Abstract
Immunotherapy has greatly changed the status of cancer treatment, and many patients do not respond or develop acquired resistance. The related research is blocked by lacking of comprehensive resources for researchers to discovery and analysis signatures, then further exploring the mechanisms. Here, we first offered a benchmarking dataset of experimentally supported signatures of cancer immunotherapy by manually curated from published literature works and provided an overview. We then developed CiTSA (http://bio-bigdata.hrbmu.edu.cn/CiTSA/) which stores 878 entries of experimentally supported associations between 412 signatures such as genes, cells, and immunotherapy across 30 cancer types. CiTSA also provides flexible online tools to identify and visualize molecular/cell feature and interaction, to perform function, correlation, and survival analysis, and to execute cell clustering, cluster activity, and cell–cell communication analysis based on single cell and bulk datasets of cancer immunotherapy. In summary, we provided an overview of experimentally supported cancer immunotherapy signatures and developed CiTSA which is a comprehensive and high-quality resource and is helpful for understanding the mechanism of cancer immunity and immunotherapy, developing novel therapeutic targets and promoting precision immunotherapy for cancer.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00262-023-03414-6.
Keywords: Cancer immunotherapy, Biomarkers, Tumor microenvironment, scRNA-seq
Introduction
Cancer immunotherapy is a breakthrough treatment strategy, rapidly developing research direction in oncology and immunity, and it currently has been applied for many cancer types such as melanoma [1, 2], non-small cell lung cancer (NSCLC) [3], gastric cancer [4, 5], and renal cell carcinoma [6]. Immunotherapy can be classified as immune checkpoint inhibition, adoptive cell transfer, oncolytic virus therapies, cancer vaccines, and cytokine therapies [7]. Compared with traditional treatment methods, immunotherapy has better and longer efficacy with fewer side effects [8]. However, many patients do not respond or resistance to immunotherapy and the mechanisms is highly heterogeneous [9, 10]. High-quality signatures, which enable screening of patients who can benefit from immunotherapy before treatment and contributing to mechanisms that influence the efficacy of immunotherapy, are urgently needed.
Tumor microenvironment (TME) cells and alterations of multiple types of biomolecules are associated with the benefit of tumor immunotherapy. Plasma cells can predict outcomes to PD-L1 blockade immunotherapy in NSCLC [11]. Rzhevskiy et al. [12] highlighted the potential applications of circulating tumor cells for identifying biomarkers of immunotherapy. PD-L1 expression is a predictive biomarker for cancer immunotherapy [13]. Tumor mutation burden (TMB) has been regarded as biomarker for cancer immunotherapy [14, 15]. Liu et al. [16] revealed the combination of TMB and copy number alteration can jointly stratify predictive responses to immunotherapy across metastatic tumors. These findings provide important guidance for improving the efficiency of immunotherapy. Cancer immunity and immunotherapy involve complex processes, and it is difficult to capture comprehensive information with a single marker [14]. Thus, combining markers for immunotherapy is needed [17, 18]. There are quite a few experimentally validated biomarkers of cancer immunotherapy, which are fragmented and hidden in thousands of published studies, and this poses a challenge for predicting response of immunotherapy and investigating related mechanisms by combining markers. Thus, a comprehensive and high-quality resource is urgently needed for cancer immunotherapy. In addition, some studies used computational methods to identify markers for cancer immunotherapy. For example, Chen et al. [19] developed TIRSF to identify gene signatures to predict immunotherapy response of cancer. Miao et al. [20] proposed ImmuCellAI to estimate the abundance of immune cells and then construct model for predicting response of tumor immunotherapy. These works have important guiding significance for the discovery of novel immunotherapy markers. However, a benchmarking dataset to effectively evaluate the accuracy and reliability of these computational methods is missing. To sum up, a comprehensive and high-quality data source for cancer immunotherapy signature is needed to provide important support for predicting response of individual immunotherapy and elucidating mechanism of therapeutic resistance.
Meanwhile, rapid expansion of the available expression profiles for tumor immunotherapy is obtained by high-throughput technologies, the development of an efficient platform for analyzing large amounts of datasets is essential. TISMO, a database collection of syngeneic mouse model profiles, allows users to investigate immunotherapy response-related gene expression, pathway enrichment, or immune infiltration level [21]. Lin et al. [22] developed a Web server for analysis on multi-omics of immunotherapy in cancer. However, our understating of tumor immunotherapy is still limited. Furthermore, single-cell technologies will dramatically enhance the ability to discovering novel target, investigating mechanism and screening the rational agent for immunotherapy [23]. Increasing single cell sequencing data are available for cancer immunotherapy. There is currently lack of a comprehensive database to effectively integrate these data sources and to provide support and guarantee for researches of cancer immunity and immunotherapy.
Here, we first manually collected and summarized experimentally confirmed markers associated with tumor immunotherapy. Then, we developed CiTSA (http://bio-bigdata.hrbmu.edu.cn/CiTSA/), which documents 878 entries of experimentally supported associations between genes at transcription or protein level, cells, etc., signatures and cancer immunotherapy, and also provides analysis tools based on single cell and bulk datasets. CiTSA can serve as a valuable resource for marker discovery and elucidating the mechanism of both tumor immunity and immunotherapy.
