Abstract
Background
Tumor PD-L1 expression is a predictive biomarker for patients with NSCLC receiving PD-(L)1 blockade agents. However, although increased tumor PD-L1 expression predicts responsiveness, clinical benefit has been observed regardless of tumor PD-L1 expression, suggesting the existence of other PD-L1 sources. The aim of our study was to analyze whether integrating systemic and tumor PD-L1 is more predictive of efficacy in patients with advanced NSCLC receiving PD-(L)1 blockade agents.
Material and methods
Twenty-nine healthy donors and 119 consecutive patients with advanced NSCLC treated with PD-(L)1 drug were prospectively included. Pretreatment blood samples were collected to evaluate PD-L1 levels on circulating immune cells, platelets (PLTs), platelet microparticles (PMPs), and the plasma soluble PD-L1 concentration (sPD-L1). Tumor PD-L1 status was assessed by immunohistochemistry. The percentages of circulating PD-L1 + leukocytes, sPD-L1 levels, and tumor PD-L1 were correlated with efficacy.
Results
No differences in the percentages of circulating PD-L1 + leukocytes were observed according to tumor PD-L1 expression. Significantly longer progression-free survival was observed in patients with higher percentages of PD-L1 + CD14 + , PD-L1 + neutrophils, PD-L1 + PLTs, and PD-L1 + PMPs and significantly longer overall survival was observed in patients with higher percentages of PD-L1 + CD14 + and high tumor PD-L1 expression. Integrating the PD-L1 data of circulating and tumor PD-L1 results significantly stratified patients according to the efficacy of PD-(L1) blockade agents.
Conclusions
Our results suggest that integrating circulating PD-L1 + leukocytes, PLT, PMPs, and sPD-L1 and tumor PD-L1 expression may be helpful to decide on the best treatment strategy in patients with advanced NSCLC who are candidates for PD-(L)1 blockade agents.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00262-021-03107-y.
Keywords: Immunotherapy, NSCLC, Systemic PD-L1, Soluble PD-L1, Predictive biomarker
Introduction
Programmed death-1 (PD-1) or programmed death-ligand 1 (PD-L1) blockade agents have improved treatment outcomes in advanced non-small-cell lung cancer (NSCLC), either as monotherapy or in combination with chemotherapy and/or other immune-checkpoint blockade agents [1]. However, up to 60% of patients with advanced NSCLC will not benefit from PD-(L)1 blockade agents [1]. Moreover, around 11–30% of patients develop serious immune-related adverse events (irAEs) [2]. For those reasons, and given the high cost of those treatments, the development of predictive biomarkers to accurately select patients remains an ongoing challenge.
To date, tumor PD-L1 expression by immunohistochemistry (IHC) is the only approved predictive biomarker for PD-(L)1 blockade agents in advanced NSCLC. Several studies have demonstrated an improvement in overall survival (OS) in patients with previously untreated advanced NSCLC with a PD-L1 tumor proportion score (TPS) of 50% or greater [3, 4]. Nevertheless, tumor PD-L1 expression does not always correlate with efficacy in pretreated NSCLC patients and several trials have shown a survival benefit of PD-(L)1 blockade agents regardless of PD-L1 expression levels [5]. Additionally, tumor PD-L1 expression has other limitations: heterogeneous intra-tumor expression variability, variability between metastatic sites and the primary tumor, sampling methodology (biopsy or cytology), and dynamic tumor PD-L1 expression [6, 7]. This last limitation may be significant when archival samples from pretreated patients collected months or years before starting PD-(L)1 inhibitor are used. It is known that chemotherapy can upregulate PD-L1 expression, not reflecting tumor PD-L1 expression in archival samples [6, 8]. Finally, another very important concern is that tumor biopsies for PD-L1 testing may not always be available.
Retrospective studies of response to PD-(L)1 blockade agents have provided new evidence of possible circulating immunological biomarkers [9]. Different innate and adaptive immune cells are involved in cancer immune surveillance, which can potentially determine their efficacy [10]. The analysis of circulating immune cells also has the advantage of being permanently available and easy to obtain. Moreover, it can be used for the real-time monitoring of treatment response. The fact that patients with advanced NSCLC with negative tumor PD-L1 expression may also benefit from PD-(L)-1 blockade agents suggests that response may be mediated by other relevant sources of PD-L1, such as immune-related blood markers. In patients with advanced NSCLC, PD-L1can be constitutively expressed on the surface of different immune cells [11–13]. In patients with head and neck carcinoma, expression of PD-L1 was also found in platelets [14]. Although little is known regarding the relevance of systemic PD-L1 + cells as a predictor biomarker for PD-(L)1 blockade agents, a role for PD-L1 + myeloid cells in clinical responses to PD-(L)1 blockade agents in advanced NSCLC has been described [13]. Therefore, the expression of PD-L1 on immune cells, which can also compromise the immune response against the tumor, is probably underestimated. In addition, PD-L1 can also be detected as a soluble protein in plasma [11, 12, 15]. Recent evidence have demonstrated that soluble PD-L1 (sPD-L1) derives from malignant cells through different mechanisms: secreting sPD-L1 by alternative splicing [16]; shedding PD-L1 by tumor surface proteases [17] and generation of extracellular vesicles carrying PD-L1 [18, 19]. Current evidence indicated that sPD-L1 has the capacity to compromise anti-tumor response and sPD-L1 is a predictor for patients with cancer that received PD-(L)1 blockade agents [15, 16, 20, 21]. We hypothesized that the integration of pretreatment systemic and tumor PD-L1 expression may prove to be a better predictor of clinical outcomes in patients with advanced NSCLC receiving PD-(L)1 blockade agents. Therefore, the aim of our study was to analyze the role of systemic PD-L1 from different sources as a potential immune-related blood biomarker in patients with advanced NSCLC treated with PD-(L)1 inhibitors.
Material and methods
Patient population
This was a prospective, single-center observational, non-interventional study. We included 29 healthy donors (HD) and 119 consecutive patients with histologically or cytologically confirmed advanced NSCLC treated at our institution with PD-(L)1 blockade agents alone (N = 104) or in combination (n = 15), irrespective of treatment line, from May 2015 to September 2019. The end of follow-up was June 2020. Treatment was discontinued until disease progression by Response Evaluation Criteria in Solid Tumors (RECIST) or Immune-Response Evaluation Criteria In Solid Tumors (iRECIST), treatment completion, or unacceptable toxic effects. Treatment could be continued beyond disease progression if a clinical benefit was maintained.
