Abstract
Animal intestines is considered as a source of lactic acid bacteria (LAB) that have potential to decrease the nitrite level during fermentation of food such as pickles. It was hypothesized that optimized level of LAB has a high capacity to degrade nitrite during Chinese pickle fermentation and benefit a higher acceptability of the Chinese pickle product. This study aims to investigate the performance of a goose intestine-isolated LAB strain G6 under the species Lactiplantibacillus plantarum as a starter culture of Chinese pickles. The results showed that Lactiplantibacillus sp. G6 had a nitrite degradation rate close to 100% under the MRS broth condition of 25 °C, 2% inoculum volume and pH at 5. As a starter culture for Chinese pickle, this strain was able to achieve a higher LABs amount, lower nitrite residue after fermentation, compared with the group without the starter, which implicates its feasibility of applying on fermented food for reducing nitrite level.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10068-023-01433-8.
Keywords: Nitrite degradation, Lactiplantibacillus, Chinese pickle, Goose intestine
Introduction
Chinese pickle (Paocai) is an important probiotic product that is widely consumed in China (Panghal et al., 2018). It is produced by immersing the vegetables, namely Chinese cabbages, in salt brine with or without seasoning and fermented spontaneously by lactic acid bacteria (LABs) (Xiong et al., 2016). Currently, LABs as starter cultures, primarily Lactobacillus, has been increasingly utilised in the fermentation process to elevate the quality of the products (Alan, 2019; Çon and Karasu, 2009; Kim et al., 2017b; Liu et al., 2017; Xia et al., 2017). However, the naturally high concentration of nitrate within the cabbages would transform to nitrite during the pickling process, leading to the accumulation of nitrite, whereas nitrite, as a precursor of the carcinogenic N-nitroso compounds, can induce serious health problems (Tamme et al., 2006). Nonetheless, the fermentation process performed by the lactic acid bacteria is able to deplete nitrite in the production of pickles (Kim et al., 2017b; Liu et al., 2017; Oh et al., 2004; Xia et al., 2017). Therefore, selecting strains of lactic acid bacteria efficient in degrading nitrite would highly contribute to removing the nitrite and thus improving the food safety of the processed pickles.
Recently, increasing studies reported that lactic acid bacteria isolated from animal intestines constitute an important source of probiotic which develops various biological roles during food processing (Bakari et al., 2011; Lokapirnasari et al., 2018; Musikasang et al., 2009; Rajoka et al., 2018). Goose, as a common herbivore, has relatively high diversity of gut microbiota owing to its high dietary fibre level and meanwhile phylum Firmicutes, which lactic acid bacteria belong to, are dominant in its gut (Liu et al., 2018; Xu et al., 2017). These characteristics enable the goose’s intestine to be an ideal source of nitrite-degrading lactic acid bacteria.
Sufficient studies indicated that lactic acid bacteria isolated from Chinese pickles and Kimchi, especially Lactobacillus spp., are effective in degrading nitrite during the fermentation of cabbages (Liu et al., 2017; Xia et al., 2017; Yan et al., 2008). However, the research on isolating lactic acid bacteria from animal intestines as a starter culture of Chinese pickles is limited. Selecting LABs from goose intestine with high efficiency in nitrite depletion can restrain the accumulation of nitrite, which has the potential to shorten the fermentation period without compromising food safety. Hence, this study aims to isolate and optimize a LAB for degrading nitrite in Chinese pickles fermentation, contributing to better product safety and quality consistency. The optimized LAB is hypothesized to have a high capacity to degrade nitrite during Chinese pickle fermentation and benefit a higher acceptability of the Chinese pickle product.
Materials and methods
Analysis methods
Nitrite determination
The nitrite residue was determined by referencing the Determination of Nitrite and Nitrate in Foods and the National Food Safety Standard GB 5009.33-2010 and GB 5009.33-2016 using the N-(1-naphthyl)-ethylenediamine dihydrochloride spectrophotometric method, excluding the protein precipitation and lipid depletion steps (Fei et al., 2014). Nitrite reacts with sulphanilamide and N-(1-naphthyl)-ethylene-diamine dihydrochloride, giving a red-violet diazo dye, which was measured based on its absorbance in the visible range at 538 nm measured by UV Spectrophotometer (UV-1100, Shanghai Mapade Instruments Co, Ltd). Firstly the nitrite calibration curve was generated, by which the absorbance at 538 nm of standard sodium nitrite solution was plotted linear to the corresponding concentration. Then all the samples containing liquid, including pickle and LABs inoculated MRS broth, were centrifuged at 6000 rpm for 5 min at 4 °C (KDC-140HR High-speed Refrigerated Centrifug, Anhui North Electro Mechanical Co., Ltd, China) and the supernatants were used for nitrite analysis. The standard curve y = 3.1461x − 0.071 with R2 = 0.999, in which the x represents the absorbance of at 538 nm and y reflects the corresponding nitrite concentration was obtained for calculating the nitrite concentration.
The nitrite degradation rate of strain was calculated by Eq. (1) as shown and was reported as the mean value of triplicate.
| 1 |
Microbiological analysis
The growth of LABs in MRS broth was measured using the UV Spectrophotometer (UV-1100, Shanghai Mapade Instruments Co., Ltd.) with OD600nm absorbance. While manufacturing the pickles, 2.5 g cut pickles were diluted and 1 mL of supernatant was added into the medium. The LABs and total bacteria during pickles manufacturing process were enumerated on MRS agar or Nutrient agar in anaerobic conditions after 24 h or 48 h at 37 °C.
pH measurements
The pH was determined by immersing a Sartorius PB-10 pH meter probe (Sartorius AG, Germany) into the MRS broth, bacterial inoculum samples or the pickle brine.
Statistical analysis
All experiments were conducted in triplicate and the mean value and standard error were deduced. The significant difference test was performed using IBM SPSS Statistics Version 23. An alpha of 0.05 was used as the cutoff for significance.
