4 and 4-1BB are the main costimulatory molecules in NK cells. Therefore, we designed a chimeric TCR that consisted of the extracellular domains of the TCR α and β chains specific for NYESO-1 fused to the CD28 transmembrane domain followed by the 41BB and CD3ζ signaling domains as well as the 2B4 and DAP10 signaling domain, respectively. The chimeric TCR genetically engineered NK-92 cells exhibit antigen-specific recognition and lysis of tumor cells both in vitro and in vivo. In addition, TCR-28-2B10/BBζ can be feasibly expressed in primary NK cells and exhibit antigen-reactive recognition and effect function. The overall encouraging data highlight the value of NK-92 cells and primary NK cells engineered to express therapeutic chimeric TCR for adoptive immunotherapies.
Keywords: Natural killer, TCR, TCR-NK
Introduction
Chimeric antigen receptor (CAR) or T cell receptor (TCR) engineered T cells has demonstrated remarkable clinical outcome in the treatment of cancer [1], but CAR-T and TCR-T cells have faced multiple challenges, such as cytokine release syndrome (CRS), on-target off-tumor effects, limited application of allogeneic therapies and the generation of off-the-shelf cellular products due to the risk of graft versus host disease, which have resulted in the investigation of other immune cells as treatment alternative for CAR and TCR engineered modification [2–4].
As opposed to T cells, NK cells have gained much attention as a valuable substituted platform for CAR and TCR engineering because of the specialized biological characteristics, the favorable clinical safety profile with minimal toxicities, and the potential use as an off-the-shelf cellular therapy [2–4]. Although many previous studies have reported that CAR-NK cells demonstrate preclinical and clinical efficacy [2–5], only three studies reported the antitumor activity of TCR-NK cells. Since TCR complex requires assembly of the TCR α and β chains with CD3 molecules (CD3ζ, CD3δ, CD3γ and CD3ε) to be correctly expressed on the cell surface and yet NK cells lack the expression of these CD3 molecules besides CD3ζ, these three studies introduced TCR α / β chains into NK-92 cells (human natural killer cell line), along with CD3δ, γ, and ε molecules, facilitating the cell surface expression of a functional TCR complex [3, 4, 6], which are not only laborious to generate TCR genetically engineered NK cells, but difficult for stable and efficient transduction of primary NK cells. Thus, it is essential to establish a simple and efficient approach to correctly express TCRs at the membrane of NK cells. Since the transmembrane domains of TCR α / β chains are involved in TCR complex assembly, transmembrane regions of TCR α and β chains replaced by the CD28 transmembrane domain could result in the expression of TCR independent of its companion CD3 molecules. However, since the absence of CD3 signaling components can influence the transmission of TCR signals to NK cells, it is essential to add some signaling molecules of NK cells followed by the CD28 transmembrane domain [7]. CD3ζ, a signal transducing molecule, includes three immunoreceptor tyrosine activation motifs and is involved with several activating receptors of NK cells. DAP10, associated with the activating receptor NKG2D, has been demonstrated to trigger NK cytotoxicity [7]. Thus, both CD3ζ and DAP10 play an important role in the activation and cytotoxicity of NK cells. Moreover, previous studies have demonstrated that the addition of costimulatory molecules to chimeric antigen receptors could enhance the survival and proliferation of T cells [8]. The costimulatory molecules in NK cells mainly include 2B4 and 4-1BB [7]. In addition, several previous studies reported that the addition of 4-1BB or 2B4 to CAR significantly mediated more vigorous activation signals and enhanced NK cytotoxicity [7, 9, 10]. In this study, we found that NK-92 cells efficiently expressing the chimeric TCR exhibited antigen-specific recognition and cytotoxicity of tumor cells in vitro and in vivo. The proof-of-concept study demonstrates the value of NK-92 cells engineered to express therapeutic chimeric TCR for adoptive immunotherapies.
