Abstract
Introduction
Endometriosis is a risk factor for low-grade serous, clear cell, and endometroid ovarian carcinoma. In both endometriosis and ovarian carcinoma, immunological factors are associated with clinical outcome. Chronic inflammation in endometriosis may be linked to tumorigenesis, but exact processes contributing to endometriosis-associated ovarian carcinoma remain unknown. This review aims to describe potential immunological factors involved in the malignant transformation of endometriosis into ovarian carcinoma.
Methods
PubMed and Embase were searched from inception up to October 2020 for studies comparing immunological processes in endometriosis and endometriosis-associated ovarian carcinoma.
Results
Detailed analysis of immune components in the malignant transformation of endometriosis into endometriosis-associated ovarian carcinoma is lacking. Altered levels of chemokines and cytokines as IL-6, IL-8, IL-10, and TNF-α are reported and the function, number and polarization of NK cells, dendritic cells, and monocytes differ between endometriosis and associated ovarian carcinoma compared to healthy tissue. In addition, altered inflammasome and complement systems, indicate a role for the immune system in the carcinogenesis of endometriosis.
Conclusion
Chronic inflammation in endometriosis may potentially drive inflammation-induced carcinogenesis in endometriosis-associated ovarian carcinoma. Exact immunological pathways and cellular processes remain unknown and require more thorough investigation.
Keywords: Endometriosis, Ovarian carcinoma, Microenvironment, Immunology, Cytokines
Introduction
Ovarian carcinoma (OvCa) is world’s most lethal gynecological malignancy, with an incidence of 6.6 per 100,000/year and a mortality rate of 3.8 per 100,000/year [1]. Histologically, 90% of OvCa is epithelial (EOC) including serous (70%), endometroid (EC, 10%), clear-cell (CCC, 10%), mucinous (3%), and non-specified carcinoma. The 10% non-epithelial carcinoma consists of germ-cell and sex cord tumors [2]. Risk factors vary between histological types but include, for EOC, increased age, low parity number, and incessant ovulation (non-mucinous), obesity, smoking (mucinous), low socioeconomic status, BRCA mutations (mainly serous), Lynch syndrome (mainly EC and CCC), and endometriosis (EC and CCC) [2, 3]. Five year survival in EOC varies from 17 to 90%, depending on histological subtype and tumor stage upon diagnosis [1]. Due to atypical presentation and lack of accurate screening tools, OvCa is often found at a late stage with high-tumor burden and poor survival rate.
Endometriosis is associated with certain histological OvCa subtypes, known as endometriosis-associated ovarian carcinoma (EAOC). Approximately 1% of endometriosis patients develop EAOC, with associated carcinomas being CC (OR of 3.73), EC (OR of 2.32), and low-grade serous (LGSC, with an OR of 2.02) [4, 5].
Endometriosis itself is estrogen-dependent and characterized by deposition of endometrial glandular tissue, epithelium, and stroma outside of the uterine cavity. Inflammation in lesions results in pelvic pain, dysmenorrhea, dyspareunia, and even infertility. The most widely accepted pathophysiological explanation is Samsons ‘retrograde menstruation theory’ describing ‘backflow’ of blood and endometrium through the oviducts into the peritoneal cavity during menstruation [6]. However, this theory fails to explain why endometriosis affects only 10% of women of reproductive age [7]. Genetics and local environmental factors such as steroid signaling, redox pathways, angiogenesis, and inflammatory markers can induce an endometriosis-friendly environment [7]. Endometriotic tissue depositions increase breakdown products such as hemoglobin, heme, and iron species, causing severe oxidative stress, depletion of antioxidant response, and disruption of the redox balance. This supports chronic inflammation, uncontrolled proliferation, and potentially malignant transformation [8]. Furthermore, immune dysfunction has been theorized to facilitate ectopic lesion development and growth [7, 9–11].
About 10–15% of women with OvCa have previously been diagnosed with endometriosis, further substantiating the relation between the two diseases [12]. Histologically, EAOC is defined by (1) endometriotic lesions near the tumor site, (2) a malignancy not metastasized from elsewhere, (3) tissue present resembling endometrial stroma, and (4) a visible microscopic transition from endometriosis to carcinoma [13, 14]. Risk factors for EOAC, in patients with endometriosis, are age over 45, nulliparity, postmenopausal state, endometriomas larger than 9 cm, hyper-estrogenism, and cysts with solid compartments [15].
Development of EAOC from endometriosis may be a gradual process in which atypical endometriosis (AE) develops from endometriosis and advances into EAOC [16]. Histologically, AE is defined by presence of large hyperchromatic or pale nuclei with pleomorphism, increased nuclear-to-cytoplasmic ratio and cellular crowding and stratification [17]. Incidence rates are poorly documented, but the occurrence of AE is higher in patients with EAOC than controls with endometriosis, suggesting involvement in malignant transformation [16]. It is hypothesized that chronic inflammation in endometriosis generates mutational changes and acts in tumor initiation, generating a shift from endometriosis to AE to EAOC. Inflammatory processes are recognized in the hall marks of cancer as key players in carcinogenesis through direct toxicity, aberrant DNA repair, and stimulation of proliferation. They act on tumor initiation (carcinogens causing DNA mutations generating oncogene activation and tumor-suppressor gene inactivation), promotion (proliferation and reduced cell death) and progression (invasion and metastasis) [18]. Previous studies showed increased risk of malignant transformation due to somatic mutations in tumor-suppressor genes (ARID1A and PTEN), oncogenes (K-RAS and PIK3A), and β-catenin/Wnt signaling pathway; increased levels of VEGF and Ki-67; specific miRNA’s; hormone receptor changes; and the effect of iron metabolism and excessive stress from reactive oxygen species (ROS) [5, 8]. Chronic inflammation in endometriosis may drive carcinogenesis and inversely, tumors modulate the immune microenvironment directly by suppressing anti-tumor response mechanisms and secreting soluble factors and chemoattractants that shifts immune cell balance [19, 20].
