Abstract
Acute megakaryocytic leukemia (AMKL) is one of the rarest sub-types of acute myeloid leukemia (AML). AMKL is characterized by high proliferation of megakaryoblasts and myelofibrosis of bone marrow, this disease is also associated with poor prognosis. Previous analyses have reported that the human megakaryoblastic cells can be differentiated into cells with megakaryocyte (MK)-like characteristics by phorbol 12-myristate 13-acetate (PMA). However, little is known about the mechanism responsible for regulating this differentiation process. We performed long non-coding RNA (lncRNA) profiling to investigate the differently expressed lncRNAs in megakaryocyte blast cells treated with and without PMA and examined those that may be responsible for the PMA-induced differentiation of megakaryoblasts into MKs. We found 30 out of 90 lncRNA signatures to be differentially expressed after PMA treatment of megakaryoblast cells, including the highly expressed JPX lncRNA. Further, in silico lncRNA-miRNA and miRNA-mRNA interaction analysis revealed that the JPX is likely involved in unblocking the expression of TGF-β receptor (TGF-βR) by sponging oncogenic miRNAs (miR-9-5p, miR-17-5p, and miR-106-5p) during MK differentiation. Further, we report the activation of TGF-βR-induced non-canonical ERK1/2 and PI3K/AKT pathways during PMA-induced MK differentiation and ploidy development. The present study demonstrates that TGF-βR-induced non-canonical ERK1/2 and PI3K/AKT pathways are associated with PMA-induced MK differentiation and ploidy development; in this molecular mechanism, JPX lncRNA could act as a decoy for miR-9-5p, miR-17-5p, and miR-106-5p, titrating them away from TGF-βR mRNAs. Importantly, this study reveals the activation of ERK1/2 and PI3K/AKT pathway in PMA-induced Dami cell differentiation into MK. The identified differentially expressed lncRNA signatures may facilitate further study of the detailed molecular mechanisms associated with MK development. Thus, our data provide numerous targets with therapeutic potential for the modulation of the differentiation of megakaryoblastic cells in AMKL.
Keywords: Megakaryocyte, Leukemia, PMA, LncRNA, miRNA, TGF-βR, ERK1/2, PI3K, AKT
Introduction
Leukemia is a metastasizing blood cell disease characterized by abnormal proliferation, apoptosis repression, and differentiation blockage in hematopoietic stem/progenitor cells [1–3]. Acute megakaryocytic leukemia (AMKL) is one of the rarest sub-type of acute myeloid leukemia (AML). AMKL is characterized by abnormal megakaryoblasts with high cell proliferation and extensive myelofibrosis [3]. This disease is rare in adult population, only 1% of all AML patients, but this disease is more common in children, comprises between 4-15% of all AML cases [4]. AMKL is associated with poor prognosis, diagnosis and clinical management are also the challenges which are associated with AMKL.
One approach for the treatment of different leukemia cases is chemical-induced differentiation of leukemia blasts, this approach is also referred to as differentiation therapy [5]. Retinoic acid (RA) is one of the examples of the successful differentiation therapy for acute promyelocytic leukemia. In recent studies, different transcriptions factors, cell cycle regulators, and non-coding RNAs have been recognized with aberrant expression in leukemia blasts, the aberrant expression of these components is most likely involved in leukemogenesis via blocking leukemia blasts differentiation [6].
The treatment of megakaryoblastic leukemia cells with phorbol myristate acetate (PMA) can overcome blocks in differentiation and terminal maturation of MKs. In previous studies, it has been observed that upon PMA treatment Dami [7] and K562 [8] cells result in growth arrest and differentiation into adherent megakaryocytic phenotype.
Understanding the dynamics of gene expression profiles during PMA-induced MK differentiation will allow us to identify novel targets with therapeutic potential for the clinical management of megakaryocytic leukemia. In different studies, the transcriptional profiling has been identified to be very useful to determine the differential gene expression patterns among samples of different lineages. Recent reports have shown non-coding RNAs (ncRNAs) such as miRNAs and long ncRNAs (lncRNAs) with functional role in hematopoiesis and leukemia [9]. LncRNAs are defined as non-protein coding RNA transcripts of > 200 nucleotides in length. LncRNAs participate in multiple regulatory functions at transcriptional and post-transcription levels by interacting with mRNA, miRNAs and proteins [9–12]. Notably, several lncRNAs have been identified with clinical potential in the diagnosis, prognosis, and treatment of leukemia [13]. However, there are only few studies which have identified and characterized the lncRNA expression profiles in myeloid leukemia cells [14–16], also the expression of lncRNAs has not been explored in megakaryoblastic leukemia cells.
The aim of this study was to gain an understanding on the lncRNA signatures and associated molecular mechanisms involved in PMA-induced megakaryocytic differentiation of megakaryoblastic Dami cells and establish an lncRNA signature-based foundation for future molecular studies of megakaryoblasts.
Materials and methods
Cell culture
Dami cells (Megakaryoblastic cells) were used to investigate the megakaryocytic effects of PMA. Cells were cultured in RPMI-1640 media (Gibco, Thermo Fisher) supplemented with 10% fetal bovine serum (FBS; Gibco) and 1% antibiotic–antimycotic (Sigma Aldrich) at 37 °C in 5% CO2. To investigate the effects of PMA on cell growth and differentiation, cells were treated with 100 nM concentration of PMA (Sigma–Aldrich) followed by quantification of cells by hemocytometer using trypan blue stain (Mediatech).
Human-induced pluripotent stem cells (hiPSCs)-derived CD34+ hematopoietic progenitor cells (HPCs) were attained from inStem CSCR, Vellore, India. For MKs differentiation, HPCs were cultured in MK differentiation media: StemPro-34TM basal media supplemented with PenStrep (1X), L-Glutamine (2 mM), FGF2 (10 ng/ml), BMP4 (10 ng/ml), SCF (20 ng/ml), VEGF-2 (10 ng/ml), TPO (50 ng/ml), IL-11 (10 ng/ml), and IL-3 (10 ng/ml). This standardized differentiation system provides a simple platform to produce MKs in feeder-free condition within 5–7 days. Half media was replaced at every 2-day intervals.