Written informed consent was obtained from each patient and ethical approval for the study was granted by the Institutional Ethics Committee. Patient data were collected from electronical medical records.
OS was defined as the time between the first dose of PD-(L)1 blockade agent and death from any cause. Progression-free-survival (PFS) was defined as the time from the first dose of the PD-(L)1 blockade agent to radiological progression assessed by local investigators according to iRECIST and RECISTversion 1.1. The PFS of five patients was not included since their progression was not evaluable.
Sample collection and whole blood staining
Whole blood samples from HD and patients with advanced NSCLC were collected in heparinized BD Vacutainer tubes (BD, Franklin Lakes, NJ) before starting anti-PD-(L)1 therapy. One-hundred microliters (µL) of whole blood were incubated with anti-CD3-PECy7 (clone HIT3a), anti-CD8-PeCy5 (clone SK1), anti-PD-L1-PE (clone 29E.2A3) (Biolegend, San Diego, USA), anti-CD41a-FITC (clone HIP8)(Immunotools, Friesoythe, Germany), and anti-CD14-PECy7 (clone M5E2) (BD) monoclonal antibodies. Additionally, fluorescence minus one (FMO) controls were prepared for each cell marker to define negative gates. Then, red blood cells were lysed and white cells fixed using BD FACS lysing solution (BD Bioscience), washed with two milliliters (mL) of PBS 1X, and resuspended in 400 µL of PBS 1X to be analyzed by flow cytometry.
Flow cytometry analysis
Lymphocytes were gated according to Forward scatter (FSC) and Side scatter (SSC). CD4 + and CD8 + T lymphocytes were identified on gated lymphocytes as CD3 + CD8- and CD3 + CD8 + respectively. CD8 + NK cell subset was identified on gated lymphocytes as CD3-CD8 + [22, 23]. Monocytes were gated according to CD14 expression (CD14 +) and SSC. Neutrophils were gated as SSC high CD14- cells. To analyze platelets (PLTs) and remainder platelet microparticles (PMPs) in blood after centrifugation, samples were acquired using FSC and SSC on logarithmic scale. Blood PLTs (> 1 µm) and PMPs (< 1 µm) were identified as CD41a + events in corresponding gate regions previously established using a blend of size-calibrated fluorescent beads with sizes ranging from 0.22 to 1.35 µm (Spherotech, IL, USA). Samples were acquired with the MACSQuant Analyzer 10 flow cytometer (Miltenyi Biotec, Bergisch Gladbach, Germany). The percentage of PD-L1 positive cells (PD-L1 +) according to FMO controls and event/µL for each population was obtained using FlowJo version X (FlowJo LLC, Ashland, USA).
The percentage of PD-L1 + immune cells was analyzed in all patients and the percentage of PD-L1 + PLTs and PMPs was analyzed in 76 patients. To determine patients with high or low percentages of PD-L1 + CD4 + , PD-L1 + CD8 + , PD-L1 + CD8 + NK, PD-L1 + CD14 + , PD-L1 + Neutrophils, PD-L1 + PLTs, and PD-L1 + PMPs, we calculated confidence interval (CI) cut-offs of 95% of HD values. These cut-offs excluded 95% of HD values in a normal distribution. Statistically, a CI of 95% is equal to the mean plus 1.96 standard deviations.
Determination of soluble PD-L1
Plasma concentrations of sPD-L1 were determined using a specific ELISA kit (Invitrogen, California, USA) according to the manufacturers’ instructions and using the specific standard curves of recombinant molecules. The 95% CI of HD values was calculated to identify patients with high and low levels of sPD-L1. sPD-L1 was determined in 118 patients. The lower limit of detection was 4.69 pg/ml.
Tumor PD-L1 expression
PD-L1 status was assessed on tumor cells by immunohistochemistry using the anti-PD-L1 antibody clone 22C3 (pharmDxDakokit) as per local practice. The expression of PD-L1 by TPS (Tumor Proportion Score) was valid when evaluated in more than 100 cells and categorized as negative (< 1%), low PD-L1 expression (1–49%), and high expression (≥ 50%), which are the standard cutoffs for clinical use. Tumor PD-L1 expression was determined in 102 patients.
Radiological assessment
Radiological evaluation was determined by investigators according to RECIST version 1.1 and iRECIST, at baseline and approximately every three months (in line with clinical practice) or whenever the physician deemed it necessary.
Statistical analysis
The Kolmogorov–Smirnov test was used to analyze the normal distribution of data. To describe our population, numbers and percentages were used for qualitative variables, while the median (interquartile ranges, IQR) was calculated for ordinal and quantitative variables with asymmetric distribution. Comparisons between groups were tested with the Student’s t or the Mann–Whitney test, according to a Gaussian distribution. ANOVA and Kruskal–Wallis tests were used for comparisons between more than two groups. Correlation analyses were carried out with Pearson’s or Spearman correlations. The Kaplan-Meier method, along with the long-rank Mantel-Cox test, was used to analyze differences in PFS and OS during the follow-up period. Univariate and multivariate (forward stepwise) Cox regression models were performed to calculate the hazard ratio (HR), the CI of 95%, and to evaluate the predictive impact of systemic and local PD-L1 expression in OS and PFS. A Receiver Operating Characteristic (ROC) curve was performed to establish the optimal cut-off point for integrated PD-L1 data in order to discriminate patients with progressive vs non-progressive disease.
All p values were based on a 2-sided hypothesis, and those under 0.05 were considered statistically significant. Analyses were performed using Graph Pad Prism 7 software except Cox regression model analysis, which was performed using the SPSS statistical software package (version 22, SPSS, Inc Chicago, Illinois, USA).
Results
Patient characteristics
Patient characteristics are shown in Table 1.
Table 1.
