Abstract
Neoantigens are T-cell antigens derived from protein-coding mutations in tumor cells. Although neoantigens have recently been linked to anti-tumor immunity in long-term survivors of cancers such as melanoma, their prognostic and immune-modulatory role in many cancer types remain unexplored. We investigate neoantigens in hepatocellular carcinoma (HCC) through a combination of whole exome sequencing (WES), RNA sequencing (RNA-seq), computational bioinformation, and immunohistochemistry. Our analysis reveals that patients carried with TP53 neoantigen have a longer overall survival than others (p = 0.0371) and they showed higher Immune score (p = 0.0441), higher cytotoxic lymphocytes infiltration (p = 0.0428), and higher CYT score (p = 0.0388). In contrast, the prognosis is not associated with TMB and neoantigen load. Our study draws a preliminary conclusion that it is not TMB or neoantigen load but the TP53 specific neoantigen is related to overall survival of HCC patients. We suggest that the TP53 neoantigen may affect prognosis by regulating anti-tumor immunity and that the TP53 neoantigen may be harnessed as potential targets for immunotherapies of HCC.
Electronic supplementary material
The online version of this article (10.1007/s00262-020-02711-8) contains supplementary material, which is available to authorized users.
Keywords: Hepatocellular carcinoma, TP53 neoantigen, Immune microenvironment, Immunotherapy
Introduction
Hepatocellular carcinoma (HCC), the major pathological type of liver cancer (comprising 75–85% of cases), is still a leading cause of cancer-related death worldwide [1] and with very limited therapeutic options. Particularly in advanced stage, long-term survival is uncommon [2, 3].
Since cancer survival is generally influenced by different prognostic factors such as cytogenetic abnormalities, immune cell infiltration, and stage [4–6], the identification of prognostic factors in HCC may lead to novel and more effective treatments.
Neoantigens are epitopes derived from abnormal proteins encoded by mutant cancer genomes and are considered important targets for cancer immunotherapy because of their immunogenicity and lack of expression in normal tissues. The epitopes are displayed by major histocompatibility complex (MHC) molecules on tumor cells to trigger the activation of cytotoxic T cells [7, 8]. Next generation sequencing technologies and computational analysis have recently made neoantigen discovery possible. Immune cells play a dual role in mediating the anti-tumor immune responses, which can either suppress cancer development by generating cytotoxic T cells or promote cancer through the expression of immunosuppressive factors [9]. Neoantigens have been strongly linked to tumor mutation burden (TMB) and the clinical benefit of checkpoint blockade therapy in melanoma, non-small cell lung cancer (NSCLC) and other cancer [10–13]. Furthermore, immunogenic personal vaccines based on neoantigens have shown dramatic efficacy in melanoma and glioblastoma, providing tumor-specific immune targets that enhance the efficacy of cancer immunotherapy [14, 15].
Despite recent studies implicating neoantigens in the treatment and prognosis of several cancer types [16–18], the relationships between tumor neoantigens, immune cells and prognosis in HCC have not been reported. Here we fill this gap by identify prognostic markers in long-term HCC survivors and evaluate the relationship of neoantigen load, unique neoantigens, immune cell infiltration and prognosis.
Materials and methods
Patient and sample characteristics
Twenty two HCC participants were recruited at Peking Union Medical College Hospital (PUMCH) between March 2019 and July 2019, with ages ranging from 29 to 77 years old (median age: 56 years). All pathology specimens (stages ranged from IIIA to IVA) were reviewed by experienced gastrointestinal pathologists. All patients had received standard therapy with surgery alone or surgery plus chemoradiotherapy. No patients had received immunotherapy treatment prior to surgery. All tumor tissues were surgically resected primary HCC and confirmed by pathological and histological re-review by two expert HCC pathologists before analysis. Informed consent was obtained from all participants. This study was approved by the Ethical Committee of PUMCH and was performed in accordance with the ethical standards of the World Medical Association Declaration of Helsinki.
Whole-exome sequencing and somatic mutation calling
Tumor and matched normal DNA were extracted using the GeneRead DNA FFPE Kit from formalin-fixed paraffin-embedded (FFPE) tissues. Libraries were constructed by the Agilent SureSelect v.4 Kit (Agilent) and sequenced with next-generation sequencing. Genomic DNA was fragmented, end-repaired, adenylated at the 3′ ends, end-connected, amplified, purified, and size-selected in the process of library construction, then was sequenced on the Illumina X10 platform (Illumina Inc., San Diego, CA, USA).
WES data underwent mutation analysis and human genome build hg19 was used as the reference genome. Somatic SNVs and InDels were analyzed via GATK MuTect2. The sequenced reads were realigned to the hg19 by Burrows–Wheeler Aligner BWA-MEM (https://biobwa.sourceforge.net/) to enhance valid SNVs.
RNA sequencing
Tumor RNA was extracted using the RNeasy FFPE Kit (Qiagen) from FFPE tissues. Libraries were constructed using a TruSeq RNA Exome kit and sequenced with NGS. Total RNA was fragmented, reverse transcribed into complementary DNA, base ‘A’ added in the 3′ ends, adapter connected, amplified and purified, and then sequenced on Illumina X10 platform (Illumina Inc., San Diego, CA, USA).
Clean reads were mapped to the hg19 human genome using Bowtie2 (version 2.2.4) software from Tophat2 (version 2.0.10) with default parameters. The program Cufflinks (version 2.2.1) was used to calculate the expression levels of genes in terms of reads per kilobase per million reads (FPKM).
