Abstract
The dynamic interactions between macrophages and T-lymphocytes in the tumor microenvironment exert both antagonistic and synergistic functions affecting tumor growth. Extensive experimental effort has been expended to investigate immunotherapeutic strategies targeting macrophage polarization as well as T-cell activation with the goal to promote tumor cell killing and cancer elimination. However, these interactions remain poorly understood, and cancer immunotherapeutic strategies are often disappointing. The complex system encompassing innate and adaptive immune cell activity in response to tumor growth could benefit from a systems perspective built upon mathematical modeling. This study develops a modeling system to help evaluate the effects of macrophage and T-lymphocyte interactions on tumor growth. The system enables simulating the combined cytotoxic and tumor-promoting interactions of these two immune cell populations in a vascularized organ microenvironment, such as in liver metastases. A hypothetical immunotherapeutic strategy is simulated to increase the number of tumor-suppressive (M1-phenotype) vs. tumor-promoting (M2-phenotype) macrophages to gauge their effects on CD8+ T-cells and CD4+ T-helper cells, which in turn affect the macrophage functions. The results highlight the dynamic interactions between macrophages and T-lymphocytes in the tumor microenvironment and show that with the chosen set of parameter values, the overall cytotoxic effect from macrophages and T-lymphocytes obtained by driving the M1:M2 ratio higher could saturate and fail to achieve tumor regression. Further expansion of this modeling platform to include additional tumor-immune cell interactions, coupled with parameters representing particular tumor characteristics, could enable systematic evaluation of immunotherapeutic strategies tailored to patient-tumor specific conditions, including metastatic disease.
Electronic supplementary material
The online version of this article (10.1007/s00262-020-02785-4) contains supplementary material, which is available to authorized users.
Keywords: Tumor-associated macrophages, T lymphocytes, Cancer immunotherapy, Liver metastasis, Mathematical modeling, Cancer simulation
Introduction
To state that tumor-immune system interactions are complex is an understatement. Both cancer behavior and immune system function are still insufficiently understood, particularly in the context of metastasis. In general terms, it is well known that the advent of a cancerous lesion triggers both pro- and anti-inflammatory responses from the surrounding local host tissue. These responses are driven by stromal cells, including fibroblasts and immune cells [1]. The innate immune response includes natural killer cells [2], neutrophils [3], dendritic cells [4] and monocytes [5], whose task is to essentially restrain tumor cells by killing them on contact. However, the microenvironment within and in the vicinity of tumors can modulate the effector function of these innate immune cells. In the case of monocytes that differentiate into tumor-associated-macrophages (TAM), which can polarize to a M1 tumor-suppressing macrophage phenotype [6] to restrain tumor growth, microenvironment cues may instead favor polarization to an M2 pro-tumorigenic macrophage phenotype [7, 8]. This polarization is dynamic [9, 10], as it can occur and adjust along with a range of pro- and anti-tumor functions dependent on the local microenvironment conditions [11, 12]. Monocyte polarization is of critical importance to tumor containment, as macrophages constitute the majority of immune cells in the primary as well as the metastatic tumor microenvironment [13], and especially in the liver [14].
The adaptive immune response has also been heavily implicated in cancer growth. The adaptive response includes B lymphocytes (B cells) and T lymphocytes (T cells), presenting as in the case of the innate response with both anti- and pro-tumor effects modulated by local tumor microenvironmental conditions. Although the interaction of tumor cells with B cells is not as well understood, with activated B effector cells able to kill tumor cells on contact while resting B effector cells downstage the immune response [15], much focus has been dedicated to the potential role of T cells in restraining tumor growth. The T cell population includes T memory cells, which retain pathogen memory; cytotoxic (CD8+) T cells, which secrete pro-inflammatory cytokines (e.g., TNF-α and IFN-γ) as well as cytotoxic mediators (e.g., perforin), and express death ligands that can kill tumor cells on contact [16]; T helper (CD4+) cells, which release a plethora of factors that activate macrophages, B cells, and cytotoxic T cells; regulatory T (CD4+Foxp3+) cells, which inhibit proliferation and activity of T helper cells [17]. In tumors, cytotoxic T cells apparently can become anergic (exhausted), and thus ineffective in their anti-tumor action because of the interaction of PD-1 expressed on CD8+ T cells and its ligand PD-L1 which can be expressed by both tumor cells and tumor-associated macrophages [18]. Thus, recent therapeutic approaches have aimed to reverse this situation by implementing PD-1 or PD-L1 inhibitors in the treatment of various cancer types with varying degrees of success [19]. Additionally, macrophages have been shown to restrain CD8+ T cells, e.g., by inhibiting their activation via secretion of TGF-β and IL-10, and thus limiting the efficacy of immunotherapeutic strategies targeting T cells [20].
T helper cells can polarize into T helper 1 (Th1) cells, which release pro-inflammatory cytokines (such as IFN-γ, IL-2, IL-12) and promote dendritic cell function, which in turn activate T lymphocytes. On the hand, T helper cells can polarize into T helper 2 (Th2) cells, which restrain cytotoxic T cell proliferation and promote polarization of macrophages into the M2 phenotype, e.g., via secretion of IL-5 and IL-13 [21]. It has been recently shown that the proportion of anti-tumor Th1 to pro-tumor Th2 cells can be of prognostic value in cancer [22]. Particularly, the interaction between T helper cells and macrophages is complex; M1 macrophages promote polarization to Th1, e.g., via secretion of IL-12 [6], while M2 macrophages promote a Th2 response [23]. The adaptive immune response is thus influenced by the dynamic tumor microenvironment-induced polarization of macrophages.
The immense experimental effort to understand macrophage polarization and tumor-associated macrophage interactions within the tumor microenvironment has recently been complemented by mathematical modeling and computational simulation, as these complex interactions may be better elucidated by a system-level approach that allows for evaluation of relevant parameters. Interactions between macrophages and T helper cells in determining a general immune response were modeled in [24]. More recent modeling studies have focused on the effects of macrophage polarization on tumor growth [25–27] and drug therapy [28–31]. The natural phagocytic function of macrophages has been explored to synergistically maximize the drug response [32–35]. The activity of T lymphocytes in the tumor microenvironment has also been extensively modeled, as recently reviewed in [36]. In this study, we apply a spatial model of tumor growth [37, 38] to simulate the interactions between macrophages and T lymphocytes in the metastatic context of the liver. The liver is a richly vascularized organ, to which many cancer types metastasize during advanced stages of the disease, including colon, breast, prostate, and ovarian cancer. In particular, we evaluate the dynamic relationship between macrophage polarization in the tumor microenvironment, cytotoxic T cell and T helper cell activity, and the combination of their effects on tumor growth.
Materials and methods
The computational model builds upon recent work in which a tumor growth model [37, 38] was coupled with a macrophage model representing the innate immune system [26] to evaluate the response of breast cancer liver metastasis to nanotherapy [32–34]. Here, the modeling of the immune system is expanded to include an adaptive response, namely CD8+ cytotoxic T cells, CD4+ Th1 cells, and CD4+ Th2 cells, and their differentiation, cell–cell interactions, and effects on the vascularized tumor growth. Briefly, the model is composed of a tumor lesion in a 2D grid of preexisting vasculature representing a well-perfused organ, as previously described in [26]. A spatial component enables modeling the movement of immune cells as well as the transport of cytokines and other substances in the tumor microenvironment.
