Abstract
Dendritic cells have been at the forefront of cancer-immunotherapy research for over 2 decades. They elicited that attention by having an unprecedented capacity to mount T cells responses against tumors. However, the clinical use of DC-based vaccination against established malignancies has resulted in limited clinical benefits. Several factors are responsible for limiting the efficacy of DC-based immunotherapy, such as the harmful influence of the tumor microenvironment on DCs activity. New insights into the inner process of DC-mediated T cell activation have supported the development of new strategies that potentiate DCs-based therapies. Herein, we identify signaling cascades that have recently been targeted by small molecules and biologicals to promote the activation of monocyte-derived DCs and decrease their susceptibility to becoming tolerogenic. While Statins can markedly enhance antigen presentation, protein kinase inhibitors can be used to increase the expression of co-receptors and adhesion molecules. STAT3 and IDO can be modulated to limit the production of regulatory factors that work against differentiation and activation. The targeting of multiple pathways simultaneously has also been found to produce synergism and drastically enhance DCs activity. Some of these strategies have recently yielded positive results in clinical settings against established malignancies such as non-small cell lung cancer. The emergence of these approaches opens the door for a new generation of potent dendritic cell-based therapeutics to fight cancer.
Keywords: Dendritic cells, Cell-based vaccination, Immunotherapy, Pharmacological inhibitors
Main
Evolution of dendritic cell-based cancer-immunotherapy
Cancer initiation is a process that occurs over many years. During that time, the immune system constantly fights to limit the expansion of tumor cells. Among notable responders, the dendritic cell (DCs) ensures the activation of cellular immunity against tumors and provides support through the secretion of pro-inflammatory cytokines [1]. There is a point, however, when cancer cells gain the upper hand by secreting factors that impede the activation of the immune system [2]. More than 2 decades ago, in an effort to rescue anti-tumoral immunity, dendritic cell vaccination made its day view [3]. The idea behind the initiative is that injection of DCs loaded with tumor-associated antigens (TAA) could, de novo, initiate naïve T cell activation leading to a cytotoxic response. While dendritic cell-based therapeutic vaccines can stabilize cancer progression [4, 5], their effectiveness in reducing tumor volume remains low. It was found that both tumor-derived factors and intrinsic flaws of the approach were the main reasons behind negative results [5, 6]. Indeed, patient-derived DCs are much less functional than their healthy counterparts, and ex vivo manufactured DCs even less so than their naturally occurring DC (nDCs) counterparts [7, 8]. Several studies have also exposed the fact that moDC-based vaccines rely on host dendritic cells for activity [9, 10]. This hypothesis was further supported when the loading of antigens in monocytes, which are not considered as potent antigen presenting cells, was able to induce T cell anti-tumor responses through antigen transfer to host nDCs [11]. An alternative emerged, where highly specialized subsets of DCs, directly isolated from blood, could be used for vaccination [12]. Autologous transfer of nDCs has been deemed safe and results in the initiation of T cell responses, however, evidence that they yield positive outcomes against established malignancies is lacking [12].
Moreover, the discovery of a conserved regulatory module that reduces the functionality of nDCs in human and mouse cancers adds significant complications [13]. Indeed, DCs were found to be susceptible of acquiring an immunosuppressive phenotype, marked by PD-L1 overexpression, upon uptake of tumor-derived antigens. The clinical failures may be due to DCs being intrinsically unsuited for immunotherapy. In the past years, several reviews have produced detailed accounts of the biology of DCs and their role in cancer-immunotherapy [14, 15]. Herein, we focus on the advances of an emerging field committed on modulating the characteristics of ex vivo generated DCs. We review the molecular pathways that can be targeted to limit the influence of tumor secreted factors on DC function, increase lymph node homing, and boost antigen presentation. Together, those modifications address the fundamental flaws of DCs and provide considerable tools for cell-based therapeutics.
The role and function of dendritic cells
DCs are professional antigen-presenting cells (APCs) that form a bridge between innate and adaptive immunity. As the most potent of all APCs, DCs have a high expression of co-stimulatory molecules, notably CD80/CD86 and CD40, and major histocompatibility complexes I and II (MHC I and MHC II) [1]. Their capacity to produce IL-12p70, IL-2 and IL-4 is fundamental to skewing T cell differentiation toward the appropriate adaptive immune response. While IL-12 and IL-2 are necessary for anti-tumoral T-helper 1 (Th1) mediated responses, IL-4 will activate T-helper 2 (Th2) response gene programs that are generally associated with immune regulation. The common progenitor of monocytes and dendritic cells originating from CD34 + stem cells is found in the bone marrow. This progenitor, the monocyte, and DC progenitor (MDPs) give rise to monocyte or DCs committed progenitors (MCPs and CDPs, respectively). CDPs then develop into pre-DCs that egress the bone marrow and eventually give rise to conventional dendritic cells and plasmacytoid dendritic cells (pDCs) [16, 17]. In steady state conditions, DCs are functionally immature and can be found in lymphoid and peripheral tissues. In this immature state, DCs have the most potent phagocytic capacities, allowing them to recognize and capture antigens potently. When faced with inflammatory “danger signals,” immature dendritic cells undergo maturation where they upregulate MHC I and II complexes, as well as adhesion/co-stimulatory molecules CD40, CD54, CD80, CD83, and CD86 [12, 18, 19]. The activation of DCs causes their migration to lymphoid organs. They will present processed exogenous peptides to naïve CD4 + T cells through the MHC class II complex and endogenous peptides to naïve CD8 + T cells via the class I MHC (Fig. 1). The most relevant axis for antigen processing and presentation in the scope of cancer immunotherapy is the cross-presentation pathway. The cross-presentation pathway is a dedicated phagocytic pathway used to process and load exogenous antigens on MHC I complexes for subsequent CD8 + T cell priming [16, 20]. DCs are the most competent cross-presenting cells out of the known APC subsets [21, 22]. Upon endocytosis or phagocytosis by DCs, exogenous antigens are targeted for proteasomal or lysosomal degradation. Antigens are then trimmed into peptides before being loaded onto MHC I molecules for presentation. Cross-presentation by moDCs is inferior to nDCs [8]. However, recent findings showed that reducing lysosomal degradation of pulsed peptides results in a superior capacity to prime T cells [23]. These findings suggest that ex vivo reactions do not require additional antigen processing by DCs, such as degradation and trimming, since the cells are pulsed with a final form of antigen peptides. Any processing would thus result in proteolysis of the desired peptide and a decrease in T cell priming.
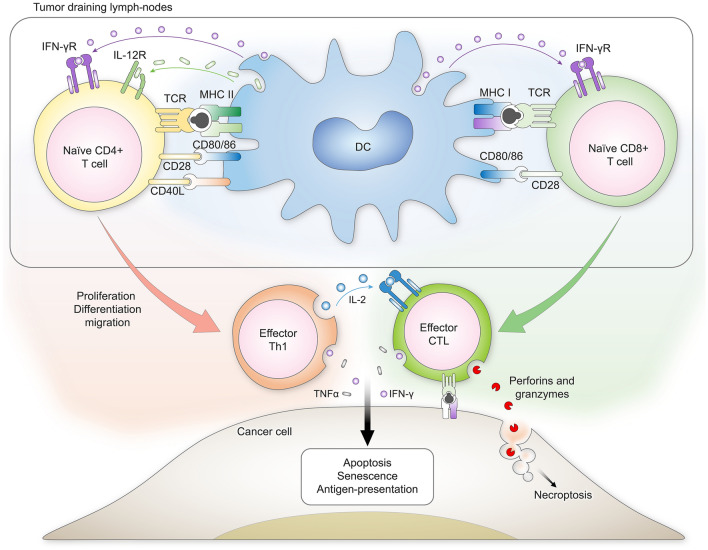
Fig. 1.