Neoantigen predictions
HLA typing was acquired from normal DNA using OptiType (version 1.3.1). The cancer immunotherapy pipeline—pVACseq, was used to identify neoantigens [19].
Peptide-MHC affinity for half maximal inhibitory concentration (IC50) values were predicted using NetMHC/NetMHCIIpan or PickPocket. IC50 < 500 nM is considered an accepted standard in the field for predicting binders, and IC50 ≤ 150 nM is accepted as a strong binder [20].
Mutated peptides with a binding affinity of IC50 < 500 nM was regarded as candidate neoantigens, and mutated peptides (IC50 ≤ 150 nM) were regarded as strong-quality neoantigens [15, 18, 21]. Neoantigen expression was confirmed in any such neoantigens with RNA-Seq counts ≥ 1 [4, 21].
Assessment of immune infiltration and immune cytolytic activity
Immune score
Based on the gene expression (FPKM), an R package—ESTIMATE [22] (https://sourceforge.net/projects/estimateproject/), was used to estimate the fraction of stromal and immune cells in tumor samples. “Immune score” aimed to represent the infiltration of immune cells in tumor tissue.
MCP-counter score
An MCP-counter [23] method (https://github.com/ebecht/MCPcounter) was used to infer the absolute intratumor cell abundance of ten cell types (eight immune and two stromal cell populations) in each tumor tissue.
CYT score
Based on the notion that effective natural anti-tumor immunity requires a cytolytic immune response, we quantified immune cytolytic activity using an expression metric of effector molecules that mediate cytolysis. Immune cytolytic activity (CYT score) was calculated as the geometric mean of GZMA and PRF1 (as expressed in TPM) [24].
Immunohistochemistry
Antibodies against CD3, CD8, CD45RO, and FOXP3 were purchased from Abcam. Immunohistochemistry (IHC) was performed according to the manufacturer’s instructions. Four 5-μm sections were cut from each case. After dewaxing, slides were boiled with 1 mM EDTA pH 8.0 followed by 15 min at a sub-boiling temperature. Slides were washed with phosphate-buffered saline at three times for 5 min each, subsequently quenched in 3% hydrogen peroxide for 15 min, and then blocked with 10% goat serum for 10 min. Slides were incubated overnight at 4 °C with the primary antibody diluent (1:100 anti-CD3 antibody, ab16669. 1:100 anti-CD8 antibody, ab4055. 1:150 anti-CD45RO antibody, ab23 and 1:200 anti-FOXP3 antibody, ab20034). The slides were then incubated with biotinylated secondary antibody per the manufacturer’s recommendation for 30 min. Antibody binding was visualized with DAB.
Statistical analysis
Statistical analysis was performed using SPSS 21.0 software (IBM Corporation, New York). The distribution test was analyzed by Shapiro–Wilk test. Parametric data were calculated by t test and one-way analysis of variance. The Mann–Whitney test and Kruskal–Wallis test were used for non-parametric data. Survival was summarized by a Kaplan–Meier survival curve. The probability value was calculated by two-sided tests. p < 0.0500 was considered statistically significant. Figures were drawn using GraphPad Prism 8.0 software (San Diego, USA).
Results
Patient characteristics
A total of 22 patients with HCC were enrolled in the study, and their clinicopathological characteristics were summarized in supplementary Table 1. The median age of the included patients was 56 (IQR: 53–64) years, and 86.4% were males. No patients received pre-operative chemotherapy. 72.7% of patients had cirrhosis. The Child–Pugh score of 45.5% patients ws A, and that of 54.5% was B. Overall, 36.4% of patients were at TNM stage II, 50.0% were at stage IIIA, 9.1% were at stage IIIB, and 4.5% were at stage IIIC. The Barcelona Clinic Liver Cancer (BCLC) score of 4.5% of patients were 0, 18.2% were A1, 4.5% were A2, 9.1% were A4, 50.0% were B, and 13.6% were C. 9.1% of patients underwent hepatic segmentectomy, 72.7% underwent combined segmentectomy, and 13.6% underwent cholecystectomy. Further, 63.6% of the patients received radical resection. 27.3% of patients received no post-operative therapy, 68.1% received simple interventional therapy, and 4.5% received interventional therapy and targeted drug.
The median OS was 51.00 (IQR: 36.50–61.75) months. 63.6% of patients at last follow-up remained alive. A total of 60.0% of patients reported recurrence.
Overall survival comparison and gene mutation landscape in HCC patients
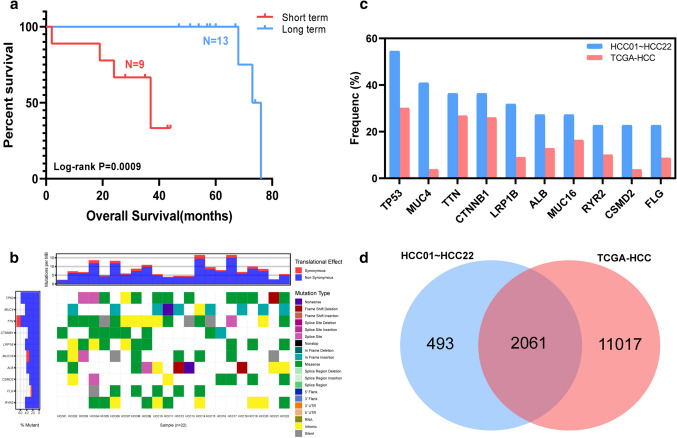
A cohort of 22 HCC patients (median OS, 51 months) undergoing treatment at Peking Union Medical College Hospital (PUMCH, Beijing, China) was analyzed for this study. Long-term survivors (n = 13, median OS, 58 months) and short-term survivors (n = 9, median OS, 35 months) group had a significant difference on survival with p = 0.0009 (Fig. 1a).