Materials and methods
Collection of experimentally supported signatures of cancer immunotherapy
To obtain high-quality dataset, all CiTSA entries were manually curated through several steps as previously described [24]. Firstly, we searched the PubMed database by using a list of keywords. In general, keywords consist of three parts: (1) the first part describes cancer, which includes ‘cancer’ or ‘carcinoma’; (2) the second part describes immunotherapy, which includes ‘immunotherapy,’ ‘Immune checkpoint therapy,’ ‘vaccination of cancer,’ ‘immunotherapy response,’ ‘immunotherapy resistance,’ etc.; (3) the third part describes signatures, which mainly includes ‘gene’ and also includes ‘microRNA,’ ‘lncRNA,’ ‘noncoding,’ etc. These keywords or their combination were inputted to the advanced search box of PubMed with the ‘Title’ field to collect literature works. The published dates of searched literature works were from March 2014 to January 2022 and approximately 10,000 literature works were returned. Next, we preliminarily screened the literature by title. As a result, nearly 5000 literature works were retained to be further reviewed. Then, experimentally supported cancer immunotherapy-associated molecules or cells were extracted manually from these literature works. In this process, we retrieved the information for cancer and immunotherapy signatures including cancer name, treatment (drug) of immunotherapy, immunotherapy type, signature (e.g., gene and cell), mode of action (mode of action for signatures in immunotherapy), experimental (e.g., clinical trial and mouse model), experimental tissue, the reference information and a brief description of the association from each literature. All records were rechecked by another researchers. Finally, in order to ensure the consistency with other databases and promote the applicability of data, we referred to The Cancer Genome Atlas (TCGA) database (https://www.cancer.gov/tcga) for uniform cancer names and abbreviations, unified gene/protein names with NCBI Entrez ID and symbol as gene signatures and map cell signature with CellMarker database [25]. After the above processes, a total of 878 entries of experimentally supported associations between 412 signatures including genes (transcription or protein level), cells, etc., and tumor immunotherapy across 30 cancer types including pan cancer (PACA) were obtained.
Collection of cancer immunotherapy-related transcriptome data
We searched cancer immunotherapy datasets by using ‘cancer,’ ‘immunotherapy’ and ‘Homo sapiens’ as keywords in GEO database [26] to collect cancer immunotherapy-related bulk and single cell data of gene expression. Finally, we obtained 35 bulk and 13 single cell datasets through manually reviewed.
For the follow-up analysis of cancer immunotherapy data, we classified samples into response (R) and non-response (NR) groups in some datasets involving cancer immunotherapy responses information. Briefly, sample classification was conducted according to the original dataset if the R/NR information about immunotherapy was available. Otherwise, we classified samples based on disease progression, survival prognosis and other information of the samples after immunotherapy. For example, we defined complete/partial response samples as immunotherapy response (N) group, and progressive/stable disease samples as immunotherapy non-response (NR) group. Details of the R/NR classification of the sample in datasets involved in this study are shown in Table S1.
Database construction
The CiTSA Web site was developed in JSP using a Struts2 framework and was deployed on a Tomcat Web server that ran under a Redhat 6.4 system. All data in CiTSA were stored and managed using MySQL (version 5.7.18). jQuery was used to manage the result views.
Results
Data collection and content of CiTSA
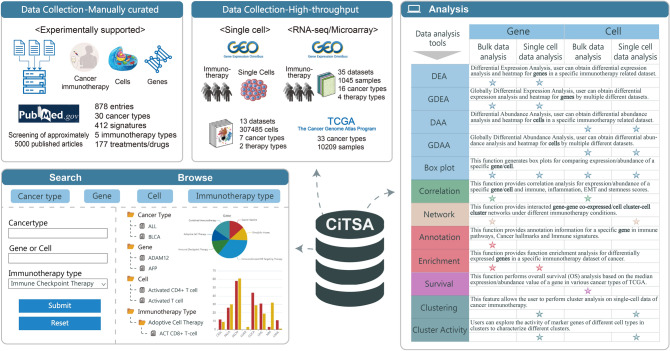
After strict screening of approximately 5000 published articles, a total of 878 entries of experimentally supported associations between 412 signatures including genes (transcription or protein level), cells, etc., and immunotherapy across 30 cancer types were manually curated. Detailed information of 30 cancer types is shown in Table S2. These experimentally supported associations involve 177 treatments/drugs mainly related to five immunotherapy types including immune checkpoint therapy, adoptive cell therapy, cancer vaccine, oncolytic viruses and immunostimulant or targeting. In CiTSA (http://bio-bigdata.hrbmu.edu.cn/CiTSA/), users can flexibly browse, search, and obtain these entries (Fig. 1). Meanwhile, CiTSA integrated gene expression profiles of cancer immunotherapy. In total, 35 bulk datasets are integrated, which contains immunotherapy treatment/non-treatment, and response/non-response samples across 16 cancer types (details see Table S3). We also obtained 13 single cell RNA-seq (scRNA-seq) datasets of immunotherapy, which contains about 300,000 cells from 7 cancer types (Table S4). For survival analysis of signatures related to tumor immunotherapy, we also integrated expression profiles and clinical information of 10,209 samples from 33 cancer types of TCGA. Based on these datasets, several panels of tools that facilitate discovery immunotherapy signatures and explore related mechanisms are also provided in CiTSA (Fig. 1).
Fig. 1.