Microscopy
To evaluate morphological changes in PMA-induced megakaryoblastic cells, cells upon 72 h of PMA treatment were visualized and imaged under inverted light microscope.
MK-specific marker, CD41 surface antigen expression was evaluated by immunostaining of cells with anti-CD41 FITC (BD Pharmingen). Cells (1 × 106) were fixed with paraformaldehyde (2%) and permeabilized with 1% Triton X-100, and blocked with 2% BSA for 60 min. After blocking, cells were incubated for one hour with anti-CD41 FITC followed by washing with PBS to remove all unbound antibodies. Finally, cells were mounted with DAPI and detected under confocal microscope (Carl Zeiss Microimaging).
Flow cytometry analysis
Cells (1 × 106) were harvested and washed with ice cold PBS. Further, cells were fixed with paraformaldehyde. After blocking with 5% mouse serum and 1% BSA, cells were stained with FITC-conjugated anti-human CD41a (BD Biosciences) or isotype-matched IgGs (negative controls). Finally, cells were washed with 0.1% FBS in 1X PBS and analyzed using FACS Aria™ III flow cytometer (BD Biosciences).
Quantitative RT-PCR
Total RNA was prepared from harvested cells by using miRNeasy mini kit (Qiagen) as per the manufacturer’s protocol. cDNA was prepared from 1 ug of total RNA using random hexamer assays of EasyScriptTM cDNA Synthesis Kit (abm) as per the instructions provided by manufacturer. Further, the cDNA was used for the qRT-PCR quantification, qRT-PCR was performed with specific primers (Table 1) and SYBR Green FAST qPCR Master Mix (Kappa Biosystems) using Step One PlusTM Applied Biosystems real time PCR system. PCR cycle conditions were: initial denaturation at 52 °C (2 min) and 95 °C (8 min), followed by 42 cycles of 56 °C (30 s) and 72 °C (30 s). Ct-values were normalized against internal control GAPDH (Glyceraldehyd-3-Phosphate-Dehydrogenase). miScript Primer Assays (Qiagen) were used to analyze the expressions of miRNAs by qRT-PCR, U6 was used as internal control for data normalization. Relative quantification of genes was calculated by the comparative ΔΔCt method (2^(-ΔΔCt)) and represented as mean ± standard deviation (SD) of three independent experiments.
Table 1.
List of primers used in the present study
| PCR Primers | Primer Sequence (5′–3′) | |
|---|---|---|
| CD41 | F: TCAACCCTCTCAAGGTGGAC | R: GCAGCACAAACTGATCCAGA |
| CD61 | F: CCTGTTGGGAGTGAGGATGT | R: AGAGCTGCCAATAAGGCAAA |
| GAPDH | F: ACCACAGTCCATGCCATCAC | R: TCCACCACCCTGTTGCTGTA |
| TGF-β1 | F: GCAACAATTCCTGGCGATAC | R: TAGTGAACCCGTTGATGTCC |
| TGFßR1 | F: GTGACAGATGGGCTCTGCTT | R: GAGGGTGCACATACAAACGG |
| TGFßR2 | F: AGACGTTGACTGAGTGCTGG | R: TTAGGGAGCCGTCTTCAG |
| Cyc D1 | F: TGAACTACCTGGACCGCT | R: GCCTCTGGCATTTTGGAG |
| Cyc D2 | F: CAGAAGGACATCCAGCCGT | R: TCGGGACTCCAGCCAAGAA |
| Cyc E | F: CTCCAGGAAGAGGAAGGCA | R: CGTGACCGTTTTTTTGCAGG |
| P16 | R: CCCAACGCACCGAATAGT | R: ACCAGCGTGTCCAGGAAG |
| JPX | F: GCACCACCAGGCTTCTGTAAC | R: GGGCATGTTCATTAATTGGCC |
| MEG9 | F: GCTGATGAACCAGGCGGAGG | R: TTTGTCTCCCCTGAGTCCAC |
| TncRNA | F: GCTGGAGTCTTGGGCACGGC | R: TCAACCGAGGCCGCTGTCTC |
| 18S RNA | F: CTCGGCAACGGATATCTCG | R: GCCCTCAACCTAATGGCTTC |
| UCA-1 | F: GCCCAAGGAACATCTCACCAATTT | R: TTGAGGGGTCAGACTTTTGACAAGG |
| UM9-5 | F: GTCTCCATTTCACAGGAAGAAACA | R: GCTAACTCAGTCTCTTACTGAGA |
| RUNX1 | F: AACCTCGAAGACATCGGCAG | R: GGCTGAGGGTTAAAGGCAGT |
Long non-coding RNA array
For lncRNA profiling, total RNA was isolated from cultured cells using the miRNeasy mini kit (Qiagen) following the manufacturer's instructions, and total RNA input (1 µg) was reverse transcribed by using Human LncProfilers™ cDNA synthesis Kit (System Biosciences), as per instructions specified by the manufacturers. For lncRNAs profiling, the Human LncProfilers™ qPCR Array was used. Ct values were normalized to 18S RNA, differentially expressed lncRNAs between control group (Dami cells) and differentiated MKs were recognized via ΔΔCt analysis software (fold change ± 2 and p < 0.05) in SBI website (www.systembio.com/LncRNA).
Western blot
Total proteins were extracted from cells by utilizing pre-chilled RIPA buffer (Sigma) supplemented with protease inhibitor cocktail (Sigma-Aldrich) and phosphatase arrest (G-Biosciences; Geno Technology). Proteins (50 μg) were separated on 6% to 12% SDS-PAGE and transferred to nitrocellulose (NC) membranes (Millipore). Membranes were blocked with 5% bovine serum albumin (BSA) and incubated overnight at 4 °C with specific primary antibodies (PI3K, AKT, Cyc D1, P16, P21, pERK1/2: Santa Cruz; β-actin: Sigma). After washing with TBST, NC membranes were incubated (1 h at rT) with suitable peroxidase-conjugated secondary antibody. A chemiluminescent solution, Pierce™ ECL Substrate (Thermo Fisher Scientific) was used to develop protein bands on NC membranes and visualized by Chemidoc Imaging System (BioRad) as per the instructions of manufacturer. ImageJ software was used to quantify the relative protein levels using western blot band density and β-actin was used for normalization.