Fig. 1.
Overall survival comparison and gene mutation landscape in HCC patients. a Overall survival of 22 HCC cohort patients. b Gene mutation landscape of 22 HCC cohort patients. c Mutation frequency comparison between TCGA-HCC and top 10 mutated genes in HCC01 ~ HCC22. d Mutated gene comparison between HCC01 ~ HCC22 and TCGA-HCC
We performed exome sequencing on genomic DNA of 22 HCC tumor and matched normal tissue. After filtering the exome sequencing data, we observed a mean of 124 somatic mutations per tumor (range 40–300). In the exome sequencing data analysis, we focused on genes that were mutated in at least two patients. This resulted in a list of 341 genes (supplementary Table 2) and most of these genes (272/341, 79.77%) were mutated in two tumors. Top 10 mutated genes of HCC were TP53, MUC4, TTN, CTNNB1, LRP1B, MUC16, ALB, CSMD2, FLG and RYR2 (Fig. 1b). The most frequently mutated gene was TP53, which was mutated in 12 tumors (12/22, 54.55%).
We compared our mutated genes with HCC patients in TCGA database and found 2061 genes were in common (Fig. 1d). Our top 10 mutated genes were all included in TCGA-HCC and had a higher mutation frequency (Fig. 1c), which may be caused by our limited patients.
Immune infiltration comparison between long term and short term
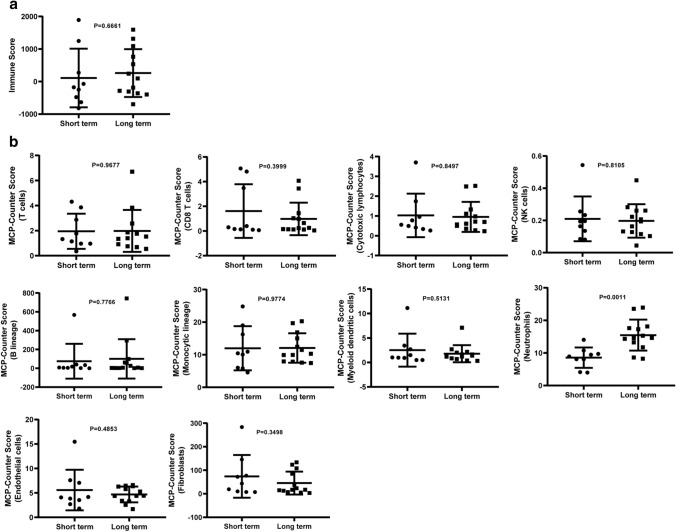
Tumors underwent RNA-seq and normalized data were analyzed to quantify immune infiltration using Immune score and MCP-Counter score. We compared the immune infiltration observed in two groups. Long-term patients had a significant higher neutrophils infiltration than Short term, p = 0.0011 (Fig. 2b). Neutrophils infiltrating tumor tissues play functions in the anti-tumor immune responses and restrict cancer growth by expressing anti-tumor and cytotoxic mediators [25–27]. Immune score and other cells’ infiltrated MCP-Counter score showed no statistically significant difference (Fig. 2a, b).
Fig. 2.
Immune infiltration difference with Long term and Short term. a Immune score comparison between Long term and Short term. b 10 kinds of cells’ infiltrated MCP-Counter score comparison between Long term and Short term
The effect of TMB, neoantigen load on patient survival
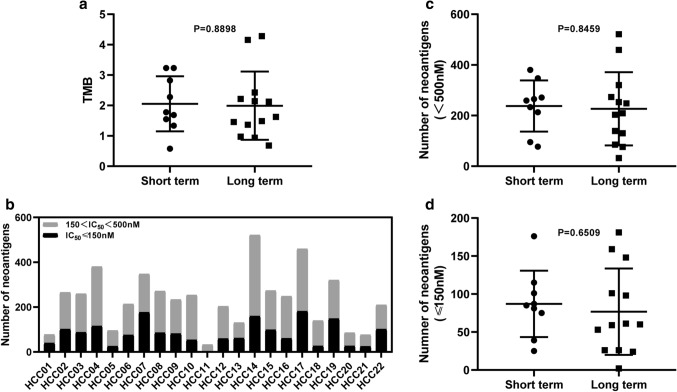
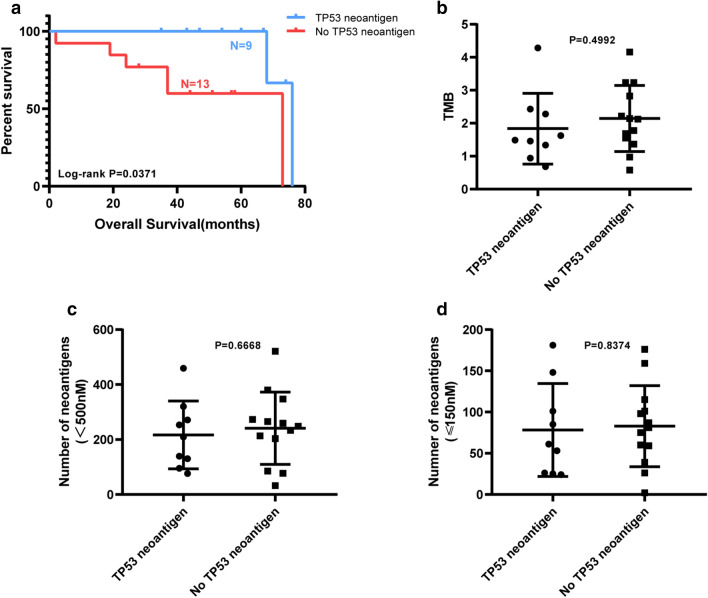
Several reports have shown that TMB and neoantigen load were associated with prognosis in lung cancer and melanoma [28–30]. To investigate whether the same was true for HCC patients, we analyzed the relationship between TMB, neoantigen load and prognosis. TMB ranged from 0.58 to 4.28 (median = 1.73) in these patients. The total neoantigen load with IC50 < 500 nM ranged from 32 to 521 (median = 241). High-quality neoantigen load with IC50 < 150 nM ranged from 2 to 181 (median = 78).
