Abstract
Plasmacytoid dendritic cells (pDCs) represent a subset of antigen-presenting cells that play an ambivalent role in cancer immunity. Here, we investigated the clinical significance of circulating pDCs and their interaction with tumor-specific T cell responses in patients with non-small cell lung cancer (NSCLC, n = 126) . The relation between intratumoral pDC signature and immune checkpoint inhibitors efficacy was also evaluated. Patients with NSCLC had low level but activated phenotype pDC compared to healthy donors. In overall population, patients with high level of pDC (pDChigh) had improved overall survival (OS) compared to patients with pDClow, median OS 30.4 versus 20.7 months (P = 0.013). This clinical benefit was only observed in stage I to III patients, but not in metastatic disease. We showed that patients harboring pDChigh profile had high amount of Th1-diffentiation cytokine interleukin-12 (IL-12) in blood and had functional T cells directed against a broad range of tumor antigens. Furthermore, a high pDC signature in the tumor microenvironment was associated with improved clinical outcome in patients treated with anti-PD-(L)1 therapy. Overall, this study showed that circulating pDChigh is associated with long-term OS in NSCLC and highlighted the predictive value of intratumor pDC signature in the efficacy of immune checkpoint inhibitors.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00262-022-03271-9.
Keywords: Plasmacytoïd dendritic cells, Tumor-specific T cell responses, Lung cancer, Prognosis, Peripheral blood
Introduction
A better understanding of immune response against cancer encompasses the analysis of immune cell within the tumor microenvironment but also in the peripheral blood [1]. Hence, evidence supports the contribution of systemic immune responses for effective antitumor immunity [2].
Dendritic cells (DCs) are professional antigen-presenting cells (APC) able to present tumor-associated antigens to naive and memory T cells in the context of MHC molecules. These cells are critical orchestrators in priming, differentiation and proliferation of antitumor CD8 and CD4 T cells [1, 3].
Plasmacytoïd DCs (pDCs) represent one of the three main subsets of DCs along with type I conventional DCs (cDC1) and type II cDCs (cDC2) [4, 5]. In human, pDCs phenotypically express CD123, BDCA2, BDCA4 and low class II MHC. Functionally, pDCs are characterized by the production of large amount of type I interferons (IFNs) and play a critical role in antiviral immunity [6, 7]. However, their role in tumor immunity is ambiguous. pDCs are involved in the efficacy of anticancer treatment, suggesting that these cells may promote antitumor immunity [8, 9]. In contrast, accumulation of pDC in the tumor microenvironment was often associated with poor prognosis in many cancers [7, 10, 11]. Indeed, according to the type of stimulation and the environment, pDCs have been shown to enhance immunogenic or tolerogenic responses [12, 13].
Many studies have focused on the role of pDC in the tumor microenvironment [1]. In the peripheral blood of cancer patients, high level of circulating pDCs seems to be associated with better clinical outcome [14–17]. However, the potential explanations of this clinical benefit are not investigated yet. Our hypothesis is that circulating pDCs may involve in the stimulation and/or persistence of tumor-specific T cell responses in peripheral blood. We and others previously have shown that spontaneous tumor-specific T cells in peripheral blood were associated with improvement of patient’s survival [18–25].
In this present study, we investigated the relationship between pDC and tumor-specific T responses in the peripheral blood and their prognosis value in NSCLC patients with 10-year follow-up.
Materials and methods
Study population
Patients with non-small cell lung cancer were enrolled from 2010 to 2014 at the University Hospital Georges Pompidou (Paris, France) and University Hospital of Besançon (France) in the TELOCAP 01 study, a prospective study of antitumor T cell immunity in lung cancer (N°EUDRACT: 2009-A00642-55). Blood samples from patients with stages I to IV were included before any anticancer therapy. All patients were enrolled after the signature of an informed consent in accordance with French laws and after approval by the local and national ethic committees. Peripheral blood mononuclear cells (PBMC) were isolated by density gradient separation on Ficoll Unisep tube (Eurobio) and frozen until use. Sera were also collected at inclusion, isolated and frozen until use. Information about patient’s outcome has been collected 10 years after their inclusion. Main patients’ clinical characteristics are described in Supplementary Table 1. Blood cells were also collected from 30 anonymous healthy donors (HD) at the Etablissement Français du Sang (EFS, Besançon France) after an informed consent signature and following EFS guidelines.
Flow cytometry
Immune analysis of DC was performed in 2015. PBMC from 126 patients and 30 healthy donors were thawed, washed in 1X PBS (Gibco) and stained with a viability marker, efluor 506 viability dye (eBiosciences, France) according to the manufacturer’s instructions. For DC analysis, PBMC were stained for 30 min at 4 °C with a mixture of the following antibodies: antihuman lineage cocktail, composed of FITC antihuman CD3/CD14/CD16/CD19/CD20/CD56 (lin1, BD Biosciences), V500 antihuman HLA-DR (BD Bioscience, clone G46-6), PE-Cy7 antihuman CD123 (Biolegend, clone 6H6), APC antihuman BDCA3 (Miltenyi, clone AD5-14H12), Pacific Blue antihuman CD86 (Biolegend, clone IT2.2) and PerCP Cy5.5 antihuman CD40 (Biolegend, clone SC3). Cells were acquired on a FACS Canto II™ cytometer (BD Biosciences) and analyzed with FACSDiva™ and Kaluza™ softwares (BD Biosciences and Beckman Coulter, respectively).
Synthetic tumor antigen-derived peptides
Telomerase-derived peptides were purchased from JPT (Germany) (purity > 80%) [26, 27]. Overlapping 15 mers peptides mixtures derived from NYESO1 and from Wilm’s tumor 1 (WT1) were also used (CTL, Cellular Technology Ltd, Germany). To assess antiviral T cell immunity, peptide mixtures derived from influenza virus, Epstein Barr virus and cytomegalovirus were used (PA-CEF-001, CTL, Cellular Technology Ltd, Germany).