Multifactorial processes potentially explain malignant progression with immune dysregulation being an important oncogenic driver [4, 5]. This review aims to elucidate the involvement of immune system in the malignant transformation of endometriosis into EAOC.
Methods
PubMed and Embase were searched from inception up to October 2020 for studies on immune processes in patients with both endometriosis and OvCa, resulting in 200 and 295 abstracts, respectively. A total of 90 double references were excluded. Studies were defined useful based on title and abstract by two authors (S. Leenen and M. Hermens) if they focused on OvCa originating from endometriosis and immune components cells or processes. This resulted in 57 abstracts, of which 36 full text articles. All 57 abstract and 36 full texts were read and scored based on their relevance. Studies with only abstracts available were either conference abstracts (19), preliminary results of a previously included paper (1) or not in English (1) and were excluded based on restricted information available. Of the 36 full texts found, another 18 articles were excluded for not comparing endometriosis and OvCa (9), not focusing on immune factors (5), focusing on immune components as biomarkers (3) or focusing on the effect of the tumor on the immune microenvironment instead of the other way around (1). The remaining 18 articles were used in this review.
Immune microenvironment in normal endometrium and endometriosis
Immune cell fluctuations in endometrium during the menstrual cycle
Endometrial immune cell fluctuations are hypothesized to result from either altered tissue-resident immune cell proliferation or adjusted differentiation of peripherally migrating immune cells and precursors [10].
The endometrium is composed of a basal and functionalis layer, with the latter being excreted during menstruation and regenerated during proliferation. Immune cells lie scattered in the stroma and between the epithelial cells of the functionalis layer, with their types and numbers depending highly on hormonal changes. Lymphoid aggregates are located in the basal layer, where they develop during the proliferative phase. They consist of B cells, cytotoxic CD8 + T cells and are surrounded by myeloid cells [10]. During the proliferative phase, the majority of leukocytes are T cells (40–60%), followed by NK cells (30–40%) and macrophages (10%). During the late secretory phase, the T cell population declines to less than 10% and the majority consists of NK cells (70–80%) and macrophages (30%) [10].
Immune cell infiltrates in endometriosis compared to healthy control tissue
Innate immunity
Natural killer (NK) cells—in healthy endometrium prevent infection and enhance vascular remodeling [10]. In both healthy individuals and patients with endometriosis, their numbers increase in the secretory phase. However, in endometriosis, cytolytic activity decreases in the local peritoneal environment and, to a lesser extent, in the peripheral blood [7, 9, 10]. In advanced endometriosis, impaired cytolytic activity could be explained by the upregulation of inhibitory receptors NKB1 and EB6 and high concentrations of IL-6, IL-10, IL-15, and TGF-β driving anti-inflammatory processes, in the peritoneal fluid [7, 9]. In addition, elevated expression levels of MHC class I polypeptide-relates sequence A (MICA) and B (MICB) inhibit NK cell function by binding NKG2D and c-type lectin-like NK cell receptor [7, 9]. Increased levels of soluble NKG2D ligands in peritoneal fluid in endometriosis compared to controls implicate proteolytic shedding in ectopic lesions resulting in evasion from NK cell recognition [21].
Neutrophils—infiltrate healthy endometrium more abundantly during the secretory and menstrual phase compared to the proliferative phase. Endometrial neutrophils express high levels of VEGF and produce IFN-γ, essential for cell proliferation and pro-inflammatory immune responses [10]. In endometriosis, neutrophils are increased in the endometrium and peritoneal cavity, where they are proposed to act in early lesion formation and disease progression by stimulating proliferation and creating an inflammatory milieu [7, 10]. Decreased lesion growth in a neutrophil-depleted endometriotic mouse model substantiates this claim [10].
Dendritic cells (DCs)—increase in numbers in healthy endometrium during the secretory and menstruation phase with high levels of immature DCs (CD1a + , CD209 + , HLA-DRLow) compared to mature DCs (CD83 + , HLA-DRHigh) [10]. In endometriosis, cyclic fluctuations are lost and high levels of immature and low levels of mature DCs are documented. In ectopic lesions, immature DCs may promote neuroangiogenesis, contributing to lesion growth and increased pain sensitization [10]. Moreover, lack of maturation may result in impaired immune activation and inefficient clearing of endometrial cells during menstruation, therefore enabling the establishment of endometriotic lesions [10].