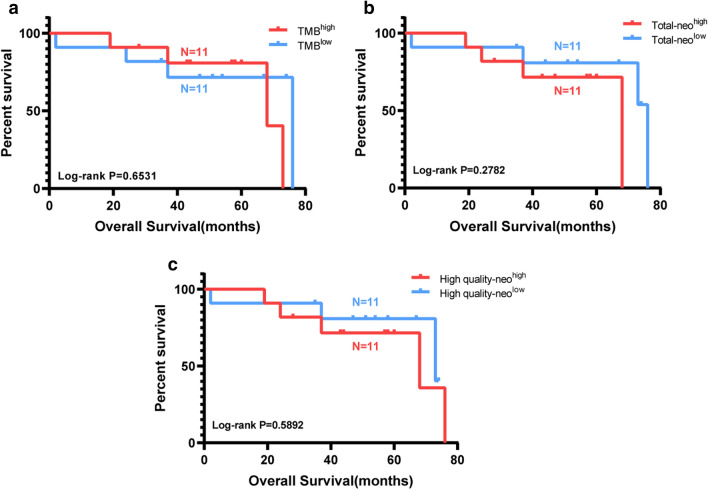
First, TMB and neoantigen load were compared based on Long term and Short term. But there was no significant difference (Fig. 3a–d). Then all the patients were divided into two groups separately based on the median TMB and neoantigen load. There was no significant correlation between TMB, neoantigen load, and survival in HCC patients (Fig. 4a–c). It means that TMB and neoantigen load may have no contribution to survival for HCC patients in our project.
Fig. 3.
TMB, neoantigen load comparison between Long term and Short term. a TMB comparison between Long term and Short term, p = 0.8898. b Neoantigen number per patient in HCC01 ~ HCC22. c, d Neoantigen load comparison between Long term and Short term. p = 0.8459 for total neoantigen load and p = 0.6509 for high-quality neoantigen load with IC50 < 150 nM
Fig. 4.
Correlation between TMB, neoantigen load and prognosis. a TMB and prognosis, p = 0.6531. b Total neoantigen load and prognosis, p = 0.2782. c High-quality neoantigen load (IC50 < 150 nM) and prognosis, p = 0.5892
TP53 neoantigen predicts better prognosis in HCC patients
Some neoantigen-specific immunity gained during tumor outgrowth could be associated with decreased relapse and prolonged survival, like MUC16 neoantigen in long-term survivors of pancreatic cancer [18].
To identify neoantigens that can be used to treat or predict prognosis in HCC, we analyzed and found that patients who carried with TP53 neoantigen had a significant longer overall survival than those who did not carry TP53 neoantigen, p = 0.0371 (Fig. 5a). However, they showed no statistically significant difference on TMB and neoantigen load (Fig. 5b–d).
Fig. 5.
OS, TMB and neoantigen load comparison between patients with the TP53 neoantigen and without TP53 neoantigen. a Overall survival comparison. b TMB load comparison. c, d Neoantigen load comparison. ‘TP53 neoantigen’ group (n = 9) include patients carrying TP53 neoantigen, and ‘No TP53 neoantigen’ group (n = 13) include patients without TP53 neoantigen
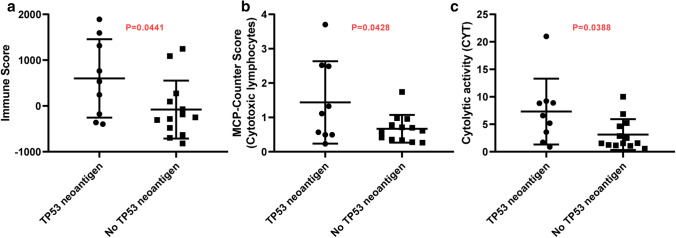
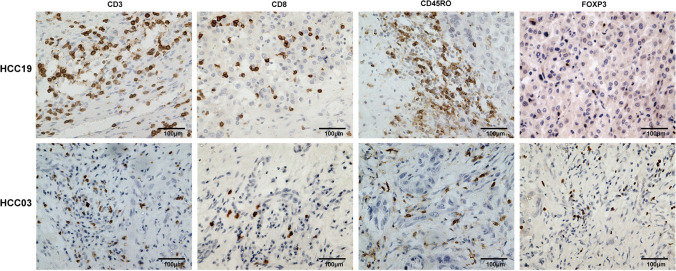
Besides, immune infiltration and immune cytolytic activity (CYT score) were estimated between ‘TP53 neoantigen’ group and ‘No TP53 neoantigen’ group. ‘TP53 neoantigen’ group had a significantly higher Immune score (Fig. 6a, p = 0.0441), higher cytotoxic lymphocytes infiltration (Fig. 6b, p = 0.0428), and higher CYT score (Fig. 6c, p = 0.0388). The immunohistochemistry results also confirmed that patients in ‘TP53 neoantigen’ group had more immune cell infiltration (Fig. 7).