Assessment of spontaneous T-cell responses against TAA by IFN-y ELISpot
T cell responses were assessed by IFN-γ ELISPOT assay after a short in vitro stimulation of PBMC as previously described [18]. Briefly, ficoll-isolated PBMCs were plated at 4.106 cells/well for 6 days in 24-well plates containing 5 μg/ml of the peptides mixture derived from TERT, NYESO1, WT1 and CEF. Recombinant interleukins, IL-7 (5 ng/ml; Preprotech) and IL-2 (20 UI/ml; Novartis) were added at days 1 and 3, respectively. At day 7, specific T cells were measured by IFN-ɣ ELISPOT according to the manufacturer’s instructions. Briefly, cells were incubated at 1 × 105 cells/well in X-Vivo 15 medium (Lonza, France) in a 96-well ELISPOT plate with the relevant peptides for 15 h. Cells cultured with medium or Phorbol Myristate Acetate (PMA, 1 ng/ml)/ionomicyn (500 ng/ml) were used as negative and positive controls, respectively. Spots were revealed, and spots forming cells were counted using the C.T.L Immunospot System™ (Cellular Technology Ltd). Responses were considered as positive when IFN-γ spots numbers were twice higher than medium control and > 10 [28].
Cytokine measurement
IL-10 was measured in patients’ serum using Cytometric Bead Array (CBA) Flex Set (BD Biosciences, France). IL-12p40/p70, TGF-β and IFN-α2 serum level were assessed by ELISA Kit assay (Diaclone and R&D system, France).
RNA sequencing
RNAseq data were generated in Dijon NGS core facility (France). The pDC signature in the tumor tissues was analyzed in a cohort of advanced NSCLC treated with anti-PD(L)1 therapy. The cohort includes 89 patients, treated in first or second line by anti-PD-1 or anti-PD-L1 as monotherapy. Raw data were pseudo-aligned, and gene counts were quantified using the kallisto software. This data set was previously published [29]. The pDC signature expression for each patient is the average of the DeSeq2 normalized counts (log2 transformation) of the CLEC4C, TCF4 and GZMB genes based on a previous publication in head and neck cancer [30]. The signature expression is then dichotomized by the optimal threshold for the cohort, calculated with R's maxstat function so that the groups formed each contain a minimum of 30% of the patients and a maximum of 70%. The hospital institutional review board approved the study in accordance with the principles of Good Clinical Practice, the Declaration of Helsinki and other applicable local regulations. Tumors were collected, stored and used with the written informed consent of the patient. This cohort falls within the scope of the biological collection authorization registered under the name AC-2014-2260.
Statistics
For two-group comparisons, the nonparametric Student t-test (Mann–Whitney U-test) was used. Frequency (percentage) was provided for the description of categorical variables. Proportions were compared using the X2-test (or Fisher’s exact test, if appropriate). For survival analysis, we used the restricted cubic spline for determine the thresholds of DCs (pDC: 6.1% and cDC1: 0.6%). Overall survival (OS) was calculated from the date of study enrollment to the date of death from any cause. Patients known to be alive were censored at the time of their last follow-up assessment. The endpoint date was July 2020. OS was estimated using the Kaplan–Meier method, described using median or rate at specific time points with 95% confidence intervals (95% CI) and compared among the groups with the log-rank test. Cox proportional hazard models were performed to estimate hazard ratio (HR) and 95% CI for factors associated with OS. The association of baseline parameters with OS was first assessed using univariate Cox analyses, and then, those with P ≤ 0.05 were entered into a final multivariate Cox regression model. When used continuously, the association between the level of pDc and cDC1 and OS was investigated using the restricted cubic splines method with graphical evaluation. All analyses were performed using Prism 6 GraphPad™ software and R software version 2.15.2 (R Development Core Team; http://www.r-project.org). Values of P ≤ 0.05 were considered statistically significant, and all tests were two-sided.
Results
High level of pDC in peripheral blood is associated with an improvement of long-term overall survival in NSCLC
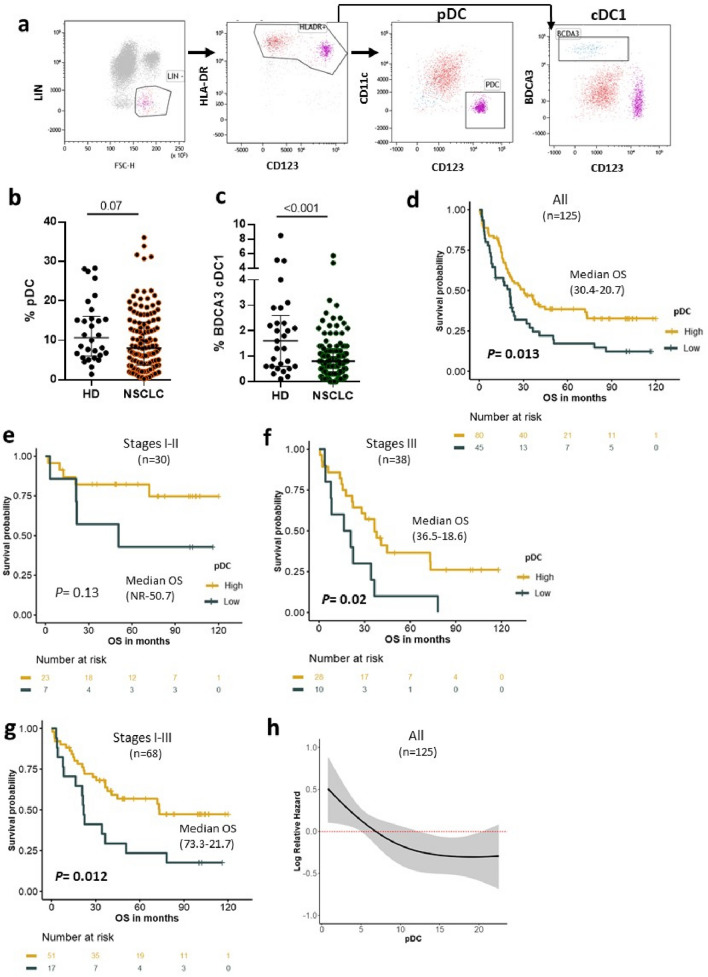
pDC was defined as Lin-HLADR+CD11c-CD123+ and was analyzed in PBMC of 126 NSCLC patients and 30 healthy donors (HD) by flow cytometry (Fig. 1a). In parallel, another DC subset, cDC1 defined as Lin-HLADR+CD11c+BDCA3high was measured in the same population (Fig. 1a). Lower frequencies of circulating pDC were found in NSCLC patients compared to HD (Fig. 1b and supplementary Fig. 1a). Similar observations were made when the pDC were analyzed within the PBMC population (0.31% in NSCLC vs 0.42% in HD, P = 0.01 and within the LIN compartment (9.8% in NSCLC vs 12.4% in HD, P = 0.07). NSCLC patients exhibited also lower levels of cDC1 compared to HD (Fig. 1c and Supplementary Fig. 1b).