Statistical analysis
All experiments were performed in triplicates and data represented from three individual experiments (n = 3). Bar represents mean ± SD of three individual experiments (n = 3). Student’s t-test was performed to examine significance variance between the analyzed groups. p value < 0.05 represent statistical significance of data.
Results and discussion
PMA-induced differentiation of Dami cells to megakaryocyte
The malignant megakaryoblasts are the hyperproliferative immature precursors of platelets and these hyperproliferative megakaryoblasts cause tissue damaging effects. Different types of leukemia have different treatment options and outlooks. However, megakaryoblastic leukemia remain frustratingly difficult to treat because of poor prognosis. Previously, several studies have reported that PMA resulted in a dose-related inhibition of growth and a stimulation of differentiated functions of different myeloid leukemia cells [6, 8]. Understanding the molecular mechanism associated with MK differentiation will allow us to identify novel therapeutic targets.
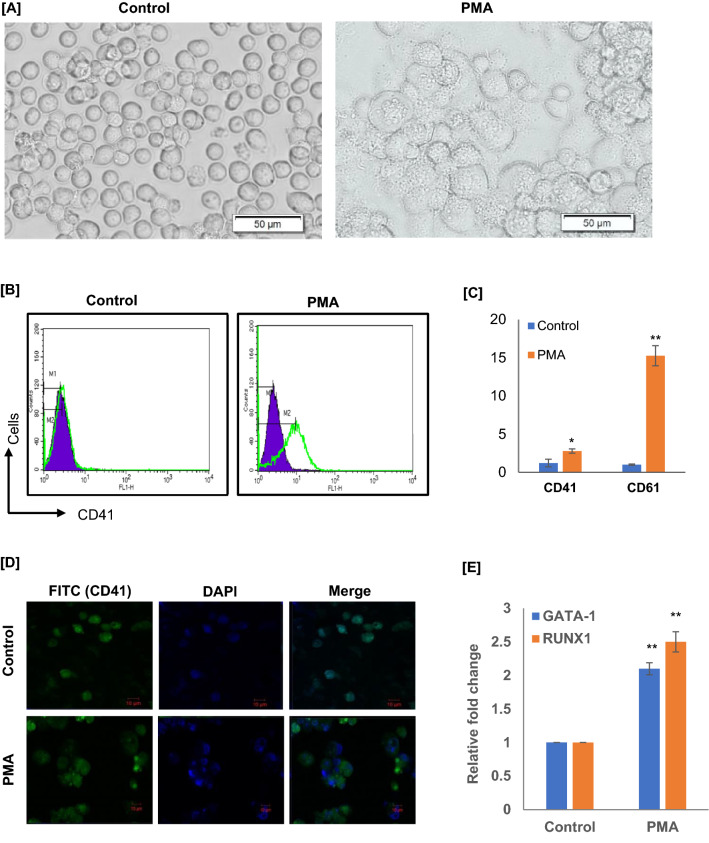
In order to access the PMA-induced MK differentiation, we have treated Dami cells with PMA (100 nM), PMA treated cells were grown for 72 h and examined for MK-related morphological features and cell surface antigens expression. Post 72 h of PMA stimulation, cell proliferation was stopped, a marked increase in cell size, adherence, and granularity was observed for PMA-treated Dami cells, which had typical signs of megakaryocytic differentiation (n = 3; Fig. 1a). The megakaryocytic differentiation was further confirmed by analyzing the MK markers. In FACS study, we analyzed megakaryocytic surface antigens, CD41, post 72 h of PMA stimulation more than 85% of cells were CD41 positive (n = 3; Fig. 1b). MK surface antigen CD41 (DAPI/CD41-FITC) expression was also visualized under fluorescence microscope; we observed more CD41-FITC positive cells with increased fluorescence and cell size upon PMA stimulation as compared to control (n = 3; Fig. 1d). In qRT-PCR study, we further confirmed that MK markers CD41 and CD61 mRNA levels were significantly increased in PMA stimulated Dami cells as compared to unstimulated Dami cells, control (n = 3; p < 0.02; Fig. 1c). Moreover, we have also analyzed the megakaryocytic transcription factor RUNX1 and GATA1 mRNA expressions; interestingly, the expression levels of both the transcription factor encoding mRNAs were significantly high in PMA-induced megakaryocytic cells as compared to uninduced Dami cells (n = 3; p < 0.02; Fig. 1e). The results of the present study confirmed that PMA stimulation of Dami cells significantly established the megakaryocytic features – increased cell size, adherence, and the expression of surface MK specific surface antigens and megakaryocytic transcription factors. Thus, PMA-induced megakaryocytic differentiating cells could be a potential tool in understanding the molecular mechanism associated with MK differentiation.
Fig. 1.
PMA-induced differentiation of megakaryoblastic Dami cells into megakaryocytes (MKs). a Photomicrograph of cells (20 × objective; scale = 50 μm) showing morphological differences (cell size and adherence) in Dami cells upon 72 h of PMA treatment as compared to unstimulated Dami cells. b Representative flow cytometry histograms of the cultured suspension cells on day 3, maximum Dami cells expressed MK markers CD41 upon PMA stimulation as compared to unstimulated Dami cells. c qRT-PCR results showing fold induction in the expression of MK markers CD41 and CD61 in Dami cells upon PMA stimulation as compared to unstimulated control cells (n = 3; *p < .05; **p < .02). d Photomicrograph of cells stained with CD41-FITC and DAPI showing the expression of MK surface antigen CD41. Bars represent mean ± SD of three independent experiments. e Differential expression analysis of megakaryocytic transcription factor RUNX1 and GATA1 mRNA
Differential expression of lncRNAs is associated with PMA-induced megakaryocytic differentiation
The molecular heterogeneity in leukemia cases is well documented, such as genetic mutations in different genes, and epigenetic regulation of gene expression at transcriptional and post-transcriptional levels. The recent applications of high-throughput sequencing and the rapid development of biological techniques have shown that lncRNAs can regulate gene expression at multiple levels, including transcriptional and post-transcriptional levels. Notably, several lncRNAs have been identified with clinical potential as tumor-suppressors and key targets in the diagnosis, prognosis, and treatment of leukemia [13]. Currently, there are only few studies which have identified and characterized the lncRNA expression profiles in myeloid leukemia cells [14–16], also the expression of lncRNAs has not been explored in megakaryoblastic leukemia cells.