Fig. 6.
Immune infiltration and cytolytic activity difference between patients with the TP53 neoantigen and without TP53 neoantigen. a Immune score comparison. b MCP-Counter score of cytotoxic lymphocytes comparison. c CYT score comparison
Fig. 7.
Immunohistochemistry results between patients with the TP53 neoantigen (HCC19) and without TP53 neoantigen (HCC03)
Discussion
To the best of our knowledge, this is the first study that identifies a prognostic marker for neoantigens in long-term HCC survivors and evaluates the relationship of neoantigen load, unique neoantigens, the infiltration of immune cells, and prognosis.
In this study, we predicted neoantigens by WES, RNA-Seq, and HLA affinity in 22 HCC patients and found that OS is not associated with TMB, neoantigen load. We discovered that unique TP53 neoantigen is associated with better prognosis, higher cytotoxic lymphocyte infiltration as well as immune cytolytic activity (CYT score). These results suggest that the TP53 neoantigen could affect prognosis by regulating anti-tumor immune responses among HCC patients.
There are discrepancies regarding the relationship between TMB, neoantigen load, and prognosis in various cancers. Several reports have shown that high TMB and neoantigen load are associated with poor prognosis in NSCLC, head and neck squamous cell carcinoma, adenoid cystic carcinoma, liver hepatocellular carcinoma, and myeloma [4, 31, 32]. On the other hand, other studies reported no relationship between TMB or neoantigen load and prognosis in clear cell renal cell carcinoma [28], which is more in line with our current study of HCC. The exact reasons for such discrepancies require future investigations but may be potentially due to different cancer types, treatment history, and patient sample sizes involved in the different studies.
Cytotoxic lymphocytes (including CD8 + cytotoxic lymphocytes, natural killer-NK cells and lymphokine-activated killer cells, etc.) depend primarily on the perforin or granzyme system to kill their targets [33, 34]. Cytotoxic T cells (CTLs) and NK cells release perforin 1 (PRF1), granzymes, and granulysin, upon their expose to infected or dysfunctional somatic cells. These cytotoxins can ultimately lead target cells to apoptosis by trigger a caspase cascade or cell–surface interaction between the CTL and the infected cell [35–37]. The expression of the toxins granzyme A (GZMA) and perforin 1 (PRF1), secreted by effector cytotoxic T cells and natural killer (NK) cells, was recently used as a denominator of the intratumoral immune cytolytic activity (CYT). These levels are significantly elevated upon CD8 + T cell activation as well as during a productive clinical response against immune-checkpoint blockade therapies [38, 39]. Another study has proposed that intrinsic tumor factors, such as mutated neoantigens, can induce local immune infiltrates that include cytolytic effector cells (expressing GZMA/PRF1) that kill tumors [24].
Our study draws a preliminary conclusion that it is not TMB or neoantigen load, but the TP53 specific neoantigen is related to overall survival of HCC patients. Patients carried TP53 neoantigen could activate immune system and trigger higher immune infiltration to lead tumor apoptosis, which could prolong patients’ life. Higher T cell infiltration was observed in TP53 neoantigen group based on the IHC and sequencing data. However, T cell did not correlate with HCC long-term prognosis, which indicates that TP53 may have other effect than the T cell infiltration. Interestingly, in previous study, Cooks et. al [40] found that TP53-mutated colon cancer cells could shed exosomes to alter tumor status with reprograming macrophages. More research is needed to understand the potential mechanisms of TP53 neoantigen.
Because our study population showed heterogeneity in patient clinicopathological characteristics and management, we evaluated the impact of these factors on patients’ survival, TP53, immune score, and CD8 T cell count. No significant correlation has been revealed under the analysis, which implies that management and patients’ status are not related to survival time, neoantigens,and immune microenvironments (supplementary Table 3–6). However, the small patients’ number limited the applicability of our study, and future researches focused on TP53 could broaden the database and further confirmed the clinical impact of TP53.
In conclusion, we propose that TP53 neoantigen ould be used as a biomarker to predict prognosis or provide a novel choice for the treatment in HCC patients someday in the future.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank all the members in the Department of Medical Oncology, Liver Surgery and Pathology of Peking Union Medical College Hospital for their help and valuable opinions.
Abbreviations
- CTLs
Cytotoxic T cells
- CYT
Cytolytic activity
- FFPE
Formalin-fixed paraffin-embedded
- GZMA
Granzyme A
- HCC
Hepatocellular carcinoma
- IHC
Immunohistochemistry
- MHC
Histocompatibility complex
- NSCLC
Non-small cell lung cancer
- PRF 1
Perforin 1
- PUMCH
Peking Union Medical College Hospital
- TMB
Tumor mutation burden
- WES
Whole-exome sequencing
Author contributions
Conceptualization, HY, LS and YM; methodology, HY, ML, HX, HZ, XL, XS, SZ, XM; software, LS, QC; validation, YM; formal analysis, LS, QC; writing—original draft preparation, HY, LS; writing—review and editing, AG; visualization, XM; supervision, YM.
Funding
This study was supported by Grants from the Chinese Academy of Medical Sciences (CAMS) Initiative for Innovative Medicine (No. 2017-I2M-4-002; No. 2016-I2M-1-001), PUMC Youth Fund (No. 2017320001) and National Natural Science Foundation of China (No. 81472785; No. 61435001).