Isolation of LABs from goose intestine
The intestines of domestic geese (Anser anser), purchased from Guangzhou, China, were collected after slaughtering and kept at 4 °C. Since the geese were fed from the intensive system, the diet of geese was assumed as a high grain diet. Collected intestinal contents of the large intestines were mixed with 15 mL distilled water and homogenized as a stock sample. Then the stock sample of 10−1 dilution was prepared by adding a 1 mL stock sample to 9 mL distilled water and mixed vigorously. Following the same procedure, the samples of up to 10−8 dilution were prepared. 1 mL dilutions of 10−4 to 10−8 were suspended on De Man, Rogosa and Sharpe (MRS) agar with the addition of 150 µg/mL nitrite to obtain a single colony with nitrite degradation ability. 11 purified colonies were transferred to the MRS broth and incubated as seed cultures. The culture condition was anaerobically grown at 37 °C for 24 h. 5% freshly starter LAB cultures were added to culture tubes with 9 mL of MRS broth containing 150 µg/mL sodium nitrite. These bacterial inoculum samples were incubated at 37 °C for 24 h and then centrifuged at 6000 rpm for 5 min at 4 °C. The supernatants of these samples were measured for the nitrite residue. The strains with low nitrite residue were selected and cultured consequently.
Monitoring the strains for nitrite residue, LAB growth and pH within 24 h
The selected strains were inoculated at 2% with 100 mL MRS broth containing 150 µg/mL sodium nitrite and incubated for 24 h at 37 °C. The nitrite remaining concentration, bacteria concentration and pH were measured with an interval of 4 h. The MRS broth without strains and sodium nitrite was used as a control.
Identification of the LAB strains
The morphology of LAB strains was studied by Gram staining, then the selected LABs were identified molecularly.
After 24 h of incubation at 37°C, these bacterial inoculum samples were centrifuged at 6000 rpm for 5 min to harvest the selected LABs to be processed for genomic DNA extraction using the Universal Genomic DNA Extraction Kit (Beijing Solarbio Science & Technology Co., Ltd., China).
The amplification of the 16S rRNA gene of the selected LABs were carried out using Polymerase Chain Reaction (PCR) by universal primers 1492R and 27F. The PCR products were cloned and then sequenced. The DNA sequences were analysed with the Internet BLAST Gene database (http://www.ncbi.nlm.nih.gov) and submitted to GenBank. Mega X was used to build the phylogenetic tree.
Effect of incubation conditions on the selected LABs
The effect of incubation conditions on the growth and nitrite depletion ability of LABs was examined with the incubation of the inoculum samples for 24 h. During the incubation, the nitrite concentration and bacteria concentration were examined twice at an interval of 12 h. All the inoculum samples were prepared in triplicate.
Temperature
9 mL MRS broth containing 150 µg/mL sodium nitrite inoculated with 5% selected strains and incubated at 15 °C, 20 °C, 25 °C, 30 °C, 35 °C and 40 °C for 24 h. Then the bacteria concentration and remaining nitrite concentration were examined.
pH
Effect of added lactic acid on the nitrite degradation
Lactic acid was added into deionized water to adjust the pH of the samples of 6.0, 5.5, 5.0, 4.5, 4.0 and 3.5, followed by the addition of 150 µg/mL sodium nitrite. Then the samples were incubated at 37 °C for 24 h and the nitrite concentration was examined at an interval of 12 h.
Effect of MRS broth pH on the nitrite degradation
The citric acid-sodium citrate buffer was prepared as the pH of 3.0, 4.0, 5.0 and 6.0. Then standardized MRS broth containing 150 µg/mL sodium nitrite was mixed with 5% selected strains. The temperature of incubation was 37 °C and the concentrations of nitrite and bacteria were examined.
Inoculum volume
The volume 2%, 4%, 8% and 10% of selected strains were inoculated into the MRS Broth with 150 µg/mL sodium nitrite. The same MRS broth without the strain inoculum was used as control and the incubation temperature was 37 °C. The concentrations of nitrite and bacteria were then measured.
Nitrite addition
The MRS broths with 50, 100, 150, 200 and 250 µg/mL sodium nitrite were used to inoculated the strains with the optimized inoculum volume at 37 °C. The concentrations of nitrite and bacteria were then measured.
Chinese pickles manufacturing process
Fresh Chinese cabbages were washed with distilled water and drained, then cut into strips. 50 g of cabbage strips were added to approximately 75 g of sterilized and cooled 8% (w/v) NaCl solution. The selected strain of LABs cultured in the 1 mL MRS broth was added into the cabbage and brine mixture at 0 h or 24 h. The control pickles without LABs contained salt solution and cabbage. All the Chinese pickles were prepared in triplicate, sealed and stored at 37 °C for 7 days. During fermentation, the changes of pH, nitrite residue, the growth of LAB and total bacteria were monitored. The brine of the pickles was collected each 24 h to measure the pH. 2.5 g pickles were weighed and cut into small pieces, and then diluted with 5 mL sterile distilled water and vigorously mixed for gradient dilution. 1 mL diluted solution were then added into the plate poured with MRS Agar and Nutrient Agar and cultured at 37 °C for 24–48 h in order to count the viable LAB and aerobic plate count.
The fermented Chinese pickles were assessed following the People’s Republic of China domestic trade industry-standard SB/T 10756-2012. Physicochemical analyses were performed to determine total acidity (% lactic acid), total solid (%) and salt (%NaCl) in the Chinese pickles. The total acidity was measured by titration using 0.05 mol/L sodium hydroxide solution with phenolphthalein indicator and was calculated as lactic acid and acetic acid percentages. In order to measure the amount of salt in the brine, 50 g/L potassium butyrate solution was added to samples and the samples were titrated by 0.01 mol/L AgNO3.