Macrophages—invade healthy endometrium more abundantly during the secretory and menstrual phase. They promote shedding of the endometrium during menstruation by initiating apoptosis and acting as scavengers of cell debris. Furthermore, they may act in endometrium rebuilding through induction of angiogenic regeneration [10]. The majority of macrophages (CD68 + , CD14High/Low) in the endometrium of healthy controls is M2 with an anti-inflammatory profile (CD68 + , CD163 + , CD14Low), while M1, pro-inflammatory macrophages (CD68 + , CD14High), dominate in endometriosis [9, 10]. However, in ectopic endometriotic lesions, M2s dominate, possibly facilitating lesion growth [9, 10]. In endometriosis, an increase in macrophages in the secretory phase is not observed, conceivably resulting in incomplete endometrial excretion [7, 9]. In addition to enhanced activation and numbers, stimulatory factors nuclear factor κB (NF-κB), TNFα, IL-Iβ and IL-6 are significantly increased, in both the peritoneal fluid and endometriotic ectopic lesions in endometriosis patients, generating a pro-inflammatory environment [7, 9, 10]. Despite high-activation state, phagocytic ability is reduced, through downregulation of the class B scavenger receptor CD36 via the prostaglandin E2-dependent signaling pathway. A therapeutic effect by prostaglandin antagonists such as non-steroidal anti-inflammatory drugs (NSAIDs) is suggested [7, 9]. In summary, M1 polarization in the endometrium may induce early lesion formation and inadequate shedding, while subsequent switch to an M2-rich environment generates lesion growth and fibrosis [10].
Adaptive immunity
T cells—are more abundant in healthy endometrium in the proliferative compared to the secretory phase. They are mainly located in the basal layer at a 2:1 ratio of CD8 + T cell and CD4 + T cells [10]. In ectopic endometriotic lesions, CD8 + T cell numbers are increased with loss of fluctuation and an increased CD4 + /CD8 + ratio is observed, with increased CD4 + cells mainly represented by anti-inflammatory subtypes (e.g., Treg) [9, 10]. Both Th1 (pro-inflammatory) and Th2 (anti-inflammatory) cytokines are increased in the peritoneal fluid and peripheral blood of endometriosis patients with a slight skew toward Th2 cytokines (e.g., IL-4 and IL-10) [7, 10]. However, an increase of Th2 cells over Th1 cells has not been documented, as exact cell numbers have not been measured [10]. CD3 + CD4 + IL-17 -producing cells are increased in endometriosis in ectopic lesions, peritoneal fluid and peripheral blood, with numbers positively correlating with disease stage. In healthy endometrium, their function and possible cyclic fluctuation are still unknown. However, they may act in chronic inflammation through IL-17 production, triggering IL-8 release to stimulate inflammation and angiogenesis [7, 10]. Regulatory CD4 + T cells (Tregs, expressing FoxP3) are generally increased during proliferation and decreased at the end of the secretory phase. Many studies report enhanced Tregs in endometriosis patients in endometrium, ectopic lesions, and peritoneal fluid, possibly resulting in local immune suppression and induced lesion development and growth [7, 10].
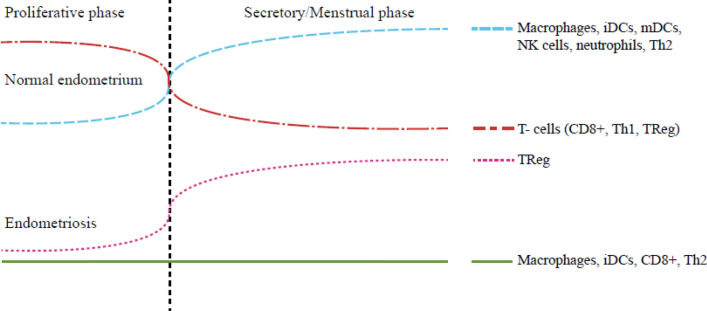
B cells—have both been documented to be increased and decreased in endometriosis, shedding no light on B cell function in endometriosis development or progression. However, increase of B lymphocyte stimulator (BLyS) in ectopic endometriosis, suggests an induced state [9, 10]. Increased B cells can alter CD4 + maturation through cytokine release and ligand-receptor interactions and produce auto-antibodies against components of the endometrium [7, 10, 22]. Overall, B cell influence is unclear and may depend on disease stage (Fig. 1).
Fig. 1.
Fluctuations in immune cells in normal endometrium and endometriosis during the menstrual cycle. iDCm immature dendritic cells, mDCs mature dendritic cells, NK cells natural killer cells, Th2 T-helper cells type 2, CD8+ cytotoxic T-cell, Th1 T-helper cell type 1, TReg regulatory T cell
Immune responses in EOAC compared to endometriosis
In ectopic endometriosis, the immune microenvironment contains increased levels of immature DCs, M2 macrophages, and Tregs, enhanced CD4 + /CD8 + T cell ratio, with mainly anti-inflammatory CD4 + subtypes, and impaired NK cell function. The chronic inflammation with an exhausted phenotype in endometriosis is associated with evasion of immune surveillance and with tumor development through reduced apoptosis [7]. Altered immune responses, induction of angiogenesis, and genetic changes may act in the transformation of endometriosis to EAOC. The subsequent paragraphs combine current scarce knowledge about the role of the immune system in this transformation.
Systemic immune responses in peripheral circulation
To this date, no systemic cellular responses of innate and adaptive immunity in endometriosis and EAOC are documented. Identified studies only report on cytokine levels and HLA-G expression.