Fig. 1.
Circulating pDC on NSCLC patients: distribution and correlation with clinical outcome of NSCLC patients. a Gating strategy for pDC (Lin-HLADR+CD11c-CD123+) and cDC1 (Lin-HLADR+BDCA3+) analysis on PBMC from NSCLC and HD. b Level of circulating pDC within Lin compartment in HD (n = 30) and in NSCLC (n = 126) (Median and interquartile range are indicated, Mann–Whitney test). c Level of circulating cDC1 within Lin compartment in HD (n = 30) and in NSCLC (n = 126) (median and interquartile range are indicated, Mann–Whitney test). d–g Association between the percentage of circulating pDC and overall survival (OS). Kaplan–Meier curves according to pDClow and pDChigh in all population (d), in stages I–II (e), in stages III (f) and in stages I to III (g) (log-rank tests). h Risk of death according to the level of pDC. Restricted cubic splines modeling of hazard ratio (HR) for OS as function of pDC level in all population. The gray area around the red line represents the 95% confidence interval. NR not reached
In this cohort, there were 31 patients in stages I/II, 38 in stage III; among them, 22 were IIIA, and 16 patients in stage IIIB and 57 patients had metastatic disease (stage IV) (Supplementary Table 1). A low frequency of pDC was observed in the blood of metastatic stage IV patients compared to stage III and stages I-II (8.3%, 11% and 10.7%, respectively, P = 0.048). This inverse correlation with the disease stage was not observed with cDC1 cells (Table 1).
Table 1.
Level of circulating pDC and cDC1 according to main patients’ characteristics
| N | pDC | cDC1 | |||
|---|---|---|---|---|---|
| Mean | P | Mean | P | ||
| Overall population | |||||
| 126 | 9.85 | 0.92 | |||
| Age—years | |||||
| < 65 | 72 | 10.49 | 0.293 | 1.04 | 0.0612 |
| ≥ 65 | 54 | 8.99 | 0.75 | ||
| Sex | |||||
| Men | 82 | 9.84 | 0.984 | 0.98 | 0.319 |
| Women | 44 | 9.87 | 0.81 | ||
| Histologic subtype | |||||
| Adenocarcinoma | 65 | 9.2 | 0.9 | ||
| Squamous cell carcinoma | 29 | 10.17 | 0.586 | 0.8 | 0.545 |
| Other | 9 | 9.09 | 0.67 | ||
| Stage | |||||
| I–II | 31 | 10.7 | 0.94 | ||
| IIIA–B | 38 | 11.4 | 0.048 | 0.84 | 0.98 |
| IV | 57 | 8.35 | 0.95 | ||
Next, we investigated the prognostic value according to the levels of DC subsets in NSCLC patients with 10-year follow-up. Regardless pDC level, the median overall survival (OS) of the overall population was 22 months, not reached in stages I–II, 32 months in stages III and 16 months in metastatic stages (not shown). In overall population, patients who exhibited high level of pDC (pDChigh) had better OS compared with patients with pDClow (30.4 vs 20.7 months, P = 0.013) (Fig. 1d). Most patients (76%, 23/30) in stage I/II had pDChigh profile, and it was the same trend in stage III (73% of pDChigh 28/38). We found that the clinical benefit associated with pDChigh status was preserved in both groups although the statistical significance was not reached in stage I/II due to the small size of this population (Fig. 1e,f). Hence, the median OS was not reached versus 50 months in pDChigh and pDClow group, respectively (P = 0.13) (Fig. 1e). In the stage III, pDChigh patients had median OS of 36.5 months compared to 18.6 months in pDClow (P = 0.02) (Fig. 1f). Compared to stages I to III, this association was not found in the metastatic stage IV patients (Supplementary Fig. 2). Accordingly, the restricted cubic spline (RCS) analysis showed that patients with pDChigh displayed a low risk of death (Fig. 1h). The multivariate Cox analysis showed that pDChigh and stages were independent prognostic factor for OS in NSCLC patients (Supplementary Table 2). In contrast to pDC subset, no association between the circulating level of cDC1 and survival outcome was found (Supplementary Fig. 3).
pDChigh is related to a Th1-polarized cytokines environment
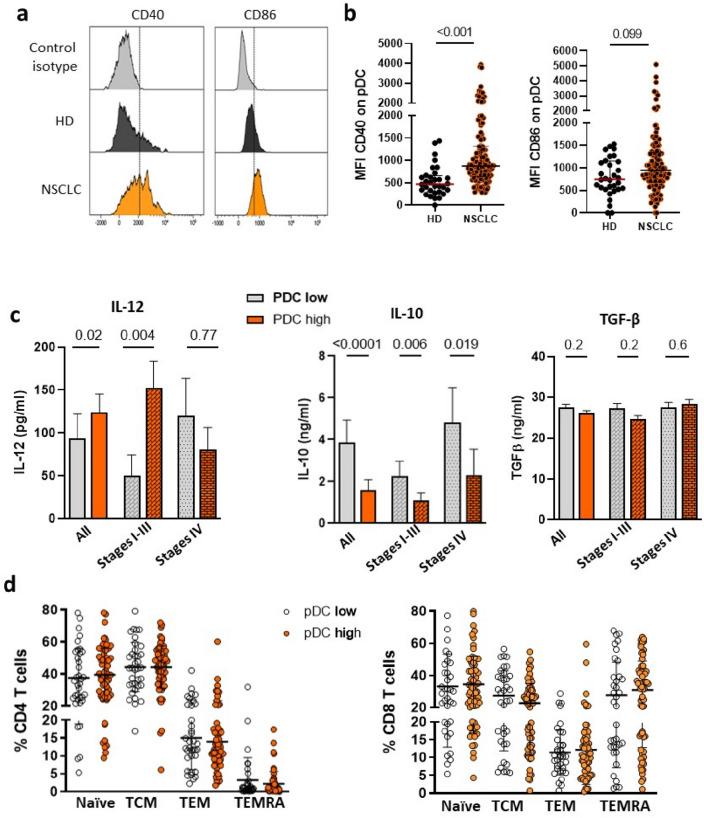
Next, we addressed the immune environment associated with pDChigh in blood. First, we analyzed the phenotype of pDC using the activation markers such as CD40 and CD86/B7-2. The mean of flurorescence (MFI) of CD40 and CD86 was significantly higher on NSCLC patients’ pDC compared to HDs’ one. Moreover, NSCLC patients showed high level of activated CD40/CD86+ pDC (Fig. 2a, b). Similar trend was observed in patients’ cDC1 (Supplementary Fig. 4 a, b).