Overview of the three critical signals that DCs use to prime T cells against cancer. DCs present antigens to naïve CD4 + and CD8 + T cells through class II and class I major histocompatibility complexes, respectively. Co-receptor engagement and cytokine signaling are also required to fully prime the activation, proliferation, and differentiation of T cells. Upon their differentiation to effector cells, T cells migrate from the tumor-draining lymph nodes to the tumors themselves. Recognition of cognate antigens on the surface of cancer cells leads to the secretion of perforins, granzymes, and pro-inflammatory cytokines by effector cytotoxic T lymphocytes (CTLs). These factors will induce the death of cancer cells by apoptosis or necrosis. In the context of anti-tumoral immunity, CTLs are also assisted by Th1 cells that produce IL-2 to maintain the responsiveness and proliferation of CTLs
Naturally occurring dendritic cells (nDCs) and their use in cancer therapeutics
Two distinct families of dendritic cell populations are found in human tissues: conventional (cDCs) and plasmacytoid dendritic cells (pDCs). cDCs and pDCs are phenotypically and functionally divergent. The most prevalent form of naturally occurring dendritic cells is the conventional dendritic cell type (cDC). cDCs can be further categorized into cDC1s and cDC2s based on the respective surface markers they express. In addition to the expression of CLEC9/DNGR-1 and XCR1, which are also found at the surface of murine cDC1s counterparts, human cDC1s express the cell surface BDCA3, also known as CD141 [24–26]. Studies have also demonstrated that the transcription factors BATF3 and IRF8 are important for cDC1s capacity to induce potent T cell responses [24]. Conversely, human cDC2s express BDCA1 (CD1c), CD11b, and CD172a [24, 27]. The two populations of cDCs have complementary roles in establishing T cell responses against cancers. cDC2s are involved in, but not limited to, antigen presentation to CD4 + T cells through MHC class II complex in tumor-draining lymph nodes, whereas cDC1s have a superior capacity to cross-present tumor-derived antigens to CD8 + cytotoxic T cells [28, 29]. cDC1s have also been recently found to be critical for CD4 + T Cell activation and are thus not limited to CD8 + T cell priming as previously believed [30]. Studies have also demonstrated that priming naïve CD8 + T cells by cDC1s within tumors correlates with increased patient survival [31]. Chemokines secreted from the tumor microenvironment (TME), such as CCL4, CCL5, and XCL1, contribute to cDC1s accumulation in the tumors [32, 33]. However, other factors secreted by the tumor microenvironment can impair the anti-tumoral activity of dendritic cells and the activation of T cells [3]. Host dendritic cells can become tolerogenic and even promote the stimulation of regulatory T cells in the presence of Arginase I [34], IL-10 [35], IDO [36], TGF-β [37], PGE2 [38], and VEGF [39]. Tolerogenic DCs were also shown to directly contribute to tumor escape [40]. The second type of naturally occurring DCs is the plasmacytoid DC (pDCs). Intra-tumoral presence of pDCs has been associated with positive and negative prognostic values for various cancers [41–43]. From a clinical perspective, various trials have identified that injections of autologous pDCs are safe and result in mounting T cells responses [12, 44, 45]. Since both naturally occurring and adoptively transferred dendritic cells are vulnerable to becoming tolerogenic, there is a need for interventions that can prevent their conversion. Without such intervention, achieving prolonged response from adoptively transferred dendritic cells is unlikely. For a long time, the identities of several DC subsets was unknown, let alone which subsets are most suitable for dendritic cell vaccination. Recent advances suggest that nDC subsets of the cDC1s phenotype or that bear the XCR1 chemokine receptor are the best candidates for nDC vaccination against cancers [12, 24, 46, 47]. This subset appears to fulfill that role by having greater cross-presentation capacities than any other subset of APC [46]. Injection of antigen loaded cDC1s, but not GM-CSF/ IL-4 generated DCs, into tumor bearing and cDC1s deficient mice (Irf8 32−/−) yielded tumor regression [48]. This body of evidence supports the fact that cDC1s are the most suitable DC subset for therapeutic vaccination. The major limitation for clinical use is the low frequency of those subsets, with about 0.05% of all PBMCs being cDC1s [26]. The clinical landscape of nDC therapeutics has already been covered in depth and will not be the focus of this review [12].
Ex vivo generated dendritic cells
Although promising, naturally occurring DC interventions can only utilize a relatively limited number of naturally occurring DCs, given the rarity of those populations. This limitation is made more pronounced in the case of advanced cancers, where the proportion of cDCs and pDCs in circulation is significantly decreased [7]. The other inconvenience of using dendritic cells as therapeutic material is their incapacity to proliferate upon activation. Therefore, extensive blood draws are required to harvest the biological material necessary to perform the intervention, but is inadequate in the case of severely ill patients. One potential solution to this limitation is to artificially produce dendritic cells from a more common cell type, such as monocytes.
Monocyte-derived dendritic cells (moDCs)
Monocyte-derived dendritic cells (moDCs) were initially developed as a manufacturable alternative to naturally occurring DCs to initiate T cell responses in vivo or ex vivo [49]. Most protocols use monocytes from leukapheresis as the starting material for moDC production, but CD34 + stem cells are also readily used. In the case of monocytes isolated from peripheral blood mononuclear cells (PBMCs), protocols usually have a 6–8 days differentiation period using GM-CSF and IL-4, which precedes a 24–48 h maturation period [50]. It was also demonstrated that DCs could be differentiated from monocytes in only three days [51]. The two most commonly used maturation protocols rely on a combination of LPS/IFN-γ [52] or TNF-α/IL-1β/IL-6/PGE2 [53]. Differentiation and maturation of monocytes into DCs upregulates the three key immunogenic signals required for potent T cell priming. First, antigen presentation through class I and II MHC molecules; second, co-stimulatory signals by CD80/86, CD40, and ICOS-L and third, cytokine secretion [3] (Box 2). Among the constituents of maturation cocktails, IFN-γ was crucial for producing increased amounts of IL-12p70, a cytokine that will favor the development of cytotoxic cellular immunity [1]. Several transcription factors were found to be crucial for the formation of mature moDCs, most notably NF-κB, PU1, IRF4, and STAT6 [54].
Antigen cross-presentation by MHC I molecules on the surface of DCs is required to generate de novo cytotoxic T lymphocytes. While cross-presentation does occur within the TME, T cell priming mainly occurs in tumor-draining lymph nodes [55]. DC retention in lymph nodes relies on the presence of chemokine receptor CCR7 [56]. Comparisons of transcriptional profiles between ex vivo generated DCs, and their naturally occurring counterparts revealed that the former has a lower expression of CCR7, which might decrease their capacity to reach tumor-draining lymph nodes efficiently [8, 13].
moDCs still represent a viable alternative to substitute naturally occurring DCs in the context of vaccination. Notably, they are phenotypically and functionally different from their naturally occurring cDC counterparts [8, 57]. Monocytes do not give rise to tissue residing DCs in steady–state conditions. Indeed, monocytes only acquire functional and phenotypic resemblance to cDCs under specific environmental cues [58–60]. Mounting evidence for the inferior activity of moDCs to elicit immune responses compared to nDCs gave rise to a paradigm shift in dendritic cell therapeutics [61]. Years of clinical failures have made clear that there are underlying problems with dendritic cell-based cancer immunotherapy: (1). Ex vivo generated dendritic cells are less functional than their naturally occurring counterparts in terms of T cell priming for cancer neo-antigens, (2). DCs from cancer patients have poor immunological function due to the influence of tumor-derived signals, (3). The tumor secreted factors can drastically reduce the efficacy of cell-based therapeutics. Recent findings have also identified the presence of a regulatory module that reduces cDC1 functionality in human and mouse cancers, indicating that the flaws of therapeutic interventions are possibly intrinsic to DCs themselves [13]. Herein, we review research that proposed modulating various signaling cascades in DCs to address the shortcomings of DC-based therapy. Each strategy can be further regrouped by the DC-T cell signal it most potently augments: Antigen presentation, adhesion molecules and co-receptor expression and cytokine secretion (Table 1).