Compliance with ethical standards
Conflict of interest
The authors have declared no conflicts of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Ethical Committee of PUMCH (S-K908) and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Huayu Yang and Lejia Sun contributed equally to this article.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.European Association For The Study Of The L, European Organisation For R, Treatment Of C EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56(4):908–943. doi: 10.1016/j.jhep.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 3.Wen T, Jin C, Facciorusso A, Donadon M, Han HS, Mao Y, Dai C, Cheng S, Zhang B, Peng B, Du S, Jia C, Xu F, Shi J, Sun J, Zhu P, Nara S, Millis JM, Hospital* MDToWC Multidisciplinary management of recurrent and metastatic hepatocellular carcinoma after resection: an international expert consensus. Hepatobiliary Surg Nutr. 2018;7(5):353–371. doi: 10.21037/hbsn.2018.08.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miller A, Asmann Y, Cattaneo L, Braggio E, Keats J, Auclair D, Lonial S, Network MC, Russell SJ, Stewart AK. High somatic mutation and neoantigen burden are correlated with decreased progression-free survival in multiple myeloma. Blood Cancer J. 2017;7(9):e612. doi: 10.1038/bcj.2017.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li ZL, Han J, Liu K, Xing H, Wu H, Lau WY, Pawlik TM, Li C, Wang MD, Yu JJ, Wu MC, Shen F, Yang T. Association of family history with long-term prognosis in patients undergoing liver resection of HBV-related hepatocellular carcinoma. Hepatobiliary Surg Nutr. 2019;8(2):88–100. doi: 10.21037/hbsn.2018.11.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Erridge S, Sodergren MH. The Chengdu system for recurrent hepatocellular carcinoma: a step in the right direction. Hepatobiliary Surg Nutr. 2019;8(3):298–300. doi: 10.21037/hbsn.2019.01.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lu YC, Robbins PF. Cancer immunotherapy targeting neoantigens. Semin Immunol. 2016;28(1):22–27. doi: 10.1016/j.smim.2015.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science. 2015;348(6230):69–74. doi: 10.1126/science.aaa4971. [DOI] [PubMed] [Google Scholar]
- 9.Klink M. Interaction of immune and cancer cells. Anticancer Res. 2014;34(6):203–206. [Google Scholar]
- 10.Li L, Goedegebuure SP, Gillanders WE. Preclinical and clinical development of neoantigen vaccines. Ann Oncol. 2017;28(suppl_12):xii11–xii17. doi: 10.1093/annonc/mdx681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Snyder A, Makarov V, Merghoub T, Yuan J, Zaretsky JM, Desrichard A, Walsh LA, Postow MA, Wong P, Ho TS, Hollmann TJ, Bruggeman C, Kannan K, Li Y, Elipenahli C, Liu C, Harbison CT, Wang L, Ribas A, Wolchok JD, Chan TA. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med. 2014;371(23):2189–2199. doi: 10.1056/NEJMoa1406498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van Allen EM, Miao D, Schilling B, Shukla SA, Blank C, Zimmer L. Genomic correlates of response to CTLA-4 blockade in metastatic melanoma. Science. 2015;350(6257):207–211. doi: 10.1126/science.aad0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, Skora AD, Luber BS, Azad NS, Laheru D, Biedrzycki B, Donehower RC, Zaheer A, Fisher GA, Crocenzi TS, Lee JJ, Duffy SM, Goldberg RM, de la Chapelle A, Koshiji M, Bhaijee F, Huebner T, Hruban RH, Wood LD, Cuka N, Pardoll DM, Papadopoulos N, Kinzler KW, Zhou S, Cornish TC, Taube JM, Anders RA, Eshleman JR, Vogelstein B, Diaz LA., Jr PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372(26):2509–2520. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sahin U, Derhovanessian E, Miller M, Kloke BP, Simon P, Lower M, Bukur V, Tadmor AD, Luxemburger U, Schrors B, Omokoko T, Vormehr M, Albrecht C, Paruzynski A, Kuhn AN, Buck J, Heesch S, Schreeb KH, Muller F, Ortseifer I, Vogler I, Godehardt E, Attig S, Rae R, Breitkreuz A, Tolliver C, Suchan M, Martic G, Hohberger A, Sorn P, Diekmann J, Ciesla J, Waksmann O, Bruck AK, Witt M, Zillgen M, Rothermel A, Kasemann B, Langer D, Bolte S, Diken M, Kreiter S, Nemecek R, Gebhardt C, Grabbe S, Holler C, Utikal J, Huber C, Loquai C, Tureci O. Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature. 2017;547(7662):222–226. doi: 10.1038/nature23003. [DOI] [PubMed] [Google Scholar]