Content of CiTSA. The figure summarizes content of CiTSA, which includes data collection and user interface. The upper left area contains the collected data content including experimentally supported signatures of cancer immunotherapy, integration of scRNA-seq, bulk datasets of cancer immunotherapy and TCGA dataset. The lower left and right areas exhibit user interface to access and acquire experimentally supported signatures of cancer immunotherapy and several panels of analysis tools
Overview of the experimentally supported signatures of cancer immunotherapy
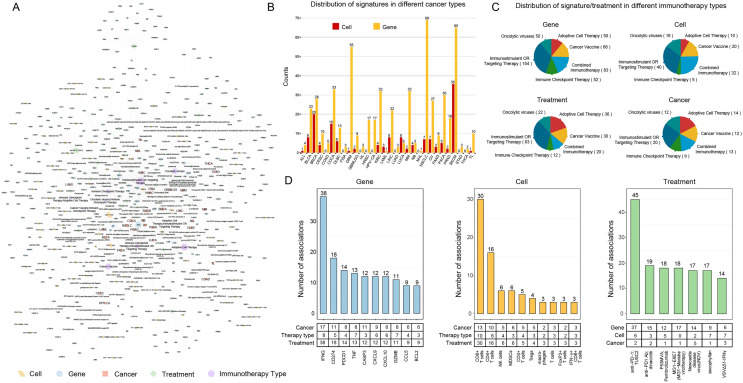
We performed a systematic analysis for these experimentally supported signatures of cancer immunotherapy. First, the cancer immunotherapy type-treatment-signature (gene/cell) association network was constructed (Fig. 2A), and provides an overview landscape of cancer immunotherapy signatures. These experimentally confirmed associations involved immunotherapy signatures for a total of 30 cancer types (Table S2). An inspection of the distribution of gene/cell signatures in each cancer type found that cancer types such as NSCLC, melanoma (SKCM) and colon cancer (COCA) have relative high number of associated signatures (Fig. 2B). This may due to the good effect and wide application of immunotherapy on these cancer types. Next, we dissected different immunotherapy types in terms of gene/cell signatures, treatments, and cancer types. Here, we unified antibody targeted therapy and immune stimulation as ‘Immunostimulant OR Targeting Therapy.’ Considering that combination of immunotherapy is often used in clinical practice, we referred combination therapy types such as ‘Cancer Vaccine; Immune Checkpoint Therapy,’ ‘Immune Checkpoint Therapy; Immunostimulant OR Targeting Therapy’ as ‘Combined Immunotherapy.’ Thus, cancer immunotherapy types are divided into six main categories (Fig. 2C). We found that ‘Immunostimulant OR Targeting Therapy’ has the highest number of associated gene/cell signatures and cancer types (Fig. 2C). We further focused on genes, cells, and treatments with relative high degree in the network. The results shown that genes with high degree (the number of associated treatments) including IFNG, CD274 (PD-L1), PDCD1 (PD-1), etc. (Fig. 2D). Most of these gene signatures are well-known or potential target of cancer immunotherapy. For example, IFNG induces tumor resistance to anti-PD-1 immunotherapy by promoting YAP phase separation [27]. Inhibition of PD-1-PD-L1 signaling plays important roles in cancer immune evasion, and thus has been regarded as one of the major targets in cancer immunotherapy [28]. T cells are associated with more treatments (relative high degree) (Fig. 2D). This further indicates the important role of T cells in immunotherapy. We also found treatments that target or mediate PD-1 and IFNG (IFNγ) have relative high number of gene/cell signatures (Fig. 2D). This suggests that these treatments are currently the dominant immunotherapy strategies.
Fig. 2.
An overview of the experimentally supported signatures of cancer immunotherapy. A Overview landscape of cancer-immunotherapy type-treatment-signature (gene/cell) association. The size of nodes on the network is proportional to their degree. B Distribution of immunotherapy gene/cell signatures in different cancer types. C Distribution of gene, cell signature and treatment in six immunotherapy types. D Statistics of cancer immunotherapy-signature (gene/cell) associations in CiTSA. Left: the number of associations (treatments) for top ranked gene signatures. Middle: the number of associations (treatments) for top ranked cell signatures. Right: the number of associations (gene/cell signatures and immunotherapy types) for top ranked treatments that are related to cancer immunotherapy
In summary, we provided an overview landscape of cancer immunotherapy signatures, and the analysis revealed detailed information about experimentally supported signatures of cancer immunotherapy, which will further promote the related researches of cancer immunity and immunotherapy.
Feature and utility of CiTSA
Flexible ways to access and obtain the experimentally supported signatures of cancer immunotherapy
The CiTSA provides a user-friendly Web interface that enables users to search, browse, submit, and obtain experimentally supported signatures of cancer immunotherapy (Fig. 3). On the ‘Search’ page, users can search by inputting interested ‘cancer name,’ ‘gene name,’ ‘cell name’ or ‘immunotherapy type.’ CiTSA also provides ‘Quick Search’ function that enables users to search information easily by clicking the cancer name (Fig. 3A). On the ‘Browse’ page, users can browse all experimentally supported records of signatures in CiTSA by selecting a specific cancer type, gene/cell name or treatment/drug underling the corresponding immunotherapy type (Fig. 3B). The search/browse results include information about cancer immunotherapy signatures, such as cancer name, treatment/drug of cancer immunotherapy, signature name, experimental, PubMed ID (a hyperlink to original reference) (Fig. 3C). A hyperlink named ‘Details’ in the result page provides detailed information about the corresponding records, besides the above still including immunotherapy type, drug status and DrugBank ID for immunotherapy treatment, immunotherapy checkpoints (if available), signature type, official symbol for signature, mode of action (describes the act of signature in immunotherapy), description (describes the evidence of association), and the title of reference (Fig. 3D). The result page also provides links for analyzing the corresponding signature based on bulk datasets. Users can copy or download the searched/browsed results as file. In addition, a ‘Statistics’ page is also provided to facilitate users for understanding datasets in CiTSA (Fig. 3E). In order to maintain and facilitate data update, a ‘Submit’ page is provided in CiTSA for researchers to submit experimentally supported signatures for cancer immunotherapy. The submitted record, once approved by the submission review committee, will be made available to the updated releases of CiTSA. A detailed guide showing users how to use CiTSA is available on the ‘Help’ page.
Fig. 3.