Table 1.
Summary of the targetable enzymes that modulate the activation status of moDCs
| DC signal potentiated | Pathway | Enzyme(s) targeted | Compound | Readout | References |
|---|---|---|---|---|---|
| Antigen presentation | Mevalonate | HMG-CoA reductase |
Simvastatin (FDA approved as lipid-lowering medication) |
↑ Surface MHC I:Ag. complex ↑ Surface MHC II:Ag. Complex In vivo efficacy against B16 (OVA) melanoma |
[46] |
| Mevalonate | GGPP synthase | TH-Z145 |
↑ Surface MHC I:Ag. complex ↑ Surface MHC II:Ag. Complex In vivo efficacy against B16 (OVA) melanoma |
[46] | |
| Mevalonate | FPP synthase | TH-Z93 |
↑ Surface MHC I:Ag. complex ↑ Surface MHC II:Ag. Complex In vivo efficacy against B16 (OVA) melanoma |
[46] | |
| Co-receptors | PI3K/AKT | AKT | MK2206 |
↑ Surface MHC I ↑ CCR7 |
[59] |
| DNA-PK | DNA-PK | NU7441 |
↑ Surface MHC I ↓ PD-L1/2 |
[59] | |
| MAPK | MEK | Trametinib (FDA approved for metastatic NSCLC) | ↑ Surface MHC I | [59] | |
|
PI3K/AKT + DNA-PK + MAPK |
AKT + DNA-PK + MEK |
MK2206 + NU7441 + Trametinib |
↑ Surface MHC I ↑ CCR7 ↓ PD-L1/2 ↑ CD40, CD83, CD86 ↑ In vivo efficacy versus U87 GBM |
[59] | |
| MAPK | P38 | SB202190 |
↑ OX40L ↑ In vivo efficacy versus B16 (OVA) melanoma |
[64] | |
| Cytokines |
JAK/STAT/ PTPs |
PTPN1 PTPN2 |
L598 |
moDC differentiation: ↑ Surface MHC I & II, CD40, CD80, CD86 ↑ IL-12p70 and IFN-γ |
[69] |
|
JAK/STAT/ SOCS |
SOCS1 | SOCS1 siRNA | ↑ IL-12p70 | [72] | |
| Autocrine IL-10 signaling | BTK | Ibrutinib (FDA approved for various leukemias and lymphomas) |
↑ Surface MHC II ↑ CD80 |
[78] | |
| BTK-IDO-GATOR2-mTOR |
BTK + IDO |
Ibrutinib + indoximod |
↑ moDC differentiation toward cDC1 phenotype ↑ In vivo efficacy versus B16F10 and EL4-OVA |
[79] |
Increasing antigen presentation by targeting the mevalonate pathway
Reduced degradation by phagocyting compartments is a characteristic trait of cDC1s that allows superior cross-presentation capacity [62]. Molecules that reduce the vesicular degradation of antigens are therefore promising adjuvants to increase antigen cross-presentation during adoptive transfers. The study by Xia et al. identified that the mevalonate pathway (MVA), also known as the isoprenoid pathway, is a druggable target for vaccine adjuvant discovery [23]. Their research shows that blocking protein prenylation and geranyl-geranylation with Simvastatin, an inhibitor of HMG-CoA reductase, increases antigen preservation and presentation in mouse dendritic cells leading to superior T cell priming [23]. Mechanistically, simvastatin and other inhibitors of the mevalonate pathway prevent the geranyl-geranylation of small GTPase Rab 5. Lipid-post-translational modification of the family of Rab GTPases is essential to mediate their localization to endosomal membranes, which induces the fusion between endosomes and lysosomes [23]. Systemic administration of statins together with immunization also confers superior anti-cancer responses, indicating that host DCs also benefit from the intervention [23]. It was also demonstrated that CLEC9A + dendritic cells are responsible for conducting anti-tumoral immune activation during MVA inhibition. This further supports evidence that cDC1s are the most critical DC subset for anti-tumoral immunity. Inhibition of the mevalonate pathway directly in cancer cells also potentiates immune activation by inhibiting the small GTPase Rac1 geranyl-geranylation [63]. Using antagonists of enzymes downstream in the mevalonate cascade could also provide the same benefits but with fewer off-target effects than upstream inhibitors. Other molecules that target farnesyl and geranyl transferases, the enzymes responsible for linking lipid modifications to proteins, could also be valuable candidates. The lengthy clinical track record of statins makes them suitable for use as systemic DC cell-based vaccine adjuvants. However, high doses of mevalonate pathway inhibitors have also shown to have the opposite effect on MHC class II derived antigen presentation, thereby limiting the therapeutic window for adjuvant use [64]. In brief, statins, and other inhibitors of the mevalonate pathway have a distinct capacity to reduce endosomal trafficking of capture antigens, which is highly beneficial for DC, or other APC-based therapeutics. Several other pathways can also be modulated to increase the surface expression of both MHC I and II molecules, most of which are covered subsequently (Table 1). Those pathways, however, are distinct in their effect compared to statins since they increase antigen presentation by augmenting the maturation status of DCs.
Potentiators of adhesion molecules and co-stimulatory receptor-expression
PI3K/AKT pathway antagonists
Although kinases are often perceived as positive regulators of cellular functions, a large group of kinases acts as negative regulators in the context of dendritic cell activation. In dendritic cells, the PI3K/AKT cascade acts as a negative regulator of TLR-4 signaling [65, 66] (Fig. 2). It was therefore proposed that AKT inhibitors could be employed to increase further the maturation status of ex vivo generated dendritic cells that rely on TLR signals for maturation. It was shown that MK2206, a small molecule inhibitor of AKT, significantly increased the surface expression of CCR7 on the moDCs [67]. Whether or not this approach could increase the lymph node transit time of injected DCs remains unknown.
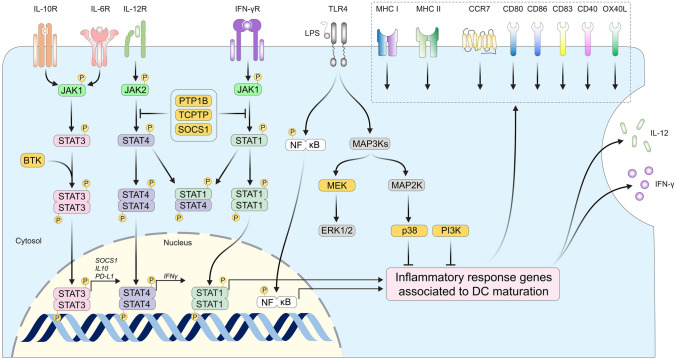
Fig. 2.