Understanding the dynamics of lncRNAs expression during MK differentiation will allow us to identify novel therapeutic targets for megakaryoblastic leukemia. In an effort to explore the expression profile of differentially expressed lncRNAs, a qRT-PCR-based lncRNA Profiler, qRT-PCR array was used, which contained orderly 90 lncRNAs. The differential expression analysis was performed in unstimulated Dami cells (control) versus PMA stimulated megakaryocytic differentiating Dami cells (MKs). Heat map shows the differentially expressed lncRNA profiles of control and differentiated megakaryocytic cells (n = 3; Fig. 2a). Further, the data was presented as bar graph which shows statistically significant (p value < 0.05) differentially expressed (fold-change of ± two-fold) lncRNA genes (n = 3; Fig. 2b). For the first time, we have investigated the differential expression profile of 90 lncRNAs in megakaryoblastic cells versus PMA-induced MKs. A total of 30 lncRNAs were identified with statistically significant differential expression after PMA treatment (n = 3; p < 0.05; Fig. 2b); the JPX was the highest upregulated lncRNA in PMA-induced MKs as compared to control megakaryoblastic cells. Further, we did validation of array-based gene expression profile by qRT-PCR using lncRNA (JPX, MEG9, TncRNA, UM9-5, and UCA1)-specific primers, all tested lncRNAs were showing significantly high expression levels in PMA-induced MKs as compared to control (n = 3; *p < 0.05; Fig. 2c). Moreover, the expression levels of these lncRNAs were similar to that observed in array profile. Furthermore, the expression levels of the highest upregulated JPX lncRNA were investigated in cytokine-induced HPCs-MKs (n = 3; *p < 0.02; Fig. 2d) and the results were consistent with our array findings. The increased expression of these lncRNAs after PMA induction of megakaryoblastic cells and cytokine induction of HPCs emphasize a comprehensive understanding of their functional role in MK development. Moreover, in new megakaryoblastic leukemia treatment strategies these lncRNAs could be the potential therapeutic options, and prognostic markers for monitoring the pathogenesis of megakaryoblastic leukemia.
Fig. 2.
lncRNA profiling and analysis of differentially expressed lncRNAs in PMA-induced MKs. a Visualization of delta-Ct values of lncRNA genes in control (Dami) and PMA-induced MKs (Dami-MKs) using heat map (n = 3). b Array Data showing top 30 most differentially expressed genes in Dami and Dami-MKs (n = 3; *p < 0.05). c Validation of lncRNA array data using qRT-PCR. qRT-PCR reactions with differential expressions of JPX, MEG9, TncRNA, UM9-5, and UCA1 in Dami and Dami-MKs (n = 3; *p < .05). d Further, the expression levels of the highest upregulated JPX lncRNA were validated in HPCs derived MKs by using qRT-PCR (n = 3; *p < 0.02). Bars represent mean ± SD of three independent experiments
lncRNA JPX/miRNAs/TGF-βR axis correlates with the growth and differentiation of megakaryoblastic leukemia cells
The present study results have confirmed PMA as a potential drug that can inhibit the proliferation of megakaryoblastic cells and induce MK differentiation. Interestingly, we have also identified that the differentiation expression of lncRNAs is associated with megakaryocytic differentiation, JPX was the highest upregulated lncRNA in PMA-induced MKs as compared to uninduced megakaryoblastic cells. In the previous study, JPX lncRNA was identified with differential expression between leukemia patients in favorable and intermediate/normal risk categories, the expression was reported with statistically significant upregulation in the cytogenetically favorable risk category as compared to intermediate/normal risk category [17].