- 15.Keskin DB, Anandappa AJ, Sun J, Tirosh I, Mathewson ND, Li S, Oliveira G, Giobbie-Hurder A, Felt K, Gjini E, Shukla SA, Hu Z, Li L, Le PM, Allesoe RL, Richman AR, Kowalczyk MS, Abdelrahman S, Geduldig JE, Charbonneau S, Pelton K, Iorgulescu JB, Elagina L, Zhang W, Olive O, McCluskey C, Olsen LR, Stevens J, Lane WJ, Salazar AM, Daley H, Wen PY, Chiocca EA, Harden M, Lennon NJ, Gabriel S, Getz G, Lander ES, Regev A, Ritz J, Neuberg D, Rodig SJ, Ligon KL, Suva ML, Wucherpfennig KW, Hacohen N, Fritsch EF, Livak KJ, Ott PA, Wu CJ, Reardon DA. Neoantigen vaccine generates intratumoral T cell responses in phase Ib glioblastoma trial. Nature. 2019;565(7738):234–239. doi: 10.1038/s41586-018-0792-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hilf N, Kuttruff-Coqui S, Frenzel K, Bukur V, Stevanovic S, Gouttefangeas C, Platten M, Tabatabai G, Dutoit V, van der Burg SH, Thor Straten P, Martinez-Ricarte F, Ponsati B, Okada H, Lassen U, Admon A, Ottensmeier CH, Ulges A, Kreiter S, von Deimling A, Skardelly M, Migliorini D, Kroep JR, Idorn M, Rodon J, Piro J, Poulsen HS, Shraibman B, McCann K, Mendrzyk R, Lower M, Stieglbauer M, Britten CM, Capper D, Welters MJP, Sahuquillo J, Kiesel K, Derhovanessian E, Rusch E, Bunse L, Song C, Heesch S, Wagner C, Kemmer-Bruck A, Ludwig J, Castle JC, Schoor O, Tadmor AD, Green E, Fritsche J, Meyer M, Pawlowski N, Dorner S, Hoffgaard F, Rossler B, Maurer D, Weinschenk T, Reinhardt C, Huber C, Rammensee HG, Singh-Jasuja H, Sahin U, Dietrich PY, Wick W. Actively personalized vaccination trial for newly diagnosed glioblastoma. Nature. 2019;565(7738):240–245. doi: 10.1038/s41586-018-0810-y. [DOI] [PubMed] [Google Scholar]
- 17.Ott PA, Hu Z, Keskin DB, Shukla SA, Sun J, Bozym DJ, Zhang W, Luoma A, Giobbie-Hurder A, Peter L, Chen C, Olive O, Carter TA, Li S, Lieb DJ, Eisenhaure T, Gjini E, Stevens J, Lane WJ, Javeri I, Nellaiappan K, Salazar AM, Daley H, Seaman M, Buchbinder EI, Yoon CH, Harden M, Lennon N, Gabriel S, Rodig SJ, Barouch DH, Aster JC, Getz G, Wucherpfennig K, Neuberg D, Ritz J, Lander ES, Fritsch EF, Hacohen N, Wu CJ. An immunogenic personal neoantigen vaccine for patients with melanoma. Nature. 2017;547(7662):217–221. doi: 10.1038/nature22991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Balachandran VP, Luksza M, Zhao JN, Makarov V, Moral JA, Remark R, Herbst B, Askan G, Bhanot U, Senbabaoglu Y, Wells DK, Cary CIO, Grbovic-Huezo O, Attiyeh M, Medina B, Zhang J, Loo J, Saglimbeni J, Abu-Akeel M, Zappasodi R, Riaz N, Smoragiewicz M, Kelley ZL, Basturk O, Australian Pancreatic Cancer Genome I, Garvan Institute of Medical R, Prince of Wales H, Royal North Shore H, University of G, St Vincent's H, Institute QBMR, University of Melbourne CfCR, University of Queensland IfMB. Bankstown H, Liverpool H, Royal Prince Alfred Hospital COBL. Westmead H, Fremantle H, St John of God H, Royal Adelaide H, Flinders Medical C. Envoi P, Princess Alexandria H, Austin H, Johns Hopkins Medical I, Cancer AR-NCfARo. Gonen M, Levine AJ, Allen PJ, Fearon DT, Merad M, Gnjatic S, Iacobuzio-Donahue CA, Wolchok JD, DeMatteo RP, Chan TA, Greenbaum BD, Merghoub T, Leach SD. Identification of unique neoantigen qualities in long-term survivors of pancreatic cancer. Nature. 2017;551(7681):512–516. doi: 10.1038/nature24462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hundal J, Carreno BM, Petti AA, Linette GP, Griffith OL, Mardis ER, Griffith M. pVAC-Seq: a genome-guided in silico approach to identifying tumor neoantigens. Genome Med. 2016;8(1):11. doi: 10.1186/s13073-016-0264-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen F, Zou Z, Du J, Su S, Shao J, Meng F, Yang J, Xu Q, Ding N, Yang Y, Liu Q, Wang Q, Sun Z, Zhou S, Du S, Wei J, Liu B. Neoantigen identification strategies enable personalized immunotherapy in refractory solid tumors. J Clin Invest. 2019;129(5):2056–2070. doi: 10.1172/JCI99538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bulik-Sullivan B, Busby J, Palmer CD, Davis MJ, Murphy T, Clark A, Busby M, Duke F, Yang A, Young L, Ojo NC, Caldwell K, Abhyankar J, Boucher T, Hart MG, Makarov V, Montpreville VT, Mercier O, Chan TA, Scagliotti G, Bironzo P, Novello S, Karachaliou N, Rosell R, Anderson I, Gabrail N, Hrom J, Limvarapuss C, Choquette K, Spira A, Rousseau R, Voong C, Rizvi NA, Fadel E, Frattini M, Jooss K, Skoberne M, Francis J, Yelensky R. Deep learning using tumor HLA peptide mass spectrometry datasets improves neoantigen identification. Nat Biotechnol. 2018 doi: 10.1038/nbt.4313. [DOI] [PubMed] [Google Scholar]