Tumor metastasis in a vascularized organ
Tumor growth is modeled according to [37] and builds upon [33, 34, 38, 39]. A small lesion is simulated in a Cartesian grid of regularly-spaced (250 μm) pre-existing capillaries in the surrounding host tissue. The tumor microenvironment is described based on oxygen and cell proliferation: normal oxygenation, non-proliferating host tissue; normal oxygenation, proliferating tumor tissue; hypoxia, viable but not proliferating (quiescent) tumor tissue; hypoxia, necrotic (non-viable) tumor tissue. Hypoxic tissue releases tumor angiogenic factors (TAF), which diffuse into the surrounding microenvironment and stimulate sprouting of nearby capillaries via angiogenesis. These growth factors also promote vascular extravasation of macrophages and T lymphocytes at the tumor location, analogous to the action of vascular endothelial growth factor (VEGF) on macrophage recruitment [40]. The main parameters of the model are in Supplementary Table 1.
Angiogenesis is as described in [37, 38, 41], and implements the development of tumor-induced vasculature from the pre-existing capillaries in response to the release of TAF by the tumor tissue. The model accounts for blood flow coupled with pressures internal and external to the vessels. The inhomogeneous tumor growth induces heterogeneity in the level of oxygen, nutrients, and growth factors diffusing from the vasculature within and in the vicinity of the tumor tissue.
Assuming steady-state, the mass balance for the concentration of a particular cytokine or chemokine C (dimensionless units, ranging from 0 to 1) secreted by tumor or immune cells is as described in [42].
For all the diffusion and pressure equations, zero Neumann conditions are taken at the boundaries [37].
Immune cells
Monocytes and T lymphocytes (CD8+, Th1 and Th2 T cells) are simulated as discrete entities to exit the vasculature in proportion to the local concentration of chemoattractants, such as pro-angiogenic factors released by tumor cells, and to preferentially migrate along the increasing gradient of these chemoattractants [26]. Monocytes can be polarized to M1 or M2 macrophages in the vicinity of the tumor microenvironment based on the concentration of macrophage-polarizing factors released by proliferating/normoxic and quiescent/hypoxic tumor tissue [26]. Lymphocytic precursors are assumed to differentiate at secondary lymphoid tissues systemically. As the number of tumor cells is proportional to tumor size, a reasonable bound for a 1 mm3 lesion is ~ 3 × 106 cells [43], with 10% of these cells being macrophages. A conservative estimate of the number of macrophages recruited to the tumor is ~ 25% of that observed in vivo (2.78 × 104 macrophages/mm3) [34]. As the number of T lymphocytes can vary widely based on specific tumor conditions, for the purposes of this study, the initial proportion of CD8+, Th1 and Th2 T cells arriving at the tumor site was kept similar, with initial numbers being on the same order of magnitude as the macrophages.
Macrophage differentiation
As monocytes exit the vasculature in the tumor vicinity, they are exposed to soluble factors diffusing from the tumor tissue that influence their differentiation. The concentration of proteins, such as interleukins that induce differentiation into particular subtypes (M1 or M2), modulates the respective differentiation probabilities Ri dependent on the size of the interval within which a randomly generated number occurs. Differentiation probabilities are thus proportional to the local concentration of proteins, :
| 1 |
where are intensity coefficients calibrated to reflect the relative prevalence of M1 vs. M2 macrophages in the tumor tissue, , , and are local concentrations of cytokines and other factors promoting differentiation to M1 or M2, released by viable (proliferating and quiescent) tumor tissue, and is an intensity coefficient to tune the effect of the Th2 cells-released factor favoring M2 differentiation. For simplicity, a binary M1 or M2 state is simulated, although this polarization, in reality, occurs along with a range of phenotypic characteristics shared between M1 and M2 [44].
T lymphocyte differentiation
Similar to the macrophage model, the respective production probability Ri of each T cell type depends on the size of an interval that a randomly generated number may fall into. Thus, the differentiation probabilities depend on the local concentration of cytokines and factors influencing this differentiation. T lymphocytes have a baseline probability of differentiation, respectively kTH1, kTH2, kCD8, with differentiation further influenced by the systemic concentration of cytokines Cvasc,i released by immune cells. This baseline production saturates at ksat,i as the respective total cell counts, TTH1, TTH2, TCD8, increase at each simulation time step:
| 2 |
The terms , , control the rates at which circulating cytokines influence relative production of each lymphocyte at secondary lymphoid tissues. M1 macrophages promote Th1 differentiation through release of cytokines such as IL-12 at rate . These differentiated Th1 cells promote CD8+ T cells through characteristic cytokines like IFN-γ described as at rate . Similarly, M2 macrophages favor Th2 differentiation through circulating cytokines such as IL-4 at rate .
T lymphocytes and macrophages are given a maximum lifetime in the tumor microenvironment with a probability of earlier death, Gi, proportional to :
| 3 |
IL-10 is modeled to act locally to suppress the effect of CD8+ T cells on the tumor at rate , thus lowering the number of fit CD8+ cells.
Immune cell movement
Immune cells migrate through the interstitium along gradients of oxygen, pressure, and chemoattractants. Movement in one of four directions along the 2D computational grid is calculated semi-stochastically, similar to the immune cell production rates. Probability of movement in x + 1 direction is [26]:
| 4 |
where and are intensity coefficients accounting for the effect of oxygen concentration, pressure, and chemoattractant on movement, and and are the difference in concentration of the factor of interest from the current point to the direction of movement under consideration. Similar calculations are done for the other three directions in the Cartesian grid. Each probability is divided by the total sum of the four probabilities, and intervals are established proportional to the respective magnitude of these scaled probabilities [26]. A random number of value between 0 and 1 is generated, and movement in a particular direction is decided based on which interval the number falls into. The cell remains in place if no interval qualifies. Immune cells can share the same location on the grid as a vessel or tumor tissue, but not with each other.
The deeper penetration of M1 macrophages compared to M2 into tumor tissue observed in experiments [33] was simulated to replicate this effect. This was implemented as a concentric field (value 1 at center of lesion and 0 at the boundary), biasing the M1 movement into the core of the lesion.
Coupling of tumor tissue and immune cells
The cytotoxicity of M1 macrophages, CD8+ T cells and Th1 cells are modeled as acting locally to induce cell death. The respective effects, and are linked to the tumor proliferation term in the tumor velocity [37]:
| 5 |
where is the tumor native mitosis rate, is the local oxygen concentration and is the tumor natural apoptosis rate. The non-dimensionalized rate of cell degradation in necrotic tissue () assumes that cell debris is continuously degraded and the associated fluid is removed. Cytotoxicity is modeled to affect both normoxic (cycling) and hypoxic (quiescent) tissue since the death mechanism is assumed to be cell-cycle independent.