Results and discussions
Isolation of LABs from goose intestine
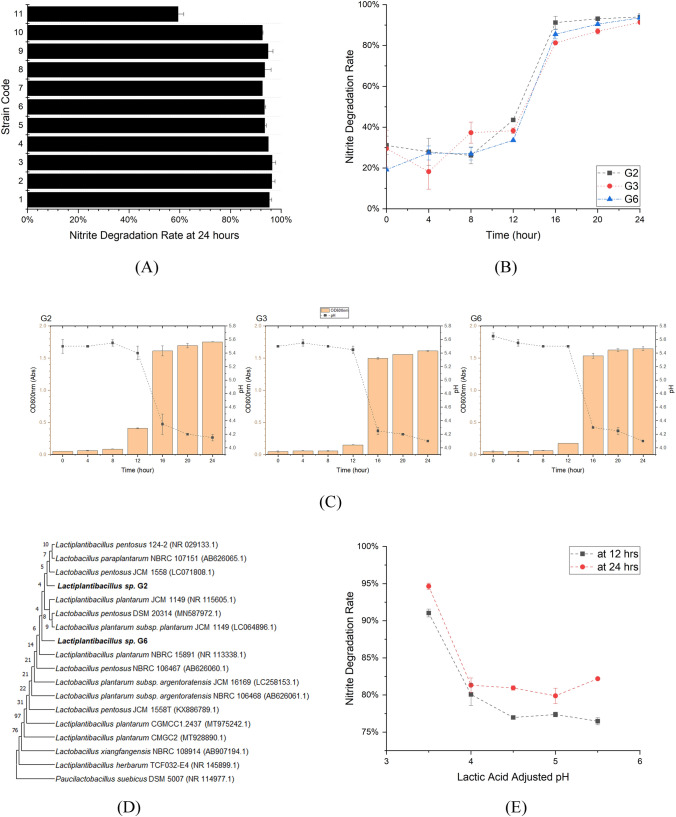
The nitrite degradation rate was acquired in reference to the Eq. 1. The 5% selected bacterial inoculum samples with 150 µg/mL sodium nitrite were incubated for 24 h and examined for the nitrite degradation rate, as shown in Fig. 1(A). All strains demonstrated high nitrite degradation rates exceeding 90%, except for strain 11. Strain 3 exhibited the highest degradation rate of 96.4% while strain 11 of 59.3%. It is also reported by Yan et al. (2008) that the lactic acid bacteria isolated from Chinese pickles degraded more than 97% of nitrite in 72 h from the initial sodium nitrite content of 1000 µg/mL in MRS broth. In contrast, Jiang et al. (2008) reported relatively low nitrite degradation of 71% and 72% for Lb. sakei while high nitrite degradation exceeded 97% for Lb. plantarum in the same culture time from the initial sodium nitrite content 250 µg/mL. This implied the difference in nitrite degradation rate between strains may result from the various species of strains. The ability of nitrite degradation of the strains is in the order of: 3 > 2 > 1 > 4 > 9 > 5 = 8 > 6 > 7 = 10 > 11. Apart from strain 11, the differences between strains are relatively small. In order to select the strains, the 11 strains were divided into three clusters, using K-Means Cluster Analysis based on nitrite degradation rate, shown in Table 1.
Fig. 1.
The screening of strains. Nitrite degradation rate at 24 h after inoculum of different strains isolated from goose intestine (A); Nitrite degradation rate (B), pH and OD600nm absorbance (C) of the strain G2, G3 and G6 within 24 h; Phylogenetic tree showing the relative position of strain G6 as inferred by the neighbour-joining method with the 16S rRNA gene sequences (D). Paucilactobacillus suebicus DSM 5007 (NR 114977.1) is used as an outgroup; Nitrite degradation rate of lactic acid solution with different pH, measured at 12 and 24 h (E)
Table 1.
Classification of strains based on the nitrite degradation rate
| Cluster code | a | b | c |
|---|---|---|---|
| Strain code | 11 | 6, 7, 8, 10 | 1, 2, 3, 4, 9 |
The representative strains of both Cluster b and c were selected, respectively, that of, strain 3 and 6. However, strain 2 was also selected, as the nitrite rate of strain 2, which is 96.3%, was regarded as the same as strain 3 which is 96.4% in Cluster c. The selected three strains 2, 3, 6 were coded G2, G3, and G6 respectively for further study.
Monitoring the LAB strains for nitrite residue, growth and pH within 24 h
In order to clarify the dynamic changes of nitrite degradation, the 2% seed cultures of selected bacteria concentration were inoculated into the medium with sodium nitrite and incubated for 24 h. During the incubation, nitrite residue, bacteria concentration, and pH were measured at an interval of 4 h. As shown in Fig. 1(B) and (C), all strains indicated a sharp increase of nitrite degradation rate and bacteria, and a quick decline of pH between 12 and 16 h, demonstrating that the bacteria grew rapidly along with the acid production. This suggested the production of acid and the consequent decrease in pH could dominantly result in nitrite loss. Dodds and Collins-Thompson (1984) also have reported two mechanisms of nitrite depletion for lactic acid bacteria: chemical depletion due to acid production during growth and enzymatic depletion. It should be also noticed that before 12 h, the trend of nitrite degradation was not corresponding to pH and bacteria growth, indicating that the nitrite degradation in this period is not only responsible for chemical depletion.
Almost all added sodium nitrite was depleted by the selected LABs after 24-h incubation and did not inhibit the growth of LABs. This result agrees with the work of Oh et al. (2004) who reported that the isolated strains from kimchi can degrade 150 µg/mL nitrite within 1–2 days at 36 °C, and Korkeala et al. (1992) who only observed the pronounced inhibitory effects caused by sodium nitrite at 200 µg/mL and higher concentration.
Interestingly, G2 and G3 showed a lower nitrite degradation rate at 24 h compared to the result of the 5% bacteria inoculum sample as seen in Fig. 1(A), implying the nitrite degradation ability of G2 and G3 are more sensitive to the increase of inoculum volume.
Considering the higher nitrite degradation rate of G2 got in the lower bacteria inoculum, the G2 was retained to represent Cluster c. G6, which have the highest nitrite degradation rate among three strains, was also retained for the identification further.