Molecular pathways that can be targeted to potentiate the function of moDCs. The binding of interferon-gamma (IFN-γ) and IL-12p70 to their respective receptors induce the activation of JAK1 and 2, which phosphorylate STAT1 and STAT4 transcription factors. Tyrosine phosphorylation of STAT proteins leads to their homo or heterodimerization and promotes their translocation to the nucleus. STAT1 and STAT4 homo-dimers promote the transcription of various genes involved in DC activity, such as IFN-γ, CD80, and CD86. PTP1B, TCPTP, and SOCS1 dephosphorylate both JAK1/2 and STATs to limit their activation. Among STAT factors, STAT3 is the most important signal transducer for IL-10 and IL-6 mediated signaling. Bruton’s tyrosine kinase (BTK) phosphorylates STAT3 to promote its nuclear translocation. Activation of this pathway leads to the transcription of genes with a regulatory function such as SOCS1, IL-10, and checkpoint receptor PD-L1 that are deleterious to the priming of T cells by DCs. Pattern recognition receptors such as TLR-4 also play a central role in the maturation of dendritic cells. Activation of TLR-4 by bacterial Lipopolysaccharides (LPS) induces the phosphorylation of NF-κB. This transcription factor also orchestrates the transcription of inflammatory response genes associated with DC maturation. In parallel of NF-κB, TLR-4 signaling induces mitogen-activated protein kinase-kinase-kinase phosphorylation (MAP3Ks), which in turn phosphorylates mitogen-activated protein kinase-kinases (MAP2Ks) such as MEK. MAP2Ks phosphorylate mitogen-activated protein kinases (MAPKs) such as p38. PI3K is also active during the maturation of dendritic cells, although independently of MAPK reactions. MEK, p38, and PI3K have been found to circumstantially repress the transcription of genes important to DC maturation. Together, the function of those enzymes (yellow label) can be inhibited to potentiate the differentiation and maturation of dendritic cells ex vivo
Mitogen-activated protein kinase (MAPK) pathway inhibitors
Mitogen-activated protein kinase-kinase MEK is another enzyme that negatively impacts the function of mature DCs. MEK activity limits NF-KB-dependent gene transcription during activation and contributes to maintaining DCs in an immature state [68, 69]. Inhibition of MEK with trametinib in combination with AKT and DNA-PK inhibitors results in superior human DC activation as marked by increased expression of MHC I, CD83, and CCR7 [67]. However, MEK inhibition alone results in upregulation of PD-L1 and PD-L2, which can counteract other mechanisms leading to superior T cell priming. Indeed, several studies have found that the systemic use of MEK inhibitors against cancer collaterally diminishes dendritic and T cell responses [70, 71]. Further investigation is therefore required to fully understand how MEK inhibitors can be employed to potentiate dendritic cell-based vaccines.
p38 MAPK inhibition
Inhibition of p38 MAPK increased immunostimulatory potency by upregulating the surface expression of OX40L, a co-stimulatory molecule involved in skewing effector T cell responses [72] (Fig. 2). Other reports have demonstrated that p38 activity is crucial for DC cross-presentation and the production of IL-12p70 [73]. Autocrine production of IL-6 and subsequent activation of p38 MAPK have a negative impact on the CCR7-dependent DC migration [59, 74]. Whether or not p38 inhibition increases CCR7 expression remains unknown.
Increasing cytokine production and signaling
Inhibiting protein tyrosine phosphatases targeting JAK/STAT signaling
JAK/STAT signaling pathways are significant regulators of DC differentiation, cytokine secretion, and DC-mediated T cell activation required for effective anti-tumor response [75]. The activity of JAK/STAT is counterbalanced by the action of protein tyrosine phosphatases (PTP), most notably PTP1B and TCPTP, respectively, encoded by the PTPN1 and PTPN2 genes [76] (Fig. 2). Antagonists of PTP1B and TCPTP were shown to increase the production of IL-12p70 and IFN-γ, leading to superior T cell priming against cancer antigens in human and mouse moDCs [77]. Furthermore, the inhibition of PTP1B and TCPTP significantly improved the maturation and antigen-presenting properties of defective moDCs derived from pancreatic cancer patients [77].
SOCS1 silencing
The amplitude of DC maturation and initiation of adaptive immune responses is negatively regulated by the suppressor of cytokine signaling 1 (SOCS1) gene [78] (Fig. 2). It was found that SOCS1 silencing increases IL-12 response and production, increasing anti-tumor immunity [79]. The challenge with targeting SOCS1 is the unavailability of small molecule inhibitors making transcriptional interference the most suitable option for cell-based therapeutics presently. Phase I results in patients suffering from resected Non-small cell lung cancer (NSCLC) treated with human moDCs pulsed with two TAAs, silenced with SOCS1, and stimulated with flagellin, showed a modest response [80].
Antagonizing regulatory cytokine receptor signaling
STAT3 Inhibition and IL-6
JAK activates Signal transducer and activator of transcription 3 (STAT3) to induce the production of factors that deleteriously affect DC activity [40, 81]. STAT3 modulates the signaling from cytokines that limit the activity of DCs such as IL-6, IL-10, and TGF-ß [82] (Fig. 2). STAT3 null dendritic cells were shown to have increased activity and a superior capacity to activate T cells in glial malignancies [83, 84]. STAT3 signaling is also required for the autocrine production of IL-6, a cytokine associated with DC dysfunction [72]. Interestingly, modification of conventional moDC differentiation protocols by replacing IL-4 with IL-6 resulted in moDCs resembling cDC1s [85]. IL-6 might thus be favorable for differentiating DCs with a high cross-presentation capacity but deleterious for functions realized by mature DCs.
Brutons tyrosine kinase
Bruton tyrosine kinase (BTK) positively regulates autocrine IL-10 signaling in DCs, promoting tolerogenicity. BTK Inhibition in mouse DCs was shown to increase the proliferation of CD4 + T cells by increasing the surface expression of MHC class II molecules and CD80 [86] (Fig. 2). Inhibition of BTK and IDO resulted in the robust promotion of anti-tumor T cell responses by CD103 + cDCs [87]. BTK and IDO are promising targets to increase the differentiation of inflammatory dendritic cells ex vivo and in vivo.
Essential considerations for drug discovery
With the evolution of this dendritic immunotherapy, the field comes essential lessons from past failures. It has become evident that DC therapeutics rely on host DCs for efficacy [9, 10, 48]. The effects of host DCs must thus be considered carefully during the evaluation of therapeutic vaccines. DC deficient mouse models are thus valuable tools for demonstrating the direct capacity of injected DCs to mount T cell responses independently of the host DCs [48, 88–90]. The interpretation of assays designed to evaluate the effect of small molecules on DC function must be made with solid control over false positives. A significant source of false positivity is the dendritic cell stress response itself. In response to surrounding necrotic/apoptotic cells and activation of molecular stress pathways, DCs can upregulate MHC and co-stimulatory molecules at their surface [91]. It is therefore imperative to evaluate compounds in a setting that provides optimal viability to ensure the specificity of findings. Quantification of co-stimulatory molecules (CD80/86, CD83, CD40, OX40L) should also be accompanied by PD-L1/2 and TIM3 to ensure that co-stimulatory signals do not occur with the upregulation of checkpoint receptors. There is currently a lack of information surrounding the transcriptional impact of the interventions. The notion of antagonism, synergism, and additivity is also highly relevant when using molecules that can have off-target effects. An encouraging fact is that combinations of molecules targeting different pathways have already been shown to act in synergy [67, 87].