Baseline characteristics of patients with NSCLC
| N = 119 (%) | |
|---|---|
| Median Age (range) | 65 (36–84) |
| Sex | |
| Male | 93 (78.1) |
| Female | 26 (21.9) |
| Smoking habit | |
| Non-smoker | 11 (9.2) |
| Smoker | 34 (28.6) |
| Former smoker | 74 (62.2) |
| ECOG PS | |
| 0 | 15 (12.6) |
| 1 | 87 (73.1) |
| 2 | 17 (14.3) |
| Histology | |
| Squamous | 46 (38.7) |
| Non-squamous | 73 (61.3) |
| PD-L1 TPS | |
| < 1% | 30 (25.2) |
| 1–49% | 35 (29.4) |
| ≥ 50% | 37 (31.1) |
| NE | 17 (14.3) |
| Treatment line | |
| First Line | 37 (31.1) |
| Second Line | 64 (53.8) |
| Third Line or beyond | 18 (15.1) |
| Treatment | |
| Combination IO-IO | 4 (3.4) |
| Combination IO-CT | 11 (9.2) |
| Monotherapy | 104 (87.4) |
| Drug | |
| Anti-PD-1 | 91 (76.5) |
| Anti-PD-L1 | 28 (23.5) |
TPS tumor proportion score, NE not evaluable, IO immunotherapy, CT chemotherapy, ECOG PS Eastern Cooperative Oncology Group performance status
Median age was 65 years [IQR 36–84], 78.1% of patients were male and 21.9% were female. Most patients were current or former smokers (90.8%) and baseline Eastern Cooperative Oncology Group (ECOG) performance status (PS) was 0–1 in 85.7% of patients. The most common histology was non-squamous (61.3%). PD-L1 expression was < 1% in 30 patients (25.2%), 1–49 in 35 (29.4%), ≥ 50% in 37 (31.1%) and not evaluable in 17 (14.3%). Thirty-seven patients (31.1%) received PD-(L)1 blockade agents as first-line treatment and 82 (68.9%) as second-line or beyond. PD-(L)1 blockade agents were administered alone in 104 patients (87.4%), in combination with chemotherapy in 11 patients (9.2%), and in combination with other immunotherapy agents in four patients (3.4%).
Median follow-up was 10.97 months [IQR: 6.06–21.87]. At the data cut-off on June 2020, a total of 24 patients were still receiving immunotherapy. The most common reasons for permanent discontinuation of treatment were progressive disease (68 patients), toxicity (10 patients), and treatment completion (6 patients).
Percentages of circulating PD-L1 + subpopulations of leukocytes, PD-L1 + PLTs and PMPs, and plasma sPD-L1 levels were independent of tumor PD-L1 expression
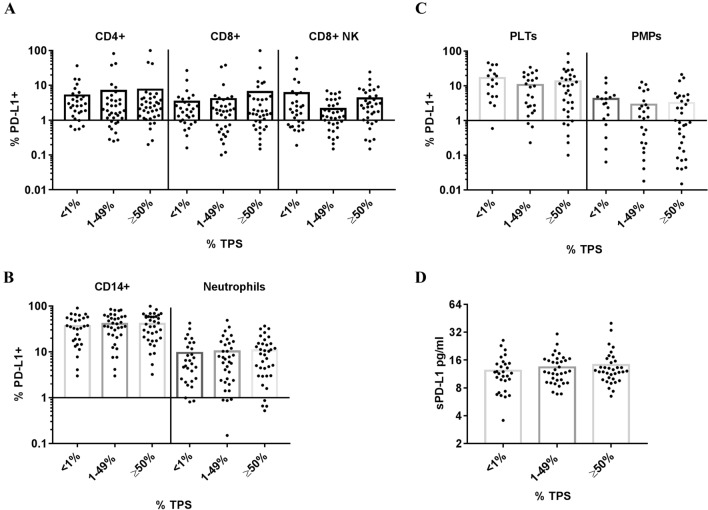
Higher percentages of circulating PD-L1 + leukocytes (CD4 + and CD8 + T lymphocytes, CD8 + NK cells, CD14 + and neutrophils) and PD-L1 + PLTs and PMPs were found in patients with advanced NSCLC compared to HD (Fig. 1A and B). Higher plasma sPD-L1 levels were also found in patients with NSCLC (Fig. 1c). We did not observe differences in the percentages of circulating PD-L1 + subpopulations when we segregated patients with NSCLC according to tumor PD-L1 expression (Fig. 2), suggesting that systemic and tumor PD-L1 expression are independent variables in patients with NSCLC.
Fig. 1.
Percentage of baseline circulating PD-L1 + leukocytes, PD-L1 + PLTs and PMPs and plasma sPD-L1 levels in patients with advanced NSCLC and HD prior to PD-(L)1 therapy. a Representative image of PD-L1 expression (PD-L1 +) on circulating CD4 + and CD8 + T lymphocytes, CD8 + NK cells, CD14 + monocytes, Neutrophils, platelets (PLTs), and platelets microparticles (PMPs) from HD and patients with NSCLC. b Dot plots show the percentage of circulating PD-L1 + leukocytes subpopulations (HD and 119 patients with NSCLC), PLTs and PMPs (HD and 76 patients with NSCLC), and c plasma soluble PD-L1 (sPD-L1) levels (HD and 118 NSCLC patients). * p < 0.05, **p < 0.01 and ***p < 0.001
Fig. 2.
Percentage of circulating PD-L1 + leukocytes, PD-L1 + PLTs and PMPs, and plasma sPD-L1 levels according to tumor PD-L1 expression. Dot plots show percentage of a PDL1 + CD4 + , PD-L1 + CD8 + and PD-L1 + CD8 + NK cells (102 patients with NSCLC), b PD-L1 + CD14 + and PD-L1 + Neutrophils (102 patients with NSCLC), c Platelets (PLTs) and platelets microparticles (PMPs) (73 patients with NSCLC) and d plasma soluble PD-L1 (sPD-L1) (101 patients with NSCLC) according to PD-L1 tumor expression (TPS: tumor proportion score)
The percentages of PD-L1 + CD4 + and CD8 + T lymphocytes correlated with the percentage of PD-L1 + CD8 + NK cells. In addition, there was a significant correlation between PD-L1 + CD14 + and PD-L1 + neutrophils and between PD-L1 + PLTs and PD-L1 + PMPs. We observed a weak correlation between PD-L1 + lymphoid cells, PD-L1 + myeloid cells, and PD-L1 + PLTs and PMPs (Supplementary Table 1).
No differences in the percentages of PD-L1 + CD8 + T lymphocytes, CD8 + NK cells, CD14 + , neutrophils, PLTs, and PMPs were observed according to gender, smoking status, time from diagnosis, histology, treatment line, or ECOG PS. However, higher percentages of PD-L1 + CD4 + were observed in females and in patients with two or more previous treatments. We also observed higher plasma sPD-L1 levels in females and in patients with a high PD-L1 tumor expression and no previous treatment (Supplementary Table 2).