The cytotoxic effect of the M1 subtype is simulated to affect tumor tissue in proportion to the release rate of nitric oxide in the immediate vicinity of the macrophage (), since nitric oxide has a short half-life in vivo with limited diffusion distance [26]:
| 6 |
The CD8+ T cells and Th1 cells are stimulated to induce their respective cytotoxic effects ( and ) through direct cell–cell interactions. Tissue death occurs in the immediate T cell vicinity (1CD8 and 1TH1) in proportion to the level of cytotoxic mediators and death ligands ( and , respectively) targeting the tumor tissue:
| 7 |
| 8 |
In contrast to the cytotoxic effects of M1 macrophages, CD8+ T cells and Th1 cells, the M2 macrophages are simulated to favor tumor growth by secreting growth factors [26] that transiently increase the proliferation rate of tumor tissue in proportion to the local concentration of these factors. These growth factors diffuse in the tumor microenvironment as described in [42]. The positive effect on the proliferating region in Eq. 5 is calculated in time as:
| 9 |
where is the proliferation rate at a given location due to the local concentration of diffusible M2 growth factors, which enhances the native proliferation , while is the actual effect of these factors on the proliferation. The overall effect decreases as the net proliferation approaches a maximum value of 1 day−1, and it decays at a constant rate .
A hypothetical immunotherapeutic regimen that favors monocyte polarization towards the M1 phenotype in the tumor microenvironment is simulated by adjusting the macrophage intensity coefficients (raising and lowering in Eq. 1) so that the overall M1:M2 ratio is increased.
Implementation
Values for parameters associated with the immune cells are described in Supplementary Table 2. These parameters were calibrated so that the simulated tumor growth would match experimentally measured values for breast cancer metastasis to the liver [33, 34]. Characteristics of the representative cytokines secreted by the immune cells simulated in this study are shown in Supplementary Table 3, Values for the parameters associated with these cytokines are in Supplementary Table 4, based on previous work that evaluated protein diffusivity as a function of molecular weight [42]. The numerical implementation of the model system is as described in [37, 38] and references therein. All simulations were run n = 3. The data were statistically analyzed using Student t test, with p < 0.05 considered significant.
Results
Simulations were performed to explore the dynamic relationship between macrophage polarization in the metastatic tumor microenvironment, CD8+ T cell and T helper cell activity, and the resulting effects on tumor growth.
The tumor tissue is simulated in 2D space growing within a highly vascularized organ, such as the liver. Figure 1 shows a representative lesion at 18 days after inception. The interior of the lesion is simulated to be highly hypoxic, as can be seen from the oxygen concentration, consistent with experimental observations of breast [33, 34] and colon cancer [45, 46] metastatic lesions to the liver. The hypoxic tissue releases a net balance of pro-angiogenic factors (e.g., vascular endothelial growth factor or VEGF), collectively denoted as tumor angiogenic factors (TAF), as well as cytokines and chemokines (e.g., M-CSF and CCL2), which diffuse from the tissue into the surrounding microenvironment. Both the TAF and the cytokines act as macrophage chemoattractants and polarizing factors, causing monocytes to migrate towards the tumor tissue and differentiate into distinct phenotypes. The TAF trigger the vascular endothelial cells in the nearby capillaries to proliferate and form new vessels, which grow along the gradient of the TAF towards the tumor. These vessels anastomose so that blood flow is established, which occurs primarily in the lesion periphery and proliferating tissue regions, which enables these regions to grow further.
Fig. 1.
Representative simulation of a tumor lesion at 18 days after inception. Left panel shows metastatic tumor proliferating (red) and hypoxic (blue) tissue embedded in a regularly vascularized capillary bed (grid lines). Irregular lines indicate vessel growth in response to angiogenic stimuli by the tumor tissue. Middle panel illustrates the oxygen concentration (normalized to the maximum in the vasculature), indicating hypoxia in the tumor interior and a ring of higher oxygenation due to developing angiogenesis surrounding the growing tumor. Right panel shows the concentration (non-dimensionalized) of immune cell chemoattractants, such as VEGF, secreted by tumor cells and diffusing into the surrounding microenvironment. Bar = 200 μm
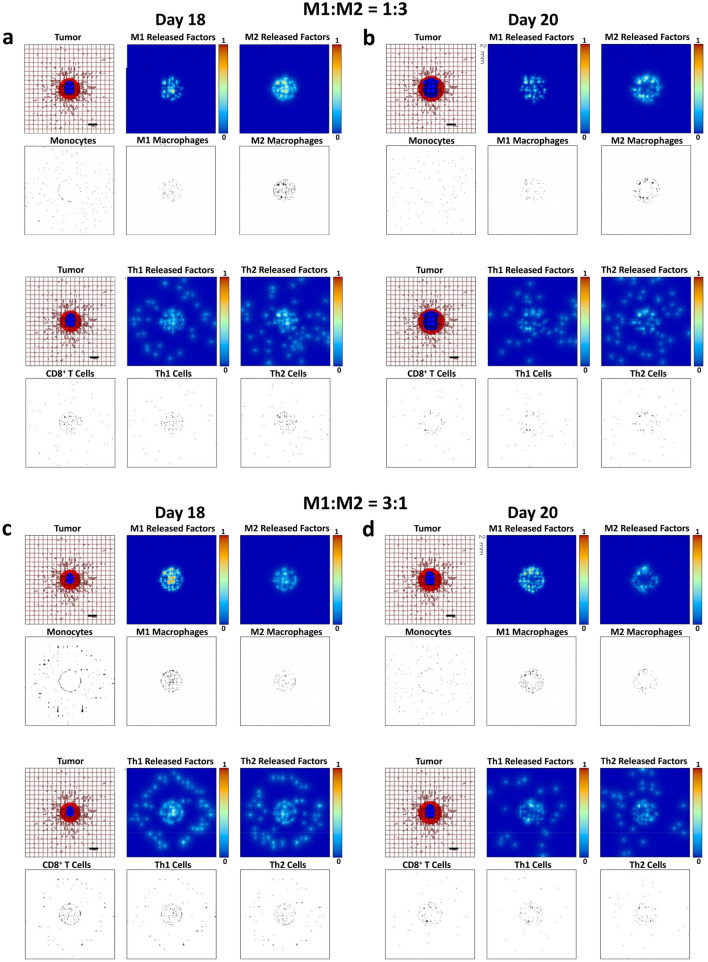
Figure 2a, b qualitatively illustrates the macrophage activity over the course of 2 days in response to a growing lesion (18 days after inception). Monocytes extravasate from nearby vessels, including the dynamically evolving neovasculature, and migrate along the chemoattractant gradient towards the tumor. Based on the balance of the factors in the microenvironment, some of these monocytes differentiate towards an M1 (inflammatory and tumoricidal) phenotype, while others differentiate towards an M2 (anti-inflammatory and tumor-promoting) phenotype, yielding an approximate M1:M2 ratio of 1:3. The model was calibrated to simulate the macrophage behavior experimentally observed in response to breast cancer metastases to the liver [33, 34], for which the M1 subtype was shown to penetrate deeper into the tumor tissue than M2 macrophages. M1 macrophages release cytotoxic substances, e.g., nitric oxide, which kills cells in close proximity, while M2 macrophages release diffusible growth factors that promote tumor proliferation. In this simulation with the M1:M2 ratio at 1:3, the net balance of these effects leads to continued tumor growth.
Fig. 2.