Concluding remarks
While DC-based research focuses on identifying subsets most suitable for immunotherapy, many groups are attempting to potentiate ex vivo generated DCs. There is evidence, merging from the two parallel fields, that increasing the transcriptional and phenotypical resemblance between ex vivo DCs and naturally occurring cDC1s could induce more potent anti-cancer responses. Targeting pathways that upregulate CCR7, antigen presentation, co-stimulatory molecules, and production of IL-12 and IFN-γ, results in DCs with superior capacities for immunotherapy. Targeting all those pathways using a combination of molecules could resolve some of the issues of dendritic cell-based cancer immunotherapy by decreasing the intrinsic limitations of DCs themselves. Very little is known about the clinical applicability of those interventions and if they perform as well as their unmodified nDCs counterparts. Furthermore, it remains unknown whether those interventions decrease the reliance on host DCs for efficacy. While ex vivo manufacture is an appealing approach for yielding large quantities of DCs, it remains a puzzle how we can achieve it without decreasing their function. Furthermore, can ex vivo artificial programming ever perform as well as in vivo differentiation and maturation in the case of DCs? The evidence is clear, however, that we need to evolve our approach to DC-based therapeutics to make progress. Perhaps the way forward is by sculpting a new form of DCs using a combination of inhibitors and gene editing.
Acknowledgments
We thank Dr. Noriko Uetani for her help with the original figures. AP is a Canderel Studentship and FRSQ doctoral scholarship recipient. MLT is a Distinguished James McGill Professor and the holder of the J. and J.L. Levesque Chair in Cancer Research. This work was supported by a Canadian Institute of Health Research Foundation grant to MLT (CIHR FDN-159923), the Richard and Edith Strauss Canada Foundation, the Aclon Foundation and the FRQS Oncopole program.
Author contributions
AP and MLT had the idea for the article, AP performed the literature search, data analysis, and drafted the work. MLT critically revised the work and provided supervision. All authors reviewed the manuscript.
Declarations
Conflict of interest
The authors declare they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Steinman RM. Decisions about dendritic cells: past, present, and future. Annu Rev Immunol. 2012;30:1–22. doi: 10.1146/annurev-immunol-100311-102839. [DOI] [PubMed] [Google Scholar]
- 2.Gonzalez H, Hagerling C, Werb Z. Roles of the immune system in cancer: from tumor initiation to metastatic progression. Genes Dev. 2018;32(19–20):1267–1284. doi: 10.1101/gad.314617.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mastelic-Gavillet B, Balint K, Boudousquie C, Gannon PO, Kandalaft LE. Personalized dendritic cell vaccines: recent breakthroughs and encouraging clinical results. Front Immunol. 2019;10:766. doi: 10.3389/fimmu.2019.00766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peoples GE, Holmes JP, Hueman MT, Mittendorf EA, Amin A, Khoo S, et al. Combined clinical trial results of a HER2/neu (E75) vaccine for the prevention of recurrence in high-risk breast cancer patients: U.S. military cancer institute clinical trials group study I-01 and I-02. Clin Cancer Res. 2008;14(3):797–803. doi: 10.1158/1078-0432.CCR-07-1448. [DOI] [PubMed] [Google Scholar]
- 5.Okada H, Kalinski P, Ueda R, Hoji A, Kohanbash G, Donegan TE, et al. Induction of CD8+ T-cell responses against novel glioma-associated antigen peptides and clinical activity by vaccinations with {alpha}-type 1 polarized dendritic cells and polyinosinic-polycytidylic acid stabilized by lysine and carboxymethylcellulose in patients with recurrent malignant glioma. J Clin Oncol. 2011;29(3):330–336. doi: 10.1200/JCO.2010.30.7744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Small EJ, Schellhammer PF, Higano CS, Redfern CH, Nemunaitis JJ, Valone FH, et al. Placebo-controlled phase III trial of immunologic therapy with sipuleucel-T (APC8015) in patients with metastatic, asymptomatic hormone refractory prostate cancer. J Clin Oncol. 2006;24(19):3089–3094. doi: 10.1200/JCO.2005.04.5252. [DOI] [PubMed] [Google Scholar]
- 7.Almand B, Resser JR, Lindman B, Nadaf S, Clark JI, Kwon ED, et al. Clinical significance of defective dendritic cell differentiation in cancer. Clin Cancer Res. 2000;6(5):1755–1766. [PubMed] [Google Scholar]
- 8.Lundberg K, Albrekt AS, Nelissen I, Santegoets S, de Gruijl TD, Gibbs S, et al. Transcriptional profiling of human dendritic cell populations and models–unique profiles of in vitro dendritic cells and implications on functionality and applicability. PLoS ONE. 2013;8(1):e52875. doi: 10.1371/journal.pone.0052875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yewdall AW, Drutman SB, Jinwala F, Bahjat KS, Bhardwaj N. CD8+ T cell priming by dendritic cell vaccines requires antigen transfer to endogenous antigen presenting cells. PLoS ONE. 2010;5(6):e11144. doi: 10.1371/journal.pone.0011144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kleindienst P, Brocker T. Endogenous dendritic cells are required for amplification of T cell responses induced by dendritic cell vaccines in vivo. J Immunol. 2003;170(6):2817–2823. doi: 10.4049/jimmunol.170.6.2817. [DOI] [PubMed] [Google Scholar]
- 11.Huang MN, Nicholson LT, Batich KA, Swartz AM, Kopin D, Wellford S, et al. Antigen-loaded monocyte administration induces potent therapeutic antitumor T cell responses. J Clin Invest. 2020;130(2):774–788. doi: 10.1172/JCI128267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bol KF, Schreibelt G, Rabold K, Wculek SK, Schwarze JK, Dzionek A, et al. The clinical application of cancer immunotherapy based on naturally circulating dendritic cells. J Immunother Cancer. 2019;7(1):109. doi: 10.1186/s40425-019-0580-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maier B, Leader AM, Chen ST, Tung N, Chang C, LeBerichel J, et al. A conserved dendritic-cell regulatory program limits antitumour immunity. Nature. 2020;580(7802):257–262. doi: 10.1038/s41586-020-2134-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gardner A, de Mingo PÁ, Ruffell B. Dendritic cells and their role in immunotherapy. Front Immunol. 2020;11:924. doi: 10.3389/fimmu.2020.00924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wculek SK, Cueto FJ, Mujal AM, Melero I, Krummel MF, Sancho D. Dendritic cells in cancer immunology and immunotherapy. Nat Rev Immunol. 2020;20(1):7–24. doi: 10.1038/s41577-019-0210-z. [DOI] [PubMed] [Google Scholar]
- 16.Sabado RL, Balan S, Bhardwaj N. Dendritic cell-based immunotherapy. Cell Res. 2017;27(1):74–95. doi: 10.1038/cr.2016.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu K, Nussenzweig MC. Origin and development of dendritic cells. Immunol Rev. 2010;234(1):45–54. doi: 10.1111/j.0105-2896.2009.00879.x. [DOI] [PubMed] [Google Scholar]