Identification of selected LAB strains
With round or oval colonies, the strain G2 and G6 were Gram-positive and rod-shaped. As found by BLAST comparison on NCBI (http://www.ncbi.nlm.nih.gov/) that G2 and G6 had 97.85% sequence similarity in the 16SrRNA. Then the phylogenetic analysis was performed, and representative strains were selected for G2 and G6, on the basis of similarity according to the sequences alignment and analysis of comparability in Genbank. Consequently, the phylogenetic tree was constructed to reveal the species of G2 and G6 as seen in Fig. 1(D). Both G2 and G6 showed the closest relationship with a similarity of 99.78% and 98.72% in Lactiplantibacillus plantarum JCM 1149 (NR.115605.1), respectively. Lactiplantibacillus sp. G2 and Lactiplantibacillus sp. G6 belonged to the species Lactiplantibacillus plantarum, which was reported as a dominant member of the microbiota in spontaneous vegetable and olive fermentations (Zheng et al., 2020). The other species close to Lactiplantibacillus sp. G2 and Lactiplantibacillus sp. G6 are Lactiplantibacillus plantarum subsp. plantarum, Lactiplantibacillus pentosus, Lactiplantibacillus paraplantarum and Lactiplantibacillus argentoratensis. The former three species have been involved in several food and biotechnological applications. Lactiplantibacillus argentoratensis, was validly published in 2005 and reported that isolated from starchy food, fermented cereals and vegetables (Bringel et al., 2005). All these four species have close relationships with G2 and G6 belong to genus Lactiplantibacillus on the phylogenetic tree.
The occurrence of Lactiplantibacillus sp. G2 and Lactiplantibacillus sp. G6 in the goose intestine is likely to be responsible for plant feed fermentation. However, for further accurate discrimination of Lactiplantibacillus sp. G2 and G6, whole genome sequencing was proposed due to the limited discriminating power for identifying Lactiplantibacillus plantarum and Lactiplantibacillus pentosus (Liu et al., 2012) and sequencing of the recA and cpn60 genes or AFLP profiling (Zheng et al., 2020) was needed to distinguish the subspecies.
Optimisation of nitrite degrading ability
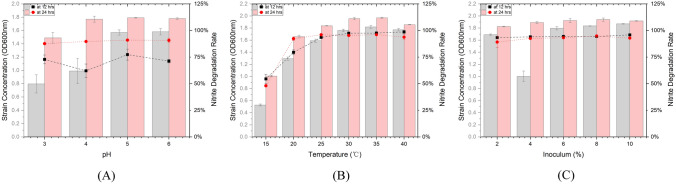
The effect of temperature, pH, inoculum volume and sodium nitrite addition on the nitrite degrading ability of G2 and G6 were examined. The sodium nitrite added ranging from 50 to 250 μg/mL did not significantly affect the nitrite degradation rate of both G2 and G6, as shown in Fig. S1. For the other optimised conditions, compared with G2 seen in Fig. S2, strain G6 exhibited relatively high strain concentration and nitrate degradation rate at 12 h, implying its faster growth and nitrite degradation at all optimisation conditions, which is more feasible and economical for industry production. Therefore, the discussion of optimisation and the subsequent application would be focused on strain G6.
Effect of pH
The lactic acid was proven to have the ability to degrade up to 95% of nitrite as seen in Fig. 1(E). It is well noted that the majority of added nitrite was degraded by lactic acid in the first 12 h, especially for those with pH lower than 4, suggesting a high concentration of lactic acid was effective for nitrite degradation. This result is consistent with what Li et al. (2010) reported that the lactic acid itself showed an ability to degrade nitrite when the pH was lower than 6 and the nitrite degradation increased with the decrease of pH.
In contrast, for strain G6 as shown in Fig. 2(A), the nitrite degradation rate reached up to only 90% observed at 24 h, and the relatively low final nitrite residue agreed with the conclusion that lactic acid production is not the only factor for nitrite degradation of lactic acid bacteria. However, the nitrite degradation was weakly correlated to the pH of the medium at 12 h, implying that the enzymatic depletion, rather than chemical depletion, of nitrite may be dominated in the fermentation. This result partly aligns with the work of Zhang et al. (2002) who reported that the correlation between pH and nitrite degradation was significant only when the pH of MRS broth was lower than 3.5 at the early stage of lactic acid bacteria fermentation (before 12 h). Figure 2(A) also confirmed that the citric acid-sodium citrate buffer used for pH adjustment does not have the ability to degrade nitrite directly. Some scientists have reported the addition of some organic acid such as citric acid and oxalic acid during pickled vegetable fermentation could reduce nitrite (Liu, 2013; Shang et al., 2018), but the mechanism is yet to be clarified.
Fig. 2.
Nitrite degradation rate and OD600nm absorbance of strain G6 with different medium pH (A), at different medium temperatures (B) and added to different inoculum volume (C) measured at 12 and 24 h
Compared to degradation rate, the growth of G6 was closely correlated to the increase of pH, reaching the highest absorbance at pH 6. This is not surprising because the external acid can hinder the growth of LABs, as Giraud et, al reported the optimum pH for the growth of Lb. plantarum close to 6.0 (Giraud et al., 1991) while Fu and Mathews indicated that the optimum pH for cell growth and acid production was between 5 and 6 (Fu and Mathews, 1999). However, G6 could still grow even at pH 3 as seen in the comparison of OD600nm between 12 and 24 h, this supported McDonald et al. (1990) finding that Lb. plantarum can maintain pH homeostasis down to an external pH of 3.0.