Fig. 2.
Association between circulating pDC, cytokine environment and T cell differentiation in peripheral blood of NSCLC. a Representative histograms of mean fluorescence intensity (MFI) of CD40 and CD86 on pDC from one HD and one patient. b MFI distribution of CD40 (left) and CD86 (right) on pDC from 30 HD and 126 NSCLC. (Median and interquartile range are indicated, Mann–Whitney test.) c Concentrations of IL-12, IL-10 and TGF-β in all population, in stages I–III and stages IV NSCLC exhibited high or low levels of pDC (Mann–Whitney test). d Circulating percentage of CD4 and CD8 T cell subsets (naive, central memory (CM), effector memory (EM), TEMRA) according to the level of pDC in the blood of NSCLC
pDCs produce various cytokines involve in the cross talk between immune cells [31]. Therefore, we measured cytokines such as IFN alpha, IL-12, IL-10 and TGF-β in the sera of pDChigh and pDClow patients. Although IFN alpha was not detectable in the sera of patients, pDChigh group showed increase level of IL-12 especially in localized patients (P = 0.004) and diminution of IL-10 regardless of the disease staging. In contrast, TGF-β concentrations were similar between the two groups (Fig. 2c). No obvious association was observed with cDC1 level and these cytokines (Supplementary Fig. 4c). Furthermore, there was no association between pDC level and the subsets of naive, central memory (CM), effector memory (EM) or TEMRA CD4 and CD8 T cells (Fig. 2d). Thus, circulating pDC exhibited activated phenotype in NSCLC and pDChigh profile was associated with a Th1-polarized cytokine milieu.
Increase of circulating tumor-specific T cell responses is associated with pDChigh profile
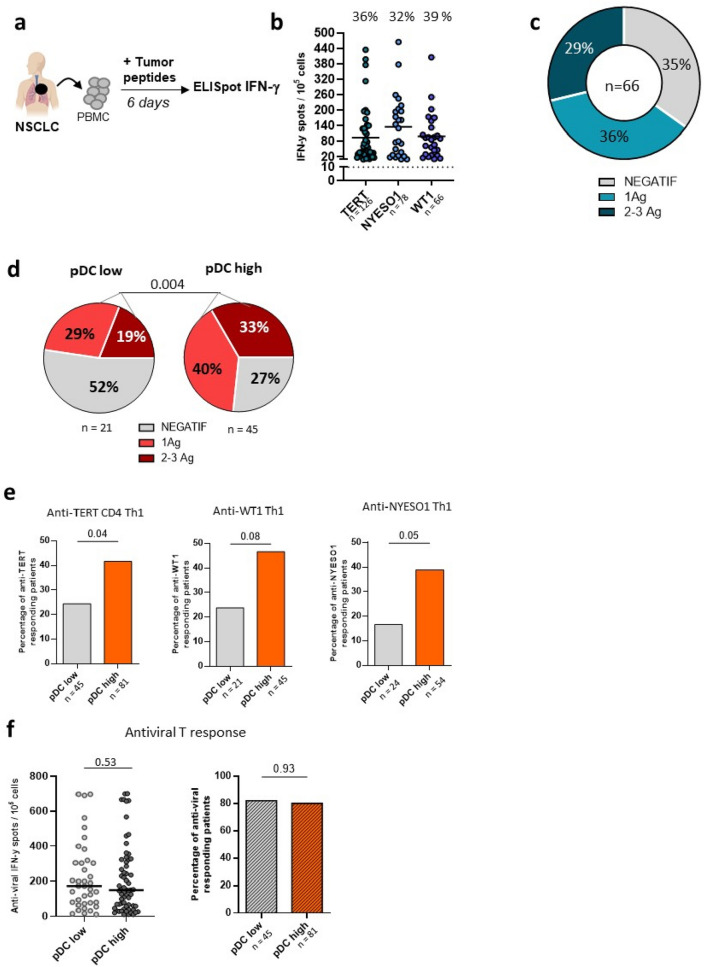
Based on above results, we assumed that pDChigh could be involved in priming of antigen specific T cells. To test this assumption, we evaluated T cell reactivity against three tumor antigens such as TERT, NYESO1 and WT-1 by IFN-γ ELISpot assay (Fig. 3a). The proportion of patients displaying spontaneous T-cell responses against TERT, NYESO1 or WT-1 was around 35% (Fig. 3b, c). The antitumor T cell responses were more prevalent in pDChigh than pDClow (73.3 vs 47.6%, P = 0.004) (Fig. 3d). The frequency of immune responder to two or three antigens was 33% in pDChigh compared to 19% in pDClow (Fig. 3d) and most patients (8/9) who had T cells against all the three tumor antigens belonged in pDChigh group (not shown). This observation was confirmed when considering each tumor antigen individually (Fig. 3e). In contrast, no relationship was observed with the antiviral T cell responses (CMV, EBV, influenzae) (Fig. 3f). The level of peripheral cDC1 was not related to antitumor response in this cohort (Supplementary Fig. 5).
Fig. 3.