Method and materials
Cell lines and primary NK cells
T2 cell line, a lymphoblastoid cell line deficient in TAP function whose HLA/A2 protein can be easily loaded with exogenous peptides, NK-92 and 293 T cell lines (human embryonic kidney cells, HEK) were obtained from the American Type Culture Collection. Primary NK cells (CD56 + CD3-) were isolated from peripheral blood mononuclear cells (PBMC) using by flow cytometric cell sorting. Primary NK cells were cultured in X-VIVO 15 medium (Lonza, USA) supplemented with Glutamax (Life Technologies, USA), 5% FBS, IL-2 (100 U/mL, Perprotech, USA), IL-15 (10 ng/mL, Perprotech, USA).
DNA constructs, generation of lentivirus and transduction of NK-92 cells and primary NK cells
Construction of lentiviral vectors encoding TCRs was previously described [11]. Briefly, we generated TCR in a β / α chain order, and replaced the TCR constant regions by murine ones modified with interchain disulfide bonds and hydrophobic substitution, which not only facilitates identification of TCR but also promotes pairing of the introduced TCR (Fig. 1A). TCR-28-2B10/BBζ was constructed extracellular domains of TCR α and β chains were fused to an artificial transmembrane and signaling domain (Fig. 1A): namely, CD28 transmembrane coding sequence followed by 2B4 costimulatory domain and DAP10 signaling domain as well as 41BB costimulatory domain and CD3ζ signaling domains, respectively (Fig. 1A). The full length of NYESO-1 TCR and TCR-28-2B10/BBζ were codon-optimized and synthesized by GenScript and subcloned into pCDH lentivirus vector [11].
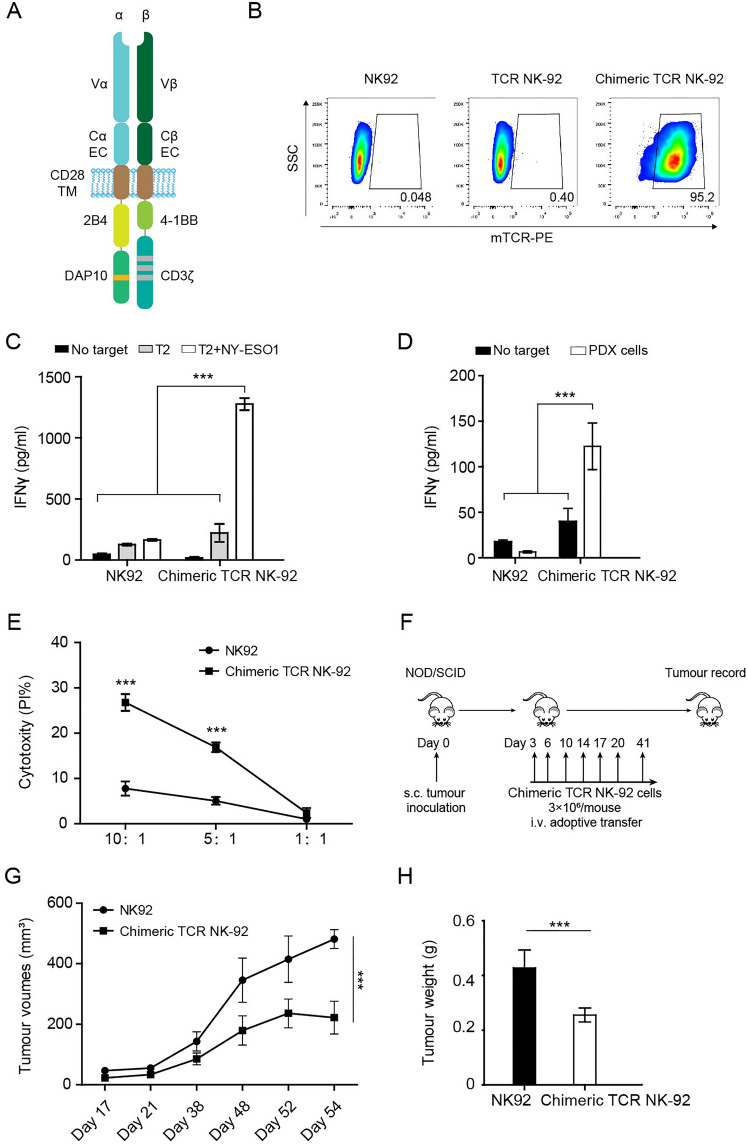
Fig. 1.