User interfaces to access and obtain experimentally supported signatures of cancer immunotherapy. A Interface of the search/quick search function. B Interface of the browse function. C The search/browse result page. D Result page for the ‘Details’ link. E The ‘Statistics’ page
Web tools for deciphering gene/cell and TME in cancer immunotherapy
The rapid growth of cancer immunotherapy single cell and bulk datasets provides new opportunities for discovery signatures and explore related mechanisms of cancer immunotherapy, and the development of an efficient platform for integration and analysis of these datasets is urgently needed. CiTSA offers a series of analysis tools including identification and visualization of molecular/cell features, molecular interaction analysis and visualization, function, correlation and survival analysis, etc., based on bulk datasets (Fig. 4). CiTSA also provides cell clustering, identification and visualization of molecular/cell features, function analysis, cluster activity, and cell–cell communication analysis underlying different immunotherapy conditions based on single cell datasets (Fig. 4). By using these analysis tools, users can explore experimentally validated signatures, discover novel immunotherapy signatures, and dissect related mechanisms.
Fig. 4.
Web tools in CiTSA. A–B The panel of gene tools based on single cell A and bulk B datasets of cancer immunotherapy. C–D The panel of cell tools based on single cell C and bulk D datasets of cancer immunotherapy
A panel of gene tools based on single cell datasets
CiTSA integrated large-scale scRNA-seq data to provide a series of tools for discovering novel gene signatures and exploring the mechanism of cancer immunotherapy. The panel of gene tools based on scRNA-seq datasets is under the ‘Analysis–> Gene Tools–> Single cell data analysis’ directory, which involves six functions (DEA, GDEA, Box Plot, Enrichment, Clustering and Cluster Activity) (Fig. 4A). For each scRNA-seq dataset, CiTSA provides the differential analysis between the corresponding immunotherapy condition (T/NT: immunotherapy treatment vs no treatment; R/NR: immunotherapy response vs no response). The ‘DEA’ function allows user to obtain the differences of genes in two groups by selecting immunotherapy condition and dataset. The result of ‘DEA’ function including Entrez ID, gene symbol, P value and fold change (FC) of genes. In order to comprehensively consider the consistency of genes across different immunotherapy datasets, the ‘GDEA’ function provides differential expression analysis and heatmap visualization by globally considering multiple single cell datasets. After selected the immunotherapy condition and input parameters related to the number of datasets that genes differential in, the result of ‘GDEA’ function will generate a heatmap visualization graph. In the graph, each row represents a gene that meets the inputted criteria, each column represents a single cell dataset, red (blue) color in heatmap represents the gene high (low) expression in immunotherapy treatment/response group of the corresponding dataset. This feature allows user to compare the degree and direction of differential expression of genes across different datasets of cancer immunotherapy. CiTSA also involves a ‘Box Plot’ function to visualize expression of a specific gene between cells of samples in different immunotherapy status (T/NT or R/NR) of the selected dataset. The ‘Enrichment’ function allows users to explore biological function (GO/KEGG, immune pathways, cancer hallmarks and immune signature sets from MsigDB) that differentially expressed genes in cancer immunotherapy participate in. Special for single cell datasets, CiTSA offers ‘Clustering’ and ‘Cluster Activity’ functions to explore the expression activity of genes in TME cells of immunotherapy datasets. CiTSA classified cells into different cell populations based on gene expression profile and provides visualization and characterization of cell populations underlying different immunotherapy conditions. Users can use the ‘Clustering’ function to obtain and visualize cell populations for the selected dataset and also to explore the inputted gene expression activities in TME cell populations. In order to facilitate users to further understand the TME cell population underlying immunotherapy, CiTSA also provides ‘Cluster Activity’ function. In this section, users can explore the activity of different cell types to characterize cell populations (clusters) in TME of immunotherapy-related individuals. CiTSA will perform ‘GSVA’ strategy [29] to evaluate the activity of marker genes of cell types in CellMarker database [25] for different cell populations (clusters) of the selected immunotherapy dataset.
A panel of gene tools based on bulk datasets
CiTSA also provides a panel of gene tools based on bulk datasets of cancer immunotherapy. These tools are under the ‘Analysis–> Gene Tools–> Bulk data analysis’ directory, which includes eight functions (Fig. 4B): (1–3) The ‘DEA,’ ‘GDEA’ and ‘Box Plot’ functions are focused on differential analysis and visualization under the corresponding immunotherapy condition, which are similar with the above tools based on single cell datasets. (4) The ‘Correlation’ function provides correlation analysis for expression of a specific gene and immune, inflammation, EMT and stemness scores in immunotherapy T/NT (R/NR) samples, respectively. The correlation was estimated by using spearman method and the immune, inflammation, EMT and stemness scores in samples were estimated by using GSVA method [29]. In addition, this function also provides box plot for comparing theses scores between immunotherapy T/NT (R/NR) samples. In this section, users can obtain a panel visualization graph which includes correlations and box plots by selecting immunotherapy conditions and datasets, and entering interested gene name. (5) The ‘Network’ function provides interacted gene–gene co-expression networks in different immunotherapy conditions including T/NT or R/NR. This feature allows user to explore the direct associations for a specific gene under different immunotherapy conditions. User can also flexibly select correlation threshold for the network construction. (6) The ‘Annotation’ function provides annotation information for a specific gene (e.g., immunotherapy-related gene) in immune pathways, cancer hallmarks and immune signatures by inputting gene name. (7) The ‘Enrichment’ function also similar with the above tool based on single cell datasets. (8) The ‘survival’ function performs overall survival (OS) analysis based on the median expression value of a gene in various cancer types of TCGA. User can explore the clinical relevance of an interested gene (e.g., immunotherapy-related gene) across different cancer types by selecting cancer type and inputting a gene name.