Effect of temperature
The increase of temperature was consistent with the increase of nitrite rate for this strain as shown in Fig. 2(B). The nitrite degradation rate was close to 100% when the temperature was between 25 and 40 °C, whereas the rate was only 50% at 15 °C and around 75% at 20 °C. Overall, as temperature increased (15–25 °C), the nitrite degradation rate and strain concentration increased. At the temperature over 25 °C, the degradation rate reached the optimum while the strain concentration slightly fluctuated. It is not surprising because the trend of nitrite degradation rate and strain concentration is likely to be associated with the Lactiplantibacillus activity under different temperature conditions. As reported by Syrokou et al. (2021), the activity of Lactiplantibacillus was significantly improved under a 30 °C incubation temperature compared with 20 °C, while the strain activity at 37 °C was similar with those at 30 °C, which is consistent with the current results on nitrite degradation rate and strain concentration. Particularly, Jiang et al. (2021) studied the combined effects of different factors on the nitrite degradation rate and reported that the optimal temperature for the nitrite degradation rate was 30.19 °C, within a range of 15–35 °C. Specifically, in present studies, the growth rate and concentration of LABs were relatively low which compromised the nitrite degradation at lower temperatures. As the temperature increased, the nitrite degrading rate increased at 12 h, corresponding to the increase of strain activity. With almost 100% nitrite being degraded at 12 h, no further increase in degrading nitrite at 24 h was shown. Oh et al. (2004) also reported that the depletion of nitrite by Lb. plantarum was greatly affected by increasing temperature which agreed with the current results.
Effect of inoculum volume
As shown in Fig. 2(C), the inoculum volume (2–8%) did not have significant effect on the nitrite degradation rate, with all of them close to 100%.
Nonetheless, the strain concentration at 12 h and 24 h showed an overall increasing trend with the addition of inoculum volume, but no significant effect was shown (P > 0.05).
The exception of the 4% inoculum group might be due to the variation caused by limited sample size or different experiment operations. From the prospective of strain concentration, the 8% and 10% inoculum group performed well, while the nitrite degradation maintained at a relatively high level. This result is consistent to the previous study of the effects on the nitrite degradation rate which reported that the optimal inoculation quantity for nitrite degrading rate was 9.13% out of five groups ranging from 3 to 15% (Jiang et al., 2021). Hence, in the current study, 8% inoculum could be a promising candidate for the upcoming food fermentation experiment.
Chinese pickles fermentation
Based on the optimized conditions and the identification of G6, Chinese pickles without seasoning were produced to examine the feasibility of applying G6 in the food product. The Lb. plantarum species and their subspecies Lb. subsp. plantarum, Lb. pentosus, Lb. paraplantarum and Lb. argentoratensis can both be isolated from fermented vegetables, disguising them for usage in pickle fermentation. In addition, many researchers have reported applying Lb. plantarum as a nitrite reducing agent as well as starter culture in paocai (Yan et al., 2008), kimchi (Kim et al., 2017a), pickle (Yu and Zhang, 2013) and vegetable juice (Buckenhüskes, 1993).
Nitrite concentration, pH and aerobic plate counts during Chinese pickle fermentation
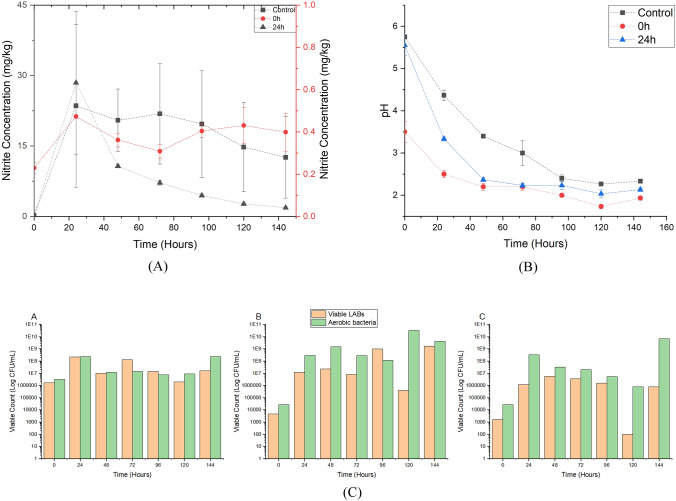
The optimized 8% strain G6 was inoculated into pickles with 8% brine, in comparison with a control (Group Control) in which only an equal amount of sterilized distilled water was inoculated into the Chinese pickles. G6 was inoculated into the pickle at the start of the fermentation (Group 0 h) or after 24 h of spontaneous fermentation without bacteria added (Group 24 h). All three groups of samples with triplicate were fermented at 37 °C for 7 days. During the fermentation, the pickles and brine were acquired for the determination of nitrite concentration, pH and viable bacteria counts, and the results were shown in Fig. 3(A–C), respectively. It was obvious that the standard error of nitrite concentration was high for Group Control and low for Group 24 h, implying that the various bacteria existing in raw cabbages in different samples differed the nitrite concentration.
Fig. 3.
Pickles fermentation. Nitrite concentration (A) and pH (B) of pickles with or without G6 at the start or at 24 h of the 7-days fermentation; Aerobic bacteria and viable LABs (C) counted in logarithmic scale during the fermentation of pickles of Group A-Control, Group B-0 h and Group C-24 h
With the fermentation, all the Chinese pickles exhibited an decrease in pH and the highest nitrite concentration at 24 h. As for the aerobic bacteria, all the samples in almost all time points were dominated by LABs. However, the low aerobic plate count and high viable LABs cannot predict the nitrite concentration, as seen in the comparison between Control and 0 h shown in Fig. 3(A) and (C). This demonstrated that although spontaneous fermentation of Chinese pickles lead to the growth of LABs, not all LABs have the high capacity to reduce nitrite.
It is reported by Walker (1996) that nitrite occurs in fresh plants at low concentrations, usually between 1 and 2 mg/kg and rarely in excess of 10 mg/kg. Nonetheless, nitrite can be accumulated during vegetable storage, in which the concentration of nitrite rises over time to reach a peak value after 4 days of storage and then decreases at ambient temperature for Chinese cabbage (Chung et al., 2004), posing safety threat for spontaneous vegetable fermentation. The addition of G6 can be a promising solution to control the nitrite content during fermentation, as seen in Fig. 3(A). Compared to Control, the 0 h and 24 h demonstrated significant lower nitrite concentration at the end of fermentation, especially at 0 h, of which nitrite was 32 times lower than Control, reaching the final nitrite content at 0.39 ± 0.09 mg/kg. This result determined the applicability of G6 as a nitrite-reducing agent because of the higher nitrite degradation capacity for Chinese pickle fermentation, as compared with other LABs which were used for pickled vegetable fermentation shown in Table 2. Various strains which belong to the genes Lactiplantibacillus such as Lb. plantarum, Lb. pentosus, Lb. brevis, Lb. sakei, Lb. curvatus were reported that they can be used to reduce nitrite in vegetable-based fermentation, but the final nitrite residue at the end of the fermentation rarely fell to 1 mg/kg.
