Abstract
Various cancer therapies, such as surgery, radiotherapy, chemotherapy, and immunotherapy, have been used to treat cancer. Among cancer immunotherapies, stimulators of interferon genes (STING) activate various immune cells and induce them to attack cancer cells. However, the secretion of type I interferon (IFN α and β) increases after stimulation of the immune cell as a side effect of STING agonist, thereby increasing the expression of programmed death-ligand 1 (PD-L1) in the tumor microenvironment (TME). Therefore, it is necessary to reduce the side effects of STING agonists and maximize cancer treatment by administering combination therapy. Tumor-bearing mice were treated with cisplatin, tumor-specific peptide, neoantigen, DMXAA (STING agonist), and immune checkpoint inhibitor (ICI). The combination vaccine group showed a reduction in tumor mass, an increased survival rate, and IFN-γ+ (interferon gamma) CD8+ (cluster of differentiation 8) T cells in the spleen and TME. The distribution of immune cells in the spleen and TME was confirmed, and the number of active immune cells increased, whereas that of immunosuppressive cells decreased. When measuring cytokine levels in the tumor and serum, the levels of pro-inflammatory cytokines increased and anti-inflammatory cytokines decreased. This study demonstrated that when various cancer therapies are combined to treat cancer, it can lead to an anticancer immune synergistic effect by increasing the immune response and reducing side effects.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00262-022-03220-6.
Keywords: Cancer immunotherapy, STING, Immune checkpoint inhibitor, Tumor microenvironment, Type I IFN, CD8+ T cell
Introduction
Various cancer therapies (e.g., surgery, chemotherapy, and immunotherapy) have been developed to treat cancer and improve patient survival [1]. Surgery, the most common cancer treatment, has a risk of recurrence and metastasis; therefore, it must be combined with various cancer treatments [2]. For example, cancer therapies such as chemotherapy (e.g., cisplatin, doxorubicin), immunotherapy (e.g., DNA or RNA vaccine, antibody-based therapy), and adjuvant therapy have been reported to decrease cancer recurrence rate and increase the survival rate of cancer patients in actual clinical practice [3]. However, each cancer therapy has various side effects [4]. Therefore, further research is necessary to compensate for the side effects of appropriate co-administration of various cancer treatments [5].
Immunotherapy is a new cancer treatment that utilizes the immune system [6] and reduces tumor-induced immunosuppression. Therefore, anticancer immunotherapy using T cell immunity is being studied as the safest and most efficient cancer treatment [7]. T cell immunity causes antigen-presenting cells (APCs; e.g., dendritic cells) to take up and present tumor antigens, activate T cells, and eventually kill the tumor [8–10]. Neoantigen vaccines use cancer-specific neoantigens extracted from cancer patients for T cell immunity [11]. Therefore, it is actively used in clinical practice because it can be applied simultaneously with other cancer therapies [12]. For anticancer immunotherapy that efficiently uses T cell immunity, it is important to confirm the presence of tumor-specific antigens and induce them in an abundant environment [11].
Chemotherapy can induce high antigen exposure to the tumor microenvironment (TME), which increases the anticancer immune response in combination with immunotherapy [13, 14]. After chemotherapy, damage-associated molecular patterns (DAMPs) are released from dying cells; their stimulating responses induce T cell activation and produce and secrete cytokines, such as type I interferon (IFN). These cytokines are known as major regulators of anticancer immune response activation, but the effect of type I IFN secreted by chemotherapy alone is insufficient to treat cancer [15–17]. The stimulators of interferon genes (STING) agonist was developed as a method to increase type I IFN, and synergistic anticancer immune response can be induced through chemotherapy and co-administration [18, 19].
STING-mediated type I IFN binds to interferon α/β receptors to protect infected and surrounding cells from local infection [20]. Recently, many studies have shown that activation of STING and stimulation of type I IFN production are important for anticancer immune responses and for promoting the release of cancer cell antigens [21–23]. Moreover, STING activation enhances cancer antigen presentation to contribute to the priming and activation of T cells and promotes trafficking and infiltration of T cells into tumors [19, 21, 24]. However, it is possible to induce an immune suppression response by upregulating the expression of the immune checkpoint programmed death-ligand 1 (PD-L1) in tumor cells as a side effect of type I IFN [25, 26]. Accordingly, it is important to decrease side effects by administering a combination of immune checkpoint inhibitor (ICI) that block the immune checkpoint [25].
In conclusion, various cancer therapies must be administered simultaneously to induce a synergistic effect for safe and efficient tumor treatment and reduce side effects [18, 27, 28]. Combination therapy can increase the exposure of tumor antigens, generate tumor-specific T cell immune responses, change the TME into immune activity responses, and downregulate immune suppression responses by tumors [9, 29, 30]. In our study, tumor-specific antigens and neoantigens were used as vaccines in various mouse models for the diversity of the MHC I class [11, 31, 32]. In addition, the combination of cisplatin and tumor antigen, STING agonist, and ICIs induces priming of tumor-specific CD8+ T cells to amplify the immune response, thereby demonstrating antitumor effects that can kill tumors [19, 33, 34].
Materials and methods
Mice and cells
C57BL/6 and BALB/c mice were purchased from Orient Bio Inc. (Seongnam, Korea). STING knockout mice (C57BL/6J-Tmem173<gt>/J) were purchased from Jackson Laboratories (Maine, USA). Six-week-old female mice were used, and all protocols were approved by the Institutional Animal Care and Use Committee (IACUC) of Konkuk University. TC-1 cells (murine tumor cells expressing the E6/E7 gene of human papillomavirus (HPV) 16) and CT26 cell line (murine colon carcinoma) were incubated in RPMI-1640 medium (Biowest, France) supplemented with 10% fetal bovine serum (FBS, Biowest, France) and 50 U/mL penicillin streptomycin (P/S, Biowest, France) at 37 °C and 5% CO2. Mouse bone marrow dendritic cells (BMDCs) and plasmacytoid dendritic cells (pDCs) were isolated from mouse bone marrow and cultured in RPMI-1640 medium supplemented with 10% FBS, 50 U/mL P/S, 1000 μM 2-mercaptomethanol (2-mer, Gibco, USA), and granulocyte–macrophage colony-stimulating factor (GM-CSF; JW CreaGene, Korea); the pDC culture medium was further supplemented with 20 ng recombinant mouse IL-4 and 1 µg Flt3-Ligand (Pepro Tech, Korea). The culture medium was added every two days, and the cells were differentiated for six days.