Simulated immune cell activity in response to a growing tumor at 18 days and 20 days after inception. Panels a, b Untreated condition. Top row: monocytes extravasate from vasculature and up the gradient of macrophage chemoattractants towards the tumor tissue. Monocytes differentiate into an M1 or M2 phenotype in response to microenvironmental conditions favoring a ratio of 1:3. The M1 macrophages release cytotoxic factors, such as nitric oxide, while M2 macrophages secrete growth factors promoting tumor proliferation. Bottom row: T lymphocytes exit the vasculature in the vicinity of the tumor and migrate towards it, with CD8+ T cells and CD4+ T helper 1 (Th1) cells having cell killing properties and CD4+ T helper 2 (Th2) cells releasing factors promoting tumor growth. The macrophages and T lymphocytes interact with each other in the microenvironment, influencing their respective response to the tumor. Panels c, d Simulation of a hypothetical immunotherapeutic strategy that increases the M1:M2 ratio to 3:1. In comparison to panels a, b. the therapy leads to higher numbers of cytotoxic CD8+ T cells and CD4+ T helper 1 (Th1) cells, with visibly diminished tumor growth. The Th2 cell number seems to slightly increase. Colors as in Fig. 1. Bar = 200 μm
The simulated T lymphocyte response associated with the macrophage activity is also shown in Fig. 2a, b. The M1 macrophages promote naïve lymphocyte differentiation towards a Th1 cell phenotype, while the M2 macrophages help to drive a Th2 cell response. Like the M1 macrophages, Th1 cells also release cytotoxic cytokines, such as IFN-γ, which kill tumor cells in the immediate vicinity. In contrast, Th2 cells drive macrophage differentiation towards the tumor-promoting M2 phenotype. The Th2 cell activity also restrains the differentiation of naïve lymphocytes to the CD8+ T cell phenotype. Once activated, CD8+ T cells can be highly effective at killing tumor cells on contact, but the restraining of their activity, either by Th2 cells or the tumor cells, can thwart their efficacy. Overall, in this representative simulation, the tumor-suppressive activity of the M1 macrophages, Th1 cells and CD8+ T cells is unable to restrain the growth of the lesion promoted by the activity of the M2 macrophages, Th2 cells, and the ongoing angiogenesis. Simulating the administration of a hypothetical immunotherapy favoring macrophage polarization towards the M1 phenotype, as could occur with a regimen targeting macrophages [32, 47], is shown by the model to consequently affect the T lymphocyte activity. Figure 2c, d qualitatively shows this activity for the case of a therapy achieving an M1:M2 ratio of 3:1.
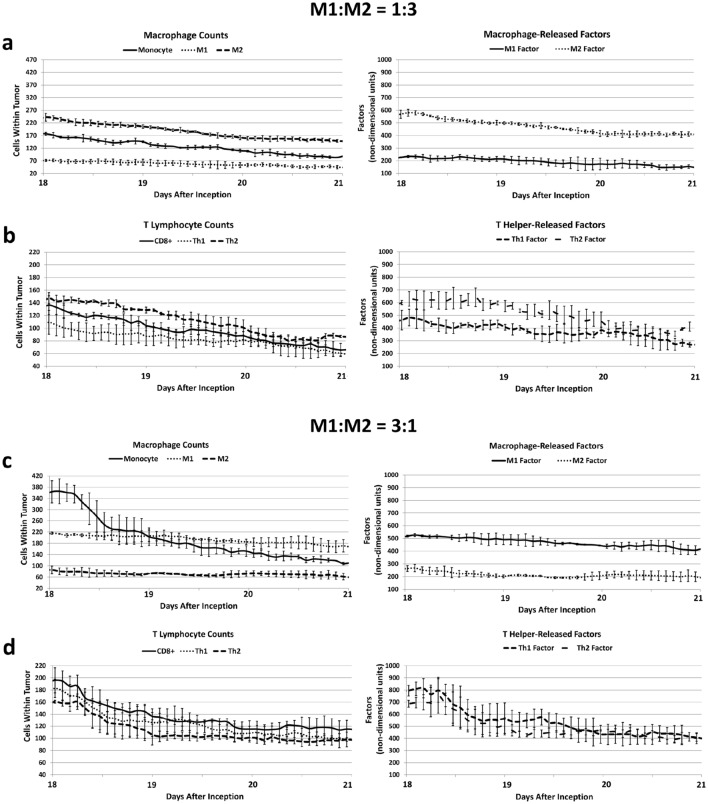
Figure 3a, b quantifies the macrophage and T lymphocyte numbers over a period of tumor growth in the case of microenvironmental conditions favoring tumorigenic activity. The ratio of M1:M2 is approximately 1:3, which helps to promote tumor proliferation as well as angiogenesis via a high level of growth factors (Fig. 3a). In turn, a higher M2 number and increased tumor growth favor greater Th2 cell over Th1 and CD8+ T cell numbers, which lead to lower tumoricidal activity as well as higher levels of pro-tumorigenic Th2 cytokines (Fig. 3b). In the case of immunotherapeutic treatment, the M1:M2 macrophage ratio is shifted to be closer to 3:1 (Fig. 3c, d) with a corresponding decrease in M2-secreted tumor growth-promoting factors (Fig. 3c). In this situation, the Th1 cell numbers increase while the Th2 cell number is lowered, along with corresponding changes in their released factors (Fig. 3d). From the combination of these effects, the CD8+ T cell numbers increase compared to the untreated condition (Fig. 3b). Supplementary Fig. 1 presents the trends of increased Th1 and CD8+ T cell activity while lowering the Th2 response for an immunotherapy yielding an M1:M2 ratio of approximately 1:1 (qualitatively shown in Supplementary Fig. 2).
Fig. 3.
Simulated macrophage and T lymphocyte cell numbers over a period of tumor growth. Panels a, b Microenvironmental conditions favoring tumorigenic activity. a The ratio of M1:M2 macrophages is approximately 1:3, which promotes tumor tissue proliferation and angiogenesis via a high level of M2-secreted growth factors. b The increased M2 macrophage and tissue activity in turn promotes higher Th2 cell over Th1 and CD8+ T cell numbers, which lead to higher levels of Th2-secreted cytokines promoting tumor growth. Panels c, d Simulation of a hypothetical immunotherapeutic regimen favoring macrophage polarization towards the M1 phenotype. This therapy shifts the M1:M2 ratio to 3:1. c Compared to the untreated condition (panels a, b), M1 macrophage secreted factors are increased while M2 macrophage secreted factors are lowered. d In response, Th1 cell numbers further increase while Th2 cell numbers decrease, and their, respectively, released factors correspondingly change. The combination of these effects increases the CD8+ T cell numbers. All values: mean ± SD, n = 3
Altogether, these results highlight the dependence of the interactions between macrophages and lymphocytes on their spatial location, which is influenced among other things by the irregular angiogenic process, tumor oncotic pressure, interstitial fluid flow, and the heterogeneous diffusion of factors secreted intra-tumorally and in the surrounding microenvironment by tumor and immune cells.
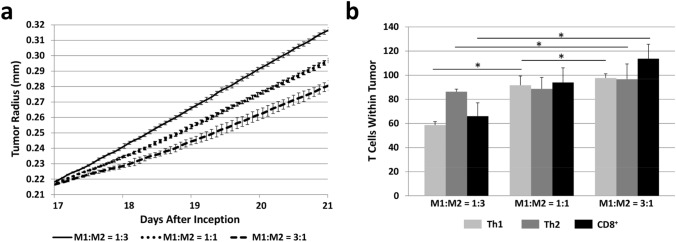
Figure 4a quantifies the tumor growth trajectory corresponding to the untreated (Fig. 3a, b) as well as the treated conditions (Fig. 3c, d; Supplementary Fig. 1). It is apparent that the shift in macrophage polarization towards a higher number of M1 macrophages, which influences the T lymphocyte as well as tumor and vascular behavior coupled to the macrophage activities, yields an overall significant decrease in the rate of tumor growth, but which is insufficient to restrain it. The number of T cells within the tumor as the M1:M2 ratio shifts due to the hypothetical immunotherapeutic treatment is shown in Fig. 4b, highlighting the nonlinear nature of the T lymphocyte response to the shifting macrophage polarization. Whereas the Th2 cells seem relatively stable with respect to the M1:M2 ratio, the Th1 cell numbers exhibit saturation as the M1 macrophage numbers increase.