Cytokines and HLA-G
Levels of IL-6 are increased in endometriosis and EAOC [23, 24] and IL-10 was found to be gradually increased from benign cysts to endometriosis to OvCa (subtypes not specified). IL-10 in serum correlates with peritoneal fluid levels in OvCa [25]. TNF-α levels were increased in cyst fluid and sera of endometriosis patients and OvCa (subtypes not specified) [23]. IL-10 is one of the cytokines which enhances HLA-G transcription [12]. HLA-G is expressed in healthy endometrium during the menstrual phase and is increased in endometriosis [12]. Soluble HLA-G (sHLA-G) levels in sera of women with endometriosis (ovarian endometrioma and deep endometriosis) and OvCa (subtypes not specified) were measured and levels were high in patients with deep endometriosis and OvCa compared to endometrioma. Additionally, in deep endometriosis, levels are increased in the secretory phase, while levels fluctuated minimally in patients with endometrioma. Investigators speculated that rise in sHLA-G can partially be explained by increased IL-10 levels [12]. They proposed that sHLA-G contributes to a suppressive immune microenvironment through induction of apoptosis and functional inhibition of NK and cytotoxic T cells [12]. However, others reported no differences in sHLA-G concentrations between OvCa and endometriosis [25].
Local immune responses in the microenvironment
Molecular and genetic pathways
A function-based data-driven analysis comparing gene sets from endometriosis with EAOC (EC and CCC) to normal ovarian control samples showed immune-related deregulations in ‘MAPK3 activity’ and ‘immune response activation’ in both endometriosis and EAOC [26]. In a subsequent analysis, altered inflammasome pathway functioning was found in endometriosis and EAOC compared to healthy ovarian tissue. Expression levels of genes involved in the inflammasome (core proteins PYCARD and NAIP) and inflammasome-related pathway (initiators NLRP3 and AIM2) were altered in CCC and EC compared to endometriosis and in all groups compared to controls [27].
Microsatellite instability (MSI) generates frameshift-mutated antigens by exposing C-terminal polypeptides of DNA, triggering T-cell response and antibody production. MSI was increased in 14.6% of EC and 23.3% of co-existing endometriosis. MSI-high endometriosis is associated with high-endometriosis-inflammatory score, based on hemorrhagic area, accumulation of macrophages, degree of erosive change and level of inflammatory cell infiltration. Inflammatory scores are diminished from endometriosis to endometriosis with EC and endometriosis with CCC [28].
Inflammatory mediators
Complement pathway—An immune transcriptome profiling of 511 immune gene probes of the tissue microenvironment was performed by NanoString examination. Endometriosis showed a mixed immune gene profile with 37% of cases clustering with healthy controls whereas the rest displayed a cancer-related profile. In addition, in an AE cohort, they found ‘cancer like’ immune environments in 85% of cases, supporting the hypothesis of AE as a precursor of EAOC [4]. Furthermore, they reported significant upregulation of several genes involved in the complement pathway, a finding confirmed by immunohistochemistry of complement factors (C7, CFD, CFB, CFH, and MASP1) in endometriosis and EAOC [4]. Knockdown and blockage of complement factor C7 in vitro inhibited ovarian cell proliferation [4]. C7 functions as part of the membrane attack complex that perforates cell membranes and resulting in cell lysis. Paradoxically, suboptimal formation of the complex induces proliferation, differentiation, and inhibits apoptosis by acting as a downstream activation receptor [29].
Chemokines and Cytokines—CXCR3 receptor and CXC chemokines contribute to anti-tumor responses by promoting Th1-dependent anti-tumoral T cell responses through recruitment of CXCR3-expressing T and NK cells, and via inhibition of angiogenesis [30]. CXCR3 expression was upregulated in OvCa and endometriosis. The proportion of CXCR3-positive lymphocytes gradually decreased from benign, to endometriosis, AE and to EAOC (CCC), whereas the CXCR3-positive tumor cells increased [30]. CXCR3 lymphocytes are associated with pro-inflammatory cytokine responses. This study postulated that IFN-γ inducible chemokines might not exert an effective anti-tumor immune responses in EAOC, as CXCR3-positive tumor cells are associated with reduced CXCR3-positive lymphocytes [30]. Expression of CXCR3A was high on tumor cells and infiltrating lymphocytes in CCC, while CXCR3B and corresponding ligand CXCL4, inducing apoptosis and inhibiting cellular proliferation, were downregulated in CCC compared to endometriosis [31]. Chemokine CXCL4 interacts directly with VEGF and fibroblast growth factor, exerting inhibitory effects on angiogenesis. Expression of CXCL4 by CD68 + macrophages was found in endometriosis, but gradually decreased in macrophages from transition zone to EAOC to non-EAOC [32]. Impaired expression of the CXCL4-CXCR3B axis may act in malignant transformation by inhibition of effective anti-tumor responses [32]. Increased levels of VEGF as well as IL-8 are described in cyst fluid of OvCa (adenocarcinoma and EC) and endometrioma compared to benign cysts [33].