- 22.Yoshihara K, Shahmoradgoli M, Martinez E, Vegesna R, Kim H, Torres-Garcia W, Trevino V, Shen H, Laird PW, Levine DA, Carter SL, Getz G, Stemke-Hale K, Mills GB, Verhaak RG. Inferring tumour purity and stromal and immune cell admixture from expression data. Nat Commun. 2013;4:2612. doi: 10.1038/ncomms3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Becht E, Giraldo NA, Lacroix L, Buttard B, Elarouci N, Petitprez F, Selves J, Laurent-Puig P, Sautes-Fridman C, Fridman WH, de Reynies A. Estimating the population abundance of tissue-infiltrating immune and stromal cell populations using gene expression. Genome Biol. 2016;17(1):218. doi: 10.1186/s13059-016-1070-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rooney MS, Shukla SA, Wu CJ, Getz G, Hacohen N. Molecular and genetic properties of tumors associated with local immune cytolytic activity. Cell. 2015;160(1–2):48–61. doi: 10.1016/j.cell.2014.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim Y, Lee D, Lee J, Lee S, Lawler S. Role of tumor-associated neutrophils in regulation of tumor growth in lung cancer development: A mathematical model. PLoS ONE. 2019;14(1):e0211041. doi: 10.1371/journal.pone.0211041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saha S, Biswas SK. Tumor-associated neutrophils show phenotypic and functional divergence in human lung cancer. Cancer Cell. 2016;30(1):11–13. doi: 10.1016/j.ccell.2016.06.016. [DOI] [PubMed] [Google Scholar]
- 27.Gregory AD, Houghton AM. Tumor-associated neutrophils: new targets for cancer therapy. Cancer Res. 2011;71(7):2411–2416. doi: 10.1158/0008-5472.CAN-10-2583. [DOI] [PubMed] [Google Scholar]
- 28.Matsushita H, Sato Y, Karasaki T, Nakagawa T, Kume H, Ogawa S, Homma Y, Kakimi K. Neoantigen load, antigen presentation machinery, and immune signatures determine prognosis in clear cell renal cell carcinoma. Cancer Immunol Res. 2016;4(5):463–471. doi: 10.1158/2326-6066.CIR-15-0225. [DOI] [PubMed] [Google Scholar]
- 29.Goodman AM, Kato S, Bazhenova L, Patel SP, Frampton GM, Miller V, Stephens PJ, Daniels GA, Kurzrock R. Tumor mutational burden as an independent predictor of response to immunotherapy in diverse cancers. Mol Cancer Ther. 2017;16(11):2598–2608. doi: 10.1158/1535-7163.MCT-17-0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Devarakonda S, Rotolo F, Tsao MS, Lanc I, Brambilla E, Masood A. Tumor mutation burden as a biomarker in resected non-small-cell lung cancer. Clin Oncol J. 2018 doi: 10.1200/JCO10.1200/JCO.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Owada-Ozaki Y, Muto S, Takagi H, Inoue T, Watanabe Y, Fukuhara M, Yamaura T, Okabe N, Matsumura Y, Hasegawa T, Ohsugi J, Hoshino M, Shio Y, Nanamiya H, Imai JI, Isogai T, Watanabe S, Suzuki H. Prognostic impact of tumor mutation burden in patients with completely resected non-small cell lung cancer: brief report. J Thorac Oncol. 2018;13(8):1217–1221. doi: 10.1016/j.jtho.2018.04.003. [DOI] [PubMed] [Google Scholar]
- 32.Wang X, Li M. Correlate tumor mutation burden with immune signatures in human cancers. BMC Immunol. 2019;20(1):4. doi: 10.1186/s12865-018-0285-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shresta S, Pham CT, Thomas DA, Graubert TA, Ley TJ. How do cytotoxic lymphocytes kill their targets? Curr Opin Immunol. 1998;10(5):581–587. doi: 10.1016/s0952-7915(98)80227-6. [DOI] [PubMed] [Google Scholar]
- 34.Heusel JW, Wesselschmidt RL, Shresta S. Cytotoxic lymphocytes require granzyme B for the rapid induction of DNA fragmentation and apoptosis in allogeneic target cells. Cell. 1994;76(6):977–987. doi: 10.1016/0092-8674(94)90376-X. [DOI] [PubMed] [Google Scholar]
- 35.Peters PJ, Borst J, Oorschot V. Cytotoxic T lymphocyte granules are secretory lysosomes, containing both perforin and granzymes. J Exp Med. 1991;173(5):1099–1109. doi: 10.1084/jem.173.5.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Trapani JA, Smyth MJ. Functional significance of the perforin/granzyme cell death pathway. Nat Rev Immunol. 2002;2(10):735–747. doi: 10.1038/nri911. [DOI] [PubMed] [Google Scholar]
- 37.Suda T. Molecular cloning and expression of the Fas ligand, a novel member of the tumor necrosis factor family. Cell. 1993;75(6):1169–1178. doi: 10.1016/0092-8674(93)90326-L. [DOI] [PubMed] [Google Scholar]
- 38.Duraiswamy J, Ibegbu CC, Masopust D, Miller JD, Araki K, Doho GH, Tata P, Gupta S, Zilliox MJ, Nakaya HI, Pulendran B, Haining WN, Freeman GJ, Ahmed R. Phenotype, function, and gene expression profiles of programmed death-1(hi) CD8 T cells in healthy human adults. J Immunol. 2011;186(7):4200–4212. doi: 10.4049/jimmunol.1001783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parish IA, Rao S, Smyth GK, Juelich T, Denyer GS, Davey GM, Strasser A, Heath WR. The molecular signature of CD8+ T cells undergoing deletional tolerance. Blood. 2009;113(19):4575–4585. doi: 10.1182/blood-2008-10-185223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cooks T, Pateras IS, Jenkins LM, Patel KM, Robles AI, Morris J, Forshew T, Appella E, Gorgoulis VG, Harris CC. Mutant p53 cancers reprogram macrophages to tumor supporting macrophages via exosomal miR-1246. Nat Commun. 2018;9(1):771. doi: 10.1038/s41467-018-03224-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.