In vivo tumor treatment experiments
In the TC-1 tumor model, C57BL/6 mice were subcutaneously injected with 2 × 105 TC-1 cells/mice on day 0. Mice were then treated intraperitoneally with 5 mg/kg cisplatin (Sigma-Aldrich, Germany) on days 15 and 18 (18 and 21 for large tumors), intratumorally with E7 42–63 long peptide (AGQAEPDRAHYNIVTFCCKCDS) (20 µg/mouse) and DMXAA (100 µg/mouse; InvivoGen, USA) on days 16 and 19 (19 and 22 for large tumors), and intraperitoneally with anti-PD-1 (clone RMP1-14) and anti-PD-L1 (clone 10F.9G2) antibodies (both 100 µg/mouse, BioXcell, USA) on days 19, 21, 23, 25, and 27. For the type 1 IFN blocking experiment, IFNAR1 (clone MAR1-5A3) and isotype mouse IgG1 (clone MOPC-21) antibodies (100 µg/mouse, BioXcell, USA) were injected intraperitoneally on days 17, 19, 21, 23, and 25. In the CT26 tumor model, BALB/c mice were subcutaneously injected with 2 × 105 CT26 cells/mice on day 0. Mice were then treated intraperitoneally with 5 mg/kg cisplatin on days 12 and 15, intratumorally with neoantigen WT (PAPRAVLTGHDHEVVCVSVCAELGLVI) and MT (PAPRAVLTGHDHEIVCVSVCAELGLVI) peptides (20 µg/mouse) on days 13, 16, 19, 22, and 25, intratumorally with DMXAA (100 µg/mouse) on days 13 and 16; and intraperitoneally with anti-PD-1 and anti-PD-L1 antibodies (100 µg/mouse) on days 13, 15, 17, and 19. Euthanasia, via slow filling of the euthanasia chamber with CO2 gas (injection rate, 10–20% per minute), was performed when the tumor exceeded 10% of bodyweight or the tumor size reached 2 cm or more.
Analysis of antigen-specific IFN-γ+ CD8+ T cell response
Tumor tissues and spleens of mice were harvested. TME cells and splenocytes were treated with ammonium-chloride-potassium (ACK) solution (Quality Biological, Gaithersburg, MD, USA) for red blood cell lysis. The cells were incubated for 16 h with 1 µg/ml E7 49–57 peptide (RAHYNIVTF) and 1 µl/ml GolgiPlug (BD Cytofix/Cytoperm Kit). After incubation, the cells were stained with PE anti-CD8 (clone 53–6.7, Biolegend, Korea) antibody at 4 °C for 30 min, washed with PBS, incubated in BD Cytofix/Cytoperm solution at 4 °C for 20 min, and stained with APC anti-IFN-γ (XMG1.2, Invitrogen, USA) monoclonal antibodies (1:100 dilution). All cells were analyzed using a BD Accuri C6 Plus flow cytometer.
NK, CD4, and CD8 T cell depletion experiments
C57BL/6 mice were injected subcutaneously with 2 × 105 TC-1 cells/mouse on day 0. Mice were then treated intraperitoneally with 5 mg/kg cisplatin on days 15 and 18, intratumorally with E7 long peptide (20 µg/mouse) and DMXAA (100 µg/mouse) on days 16 and 19, and intraperitoneally with control isotype IgG (Rat IgG2b, clone LTF-2), anti-CD4 (clone GK1.5), anti-CD8 (clone 2.43), or anti-NK (clone PK136) depletion antibodies (BioXcell, USA) (all 100 µg/mouse) on days 17, 19, 21, and 23.
Analysis of spleen and TME cells
Three days after the last vaccination, spleen and tumor tissues were harvested, and the tumor tissues were digested using the gentleMACS Dissociator and MACS Tumor Dissociation kits (Miltenyi Biotec, Germany). Splenocytes and digested TME cells were treated with an ACK solution. To evaluate the distribution of the T cell population, cells were stained with APC anti-CD3 (927), PE anti-CD8, and FITC anti-CD4 antibodies (GK1.5). Myeloid-derived suppressor cells (MDSCs) were stained with PE anti-CD11b (M1/70, Invitrogen) and FITC anti-Gr1 (RB6-8C5) antibodies. Macrophages were stained with PE anti-CD11b, APC F4/80(BM8, Invitrogen), and FITC CD206 (C068C2) antibodies. Tregs were stained with FITC anti-CD4 and APC anti-CD25 antibodies (PC61.5) at 4 °C for 30 min, washed with PBS, incubated in Fixation/Permeabilization solution (Invitrogen) at 4 °C for 20 min, and stained for PE anti-Foxp3 antibodies (FJK-16S). To evaluate the distribution of PD-L1 expression in immune and tumor cells, cells were stained with FITC anti-CD45 (30-F11) and PE anti-PD-L1 antibodies (10F.9G2) (Invitrogen, all 1:100 dilution).
Neoantigen peptide screening and synthesis
Another group studied mutant neoantigens that drive therapeutic immune responses to cancer [31]. We confirmed the mutations of 17 genes in the CT26 tumor cell line by total RNA isolation from tumor cells, followed by cDNA synthesis and amplification, and mutant gene identification through direct sequence analysis (Supplementary Table. S1). Through preliminary experiments, mutant CD4+ T cell neoantigen peptides (Nbea) were selected for use in this study (Supplementary Fig. S1). Neoantigen peptides used in our experiments were custom-made by Anygen (Gwangju, Korea).
Enzyme-linked immunosorbent assay (ELISA)
For cytokine analysis, tumor tissues, serum, and splenocytes were harvested. The tumor tissue was chopped, immersed in ice with RIPA protein extraction buffer (150 nmol/L NaCl, 50 nmol/L Tris–Cl (pH 8.0), 1 mmol/L phenylmethylsulfonyl fluoride (PMSF), 1% Nonidet P-40 (NP-40), 0.1% sodium dodecyl sulfate (SDS), and 0.5 mM EDTA) for 2 h and then centrifuged at 13,000 rpm for 15 min. Serum was extracted from mice, placed at room temperature for 40 min, and then centrifuged at 13,000 rpm for 15 min to separate the supernatant. The splenocytes were then treated with ACK solution and incubated with WT or MT neoantigen peptides for 48 h. After incubation, the supernatants were harvested, and cytokine levels were assessed using a mouse IFN-γ ELISA kit (Invitrogen, USA). DCs of all mice and tumor cells were treated with 5 µg/ml DMXAA for 24 h. After incubation, the supernatants were harvested, and cytokine levels were assessed using a mouse IL-6, TNF-α, IL-10, TGF-β, IFN-γ, and IFN-β ELISA kit (Invitrogen, USA), following the manufacturer’s protocol.
PD-L1 expression analysis
To evaluate PD-L1 expression, paraffin block production and sectioning by isolation of tumor tissues were followed by H&E staining (Logone Bio, Korea) and PD-L1 (anti-PD-L1, Cell Signaling, USA) immunohistochemistry (IHC). Slides of the stained tumor tissues were analyzed using photomicrographs, NIS elements, and Image J software. Mice were intravenously injected with 100 µg/mouse PD-L1 antibody labeled with Cy5.5-NHS ester (Click Chemistry Tools, USA), and 18 h later, tumor tissue was isolated and analyzed using the IVIS Spectrum Imaging System. To evaluate PD-L1 expression in tumor cells, BMDCs and pDCs isolated and differentiated from naive mice were treated with or without 10 μg/ml DMXAA, and the supernatant was incubated with tumor cells overnight. To evaluate the direct cytokine-inducing PD-L1 expression, tumor cells were treated overnight with 100 ng/ml recombinant proteins (recombinant mouse IL-6, TNF-α, IL-10, TGF-β, IFN-γ, and IFN-β; BioXcell, USA). To evaluate PD-L1 expression following cytokine blockage, tumor cells were treated with 10 ng/ml blocking antibody (anti-mouse IL-6, IL-10, TNF-α, TGF-β, IFN-γ, and IFNAR1; BioXcell, USA) for 30 min, washed, and then incubated overnight with the supernatants of DMXAA-treated BMDCs or pDCs. Tumor cells were stained with PE anti-PD-L1 antibody (diluted 1:100) and analyzed using a BD Accuri C6 Plus flow cytometer.