Innate and adaptive immunity in the microenvironment
Macrophages are increased in endometriosis and EAOC compared to benign lesions and unspecified OvCa through increased recruitment of monocytes by upregulation of the CCL14/SICA2 axis [34]. Macrophages are polarized toward M2 (CD163 + and CD68 +) in ectopic endometriosis and even more in EAOC [35, 36]. A gradual decrease in expression of CDC42, involved in cellular transformation and phagocytosis, by CD163 + macrophages, was reported from endometriosis not associated with OvCa (58%), to endometriosis with EAOC (28%) to EAOC alone (5%), suggesting a role for CDC42 expressing macrophages in the transition of endometriosis to EAOC [35]. In addition, anti-oxidant marker heme-oxygenase (HO)-1, expressed by M2 cells, was downregulated in EAOC, possibly due to decreased oxidative stress. Reduced HO-1 may favor malignant progression from ovarian endometriosis to EAOC [36]. Regarding adaptive immunity, studies on the microenvironment of EAOC are lacking. In endometriosis, a Th2 cytokine (IL-4 and IL-10) increase in peritoneal fluid illustrates an anti-inflammatory response that may favor tumor growth [7, 10] (Table 1).
Table 1.
Reported changes in immunological factors compared to healthy controls
| Endometriosis | EAOC | |||||
|---|---|---|---|---|---|---|
| Cellular | B-cell | ? | ? | |||
| Pro-inflammatory | CD4 + Th1 | ↑ | ? | |||
| CD8 + | ↑ | ? | ||||
| Th17 | ↑ | ? | ||||
| M1 | ↑ | ? | ||||
| iDC | ↑ | ? | ||||
| mDC | ↓ | loss of function | ||||
| Neutrophil | ↑ (loss of function) | ? | ||||
| NK-Cell | ~ (loss of function) | ? | ||||
| Anti-inflammatory | CD4 + Th2 | ↑ | ? | |||
| TReg | ↑ | ? | ||||
| M2 | ↓ (eutopic) ↑ (ectopic) | ↑ (M2/M1) | ||||
| Cytokines | Local | Serum | Local | Serum | ||
| IL-6 | ↑ | ↑ | ? | ↑ | ||
| IL-8 | ↑ | ~ | ↑ | ~ | ||
| IL-10 | ↑ | ↑ | ↑? | ↑? | ||
| TNF-α | ↑ | ↑ | ? | ↑? | ||
? unknown, ↑ increased, ↓ decreased, ~ similar as control
Future potentials/research
Immunological reports in endometriosis highlight chronic inflammation and changes in immune microenvironment in endometriotic lesions. As inflammation can initiate tumor formation and support subsequent promotion and progression, chronic inflammation in endometriosis may contribute to the development of EAOC [18]. To this date, the exact mechanisms generating EAOC are unknown, and complex interactions between the endometriotic cells, cancer cells and the innate and adaptive immunity are proposed.
Preventive strategies may be deducted from other tumors. For example, in gastrointestinal, lung, ovarian cancer and Hodgkin’s lymphoma, reduced cancer rates are described following use of NSAIDs, inhibiting NF-κB activation. As most commonly used painkillers in endometriosis, a preventive role is of interest and needs to be investigated [18]. Furthermore, in many tumors, oncogenic activation of the NF-κB -IL-6-STAT3 signaling cascade results in tumor initiation and progression. This pathway needs to be determined in EAOC, as levels of NF-κB and IL-6 were found to be increased [9, 10, 19]. Oral contraceptives (OAC) are one of the pillars of endometriosis treatment, creating decidualization and subsequent atrophy of the endometrium. OAC also has an effect on the immunity, with an increased systemic CD3 + CD8 + and decreased NK cell response in women using contraceptives compared to controls [37]. The effects of the OAC on the immune system in endometriosis and the prevention of EAOC are areas that need further investigation. OAC decreases the risk of endometrium carcinoma and OvCa and improves survival. Oral Contraceptives and Cancer Risk was originally published by the National Cancer Institute [38].
A crucial event in tumor initiation is genomic instability caused by DNA damage that affects DNA repair mechanisms. In EAOC somatic mutations in tumor-suppressor genes (ARID1A and PTEN), oncogenes (K-RAS and PIK3a) and b-catenin/Wnt signaling are described [5, 8]. Of special interest is the role of ROS and MSI in EAOC, with MSI significantly increased in endometriosis and EAOCs compared to healthy controls [28]. ROS and reactive nitrogen species (RNS), produced by activated neutrophils and macrophages, increase genomic DNA mutations. ROS additionally reduces enzymatic activity of mismatch repair proteins (MMR) that prevent genomic instability, resulting in increased DNA replication errors and MSI, promoting tumor development [19]. Additionally, TNF, IL1β, and prostaglandin E2 induce downregulation of MMR genes MSH2 and MSH6 and inflammation-induced DNA hypermethylation causes epigenetic silencing of MMR protein MLH1 and tumor-suppressor genes such as TP53 [18, 19]. In colorectal carcinoma, MSI-high tumors are associated with good responsiveness to PD-L1 immunotherapy [39]. In endometrial carcinoma, enhanced immune cell infiltration in MSI-high subtypes was observed, resulting in a rationale for immunotherapy [40]. It is of interest for future immunotherapeutic strategies to determine if MSI-high tumors form a distinct group of EAOC.