The design and antitumor ability of chimeric TCR (TCR-28-2B10/BBζ) engineered NK-92 cells A, The chimeric TCR (TCR-28-2B10/BBζ) consisted of the extracellular domains of the TCR α and β chains specific for the HLA-A2-restricted NYESO-1-derived “SLLMWITQV” epitope (NYESO-1157-165 V) fused to the CD28 transmembrane domain (TM) followed by the 2B4 costimulatory domain and DAP10 signaling domain as well as the 41BB costimulatory domain and CD3ζ signaling domains, respectively. B, Representative flow cytometry of transduction efficiencies measured by staining chimeric TCR NK-92 cells and TCR NK-92 cells with an anti-murine TCR- β chain constant region antibody. C, IFN- γ ELISA measurement after coculture of chimeric TCR NK-92 cells and NK-92 cells with NYESO-1157-165 V epitope pulsed T2 cells. D, IFN- γ ELISA measurement after coculture of chimeric TCR NK-92 cells and NK-92 cells with NYESO-1-positive tumor cells. E, Cytolytic activity of chimeric TCR NK-92 cells against NYESO-1-positive tumor cells at different E: T ratios. F, Schematic experimental plan. Each NOD/SCID mouse subcutaneously received NYESO-1-positive tumor cells on day 0. NK-92 cells or chimeric TCR NK-92 cells (3 × 106 cells per mouse) were infused seven times. G, Tumor volume in NOD/SCID mice over time in chimeric TCR NK-92 cell group and NK-92 cell group (n = 5 per group, mean and SEM are shown). H, Tumor weight in NOD/SCID mice on day 54 following the inoculation of PDX tissue fragments in chimeric TCR NK-92 cell group and NK-92 cell group (n = 5 per group, mean and SEM are shown). All in vitro experiments were performed with at least three biological replicates, and data shown are representative of at least three independent experiments. The in vivo data are representative of two independent experiments. Data are presented as mean ± SEM. *** P < 0.001
Lentivirus was produced as previously delineated [11]. In brief, lentiviruses of NYESO-1 TCR and TCR-28-2B10/BBζ were generated by cotransfection of 293 T cells using lentivector and packaging plasmids as well as PEI MAX 40,000 (Polysciences Inc. USA). The lentivirus was harvested at 48 h and 72 h after transfection and further concentrated utilizing an optimized ultracentrifugation approach at 20,000 g for 90 min at 4 °C. NK-92 cells and primary NK cells were transduced by concentrated lentivirus particles with 8 μg/mL polybrene (Sigma–Aldrich, USA).
IFN- γ ELISA
A total of responder cells (2.5 × 105 primary NK cells or 5 × 105 NK-92) and target cells (5 × 104 or 1 × 105) were cocultured in a 0.2-mL culture volume in individual wells of 96-well plates without exogenous cytokines, respectively. Following 16–20 h, the supernatants were collected, and IFN- γ levels were determined using a human IFN-γ ELISA kit (ExCell Bio, China) as per the manufacturer’s protocols.
Generation of tumor cells and PDX models
This project was carried out following protocols approved by the Institutional Review Board of the Peking University School of Oncology, China. The patient gave written informed consent for using their tumor tissues for study purposes. Cancer patient-derived tumor xenografts (PDXs) were established following the published protocol [10]. Briefly, surgical tumor fragments were cut into approximately 15 mm3 pieces and mixed with Matrigel subcutaneously and then implanted into NOD/SCID mice to obtain a PDX model. Primary tumor cells were generated from tumor tissues of a PDX model. Dissected tumor tissues of the PDX model were dissociated into single-cell suspensions utilizing gentleMACS Octo Dissociator with Heaters (Miltenyi Biotech, Germany) following the manufacturer’s protocols. Single-cell suspensions were harvested and washed with PBS, and then resuspended in DMEM with 20% FBS and 2 mM GlutaMAX.