Western blotting
To confirm the activation of signal transducer and activator of transcription 1 (STAT1) induced by STING and type I IFN, TC-1 tumor cells were treated cisplatin (20 µg/ml) for 2–3 h, washed, and then incubated overnight with DMXAA (10 µg/ml) or the supernatant of DMXAA-treated BMDCs. Cells were harvested and lysed with RIPA protein extraction buffer on ice for 1 h. The extracted protein concentration was measured using a Bradford protein assay kit (Pierce). Proteins were solubilized in sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) loading buffer (250 mM Tris–HCl, pH 6.8, 0.5 M DTT, 10% SDS, 0.25% bromophenol blue, 50% glycerol) and boiled for 10 min at 100 °C. The solubilized proteins were then separated by SDS-PAGE and transferred to PVDF membranes (Roche). Primary antibodies against phospho-STAT1, total STAT1 (Cell Signaling Technology), and β-actin (Santa Cruz) were used at 1:1000 dilution in 5% BSA. Appropriate horseradish peroxidase (HRP)-conjugated secondary antibodies (Enzo and Abbiotech) were used at 1:5000 dilution in 5% skim milk. Immunoreactive bands were confirmed using Immobilon Western Chemiluminescent HRP Substrate (Merck Millipore) (Supplementary Fig. S2).
Statistical analysis
The t tests used represented statistical significance as follows: *P < 0.05, **P < 0.01, ***P < 0.001. All experiments were performed three times independently, and IBM (International Business Machines Co.) Statistical Package for the Social Sciences (SPSS) Statistics Base 22.0 was used as a statistical tool to analyze the differences between the groups in survival experiments.
Results
Co-administration of tumor-specific antigen and STING agonist in cisplatin-treated tumor-bearing mouse models induces anticancer effects
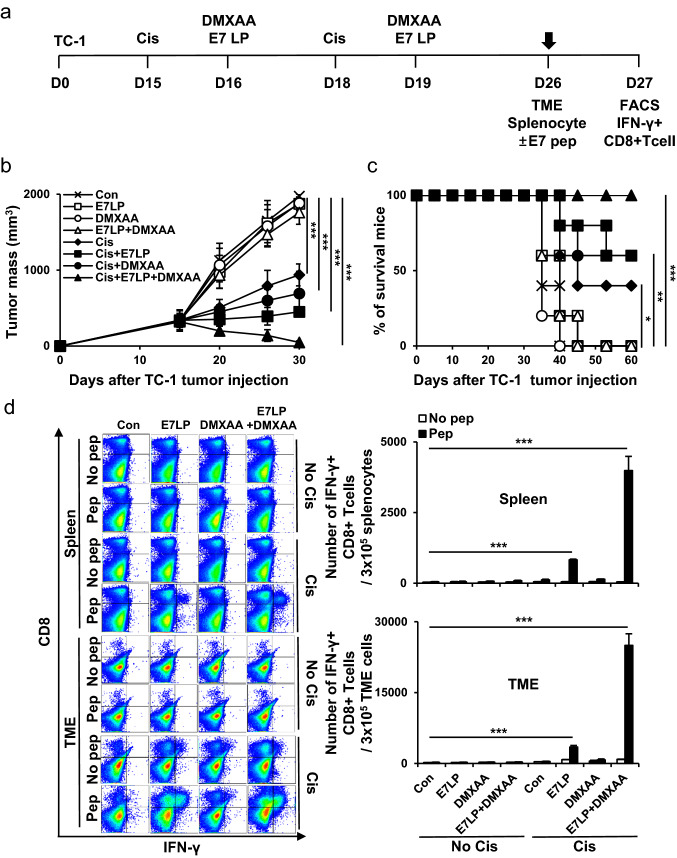
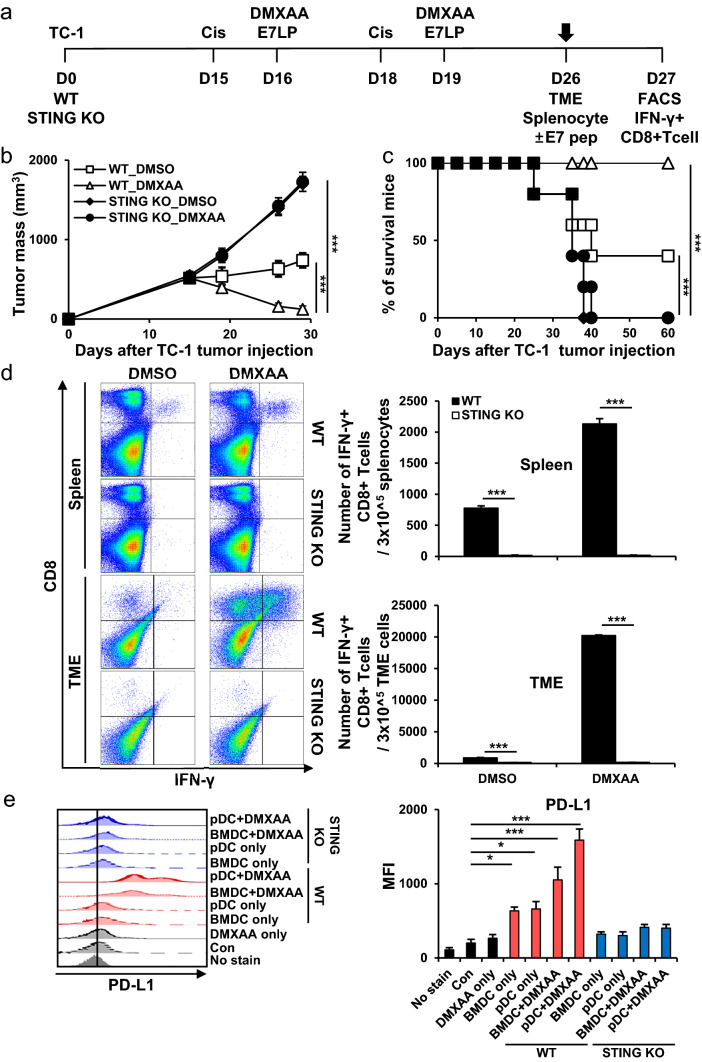
TC-1 tumor cells were subcutaneously inoculated into mice to evaluate the anticancer effects of the tumor-specific antigen and STING agonist vaccination in a cisplatin-treated tumor-bearing mouse model (Fig. 1a). The combination vaccine group strongly controlled tumor growth compared with the other groups (Fig. 1b). In addition, the vaccine groups survived for more than 60 days after tumor injection (Fig. 1c). The tumor mass was confirmed by day 60, and the tumor did not grow again in the vaccine group. Similar to previous studies, we expected that tumor rechallenge will not regrow [35]. When evaluating the E7-specific immune response, a much stronger systemic and tumor-infiltrating E7-specific IFN-γ+ CD8+ T cell response was detected in the vaccine groups (Fig. 1d). According to previous studies, we expect that IFNr+ CD4+ T cells will increase since IFNr+ CD8+ T cells increased [36–38]. In addition, it was confirmed that the direct tumor-killing effect of CD8+ T cells was confirmed to be the most important function when immune cell depletion is involved in tumor death (Supplementary Fig. S3). These data suggested that combining tumor-specific antigens and STING agonists improve the immunogenicity of cancer vaccines after cisplatin treatment.