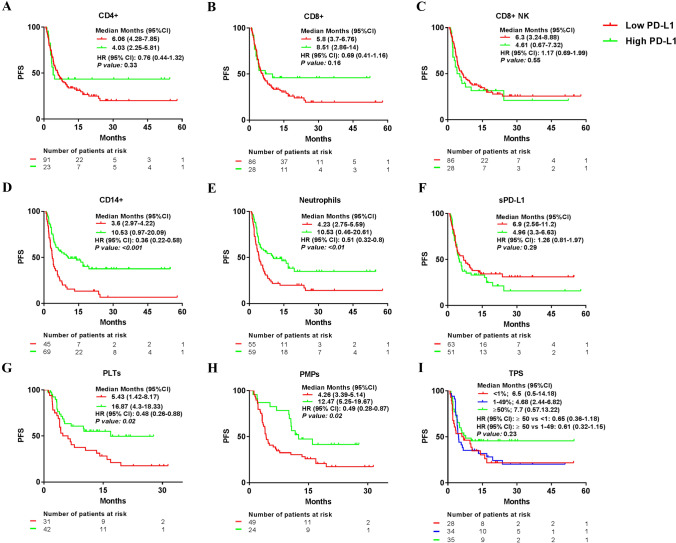
Patients with high percentages of PD-L1 + CD14 + , PD-L1 + neutrophils, PD-L1 + PLTs, and PD-L1 + PMPs had longer progression-free survival
We compared the median PFS of patients with NSCLC treated with PD-(L)1 blockade agents according to high or low percentages of PD-L1 + leukocytes, PLTs, PMPs, sPD-L1 levels, and tumor PD-L1 expression (< 1% TPS, 1–49% TPS and ≥ 50% of TPS). The cut-off obtained for each variable was 5.05% for PD-L1 + CD4 + , 3.53% for PD-L1 + CD8 + , 4.11% for PD-L1 + CD8 + NK, 30.92% for PD-L1 + CD14 + , 5.73% for PD-L1 + Neutrophils, 7.05% for PD-L1 + PLTs, 3.43% for PD-L1 + PMPs and 12.94 pg/ml for plasma sPD-L1. High or low PD-L1 percentage cut-off was not influenced by the cells/µl of each leukocyte population, PLTs, or PMPs (data not shown).
No differences in median PFS were observed between patients with high or low percentages of PD-L1 + CD4 + , PD-L1 + CD8 + , PD-L1 + CD8 + NK, and sPD-L1 level groups or according to tumor PD-L1 expression. However, median PFS was longer in patients with high percentages of PD-L1 + CD14 + , PD-L1 + neutrophils, PD-L1 + PLTs, and PD-L1 + PMPs groups (Fig. 3). Similar results were observed in patients with advanced NSCLC treated with PD-(L)1 inhibitor alone (data not shown).
Fig. 3.
Median progression-free survival comparing high and low percentage of PD-L1 + leukocytes, PD-L1 + PLTs and PMPs, plasma sPD-L1 levels, and tumor PD-L1 expression. Median progression-free survival (PFS) according to high or low group of a PD-L1 + CD4 + , b PD-L1 + CD8 + , c PD-L1 + CD8 + NK cells, d PD-L1 + CD14 + , e PD-L1 + Neutrophils, f pg/ml of sPD-L1, g PD-L1 + PLTs and h PD-L1 + PMPs. i tumor proportion score (TPS)
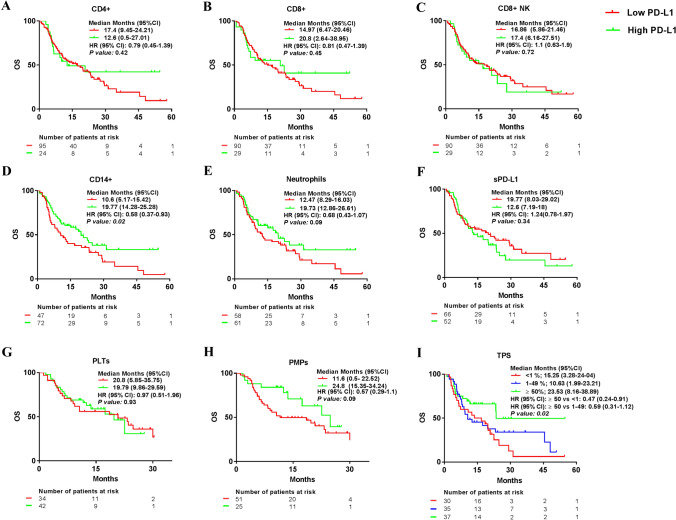
Patients with a high percentage of PD-L1 + CD14 + and high tumor PD-L1 expression had longer overall survival
We also compared the median OS of patients with NSCLC treated with PD-(L)1 blockade agents according to high or low PD-L1 + leukocytes, PLTs and PMPs percentage, sPD-L1 levels, and tumor PD-L1 expression (< 1% TPS, 1–49% TPS and ≥ 50% of TPS). No differences in median OS were observed between patients with high or low percentages of PD-L1 + CD4 + , PD-L1 + CD8 + , PD-L1 + CD8 + NK cells, and PD-L1 + PLTs or sPD-L1level.
On the contrary, an increased median OS was observed in the group of patients with a high PD-L1+ CD14+ percentage and high tumor PD-L1 expression, and a trend to longer OS was observed in the group of patients with high PD-L1+ neutrophils and PD-L1+ PMPs percentages (Figure 4). Again, similar results were observed in patients with NSCLC treated with PD-(L)1 inhibitor alone (data not shown).
Fig. 4.
Median overall survival comparing high and low percentage of PD-L1 + leukocytes, PD-L1 + PLTs and PMPs, plasma sPD-L1 levels, and tumor PD-L1 expression. Median progression-free survival (PFS) according to high or low group of a PD-L1 + CD4 + , b PD-L1 + CD8 + , c PD-L1 + CD8 + NK cells, d PD-L1 + CD14 + , e PD-L1 + Neutrophils, f pg/ml of sPD-L1, g PD-L1 + PLTs and h PD-L1 + PMPs. i Tumor proportion score (TPS)
Integration of circulating and tumor PD-L1 data predicts outcome with PD-(L)1 therapy
A univariate Cox regression model was performed to determine the effect of different PD-L1 sources on PFS and OS. High percentages of PD-L1 + CD14 + , PD-L1 + neutrophil, and PD-L1 + PMPs were predictors of PFS in patients with advanced NSCLC receiving immunotherapy. In a multivariate Cox regression model, high percentages of PD-L1 + CD14 + and PD-L1 + PMPs were two independent variables to predict PFS (Table 2). Additionally, high percentages of PD-L1 + CD14 + , PD-L1 + neutrophil and high tumor PD-L1 expression were predictors of OS in the univariate Cox regression, while high percentages of PD-L1 + CD14 + and high tumor PD-L1 expression were independent variables to predict OS using the multivariate Cox regression model (Table 2).