A panel of cell tools based on single cell datasets
TME cells are also key factors affecting cancer immunotherapy. Thus, CiTSA provides cell tools under the ‘Analysis–> Cell Tools–> Single cell data analysis’ directory based on single cell datasets (Fig. 4C). CiTSA provides six key interactive and customizable functions including clustering, differential abundance analysis (DAA), global differential abundance analysis (GDAA), Box Plot, Cluster Activity and Network based on scRNA-seq datasets from 7 cancer types. CiTSA classified cells into different cell populations (clusters) based on gene expression profile of each single cell dataset. The ‘clustering’ function provides global map of cell clusters in the selected dataset of immunotherapy, and also provides the map of immunotherapy condition information. The ‘DAA’ function allows user to obtain differential abundance analysis and heatmap for cell clusters in a specific dataset of cancer immunotherapy. Users can compare the abundance of cell clusters between T/NT (R/NR) samples and discovery cancer immunotherapy-related cell populations. The ‘Box plot’ function generates box plot to visualize the abundance difference of a specific cell cluster by inputting cell cluster ID within the selected dataset. The ‘Cluster Activity’ function allow users to explore the activity and characterize different cell clusters in cancer immunotherapy. The ‘GDAA’ function provides differential activity analysis and heatmap visualization for cell types by globally considering multiple scRNA-seq datasets. Single-sample GSEA algorithm was used to evaluate the activity of different cell types. This feature allows user to compare the degree and direction of differential activity of cell types from CellMarker database [25] in different datasets. The ‘Network’ function generates cell–cell interaction map under immunotherapy T/NT (R/NR) status, respectively.
A panel of cell tools based on bulk datasets
CiTSA also provides cell analysis tools based on bulk datasets of cancer immunotherapy. For each dataset, we constructed the cell abundance profile based on gene expression of samples. Cell abundance (proportion) in samples was assessed using single-sample GSEA algorithm based on marker genes of different cell types from CellMarker [25]. Thus, we obtained cell abundance profile, in which each row (column) represents a cell type (sample). Under the ‘Analysis–> Cell Tools–> Bulk data analysis’ directory, CiTSA provides five key functions for mining and characterizing cancer immunotherapy-related cells based on these cell abundance profiles (Fig. 4D). These five functions include differential abundance analysis (DAA), global differential abundance analysis (GDAA), box plot, correlation, and survival. The ‘DAA’ function provides differential abundance analysis and heatmap for cells in a specific dataset of cancer immunotherapy. The ‘GDAA’ function allows user to obtain differential abundance analysis and heatmap for cells by globally considering multiple datasets. This feature allows user to compare the degree and direction of differential abundance of cells in different datasets of cancer immunotherapy. The ‘Box Plot’ function generates box plot for comparing abundance of a specific cell type between samples of different immunotherapy status by selecting interesting dataset and cell type. The ‘Correlation’ function provides correlation analysis for abundance of a specific cell type and immune, inflammation, EMT and stemness scores in T/NT (R/NR) samples, respectively. This function also provides box plot for comparing immune, inflammation, EMT, and stemness scores between different sample groups of cancer immunotherapy. The ‘survival’ function performs OS analysis based on the abundance value of a cell type in various TCGA cancer types.
Example application
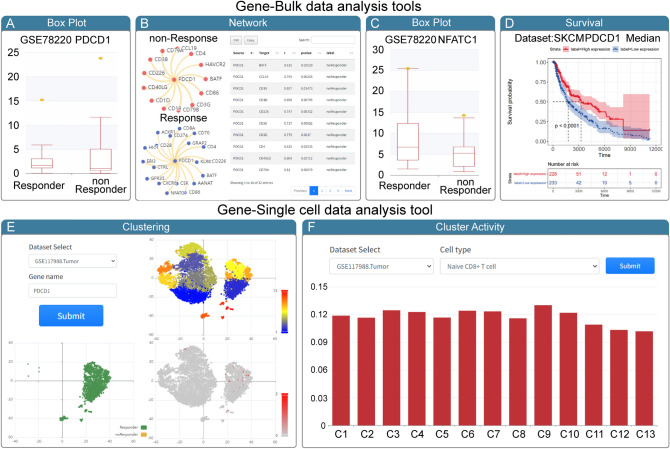
To demonstrate the usage and potential application of CiTSA, we used PD-1(PDCD1) as input for CiTSA (Fig. 5). PD-1 is a well-known target for immune checkpoint blockade (ICB) and the expression is a signature currently in use for immunotherapy applications [30, 31]. With PD-1(PDCD1) as the search input, CiTSA shown that PDCD1 is an experimentally supported signature for anti-PD-1 treatment. Then, we further explored PDCD1 in cancer immunotherapy using analysis tools in CiTSA. With PDCD1 as input in the Box plot function under ‘Analysis–> Gene Tools–> Bulk data analysis’ directory, the result shows that expression of PDCD1 is higher in responder group of anti-PD-1 immunotherapy dataset (GSE78220) which is about mRNA expressions in pre-treatment melanoma (Fig. 5A). The result of network function reveals differential interactions of PDCD1 in immunotherapy R/NR status. For example, interaction between NFACT1 and PDCD1 was specially appears in the network of response (R) group (Fig. 5B). Meanwhile, exploring the expression of NFATC1 by the Box plot function, we found that the expression of NFATC1 is also higher in responder group of GSE78220 (Fig. 5C). Some studies have demonstrated that transcription factor NFATC1 can promote the expression of PD-1 [32], and blocking the activation of NFATC1 can inhibit the expression of PD-L1 [33]. In addition, survival function also suggests that high expression of PDCD1 contributes to a better survival prognosis in melanoma (Fig. 5D). Based on the above analysis results of CiTSA, we could infer that transcription factor NFATC1 promote the expression of PD-1 and thus may make individuals sensitivity to ICB therapy and NFATC1 may be a potential target/signature for anti-PD-1/anti-PD-L1 immunotherapy. We also dissect the expression of PDCD1 in TME based on single cell analysis tools in CiTSA. In the Clustering function under ‘Analysis–> Gene Tools–> Single cell data analysis’ directory, with dataset GSE117988.Tumor of R/NR condition and PDCD1 as input, the result shows that PDCD1 tends to express in cells (cluster 7 and cluster 9) of response individuals (Fig. 5E). We next use the Cluster Activity function to characterize cluster 7 and cluster 9 and found they have relatively high Naive CD8 + T cell activity (Fig. 5F). This indicates that cluster 7 and cluster 9 likely to be key cell subset that affects immunotherapy. The above results suggest that CiTSA not only provide experimentally supported immunotherapy signature benchmarking dataset, but also is a useful platform to reveal relevant mechanisms of cancer immunotherapy, and to discover new targets and signatures.