Fig. 4.
Quantification of simulated tumor growth subjected to a hypothetical immunotherapy that raises the M1:M2 ratio. a Tumor growth trajectory corresponding to untreated (M1:M2 = 1:3) as well as under treatment that increases M1:M2 to 1:1 and M1:M2 to 3:1. The macrophages interact with T lymphocytes in the vascularized tumor microenvironment to both counteract and promote the tumor growth. Increasing the M1:M2 ratio decreases the tumor growth but fails to restrain it. b T cells within the tumor tissue at 21 days after inception. All values: mean ± SD, n = 3, *p < 0.05
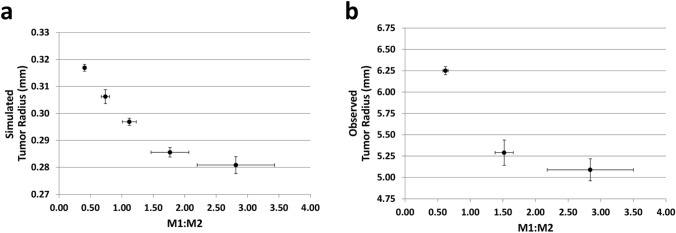
The corresponding change in tumor radius (Fig. 5a) indicates that with the chosen set of parameter values, the treatment is able to slow down but not to reverse the tumor growth as the M1:M2 ratio increases, with the effect on the radius diminishing as this ratio increases. These model results are consistent with recent experimental observations with tumors grown in immunogenic mice subjected to M2 macrophage ablation treatment to increase the intra-tumoral M1:M2 ratio [48]. The experimental data (Fig. 5b) shows the effect on the tumor radius weakening as this ratio increases. To examine this phenomenon further, the change of tumor radius in time (i.e., the tumor growth velocity) from the simulations is plotted as a function of the M1:M2 ratio in Fig. 6, indicating that the nonlinear decrease in radius attenuates at higher M1 macrophage numbers. Overall, these results suggest that under the conditions simulated, immunotherapeutic manipulation of the macrophage phenotype to potentiate the T lymphocyte mediated anti-tumor activity would be unable to eradicate the tumor lesion.
Fig. 5.
Tumor tissue response to an immunotherapeutic strategy that raises the M1:M2 macrophage ratio. a Simulated change in tumor radius as a function of the M1:M2 ratio (mean ± SD, n = 3). b Experimentally observed change in tumor radius as a function of the M1:M2 ratio in Lewis lung carcinoma xenografts grown in immunogenic mice (data from [48], mean ± SEM, n = 5). Tumors were subjected to M2-ablation treatment to raise this ratio
Fig. 6.
Simulated change of tumor radius in time (growth velocity) plotted as a function of the M1:M2 ratio being shifted by a theoretical immunotherapy targeting macrophage polarization (mean ± SD, n = 3)
Discussion
This study establishes a modeling framework to evaluate the complex interactions between macrophages and T lymphocytes in the dynamic tumor microenvironment. A small metastatic tumor is simulated to release angiogenic factors, promoting vascularization, as well as immune chemoattractants, driving immune cell migration towards the tumor tissue. The local balance of these cytokines diffusing in the microenvironment influences macrophage polarization towards either the cytotoxic M1 phenotype or the tumor-promoting M2 phenotype. In turn, M1 macrophages promote T helper cell polarization towards the cytotoxic Th1 subtype. In this manner, both M1 macrophages and Th1 cells act to restrain tumor growth. On the other hand, macrophage polarization towards the M2 phenotype promotes tumor growth, as the M2 macrophages are stimulated to release growth factors favoring cell proliferation. Furthermore, the M2 macrophages help to drive the response of Th2 subtype T helper cells, which is a feedforward manner further influence macrophage polarization towards the M2 phenotype.
The model simulates the CD8+ T cell function by having the Th1 cells help to activate CD8+ T cells (which biologically occurs via the promoting of dendritic cell function). The CD8+ T cells are cytotoxic and embody the brunt of the adaptive immune response against the tumor. This action is tempered by the Th2 cells, which are simulated to restrain the CD8+ T cell proliferation. Model parameters were calibrated where available with experimental data of breast cancer liver metastasis [33, 34]. The system was used to simulate a hypothetical immunotherapeutic that shifts the macrophage polarization towards the M1 phenotype, and the resulting effects on the T lymphocytes (Figs. 2, 3; Supplementary Figs. 1, 2) and the tumor growth (Fig. 4a) were evaluated. The results show that with the chosen parameter set, an increasing M1:M2 ratio leads to a higher number of immune cells within the tumor tissue (Fig. 4b) and to a decrease in the tumor radius (Fig. 5a), which is consistent with experimental observations of tumor xenografts in vivo (Fig. 5b) [48]. However, the simulations indicate that this decrease is unable to induce tumor regression. The number of Th1 cells seems to saturate at higher ratios (Fig. 4b), possibly due to the spatially localized (short-range) influence of the M1 macrophages promoting Th1 and CD8+ cell activity. The representative cytokine secreted by M1 (here, IL-12) that promotes Th1 cell differentiation is a relatively large molecule with lower diffusivity than the cytokines affecting Th2 differentiation or CD8+ T cell activation (Supplementary Table 3). A more exhaustive analysis of cytokine transport and persistence in the tumor microenvironment and their potential effects on lymphocyte activities would be warranted in follow-up studies.
Consistent with this interpretation, the model results further indicate that the influence of the M1:M2 ratio on the rate of tumor growth diminishes as this ratio increases (Fig. 6), suggesting a ceiling on the overall tumor-restraining benefit (from macrophage as well as T cells) that can be achieved by driving the number of cytotoxic M1 cells higher. Interestingly, the variation in the M1:M2 ratio also increases as this ratio is raised, as shown by the simulations (Fig. 5a) and the experimental data (Fig. 5b). This occurs due to an increased variation in M1 macrophage numbers when the ratio is skewed towards the M1 phenotype, which may reflect the challenge of raising the M1 numbers in the M2-phenotype promoting tumor microenvironment.
The model results are consistent with clinical data with colon cancer liver metastases. Two years after undergoing surgery for Stage III colorectal cancer, patients had a high number of liver metastases when the median M1:M2 ratio was 0.5, new liver metastases within the 2 years when the median ratio was 1.3, and no evidence of metastasis when the median ratio was 2.5, indicating that a significant (exponential) jump in the M1:M2 ratio was associated with restrained metastatic disease [49]. The results may also be consistent with disease staging of other cancer types. In patients with Stage I (with M1:M2 = 1.4 ± 0.5), Stage II (M1:M2 = 1.5 ± 0.5), or Stage III (M1:M2 = 1.3 ± 0.6) ovarian cancer, the differences in the overall M1:M2 ratios between stages were significantly smaller when compared to Stage IV patients (M1:M2 = 1.0 ± 0.5) [50]. These data suggest that although a lower ratio is associated with the worst prognosis (Stage IV), higher (and relatively similar) ratios do not necessarily reflect a restrained disease progression from Stage I (cancer is contained within the ovaries or fallopian tubes) to Stage III (cancer has spread to other organs in the peritoneal cavity).