Table 2.
LABs as starter culture to reduce nitrite in vegetable-based fermentation
| Strains | Food products | Raw material | Final nitrite content | Fermentation conditions | Peak nitrite | References |
|---|---|---|---|---|---|---|
| Lb. plantarum | Pickle | Cucumber | < 2 mg/kg | 30 °C, 5 days | At 3 days | Jiang et al. (2021) |
| Lb. plantarum | Pickled Chinese cabbage | Cabbages | ND | 20 °C, 8 days | – | Wang et al. (2010) |
| Lb. plantarum | Pickled Chinese cabbage | 0.1 ± 0.2 mg/kg | 18 °C, 7 days | – | Ji et al. (2009) | |
| Lb. pentosus, Leu. mesenteroides | Paocai | < 5 mg/kg | 30 °C, 10 days | ND | Yan et al. (2008) | |
| Lb. plantarum, Lb. brevis, Leu. mesenteroides | Paocai | Below the maximum limit 20 mg/kg | 25 °C, 7 days | Lb. plantarum: 0.25 mg/mL at 30 h; Lb. brevis: 0.13 mg/mL at 1 day; Leu. Mesenteroides: 0.33 mg/mL at 30 days | Huang et al. (2021) | |
| Lb. sakei, Lb. curvatus, Lb. brevis | Kimchi | < 2.5 mg/kg | Fermented at 13.5 °C for 2 days, and stored at − 1.0 °C for 20 days | ND | Kim et al. (2017a) | |
| Lb. delbrueckii and Lb. sparacasei (1:1 v/v) | Pickled Chinese cabbage | 3 mg/kg | 37 °C, 7 days | – | Han et al. (2014) | |
| Lb. plantarum and Leu. mesenteroides (2:1 v/v) | Paocai | 1 mg/kg | 10 °C, 20 days | ≈ 6.5 mg/kg at 1 day | Liu et al. (2017) |
ND not detectable
The difference of Group 0 h and 24 h in the nitrite content as well as the aerobic plate count should also be noted. In Group 24 h, the nitrite residue was higher than 0 h at all times, even at the end of the fermentation when it reached 1.86 mg/kg. In addition, although the G6 was added at 24 h, the viable LABs in Group 24 h did not rapidly increase, which might be due to the period of strain adaptation or the partial consumption of the nutrients. During the later period of fermentation, G6 seems to be the dominant bacteria because the nitrite residue declined and was lower than Control. Varma et al. (2010) also indicated the addition of Lb. fermentum isolated from human colonic mucosal biopsy samples can not only increase acidity by secreting lactic acid, but produce antimicrobial compounds and surface-associated proteins (SAPs) to inhibit the growth and adhesion of foodborne pathogens, respectively. This result also presented the advantages of LABs isolated from intestine samples, but further studies are needed for the investigation of the probiotic potential of G6, further researchers were needed.
Physicochemical analysis for Chinese pickles
The total acidity (% lactic acid), total solid (%) and salt (%NaCl) of Chinese pickle products were measured as shown in Table 3. Group Control exhibits the highest total acidity and total solid among the products, indicating high acid production as well as low loss of pickle solid. In contrast, for group Control, the lower total solid might be because some bacteria degraded pickles into small scraps which could not be counted. This might also contribute to the lower transparency of the brine (not shown). All the physicochemical parameters of all samples met the demand of standard SB/T 10756-2012 in which total acidity ≤ 1.5, total solid ≥ 50 and salt ≤ 15.
Table 3.
Physicochemical analysis of Chinese pickle products
| Pickle group | Total acidity (% lactic acid) | Total solid (%) | Salt (%NaCl) |
|---|---|---|---|
| Control | 0.12 ± 0.04 | 69.81 | 10.99 ± 0.22 |
| 0 h | 0.23 ± 0.03 | 78.60 | 10.97 ± 0.09 |
| 24 h | 0.16 ± 0.02 | 75.13 | 10.94 ± 0.37 |
In summary, the LAB strain G6 isolated from goose intestine, belongs to the species Lactiplantibacillus plantarum. Lactiplantibacillus sp. G6 can achieve nitrite degradation rate close to 100% when incubated in MRS broth with pH, temperature or inoculum volume higher than 5, 25 °C and 2%, respectively. This study also reveals the advantages of G6 as a starter culture for Chinese pickle manufacturing, achieving higher LABs amount as well as low nitrite residue. The Chinese pickle product with G6 as a starter culture reached the minimum nitrite of 0.39 mg/kg after 7 days of fermentation, meeting the requirements for Chinese pickle products and acquired better quality.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank Zhiwang Wu and Zetao Liu for the help for the experiments and construction of the phylogenetic tree, respectively.
Funding
This study was supported by Key-Area Research and Development Program of Guangdong Province (Project No.2018B020206001) and the Undergraduate Training Program for Innovation and Entrepreneurship of Guangdong Province, China (Grant No. 201610564206).