Fig. 5.
Example for the application of CiTSA. A–B The visualization result of ‘Box Plot’ A and ‘Network’ B function with ‘PDCD1’ and ‘GSE78220’ as input in the panel of gene tools based on bulk dataset. C The visualization result of ‘Box Plot’ function with ‘NFATC1’ and ‘GSE78220’ as input. D The ‘Survival’ function result with SKCM and ‘PDCD1’ as input. E The result of ‘Clustering’ function with ‘GSE117988.Tumor’ and ‘PDCD1’ as input in the panel of gene tools based on single cell dataset. F The result of ‘Cluster Activity’ function with ‘GSE117988.Tumor’ and ‘Naïve CD8 + T cell’ as input
Conclusions and discussion
Immunotherapy has revolutionized cancer treatment. However, many patients do not respond to immunotherapy or develop acquired resistance. Mechanisms that influence the efficacy of immunotherapy are still unclear. Analysis and discovery of cancer immunotherapy signatures is essential to understand the underlying mechanisms and improve the effectiveness of immunotherapy. Here, we first manually collected experimentally supported signatures of cancer immunotherapy from published literature works and provided an overview of cancer immunotherapy signatures. We then developed a comprehensive resource, CiTSA, which not only offers a benchmarking dataset for experimentally confirmed immunotherapy signatures, but also provides analysis tools based on bulk and single-cell dataset for exploring mechanisms, analyzing and discovering signatures of cancer immunotherapy.
Currently, several databases can provide cancer immunotherapy-related genes, datasets, and analysis. The TIRSF Web server can identify gene signatures to predict ICB therapy response in cancer based on gene expression [19]. TISMO provides syngeneic mouse model profiles for studying tumor immunity and immunotherapy [21]. TIGER stores bulk and single-cell transcriptome data of tumor immunotherapy [34]. These studies only focused on immunotherapy-related genes which are mainly inferred by computational methods. CiTSA not only contains gene signatures, but also contains cells, etc. The CanImmunother database contains experimentally supported cancer immunotherapy biomarkers [35]. CiTSA provides both experimentally supported signatures of five main immunotherapy types for cancer and also analysis tools to identify novel signatures and to analyze gene/cell and TME in tumor immunotherapy based on single cell and bulk datasets. Furthermore, CiTSA provides both R/NR and T/NT conditions, which is more beneficial to marker discovery and mechanism research of cancer immunotherapy. CiTSA thus has considerable potential to complement the above databases in terms of researches for cancer immunotherapy.
In summary, we offered an overview landscape of experimentally supported cancer immunotherapy signatures and developed CiTSA which provides a comprehensive and high-quality resource for studying biology molecules/cells act in tumor immunity and immunotherapy and is helpful for understanding the molecular mechanism, developing novel therapeutic targets and achieving the precision immunotherapy of cancer. The extensions of database will continue such as expanding non-coding RNA signatures and related datasets. We believe that CiTSA will be a valuable resource for the future research of cancer immunity and immunotherapy.
Supplementary Information
Below is the link to the electronic supplementary material.
Abbreviations
- BRCA
Breast cancer
- CAN
Copy number alteration
- COCA
Colon cancer
- FC
Fold change
- GBM
Glioma
- HNSC
Head and neck cancer
- LUCA
Lung cancer
- MM
Myeloma
- NSCLC
Non-small cell lung cancer
- OS
Overall survival
- R/NR
Immunotherapy response/no response
- SKCM
Skin melanoma
- STAD
Gastric cancer
- T/NT
Immunotherapy treatment/no treatment
- TCGA
The Cancer Genome Atlas
- TMB
Tumor mutation burden
- TME
Tumor microenvironment
Author contributions
YJX, XL, and YPZ contributed to conceptualization; FL, KJD, and JWW contributed to single cell and bulk data curation; FL, KJD, and CLZ performed formal analysis; KJD, JWW, YJT, KX, XZ, and XMZ contributed to the experimentally supported data curation; FL, YJX, and CLZ contributed to interpreting the results; KYS, MYL, RZ, and XLZ contributed to organization and visualization of diagrams; FL and KJD contributed to platform development; FL, YJX, CLZ, and JWW contributed to writing the manuscript. All the authors commented on the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (Grant Nos. 62101164, 62172131 and 32070622), the National Key R&D Program of China (2018YFC2000100), the China Brain Project (2021ZD0202403), the China Postdoctoral Science Special Foundation (Grant No. 2020T130162), the Heilongjiang Touyan Innovation Team Program, Heilongjiang Province Natural Science Foundation Joint guidance Project (Grant No. LH2022F042), the China Postdoctoral Science Foundation (Grant No. 2019M661295), and the Doctor Green Seedlings Breaking Ground Project of Harbin Medical University (Grant No. QMPT-2010).
Data availability
The datasets generated and/or used during the current study are available in the Gene Expression Omnibus (https://www.ncbi.nlm.nih.gov/geo/), The Cancer Genome Atlas (https://portal.gdc.cancer.gov/), and we also develop database, CiTSA (http://bio-bigdata.hrbmu.edu.cn/CiTSA/).
Declarations
Conflict of interest
All authors declare that they have no competing interests.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Feng Li, Kejing Dong, Chunlong Zhang, Jingwen Wang have contributed equally.