- 18.Schuler G, Steinman RM. Murine epidermal langerhans cells mature into potent immunostimulatory dendritic cells in vitro. J Exp Med. 1985;161(3):526–546. doi: 10.1084/jem.161.3.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Caux C, Massacrier C, Vanbervliet B, Dubois B, Van Kooten C, Durand I, et al. Activation of human dendritic cells through CD40 cross-linking. J Exp Med. 1994;180(4):1263–1272. doi: 10.1084/jem.180.4.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alloatti A, Kotsias F, Magalhaes JG, Amigorena S. Dendritic cell maturation and cross-presentation: timing matters! Immunol Rev. 2016;272(1):97–108. doi: 10.1111/imr.12432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jung S, Unutmaz D, Wong P, Sano G-I, De losSantosSparwasser KT, et al. In vivo depletion of CD11c <sup>+</sup> dendritic cells abrogates priming of CD8<sup>+</sup> T cells by exogenous cell-associated antigens. Immunity. 2002;17(2):211–220. doi: 10.1016/S1074-7613(02)00365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Joffre OP, Segura E, Savina A, Amigorena S. Cross-presentation by dendritic cells. Nat Rev Immunol. 2012;12(8):557–569. doi: 10.1038/nri3254. [DOI] [PubMed] [Google Scholar]
- 23.Xia Y, Xie Y, Yu Z, Xiao H, Jiang G, Zhou X, et al. The mevalonate pathway is a druggable target for vaccine adjuvant discovery. Cell. 2018;175(4):1059–73.e21. doi: 10.1016/j.cell.2018.08.070. [DOI] [PubMed] [Google Scholar]
- 24.Böttcher JP, Reis E, Sousa C. The role of type 1 conventional dendritic cells in cancer immunity. Trends Cancer. 2018;4(11):784–792. doi: 10.1016/j.trecan.2018.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Robbins SH, Walzer T, Dembélé D, Thibault C, Defays A, Bessou G, et al. Novel insights into the relationships between dendritic cell subsets in human and mouse revealed by genome-wide expression profiling. Genome Biol. 2008;9(1):R17. doi: 10.1186/gb-2008-9-1-r17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jongbloed SL, Kassianos AJ, McDonald KJ, Clark GJ, Ju X, Angel CE, et al. Human CD141+ (BDCA-3)+ dendritic cells (DCs) represent a unique myeloid DC subset that cross-presents necrotic cell antigens. J Exp Med. 2010;207(6):1247–1260. doi: 10.1084/jem.20092140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sosa Cuevas E, Ouaguia L, Mouret S, Charles J, Fraipont F, Manches O, et al. BDCA1 + cDC2s, BDCA2 + pDCs and BDCA3 + cDC1s reveal distinct pathophysiologic features and impact on clinical outcomes in melanoma patients. Clin Trans Immunol. 2020;9(11):e1190. doi: 10.1002/cti2.1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Merad M, Sathe P, Helft J, Miller J, Mortha A. The dendritic cell lineage: ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting. Annu Rev Immunol. 2013;31(1):563–604. doi: 10.1146/annurev-immunol-020711-074950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gutiérrez-Martínez E, Planès R, Anselmi G, Reynolds M, Menezes S, Adiko AC, et al. Cross-presentation of cell-associated antigens by MHC class I in dendritic cell subsets. Front Immunol. 2015;6:363. doi: 10.3389/fimmu.2015.00363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferris ST, Durai V, Wu R, Theisen DJ, Ward JP, Bern MD, et al. cDC1 prime and are licensed by CD4(+) T cells to induce anti-tumour immunity. Nature. 2020;584(7822):624–629. doi: 10.1038/s41586-020-2611-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Binnewies M, Miranda, Boldajipour B, Amanda J, David, et al. Dissecting the tumor myeloid compartment reveals rare activating antigen-presenting cells critical for T Cell immunity. Cancer Cell. 2014;26(5):638–652. doi: 10.1016/j.ccell.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spranger S, Bao R, Gajewski TF. Melanoma-intrinsic β-catenin signalling prevents anti-tumour immunity. Nature. 2015;523(7559):231–235. doi: 10.1038/nature14404. [DOI] [PubMed] [Google Scholar]
- 33.Zelenay S, van der Veen AG, Böttcher Jan P, Snelgrove Kathryn J, Rogers N, Acton Sophie E, et al. Cyclooxygenase-dependent tumor growth through evasion of immunity. Cell. 2015;162(6):1257–1270. doi: 10.1016/j.cell.2015.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu Q, Zhang C, Sun A, Zheng Y, Wang L, Cao X. Tumor-educated CD11bhighIalow regulatory dendritic cells suppress T cell response through arginase I. J Immunol. 2009;182(10):6207–6216. doi: 10.4049/jimmunol.0803926. [DOI] [PubMed] [Google Scholar]
- 35.Bellone G, Carbone A, Smirne C, Scirelli T, Buffolino A, Novarino A, et al. Cooperative induction of a tolerogenic dendritic cell phenotype by cytokines secreted by pancreatic carcinoma cells. J Immunol. 2006;177(5):3448–3460. doi: 10.4049/jimmunol.177.5.3448. [DOI] [PubMed] [Google Scholar]
- 36.Nguyen NT, Kimura A, Nakahama T, Chinen I, Masuda K, Nohara K, et al. Aryl hydrocarbon receptor negatively regulates dendritic cell immunogenicity via a kynurenine-dependent mechanism. Proc Natl Acad Sci U S A. 2010;107(46):19961–19966. doi: 10.1073/pnas.1014465107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cubillos-Ruiz JR, Baird JR, Tesone AJ, Rutkowski MR, Scarlett UK, Camposeco-Jacobs AL, et al. Reprogramming tumor-associated dendritic cells in vivo using miRNA mimetics triggers protective immunity against ovarian cancer. Cancer Res. 2012;72(7):1683–1693. doi: 10.1158/0008-5472.CAN-11-3160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zou W, Machelon V, Coulomb-L'Hermin A, Borvak J, Nome F, Isaeva T, et al. Stromal-derived factor-1 in human tumors recruits and alters the function of plasmacytoid precursor dendritic cells. Nat Med. 2001;7(12):1339–1346. doi: 10.1038/nm1201-1339. [DOI] [PubMed] [Google Scholar]
- 39.Takahashi A, Kono K, Ichihara F, Sugai H, Fujii H, Matsumoto Y. Vascular endothelial growth factor inhibits maturation of dendritic cells induced by lipopolysaccharide, but not by proinflammatory cytokines. Cancer Immunol Immunother. 2004;53(6):543–550. doi: 10.1007/s00262-003-0466-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rabinovich GA, Gabrilovich D, Sotomayor EM. Immunosuppressive strategies that are mediated by tumor cells. Annu Rev Immunol. 2007;25(1):267–296. doi: 10.1146/annurev.immunol.25.022106.141609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kini Bailur J, Gueckel B, Pawelec G. Prognostic impact of high levels of circulating plasmacytoid dendritic cells in breast cancer. J Transl Med. 2016;14(1):151. doi: 10.1186/s12967-016-0905-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Treilleux I, Blay JY, Bendriss-Vermare N, Ray-Coquard I, Bachelot T, Guastalla JP, et al. Dendritic cell infiltration and prognosis of early stage breast cancer. Clin Cancer Res. 2004;10(22):7466–7474. doi: 10.1158/1078-0432.CCR-04-0684. [DOI] [PubMed] [Google Scholar]