Correlation between the level of circulating pDC and antitumor T cell responses in NSCLC. a T-cell responses against TERT, NYESO1 and WT1 were measured in NSCLC by IFN-y ELISpot. b Number of IFN-γ producing T cells against TERT, NYESO1 and WT-1 in NSCLC patients, frequencies of responders patients for each TAA in the top. c Distribution of anti-TAA responders according to T cell responses against 0, 1 or > 2 TAA in patients (n = 66). d Distribution of anti-TAA responders patients according to pDC low (n = 21) versus pDChigh (n = 45) patients. e Frequencies of anti-TERT, anti-WT1 and anti-NYESO1 responders according to patients exhibited high or low pDC level. f Association between pDC level and antiviral T cell responses measured by IFN-γ ELISpot. Intensities of T-cell responses (in left) and frequencies of responders patients (in right) according to pDC low and pDChigh patients
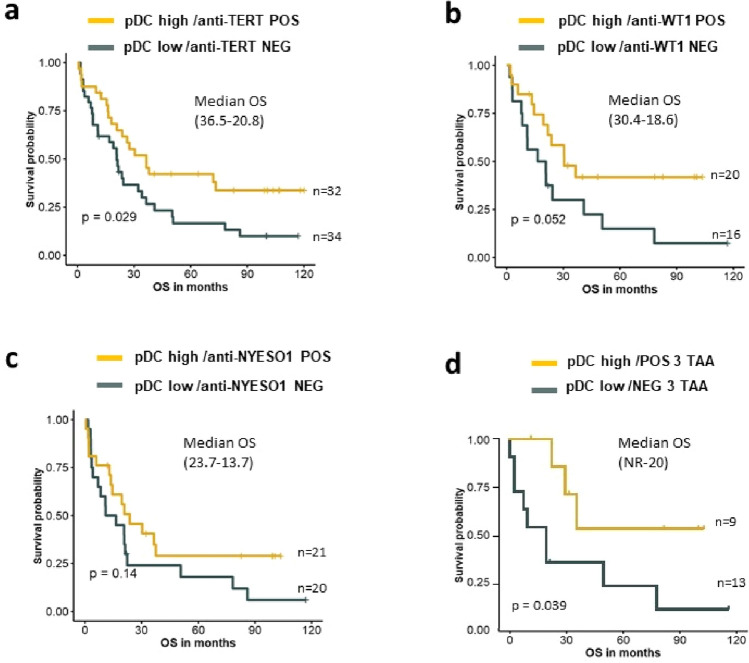
Based on these findings, we stratified patients according to the level of pDC and the antitumor T cell response. We found that patients exhibiting a pDChigh and immune responder (antitumor POS) profile had better OS than pDClow/immune non-responder (antitumor NEG). This clinical benefit was especially found in patients belonged to pDChigh/TERT and WT1 immune responders (Fig. 4a–c).
Fig. 4.
Prognostic value of NSCLC patients according to circulating pDC level and antitumor T cells responses. a–d Kaplan–Meier curves according to pDC level and anti-TERT responders or no (POS or NEG) (a), anti-WT1 responders or no (POS or NEG) (b), anti-NYESO1 responders or no (POS or NEG) (c), anti-TAA responders or no (POS or NEG) against the three TAA (d) (log-rank tests). Median OS in brackets, NR not reached
The better outcome was found in pDChigh/TERT+WT1 + NYESO1 immune responder group (10-year median OS not reached vs 20 months, P = 0.039) (Fig. 4d).
High pDC signature in the tumor was associated with improved survival to anti-PD-(L)1 therapy in NSCLC
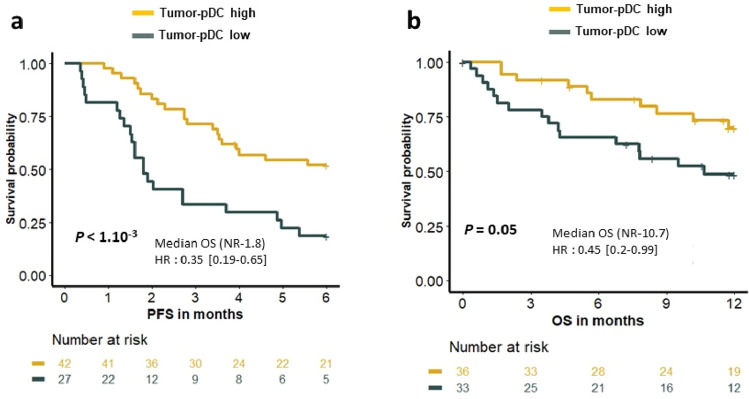
To investigate the role of pDC in the efficacy of immune checkpoint inhibitors (ICIs), we used tumor RNAseq data from a cohort of 89 patients treated with anti-PD1/PDL1 therapy [29]. Using a previously published pDC transcriptomic signature (CLEC4C, TCF4 and GZMB) [30], we showed that the 6-month progression-free survival (PFS) was not reached versus 1.8 months (P < 1.10–3) and the 12-month OS was not reached versus 10.7 months (P = 0.05) in pDChigh and pDClow, respectively (Fig. 5a, b). These results suggested that the level of pDC in the tumor could predict response to ICIs.
Fig. 5.
Association between tumor pDC signature and clinical outcome of NSCLC patients treated with anti-PD(L)-1. RNAseq was performed on tumor tissue from NSCLC patients (n = 89). Kaplan–Meier estimate PFS and OS of patients (log-rank tests)
Discussion
In this study, we showed that circulating level of pDC influences the long-term survival of patients with NSCLC by interacting with tumor-specific T cell responses. In overall population, the 5-year OS rate was around 38% in pDChigh and reached 82% in stages I–II, while it was three time lower in pDClow. These results are very interesting as the 5-year OS rate in NSCLC, all stages, is around 20% in word wild [32]. These findings are in line with previous studies reporting the clinical benefit associated with high level of pDC in peripheral blood in several cancers [14, 15, 17].
Although pDChigh appear to be an independent prognostic factor in NSCLC, no obvious correlation with the outcome at metastatic stage was observed. In line with the literature [14, 33], we found that metastatic patients have lower circulating pDC than early stages that mostly harbored pDChigh profile. The lower rate of pDC in metastatic stage could be explain by the accumulation of immunosuppressive cells such as regulatory T cell, myeloid-derived suppressive cells which can counteract the effect of pDC [34–37].