Contributor Information
Yanjun Xu, Email: xuyanjun@hrbmu.edu.cn.
Yunpeng Zhang, Email: zhangyp@hrbmu.edu.cn.
Xia Li, Email: lixia@hrbmu.edu.cn.
References
- 1.Huang AC, Zappasodi R. A decade of checkpoint blockade immunotherapy in melanoma: understanding the molecular basis for immune sensitivity and resistance. Nat Immunol. 2022;23:660–670. doi: 10.1038/s41590-022-01141-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kalaora S, Nagler A, Wargo JA, Samuels Y. Mechanisms of immune activation and regulation: lessons from melanoma. Nat Rev Cancer. 2022;22:195–207. doi: 10.1038/s41568-022-00442-9. [DOI] [PubMed] [Google Scholar]
- 3.Herbst RS, Wang M, Chen L. When immunotherapy meets surgery in non-small cell lung cancer. Cancer Cell. 2022;40:603–605. doi: 10.1016/j.ccell.2022.05.010. [DOI] [PubMed] [Google Scholar]
- 4.Foronda M. Front-line immunotherapy combinations for gastric cancer. Nat Cancer. 2021;2:1286. doi: 10.1038/s43018-021-00308-3. [DOI] [PubMed] [Google Scholar]
- 5.Takei S, Kawazoe A, Shitara K. The New era of immunotherapy in gastric cancer. Cancers (Basel) 2022;14:1054. doi: 10.3390/cancers14041054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Braun DA, Bakouny Z, Hirsch L, Flippot R, Van Allen EM, Wu CJ, Choueiri TK. Beyond conventional immune-checkpoint inhibition-novel immunotherapies for renal cell carcinoma. Nat Rev Clin Oncol. 2021;18:199–214. doi: 10.1038/s41571-020-00455-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang Y, Zhang Z. The history and advances in cancer immunotherapy: understanding the characteristics of tumor-infiltrating immune cells and their therapeutic implications. Cell Mol Immunol. 2020;17:807–821. doi: 10.1038/s41423-020-0488-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abdelbaky SB, Ibrahim MT, Samy H, Mohamed M, Mohamed H, Mustafa M, Abdelaziz MM, Forrest ML, Khalil IA. Cancer immunotherapy from biology to nanomedicine. J Control Release. 2021;336:410–432. doi: 10.1016/j.jconrel.2021.06.025. [DOI] [PubMed] [Google Scholar]
- 9.O'Donnell JS, Teng MWL, Smyth MJ. Cancer immunoediting and resistance to T cell-based immunotherapy. Nat Rev Clin Oncol. 2019;16:151–167. doi: 10.1038/s41571-018-0142-8. [DOI] [PubMed] [Google Scholar]
- 10.Vesely MD, Zhang T, Chen L. Resistance mechanisms to anti-PD cancer immunotherapy. Annu Rev Immunol. 2022;40:45–74. doi: 10.1146/annurev-immunol-070621-030155. [DOI] [PubMed] [Google Scholar]
- 11.Patil NS, Nabet BY, Muller S, Koeppen H, Zou W, Giltnane J, Au-Yeung A, Srivats S, Cheng JH, Takahashi C, et al. Intratumoral plasma cells predict outcomes to PD-L1 blockade in non-small cell lung cancer. Cancer Cell. 2022;40:289–300. doi: 10.1016/j.ccell.2022.02.002. [DOI] [PubMed] [Google Scholar]
- 12.Rzhevskiy A, Kapitannikova A, Malinina P, Volovetsky A, Aboulkheyr Es H, Kulasinghe A, Thiery JP, Maslennikova A, Zvyagin AV, Ebrahimi Warkiani M. Emerging role of circulating tumor cells in immunotherapy. Theranostics. 2021;11:8057–8075. doi: 10.7150/thno.59677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patel SP, Kurzrock R. PD-L1 Expression as a predictive biomarker in cancer immunotherapy. Mol Cancer Ther. 2015;14:847–856. doi: 10.1158/1535-7163.MCT-14-0983. [DOI] [PubMed] [Google Scholar]
- 14.Strickler JH, Hanks BA, Khasraw M. Tumor mutational burden as a predictor of immunotherapy response: is more always better? Clin Cancer Res. 2021;27:1236–1241. doi: 10.1158/1078-0432.CCR-20-3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chan TA, Yarchoan M, Jaffee E, Swanton C, Quezada SA, Stenzinger A, Peters S. Development of tumor mutation burden as an immunotherapy biomarker: utility for the oncology clinic. Ann Oncol. 2019;30:44–56. doi: 10.1093/annonc/mdy495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu L, Bai X, Wang J, Tang XR, Wu DH, Du SS, Du XJ, Zhang YW, Zhu HB, Fang Y, et al. Combination of TMB and CNA stratifies prognostic and predictive responses to immunotherapy across metastatic cancer. Clin Cancer Res. 2019;25:7413–7423. doi: 10.1158/1078-0432.CCR-19-0558. [DOI] [PubMed] [Google Scholar]
- 17.Dolgin E. Combining biomarkers for immunotherapy. Cancer Discov. 2018;8:1500–1501. doi: 10.1158/2159-8290.CD-NB2018-143. [DOI] [PubMed] [Google Scholar]