Table 2.
Univariate and multivariate analysis for prediction of PFS and OS in patients with advanced NSCLC receiving immunotherapy
| Variables | PFS | OS | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | |||||||||
| HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | |
| % PD-L1 + CD4 + | 0.98 | 0.94–1.01 | 0.27 | 0.97 | 0.93–1.01 | 0.28 | ||||||
| % PD-L1 + CD8 + | 0.95 | 0.88–1.02 | 0.18 | 0.96 | 0.9–1.03 | 0.31 | ||||||
| % PD-L1 + CD8 + NK | 0.96 | 0.91–1.02 | 0.23 | 0.97 | 0.91–1.04 | 0.42 | ||||||
| % PD-L1 + CD14 + | 0.97 | 0.96–0.99 | 0.002 | 0.98 | 0.96–0.99 | 0.001 | 0.97 | 0.95–0.99 | 0.02 | 0.98 | 0.96–0.99 | 0.02 |
| % PD-L1 + Neutrophil | 0.96 | 0.93–0.99 | 0.04 | 0.94 | 0.91–0.98 | 0.04 | ||||||
| % PD-L1 + PLTs | 0.98 | 0.95–1 | 0.06 | 0.97 | 0.94–1.1 | 0.058 | ||||||
| % PD-L1 + PMPs | 0.89 | 0.81–0.99 | 0.04 | 0.89 | 0.81–0.99 | 0.02 | 0.95 | 0.88–1.04 | 0.27 | |||
| pg/ml sPD-L1 | 0.98 | 0.94–1.03 | 0.56 | 1 | 0.96–1.05 | 0.75 | ||||||
| % TPS | 0.99 | 0.98–1 | 0.14 | 0.98 | 0.98–0.99 | 0.031 | 0.99 | 0.98–1 | 0.04 | |||
CI Confidence interval, HRHazard Ratio, OS Overall survival, PMPs Platelet microparticles, PFS Progression free survival, PLTs Platelets, TPS tumor proportion score
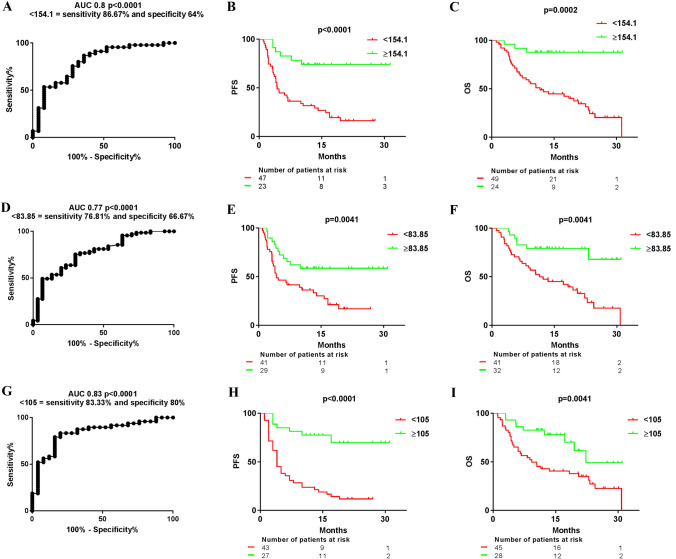
We next investigated the integration of circulating and tumor PD-L1 data to predict clinical outcomes in patients receiving PD-(L)1 blockade agents, which was named integrated PD-L1 data (IPD). First, we performed an analysis on 70 patients with all the circulating and tumor PD-L1 data through a ROC curve comparing patients with progressive versus non-progressive disease to PD-(L)1 blockade agents to generate a cut-off point. AUC was 0.8 and the cut-off point was 154.1. We found that patients with IPD ≥ 154.1 had longer median PFS than those with IPD < 154.1: median PFS not reached (NR) (95% CI not achieved) vs 4.33 months (95% CI: 3.25–5.41), (HR 0.29; 95% CI: 0.16–0.54, p < 0.0001). Similarly, median OS was longer in patients with IPD ≥ 154.1: median OS NR (95% CI not achieved) vs 11.6 months (95% CI: 5.8–17.39), (HR 0.28; 95% CI: 0.14–0.54, p = 0.0002) (Fig. 5a-c). We then analyzed the combination of PD-L1 + CD14 + and high tumor PD-L1 expression, since they were independent variables for predicting OS in the multivariate analysis (the cut-off point was 83.85 as calculated by the ROC curve). We found that patients with IPD ≥ 83.85 had longer median PFS than those with IPD < 83.85: median PFS NR (95% CI not achieved) vs 4.27 months (95% CI: 1.49–7.06), (HR 0.41; 95% CI: 0.23–0.75, p = 0.0041). Patients with IPD ≥ 83.85 also had longer median OS: median OS NR (95% CI not achieved) vs 11.44 months (95% CI: 3.41–19.46), (HR 0.32; 95% CI: 0.17–0.62, p = 0.0041) (Fig. 5d-f). Finally, we analyzed the capability of IPD from all circulating PD-L1 sources to predict PFS and OS (the cut-off point was 105 as calculated by the ROC curve). We found that patients with IPD ≥ 105 had longer median PFS than those with IPD < 105: median PFS NR (95% CI not achieved) vs four months (95% CI: 2.73–5.26), (HR 0.21; 95% CI: 0.11–0.4, p < 0.0001). A longer median OS was also observed in patients with IPD ≥ 105: median OS NR (95% CI not achieved) vs 9.23 months (95% CI: 4.18–14-28), (HR 0.38; 95% CI: 0.2–0.73, p = 0.0041) (Fig. 5g-i). Multivariate analysis for PFS and OS including clinical variables such ECOG PS, histology, or line of therapy along with all the circulating and tumor PD-L1 data was also performed. We found that high PD-L1 + CD14 + percentage was the strongest variable followed by ECOG PS for survival outcomes.
Fig. 5.