Cytotoxic assay
The ability of the transduced NK-92 cells to lyse targets was measured using a CFSE-based cytotoxicity assay as previously described [11]. Target cells were stained with CFSE for 15 min at 37℃ and then co-cultured with responder cells (NK-92 cells) at 37 °C for 4 h at different E: T ratios. After co-culture, 1 μg/mL propidium iodide was added to quantify the ratio of target cell death through analysis of flow cytometry.
In vivo antitumor studies
Tumor treatment consisted of seven intravenous injections of 3 × 106 NK-92 cells or chimeric TCR NK-92 cells on days 3, 6, 10, 14, 17, 20 and 41 following tumor inoculation. Tumor size was determined according to the formula: tumor volume (mm3) = [(length) × (width) × (width)]/2.
Statistics analysis
Statistical analysis was performed utilizing GraphPad Prism 7.0. Statistical comparisons were performed with Student’s t test. All tests were two-sided, and a p value < 0.05 was considered statistically significant.
Results and discussion
We designed a chimeric TCR (TCR-28-2B10/BBζ) that consisted of the extracellular domains of the TCR α / β chains specific for the HLA-A2-restricted NYESO-1-derived “SLLMWITQV” epitope (NYESO-1157-165 V) fused to the CD28 transmembrane domain followed by the 41BB costimulatory domain and CD3ζ signaling domains as well as the 2B4 costimulatory domain and DAP10 signaling domain, respectively (Fig. 1A). In addition, extracellular constant regions of TCR α / β chains were replaced utilizing mouse counterparts modified with hydrophobic substitution and interchain disulfide bonds, which was used for the detection of TCR and improving TCR pairing [12]. NYESO-1 TCR and chimeric TCR were introduced into NK-92 cells, and their cell surface expression was assessed by anti-murine TCR- β chain constant region antibody. We found that chimeric TCR was expressed on the surface of NK-92 cells with a transduction efficiency of more than 95%, but the NYESO-1 TCR was not (Fig. 1B).
We next evaluated antigen-reactive triggering of chimeric TCR-transduced NK-92 cells. TAP-deficient HLA-A2+ T2 cells were pulsed with 10 μg/mL of the target NYESO-1157-165 V epitope for 4 h and were then cocultured with chimeric TCR-NK-92 cells. The chimeric TCR NK-92 cells produced IFN- γ only when the T2 cells were loaded with the NYESO-1 peptide (Fig. 1C), demonstrating the functional capabilities and antigen reactivity of the chimeric TCR.
To further assess whether chimeric TCR NK-92 cells have the functionality to identify endogenously processed NYESO-1 in complex with HLA-A2, we investigated the response against HLA-A2 + and NYESO-1 + lung cancer patient-derived primary tumor cells and xenograft (PDX) models. As shown in Fig. 1D, greater amounts of IFN- γ were produced by chimeric TCR NK-92 cells than NK-92 cells in the presence of NYESO-1-positive tumor cells. An in vitro cytotoxicity assay further suggested that chimeric TCR NK-92 cells could efficiently lyse NYESO-1-positive tumor cells, whereas NK-92 cells failed to lyse the target cells (Fig. 1E). These data indicated that chimeric TCR NK-92 cells had intrinsic antigen-dependent cytotoxic reactivity. Considering the increased cytokine production capacity of chimeric TCR NK-92 cells and better in vitro antitumor activities, chimeric TCR NK-92 cells were evaluated for in vivo antitumor assay. In the lung cancer PDX model, mice were injected intravenously with NK-92 cells or chimeric TCR NK-92 cells seven times (Fig. 1F). Tumors in the chimeric TCR NK-92 group were significantly reduced in volume (P < 0.001) and tumor weight (P < 0.001) relative to the mock NK-92 group on day 54 after tumor inoculation (n = 5 mice per group, Fig. 1G-H). These results suggested that chimeric TCR NK-92 cells could efficiently eliminate NYESO-1-positive lung cancer xenografts.