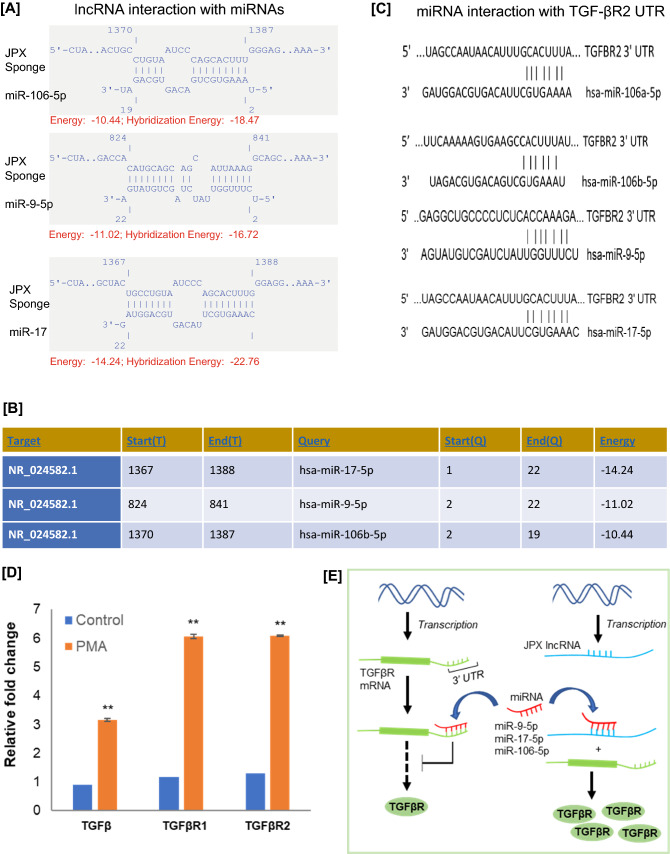
The functional role of non-coding RNAs (specifically miRNAs) has been widely studied in last decade [18]. The lncRNAs are a class of non-coding RNAs, they are involved in various biological processes such as chromatin interactions, transcription and translation regulation, and the regulation of protein interactions [11, 17]. In different studies, lncRNAs have been identified in the regulation of gene expression by acting as a competing RNA for miRNAs [19]. These lncRNAs act as a molecular sponge, they allow the protein expression by competing with miRNAs for mRNA binding. Further, in order to understand the functional role of JPX in molecular regulation of miRNAs during MK maturation, we have performed lncRNA-miRNA interaction study. In this interaction study, we have used IntaRNA online tool (http://rna.informatik.uni-freiburg.de/IntaRNA). The interactions were performed between JPX lncRNA and three commonly known oncogenic miRNAs (miR-9-5p, miR-17-5p, miR-106-5p) those were previously reported to be involved in leukemia development [20–24]. Interaction study identified significant intersections between JPX sequence and all three selected mature miRNAs sequences (Fig. 3a and b). During MK differentiation or maturation, the JPX lncRNA may function as a miRNA-sponge, thereby regulating the expression of miRNA target genes after transcription. Interestingly, in target scan analysis (http://www.targetscan.org/vert_72/) TGF-β receptor mRNA was identified as the confirmed common target of miR-9-5p, miR-17-5p, and miR-106-5p (Fig. 3c). Further, we analyzed the expression of TGF-β and TGF-β receptor (TGF-βR), the expression levels of both the TGF-β and TGF-βR mRNAs were consistently high in PMA-induced MKs as compared to uninduced megakaryoblastic cells (n = 3; p < 0.02; Fig. 3d). TGF-β receptor signaling is involved in multiple biological functions, TGF-β is a potent inhibitor of human myeloid leukemia cells [25–27], TGF-β signaling has also been recently reported to be involved in MK maturation [8]. Considering the JPX-miRNA-TGF-βR mRNA interaction data, a possible explanation may be that JPX involved in the regulation of TGF-β receptor expression by competing with TGF-βR targeting miRNAs during megakaryocytic differentiation (Fig. 3e). Thus, JPX could be a molecular signature and a potential therapeutic target in megakaryoblastic leukemia.
Fig. 3.
JPX-lncRNA-miRNAs and miRNAs-TGF-βR mRNA interaction study in PMA-induced megakaryocytic differentiating cell line model. a JPX-lncRNA-miRNAs sequence interactions. b Minimum free energy (mfe) duplex of JPX-lncRNA miRNAs analyzed by IntaRNA tool. c Target scan analysis showing miR-9-5p, miR-17-5p, and miR-106-5p interaction with TGF-βR. d qRT-PCR results showing the differential expression of TGF-β1, TGF-βR1, and TGF-βR2 in PMA-induced MKs as compared to uninduced (control) Dami cells (n = 3, **p < 0.02). e The graphical representation showing the effect of JPX- lncRNA on the expression of TGF-βR by titrating miRNAs away from TGF-βR mRNAs. Bars represent mean ± SD of three independent experiments
TGF-βR signaling activates SMAD-independent pathways during PMA-induced megakaryocytic differentiation
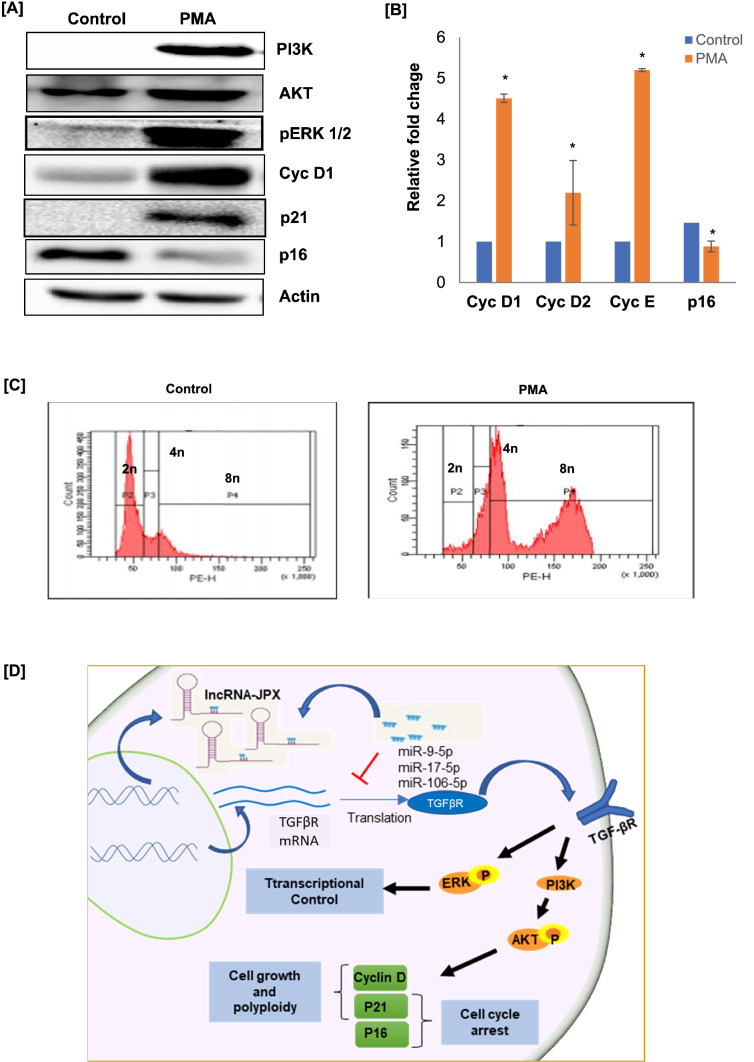
In different studies, TGF-β has been reported as one of the potential regulators of apoptosis, proliferation, differentiation, and matrix formation [28]. Specifically, in recent studies, the activation of TGF-βR/SMAD pathway has been reported with the functional significance in MK maturation [8]. The TGF-βR signaling involves various ligands, receptors, SMADs, and interacting partners, TGF-β is also known to activate various non-SMAD or SAMD-independent pathways such as ERK1/ERK2, Jun-N terminal kinase (JNK), and p38 and PI3K kinases [29, 30]. In the present study, we examined the components of SAMD-independent ERK1/ERK2 and PI3K/AKT pathways. In protein analysis by western blot, the expression levels of pERK1/2 and PI3K/AKT were observed with increased band intensity in PMA-induced MKs as compared to control (n = 3; p < 0.02; Fig. 4a). In previous studies, PI3K/AKT and ERK1/2 pathways have been associated with MK maturation, these are the main pathways activated by TPO receptor during MK development [31]. Moreover, PI3K/AKT is well known to be involved in ploidy, size, and cytoplasmic maturation of MK [31–33].