Declarations
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Alan Y. Culture fermentation of Lactobacillus in traditional pickled gherkins: Microbial development, chemical, biogenic amine and metabolite analysis. J. Food Sci. Technol. 2019;56(8):3930–3939. doi: 10.1007/s13197-019-03866-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakari D, Tatsadjieu NL, Mbawala A, Mbofung CM. Assessment of physiological properties of some lactic acid bacteria isolated from the intestine of chickens use as probiotics and antimicrobial agents against enteropathogenic bacteria. Innov. Rom. Food Biotechnol. 2011;8:33–40. [Google Scholar]
- Bringel F, Castioni A, Olukoya DK, Felis GE, Torriani S, Dellaglio F. Lactobacillus plantarum subsp. argentoratensis subsp. nov., isolated from vegetable matrices. Int. J. Syst. Evol. Microbiol. 2005;55:1629–1634. doi: 10.1099/ijs.0.63333-0. [DOI] [PubMed] [Google Scholar]
- Buckenhüskes HJ. Selection criteria for lactic acid bacteria to be used as starter cultures for various food commodities. FEMS Microbiol. Rev. 1993;12(1):253–271. doi: 10.1016/0168-6445(93)90067-J. [DOI] [Google Scholar]
- Chung JC, Chou SS, Hwang DF. Changes in nitrate and nitrite content of four vegetables during storage at refrigerated and ambient temperatures. Food Addit. Contam. 2004;21(4):317–322. doi: 10.1080/02652030410001668763. [DOI] [PubMed] [Google Scholar]
- Çon A, Karasu N. Determination of antagonistic starter cultures for pickle and olive fermentation processes. Czech J. Food Sci. 2009 doi: 10.17221/86/2008-CJFS. [DOI] [Google Scholar]
- Dodds KL, Collins-Thompson DL. Incidence of nitrite-depleting lactic acid bacteria in cured meats and in meat starter cultures. J. Food Prot. 1984;47(1):7–10. doi: 10.4315/0362-028x-47.1.7. [DOI] [PubMed] [Google Scholar]
- Fei YT, Liu DM, Luo TH, Chen G, Wu H, Li L, Yu YG. Molecular characterization of Lactobacillus plantarum DMDL 9010, a strain with efficient nitrite degradation capacity. PLoS ONE. 2014;9(11):e113792. doi: 10.1371/journal.pone.0113792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu W, Mathews AP. Lactic acid production from lactose by Lactobacillus plantarum: kinetic model and effects of pH, substrate, and oxygen. Biochem. Eng. J. 1999;3(3):163–170. doi: 10.1016/S1369-703X(99)00014-5. [DOI] [Google Scholar]
- Giraud E, Lelong B, Raimbault M. Influence of pH and initial lactate concentration on the growth of Lactobacillus plantarum. Appl. Microbiol. Biotechnol. 1991 doi: 10.1007/BF00164706. [DOI] [Google Scholar]
- Han X, Yi H, Zhang L, Huang W, Zhang Y, Zhang L, Du M. Improvement of fermented Chinese cabbage characteristics by selected starter cultures. J. Food Sci. 2014;79(7):M1387–M1392. doi: 10.1111/1750-3841.12495. [DOI] [PubMed] [Google Scholar]
- Huang Y-Y, Jia X-Z, Yu J-J, Chen Y-H, Liu D-M, Liang M-H. Effect of different lactic acid bacteria on nitrite degradation, volatile profiles, and sensory quality in Chinese traditional paocai. LWT. 2021 doi: 10.1016/j.lwt.2021.111597. [DOI] [Google Scholar]
- Ji F-D, Ji B-P, Li BO, Lu FEI. Effect of fermentation on nitrate, nitrite and organic acid contents in traditional pickled Chinese cabbage. J. Food Process. Preserv. 2009;33:175–186. doi: 10.1111/j.1745-4549.2008.00291.x. [DOI] [Google Scholar]
- Jiang J, Li N, Wang W, Xie R, Qiao Y, Wang F, Zhang C. Screening and characterization of nitrite degrading Lactobacillus plantarum in Chinese traditional pickles. Food Nutr. Sci. 2021;12(12):1287–1298. doi: 10.4236/fns.2021.1212094. [DOI] [Google Scholar]
- Jiang X, Li X, Zhang B, Ren D. Isolation and identification of nitrite-degrading lactic acid bacteria from traditional pickled vegetable. China Brew. 2008;1:13–16. doi: 10.3969/j.issn.0254-5071.2008.01.005. [DOI] [Google Scholar]
- Kim S-H, Kang KH, Kim SH, Lee S, Lee S-H, Ha E-S, Sung NJ, Kim JG, Chung MJ. Lactic acid bacteria directly degrade N-nitrosodimethylamine and increase the nitrite-scavenging ability in kimchi. Food Control 71: 101-109 (2017a) 10.1016/j.foodcont.2016.06.039
- Kim S-H, Kim SH, Kang KH, Lee S, Kim SJ, Kim JG, Chung MJ. Kimchi probiotic bacteria contribute to reduced amounts of N-nitrosodimethylamine in lactic acid bacteria-fortified kimchi. LWT 84: 196-203 (2017b) 10.1016/j.lwt.2017.05.060
- Korkeala H, Alanko T, Tiusanen T. Effect of sodium nitrite and sodium chloride on growth of lactic acid bacteria. Acta Vet. Scand. 1992;33(1):27–32. doi: 10.1186/BF03546933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Wang B, Liu L. Study on influencing factors of nitrite degradation. Food Ind. 2010;4:7–9. [Google Scholar]
- Liu A, Li X, Pu B, Ao X, Zhou K, He L, Chen S, Liu S. Use of psychrotolerant lactic acid bacteria (Lactobacillus spp. and Leuconostoc spp.) isolated from Chinese traditional paocai for the quality improvement of paocai products. J. Agric. Food Chem. 2017;65(12):2580–2587. doi: 10.1021/acs.jafc.7b00050. [DOI] [PubMed] [Google Scholar]
- Liu G, Luo X, Zhao X, Zhang A, Jiang N, Yang L, Huang M, Xu L, Ding L, Li M, Guo Z, Meng H. Gut microbiota correlates with fiber and apparent nutrients digestion in goose. Poult. Sci. 2018;97(11):3899–3909. doi: 10.3382/ps/pey249. [DOI] [PubMed] [Google Scholar]