In tumor promotion and progression, tumor-associated macrophages (TAMs) and cytokine-expression profiles are essential [18]. In endometriosis, innate immune reactions exhibit pro-tumorigenic effects by decreasing NK cells in number and function, increasing neutrophils and immature DCs and promoting M2 accumulation. Additionally, in endometriosis and OvCa, a cytokine profile comparable to TAMs is observed, with increased lesion stimulating cytokines IL-6, IL-10, TNF-α, and VEGF and decreased CXCR3 lymphocytes and chemokines such as CXCL4 [9, 32]. VEGF induces angiogenesis and suppresses DC function and maturation in endometriosis and OvCa [19, 33]. Reduced DC maturation in endometriosis and loss of function in EAOC suggest aberrant activation of adaptive immunity activity in both diseases. The adaptive immunity potentially has anti-tumorigenic effects when activation is characterized by an anti-tumor pro-inflammatory Th1 (IFN-γ associated) response. However in endometriosis, the chronic inflammation is characterized by increased CD4 + /CD8 + ratio with induced Treg influx and elevated Th2 cytokines, indicating a pro-tumorigenic immune response that supports tumor progression and invasion.
Further knowledge of the role of the innate and adaptive immune reactions in the transition of endometriosis into EOAC may provide us with directives toward preventive strategies and immunotherapeutic options for different stages of the disease. Immunotherapeutic strategies directed at improvement of NK cell function, DC maturation, and M1 polarization and induction of effector T cells are of high interest in this specific subset of ovarium cancers. Combination of these therapies with chemotherapy or either in combination with the widely used bevacizumab (anti-VEGF) is of special interest to induce an effective anti-tumor immune response [41, 42]. Detailed analysis of the chronic inflammation in endometriosis and immune modulation by the tumor is crucial to provide more insight in malignant transformation from endometriosis into EAOC. Based on the findings in this review, we are in design of a systematic study comparing immune cell changes in various stages of endometriosis, AE and EAOC to generate a better understanding of the complex processes that underlie the carcinogenesis.
Abbreviations
- AE
Atypical endometriosis
- CCC
Clear cell carcinoma
- EAOC
Endometriosis-associated ovarian carcinoma.
- EC
Endometrioid carcinoma
- EOC
Epithelial ovarian carcinoma
- LGSC
Low-grade serous carcinoma
- OvCa
Ovarian carcinoma
Appendix
Search specifics and exclusion protocol.
Author contributions
RB and EE were responsible for the study initiative and study design. SL and MH conducted the literature search and performed the selection of manuscripts. All authors contributed to the writing of the manuscript.
Funding
This study did not receive any funding.
Data availability
All articles used are published full text articles.
Compliance with ethical standards
Conflict of interest
All authors declare that they had no conflict of interest.
Ethical approval
This article does not contain any studies with human participants performed by any of the authors.
Footnotes
To our knowledge, this is the first review carried out that focusses solely on immunological factors involved in malignant transformation from endometriosis to associated ovarian carcinoma.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Reid F, Bhatla N, Jones A (2018) The world ovarian cancer coalition atlas (trans: Study TEW). World Ovarian Cancer Coalition, United Kingdom
- 2.Momenimovahed Z, Tiznobaik A, Taheri S, Salehiniya H. Ovarian cancer in the world: epidemiology and risk factors. Int J Womens Health. 2019;11:287–299. doi: 10.2147/ijwh.s197604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matulonis UA, Sood AK, Fallowfield L, Howitt BE, Sehouli J, Karlan BY. Ovarian cancer. Nat Rev Dis Primers. 2016;2:16061. doi: 10.1038/nrdp.2016.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Suryawanshi S, Huang X, Elishaev E, Budiu RA, Zhang L, Kim S, Donnellan N, Mantia-Smaldone G, Ma T, Tseng G, Lee T, Mansuria S, Edwards RP, Vlad AM. Complement pathway is frequently altered in endometriosis and endometriosis-associated ovarian cancer. Clin Cancer Res. 2014;20(23):6163–6174. doi: 10.1158/1078-0432.Ccr-14-1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wendel JRH, Wang X, Hawkins SM. The Endometriotic tumor microenvironment in ovarian cancer. Cancers (Basel) 2018;10(8):261. doi: 10.3390/cancers10080261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sampson JA, Albany NY. Peritoneal endometriosis due to the menstrual dissemination of endometrial tissue into the peritoneal cavity. Am J Obstet Gynecol. 1927;14(4):422–469. doi: 10.1016/s0002-9378(15)30003-x. [DOI] [Google Scholar]