Fig. 4.
TGF-βR signaling activates SMAD-independent pathways during PMA-induced megakaryocytic differentiation. a pERK1/2, PI3K/AKT, Cyc D1, p21, and p16, protein levels were quantified in control cells and PMA-induced MKs (n = 3; p < 0.02). b The qRT-PCR analysis of different cell cycle regulators (Cyc D1, Cyc D2, Cyc E, and p16) expression in PMA-induced MKs as compared to uninduced Dami cells. c DNA index (Cell Ploidy) levels analysis in PMA-induced and -uninduced Dami cells by Flow cytometry (n = 3). d The graphical representation of the activation of TGF-βR-induced SMAD-independent ERK1/2 and PI3K/AKT pathways during PMA-induced differentiation of Dami cells into MKs, and a crosstalk between JPX lncRNA, miRNAs, and TGF-βR during MK development. Bars represent mean ± SD of three independent experiments
Further, we have analyzed the expression levels of different cell cycle regulators which are well known to be regulated by PI3K/Akt pathway. Interestingly, the protein expression levels of cyclin D1 and p21 were increased; however, the expression levels of p16 were reduced (n = 3; p < 0.02; Fig. 4a). The similar expression profile of cyclins and Cdk inhibitor p16 was observed in our qRT-PCR study (n = 3; p < 0.05; Fig. 4b). Furthermore, to understand the PMA-induced MK maturation, we have analyzed the ploidy by flow cytometry of PI-stained cells. We observed that post-PMA stimulation most of the cells reaching a ploidy class of 4 N and 8 N compared to unstimulated control cells (n = 3; Fig. 4c). The megakaryocytic transcription factor RUNX1, which plays crucial role in MK ploidy development and maturation, was also observed with increased expression in PMA-induced cells (n = 3; p < 0.05; Fig. 1e). The PI3K/AKT pathway is a strong activator of cyclin D1, an important regulator of cell cycle (G1/S phase) progression [34]; in previous studies cyclin D1 has been well defined for its role in growth and polyploidization of MKs [35]. Whereas p16 is a well-known inhibitor of cell cycle progression, report suggests that the stability and function of p16 can be modulated by PI3K/AKT signaling. On the other hand, studies suggest that the function of p21 is more complex in the regulation of cyclin/Cdks, during cell cycle progression p21 function appears to ensure appropriate Cdk activation and has both positive and negative effects on cell cycle progression. In different studies the activity of p21 is also associated with PI3K/AKT pathway activation. The present data suggest the possible role of TGF-βR-activated SMAD-independent ERK1/2 and PI3K/AKT pathways in the regulation of the expression and activity of different transcription factors and cell cycle regulators in PMA-induced megakaryocytic maturation and ploidy development.
Conclusion
In this study, we assessed the molecular mechanisms associated with the PMA-induced differentiation of megakaryoblasts into MKs. For this purpose, MKs generated upon PMA stimulation of Dami cells (megakaryoblasts) were used (Fig. 1). Notably, several lncRNAs have been identified with clinical potential in the diagnosis, prognosis, and treatment of leukemia. Currently, there are only few studies which have identified and characterized the lncRNA expression profiles in myeloid leukemia cells; however, the expression of lncRNAs has not been explored in megakaryoblastic leukemia cells. To elucidate the functional role of lncRNAs in MK differentiation, we have performed the differential lncRNA expression profiling in megakaryoblasts and PMA-induced MKs. A total of 30 lncRNAs were identified with statistically significant differential expression after PMA treatment, JPX was the highest upregulated lncRNA in PMA-induced MKs (Fig. 2). JPX lncRNA is a molecular switch for X chromosome inactivation, JPX is also known for its activities as a suppressor or promoter of cancer cells proliferation by sponging different miRNA. Further to understand the functional role of JPX in molecular regulation during MK differentiation, we have performed interaction studies with three commonly known oncogenic miRNAs (miR-9-5p, miR-17-5p, miR-106-5p); previously, these miRNAs have been reported with their oncogenic properties in leukemia cells. The intersection study identified strong interactions between JPX and putative miRNAs (Fig. 3). Interestingly, TGF-βR, a confirmed common target of miR-9-5p, miR-17-5p, and miR-106-5p, was identified with increased expression during megakaryoblastic cells differentiation into MKs. A possible explanation of co-expression and interaction data may be that JPX involved in the regulation of TGF-βR expression by sponging TGF-βR targeting miRNA in MKs. ERK1/2 and PI3K/AKT are the main pathways activated by TPO receptor during MK development; TGF-βR signaling is also well known to activate non-SMAD pathways such as ERK1/ERK2 and PI3K/AKT. In the present study, we report the activation of SAMD-independent ERK1/ERK2 and PI3K/AKT pathways and differential regulation of their targets (cyclin D1, p16, and p21) during PMA-induced megakaryoblastic differentiation (Fig. 4a). Moreover, we have identified cells with high ploidy (Fig. 4c) and increased expression of megakaryocytic transcription factors RUNX1 and GATA1 (Fig. 1e) upon PMA stimulation. In previous studies, PI3K/AKT and ERK1/2 pathways have been associated with MK maturation. Moreover, PI3K/AKT is well known to be involved in ploidy, size, and cytoplasmic maturation of MK. In conclusion, JPX lncRNA is likely involved in the regulation of TGF-βR by sponging miRNAs. Thus, by unblocking the TGF-βR expression, JPX may augment the activity of ERK1/ERK2 and PI3K/AKT pathways and that in turn enhance the polyploidization and terminal maturation of MKs. This data present that JPX could be a molecular signature and a potential therapeutic target in megakaryoblastic leukemia. However, further experimental validations are required to confirm the interactions between TGF-βR, JPX and miRNAs and the functional effects of these interactions in MK development.