- 18.Jardim DL, Goodman A, de Melo Gagliato D, Kurzrock R. The challenges of tumor mutational burden as an immunotherapy biomarker. Cancer Cell. 2021;39:154–173. doi: 10.1016/j.ccell.2020.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen L, Chen T, Zhang Y, Lin H, Wang R, Wang Y, Li H, Zuo Z, Ren J, Xie Y. TIRSF: a web server for screening gene signatures to predict tumor immunotherapy response. Nucleic Acids Res. 2022;50(W1):W761–W767. doi: 10.1093/nar/gkac374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miao YR, Zhang Q, Lei Q, Luo M, Xie GY, Wang H, Guo AY. ImmuCellAI: a unique method for comprehensive T-cell subsets abundance prediction and its application in cancer immunotherapy. Adv Sci (Weinh) 2020;7:1902880. doi: 10.1002/advs.201902880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zeng Z, Wong CJ, Yang L, Ouardaoui N, Li D, Zhang W, Gu S, Zhang Y, Liu Y, Wang X, et al. TISMO: syngeneic mouse tumor database to model tumor immunity and immunotherapy response. Nucleic Acids Res. 2022;50:D1391–D1397. doi: 10.1093/nar/gkab804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin A, Qi C, Wei T, Li M, Cheng Q, Liu Z, Luo P, Zhang J. CAMOIP: a web server for comprehensive analysis on multi-omics of immunotherapy in pan-cancer. Brief Bioinform. 2022 doi: 10.1093/bib/bbac129. [DOI] [PubMed] [Google Scholar]
- 23.Yofe I, Dahan R, Amit I. Single-cell genomic approaches for developing the next generation of immunotherapies. Nat Med. 2020;26:171–177. doi: 10.1038/s41591-019-0736-4. [DOI] [PubMed] [Google Scholar]
- 24.Li F, Wu T, Xu Y, Dong Q, Xiao J, Xu Y, Li Q, Zhang C, Gao J, Liu L, et al. A comprehensive overview of oncogenic pathways in human cancer. Brief Bioinform. 2020;21:957–969. doi: 10.1093/bib/bbz046. [DOI] [PubMed] [Google Scholar]
- 25.Zhang X, Lan Y, Xu J, Quan F, Zhao E, Deng C, Luo T, Xu L, Liao G, Yan M, et al. Cell Marker: a manually curated resource of cell markers in human and mouse. Nucleic Acids Res. 2019;47:D721–D728. doi: 10.1093/nar/gky900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barrett T, Wilhite SE, Ledoux P, Evangelista C, Kim IF, Tomashevsky M, Marshall KA, Phillippy KH, Sherman PM, Holko M, et al. NCBI GEO: archive for functional genomics data sets–update. Nucleic Acids Res. 2013;41:D991–D995. doi: 10.1093/nar/gks1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu M, Peng Z, Qin M, Liu Y, Wang J, Zhang C, Lin J, Dong T, Wang L, Li S, et al. Interferon-gamma induces tumor resistance to anti-PD-1 immunotherapy by promoting YAP phase separation. Mol Cell. 2021;81:1216–1230. doi: 10.1016/j.molcel.2021.01.010. [DOI] [PubMed] [Google Scholar]
- 28.Huang X, Zhang Q, Lou Y, Wang J, Zhao X, Wang L, Zhang X, Li S, Zhao Y, Chen Q, et al. USP22 deubiquitinates CD274 to suppress anticancer immunity. Cancer Immunol Res. 2019;7:1580–1590. doi: 10.1158/2326-6066.CIR-18-0910. [DOI] [PubMed] [Google Scholar]
- 29.Hanzelmann S, Castelo R, Guinney J. GSVA: gene set variation analysis for microarray and RNA-seq data. BMC Bioinformatics. 2013;14:7. doi: 10.1186/1471-2105-14-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kumagai S, Togashi Y, Kamada T, Sugiyama E, Nishinakamura H, Takeuchi Y, Vitaly K, Itahashi K, Maeda Y, Matsui S, et al. The PD-1 expression balance between effector and regulatory T cells predicts the clinical efficacy of PD-1 blockade therapies. Nat Immunol. 2020;21:1346–1358. doi: 10.1038/s41590-020-0769-3. [DOI] [PubMed] [Google Scholar]
- 31.Davis AA, Patel VG. The role of PD-L1 expression as a predictive biomarker: an analysis of all US Food and Drug Administration (FDA) approvals of immune checkpoint inhibitors. J Immunother Cancer. 2019;7:278. doi: 10.1186/s40425-019-0768-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Man K, Gabriel SS, Liao Y, Gloury R, Preston S, Henstridge DC, Pellegrini M, Zehn D, Berberich-Siebelt F, Febbraio MA, et al. Transcription factor IRF4 promotes CD8(+) T cell exhaustion and limits the development of memory-like T cells during chronic infection. Immunity. 2017;47(6):1129–1141. doi: 10.1016/j.immuni.2017.11.021. [DOI] [PubMed] [Google Scholar]
- 33.Li L, Zhang J, Chen J, Xu-Monette ZY, Miao Y, Xiao M, Young KH, Wang S, Medeiros LJ, Wang M, et al. B-cell receptor-mediated NFATc1 activation induces IL-10/STAT3/PD-L1 signaling in diffuse large B-cell lymphoma. Blood. 2018;132:1805–1817. doi: 10.1182/blood-2018-03-841015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen Z, Luo Z, Zhang D, Li H, Liu X, Zhu K, Zhang H, Wang Z, Zhou P, Ren J, et al. TIGER: a web portal of tumor immunotherapy gene expression resource. Genom Proteom Bioinform. 2022 doi: 10.1016/j.gpb.2022.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang W, Zeng B, Lin H, Guan W, Mo J, Wu S, Wei Y, Zhang Q, Yu D, Li W, et al. CanImmunother: a manually curated database for identification of cancer immunotherapies associating with biomarkers, targets, and clinical effects. Oncoimmunology. 2021;10:1944553. doi: 10.1080/2162402X.2021.1944553. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or used during the current study are available in the Gene Expression Omnibus (https://www.ncbi.nlm.nih.gov/geo/), The Cancer Genome Atlas (https://portal.gdc.cancer.gov/), and we also develop database, CiTSA (http://bio-bigdata.hrbmu.edu.cn/CiTSA/).