Fig. 1.
Tumor treatment effects of tumor-specific antigen and STING agonist co-administration in cisplatin-treated mouse model. C57BL/6 mice were subcutaneously injected with 2 × 105 TC-1 cells/mouse on day 0. Mice were then treated intraperitoneally with 5 mg/kg cisplatin on days 15 and 18 and intratumorally with 20 µg/mouse E7 long peptide and 100 µg/mouse DMXAA on days 16 and 19. a Schedule flowchart. b Tumor mass was measured until the mice died or the tumor diameter was > 2 cm (n = 5). c Mouse survival was observed for 60 days (n = 5). d One week after the last vaccination, the tumor tissues and spleens of TC-1 tumor-bearing mice were harvested and re-stimulated with E7 short peptide and then analyzed for IFN-γ+ CD8+ T cells by flow cytometry (n = 5). IBM SPSS Statistics Base 22.0 was used for statistical analysis. *P < 0.05, **P < 0.01, ***P < 0.001
Co-administration of tumor-specific antigen and STING agonist in cisplatin-treated tumor-bearing mouse models induces immune activation response
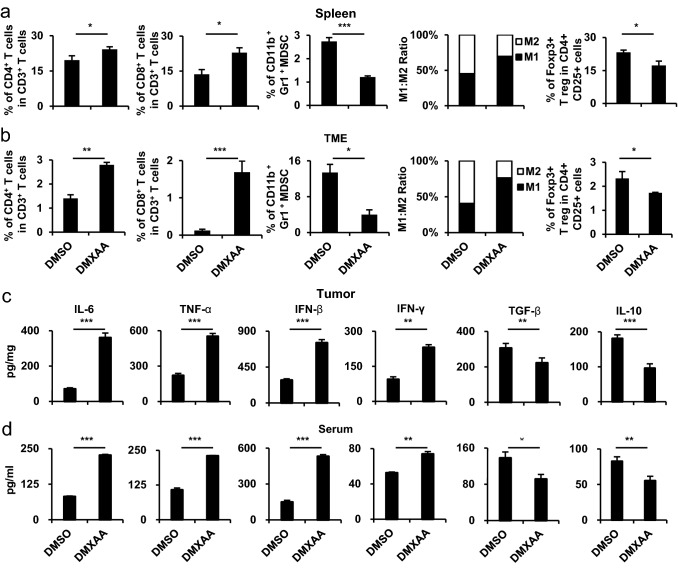
To evaluate the systemic and tumor changes after combination vaccine, the spleen and tumor tissues were harvested and the distribution of various immune cell populations was analyzed (Fig. 2a, b). In the DMXAA group, the number of immune-activating CD4+ T cells, CD8+ T cells, and M1 macrophages in the spleen and TME was higher than those in the other group. In addition, the DMXAA group showed a reduced distribution of immunosuppressive regulatory T cells (Tregs), myeloid-derived suppressive cells (MDSCs), and M2 macrophages. Similarly, an increase in the pro-inflammatory cytokines IL-6, TNF-α, IFN-γ, and IFN-β (type I IFN) levels and a decrease in TGF-β and IL-10 levels were observed in the tumor and serum of the DMXAA group (Fig. 2c, d). These data suggest that the combination vaccine has an anticancer effect by reducing the immunosuppressive response and increasing the immune activation response in systemic and TME.
Fig. 2.
Characterization after the co-administration of tumor-specific antigen and STING agonist in cisplatin-treated mouse model. In the in vivo experiments, the groups were as follows: cisplatin treatment with E7 long peptide vaccination (DMSO) and cisplatin treatment with E7 long peptide and DMXAA vaccination (DMXAA). a Tumor mass was measured until the mice died or the tumor diameter reached > 2 cm (n = 5). b Mouse survival was observed for 60 days (n = 5). c One week after the last vaccination, the tumor tissues and spleens of TC-1 tumor-bearing mice were harvested and re-stimulated with E7 short peptide and then analyzed for IFN-γ+ CD8+ T cells by flow cytometry (n = 5). d, e Tumor tissues and spleens of the mice were harvested on day 22. Bar graphs depict the presence of CD4+ T cells, CD8+ T cells, MDSCs, and Treg cells and the M1 and M2 distribution percentages of CD11b+ F4/80+ macrophages, as evaluated by flow cytometry analysis (n = 5). f, g One week after the last vaccination, the tumor tissues and serum from the same mice in c were harvested. Bar graphs represent the levels of cytokines in the tumor tissue and serum of mice as measured by ELISA (n = 5). IBM SPSS Statistics Base 22.0 was used for statistical analysis. *P < 0.05, **P < 0.01, ***P < 0.001
Type 1 IFN induced by STING agonist increases PD-L1 expression
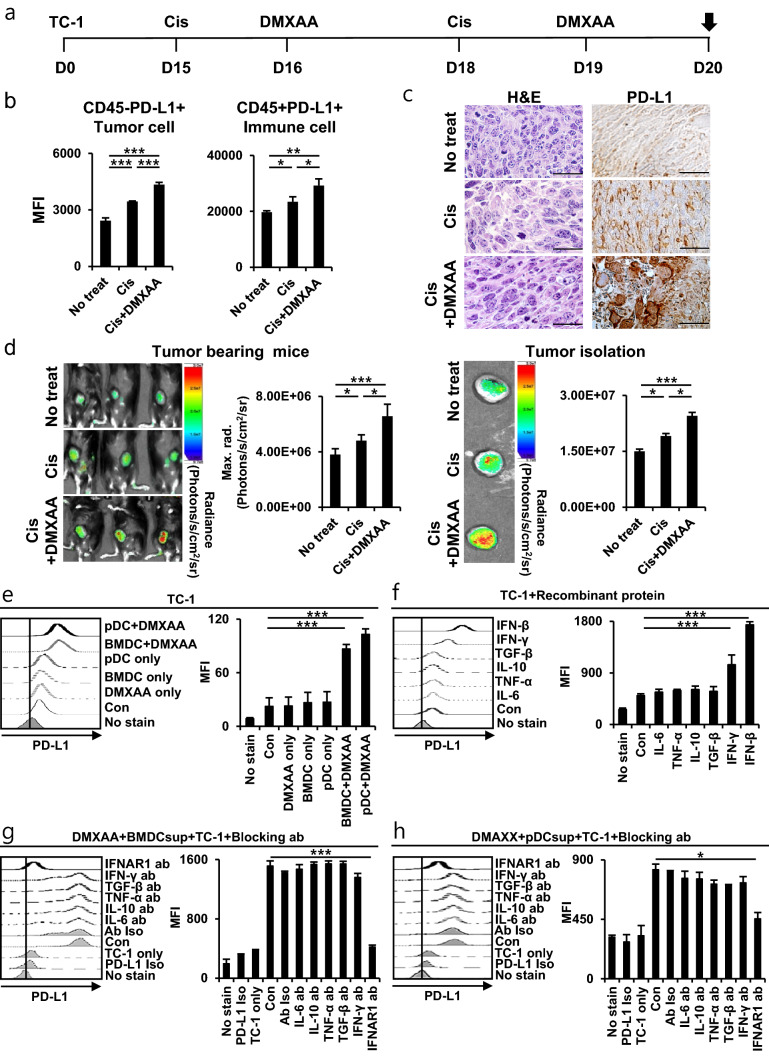
After STING agonist vaccination, the degree of immune checkpoint PD-L1 expression in tumor cells was evaluated. Tumor-bearing mice were injected with cisplatin and DMXAA (Fig. 3a). Compared to the other groups, tumors in the DMXAA group showed increased PD-L1 of immune cells and tumor cells in the TME (Fig. 3b). Additionally, the enhancement of PD-L1 was confirmed by PD-L1 IHC tissue staining after tumor isolation in the DMXAA group (Fig. 3c). We confirmed the level of PD-L1 in the tumors of the mouse model with the degree of fluorescence expression via the IVIS spectrum imaging system, and the PD-L1 increased in the DMXAA group (Fig. 3d). These data suggest that the activation of STING and chemotherapy induces increased of tumor PD-L1. Add to, it was confirmed that when the STING pathway of DCs was activated by DMXAA, the PD-L1 in tumor cells increased (Fig. 3e). In addition, when recombinant proteins of pro-inflammatory and anti-inflammatory cytokines were used in tumor cells, PD-L1 in cells treated with IFN-β (type I IFN) and IFN-γ cytokines was increased (Fig. 3f). PD-L1 expression by IFN-β is higher than by IFN-γ in TC-1 tumor cells, and these results may increase in IFN-γ, depending on the type of tumor cell or cytokine concentration [39]. STING also increases various cytokines when activated together with DC (Supplementary Fig. S4). PD-L1 expression in tumor cells treated with IFNAR1 antibody was reduced compared to the other groups (Fig. 3g, h). Anticancer response was reduced when IFNAR1 antibody was used in tumor-bearing mice in vivo (Supplementary Fig. S5). According to these results, when STING and APC is activated, the level of type I IFN cytokines increases and induces PD-1L expression in tumor cells. In conclusion, it is important to minimize the side effects and improve the anticancer effects by blocking PD-L1 with ICI.