Acknowledgements
This work was supported by Department of Biotechnology (DBT, BUILDER), Department of Science and Technology (DST-FIST), Science and Engineering Research Board (SERB, EEQ/2018/00853), Indian Council of Medical Research (ICMR, 56/5/2019-Nano/BMS), University Grants Commission (UGC-SAP-DRS, F.5-12/2016/DRS-I (SAP-II)) and Council of Scientific and Industrial Research (CSIR, No. 27(0343)/19/EMR-II) grants of Government of India.. We appreciate the funding in form of Council of Scientific and Industrial Research (CSIR) and UGC Fellowships from Government of India.
Author contributions
Dahariya S. and Kandi R. designed and performed experiments; Sangeeth A., Malleswarapu M. and Raghuwanshi S. analyzed and interpreted the lncRNA data and prepared the manuscript; Gutti R. designed and supervised the study.
Declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Ethical approval
All investigations were performed in accordance to the local laws and regulations. This work was carried out with the Institutional Ethics Committee (IEC) approval.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Pession A, Martino V, Tonelli R, et al. MLL-AF9 oncogene expression affects cell growth but not terminal differentiation and is downregulated during monocyte-macrophage maturation in AML-M5 THP-1 cells. Oncogene. 2003;22(54):8671–8676. doi: 10.1038/sj.onc.1207125. [DOI] [PubMed] [Google Scholar]
- 2.Crans HN, Sakamoto KM. Transcription factors and translocations in lymphoid and myeloid leukemia. Leukemia. 2001;15(3):313–331. doi: 10.1038/sj.leu.2402033. [DOI] [PubMed] [Google Scholar]
- 3.Dima D, Oprita L, Rosu AM, et al. Adult acute megakaryoblastic leukemia: rare association with cytopenias of undetermined significance and p210 and p190 BCR-ABL transcripts. Onco Targets Ther. 2017;10:5047–5051. doi: 10.2147/OTT.S146973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fidler MM, Soerjomataram I, Bray F. A global view on cancer incidence and national levels of the human development index. Int J Cancer. 2016;139(11):2436–2446. doi: 10.1002/ijc.30382. [DOI] [PubMed] [Google Scholar]
- 5.Ablain J, de The H. Revisiting the differentiation paradigm in acute promyelocytic leukemia. Blood. 2011;117(22):5795–5802. doi: 10.1182/blood-2011-02-329367. [DOI] [PubMed] [Google Scholar]
- 6.Zeng C, Wang W, Yu X, Yang L, Chen S, Li Y. Pathways related to PMA-differentiated THP1 human monocytic leukemia cells revealed by RNA-Seq. Sci China Life Sci. 2015;58(12):1282–1287. doi: 10.1007/s11427-015-4967-4. [DOI] [PubMed] [Google Scholar]
- 7.Lev PR, Goette NP, Glembotsky AC, et al. Production of functional platelet-like particles by the megakaryoblastic DAMI cell line provides a model for platelet biogenesis. Platelets. 2011;22(1):28–38. doi: 10.3109/09537104.2010.515271. [DOI] [PubMed] [Google Scholar]
- 8.Raghuwanshi S, Dahariya S, Sharma DS, Kovuru N, Sahu I, Gutti RK. RUNX1 and TGF-β signaling cross talk regulates Ca2+ ion channels expression and activity during megakaryocyte development. FEBS J. 2020 doi: 10.1111/febs.15329. [DOI] [PubMed] [Google Scholar]
- 9.Morlando M, Ballarino M, Fatica A. Long Non-Coding RNAs: New Players in Hematopoiesis and Leukemia. Front Med (Lausanne) 2015 doi: 10.3389/fmed.2015.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu G, Niu F, Humburg BA, et al. Molecular mechanisms of long noncoding RNAs and their role in disease pathogenesis. Oncotarget. 2018;9(26):18648–18663. doi: 10.18632/oncotarget.24307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dahariya S, Paddibhatla I, Kumar S, Raghuwanshi S, Pallepati A, Gutti RK. Long non-coding RNA: classification, biogenesis and functions in blood cells. Mol Immunol. 2019;112:82–92. doi: 10.1016/j.molimm.2019.04.011. [DOI] [PubMed] [Google Scholar]
- 12.Zhang H, Chen Z, Wang X, Huang Z, He Z, Chen Y. Long non-coding RNA: a new player in cancer. J Hematol Oncol. 2013 doi: 10.1186/1756-8722-6-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gao J, Wang F, Wu P, Chen Y, Jia Y. Aberrant LncRNA expression in leukemia. J Cancer. 2020;11(14):4284–4296. doi: 10.7150/jca.42093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang J, Griffith M, Miller CA, et al. Comprehensive discovery of noncoding RNAs in acute myeloid leukemia cell transcriptomes. Exp Hematol. 2017;55:19–33. doi: 10.1016/j.exphem.2017.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lei L, Xia S, Liu D, et al. Genome-wide characterization of lncRNAs in acute myeloid leukemia. Brief Bioinform. 2018;19(4):627–635. doi: 10.1093/bib/bbx007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feng Y, Shen Y, Chen H, et al. Expression profile analysis of long non-coding RNA in acute myeloid leukemia by microarray and bioinformatics. Cancer Sci. 2018;109(2):340–353. doi: 10.1111/cas.13465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zimta AA, Tomuleasa C, Sahnoune I, Calin GA, Berindan-Neagoe I. Long non-coding RNAs in myeloid malignancies. Front Oncol. 2019;9:1048. doi: 10.3389/fonc.2019.01048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raghuwanshi S, Karnati HK, Sarvothaman S, et al. microRNAs: key players in hematopoiesis. Adv Exp Med Biol. 2015;887:171–211. doi: 10.1007/978-3-319-22380-3_10. [DOI] [PubMed] [Google Scholar]