Median progression-free survival (PFS) and overall survival (OS) in different groups of patients with NSCLC achieved according to the integration of PD-L1 data (IPD) from different systemic and tumor sources. a ROC curve comparing the integrated PD-L1 data (IPD) from systemic and tumor PD-L1 sources (leukocytes, PLTs and PMPs, sPD-L1 and TPS) from patients with progressive versus non-progressive disease to PD-(L)1 blockade agents to obtain the best cut-off point to discriminate them. b MedianPFS and c median OS according to the cut-off point (154.1). d ROC curve comparing the IPD from PD-L1 + CD14 + and tumor PD-L1 expression from patients with progressive versus non-progressive disease to PD-(L)1 blockade agents. e Median PFS and f median OS according to the cut-off point (83.85). g ROC curve comparing the IPD from PD-L1 + leukocytes, PD-L1 + PLTs and PMPs and plasma sPD-L1 levels from patients with progressive versus non-progressive disease to PD-(L)1 blockade agents. h Median PFS and i median OS according to the cut-off point (105)
Discussion
Our results have shown that patients with advanced NSCLC presented a high percentage of circulating PD-L1 + leukocytes, PD-L1 + PLTs and PMPs, and plasma sPD-L1. Patients with NSCLC that had higher percentage of PD-L1 + myeloid cells, PLTs, and PMPs before starting PD-(L)1 therapy had a better outcome with that treatment, suggesting that these PD-L1 sources may correlate with the efficacy of PD-(L)1 blockade agents (Fig. 6). Thus, our results suggest that the integration of tumor and circulating PD-L1 sources may be a better predictor of the clinical outcomes of PD-(L)1 blockade agents in patients with advanced NSCLC than PD-L1 tumor expression alone.
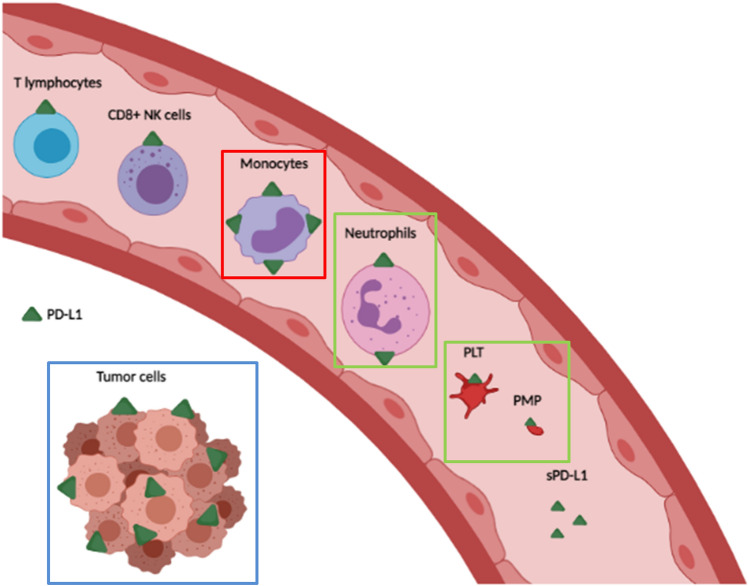
Fig. 6.
A model of the systemic and local PD-L1 sources in patients with advanced NSCLC. The PD-L1 sources that we have associated with the clinical outcome of patients with advanced NSCLC treated with anti-PD-(L)1 blockade agents are highlighted with color squares. We found that higher percentages of PD-L1 + monocytes were associated with longer PFS and OS, higher percentages of PD-L1 + neutrophils, PLTs, and PMPs with PFS, and higher percentages of PD-L1 + tumor cells with OS. Figure created with Biorender.com
We found a weak correlation of PD-L1 levels between myeloid and lymphoid cells. Although PD-L1 percentage is upregulated in the majority of leukocytes by IFN or TNF-α [4, 24–26], anti-inflammatory cytokines, such as IL-10, TGF-β, and IL-25, can induce PD-L1 expression, mainly on monocytes [27, 28]. Moreover, we observed that percentages of PD-L1 + subpopulations of leukocytes, PLTs, and PMPs, and sPD-L1 levels were not correlated with tumor PD-L1 expression in patients with advanced NSCLC. This finding has been described previously with regard to sPD-L1 concentrations and PD-L1 expression on the tumor samples of patients with advanced NSCLC [21]. We did not find differences in sPD-L1 according to response in patients with negative tumor PD-L1 expression (data not shown).
It has been shown that a higher percentage of PD-L1 + leukocytes can inhibit anti-tumor responses. Therefore, it is possible that patients with advanced NSCLC with a higher percentage of PD-L1 + leukocytes and negative PD-L1 tumor expression would beneficiate from PD-(L)1 blockade therapy [13, 29].
This is the first time that the presence of PD-L1 on PLTs and PMPs from patients with advanced NSCLC has been found independent of tumor PD-L1 expression. The activated status of PLTs in patients with advanced NSCLC may be the reason for that finding [30]. A high percentage of PD-L1 + PLT has previously been observed in patients with head and neck carcinoma [14]. All these findings suggest that PLT from patients with solid tumors present inhibitory molecules that may compromise antitumor responses. In line with this suggestion, we have observed that patients with advanced NSCLC with higher percentages of PD-L1 + PLTs and PD-L1 + PMPs had longer PFS when treated with PD-(L)1 blockade agents, thereby highlighting the importance of PLTs as a potential immune-regulatory element in patients with solid tumors.
We also found that patients with advanced NSCLC with higher percentages of PD-L1 + CD14 + and PD-L1 + neutrophils had longer PFS with PD-(L)1 therapy. Additionally, patients with a higher percentage of PD-L1 + CD-14 + , PD-L1 + neutrophils, and high tumor PD-L1 expression had longer OS. A higher percentage of PD-L1 + myeloid cells had previously been reported in a cohort of 32 patients with advanced NSCLC and objective response (OR) to PD-(L)1 agents [13].
In our study, no differences in terms of PFS or OS were observed according to plasma sPD-L1 levels or the percentage of PD-L1 + lymphocytes. The role of basal sPD-L1 levels as a predictive biomarker of efficacy has been tested in patients with advanced NSCLC treated with nivolumab with some controversial results. Constantini and Ando et al. did not observe statistical difference in basal sPD-L1 concentrations between responders and non-responders to nivolumab or those with clinical benefit. However, increasing of sPD-L1 concentration after anti-PD-(L)1 therapy was associated with poor response, less tumor regression, and non-clinical benefit to immunotherapy in cancer patients [20, 21]. In addition, Okuma Y. et al. found that patients with low plasma sPD-L1 had a longer OS [15] and patients with advanced NSCLC and high sPD-L1 levels had worse prognosis than those with low sPD-L1 levels [15, 31, 32].
Regarding lymphocytes, a correlation between a higher percentage of PD-L1 + CD4 + and PD-L1 + CD8 + T lymphocytes and prolonged OS and PFS in patients with melanoma treated with ipilimumab ± nivolumab has been reported [33]. However, this correlation between the expression of PD-L1 on lymphocytes and clinical outcome was probably related to the existence of a negative immune context characterized by the presence of myeloid dendritic suppressor cells and Tregs rather than the contribution of PD-L1 + lymphocytes [34].