Fig. 3.
PD-L1 expression by STING activation in vivo and in vitro. On day 20, the tumor tissues of mice were harvested. a Schedule flowchart. b Bar graphs depict the expression of PD-L1 in CD45− and CD45+ immune cells by flow cytometry (n = 5). c Photomicrograph of tumor tissue obtained by H&E staining and PD-L1 IHC staining (n = 3). d Intravenous injection of 100 µg/mouse Cy-5 labeled-PD-L1 antibody into tumor-bearing mice, and 18 h later analyzed by IVIS spectrum imaging system. The bar graphs show the fluorescence radiance of the Cy-5 labeled-PD-L1 antibody in tumor tissues (n = 5). e–h Bar graphs depict the in vitro expression of PD-L1 in TC-1 tumor cells determined by flow cytometry. e BMDCs and pDCs isolated and differentiated from C57BL/6 mice were treated with or without 10 μg/ml DMXAA, and the supernatant was treated with TC-1 cells overnight. f TC-1 cells were treated with 100 ng/ml recombinant protein. g, h TC-1 cells were treated with 10 ng/ml blocking antibody for 30 min, washed, and then treated overnight with the supernatant of DMXAA-treated BMDCs or pDCs. IBM SPSS Statistics Base 22.0 was used for statistical analysis. *P < 0.05, **P < 0.01, ***P < 0.001
Combination of immune checkpoint inhibitors exerts a synergistic therapeutic effect in large tumors
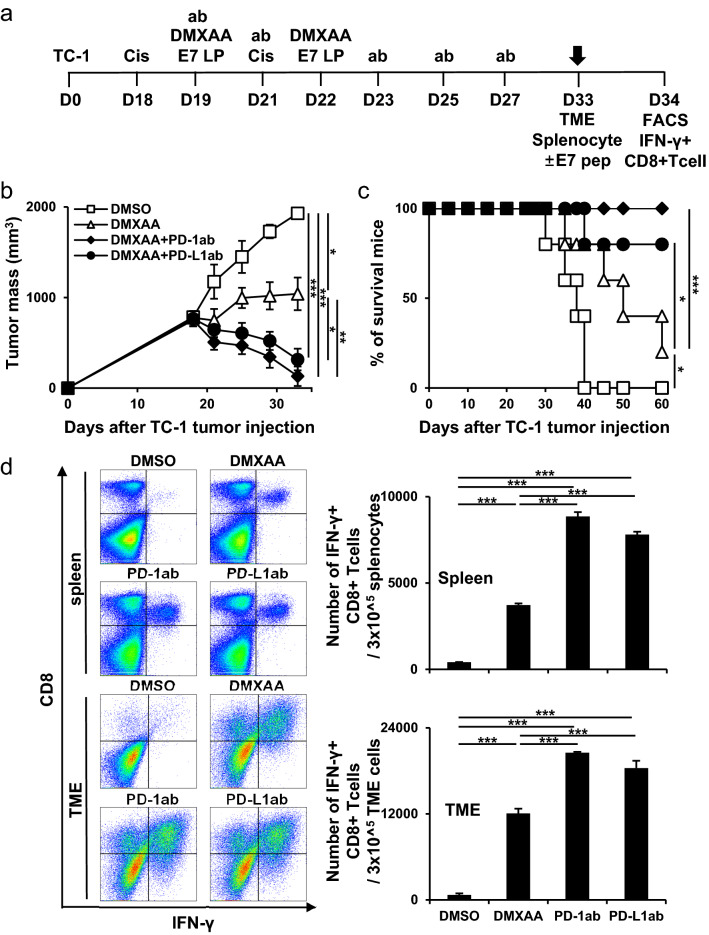
The anticancer immune effect after co-administration of an ICI, which is a side effect of cisplatin and STING activation was evaluated. On day 18, for large tumor challenge greater than 1 cm, mice were injected with combination vaccines (Fig. 4a). The ICI vaccine groups strongly controlled tumor growth compared with the other groups (Fig. 4b). In addition, the mice in the ICI vaccine groups survived for more than 60 days after tumor injection (Fig. 4c). In the ICI vaccine groups, systemic and tumor-infiltrating E7-specific IFN-γ+ CD8+ T cell responses were increased compared to those without ICI (Fig. 4d). These data showed that using an ICI vaccine can reduce the side effects of other cancer therapies and increase the immunogenic effect in large tumors.
Fig. 4.