- 7.Symons LK, Miller JE, Kay VR, Marks RM, Liblik K, Koti M, Tayade C. The immunopathophysiology of endometriosis. Trends Mol Med. 2018;24(9):748–762. doi: 10.1016/j.molmed.2018.07.004. [DOI] [PubMed] [Google Scholar]
- 8.Iwabuchi T, Yoshimoto C, Shigetomi H, Kobayashi H. Oxidative stress and antioxidant defense in endometriosis and its malignant transformation. Oxid Med Cell Longev. 2015;2015:1–7. doi: 10.1155/2015/848595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Riccio LDGC, Santulli P, Marcellin L, Abrão MS, Batteux F, Chapron C. Immunology of endometriosis. Best Pract Res Clin Obstet Gynaecol. 2018;50:39–49. doi: 10.1016/j.bpobgyn.2018.01.010. [DOI] [PubMed] [Google Scholar]
- 10.Vallve-Juanico J, Houshdaran S, Giudice LC. The endometrial immune environment of women with endometriosis. Hum Reprod Update. 2019;25(5):564–591. doi: 10.1093/humupd/dmz018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Drakes ML, Stiff PJ. Regulation of ovarian cancer prognosis by immune cells in the tumor microenvironment. Cancers (Basel) 2018;10(9):302. doi: 10.3390/cancers10090302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mach P, Blecharz P, Basta P, Marianowski P, Skret-Magierlo J, Kojs Z, Grabiec M, Wicherek L. Differences in the soluble HLA-G blood serum concentration levels in patients with ovarian cancer and ovarian and deep endometriosis. Am J Reprod Immunol. 2010;63(5):387–395. doi: 10.1111/j.1600-0897.2009.00806.x. [DOI] [PubMed] [Google Scholar]
- 13.Sampson JA. Endometrial carcinoma of de ovary, arising in Endometrial tissue in that organ. Arch Surg. 1925 doi: 10.1001/archsurg.1925.01120100007001. [DOI] [Google Scholar]
- 14.Scott RB. Malignant changes in endometriosis. Obstet Gynecol. 1953;2(3):283–289. [PubMed] [Google Scholar]
- 15.Thomsen LH, Schnack TH, Buchardi K, Hummelshoj L, Missmer SA, Forman A, Blaakaer J. Risk factors of epithelial ovarian carcinomas among women with endometriosis: a systematic review. Acta Obstet Gynecol Scand. 2017;96(6):761–778. doi: 10.1111/aogs.13010. [DOI] [PubMed] [Google Scholar]
- 16.Fukunaga M, Nomura K, Ishikawa E, Ushigome S. Ovarian atypical endometriosis: its close association with malignant epithelial tumours. Histopathology. 1997;30(3):249–255. doi: 10.1046/j.1365-2559.1997.d01-592.x. [DOI] [PubMed] [Google Scholar]
- 17.Thomas EJ, Campbell IG. Molecular genetic defects in endometriosis. Gynecol Obstet Invest. 2000;50(Suppl. 1):44–50. doi: 10.1159/000052878. [DOI] [PubMed] [Google Scholar]
- 18.Karin M, Greten FR. NF-κB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol. 2005;5(10):749–759. doi: 10.1038/nri1703. [DOI] [PubMed] [Google Scholar]
- 19.Elinav E, Nowarski R, Thaiss CA, Hu B, Jin C, Flavell RA. Inflammation-induced cancer: crosstalk between tumours, immune cells and microorganisms. Nat Rev Cancer. 2013;13(11):759–771. doi: 10.1038/nrc3611. [DOI] [PubMed] [Google Scholar]
- 20.Hanahan D, Robert A. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 21.González-Foruria I, Santulli P, Chouzenoux S, Carmona F, Batteux F, Chapron C. Soluble ligands for the NKG2D receptor are released during endometriosis and correlate with disease severity. PLoS ONE. 2015;10(3):e0119961. doi: 10.1371/journal.pone.0119961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shen P, Fillatreau S. Antibody-independent functions of B cells: a focus on cytokines. Nat Rev Immunol. 2015;15(7):441–451. doi: 10.1038/nri3857. [DOI] [PubMed] [Google Scholar]
- 23.Darai E, Detchev R, Hugol D, Quang NT. Serum and cyst fluid levels of interleukin (IL) -6, IL-8 and tumour necrosis factor-alpha in women with endometriomas and benign and malignant cystic ovarian tumours. Hum Reprod. 2003;18(8):1681–1685. doi: 10.1093/humrep/deg321. [DOI] [PubMed] [Google Scholar]
- 24.Schroder W, Ruppert C, Bender HG. Concomitant measurements of interleukin-6 (IL-6) in serum and peritoneal fluid of patients with benign and malignant ovarian tumors. Eur J Obstet Gynecol Reprod Biol. 1994;56(1):43–46. doi: 10.1016/0028-2243(94)90152-x. [DOI] [PubMed] [Google Scholar]