Searching an explanation for the clinical benefit associated with pDChigh, we found that there was a close relationship between these APC and tumor-reactive T cells naturally detected in peripheral blood. We found a positive correlation between the presence of poly-specific antitumor T cell responses and pDChigh status, suggesting that pDC may involve in the activation and persistence of these immune responses. In contrast, there was no relationship between pDChigh and virus-specific T cell responses measured at the same time, supporting our assumption. We also exclude the possibility that pDChigh group includes a greater number of the patients whose HLA alleles match to tumor-derived peptides as the prevalence of HLA class I expression such as HLA-A2 was similar in PDChigh and pDClow group, around 40% (not shown). Bailur et al. have previously reported a positive link between circulating pDC and the presence of HER-2-specific CD8 T cell response in breast cancer and showed their association with better clinical outcome [14].
Although we have not formally demonstrated a direct interaction between pDCs and T cell in patients, the ability of pDCs to prime tumor-specific T cells has been already demonstrated [38]. Besides, we did not found relationship between pDChigh and T cell differentiation phenotype (naive, effector and memory CD4/CD8 T cells), suggesting that pDCs were mostly involved in T cell function and Th1 polarization, according to the main role of pDC in cancer immunity [10]. Indeed, these cells exhibited activated phenotype with the expression of costimulatory molecules such as CD86 and CD40 were associated with Th1-induced cytokine milieu IL-12 which are known to promote cell-mediated immune response [39]. These results are consistent with the described role of activated pDC on adaptive immunity by producing a large amount of type I IFNs and promote T cell activation and polarization, whereas immature pDCs predominantly induce T cell anergy [40–43].
In contrast to pDC, no association between systemic level of cDC1 and clinical outcome was found. Indeed, cDC1 subset is mostly involved in the cross-priming and preferentially act in the tissue (lymphoid organ and tumor microenvironment) instead of the blood [44, 45].
DCs constitute a rare immune cell population especially in the peripheral blood, and recently, their classification and characterization have been redefined [5, 46]. In our study, immune analysis of pDCs were performed before these new classifications, so that we did not include BDCA2 and BDCA4 markers. Nevertheless, the expression of the IL-3 receptor alpha chain CD123 remains a prototypic marker of pDC [5].
Recent evidence suggests the role of DC subset in the efficacy of immune checkpoint inhibitors (ICIs) [30, 47–49]. Because we had no blood samples from patients treated by ICIs for pDC monitoring, we investigated the relation between pDC and the efficacy of ICIs in the tumor and found that a high pDC signature in the tumor microenvironment could predict the response to ICIs in NSCLC. Nevertheless, the prognostic value associated with circulating pDC also deserves its future assessment as a dynamic biomarker in immuno-oncology.
In conclusion, high circulating level of pDC interplays with antitumor T cell immunity to improve long-term survival of patients with NSCLC.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank all patients who contributed to this study. We thank all of the medical doctors and nurses, from oncologic department of University Hospital of Besançon and European Georges Pompidou hospital in Paris, for their contributions. The authors also thank the Biomonitoring platform (EFS, CIC-1431) for their technical support.
Author contributions
OA was involved in conceptualization and acquired the funding; OA, BG, PS and CL were responsible for methodology; CL, EG, AR, LB and MM acquired the data; CL, ES, AM, BL and OA carried out formal analysis and investigation; JL, CT, FG conducted RNA-Seq analysis. CL and OA wrote and prepared the original draft; FG, CL and OA wrote, reviewed and edited the manuscript; OA contributed to supervision.
Funding
This work was supported by grants from, La Ligue Contre le Cancer Grand Est 2020, the Conseil Regional de Franche-Comte, INCa-PLBio-2018.
Declarations
Conflict of interest
The authors have no potential conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hiam-Galvez KJ, Allen BM, Spitzer MH. Systemic immunity in cancer. Nat Rev Cancer. 2021;21:345–359. doi: 10.1038/s41568-021-00347-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Spitzer MH, Carmi Y, Reticker-Flynn NE, Kwek SS, Madhireddy D, Martins MM, et al. Systemic immunity is required for effective cancer immunotherapy. Cell. 2017;168:487–502.e15. doi: 10.1016/j.cell.2016.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Binnewies M, Mujal AM, Pollack JL, Combes AJ, Hardison EA, Barry KC, et al. Unleashing type-2 dendritic cells to drive protective antitumor CD4+ T cell immunity. Cell. 2019;177:556–571.e16. doi: 10.1016/j.cell.2019.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cancel J-C, Crozat K, Dalod M, Mattiuz R. Are conventional type 1 dendritic cells critical for protective antitumor immunity and how? Front Immunol. 2019;10:9. doi: 10.3389/fimmu.2019.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Villani A-C, Satija R, Reynolds G, Sarkizova S, Shekhar K, Fletcher J, et al. Single-cell RNA-seq reveals new types of human blood dendritic cells, monocytes, and progenitors. Science. 2017;356:eaah4573. doi: 10.1126/science.aah4573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lande R, Gilliet M. Plasmacytoid dendritic cells: key players in the initiation and regulation of immune responses. Ann N Y Acad Sci. 2010;1183:89–103. doi: 10.1111/j.1749-6632.2009.05152.x. [DOI] [PubMed] [Google Scholar]