Although the model implements key interactions between macrophages and T lymphocytes from the innate and adaptive immune systems, respectively, it lacks components that are known to have important roles in tumor progression. Dendritic cells were not directly simulated, yet they are also cytotoxic and would lead to tumor cell death [4]. Neutrophils and natural killer cells are not included, which would further promote tumor death [2, 3]. T regulatory cells are not modeled; these cells regulate T helper cell to avoid a rampant host-destroying adaptive immune response [17], and could thus restrain the activity of both Th1 cytotoxic and Th2 tumor-promoting cells. In addition to promoting dendritic cell function, Th1 cells are also known to activate natural killer cells [21]. Further, myeloid-derived suppressor cells (MDSCs) can block T lymphocyte function, thus potentially facilitating tumor growth [51]. B lymphocytes, which represent the other major arm of the adaptive response along with T lymphocytes, induce angiogenesis and regulate the immunosuppressive and pro-metastatic functions of MDSCs [52]. In the B cell category, activated B effector cells are cytotoxic while resting B effector cells release growth factors and thereby promote tumor proliferation [53]. Tumor tissue well-being can be further maintained by B regulatory cells, which attenuate the anti-tumor response of the immune system, including the cytotoxic activity of T lymphocytes [54].
The inclusion of additional immune-tumor cell interactions would be expected to offer the possibility of evaluating different types of tumors and conditions than the ones simulated in this study. Since cancer-associated fibroblasts (CAFs) and extracellular matrix (ECM) are key components regulating tumor metastasis [55], especially in the context of injured liver tissue [56, 57], modeling the interactions with fibroblasts and ECM may provide a more realistic representation of microenvironment-influenced tumor growth and immune response. A larger repertoire of inflammatory mediators released in the microenvironment could be included to study their effects. Further, different types of immunotherapies, such as programmed cell death receptor ligand 1 (PD-L1) inhibition [58] or Chimeric Antigen Receptor (CAR) T cell therapy [59], could be simulated. In particular, PD-L1 expression has recently been shown to be highly heterogeneous within tumor tissue [60] and in relation to CD8+ T cell activity [61]. Therapeutic intervention has been shown to affect the interactions between macrophages and T lymphocytes [62, 63]. Calibration of the model parameters to represent varying tumor types and conditions is expected to lead to differing outcomes with such therapies. Future iterations of the model will also need to consider the effect and interaction of standard-of-care therapies in combinational approaches alongside immunomodulatory drugs [47]. Overall, this study represents a first step focused on the evaluation of macrophage-influenced T lymphocyte activity in the metastatic tumor microenvironment. The expansion of this modeling framework coupled with parameters representing clinically-relevant values could longer-term offer the possibility of customizing immunotherapeutic regimens to patient tumor-specific conditions.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Abbreviations
- CCL-2
C–C motif ligand 2
- 2D
Two-dimensional
- IFN-γ
Interferon γ
- IL
Interleukin
- M-CSF
Macrophage colony-stimulating factor
- TAF
Tumor angiogenic factors
- TAM
Tumor-associated macrophages
- Th1 cell
T Helper 1 cell
- Th2 cell
T Helper 2 cell
- TNF-α
Tumor necrosis factor α
- VEGF
Vascular endothelial growth factor
Author contributions
Study conception and design: HF; Mathematical model implementation and testing: LC; Data collection and analysis: LC, SS, HF; Manuscript preparation and revision: LC, SS, HF.
Compliance with ethical standards
Conflict of interest
The authors have no conflicts to disclose.
Footnotes
The original online version of this article was revised: The "Grant Support" in the Pubmed database is showing a grant (R15CA203605/CA/NCI NIH HHS/United States).
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
1/5/2021
A Correction to this paper has been published: 10.1007/s00262-020-02830-2
References
- 1.Bremnes RM, Donnem T, Al-Saad S, Al-Shibli K, Andersen S, Sirera R, Camps C, Marinez I, Busund LT. The role of tumor stroma in cancer progression and prognosis: emphasis on carcinoma-associated fibroblasts and non-small cell lung cancer. J Thorac Oncol. 2011;6(1):209–217. doi: 10.1097/JTO.0b013e3181f8a1bd. [DOI] [PubMed] [Google Scholar]
- 2.Wu J, Lanier LL. Natural killer cells and cancer. Adv Cancer Res. 2003;90:127–156. doi: 10.1016/s0065-230x(03)90004-2. [DOI] [PubMed] [Google Scholar]
- 3.Wu L, Saxena S, Awaji M, Singh RK. Tumor-associated neutrophils in cancer: going pro. Cancers (Basel) 2019 doi: 10.3390/cancers11040564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ma Y, Shurin GV, Peiyuan Z, Shurin MR. Dendritic cells in the cancer microenvironment. J Cancer. 2013;4(1):36–44. doi: 10.7150/jca.5046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Olingy CE, Dinh HQ, Hedrick CC. Monocyte heterogeneity and functions in cancer. J Leukoc Biol. 2019;106(2):309–322. doi: 10.1002/JLB.4RI0818-311R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Disis ML. Immune regulation of cancer. J Clin Oncol. 2010;28(29):4531–4538. doi: 10.1200/JCO.2009.27.2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yao R-R, Li J-H, Zhang R, Chen R-X, Wang Y-H. M2-polarized tumor-associated macrophages facilitated migration and epithelial–mesenchymal transition of HCC cells via the TLR4/STAT3 signaling pathway. World J Surg Oncol. 2018;16:9. doi: 10.1186/s12957-018-1312-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yuan A, Hsiao Y-J, Chen H-Y, Chen H-W, Ho C-C, Chen Y-Y, Liu Y-C, Hong T-H, Yu S-L, Chen JJW, Yang P-C. Opposite effects of M1 and M2 macrophage subtypes on lung cancer progression. Sci Rep. 2015;5:14273. doi: 10.1038/srep14273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Italiani P, Boraschi D. From monocytes to M1/M2 macrophages: phenotypical vs. functional differentiation. Front Immunol. 2014;5:514. doi: 10.3389/fimmu.2014.00514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Laoui D, Movahedi K, Van Overmeire E, Van den Bossche J, Schouppe E, Mommer C, Nikolaou A, Morias Y, De Baetselier P, Van Ginderachter JA. Tumor-associated macrophages in breast cancer: distinct subsets, distinct functions. Int J Dev Biol. 2011;55(7–9):861–867. doi: 10.1387/ijdb.113371dl. [DOI] [PubMed] [Google Scholar]