- 25.Sipak-Szmigiel O, Wlodarski P, Ronin-Walknowska E, Niedzielski A, Karakiewicz B, Sluczanowska-Glabowska S, Laszczynska M, Malinowski W. Serum and peritoneal fluid concentrations of soluble human leukocyte antigen, tumor necrosis factor alpha and interleukin 10 in patients with selected ovarian pathologies. J Ovarian Res. 2017;10(1):25. doi: 10.1186/s13048-017-0320-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chang CM, Yang YP, Chuang JH, Chuang CM, Lin TW, Wang PH, Yu MH, Chang CC. Discovering the deregulated molecular functions involved in malignant transformation of endometriosis to endometriosis-associated ovarian carcinoma using a data-driven, function-based analysis. Int J Mol Sci. 2017;18(11):2345. doi: 10.3390/ijms18112345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chang CM, Wang ML, Lu KH, Yang YP, Juang CM, Wang PH, Hsu RJ, Yu MH, Chang CC. Integrating the dysregulated inflammasome-based molecular functionome in the malignant transformation of endometriosis associated ovarian carcinoma. Oncotarget. 2018;9(3):3704–3726. doi: 10.18632/oncotarget.23364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tomohiro M, Matsumoto T, Miura R, Oguri Y, Yokoi A, Tochimoto M, Saegusa M. Alterations in beta-catenin, microsatellite instability, and HNF-1beta levels are independently associated with ovarian endometriosis-associated tumorigenesis. Hum Pathol. 2019;89:10–23. doi: 10.1016/j.humpath.2019.04.001. [DOI] [PubMed] [Google Scholar]
- 29.Afshar-Kharghan V. The role of the complement system in cancer. J Clin Invest. 2017;127(3):780–789. doi: 10.1172/jci90962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Furuya M, Suyama T, Usui H, Kasuya Y, Nishiyama M, Tanaka N, Ishiwata I, Nagai Y, Shozu M, Kimura S. Up-regulation of CXC chemokines and their receptors: implications for proinflammatory microenvironments of ovarian carcinomas and endometriosis. Hum Pathol. 2007;38(11):1676–1687. doi: 10.1016/j.humpath.2007.03.023. [DOI] [PubMed] [Google Scholar]
- 31.Furuya M, Yoneyama T, Miyagi E, Tanaka R, Nagahama K, Miyagi Y, Nagashima Y, Hirahara F, Inayama Y, Aoki I. Differential expression patterns of CXCR3 variants and corresponding CXC chemokines in clear cell ovarian cancers and endometriosis. Gynecol Oncol. 2011;122(3):648–655. doi: 10.1016/j.ygyno.2011.05.034. [DOI] [PubMed] [Google Scholar]
- 32.Furuya M, Tanaka R, Miyagi E, Kami D, Nagahama K, Miyagi Y, Nagashima Y, Hirahara F, Inayama Y, Aoki I. Impaired CXCL4 expression in tumor-associated macrophages (TAMs) of ovarian cancers arising in endometriosis. Cancer Biol Ther. 2012;13(8):671–680. doi: 10.4161/cbt.20084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fasciani A, D'Ambrogio G, Bocci G, Luisi S, Artini PG, Genazzani AR. Vascular endothelial growth factor and interleukin-8 in ovarian cystic pathology. Fertil Steril. 2001;75(6):1218–1221. doi: 10.1016/s0015-0282(01)01804-0. [DOI] [PubMed] [Google Scholar]
- 34.Banz C, Ungethuem U, Kuban RJ, Diedrich K, Lengyel E, Hornung D. The molecular signature of endometriosis-associated endometrioid ovarian cancer differs significantly from endometriosis-independent endometrioid ovarian cancer. Fertil Steril. 2010;94(4):1212–1217. doi: 10.1016/j.fertnstert.2009.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Canet B, Pons C, Espinosa I, Prat J. CDC42-positive macrophages may prevent malignant transformation of ovarian endometriosis. Hum Pathol. 2012;43(5):720–725. doi: 10.1016/j.humpath.2011.06.020. [DOI] [PubMed] [Google Scholar]
- 36.Yamada Y, Ogawa K, Kawahara N, Yoshimoto C, Kawaguchi R, Sado T, Kobayashi H. Clinical significance of m2 macrophages expressing heme oxygenase-1 in malignant transformation of ovarian en-dometrioma. J Obstet Gynaecol Res. 2019;45(8):1734. doi: 10.1111//jog.14030. [DOI] [Google Scholar]
- 37.Auerbach L, Hafner T, Huber JC, Panzer S. Influence of low-dose oral contraception on peripheral blood lymphocyte subsets at particular phases of the hormonal cycle. Fertil Steril. 2002;78(1):83–89. doi: 10.1016/s0015-0282(02)03173-4. [DOI] [PubMed] [Google Scholar]
- 38.National Cancer Institute (2018) Oral contraceptives and cancer risk. https://www.cancer.gov/about-cancer/causes-prevention/risk/hormones/oral-contraceptives-fact-sheet
- 39.Ganesh K, Stadler ZK, Cercek A, Mendelsohn RB, Shia J, Segal NH, Diaz LA. Immunotherapy in colorectal cancer: rationale, challenges and potential. Nat Rev Gastroenterol Hepatol. 2019;16(6):361–375. doi: 10.1038/s41575-019-0126-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gargiulo P, Della Pepa C, Berardi S, Califano D, Scala S, Buonaguro L, Ciliberto G, Brauchli P, Pignata S. Tumor genotype and immune microenvironment in POLE-ultramutated and MSI-hypermutated endometrial cancers: new candidates for checkpoint blockade immunotherapy? Cancer Treat Rev. 2016;48:61–68. doi: 10.1016/j.ctrv.2016.06.008. [DOI] [PubMed] [Google Scholar]
- 41.Wu YS, Shui L, Shen D, Chen X. Bevacizumab combined with chemotherapy for ovarian cancer: an updated systematic review and meta-analysis of randomized controlled trials. Oncotarget. 2017;8(6):10703–10713. doi: 10.18632/oncotarget.12926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang W, Liu JR, Zou W. Immunotherapy in ovarian cancer. Surg Oncol Clin N Am. 2019;28(3):447–464. doi: 10.1016/j.soc.2019.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All articles used are published full text articles.