Tumor treatment effect of combination therapy using immune checkpoint inhibitor in large tumors. C57BL/6 mice were subcutaneously injected with 2 × 105 TC-1 cells/mouse on day 0. Mice were then treated intraperitoneally with 5 mg/kg cisplatin on days 18 and 21 and intratumorally with 20 µg/mouse E7 long peptide and/or with 100 µg/mouse DMXAA on days 19 and 22. The cells were then treated and/or intraperitoneally with 100 µg/mouse PD-1/PD-L1 antibody on days 19, 21, 23, 25, and 27. a Schedule flowchart. b Tumor mass was measured until the mice died or the tumor diameter reached > 2 cm (n = 5). c Mouse survival observed for 60 days (n = 5). d One week after the last vaccination, the tumor tissues and spleens of TC-1 tumor-bearing mice were harvested and re-stimulated with E7 short peptide and then analyzed for IFN-γ+ CD8+ T cells by flow cytometry (n = 5). IBM SPSS Statistics Base 22.0 was used for statistical analysis. *P < 0.05, **P < 0.01, ***P < 0.001
Presence and activation of STING induces an anticancer immune response
To evaluate the differences in anticancer immunity effects on cancer therapies according to the presence or absence of STING, tumor cells were subcutaneously inoculated into C57BL/6 (WT) and STING knockout (KO) mice (Fig. 5a). When STING KO mice were treated with combination vaccines, tumor growth did not decrease (Fig. 5b). In addition, the KO mice survived for up to 40 days after tumor injection (Fig. 5c). IFN-γ+ CD8+ T cell responses were not detected in the KO mice groups (Fig. 5d). The use of DCs from KO mice did not increase the PD-L1 in tumor cells because they were not activated due to the deficiency of the STING pathway (Fig. 5e). These data showed that the presence of STING an important role in inducing the anticancer immune response and that the immunogenic response decreased in the absence of STING. In addition, in the absence of STING, the PD-L1 in tumors does not increase, but the anticancer immunotherapy effect due to ICI cannot be increased, so the presence and activation of STING is important for implementing cancer therapies.
Fig. 5.
Suppression of tumor treatment effect due to STING deficiency. In the in vivo experiments, C57BL/6 (wild-type; WT) mice and STING KO (knockout) mice were used. a Schedule flowchart. b Tumor mass was measured until the mice died or the tumor diameter was > 2 cm (n = 5). c Mouse survival observed after 60 days (n = 5). d One week after the last vaccination, the tumor tissues and spleens of TC-1 tumor-bearing mice were harvested and re-stimulated with E7 short peptide and then analyzed for IFN-γ+ CD8+ T cells by flow cytometry (n = 5). e Bar graphs depicting the in vitro expression of PD-L1 in TC-1 tumor cells determined by flow cytometry. BMDCs and pDCs isolated and differentiated from C57BL/6 and STING KO mice were treated with or without 10 μg/ml DMXAA and the supernatant was treated with TC-1 cells overnight. IBM SPSS Statistics Base 22.0 was used for statistical analysis. *P < 0.05, **P < 0.01, ***P < 0.001
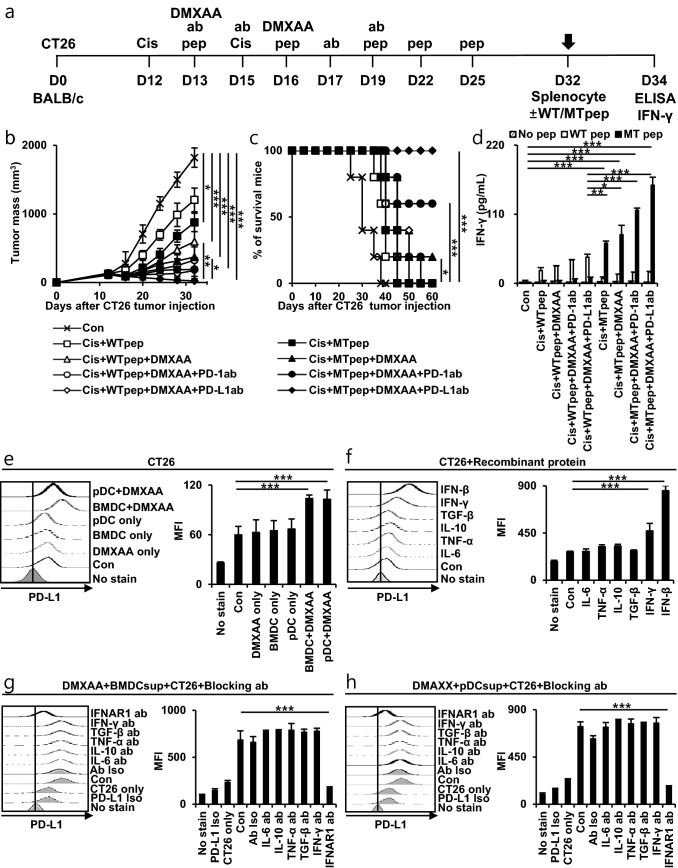
Combination of neoantigen, STING agonist, and ICI vaccination exerts a synergistic therapeutic effect in cisplatin-treated CT26 tumor-bearing mouse models
Anticancer immune efficacy was evaluated by vaccinating neoantigen, DMXAA, and ICI in a cisplatin-treated CT26 tumor-bearing mouse model. CT26 tumor cells were subcutaneously inoculated into BALB/c mice for MHC class I diversity. Mice were then injected with cisplatin, neoantigen (WT or MT peptide), DMXAA, and ICI vaccines (Fig. 6a). WT peptide groups were used as controls for the MT peptide groups. When treated with cisplatin, MT peptide, DMXAA, and ICI, tumor growth decreased compared to that in the other groups (Fig. 6b). In addition, mice in this group survived up to 60 days after tumor injection (Fig. 6c). We assessed the neoantigen peptide-induced T cell activity by IFN-γ level of splenocytes (Fig. 6d). It was concluded that neoantigen vaccines using the MT peptide, DMXAA, and ICI can improve the immunogenicity for antitumor effects. In addition, PD-L1 in CT26 tumor cells was most increased by STING agonist and type I IFN in vitro. (Fig. 6e, f). IFNAR1 antibody reduced PD-L1 in CT26 tumor cells (Fig. 6g, h). According to the in vitro results, since the increase in type I IFN by STING activation can increase the PD-L1 in tumor cells, the anticancer immunogenic effect can be enhanced by combination treatment with ICI.
Fig. 6.
Tumor treatment effects of neoantigen and combination therapy in CT26 murine colon carcinoma tumor model in vivo and in vitro. a–d BALB/c mice were injected with 2 × 105 CT26 cell/mouse subcutaneously on day 0. Mice were then treated intraperitoneally with 5 mg/kg cisplatin on days 12 and 15 and/or intratumorally with neoantigen WT (wild-type) or MT (mutant) peptide (20 µg/mouse) on days 13, 16, 19, 22, and 25. Next, mice were treated intratumorally with DMXAA (100 µg/mouse) on days 13 and 16 and/or intraperitoneally with PD-1/PD-L1 antibody (100 µg/mouse) on days 13, 15, 17 and 19. a Schedule flowchart. b Tumor mass was measured until the mice died or the tumor diameter reached > 2 cm (n = 5). c Mouse survival was observed for 60 days (n = 5). d One week after the last vaccination, splenocytes were isolated and re-stimulated with neoantigen WT/MT peptide for 2 days, and the levels of IFN-γ were estimated by ELISA (n = 5). e–h Bar graphs depict the in vitro expression of PD-L1 in CT26 tumor cells determined by flow cytometry. e BMDCs and pDCs isolated and differentiated from BALB/c mice were treated with or without 10 μg/ml DMXAA, and the supernatant was treated with CT26 cells overnight. f CT26 cells were treated with 100 ng/ml recombinant proteins. g–h CT26 cells were treated with 10 ng/ml blocking antibody for 30 min, washed, and then incubated overnight with supernatants of DMXAA-treated BMDCs or pDCs. IBM SPSS Statistics Base 22.0 was used for statistical analysis. *P < 0.05, **P < 0.01, ***P < 0.001
Discussion
In this study, a tumor antigen and STING agonist vaccine were used to confirm the anticancer effect in a cisplatin-treated mouse model. An antigen abundance environment is created by injecting cisplatin and tumor antigen, which increases type I IFN and immunogenic responses by activating the STING pathway due to the STING agonist. As a result, activation of CD8+ T cells increases, indicating an anticancer immune response. However, as a side effect of type I IFN induced by STING and chemotherapy, the expression of PD-L1 in cancer cells increased. To suppress the decrease in immune response due to the PD-L1, ICI antibodies were used to increase the survival rate of mice and achieve a larger tumor therapeutic effect. Vaccination with ICIs elicited a synergistic anticancer immune effect by amplifying the immune activation response.