- 11.Chanmee T, Ontong P, Konno K, Itano N. Tumor-associated macrophages as major players in the tumor microenvironment. Cancers. 2014;6(3):1670–1690. doi: 10.3390/cancers6031670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roca H, Varsos ZS, Sud S, Craig MJ, Ying C, Pienta KJ. CCL2 and interleukin-6 promote survival of human CD11b(+) peripheral blood mononuclear cells and induce M2-type macrophage polarization. J Biol Chem. 2009;284(49):34342–34354. doi: 10.1074/jbc.M109.042671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nielsen SR, Schmid MC. Macrophages as key drivers of cancer progression and metastasis. Mediat Inflamm. 2017;2017:11. doi: 10.1155/2017/9624760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krenkel O, Tacke F. Liver macrophages in tissue homeostasis and disease. Nat Rev Immunol. 2017;17(5):306–321. doi: 10.1038/nri.2017.11. [DOI] [PubMed] [Google Scholar]
- 15.Yuen GJ, Demissie E, Pillai S. B lymphocytes and cancer: a love–hate relationship. Trends Cancer. 2016;2(12):747–757. doi: 10.1016/j.trecan.2016.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maher J, Davies ET. Targeting cytotoxic T lymphocytes for cancer immunotherapy. Br J Cancer. 2004;91(5):817–821. doi: 10.1038/sj.bjc.6602022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kondelkova K, Vokurkova D, Krejsek J, Borska L, Fiala Z, Ctirad A. Regulatory T cells (TREG) and their roles in immune system with respect to immunopathological disorders. Acta med (Hradec Kral) 2010;53(2):73–77. doi: 10.14712/18059694.2016.63. [DOI] [PubMed] [Google Scholar]
- 18.Jiang Y, Li Y, Zhu B. T-cell exhaustion in the tumor microenvironment. Cell Death Dis. 2015;6:e1792. doi: 10.1038/cddis.2015.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zarour HM. Reversing T-cell dysfunction and exhaustion in cancer. Clin Cancer Res. 2016;22(8):1856–1864. doi: 10.1158/1078-0432.CCR-15-1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peranzoni E, Lemoine J, Vimeux L, Feuillet V, Barrin S, Kantari-Mimoun C, Bercovici N, Guerin M, Biton J, Ouakrim H, Regnier F, Lupo A, Alifano M, Damotte D, Donnadieu E. Macrophages impede CD8 T cells from reaching tumor cells and limit the efficacy of anti-PD-1 treatment. Proc Natl Acad Sci U S A. 2018;115(17):E4041–E4050. doi: 10.1073/pnas.1720948115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu X, Wang R, Su Q, Huang H, Zhou P, Luan J, Liu J, Wang J, Chen X. Expression of Th1-, Th2- and Th17-associated cytokines in laryngeal carcinoma. Oncol Lett. 2016;12(3):1941–1948. doi: 10.3892/ol.2016.4854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Monte L, Reni M, Tassi E, Clavenna D, Papa I, Recalde H, Braga M, Di Carlo V, Doglioni C, Protti MP. Intratumor T helper type 2 cell infiltrate correlates with cancer-associated fibroblast thymic stromal lymphopoietin production and reduced survival in pancreatic cancer. J Exp Med. 2011;208(3):469–478. doi: 10.1084/jem.20101876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Muraille E, Leo O, Moser M. TH1/TH2 paradigm extended: macrophage polarization as an unappreciated pathogen-driven escape mechanism? Front Immunol. 2014;5:603. doi: 10.3389/fimmu.2014.00603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bar-Or RL. Feedback mechanisms between T helper cells and macrophages in the determination of the immune response. Math Biosci. 2000;163(1):35–58. doi: 10.1016/s0025-5564(99)00046-2. [DOI] [PubMed] [Google Scholar]
- 25.den Breems NY, Eftimie R. The re-polarisation of M2 and M1 macrophages and its role on cancer outcomes. J Theor Biol. 2016;390:23–39. doi: 10.1016/j.jtbi.2015.10.034. [DOI] [PubMed] [Google Scholar]
- 26.Mahlbacher G, Curtis LT, Lowengrub J, Frieboes HB. Mathematical modeling of tumor-associated macrophage interactions with the cancer microenvironment. J Immunother Cancer. 2018;6(1):10. doi: 10.1186/s40425-017-0313-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Norton KA, Jin K, Popel AS. Modeling triple-negative breast cancer heterogeneity: effects of stromal macrophages, fibroblasts and tumor vasculature. J Theor Biol. 2018;452:56–68. doi: 10.1016/j.jtbi.2018.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bracci L, Schiavoni G, Sistigu A, Belardelli F. Immune-based mechanisms of cytotoxic chemotherapy: implications for the design of novel and rationale-based combined treatments against cancer. Cell Death Differ. 2014;21(1):15–25. doi: 10.1038/cdd.2013.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen D, Bobko AA, Gross AC, Evans R, Marsh CB, Khramtsov VV, Eubank TD, Friedman A. Involvement of tumor macrophage HIFs in chemotherapy effectiveness: mathematical modeling of oxygen, pH, and glutathione. PLoS ONE. 2014 doi: 10.1371/journal.pone.0107511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kareva I, Waxman DJ, Klement GL. Metronomic chemotherapy: an attractive alternative to maximum tolerated dose therapy that can activate anti-tumor immunity and minimize therapeutic resistance. Cancer Lett. 2015;358(2):100–106. doi: 10.1016/j.canlet.2014.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun Y, Campisi J, Higano C, Beer TM, Porter P, Coleman I, True L, Nelson PS. Treatment-induced damage to the tumor microenvironment promotes prostate cancer therapy resistance through WNT16B. Nat Med. 2012;18(9):1359–1368. doi: 10.1038/nm.2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leonard F, Curtis LT, Hamed AR, Zhang C, Chau E, Sieving D, Godin B, Frieboes HB. Nonlinear response to cancer nanotherapy due to macrophage interactions revealed by mathematical modeling and evaluated in a murine model via CRISPR-modulated macrophage polarization. Cancer Immunol Immunother. 2020;69(5):731–744. doi: 10.1007/s00262-020-02504-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leonard F, Curtis LT, Ware MJ, Nosrat T, Liu X, Yokoi K, Frieboes HB, Godin B. Macrophage polarization contributes to the anti-tumoral efficacy of mesoporous nanovectors loaded with albumin-bound paclitaxel. Front Immunol. 2017;8:693. doi: 10.3389/fimmu.2017.00693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leonard F, Curtis LT, Yesantharao P, Tanei T, Alexander JF, Wu M, Lowengrub J, Liu X, Ferrari M, Yokoi K, Frieboes HB, Godin B. Enhanced performance of macrophage-encapsulated nanoparticle albumin-bound-paclitaxel in hypo-perfused cancer lesions. Nanoscale. 2016;8(25):12544–12552. doi: 10.1039/C5NR07796F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Owen MR, Stamper IJ, Muthana M, Richardson GW, Dobson J, Lewis CE, Byrne HM. Mathematical modeling predicts synergistic antitumor effects of combining a macrophage-based, hypoxia-targeted gene therapy with chemotherapy. Cancer Res. 2011;71(8):2826–2837. doi: 10.1158/0008-5472.can-10-2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mahlbacher GE, Reihmer KC, Frieboes HB. Mathematical modeling of tumor-immune cell interactions. J Theor Biol. 2019;469:47–60. doi: 10.1016/j.jtbi.2019.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Macklin P, McDougall S, Anderson ARA, Chaplain MAJ, Cristini V, Lowengrub J. Multiscale modelling and nonlinear simulation of vascular tumour growth. J Math Biol. 2009;58(4–5):765–798. doi: 10.1007/s00285-008-0216-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu M, Frieboes HB, McDougall SR, Chaplain MAJ, Cristini V, Lowengrub J. The effect of interstitial pressure on tumor growth: coupling with the blood and lymphatic vascular systems. J Theor Biol. 2013;320:131–151. doi: 10.1016/j.jtbi.2012.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van de Ven AL, Wu M, Lowengrub J, McDougall SR, Chaplain MA, Cristini V, Ferrari M, Frieboes HB. Integrated intravital microscopy and mathematical modeling to optimize nanotherapeutics delivery to tumors. AIP Adv. 2012;2(1):11208. doi: 10.1063/1.3699060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lewis C, Murdoch C. Macrophage responses to hypoxia: implications for tumor progression and anti-cancer therapies. Am J Pathol. 2005;167(3):627–635. doi: 10.1016/S0002-9440(10)62038-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McDougall SR, Anderson ARA, Chaplain MAJ. Mathematical modelling of dynamic adaptive tumour-induced angiogenesis: clinical implications and therapeutic targeting strategies. J Theor Biol. 2006;241(3):564–589. doi: 10.1016/j.jtbi.2005.12.022. [DOI] [PubMed] [Google Scholar]