Collection of whole blood is a non-invasive method and can easily be obtained, and this fact supports the potential of systemic PD-L1 expression as a complementary predictive biomarker. Since we observed that systemic and local PD-L1 were independent predictive biomarkers of clinical outcome in patients with advanced NSCLC, we have confirmed our hypothesis that global PD-L1 expression has the potential capacity to suppress anti-tumor response. Although our multivariate analysis showed that the combination of a high percentage of PD-L1 + CD14 + and high tumor PD-L1 expression was the most significant predictive biomarkers of OS, the best prediction of treatment outcome was obtained when we included all systemic and local sources of PD-L1 in the IPD.
This study has several limitations. Although the sample size is not small, a larger cohort of patients and a longer follow-up would be required to validate our findings. Nevertheless, we did observe significant results regarding survival. Furthermore, it would be necessary to use a validation cohort with a control group to confirm the predictive value of systemic PD-L1. We have included patients receiving PD-(L)1 blockade agents in different treatment lines, and in combination with chemotherapy or other immunotherapeutic agents, which may impact the patients’ outcomes. However, similar results were observed in patients with NSCLC treated with PD-(L)1 alone. Lastly, the percentages of circulating PD-L1 + leukocytes, PLTs, PMPs, and plasma sPD-L1 were only analyzed at baseline, and the evaluation of the dynamic changes during treatment with PD-(L)1 blockade agents would be more helpful to predict the treatment outcomes of patients with NSCLC.
In conclusion, an approach based on integrating systemic immune-related biomarkers and tumor PD-L1 expression could better stratify patients according to the potential efficacy of PD-(L1) blockade agents. These findings could be helpful when deciding on the best treatment strategy for patients with advanced NSCLC who are candidates for PD-(L)1 blockade agents.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgments
SV was supported by “Fondo Investigaciones Sanitarias” and a participant in the Program for the Stabilization of Investigators from the “Direcció i d’Estrategia I Coordinació del Departament Salut de la Generalitat de Catalunya.”
Abbreviations
- CH
Chemotherapy
- CI
Confidence interval
- ECOG PS
Eastern Cooperative Oncology Group performance status
- FITC
Fluorescein
- HD
Healthy donors
- HR
Hazard Ratio
- IO
Immunotherapy
- IPD
Integrated PD-L1 data
- irAEs
Immune-related adverse events
- IHC
Immunohistochemistry
- iRECIST
Immune-Response Evaluation Criteria In Solid Tumors
- NSCLC
Non-small cell lung cancer
- PD-1
Programmed death-1
- PD-L1
Programmed death-ligand 1
- PLTs
Platelets
- PMPs
Platelet microparticles
- sPD-L1
Plasma concentrations of soluble PD-L1
- OS
Overall survival
- PFS
Progression-free-survival
- TPS
Tumor proportion score
- RECIST
Response Evaluation Criteria in Solid Tumors
- NE
Not evaluable
- PE
Phycoerythrin
- PECy7
Phycoerythrin Cyanine 7
- PECy5
Phycoerythrin Cyanine 5
Authors’ contributions
All authors were involved in revising intellectual content, and all authors approved the final version for publication. CZ, MM, LA, and MAO performed cellular staining and flow cytometry analysis and ELISAs. CZ and MM and MAO analyzed results of flow cytometry and ELISAS; GA, MR, IS, AB, JS, OG, JG, and MM collected samples and clinical data; CZ and SV performed statistical analysis.GA, CZ, MM and SV wrote the manuscript. MM, and SV designed the study.
Funding
This work was supported by the Bristol Myers Squibb.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The study was conducted in accordance with the Helsinki Declaration and approved by the Research Ethics Board of Hospital de la Santa Creu I Sant Pau, Barcelona (IIBSP-PDL-2017–82).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Carlos Zamora Atenza and Geòrgia Anguera have contributed equally to this work. Sílvia Vidal and Margarita Majem Tarruella have contributed equally to this work.
References
- 1.Nixon NA, Blais N, Ernst S, et al. Current landscape of immunotherapy in the treatment of solid tumours, with future opportunities and challenges. Curr Oncol. 2018;25:e373–e384. doi: 10.3747/co.25.3840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bocanegra A, Blanco E, Fernandez-Hinojal G, et al. PD-L1 in systemic immunity: unraveling its contribution to PD-1/PD-L1 blockade immunotherapy. Int J Mol Sci. 2020;21:1–17. doi: 10.3390/ijms21165918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hui R, Gandhi L, Carcereny Costa E, et al. Long-term OS for patients with advanced NSCLC enrolled in the KEYNOTE-001 study of pembrolizumab (pembro) J Clin Oncol. 2016;34:9026–9026. doi: 10.1200/jco.2016.34.15_suppl.9026. [DOI] [Google Scholar]
- 4.Hartley G, Regan D, Guth A, Dow S. Regulation of PD-L1 expression on murine tumor-associated monocytes and macrophages by locally produced TNF-α. Cancer Immunol Immunother. 2017;66:523–535. doi: 10.1007/s00262-017-1955-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hallqvist A, Rohlin A, Raghavan S (2020) Immune checkpoint blockade and biomarkers of clinical response in non–small cell lung cancer. Scand J Immunol 92. 10.1111/sji.12980 [DOI] [PMC free article] [PubMed]
- 6.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in Cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/nejmoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cottrell TR, Taube JM. PD-L1 and emerging biomarkers in immune checkpoint blockade therapy. Cancer J (United States) 2018;24:41–46. doi: 10.1097/PPO.0000000000000301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prelaj A, Tay R, Ferrara R, et al. Predictive biomarkers of response for immune checkpoint inhibitors in non-small-cell lung cancer. Eur J Cancer. 2019;106:144–159. doi: 10.1016/j.ejca.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 9.Riudavets M, Auclin E, Mezquita L. Host circulating biomarkers for immune-checkpoint inhibitors: single-agent and combinations. Futur Oncol. 2020;16:1665–1668. doi: 10.2217/fon-2020-0182. [DOI] [PubMed] [Google Scholar]