- 7.Swiecki M, Colonna M. The multifaceted biology of plasmacytoid dendritic cells. Nat Rev Immunol. 2015;15:471–485. doi: 10.1038/nri3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tel J, Aarntzen EHJG, Baba T, Schreibelt G, Schulte BM, Benitez-Ribas D, et al. Natural human plasmacytoid dendritic cells induce antigen-specific T-cell responses in melanoma patients. Cancer Res. 2013;73:1063–1075. doi: 10.1158/0008-5472.CAN-12-2583. [DOI] [PubMed] [Google Scholar]
- 9.Bol KF, Schreibelt G, Rabold K, Wculek SK, Schwarze JK, Dzionek A, et al. The clinical application of cancer immunotherapy based on naturally circulating dendritic cells. J Immunother Cancer. 2019;7:109. doi: 10.1186/s40425-019-0580-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mitchell D, Chintala S, Dey M. Plasmacytoid dendritic cell in immunity and cancer. J Neuroimmunol. 2018;322:63–73. doi: 10.1016/j.jneuroim.2018.06.012. [DOI] [PubMed] [Google Scholar]
- 11.Conrad C, Gregorio J, Wang Y-H, Ito T, Meller S, Hanabuchi S, et al. Plasmacytoid dendritic cells promote immunosuppression in ovarian cancer via ICOS costimulation of Foxp3(+) T-regulatory cells. Cancer Res. 2012;72:5240–5249. doi: 10.1158/0008-5472.CAN-12-2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wculek SK, Cueto FJ, Mujal AM, Melero I, Krummel MF, Sancho D. Dendritic cells in cancer immunology and immunotherapy. Nat Rev Immunol. 2020;20:7–24. doi: 10.1038/s41577-019-0210-z. [DOI] [PubMed] [Google Scholar]
- 13.Nam J-H, Lee J-H, Choi S-Y, Jung N-C, Song J-Y, Seo H-G, et al. Functional ambivalence of dendritic cells: tolerogenicity and immunogenicity. Int J Mol Sci. 2021;22:4430. doi: 10.3390/ijms22094430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kini Bailur J, Gueckel B, Pawelec G. Prognostic impact of high levels of circulating plasmacytoid dendritic cells in breast cancer. J Transl Med. 2016;14:151. doi: 10.1186/s12967-016-0905-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chevolet I, Speeckaert R, Schreuer M, Neyns B, Krysko O, Bachert C, et al. Clinical significance of plasmacytoid dendritic cells and myeloid-derived suppressor cells in melanoma. J Transl Med. 2015;13:9. doi: 10.1186/s12967-014-0376-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee YS, Radford KJ. Chapter three—the role of dendritic cells in cancer. In: Lhuillier C, Galluzzi L, editors. Immunobiology of dendritic cells part A. New York: Academic Press; 2019. pp. 123–178. [Google Scholar]
- 17.Sosa Cuevas E, Ouaguia L, Mouret S, Charles J, De Fraipont F, Manches O, et al. BDCA1+ cDC2s, BDCA2+ pDCs and BDCA3+ cDC1s reveal distinct pathophysiologic features and impact on clinical outcomes in melanoma patients. Clin Transl Immunol. 2020;9:e1190. doi: 10.1002/cti2.1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laheurte C, Dosset M, Vernerey D, Boullerot L, Gaugler B, Gravelin E, et al. Distinct prognostic value of circulating anti-telomerase CD4+ Th1 immunity and exhausted PD-1+/TIM-3+ T cells in lung cancer. Br J Cancer. 2019;121:405–416. doi: 10.1038/s41416-019-0531-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nardin C, Laheurte C, Puzenat E, Boullerot L, Ramseyer M, Marguier A, et al. Naturally occurring telomerase-specific CD4 T-cell immunity in melanoma. J Investig Dermatol. 2021;142:435–444. doi: 10.1016/j.jid.2021.07.160. [DOI] [PubMed] [Google Scholar]
- 20.Kim S, François E, André T, Samalin E, Jary M, El Hajbi F, et al. Docetaxel, cisplatin, and fluorouracil chemotherapy for metastatic or unresectable locally recurrent anal squamous cell carcinoma (Epitopes-HPV02): a multicentre, single-arm, phase 2 study. Lancet Oncol. 2018;19:1094–1106. doi: 10.1016/S1470-2045(18)30321-8. [DOI] [PubMed] [Google Scholar]
- 21.Safi S, Yamauchi Y, Rathinasamy A, Stamova S, Eichhorn M, Warth A, et al. Functional T cells targeting tumor-associated antigens are predictive for recurrence-free survival of patients with radically operated non-small cell lung cancer. Oncoimmunology. 2017;6:e1360458. doi: 10.1080/2162402X.2017.1360458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tatsumi T, Kierstead LS, Ranieri E, Gesualdo L, Schena FP, Finke JH, et al. Disease-associated bias in T helper type 1 (Th1)/Th2 CD4(+) T cell responses against MAGE-6 in HLA-DRB10401(+) patients with renal cell carcinoma or melanoma. J Exp Med. 2002;196:619–628. doi: 10.1084/jem.20012142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peng S, Trimble C, Wu L, Pardoll D, Roden R, Hung C-F, et al. HLA-DQB1*02-restricted HPV-16 E7 peptide-specific CD4+ T-cell immune responses correlate with regression of HPV-16-associated high-grade squamous intraepithelial lesions. Clin Cancer Res 2007;13:2479–2487. doi: 10.1158/1078-0432.CCR-06-2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dhodapkar KM, Gettinger SN, Das R, Zebroski H, Dhodapkar MV. SOX2-specific adaptive immunity and response to immunotherapy in non-small cell lung cancer. Oncoimmunology. 2013;2:e25205. doi: 10.4161/onci.25205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Janssen N, Fortis SP, Speigl L, Haritos C, Sotiriadou NN, Sofopoulos M, et al. Peripheral T cell responses to tumour antigens are associated with molecular, immunogenetic and cellular features of breast cancer patients. Breast Cancer Res Treat. 2017;161:51–62. doi: 10.1007/s10549-016-4037-z. [DOI] [PubMed] [Google Scholar]
- 26.Godet Y, Fabre E, Dosset M, Lamuraglia M, Levionnois E, Ravel P, et al. Analysis of spontaneous tumor-specific CD4 T-cell immunity in lung cancer using promiscuous HLA-DR telomerase-derived epitopes: potential synergistic effect with chemotherapy response. Clin Cancer Res. 2012;18:2943–2953. doi: 10.1158/1078-0432.CCR-11-3185. [DOI] [PubMed] [Google Scholar]