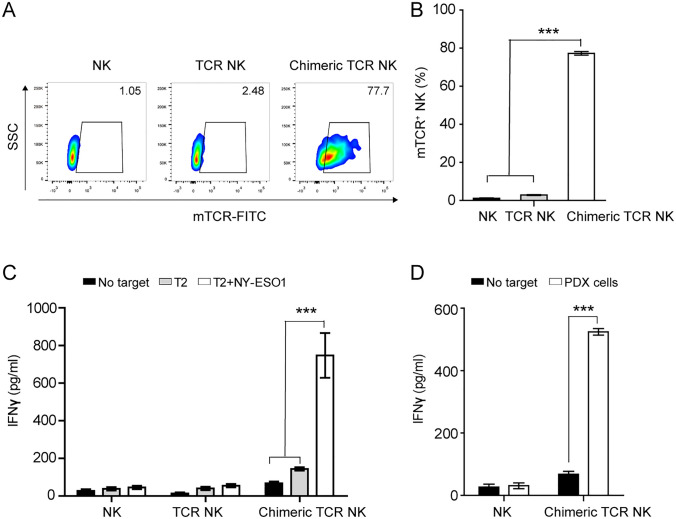
To assess whether chimeric TCR could express in primary NK cells and exhibit antigen-reactive recognition, the NYESO-1 TCR and chimeric TCR were introduced into primary NK cells from four donors and we found that the chimeric TCR was expressed on the surface of primary NK cells with a transduction efficiency of approximately 77%, but the NYESO-1 TCR was not (Fig. 2A-B). In addition, the chimeric TCR engineered NK cells produced significantly greater amounts of IFN- γ than primary NK cells in the presence of NYESO-1 peptide pulsed T2 cells and NYESO-1-positive tumor cells (Fig. 2C-D), demonstrating the functional capabilities and antigen reactivity of the chimeric TCR engineered NK cells. These data confirmed that chimeric TCR could be feasibly expressed on the surface of primary NK cells and exhibit antigen-reactive recognition and effector functions. In conclusion, TCR-28-2B10/BBζ NK-92 cells exhibit antigen-reactive recognition and lysis of tumor cells in vitro and in vivo. In addition, TCR-28-2B10/BBζ can be feasibly expressed in primary NK cells and exhibit antigen-reactive recognition and effect function. The encouraging data in this study highlight the value of NK-92 cells and primary NK cells engineered to express therapeutic TCR-28-2B10/BBζ for adoptive immunotherapies, which could pave the way for an “off-the shelf therapeutic live drug”.
Fig. 2.
The expression and antigen-reactive recognition of chimeric TCR (TCR-28-2B10/BBζ) in primary NK cells A, Representative flow cytometry of transduction efficiencies measured by staining chimeric TCR engineered primary NK cells and TCR engineered primary NK cells with an anti-murine TCR- β chain constant region antibody. B, Summary of transduction efficiencies of chimeric TCR and TCR in primary NK cells. C, IFN- γ ELISA measurement after coculture of chimeric TCR engineered primary NK cells and TCR engineered primary NK cells with NYESO-1157-165 V epitope pulsed T2 cells. D, IFN- γ ELISA measurement after coculture of primary NK cells and chimeric TCR engineered primary NK cells with NYESO-1-positive tumor cells. All experiments were performed with four biological replicates, and data shown are representative of at least three independent experiments. Data are presented as mean ± SEM. *** P < 0.001
Acknowledgements
Not applicable.
Authors’ contributions
ZML, BTY and CTZ designed the research; SCL, CTZ, LYS, XT and YFX conducted experiments; SCL, CTZ, and ZML analyzed data; and CTZ, ZML and SCL wrote the paper.
Funding
This work was supported by Natural Science Foundation of China [Grant No 81972880–ZL, Grant No 82003246–CZ]; Capital’s Funds for Health Improvement and Research (Grant No 2022-1-1022–ZL, 2020-4-1028–CZ); Open Project funded by Key laboratory of Carcinogenesis and Translational Research, Ministry of Education/Beijing (2022 Open Project-1); Cooperation Fund of Beijing Cancer Hospital and Beijing Institute for Cancer Research; Clinical Medicine Plus X—Young Scholars Project (PKU2022LCXQ036), Peking University, the Fundamental Research Funds for the Central Universities.