- 43.Labidi-Galy SI, Treilleux I, Goddard-Leon S, Combes JD, Blay JY, Ray-Coquard I, et al. Plasmacytoid dendritic cells infiltrating ovarian cancer are associated with poor prognosis. Oncoimmunology. 2012;1(3):380–382. doi: 10.4161/onci.18801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tel J, Aarntzen EH, Baba T, Schreibelt G, Schulte BM, Benitez-Ribas D, et al. Natural human plasmacytoid dendritic cells induce antigen-specific T-cell responses in melanoma patients. Cancer Res. 2013;73(3):1063–1075. doi: 10.1158/0008-5472.CAN-12-2583. [DOI] [PubMed] [Google Scholar]
- 45.van der Sluis RM, Egedal JH, Jakobsen MR. Plasmacytoid dendritic cells as cell-based therapeutics: A novel immunotherapy to treat human immunodeficiency virus infection? Front Cell Infect Microbiol. 2020;10:249. doi: 10.3389/fcimb.2020.00249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Audsley KM, McDonnell AM, Waithman J. Cross-presenting XCR1(+) dendritic cells as targets for cancer immunotherapy. Cells. 2020;9(3):565. doi: 10.3390/cells9030565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Balan S, Ollion V, Colletti N, Chelbi R, Montanana-Sanchis F, Liu H, et al. Human XCR1+ dendritic cells derived in vitro from CD34+ progenitors closely resemble blood dendritic cells, including their adjuvant responsiveness, contrary to monocyte-derived dendritic cells. J Immunol (Baltimore, Md : 1950) 2014;193(4):1622–1635. doi: 10.4049/jimmunol.1401243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ferris ST, Ohara RA, Ou F, Wu R, Huang X, Kim S, et al. cDC1 vaccines drive tumor rejection by direct presentation independently of host cDC1. Cancer Immunol Res. 2022;10(8):920–931. doi: 10.1158/2326-6066.CIR-21-0865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Santos PM, Butterfield LH. Dendritic cell-based cancer vaccines. J Immunol. 2018;200(2):443–449. doi: 10.4049/jimmunol.1701024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Posch W, Lass-Flörl C, Wilflingseder D. Generation of human monocyte-derived dendritic cells from whole blood. J Vis Exp. 2016;118:e54968. doi: 10.3791/54968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Spranger S, Javorovic M, Bürdek M, Wilde S, Mosetter B, Tippmer S, et al. Generation of Th1-polarizing dendritic cells using the TLR7/8 agonist CL075. J Immunol. 2010;185(1):738–747. doi: 10.4049/jimmunol.1000060. [DOI] [PubMed] [Google Scholar]
- 52.Hansen M, Met Ö, Svane IM, Andersen MH. Cellular based cancer vaccines: type 1 polarization of dendritic cells. Curr Med Chem. 2012;19(25):4239–4246. doi: 10.2174/092986712802884213. [DOI] [PubMed] [Google Scholar]
- 53.Jonuleit H, Kühn U, Müller G, Steinbrink K, Paragnik L, Schmitt E, et al. Pro-inflammatory cytokines and prostaglandins induce maturation of potent immunostimulatory dendritic cells under fetal calf serum-free conditions. Eur J Immunol. 1997;27(12):3135–3142. doi: 10.1002/eji.1830271209. [DOI] [PubMed] [Google Scholar]
- 54.Nuñez-Reza KJ, Naldi A, Sánchez-Jiménez A, Leon-Apodaca AV, Santana MA, Thomas-Chollier M, et al. Logical modelling of in vitro differentiation of human monocytes into dendritic cells unravels novel transcriptional regulatory interactions. Interface Focus. 2021;11(4):20200061. doi: 10.1098/rsfs.2020.0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thompson ED, Enriquez HL, Fu Y-X, Engelhard VH. Tumor masses support naive T cell infiltration, activation, and differentiation into effectors. J Exp Med. 2010;207(8):1791–1804. doi: 10.1084/jem.20092454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ohl L, Mohaupt M, Czeloth N, Hintzen G, Kiafard Z, Zwirner J, et al. CCR7 governs skin dendritic cell migration under inflammatory and steady-state conditions. Immunity. 2004;21(2):279–288. doi: 10.1016/j.immuni.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 57.Helft J, Böttcher J, Chakravarty P, Zelenay S, Huotari J, Schraml BU, et al. GM-CSF mouse bone marrow cultures comprise a heterogeneous population of CD11c(+)MHCII(+) macrophages and dendritic cells. Immunity. 2015;42(6):1197–1211. doi: 10.1016/j.immuni.2015.05.018. [DOI] [PubMed] [Google Scholar]
- 58.Jakubzick CV, Randolph GJ, Henson PM. Monocyte differentiation and antigen-presenting functions. Nat Rev Immunol. 2017;17(6):349–362. doi: 10.1038/nri.2017.28. [DOI] [PubMed] [Google Scholar]
- 59.Shinde P, Fernandes S, Melinkeri S, Kale V, Limaye L. Compromised functionality of monocyte-derived dendritic cells in multiple myeloma patients may limit their use in cancer immunotherapy. Sci Rep. 2018;8(1):5705. doi: 10.1038/s41598-018-23943-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.De Vries IJ, Krooshoop DJ, Scharenborg NM, Lesterhuis WJ, Diepstra JH, Van Muijen GN, et al. Effective migration of antigen-pulsed dendritic cells to lymph nodes in melanoma patients is determined by their maturation state. Cancer Res. 2003;63(1):12–17. [PubMed] [Google Scholar]
- 61.Wimmers F, Schreibelt G, Sköld AE, Figdor CG, De Vries IJ. Paradigm shift in dendritic cell-based immunotherapy: from in vitro generated monocyte-derived DCs to naturally circulating DC subsets. Front Immunol. 2014;5:165. doi: 10.3389/fimmu.2014.00165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Savina A, Peres A, Cebrian I, Carmo N, Moita C, Hacohen N, et al. The small GTPase Rac2 controls phagosomal alkalinization and antigen crosspresentation selectively in CD8+ dendritic cells. Immunity. 2009;30(4):544–555. doi: 10.1016/j.immuni.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 63.Xu F, Wang Z, Zhang H, Chen J, Wang X, Cui L, et al. Mevalonate blockade in cancer cells triggers CLEC9A+ dendritic cell-mediated antitumor immunity. Can Res. 2021;81(17):4514–4528. doi: 10.1158/0008-5472.CAN-20-3977. [DOI] [PubMed] [Google Scholar]
- 64.Ghittoni R, Napolitani G, Benati D, Uliveri C, Patrussi L, Laghi Pasini F, et al. Simvastatin inhibits the MHC class II pathway of antigen presentation by impairing Ras superfamily GTPases. Eur J Immunol. 2006;36(11):2885–2893. doi: 10.1002/eji.200636567. [DOI] [PubMed] [Google Scholar]
- 65.Troutman TD, Bazan JF, Pasare C. Toll-like receptors, signaling adapters and regulation of the pro-inflammatory response by PI3K. Cell Cycle. 2012;11(19):3559–3567. doi: 10.4161/cc.21572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Miao Y, Jiang M, Qi L, Yang D, Xiao W, Fang F. BCAP regulates dendritic cell maturation through the dual-regulation of NF-κB and PI3K/AKT signaling during infection. Front Immunol. 2020;11:250. doi: 10.3389/fimmu.2020.00250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Guo J, Muse E, Christians AJ, Swanson SJ, Davila E. An anticancer drug cocktail of three kinase inhibitors improved response to a dendritic cell-based cancer vaccine. Cancer Immunol Res. 2019;7(9):1523. doi: 10.1158/2326-6066.CIR-18-0684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Carter AB, Hunninghake GW. A constitutive active MEK –> ERK pathway negatively regulates NF-kappa B-dependent gene expression by modulating TATA-binding protein phosphorylation. J Biol Chem. 2000;275(36):27858–27864. doi: 10.1074/jbc.M003599200. [DOI] [PubMed] [Google Scholar]