We identified this gene in the CT26 cancer cell line and selected the neoantigen MT peptide through direct sequencing to confirm the therapeutic effect of the neoantigen vaccine. These results showed that neoantigens can achieve high immunogenicity and efficient cancer therapy. Neoantigen vaccines have been in the spotlight because they are personalized treatments that analyze the genome of cancer cells derived from cancer patients and can be treated efficiently[12, 31]. However, neoantigens have a lower immunogenic response than tumor-specific antigens such as the E7 peptide[40–42]. Therefore, it is expected that the combination therapy used in our study would be better as one of the various methods used to increase the immune response in neoantigens.
We confirmed that the distribution of immune-activated cells and pro-inflammatory cytokines synergistically increased when the STING pathway was activated. However, PD-L1 is increased in tumors by STING and type I IFN. Therefore, after Food and Drug Administration (FDA) approval for ICIs, many clinical trials have been conducted, but only 20% of cancer patients have been treated. As one of the causes, PD-L1 deficiency in tumor cells has become a hot topic because it can suppress the anticancer immune response and reduce the therapeutic effect of ICI therapy[43, 44]. As a result, type I IFN induced by STING can increase PD-L1 and immune response; it is effective for co-administration with ICI. It would be beneficial if many STING agonists could be developed in clinical trials, as in our study.
Our study showed advantages such as an increase in the anticancer immune response and reduction in side effects due to the co-administration of cancer therapy. In current clinical practice, cancer therapies are used in combination to compensate for the shortcomings of monotherapies. Moreover, in tumors with deficient PD-L1, it is expected that combination therapy with a STING agonist can increase PD-L1 expression, and ICI can be used to reduce side effects and will be widely used in the future. As such, active research on anticancer immunotherapy and the co-administration of cancer therapeutics is important and can be utilized for various anticancer immunotherapy studies in the future.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Not applicable.
Authors’ contributions
SEL, GYJ, HDH, YMP, and THK designed experiments. SEL, GYJ, JWL, and SHP conducted experiments. SEL and THK analyzed the data and wrote the manuscript. HDH verified the statistical methods used. All the authors provided critical feedback and contributed to the final manuscript. THK supervised this project.
Funding
This study was supported by National Research Foundation of Korea (NRF) Grants funded by the Korean government (NRF-2016R1A5A2012284 and NRF-2018R1A2B6008455).
Availability of data and material
The datasets analyzed in the current study are available from the corresponding author upon reasonable request.
Code availability
Not applicable.
Declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Consent to participate
In the name of all the authors, I gave my full consent to participate.
Consent for publication
I have given my full consent for publication in the name of all authors.
Ethics approval
All animal experiments were approved by the Konkuk University Institutional Animal Care Use Committee (IACUC, KU20104).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yeong-Min Park, Email: immun3023@kku.ac.kr.
Tae Heung Kang, Email: kangiron@kku.ac.kr.
References
- 1.Arruebo M, Vilaboa N, Saez-Gutierrez B, Lambea J, Tres A, Valladares M, Gonzalez-Fernandez A. Assessment of the evolution of cancer treatment therapies. Cancers. 2011;3:3279–3330. doi: 10.3390/cancers3033279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blank CU, Rozeman EA, Fanchi LF, et al. Neoadjuvant versus adjuvant ipilimumab plus nivolumab in macroscopic stage III melanoma. Nat Med. 2018;24:1655–1661. doi: 10.1038/s41591-018-0198-0. [DOI] [PubMed] [Google Scholar]
- 3.Miller KD, Nogueira L, Mariotto AB, Rowland JH, Yabroff KR, Alfano CM, Jemal A, Kramer JL, Siegel RL. Cancer treatment and survivorship statistics, 2019. CA A Cancer J Clin. 2019;69:363–385. doi: 10.3322/caac.21565. [DOI] [PubMed] [Google Scholar]
- 4.Richardson JL, Marks G, Levine A. The influence of symptoms of disease and side effects of treatment on compliance with cancer therapy. J Clin Oncol. 1988;6:1746–1752. doi: 10.1200/jco.1988.6.11.1746. [DOI] [PubMed] [Google Scholar]
- 5.Vanneman M, Dranoff G. Combining immunotherapy and targeted therapies in cancer treatment. Nat Rev Cancer. 2012;12:237–251. doi: 10.1038/nrc3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Waldman AD, Fritz JM, Lenardo MJ. A guide to cancer immunotherapy: from T cell basic science to clinical practice. Nat Rev Immunol. 2020;20:651–668. doi: 10.1038/s41577-020-0306-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rabinovich GA, Gabrilovich D, Sotomayor EM. Immunosuppressive strategies that are mediated by tumor cells. Annu Rev Immunol. 2007;25:267–296. doi: 10.1146/annurev.immunol.25.022106.141609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park HJ, Jang G-Y, Kim YS, et al. A novel TLR4 binding protein, 40S ribosomal protein S3, has potential utility as an adjuvant in a dendritic cell-based vaccine. J Immunother Cancer. 2019;7:60. doi: 10.1186/s40425-019-0539-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kang TH, Park JH, Yang A, et al. Annexin A5 as an immune checkpoint inhibitor and tumor-homing molecule for cancer treatment. Nat Commun. 2020;11:1137. doi: 10.1038/s41467-020-14821-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin Y, Xu J, Lan H. Tumor-associated macrophages in tumor metastasis: biological roles and clinical therapeutic applications. J Hematol Oncol. 2019;12:76. doi: 10.1186/s13045-019-0760-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peng M, Mo Y, Wang Y, et al. Neoantigen vaccine: an emerging tumor immunotherapy. Mol Cancer. 2019;18:128. doi: 10.1186/s12943-019-1055-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li L, Goedegebuure SP, Gillanders W. Cancer vaccines: shared tumor antigens return to the spotlight. Sig Transduct Target Ther. 2020;5:251. doi: 10.1038/s41392-020-00364-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hiam-Galvez KJ, Allen BM, Spitzer MH. Systemic immunity in cancer. Nat Rev Cancer. 2021 doi: 10.1038/s41568-021-00347-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nam G-H, Choi Y, Kim GB, Kim S, Kim SA, Kim I-S. Emerging prospects of exosomes for cancer treatment: from conventional therapy to immunotherapy. Adv Mater. 2020;32:2002440. doi: 10.1002/adma.202002440. [DOI] [PubMed] [Google Scholar]