- Liu W, Bao Q, Jirimutu, Qing M, Siriguleng, Chen X, Sun T, Li M, Zhang J, Yu J, Bilige M, Sun T, Zhang H. Isolation and identification of lactic acid bacteria from Tarag in Eastern Inner Mongolia of China by 16S rRNA sequences and DGGE analysis. Microbiol. Res. 167(2): 110-115 (2012) 10.1016/j.micres.2011.05.001 [DOI] [PubMed]
- Liu X. Research on Nitrite Degradation by Organic Acids in Pickle Fermentation. Shandong Agricultural University, Shandong (2013) http://cdmd.cnki.com.cn/Article/CDMD-10434-1014156906.htm
- Lokapirnasari WP, Sahidu AM, Soepranianondo K, Supriyanto A, Yulianto AB, Al Arif A. Potency of lactic acid bacteria isolated from balinese bovine (Bos sondaicus) intestinal waste from slaughterhouse to improve nutrient content of wheat pollard as animal feedstuff by fermentation process. Vet. World. 2018;11(8):1127–1134. doi: 10.14202/vetworld.2018.1127-1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald LC, Fleming HP, Hassan HM. Acid tolerance of Leuconostoc mesenteroides and Lactobacillus plantarum. Appl. Environ. Microbiol. 1990;56(7):2120–2124. doi: 10.1128/aem.56.7.2120-2124.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musikasang H, Tani A, H-Kittikun A, Maneerat S. Probiotic potential of lactic acid bacteria isolated from chicken gastrointestinal digestive tract. World J. Microbiol. Biotechnol. 25(8): 1337-1345 (2009) 10.1007/s11274-009-0020-8
- Oh CK, Oh MC, Kim SH. The depletion of sodium nitrite by lactic acid bacteria isolated from kimchi. J. Med. Food. 2004;7(1):38–44. doi: 10.1089/109662004322984680. [DOI] [PubMed] [Google Scholar]
- Panghal A, Janghu S, Virkar K, Gat Y, Kumar V, Chhikara N. Potential non-dairy probiotic products—A healthy approach. Food Biosci. 2018;21:80–89. doi: 10.1016/j.fbio.2017.12.003. [DOI] [Google Scholar]
- Rajoka MShR, Hayat HF, Sarwar S, Mehwish HM, Ahmad F, Hussain N, Shah SZH, Khurshid M, Siddiqu M, Shi J. Isolation and evaluation of probiotic potential of lactic acid bacteria isolated from poultry intestine. Microbiology. 2018;87(1):116–126. doi: 10.1134/s0026261718010150. [DOI] [Google Scholar]
- Shang J, Wang X, Wang J. Effect of degradation of nitrite by organic acids in pickles. Food Mach. 2018;34(3):73–78. doi: 10.13652/ji.sn.1003-5788.2018.03.015. [DOI] [Google Scholar]
- Syrokou MK, Stasinopoulou P, Paramithiotis S, Bosnea L, Mataragas M, Papadopoulos GK, Skandamis PN, Drosinos EH. The effect of incubation temperature, substrate and initial pH value on plantaricin activity and the relative transcription of PLN genes of six sourdough derived Lactiplantibacillus plantarum strains. Fermentation. 2021 doi: 10.3390/fermentation7040320. [DOI] [Google Scholar]
- Tamme T, Reinik M, Roasto M, Juhkam K, Tenno T, Kiis A. Nitrates and nitrites in vegetables and vegetable-based products and their intakes by the Estonian population. Food Addit. Contam. 2006;23(4):355–361. doi: 10.1080/02652030500482363. [DOI] [PubMed] [Google Scholar]
- Varma P, Dinesh KR, Menon KK, Biswas R. Lactobacillus fermentum isolated from human colonic mucosal biopsy inhibits the growth and adhesion of enteric and foodborne pathogens. J. Food Sci. 2010;75(9):M546–M551. doi: 10.1111/j.1750-3841.2010.01818.x. [DOI] [PubMed] [Google Scholar]
- Walker R. The metabolism of dietary nitrites and nitrates. Biochem. Soc. Trans. 1996;24(3):780–785. doi: 10.1042/bst0240780. [DOI] [PubMed] [Google Scholar]
- Wang C, Ma Y-Y, Chen M-H, Wang Y-R, Lei S, Li F-J, Liu D-W. Effect of pH on nitrite reduction of pickled Chinese cabbage. pp. 1-4. In: 2010 4th International Conference on Bioinformatics and Biomedical Engineering (iCBBE) (2010) 10.1109/ICBBE.2010.5515295
- Xia Y, Liu X, Wang G, Zhang H, Xiong Z, Sun Y, Ai L. Characterization and selection of Lactobacillus brevis starter for nitrite degradation of Chinese pickle. Food Control. 2017;78:126–131. doi: 10.1016/j.foodcont.2017.02.046. [DOI] [Google Scholar]
- Xiong T, Li J, Liang F, Wang Y, Guan Q, Xie M. Effects of salt concentration on Chinese sauerkraut fermentation. LWT. 2016;69:169–174. doi: 10.1016/j.lwt.2015.12.057. [DOI] [Google Scholar]
- Xu Q, Yuan X, Gu T, Li Y, Dai W, Shen X, Song Y, Zhang Y, Zhao W, Chang G, Chen G. Comparative characterization of bacterial communities in geese fed all-grass or high-grain diets. PLoS ONE. 2017;12(10):e0185590. doi: 10.1371/journal.pone.0185590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan P-M, Xue W-T, Tan S-S, Zhang H, Chang X-H. Effect of inoculating lactic acid bacteria starter cultures on the nitrite concentration of fermenting Chinese paocai. Food Control. 2008;19(1):50–55. doi: 10.1016/j.foodcont.2007.02.008. [DOI] [Google Scholar]
- Yu SM, Zhang Y. Effects of lactic acid bacteria on nitrite degradation during pickle fermentation. Adv. Mater. Res. 2013;781–784:1656–1660. doi: 10.4028/www.scientific.net/AMR.781-784.1656. [DOI] [Google Scholar]
- Zhang Y, Chi N, Zheng Y, Wang S, Feng Y. Effects of lactic acid bacteria on nitrite degradation during pickle fermentation. Food Ferment. Ind. 2002;28:27–31. [Google Scholar]
- Zheng Y, Chi SM, Zheng SM, Wang SM, Feng SM. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2002;70:2782–2858. doi: 10.1099/ijsem.0.004107. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.