- 10.Chen DS, Mellman I. Oncology meets immunology: The cancer-immunity cycle. Immunity. 2013;39:1–10. doi: 10.1016/j.immuni.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 11.Ilié M, Szafer-Glusman E, Hofman V, et al. Detection of PD-L1 in circulating tumor cells and white blood cells from patients with advanced non-small-cell lung cancer. Ann Oncol. 2018;29:193–199. doi: 10.1093/annonc/mdx636. [DOI] [PubMed] [Google Scholar]
- 12.Arrieta O, Montes-Servín E, Hernandez-Martinez JM, et al (2017) Expression of PD-1/PD-L1 and PD-L2 in peripheral T-cells from non-small cell lung cancer patients. Oncotarget 8:101994–102005. 10.18632/oncotarget.22025 [DOI] [PMC free article] [PubMed]
- 13.Bocanegra A, Fernandez-Hinojal G, Zuazo-Ibarra M et al (2019) PD-L1 expression in systemic immune cell populations as a potential predictive biomarker of responses to PD-L1/PD-1 blockade therapy in lung cancer. Int J Mol Sci 20. 10.3390/ijms20071631 [DOI] [PMC free article] [PubMed]
- 14.Rolfes V, Idel C, Pries R, et al (2018) PD-L1 is expressed on human platelets and is affected by immune checkpoint therapy. Oncotarget 9:27460–27470. 10.18632/oncotarget.25446 [DOI] [PMC free article] [PubMed]
- 15.Okuma Y, Wakui H, Utsumi H, et al. Soluble programmed cell death ligand 1 as a novel biomarker for nivolumab therapy for non–small-cell lung cancer. Clin Lung Cancer. 2018;19:410–417.e1. doi: 10.1016/j.cllc.2018.04.014. [DOI] [PubMed] [Google Scholar]
- 16.Zhou J, Mahoney KM, Giobbie-Hurder A, et al. Soluble PD-L1 as a biomarker in malignant melanoma treated with checkpoint blockade. Cancer Immunol Res. 2017;5:480–492. doi: 10.1158/2326-6066.CIR-16-0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Orme JJ, Jazieh KA, Xie T et al (2020) ADAM10 and ADAM17 cleave PD-L1 to mediate PD-(L)1 inhibitor resistance. Oncoimmunology 9. 10.1080/2162402X.2020.1744980 [DOI] [PMC free article] [PubMed]
- 18.Chen G, Huang AC, Zhang W, et al. (2018) Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nat. 2018;5607718(560):382–386. doi: 10.1038/s41586-018-0392-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Orme JJ, Enninga EAL, Lucien-Matteoni F, et al. Therapeutic plasma exchange clears circulating soluble PD-L1 and PD-L1-positive extracellular vesicles. J Immunother cancer. 2020;8:1–7. doi: 10.1136/jitc-2020-001113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ando K, Hamada K, Watanabe M, et al (2019) Plasma levels of soluble PD-L1 correlate with tumor regression in patients with lung and gastric cancer treated with immune checkpoint inhibitors. Anticancer Res 39:5195–5201. 10.21873/anticanres.13716 [DOI] [PubMed]
- 21.Costantini A, Julie C, Dumenil C et al (2018) Predictive role of plasmatic biomarkers in advanced non-small cell lung cancer treated by nivolumab. Oncoimmunology 7. 10.1080/2162402X.2018.1452581 [DOI] [PMC free article] [PubMed]
- 22.Ahmad F, Hong HS, Jäckel M, et al. High frequencies of polyfunctional CD8+ NK Cells in Chronic HIV-1 infection are associated with slower disease progression. J Virol. 2014;88:12397. doi: 10.1128/JVI.01420-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lindgren Å, Yun CH, Lundgren A, et al. CD8- natural killer cells are greatly enriched in the human gastrointestinal tract and have the capacity to respond to bacteria. J Innate Immun. 2010;2:294–302. doi: 10.1159/000286238. [DOI] [PubMed] [Google Scholar]
- 24.Bazhin AV, von Ahn K, Fritz J, et al. Interferon-α up-regulates the expression of PD-L1 molecules on immune cells through STAT3 and p38 signaling. Front Immunol. 2018;9:2129. doi: 10.3389/fimmu.2018.02129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Munir S, Lundsager MT, Jørgensen MA, et al (2019) Inflammation induced PD-L1-specific T cells. Cell Stress 3:319–327. 10.15698/cst2019.10.201 [DOI] [PMC free article] [PubMed]
- 26.de Kleijn S, Langereis JD, Leentjens J et al (2013) IFN-γ-stimulated neutrophils suppress lymphocyte proliferation through expression of PD-L1. PLoS ONE 8. 10.1371/journal.pone.0072249 [DOI] [PMC free article] [PubMed]
- 27.Maine CJ, Aziz NHA, Chatterjee J, et al. Programmed death ligand-1 over-expression correlates with malignancy and contributes to immune regulation in ovarian cancer. Cancer Immunol Immunother. 2014;63:215–224. doi: 10.1007/s00262-013-1503-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang WB, Yen ML, Liu KJ, et al. Interleukin-25 mediates transcriptional control of PD-L1 via STAT3 in multipotent human mesenchymal stromal cells (hMSCs) to suppress Th17 responses. Stem Cell Reports. 2015;5:392–404. doi: 10.1016/j.stemcr.2015.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389:255–265. doi: 10.1016/S0140-6736(16)32517-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gong L, Cai Y, Zhou X, Yang H. Activated platelets interact with lung cancer cells through P-selectin glycoprotein ligand-1. Pathol Oncol Res. 2012;18:989–996. doi: 10.1007/s12253-012-9531-y. [DOI] [PubMed] [Google Scholar]
- 31.Meyo MT, Jouinot A, Giroux-Leprieur E et al (2020) Predictive value of soluble PD-1, PD-L1, VEGFA, CD40 ligand and CD44 for nivolumab therapy in advanced non-small cell lung cancer: a case-control study. Cancers (Basel) 12. 10.3390/cancers12020473 [DOI] [PMC free article] [PubMed]
- 32.Zhang J, Gao J, Li Y, et al. Circulating PD-L1 in NSCLC patients and the correlation between the level of PD-L1 expression and the clinical characteristics. Thorac Cancer. 2015;6:534–538. doi: 10.1111/1759-7714.12247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jacquelot N, Roberti MP, Enot DP et al (2017) Predictors of responses to immune checkpoint blockade in advanced melanoma. Nat Commun 8. 10.1038/s41467-017-00608-2 [DOI] [PMC free article] [PubMed]
- 34.Brochez L, Meireson A, Chevolet I et al (2018) Challenging PD-L1 expressing cytotoxic T cells as a predictor for response to immunotherapy in melanoma. Nat Commun 9. 10.1038/s41467-018-05047-1 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.