- 42.Frieboes HB, Curtis LT, Wu M, Kani K, Mallick P. Simulation of the protein-shedding kinetics of a fully vascularized tumor. Cancer Inform. 2015;14:163–175. doi: 10.4137/CIN.S35374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Spinney L. Caught in time. Nature. 2006;442(7104):736–738. doi: 10.1038/442736a. [DOI] [PubMed] [Google Scholar]
- 44.Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep. 2014;6:13. doi: 10.12703/P6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reichel D, Curtis LT, Ehlman E, Mark Evers B, Rychahou P, Frieboes HB, Bae Y. Development of halofluorochromic polymer nanoassemblies for the potential detection of liver metastatic colorectal cancer tumors using experimental and computational approaches. Pharm Res. 2017;34(11):2385–2402. doi: 10.1007/s11095-017-2245-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Curtis LT, Rychahou P, Bae Y, Frieboes HB. A Computational/experimental assessment of antitumor activity of polymer nanoassemblies for pH-controlled drug delivery to primary and metastatic tumors. Pharm Res. 2016;33(10):2552–2564. doi: 10.1007/s11095-016-1981-6. [DOI] [PubMed] [Google Scholar]
- 47.Curtis LT, Frieboes HB. Modeling of combination chemotherapy and immunotherapy for lung cancer. Conf Proc IEEE Eng Med Biol Soc. 2019;2019:273–276. doi: 10.1109/EMBC.2019.8857566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee C, Jeong H, Bae Y, Shin K, Kang S, Kim H, Oh J, Bae H. Targeting of M2-like tumor-associated macrophages with a melittin-based pro-apoptotic peptide. J Immunother Cancer. 2019;7(1):147. doi: 10.1186/s40425-019-0610-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cui YL, Li HK, Zhou HY, Zhang T, Li Q. Correlations of tumor-associated macrophage subtypes with liver metastases of colorectal cancer. Asian–Pac J Cancer Prev. 2013;14(2):1003–1007. doi: 10.7314/apjcp.2013.14.2.1003. [DOI] [PubMed] [Google Scholar]
- 50.Zhang M, He Y, Sun X, Li Q, Wang W, Zhao A, Di W. A high M1/M2 ratio of tumor-associated macrophages is associated with extended survival in ovarian cancer patients. J Ovarian Res. 2014;7:19. doi: 10.1186/1757-2215-7-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Monu NR, Frey AB. Myeloid-derived suppressor cells and anti-tumor T cells: a complex relationship. Immunol Investig. 2012;41(6–7):595–613. doi: 10.3109/08820139.2012.673191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bodogai M, Moritoh K, Lee-Chang C, Hollander CM, Sherman-Baust CA, Wersto RP, Araki Y, Miyoshi I, Yang L, Trinchieri G, Biragyn A. Immunosuppressive and prometastatic functions of myeloid-derived suppressive cells rely upon education from tumor-associated B cells. Cancer Res. 2015;75(17):3456–3465. doi: 10.1158/0008-5472.CAN-14-3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Namm JP, Li Q, Lao X, Lubman DM, He J, Liu Y, Zhu J, Wei S, Chang AE. B lymphocytes as effector cells in the immunotherapy of cancer. J Surg Oncol. 2012;105(4):431–435. doi: 10.1002/jso.22093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sarvaria A, Madrigal JA, Saudemont A. B cell regulation in cancer and anti-tumor immunity. Cell Mol Immunol. 2017;14(8):662–674. doi: 10.1038/cmi.2017.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Erdogan B, Webb DJ. Cancer-associated fibroblasts modulate growth factor signaling and extracellular matrix remodeling to regulate tumor metastasis. Biochem Soc Trans. 2017;45(1):229–236. doi: 10.1042/BST20160387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hudson SV, Dolin CE, Poole LG, Massey VL, Wilkey D, Beier JI, Merchant ML, Frieboes HB, Arteel GE. Modeling the kinetics of integrin receptor binding to hepatic extracellular matrix proteins. Sci Rep. 2017;7(1):12444. doi: 10.1038/s41598-017-12691-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hudson SV, Miller HA, Mahlbacher GE, Saforo D, Beverly LJ, Arteel GE, Frieboes HB. Computational/experimental evaluation of liver metastasis post hepatic injury: interactions with macrophages and transitional ECM. Sci Rep. 2019;9(1):15077. doi: 10.1038/s41598-019-51249-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Akinleye A, Rasool Z. Immune checkpoint inhibitors of PD-L1 as cancer therapeutics. J Hematol Oncol. 2019;12(1):92. doi: 10.1186/s13045-019-0779-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Feins S, Kong W, Williams EF, Milone MC, Fraietta JA. An introduction to chimeric antigen receptor (CAR) T-cell immunotherapy for human cancer. Am J Hematol. 2019;94(S1):S3–S9. doi: 10.1002/ajh.25418. [DOI] [PubMed] [Google Scholar]
- 60.Rahn S, Kruger S, Mennrich R, Goebel L, Wesch D, Oberg HH, Vogel I, Ebsen M, Rocken C, Helm O, Sebens S. POLE Score: a comprehensive profiling of programmed death 1 ligand 1 expression in pancreatic ductal adenocarcinoma. Oncotarget. 2019;10(16):1572–1588. doi: 10.18632/oncotarget.26705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Thompson ED, Zahurak M, Murphy A, Cornish T, Cuka N, Abdelfatah E, Yang S, Duncan M, Ahuja N, Taube JM, Anders RA, Kelly RJ. Patterns of PD-L1 expression and CD8 T cell infiltration in gastric adenocarcinomas and associated immune stroma. Gut. 2017;66(5):794–801. doi: 10.1136/gutjnl-2015-310839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Weiss JM, Guerin MV, Regnier F, Renault G, Galy-Fauroux I, Vimeux L, Feuillet V, Peranzoni E, Thoreau M, Trautmann A, Bercovici N. The STING agonist DMXAA triggers a cooperation between T lymphocytes and myeloid cells that leads to tumor regression. Oncoimmunology. 2017;6(10):e1346765. doi: 10.1080/2162402X.2017.1346765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Thoreau M, Penny HL, Tan K, Regnier F, Weiss JM, Lee B, Johannes L, Dransart E, Le Bon A, Abastado JP, Tartour E, Trautmann A, Bercovici N. Vaccine-induced tumor regression requires a dynamic cooperation between T cells and myeloid cells at the tumor site. Oncotarget. 2015;6(29):27832–27846. doi: 10.18632/oncotarget.4940. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.