- 69.Aguilera-Montilla N, Chamorro S, Nieto C, Sánchez-Cabo F, Dopazo A, Fernández-Salguero PM, et al. Aryl hydrocarbon receptor contributes to the MEK/ERK-dependent maintenance of the immature state of human dendritic cells. Blood. 2013;121(15):e108–e117. doi: 10.1182/blood-2012-07-445106. [DOI] [PubMed] [Google Scholar]
- 70.Vella LJ, Pasam A, Dimopoulos N, Andrews M, Knights A, Puaux A-L, et al. MEK inhibition, alone or in combination with BRAF inhibition, affects multiple functions of isolated normal human lymphocytes and dendritic cells. Cancer Immunol Res. 2014;2(4):351. doi: 10.1158/2326-6066.CIR-13-0181. [DOI] [PubMed] [Google Scholar]
- 71.Hoyer S, Eberlein V, Schuler G, Berking C, Heinzerling L, Schaft N, et al. BRAF and MEK inhibitors affect dendritic-cell maturation and T-Cell stimulation. Int J Mol Sci. 2021;22(21):11951. doi: 10.3390/ijms222111951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lu Y, Zhang M, Wang S, Hong B, Wang Z, Li H, et al. p38 MAPK-inhibited dendritic cells induce superior antitumour immune responses and overcome regulatory T-cell-mediated immunosuppression. Nat Commun. 2014;5:4229. doi: 10.1038/ncomms5229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhou Y, Wu J, Liu C, Guo X, Zhu X, Yao Y, et al. p38α has an important role in antigen cross-presentation by dendritic cells. Cell Mol Immunol. 2018;15(3):246–259. doi: 10.1038/cmi.2016.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang S, Hong S, Yang J, Qian J, Zhang X, Shpall E, et al. Optimizing immunotherapy in multiple myeloma: Restoring the function of patients' monocyte-derived dendritic cells by inhibiting p38 or activating MEK/ERK MAPK and neutralizing interleukin-6 in progenitor cells. Blood. 2006;108(13):4071–4077. doi: 10.1182/blood-2006-04-016980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sprooten J, Agostinis P, Garg AD. Chapter Five - Type I interferons and dendritic cells in cancer immunotherapy. In: Galluzzi L, editor. Lhuillier C. Academic Press: International review of cell and molecular biology; 2019. pp. 217–262. [DOI] [PubMed] [Google Scholar]
- 76.Bourdeau A, Dubé N, Tremblay ML. Cytoplasmic protein tyrosine phosphatases, regulation and function: the roles of PTP1B and TC-PTP. Curr Opin Cell Biol. 2005;17(2):203–209. doi: 10.1016/j.ceb.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 77.Penafuerte C, Feldhammer M, Mills JR, Vinette V, Pike KA, Hall A, et al. Downregulation of PTP1B and TC-PTP phosphatases potentiate dendritic cell-based immunotherapy through IL-12/IFNγ signaling. Oncoimmunology. 2017;6(6):e1321185. doi: 10.1080/2162402X.2017.1321185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shen L, Evel-Kabler K, Strube R, Chen S-Y. Silencing of SOCS1 enhances antigen presentation by dendritic cells and antigen-specific anti-tumor immunity. Nat Biotechnol. 2004;22(12):1546–1553. doi: 10.1038/nbt1035. [DOI] [PubMed] [Google Scholar]
- 79.Evel-Kabler K, Song X-T, Aldrich M, Huang XF, Chen S-Y. SOCS1 restricts dendritic cells' ability to break self tolerance and induce antitumor immunity by regulating IL-12 production and signaling. J Clin Invest. 2006;116(1):90–100. doi: 10.1172/JCI26169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ge C, Li R, Song H, Geng T, Yang J, Tan Q, et al. Phase I clinical trial of a novel autologous modified-DC vaccine in patients with resected NSCLC. BMC Cancer. 2017;17(1):884. doi: 10.1186/s12885-017-3859-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nefedova Y, Huang M, Kusmartsev S, Bhattacharya R, Cheng P, Salup R, et al. Hyperactivation of STAT3 Is involved in abnormal differentiation of dendritic cells in cancer. J Immunol. 2004;172(1):464–474. doi: 10.4049/jimmunol.172.1.464. [DOI] [PubMed] [Google Scholar]
- 82.Johnson DE, O'Keefe RA, Grandis JR. Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat Rev Clin Oncol. 2018;15(4):234–248. doi: 10.1038/nrclinonc.2018.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ott M, Kassab C, Marisetty A, Hashimoto Y, Wei J, Zamler D, et al. Radiation with STAT3 blockade triggers dendritic cell–T cell interactions in the glioma microenvironment and therapeutic efficacy. Clin Cancer Res. 2020;26(18):4983–4994. doi: 10.1158/1078-0432.CCR-19-4092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Assi H, Espinosa J, Suprise S, Sofroniew M, Doherty R, Zamler D, et al. Assessing the role of STAT3 in DC differentiation and autologous DC immunotherapy in mouse models of GBM. PLoS ONE. 2014;9(5):e96318. doi: 10.1371/journal.pone.0096318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sharma MD, Rodriguez PC, Koehn BH, Baban B, Cui Y, Guo G, et al. Activation of p53 in immature myeloid precursor cells controls differentiation into Ly6c(+)CD103(+) monocytic antigen-presenting cells in tumors. Immunity. 2018;48(1):91–106.e6. doi: 10.1016/j.immuni.2017.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Natarajan G, Oghumu S, Terrazas C, Varikuti S, Byrd JC, Satoskar AR. A Tec kinase BTK inhibitor ibrutinib promotes maturation and activation of dendritic cells. Oncoimmunology. 2016;5(6):e1151592. doi: 10.1080/2162402X.2016.1151592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sharma MD, Pacholczyk R, Shi H, Berrong ZJ, Zakharia Y, Greco A, et al. Inhibition of the BTK-IDO-mTOR axis promotes differentiation of monocyte-lineage dendritic cells and enhances anti-tumor T cell immunity. Immunity. 2021;54(10):2354–71.e8. doi: 10.1016/j.immuni.2021.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Durai V, Bagadia P, Granja JM, Satpathy AT, Kulkarni DH, Davidson JTt, et al. Cryptic activation of an Irf8 enhancer governs cDC1 fate specification. Nat Immunol. 2019;20(9):1161–1173. doi: 10.1038/s41590-019-0450-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Loschko J, Rieke GJ, Schreiber HA, Meredith MM, Yao KH, Guermonprez P, et al. Inducible targeting of cDCs and their subsets in vivo. J Immunol Methods. 2016;434:32–38. doi: 10.1016/j.jim.2016.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cancel JC, Crozat K, Dalod M, Mattiuz R. Are conventional type 1 dendritic cells critical for protective antitumor immunity and how? Front Immunol. 2019;10:9. doi: 10.3389/fimmu.2019.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pathak SK, Sköld AE, Mohanram V, Persson C, Johansson U, Spetz A-L. Activated apoptotic cells induce dendritic cell maturation via engagement of toll-like receptor 4 (TLR4), dendritic cell-specific intercellular adhesion molecule 3 (ICAM-3)-grabbing nonintegrin (DC-SIGN), and β2 Integrins. J Biol Chem. 2012;287(17):13731–13742. doi: 10.1074/jbc.M111.336545. [DOI] [PMC free article] [PubMed] [Google Scholar]