- 15.Budhwani M, Mazzieri R, Dolcetti R. Plasticity of type I interferon-mediated responses in cancer therapy: from anti-tumor immunity to resistance. Front Oncol. 2018 doi: 10.3389/fonc.2018.00322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lu C, Klement JD, Ibrahim ML, Xiao W, Redd PS, Nayak-Kapoor A, Zhou G, Liu K. Type I interferon suppresses tumor growth through activating the STAT3-granzyme B pathway in tumor-infiltrating cytotoxic T lymphocytes. J Immunother Cancer. 2019;7:157. doi: 10.1186/s40425-019-0635-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aricò E, Castiello L, Capone I, Gabriele L, Belardelli F. Type I interferons and cancer: an evolving story demanding novel clinical applications. Cancers. 2019;11:1943. doi: 10.3390/cancers11121943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Su T, Zhang Y, Valerie K, Wang X-Y, Lin S, Zhu G. STING activation in cancer immunotherapy. Theranostics. 2019;9:7759–7771. doi: 10.7150/thno.37574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li A, Yi M, Qin S, Song Y, Chu Q, Wu K. Activating cGAS-STING pathway for the optimal effect of cancer immunotherapy. J Hematol Oncol. 2019;12:35. doi: 10.1186/s13045-019-0721-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hervas-Stubbs S, Perez-Gracia JL, Rouzaut A, Sanmamed MF, Le Bon A, Melero I. Direct effects of type I interferons on cells of the immune system. Clin Cancer Res. 2011;17:2619–2627. doi: 10.1158/1078-0432.ccr-10-1114. [DOI] [PubMed] [Google Scholar]
- 21.Zhu Y, An X, Zhang X, Qiao Y, Zheng T, Li X. STING: a master regulator in the cancer-immunity cycle. Mol Cancer. 2019;18:152. doi: 10.1186/s12943-019-1087-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiang M, Chen P, Wang L, et al. cGAS-STING, an important pathway in cancer immunotherapy. J Hematol Oncol. 2020;13:81. doi: 10.1186/s13045-020-00916-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barber GN. STING: infection, inflammation and cancer. Nat Rev Immunol. 2015;15:760–770. doi: 10.1038/nri3921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang H, Lee WS, Kong SJ, et al. STING activation reprograms tumor vasculatures and synergizes with VEGFR2 blockade. J Clin Investig. 2019;129:4350–4364. doi: 10.1172/JCI125413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patel SA, Minn AJ. Combination cancer therapy with immune checkpoint blockade: mechanisms and strategies. Immunity. 2018;48:417–433. doi: 10.1016/j.immuni.2018.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yi M, Niu M, Xu L, Luo S, Wu K. Regulation of PD-L1 expression in the tumor microenvironment. J Hematol Oncol. 2021;14:10. doi: 10.1186/s13045-020-01027-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Y, Luo J, Alu A, Han X, Wei Y, Wei X. cGAS-STING pathway in cancer biotherapy. Mol Cancer. 2020;19:136. doi: 10.1186/s12943-020-01247-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sambi M, Bagheri L, Szewczuk MR. Current challenges in cancer immunotherapy: multimodal approaches to improve efficacy and patient response rates. J Oncol. 2019;2019:4508794. doi: 10.1155/2019/4508794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tang T, Huang X, Zhang G, Hong Z, Bai X, Liang T. Advantages of targeting the tumor immune microenvironment over blocking immune checkpoint in cancer immunotherapy. Sig Transduct Target Ther. 2021;6:72. doi: 10.1038/s41392-020-00449-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murciano-Goroff YR, Warner AB, Wolchok JD. The future of cancer immunotherapy: microenvironment-targeting combinations. Cell Res. 2020;30:507–519. doi: 10.1038/s41422-020-0337-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kreiter S, Vormehr M, van de Roemer N, et al. Mutant MHC class II epitopes drive therapeutic immune responses to cancer. Nature. 2015;520:692–696. doi: 10.1038/nature14426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jiang T, Shi T, Zhang H, Hu J, Song Y, Wei J, Ren S, Zhou C. Tumor neoantigens: from basic research to clinical applications. J Hematol Oncol. 2019;12:93. doi: 10.1186/s13045-019-0787-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zheng J, Mo J, Zhu T, et al. Comprehensive elaboration of the cGAS-STING signaling axis in cancer development and immunotherapy. Mol Cancer. 2020;19:133. doi: 10.1186/s12943-020-01250-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Borst J, Ahrends T, Bąbała N, Melief CJM, Kastenmüller W. CD4+ T cell help in cancer immunology and immunotherapy. Nat Rev Immunol. 2018;18:635–647. doi: 10.1038/s41577-018-0044-0. [DOI] [PubMed] [Google Scholar]
- 35.Corrales L, Glickman LH, McWhirter SM, et al. Direct activation of STING in the tumor microenvironment leads to potent and systemic tumor regression and immunity. Cell Rep. 2015;11:1018–1030. doi: 10.1016/j.celrep.2015.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Song L, Yang MC, Knoff J, Wu TC, Hung CF. Cancer immunotherapy employing an innovative strategy to enhance CD4+ T cell help in the tumor microenvironment. PLoS ONE. 2014;9:e115711. doi: 10.1371/journal.pone.0115711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu CY, Monie A, Pang X, Hung CF, Wu TC. Improving therapeutic HPV peptide-based vaccine potency by enhancing CD4+ T help and dendritic cell activation. J Biomed Sci. 2010;17:88. doi: 10.1186/1423-0127-17-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hradilova N, Sadilkova L, Palata O, Mysikova D, Mrazkova H, Lischke R, Spisek R, Adkins I. Generation of dendritic cell-based vaccine using high hydrostatic pressure for non-small cell lung cancer immunotherapy. PLoS ONE. 2017;12:e0171539. doi: 10.1371/journal.pone.0171539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee EJ, Jang G-Y, Lee SE, Jw L, Han HD, Park Y-M, Kang TH. A novel form of immunotherapy using antigen peptides conjugated on PD-L1 antibody. Immunol Lett. 2021;240:137–148. doi: 10.1016/j.imlet.2021.10.006. [DOI] [PubMed] [Google Scholar]
- 40.Blass E, Ott PA. Advances in the development of personalized neoantigen-based therapeutic cancer vaccines. Nat Rev Clin Oncol. 2021;18:215–229. doi: 10.1038/s41571-020-00460-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen F, Zou Z, Du J, et al. Neoantigen identification strategies enable personalized immunotherapy in refractory solid tumors. J Clin Investig. 2019;129:2056–2070. doi: 10.1172/JCI99538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yarchoan M, Johnson BA, Lutz ER, Laheru DA, Jaffee EM. Targeting neoantigens to augment antitumour immunity. Nat Rev Cancer. 2017;17:209–222. doi: 10.1038/nrc.2016.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu H, Kuang X, Zhang Y, et al. ADORA1 inhibition promotes tumor immune evasion by regulating the ATF3-PD-L1 Axis. Cancer Cell. 2020;37:324–339.e8. doi: 10.1016/j.ccell.2020.02.006. [DOI] [PubMed] [Google Scholar]
- 44.Herbst RS, Soria J-C, Kowanetz M, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515:563–567. doi: 10.1038/nature14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets analyzed in the current study are available from the corresponding author upon reasonable request.
Not applicable.