- 19.Paraskevopoulou MD, Hatzigeorgiou AG. Analyzing MiRNALncRNA interactions. Methods Mol Biol. 2016;1402:271–286. doi: 10.1007/978-1-4939-3378-5_21. [DOI] [PubMed] [Google Scholar]
- 20.Chen P, Price C, Li Z, et al. miR-9 is an essential oncogenic microRNA specifically overexpressed in mixed lineage leukemia-rearranged leukemia. Proc Natl Acad Sci U S A. 2013;110(28):11511–11516. doi: 10.1073/pnas.1310144110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sugita F, Maki K, Nakamura Y, Sasaki K, Mitani K. Overexpression of MIR9 indicates poor prognosis in acute lymphoblastic leukemia. Leuk Lymphoma. 2014;55(1):78–86. doi: 10.3109/10428194.2013.790023. [DOI] [PubMed] [Google Scholar]
- 22.Fischer J, Rossetti S, Datta A, Eng K, Beghini A, Sacchi N. miR-17 deregulates a core RUNX1-miRNA mechanism of CBF acute myeloid leukemia. Mol Cancer. 2015;14:7. doi: 10.1186/s12943-014-0283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bobbili MR, Mader RM, Grillari J, Dellago H. OncomiR-17-5p: alarm signal in cancer? Oncotarget. 2017;8(41):71206–71222. doi: 10.18632/oncotarget.19331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sperb N, Krowiorz K, Grasedieck S, et al. Members of the microRNA-106a-363 cluster associate with unfavorable outcome in adult acute myeloid leukemia patients and promote leukemogenesis invivo through increased metabolic activity. Blood. 2018;132:3924–3924. doi: 10.1182/blood-2018-99-116035. [DOI] [Google Scholar]
- 25.Gong Y, Zhao M, Yang W, et al. Megakaryocyte-derived excessive transforming growth factor β1 inhibits proliferation of normal hematopoietic stem cells in acute myeloid leukemia. Exp Hematol. 2018;60(40–46):e2. doi: 10.1016/j.exphem.2017.12.010. [DOI] [PubMed] [Google Scholar]
- 26.Hu X, Cui D, Moscinski LC, Zhang X, Maccachero V, Zuckerman KS. TGFbeta regulates the expression and activities of G2 checkpoint kinases in human myeloid leukemia cells. Cytokine. 2007;37(2):155–162. doi: 10.1016/j.cyto.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 27.Jakubowiak A, Pouponnot C, Berguido F, et al. Inhibition of the transforming growth factor beta 1 signaling pathway by the AML1/ETO leukemia-associated fusion protein. J Biol Chem. 2000;275(51):40282–40287. doi: 10.1074/jbc.C000485200. [DOI] [PubMed] [Google Scholar]
- 28.Moustakas A, Heldin CH. Non-Smad TGF-beta signals. J Cell Sci. 2005;118(Pt 16):3573–3584. doi: 10.1242/jcs.02554. [DOI] [PubMed] [Google Scholar]
- 29.Bakin AV, Rinehart C, Tomlinson AK, Arteaga CL. p38 mitogen-activated protein kinase is required for TGFbeta-mediated fibroblastic transdifferentiation and cell migration. J Cell Sci. 2002;115(Pt 15):3193–3206. doi: 10.1242/jcs.115.15.3193. [DOI] [PubMed] [Google Scholar]
- 30.Engel ME, McDonnell MA, Law BK, Moses HL. Interdependent SMAD and JNK signaling in transforming growth factor-beta-mediated transcription. J Biol Chem. 1999;274(52):37413–37420. doi: 10.1074/jbc.274.52.37413. [DOI] [PubMed] [Google Scholar]
- 31.Liu ZJ, Italiano J, Jr, Ferrer-Marin F, et al. Developmental differences in megakaryocytopoiesis are associated with up-regulated TPO signaling through mTOR and elevated GATA-1 levels in neonatal megakaryocytes. Blood. 2011;117(15):4106–4117. doi: 10.1182/blood-2010-07-293092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guerriero R, Parolini I, Testa U, et al. Inhibition of TPO-induced MEK or mTOR activity induces opposite effects on the ploidy of human differentiating megakaryocytes. J Cell Sci. 2006;119(4):744–752. doi: 10.1242/jcs.02784. [DOI] [PubMed] [Google Scholar]
- 33.Raslova H, Baccini V, Loussaief L, et al. Mammalian target of rapamycin (mTOR) regulates both proliferation of megakaryocyte progenitors and late stages of megakaryocyte differentiation. Blood. 2006;107(6):2303–2310. doi: 10.1182/blood-2005-07-3005. [DOI] [PubMed] [Google Scholar]
- 34.Chang F, Lee JT, Navolanic PM, et al. Involvement of PI3K/Akt pathway in cell cycle progression, apoptosis, and neoplastic transformation: a target for cancer chemotherapy. Leukemia. 2003;17(3):590–603. doi: 10.1038/sj.leu.2402824. [DOI] [PubMed] [Google Scholar]
- 35.Muntean AG, Pang L, Poncz M, Dowdy SF, Blobel GA, Crispino JD. Cyclin D-Cdk4 is regulated by GATA-1 and required for megakaryocyte growth and polyploidization. Blood. 2007;109(12):5199–5207. doi: 10.1182/blood-2006-11-059378. [DOI] [PMC free article] [PubMed] [Google Scholar]