Data availability
The datasets used or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethics approval
This project was approved by the Institutional Review Board of the Peking University School of Oncology, China. The patient gave written informed consent for the use of their tumor tissues for research purposes.
Consent for publication
All authors have approved for publication.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Shance Li and Chaoting Zhang have contributed equally to this paper.
Contributor Information
Bentong Yu, Email: yubentong@126.com.
Zheming Lu, Email: luzheming@bjmu.edu.cn.
References
- 1.Sadelain M, Riviere I, Riddell S. Therapeutic T cell engineering. Nature. 2017;545(7655):423–431. doi: 10.1038/nature22395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Daher M, Rezvani K. Outlook for new CAR-based therapies with a focus on CAR NK cells: what lies beyond CAR-engineered t cells in the race against cancer. Cancer discov. 2020;1:45–58. doi: 10.1158/2159-8290.CD-20-0556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parlar A, Sayitoglu EC, Ozkazanc D, Georgoudaki AM, Pamukcu C, Aras M, et al. Engineering antigen-specific NK cell lines against the melanoma-associated antigen tyrosinase via TCR gene transfer. Eur J Immunol. 2019;49(8):1278–1290. doi: 10.1002/eji.201948140. [DOI] [PubMed] [Google Scholar]
- 4.Mensali N, Dillard P, Hebeisen M, Lorenz S, Theodossiou T, Myhre MR, et al. NK cells specifically TCR-dressed to kill cancer cells. EBioMedicine. 2019;40:106–117. doi: 10.1016/j.ebiom.2019.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu E, Marin D, Banerjee P, Macapinlac HA, Thompson P, Basar R, et al. Use of CAR-Transduced Natural Killer Cells in CD19-Positive Lymphoid Tumors. N Engl J Med. 2020;382(6):545–553. doi: 10.1056/NEJMoa1910607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morton LT, Wachsmann TL, Meeuwsen MH, Wouters AK, Remst DF, van Loenen MM, Falkenburg JF, Heemskerk MH. T cell receptor engineering of primary NK cells to therapeutically target tumors and tumor immune evasion. J immunother cancer. 2022;10(3):e003715. doi: 10.1136/jitc-2021-003715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shimasaki N, Jain A, Campana D. NK cells for cancer immunotherapy. Nat Rev Drug Discovery. 2020;19(3):200–218. doi: 10.1038/s41573-019-0052-1. [DOI] [PubMed] [Google Scholar]
- 8.Milone MC, Xu J, Chen SJ, Collins MA, Zhou J, Powell DJ, Jr, et al. Engineering enhanced CAR T-cells for improved cancer therapy. Nat Cancer. 2021;2(8):780–793. doi: 10.1038/s43018-021-00241-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Imai C, Iwamoto S, Campana D. Genetic modification of primary natural killer cells overcomes inhibitory signals and induces specific killing of leukemic cells. Blood. 2005;106(1):376–383. doi: 10.1182/blood-2004-12-4797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu Y, Liu Q, Zhong M, Wang Z, Chen Z, Zhang Y, et al. 2B4 costimulatory domain enhancing cytotoxic ability of anti-CD5 chimeric antigen receptor engineered natural killer cells against T cell malignancies. J Hematol Oncol. 2019;12(1):49. doi: 10.1186/s13045-019-0732-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tan Q, Zhang C, Yang W, Liu Y, Heyilimu P, Feng D, et al. Isolation of T cell receptor specifically reactive with autologous tumour cells from tumour-infiltrating lymphocytes and construction of T cell receptor engineered T cells for esophageal squamous cell carcinoma. J Immunother Cancer. 2019;7(1):232. doi: 10.1186/s40425-019-0709-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang C, Tan Q, Li S, Shen L, Zhang J, Liu Y, et al. Induction of EBV latent membrane protein-2A (LMP2A)-specific T cells and construction of individualized TCR-engineered T cells for EBV-associated malignancies. J Immunother Cancer. 2021;9(7):e002516. doi: 10.1136/jitc-2021-002516. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used or analyzed during the current study are available from the corresponding author on reasonable request.