- 27.Laheurte C, Galaine J, Beziaud L, Dosset M, Kerzerho J, Jacquemard C, et al. Immunoprevalence and magnitude of HLA-DP4 versus HLA-DR-restricted spontaneous CD4(+) Th1 responses against telomerase in cancer patients. Oncoimmunology. 2016;5:e1137416. doi: 10.1080/2162402X.2015.1137416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moodie Z, Price L, Gouttefangeas C, Mander A, Janetzki S, Löwer M, et al. Response definition criteria for ELISPOT assays revisited. Cancer Immunol Immunother CII. 2010;59:1489–1501. doi: 10.1007/s00262-010-0875-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lecuelle J, Favier L, Fraisse C, Lagrange A, Kaderbhai C, Boidot R, et al. MER4 endogenous retrovirus correlated with better efficacy of anti-PD1/PD-L1 therapy in non-small cell lung cancer. J Immunother Cancer. 2022;10:e004241. doi: 10.1136/jitc-2021-004241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Poropatich K, Dominguez D, Chan W-C, Andrade J, Zha Y, Wray B, et al. OX40+ plasmacytoid dendritic cells in the tumor microenvironment promote antitumor immunity. J Clin Investig. 2020;130:3528–3542. doi: 10.1172/JCI131992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Siegal FP, Kadowaki N, Shodell M, Fitzgerald-Bocarsly PA, Shah K, Ho S, et al. The nature of the principal type 1 interferon-producing cells in human blood. Science. 1999;284:1835–1837. doi: 10.1126/science.284.5421.1835. [DOI] [PubMed] [Google Scholar]
- 32.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 33.Failli A, Legitimo A, Orsini G, Romanini A, Consolini R. Numerical defect of circulating dendritic cell subsets and defective dendritic cell generation from monocytes of patients with advanced melanoma. Cancer Lett. 2013;337:184–192. doi: 10.1016/j.canlet.2013.05.013. [DOI] [PubMed] [Google Scholar]
- 34.Lauret Marie Joseph E, Laheurte C, Jary M, Boullerot L, Asgarov K, Gravelin E, et al. Immunoregulation and clinical implications of ANGPT2/TIE2+ M-MDSC signature in non-small cell lung cancer. Cancer Immunol Res. 2020;8:268–279. doi: 10.1158/2326-6066.CIR-19-0326. [DOI] [PubMed] [Google Scholar]
- 35.Charles J, Di Domizio J, Salameire D, Bendriss-Vermare N, Aspord C, Muhammad R, et al. Characterization of circulating dendritic cells in melanoma: role of CCR6 in plasmacytoid dendritic cell recruitment to the tumor. J Investig Dermatol. 2010;130:1646–1656. doi: 10.1038/jid.2010.24. [DOI] [PubMed] [Google Scholar]
- 36.Lenahan C, Avigan D. Dendritic cell defects in patients with cancer: mechanisms and significance. Breast Cancer Res BCR. 2006;8:101. doi: 10.1186/bcr1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beckebaum S, Zhang X, Chen X, Yu Z, Frilling A, Dworacki G, et al. Increased levels of interleukin-10 in serum from patients with hepatocellular carcinoma correlate with profound numerical deficiencies and immature phenotype of circulating dendritic cell subsets. Clin Cancer Res. 2004;10:7260–7269. doi: 10.1158/1078-0432.CCR-04-0872. [DOI] [PubMed] [Google Scholar]
- 38.Salio M, Cella M, Vermi W, Facchetti F, Palmowski MJ, Smith CL, et al. Plasmacytoid dendritic cells prime IFN-gamma-secreting melanoma-specific CD8 lymphocytes and are found in primary melanoma lesions. Eur J Immunol. 2003;33:1052–1062. doi: 10.1002/eji.200323676. [DOI] [PubMed] [Google Scholar]
- 39.Gardner A, Ruffell B. Dendritic cells and cancer immunity. Trends Immunol. 2016;37:855–865. doi: 10.1016/j.it.2016.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kadowaki N, Antonenko S, Lau JY, Liu YJ. Natural interferon alpha/beta-producing cells link innate and adaptive immunity. J Exp Med. 2000;192:219–226. doi: 10.1084/jem.192.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Noubade R, Majri-Morrison S, Tarbell KV. Beyond cDC1: emerging roles of DC crosstalk in cancer immunity. Front Immunol. 2019;10:1014. doi: 10.3389/fimmu.2019.01014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Faith A, Peek E, McDonald J, Urry Z, Richards DF, Tan C, et al. Plasmacytoid dendritic cells from human lung cancer draining lymph nodes induce Tc1 responses. Am J Respir Cell Mol Biol. 2007;36:360–367. doi: 10.1165/rcmb.2006-0284OC. [DOI] [PubMed] [Google Scholar]
- 43.Mathan TSMM, Figdor CG, Buschow SI. Human plasmacytoid dendritic cells: from molecules to intercellular communication network. Front Immunol. 2013;4:372. doi: 10.3389/fimmu.2013.00372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marciscano AE, Anandasabapathy N. The role of dendritic cells in cancer and anti-tumor immunity. Semin Immunol. 2021;52:101481. doi: 10.1016/j.smim.2021.101481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang S, Chopin M, Nutt SL. Type 1 conventional dendritic cells: ontogeny, function, and emerging roles in cancer immunotherapy. Trends Immunol. 2021;42:1113–1127. doi: 10.1016/j.it.2021.10.004. [DOI] [PubMed] [Google Scholar]
- 46.Eisenbarth SC. Dendritic cell subsets in T cell programming: location dictates function. Nat Rev Immunol. 2019;19:89–103. doi: 10.1038/s41577-018-0088-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mayoux M, Roller A, Pulko V, Sammicheli S, Chen S, Sum E, et al. Dendritic cells dictate responses to PD-L1 blockade cancer immunotherapy. Sci Transl Med. 2020;12:eaav7431. doi: 10.1126/scitranslmed.aav7431. [DOI] [PubMed] [Google Scholar]
- 48.Oshi M, Newman S, Tokumaru Y, Yan L, Matsuyama R, Kalinski P, et al. Plasmacytoid dendritic cell (pDC) infiltration correlate with tumor infiltrating lymphocytes, cancer immunity, and better survival in triple negative breast cancer (TNBC) more strongly than conventional dendritic cell (cDC) Cancers. 2020;12:E3342. doi: 10.3390/cancers12113342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kießler M, Plesca I, Sommer U, Wehner R, Wilczkowski F, Müller L, et al. Tumor-infiltrating plasmacytoid dendritic cells are associated with survival in human colon cancer. J Immunother Cancer. 2021;9:e001813. doi: 10.1136/jitc-2020-001813. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.